Abstract
The endothelial glycocalyx has a profound influence at the vascular wall on the transmission of shear stress, on the maintenance of a selective permeability barrier and a low hydraulic conductivity, and on attenuating firm adhesion of blood leukocytes and platelets. Major constituents of the glycocalyx, including syndecans, heparan sulphates and hyaluronan, are shed from the endothelial surface under various acute and chronic clinical conditions, the best characterized being ischaemia and hypoxia, sepsis and inflammation, atherosclerosis, diabetes, renal disease and haemorrhagic viral infections. Damage has also been detected by in vivo microscopic techniques. Matrix metalloproteases may shed syndecans and heparanase, released from activated mast cells, cleaves heparan sulphates from core proteins. According to new data, not only hyaluronidase but also the serine proteases thrombin, elastase, proteinase 3 and plasminogen, as well as cathepsin B lead to loss of hyaluronan from the endothelial surface layer, suggesting a wide array of potentially destructive conditions. Appropriately, pharmacological agents such as inhibitors of inflammation, antithrombin and inhibitors of metalloproteases display potential to attenuate shedding of the glycocalyx in various experimental models. Also, plasma components, especially albumin, stabilize the glycocalyx and contribute to the endothelial surface layer. Though symptoms of the above listed diseases and conditions correlate with sequelae expected from disturbance of the endothelial glycocalyx (oedema, inflammation, leukocyte and platelet adhesion, low reflow), therapeutic studies to prove a causal connection have yet to be designed. With respect to studies on humans, some clinical evidence exists for benefits from application of sulodexide, a preparation delivering precursors of the glycocalyx constituent heparan sulphate. At present, the simplest option for protecting the glycocalyx seems to be to ensure an adequate level of albumin. However, also in this case, definite proof of causality needs to be delivered.
Keywords: diabetes, inflammation, ischaemia, protease, renal failure, sepsis
Introduction
There is no longer doubt about the existence of a glycocalyx (Figure1) or, more appropriately in vivo, the presence of an endothelial surface layer (ESL) on healthy human vascular endothelial cells [1–7]. This surface layer is comprised of the ‘classical’ glycocalyx constituents, mainly heparan and chondroitin sulphate glycosaminoglycan chains covalently linked to the syndecans (transmembrane core proteins) and glypicans, and of hyaluronic acid attached via cell membrane receptors (CD44), between which plasma constituents intercalate. The exact composition and thickness of the ESL (0.2–2 µm) vary, depending on vessel type (Figure2), flow shear rate and the vascular bed [6]. However, some of the physiological functions are still under debate. Most agreement is to be found concerning the role of the glycocalyx as a transducer of shear stress from flowing blood into intracellular vascular signals [5,8,9]. Action as a component of the vascular barrier is also relatively well established [10–13], while the undeniable potential to inhibit or at least to attenuate firm adhesion of leukocytes and platelets to the vessel wall under physiological conditions is not yet generally appreciated [14–19]. Greatest uncertainty applies to whether binding of hormones, cytokines, chemokines and other kinds of signalling molecules to the glycocalyx sequesters or enhances activity of these substances [20–25]. This function will probably turn out to be ‘case sensitive’. An involvement of the glycocalyx in defining the pharmacokinetics and pharmacodynamics of pharmaceutical drugs has, to our knowledge, never been seriously investigated. Likewise, attempts specifically to protect the glycocalyx from damage (see below) or to restore its function in pathological situations are virtually absent in clinical studies [2].
Figure 1.
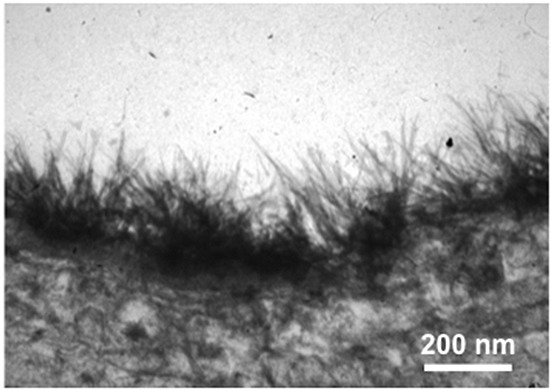
Electron microscopic view of a human mammary artery (end piece not utilized for coronary bypass grafting) after fixation with lanthanium(III)nitrate/glutaraldehyde solution. The thickness of the glycocalyx (dark fibrous zone) is approximately 200 nm. For details, see [7,28]
Figure 2.
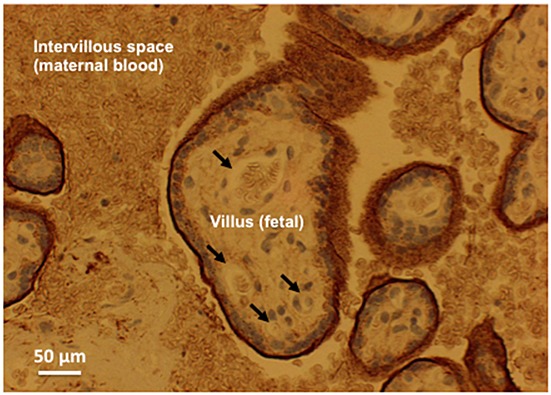
Light-microscopic picture of a slice of human placenta stained with antibody against syndecan-1 (brown stain). There is intense colouration at the villous surface, i.e. the interface between maternal blood and fetal tissue, but none in the fetal capillaries. For details, see [44]
Several pathophysiological processes have indeed been associated with structural and functional derangement of the glycocalyx. The largest bodies of evidence for a link relate to post-ischaemic organ damage [26–28], sepsis and inflammation [28–34], renal disease [32,33,35], diabetic vasculopathy [36–38] and atherosclerosis [39,40]. Symptoms such as hypertension, haemolytic uraemic syndrome and of some viral infections may also relate to deterioration of the endothelial glycocalyx, since all are accompanied by shedding of one or more of heparan sulphate, syndecans and hyaluronan, all major constituents of the glycocalyx, into blood and urine [41]. It is worth mentioning here that, in the case of syndecans, it is generally syndecan-1 (sdc1) that has been measured in studies on humans. There are four types of syndecan (numbered 1 through 4), but sdc1 seems the most prevalent type at the luminal endothelial surface. Although it can be replaced by other proteoglycans in sdc1(-/-) mice to form a hydrodynamically relevant glycocalyx [42], it is loss of sdc1 that induces a proinflammatory endothelial phenotype [43].
A relatively recent finding is that pregnancy, normal and even more so in the HELLP syndrome, displayed pronounced elevation of shedding, compatible with restructuring during gestation of the very dense glycocalyx found on the villous surface (Figure2), the interface of maternal blood and fetal/embryonal tissue in the placenta [44–47]. However, shedding of glycocalyx components into blood obviously does not prove a causal role of degradation for generating some of the clinical symptoms of the respective disease. The relationship may be incidental, merely parallel or a side effect of the underlying disease. One thing is certain though, deterioration of the endothelial glycocalyx will exacerbate phenomena of inflammation and disturbed microcirculatory physiology. Key consequences of the loss of the glycocalyx will be enhanced oedema, facilitated adhesion of leukocytes and blood platelets, and loss of flow-dependent vasodilatation [2,27]. Such complications may explain why a clinical study in which heparinase was given to patients undergoing coronary bypass heart surgery provided some negative outcomes and had to be terminated ahead of time [48].
Another general consequence of shedding of the endothelial glycocalyx will be that biologically active constituents and compounds bound in the ESL will also disappear from the immediate vicinity of the luminal vascular surface, with loss of local action and gain of systemic effects. Pertinent examples are xanthine oxidoreductase (XOR), lipoprotein lipase (LPL), tissue factor pathway inhibitor (TFPI), fibroblast growth factor (FGF2) and vascular endothelial growth factor (VEGF) [49–54]. On the one hand, loss of XOR at the endothelial surface limits local production of the important physiological antioxidant uric acid [55], displacement of LPL limits lipolysis of triglycerides, thereby reducing clearance of chylomicrons and VLDL and supply of free fatty acids to the parenchymal cells [53,54], and loss of TFPI allows initiation of blood coagulation by tissue factor at the vessel wall [3]. On the other hand, systemic effects, notably those of FGF2 and VEGF will be enhanced [54].
Clinical conditions with signs of glycocalyx degradation
Organ or whole body ischaemia followed by reperfusion, as found in cardiopulmonary bypass, repair of aortic aneurysms and deep hypothermic cardiac arrest, consistently give rise to elevated levels of sdc1 and heparan sulphate in blood [26]. This is true not only for adults, but also for infants [56]. The post-cardiac arrest syndrome is also associated with increased plasma syndecan, heparan sulphate and hyaluronan [57]. Such phenomena presumably reflect shedding of the endothelial glycocalyx and may account for the development of oedema and exacerbated leukocyte and platelet adhesion in reperfused tissue, i.e. contribute to reperfusion damage [58–61]. Interestingly, hypoxia/reoxygenation causes a similar, marked deterioration of the glycocalyx in experimental models [62]. Mediators of ischaemia- and hypoxia-induced shedding could be the nucleosides adenosine and inosine, both produced in large amounts by degradation of the high energy adenine nucleotides (ATP, ADP) under oxygen deprivation. Both are stimulants of the adenosine type-3 receptors found on human mast cells [63,64]. At least those resident mast cells found in the human myocardium contain granular stores of the enzyme heparanase, release of which into the extracellular space will cause cleavage of heparan sulphate from the endothelial glycocalyx [27,65]. Mast cells also contain a vast assortment of proteases, cytokines and chemokines, potential inducers of syndecan and hyaluronan shedding [28,62,64,66]. Blockade of adenosine A-3 receptors could, thus, be an option to inhibit post-ischaemic shedding of the glycocalyx. Also, mast cell stabilizers such as chromoglycate, ketotifen and indomethacin could prove beneficial in these settings [66]. In any case, application of adenosine during coronary reperfusion to patients with acute myocardial infarction failed to produce long term benefits in survival [67,68].
Increases in plasma sdc1, heparan sulphate and hyaluronan occur after coronary artery bypass grafting (CABG) during myocardial reperfusion [69–71]. Here shedding of the glycocalyx should lead to loss of flow-dependent vasodilatation, i.e. contribute to the ‘no-reflow’ phenomenon in formally patent coronary arteries [8,72]. Surprisingly, elevations of sdc1 and heparan sulphate of similar magnitude as in conventional CABG occur also in the ‘off-pump’ coronary bypass procedure, performed expressly to largely avoid myocardial ischaemia [69,70]. Post-ischaemic stimuli of shedding such as adenosine and inosine should not be generated in this case. In cardiac surgery, however, there is inadvertent, strong mechanical manipulation of heart tissue, including the atria. This mechanical stress serves as an adequate stimulus for release of atrial natriuretic peptide (ANP), known from experiments on isolated heart preparations to induce rapid degradation of the endothelial glycocalyx [73]. Indeed, measurements of ANP in plasma of patients undergoing on- and off-pump CABG showed identical four- to five-fold increases in ANP concentrations under both protocols. Pertinently, these increases peaked before the onset of shedding and also preceded the rise of inflammatory cytokines such as IL-6 and IL-8 [70]. From experiments comparing A-, B- and C-type natriuretic peptide, all of which proved to be capable of inducing degradation of the glycocalyx, it appears that stimulation of the B-type natriuretic peptide receptor mediates the intracellular signalling cascade ultimately giving rise to shedding in this scenario [74]. Whether application of inhibitors of the B-type receptor (such as anantin) or of signal transduction from the natriuretic peptide receptors protects the glycocalyx and improves post-CABG recovery of patients (less oedema, better reflow, less demand of catecholamines, higher cardiac output), would be worth studying. Unfortunately, to date no such drug is approved for use in man [75,76]. On the other hand, the potential of natriuretic peptides to initiate destruction of the glycocalyx may well explain the unequivocal or poor long term results obtained in clinical studies aiming to improve heart function or to limit the consequences of myocardial infarction by applying recombinant human BNP (nesiritide) or ANP (carperitide) [68,77–79].
With respect to the above described actions of natriuretic peptides (NPs), an intriguing dilemma arises from the recent PARADIGM heart failure trial [80]. This trial tested the drug sacubitril, a dual inhibitor of the angiotensin II receptor and of the enzyme neutral endopeptidase (NEP), which is considered to play an important role in degrading natriuretic peptides. Sacubitril proved so beneficial in the treatment of patients with heart failure vs. the pure ACE inhibitor enalapril that the trial was stopped prematurely (after 27 months of follow-up). The problem with trying to judge the relevance of the PARADIGM trial results in relation to the acute action of NPs on the glycocalyx is three-fold. Sacubitril has, by design, a dual inhibitor function (angiotensin II receptor and NEP), and may not even be a selective inhibitor of NEP. Even if so, natriuretic peptides are not the only peptides influenced by NEP. Endothelin and bradykinin are just two of other potent vasoactive substrates that need to be considered. Second, the benefit to patients seen in the PARADIGM trial was never causally related to changes in NP concentrations. Third, we are comparing a long term effect in patients with heart failure with an acute action on the glycocalyx. Interestingly though, there was a higher incidence of angioedema in the sacubitril group, which would fit with enhancement of NPs.
Another clinical situation probably leading to disruption of the glycocalyx is that of volume loading (VL), common practice before elective surgery in many places world-over. VL with colloidal solutions was found to provide only limited expansion of intravascular fluid. After 30 min only about 40% of the applied colloid volume remained in the vascular space [81]. In contrast, acute normovolaemic haemodilution (ANH) with similar amounts of colloidal solution in place of removed blood was not associated with vascular leak of colloid or volume after 30 min [82]. VL, but not ANH, may be expected to increase wall stress of the cardiac atria, an adequate stimulus for release of ANP. ANP, in turn, is known to cause rapid extravasation of plasma in man [83]. We have since studied these two infusion regimes closely, comparing plasma concentrations of constituents of the glycocalyx and the simultaneous changes in plasma ANP. VL, but not ANH, was associated with elevation of ANP and shedding of sdc1 and hyaluronic acid [84]. The obvious recommendation, therefore, is to avoid volume loading as far as possible in favour of haemodilution to save blood for abetting any subsequent loss [84–86]. Interestingly, heparan sulphate concentrations were not increased in either protocol, a distinction to the changes noted in the study on CABG-linked release of ANP (see above). This means that there can be only little or no release of heparanase, thus suggesting an absence of major stimulation of mast cells in the course of correctly performed volume loading or normovolaemic haemodilution.
The renal glomerulus contains a specialized microvascular bed. One important difference is that plasma ultrafiltrate is not drained by a lymphatic system from an interstitial space, but serves as the vehicle ‘primary urine’ to excrete waste products from the plasma towards the urinary tract collection system. Filtration behaviour across the glomerular capillary wall is determined by its hydraulic conductivity, the reflection coefficient for plasma proteins, the pressure within glomerular capillaries and the urinary space, and the haemodynamics established by the tone of the afferent and efferent vessels. The blood–urine barrier anatomically consists of the fenestrated glomerular endothelium that is covered by a surface layer similar to what we know from the rest of the circulatory system, and an interdigitating network of processes partially covered by the overlying cell bodies of abluminal podocytes, with a shared basement membrane in between. In addition to the negative charges of this basement membrane [87], the glomerular endothelial glycocalyx makes an important contribution to the ‘perm-selective’ filtration properties of this barrier [88]. This is owed to the tightly packed negative charges and highly organized regular architecture of the glycocalyx components at the endothelial surface, and possibly also of the podocyte surface, of glomerular capillaries [89,90]. Removing these charges experimentally led to the appearance of increased concentrations of the normally retained albumin in the primary urine [91]. Albuminuria has therefore been implicated as clinical indicator of disturbance of the glomerular capillary glycocalyx [80], in the context of either direct glomerular injury or glomerular involvement in more generalized vascular dysfunction [33,92].
Renal disease in various forms presents with endothelial dysfunction and perturbation of the glycocalyx [32,33,35,93–95]. Dialysis patients exhibited reduced glycocalyx thickness and enhanced shedding [96]. The low dimensions of the endothelial surface layer found in end stage renal disease patients normalized after successful kidney transplantation, as did elevated plasma levels of sdc1 and the endothelial marker thrombomodulin [97]. This observation is further indication of the, presumably, tight association between shedding of the glycocalyx and endothelial dysfunction in general [36,98,99]. Such a link has been described also for trauma severity and traumatic coagulopathy in patients [100,101].
Haemorrhagic viral infections such as caused by the hanta, dengue and, probably, ebola viruses are presumably accompanied by destruction of the endothelial glycocalyx [102]. In the study of hanta virus infection, sdc1 was significantly and positively related to severity of disease and loss of albumin from plasma [103]. In Crimean-Congo haemorrhagic fever, disease severity was also linked to the level of glycocalyx degradation [104]. Interestingly, both hanta and dengue viral infection led to activation of matrix-metalloproteases [105,106], which could causally account for the shedding of syndecan (see below). Recently, the glycocalyx has been brought into consideration in conjunction with the haemolytic uraemic syndrome, the atypical form of which has been related to incorrect binding of complement factor H to heparan sulphate [41].
Perhaps the clearest association between glycocalyx destruction and disease exists for sepsis. The increase of sdc1 concentrations in plasma of individual patients was negatively correlated with survival with high significance [107–109]. Likewise, serum hyaluronan concentration was dependent on disease severity in critically ill patients, most of whom had sepsis [110]. In another study, treatment of human volunteers injected with endotoxin lipopolysaccharide with etanercept, an antibody against tumour necrosis factor-alpha (TNF-α), lowered shedding of glycocalyx constituents [111]. Experiments on isolated heart preparations have revealed massive destruction of the glycocalyx after application of TNF-α. This action was prevented by pretreatment of the hearts with either hydrocortisone or antithrombin [28].
Meta-analysis of 17 clinical studies assessing resuscitation of patients with sepsis gave the result that use of albumin-containing solutions lowered mortality compared with other fluid resuscitation regimens [112]. Pertinently, human albumin affords protection of the glycocalyx in experimental models and possesses high affinity for the glycocalyx [2,10]. For example, perfusion with albumin-containing solutions elicits far more shear-stress dependent vasodilatation and lowers fluid flux across the vessel wall much more than other types of colloids in clinical use (hydroxyethyl starches and gelatines) [8,113–115]. This exceptional ability for interaction probably arises from the fact that the albumin molecule carries a net negative charge in plasma at physiological pH, as opposed to the HES and gelatine molecules. As schematically illustrated in Figure3, one potential explanation is that in vivo the glycocalyx will be highly enriched with calcium ions, electrostatically shielding the great amount of negative heparan, chondroitin and dermatan sulphate groups carried by the syndecans and glypicans at the endothelial cell surface [5,27]. Needless to say, not only Ca2+ but also Na+, K+ and Mg2+ are attracted to the negatively charged glycocalyx. Indeed, the endothelial glycocalyx poses as a buffer for sodium ions which, in turn, stabilize the structure [116]. Irrespective of charge-shielding by Na+ ions, the divalent calcium ions possess the far higher charge density. For purely electrostatic reasons, therefore, particularly Ca2+ will be enriched in the double layer of positive charges covering the negative surface molecules and there, in turn, will attract albumin molecules and allow them to intercalate in the endothelial surface layer in preference to uncharged molecular species of colloid (Figure3).
Figure 3.
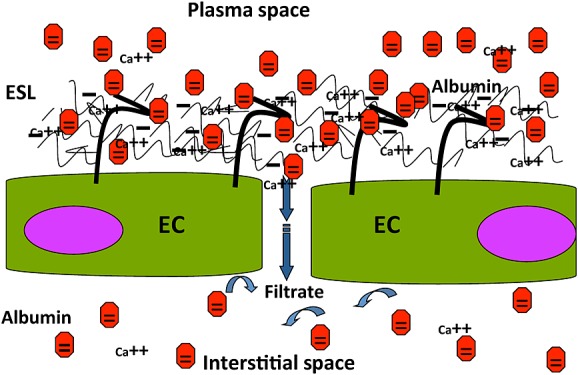
Schematic illustration of the endothelial surface layer in the presence of albumin (red dots), negatively charged at physiological plasma pH. The negative charges on heparan, chondroitin and dermatan sulphate glycosaminoglycan side chains of proteoglycans of the glycocalyx attract cations, especially the divalent Ca-ions, which shield the sulphate groups. Out of purely electrostatic considerations, this may provide an electrical double layer with a positively charged ‘outer’ face, to which albumin molecules will be attracted. Binding and enrichment of albumin in the glycocalyx results, a feature lacking for the artificial colloids in present day clinical use (all uncharged at physiological pH). For reasons of simplicity, Na+, K+ and Mg2+ ions are not included in the scheme
At this point is seems fitting to call to attention the great and ongoing controversy about which type of colloidal infusion solution should be used in volume therapy. Even the recent German Fluid Guidelines, which were consented by 14 National Societies,, were not able to put forward firm recommendations, owing to ‘contradictory results and methodological flaws’ in the trials conducted in septic patients [117]. From the sight of the endothelial glycocalyx and surface layer, the scales should tip in favour of albumin, especially in critically ill patients. In addition, albumin should better help the glycocalyx to maintain the important physiological interaction with the plasma electrolytes. There is evidence that elevation of plasma Na+ by aldosterone may lead to deterioration of the endothelial glycocalyx [116]. In this context, mineralcorticoids probably influence the stability and stiffness of the endothelial glycocalyx by affecting the plasma concentrations of Na+, Ca2+ and pH [118]. Here, again, there are no clinical studies enabling clear answers to be given regarding likely actions of mineralcorticoids or aldosterone antagonists on the endothelial glycocalyx of human patients, especially under conditions of hypo-albuminaemia.
The thickness of the endothelial surface layer in pulmonary vessels is considered to exceed that encountered in other vascular beds [119]. This indicates an important function as a barrier in lung vessels. Respiratory failure associated with sepsis is accompanied by higher heparanase activity in blood and lung tissue than in normal human biopsies and by elevated plasma heparan sulphate and hyaluronan levels [120,121]. In an experimental mouse model of sepsis, shedding of lung glycocalyx heparan sulphate was induced by lipopolysaccharide (LPS) via TNF-α. The authors presumed this occurred via activation of an endothelial heparanase. In any event, the ensuing pulmonary microvascular degradation of the glycocalyx promoted adhesion of neutrophilic granulocytes (PMN) [120]. This is an interesting finding, because PMN contain many proteolytic enzymes, including the serine proteases elastase and proteinase-3. Such enzymes could perhaps account for the cleavage of the proteinaceous cores of syndecans and the shedding of hyaluronan via proteolysis of the hyaluronan binding receptor CD 44 [5,122]. Heparanase cannot attack these molecules. To establish whether elastase is released from PMN attached to the vascular wall, we infused human PMN prestimulated with fMLP into the coronary system of guinea pig isolated hearts. This allowed us to stain with antibody against human elastase, thus avoiding any background interference. Figure4 shows an adherent human PMN in a coronary microvessel of a guinea pig heart. Immuno-histochemical examination revealed elastase, both in granula within the PMN and beginning to spread out from the PMN along the surface of the endothelial vessel lining, i.e. into the glycocalyx. It is important to relate this observation to the precautionary measure of removing leukocytes from red blood cell concentrates before transfusion. This present-day standard protocol was introduced out of necessity, pre-activated leukocytes sometimes initiating serious transfusion complications. One may postulate that destruction of the glycocalyx lay at the onset of such inflammatory complications [123].
Figure 4.
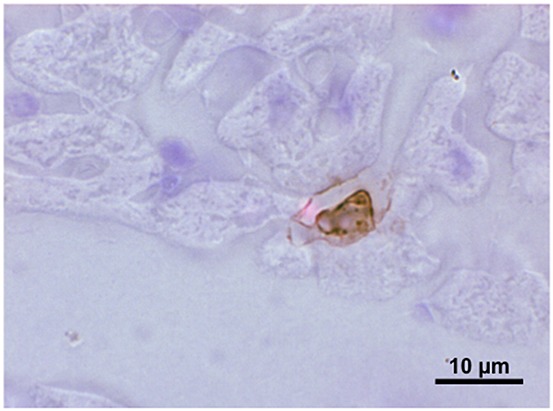
Light microscopic picture of a slice of guinea pig heart perfused with a bolus of 3 × 106 human polymorphonuclear neutrophilic granulocytes, prestimulated with formyl-Meth-Leu-Phe. The heart was fixation-perfused with formalin, sliced and stained with antibody against human elastase. The brown colouration identifies elastase in granules within a PMN attached to the wall of a small venule and beginning to spread out along the endothelial surface. For details, see [26,134]
Search for sheddases involved in cleaving glycocalyx components from the cell membrane
A therapeutic option to prevent degradation of the glycocalyx/endothelial surface layer may be to inhibit the enzymes responsible for shedding of individual components. Whereas heparanase and its bacterial counterpart heparinase are specific for cleaving heparan sulphate side chains from cell surface proteins such as syndecans and glypicans [39,65], and hyaluronidase is a prime candidate for removing hyaluronan from the glycocalyx [39,124], the precise identity of the proteases underlying cleavage of syndecans in humans under many pathological situations remains obscure. Only in the special case of tumour invasion and tumour metastasis is there good evidence for shedding of syndecans-1, -2 and -4 by matrix and membrane-type matrix metalloproteases [125,126]. Though several studies have shown that ortho-phenanthroline, doxycycline and batimastat, all rather non-selective inhibitors of metalloproteases, can attenuate syndecan and glycan shedding and glycocalyx destruction [74,127–130], there is some doubt as to whether matrix-metalloproteases are exclusively involved. According to some reports in the literature, thrombin, plasmin and elastase can induce shedding [131–133]. We have found enhanced release of the enzymes tryptase (a serine protease) and cathepsin B (a cystein protease) in the coronary venous effluent of post-ischaemic hearts, in parallel with deterioration of the glycocalyx [62,134]. These are, thus, also potential candidates as sheddases.
In an attempt to identify enzymes that may participate in shedding of constituents of the glycocalyx, especially of the syndecans, we devised a simple, straightforward test. A selection of candidate proteases was infused, singly, for 10 min into the coronary system of guinea pig isolated hearts and the coronary venous effluent collected for 20 min. This total effluent was quantified and subsequently analyzed (by immunoassay) for contents of three major components of the coronary endothelial glycocalyx, sdc1, heparan sulphate and hyaluronic acid [135]. The enzymes selected for these experiments were tryptase, elastase, proteinase-3, thrombin, tissue-type plasminogen activator, plasmin (all serine proteases), cathepsin B and hyaluronidase. The rationale behind this selection was that either there were reports in the literature about their activity as sheddases (thrombin, plasmin, elastase), or that activated mast cells, granulocytes or endothelial cells released them, i.e. they are involved in inflammatory reactions [136]. Also, experimental studies had revealed protection by antithrombin against shedding of the glycocalyx under ischaemic and inflammatory conditions [16,28,137]. Hyaluronidase actually served as a positive control, to make sure that our test system was indeed able to allow detection of shedding.
The results of these investigations are shown in Table 1. Rather unexpectedly, none of the tested enzymes caused significant elevation of coronary washout of sdc1. In fact, rates of release tended to fall in the presence of all infused enzymes vs. control hearts. Thus, none of the enzymes investigated here may be regarded as likely candidates for implementing shedding of syndecan. This finding contradicts the literature on thrombin, plasmin and elastase, where shedding was determined, albeit in other models [125,131–133]. However, it does shed an interesting light on the mechanism by which antithrombin III protects the glycocalyx against various forms of damage. The antithrombin molecule possesses, aside from its catalytic site, a heparin and heparan binding domain (D-helix), with which anti-inflammatory effects other than inhibiting serine proteases are elicited [138]. Indeed, simply by binding tightly to the heparan sulphate proteoglycans on the membrane it could hinder enzyme access and prevent degradation [2,137,139].
Table 1.
Coronary venous rates of release of glycocalyx constituents (ng min–1 g–1 heart tissue) induced by 10 min intracoronary infusion of various enzymes in guinea pig isolated perfused hearts. Values are means ± SD; for perfusion and analytical details, see [135]
| Enzyme | n | Syndecan-1 | Heparan sulphate | Hyaluronic acid |
|---|---|---|---|---|
| Control† | 9 | 11.7 ± 7.1 | 654 ± 254 (n = 8) | 7.4 ± 6.7 |
| Thrombin‡ | 8 | 5.2 ± 4.3 | 1022 ± 627 (n = 5) | 27.9 ± 32.0* |
| Tryptase§ | 11 | 4.2 ± 3.2 | 804 ± 327 (n = 5) | 19.0 ± 13.9 |
| Cathepsin B¶ | 12 | 4.3 ± 2.6 | 1556 ± 666 (n = 5)* | 30.4 ± 19.3* |
| Elastase** | 6 | 2.6 ± 2.8 | 1413 ± 70 (n = 3)* | 55.6 ± 39.3* |
| Proteinase 3†† | 3 | 2.8 ± 2.2 | (n = 0) | 27.1 ± 17.1 * |
| Plasmin‡‡ | 4 | 2.5 ± 1.3 | (n = 0) | 40.1 ± 16.5* |
| tPA§§ | 3 | 1.7 ± 0.9 | (n = 0) | 15.0 ± 10.3 |
| Hyaluronidase¶¶ | 4 | 2.4 ± 1.5 | (n = 0) | 1197.0 ± 524.7* |
†Control = time matched perfusion without exogenous enzyme, ‡ Thrombin from human plasma (Sigma,USA), 3.3-50U/heart. § Tryptase purified from human lung (Sigma, USA), 2–10 mU, ¶ Cathepsin B from bovine spleen (Sigma, USA), 1.1–5.4U, ** Elastase from human leukocytes (Sigma, USA), 0.33–0.67U, †† Proteinase 3 from human neutrophilic granulocytes (Biomol international, Hamburg,Germany), 0.47U, ‡‡ Plasmin from human plasma (Sigma, USA), 1.5U, §§ Tissue-type plasminogen activator, recombinant human, expressed in CHO cells (Sigma, USA), 1667U, ¶¶ Hyaluronidase from bovine testes (Sigma, USA), 5400U; n = number of hearts; * = P < 0.05 vs. control, ANOVA and unpaired t-test with Bonferroni correction.
Surprisingly, cathepsin B and elastase evoked slight elevations of heparan sulphate release (Table 1), although neither one of them is a heparinase or heparanase, i.e. capable of directly cleaving the glycosidic bond linking heparan sulphate side chains to protein core molecules of the glycocalyx. Piecemeal degradation of protein core molecules could explain this unexpected finding. Stimulation of resident myocardial mast cells to release heparanase represents another, indirect possibility for affecting glycocalyx degradation [139].
Massive release of hyaluronan was induced by hyaluronidase (160-fold rise vs. control), as to be expected. However, enhanced washout of hyaluronan also was induced by cathepsin B, thrombin, elastase, plasmin and proteinase-3, albeit to a lower extent (maximum seven-fold increase) than with hyaluronidase. Direct attack of these proteases on the polyglycosidic molecule of hyaluronan is impossible. On the other hand, hyaluronic acid is held at the endothelial cell surface by binding to the transmembrane receptor protein CD44 [5,122,140]. Proteolytic degradation of this molecule could explain the release of hyaluronan observed in the presence of cathepsin B, elastase, proteinase-3 and thrombin (Table 1).
Lacing of the long chain hyaluronan molecules into the endothelial glycocalyx lends elastic resilience and helps to maintain the protective layer above the adhesion molecules for leukocytes platelets and circulating tumor cells on the endothelial cell surface [140–142]. Removing just some of the hyaluronic acid by inflammatory proteases such as cathepsin B, thrombin, protease-3 and elastase may thin out the endothelial surface layer and, thus, permit a moderate rise in the adhesivity of blood constituents without requiring complete destruction of the glycocalyx. Modulation of the inflammatory response, i.e. avoidance of an all-or-nothing reaction, could rely on such a principle.
Protective measures
Protection of the endothelial glycocalyx of isolated perfused hearts against damage by ischaemic insult or TNF-α was afforded by pretreatment with hydrocortisone [16,28,135]. Antithrombin III also proved to be protective in these circumstances and in humans [57]. Both drugs are beneficial in clinical situations involving inflammation and ischaemia/reperfusion. Hydrocortisone stabilizes mast cells, inhibits leukocyte activation and down-regulates inflammatory cytokines. Antithrombin is a potent inhibitor of various serine proteases of the coagulation cascade, and also interacts with elastase [138]. However, shielding against enzymatic attack simply based on the strong binding of antithrombin to an intact endothelial glycocalyx could be an underlying explanation [2,137]. Along the same line of reasoning, stabilization of the glycocalyx by intercalation of albumin seems to be a valid clinical option to preserve the endothelial surface layer [2,114,143]. This local enrichment provides protection against redox attack (albumin contains an oxidizable sulphhydryl group) and excessive binding of leukocytes and platelets, limits fluid and colloid extravasation, and supports shear-stress dependent vasodilatation [8,114,129]. Sequestering vasoactive and inflammatory mediators such as endotoxins and LPS could also contribute to the beneficial action of albumin. Recently, the ability of albumin to carry sphingosin-1-phosphate (S1P) to the endothelial cell has been proposed as the underlying protective mechanism [144]. Pertinently, S1P itself was found to reduce shedding of glycosaminoglycans by suppression of matrix-metalloproteinase activity.
Etanercept has been successfully tested in humans against inflammation (LPS)-induced destruction of the glycocalyx [111]. Shedding of sdc1 by natriuretic peptides was strongly attenuated by ortho-phenanthroline [64]. Doxycycline inhibited glycan shedding induced by inflammatory and oxidative stress [127–129]. However, these two matrix-metalloprotease inhibitors have not been studied in humans in this respect. In any case, their specificity of action is very questionable and the therapeutic band width to toxicity narrow. Since knowledge of a specific protease responsible for shedding of the glycocalyx has not been forthcoming, indirect measures of protection seem more promising. These include stabilization of mast cells, dendritic cells and leukocytes and enhancement of anti-inflammatory signals, such as prostacyclin synthesis. Use of the anaesthetic agent sevofluran has proved protective for the glycocalyx in experimental settings [17,134]. Choice of this volatile anaesthetic in surgical procedures may, thus, be of benefit for critically ill patients.
There are a number of clinical studies testing the potential of the agent sulodexide to improve chronic venous disease, arterial disease, diabetic nephropathy and chronic kidney disease [145–147]. Sulodexide is a mixture of natural porcine heparan and dermatan sulphates (80 and 20%, respectively), which, given orally, are degraded to N-acetyl-glucosamine moieties in the digestive tract [132,148]. These presumably then serve as precursors for the synthesis of glycosaminoglycans in the endothelial glycocalyx. Whether sulodexide applied intravenously or intramuscularly acts by such a mechanism is uncertain. Experiments on endothelial cells in culture suggest that an alternative mode of action could be prevention of heparan sulphate degradation [149]. In a small study on 10 participants with type 2 diabetes vs. 10 controls, diminished dimensions of the endothelial glycocalyx and a hightened transcapillary escape rate of albumin in the diabetic group were normalized after 2 months of oral treatment [147]. However, a larger, placebo-controlled, double-blind investigation of over 1000 patients with type 2 diabetic nephropathy failed to find any renoprotective benefit of sulodexide after 1029 person-years of follow-up [150]. Sulodexide is also proposed as an endothelial-protective drug for the treatment of numerous venous and arterial disorders [151,152]. Some of the uncertainty concerning the usefulness of sulodexide may result from the fact that studies have variously employed intravenous, intramuscular and oral modes of drug application [149].
Besides prevention of degradation, the possibility of accelerated resynthesis or restoration of the glycocalyx may be a clinical option [2]. The proposed mode of action of oral sulodexide, mentioned above, exemplifies such an approach. In animal studies, recovery of a haemodynamically relevant glycocalyx in mesenterial venules following enzymatic or inflammatory degradation required 5–7 days [153]. Structure and barrier function of the glomerular endothelial surface layer recovered within 4 weeks after damage by hyaluronidase, infused into mice in vivo [154]. Conversely, human umbilical vein endothelial cells cultured under shear stress already showed regrowth of heparan sulphate in the glycocalyx 12 h after cleavage by heparanase [155]. For humans, there is little precise in vivo information. The treatment of patients with type 2 diabetes with sulodexide partially restored the dimensions of the glycocalyx after 8 weeks [147]. Owing to the design of this particular investigation (measurement of dimensions before and then not again until after 2 months of therapy), recovery may have been more complete and sooner in individual patients. Since the endothelial caveolae contain rich deposits of glycocalyx [2,27], one may speculate that externalization of these could very rapidly bring components of the glycocalyx back onto a denuded endothelial surface. However, such an approach has yet to be realized.
Conclusions
Deterioration of the endothelial glycocalyx/endothelial surface layer is currently being associated with a growing number of pathological states. Clinical studies confirming that protection of the endothelial glycocalyx from degradation benefits clinical outcomes in these scenarios are sorely lacking. This is partly due to the fact that therapeutic strategies retain a large speculative element, completing a negative cycle. At present, therefore, innovative strategies in this emerging field of experimental medicine are desperately needed. The authors are convinced that the hunt for such solutions is very much worth the effort.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare to have received no support from any organization for the submitted work, to have no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years, and that there are no other relationships or activities that could appear to have influenced the submitted work. AHJS has been supported by MRC fellowship G0802829, KRUK project grants RP18/2010 and RP45/2013, and KRUK studentship ST5/2012, which have no influence on the submitted work.
References
- 1.Pries AR, Secomb TW, Gaehtgens P. The endothelial surface layer. Pflugers Archiv: Eur J Physiol. 2000;440:653–66. doi: 10.1007/s004240000307. [DOI] [PubMed] [Google Scholar]
- 2.Becker BF, Chappell D, Bruegger D, Annecke T, Jacob M. Therapeutic strategies targeting the endothelial glycocalyx: acute deficits, but great potential. Cardiovasc Res. 2010;87:300–10. doi: 10.1093/cvr/cvq137. [DOI] [PubMed] [Google Scholar]
- 3.Jacob M, Chappell D, Stoeckelhuber M, Welsch U, Rehm M, Bruegger D, Kaczmarek I, Conzen P, Becker BF. Perspectives in microvascular fluid handling: does the distribution of coagulation factors in human myocardium comply with plasma extravasation in venular coronary segments? J Vasc Res. 2011;48:219–26. doi: 10.1159/000318795. [DOI] [PubMed] [Google Scholar]
- 4.Vink H, Duling BR. Capillary endothelial surface layer selectively reduces plasma solute distribution volume. Am J Physiol Heart Circ Physiol. 2000;278:H285–9. doi: 10.1152/ajpheart.2000.278.1.H285. [DOI] [PubMed] [Google Scholar]
- 5.Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng. 2007;9:121–67. doi: 10.1146/annurev.bioeng.9.060906.151959. [DOI] [PubMed] [Google Scholar]
- 6.Reitsma S, Slaaf DW, Vink H, van Zandvoort MA. oude Egbrink MG. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Archiv: Eur J Physiol. 2007;454:345–59. doi: 10.1007/s00424-007-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chappell D, Jacob M, Paul O, Rehm M, Welsch U, Stoeckelhuber M, Conzen P, Becker BF. The glycocalyx of the human umbilical vein endothelial cell: an impressive structure ex vivo but not in culture. Circ Res. 2009;104:1313–7. doi: 10.1161/CIRCRESAHA.108.187831. [DOI] [PubMed] [Google Scholar]
- 8.Jacob M, Rehm M, Loetsch M, Paul JO, Bruegger D, Welsch U, Conzen P, Becker BF. The endothelial glycocalyx prefers albumin for evoking shear stress-induced, nitric oxide-mediated coronary dilatation. J Vasc Res. 2007;44:435–43. doi: 10.1159/000104871. [DOI] [PubMed] [Google Scholar]
- 9.Curry FE, Adamson RH. Endothelial glycocalyx: permeability barrier and mechanosensor. Ann Biomed Eng. 2012;40:828–39. doi: 10.1007/s10439-011-0429-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacob M, Bruegger D, Rehm M, Stoeckelhuber M, Welsch U, Conzen P, Becker BF. The endothelial glycocalyx affords compatibility of Starling's principle and high cardiac interstitial albumin levels. Cardiovasc Res. 2007;73:575–86. doi: 10.1016/j.cardiores.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 11.Rehm M, Zahler S, Lotsch M, Welsch U, Conzen P, Jacob M, Becker BF. Endothelial glycocalyx as an additional barrier determining extravasation of 6% hydroxyethyl starch or 5% albumin solutions in the coronary vascular bed. Anesthesiology. 2004;100:1211–23. doi: 10.1097/00000542-200405000-00025. [DOI] [PubMed] [Google Scholar]
- 12.Adamson RH, Lenz JF, Zhang X, Adamson GN, Weinbaum S, Curry FE. Oncotic pressures opposing filtration across non-fenestrated rat microvessels. J Physiol. 2004;557:889–907. doi: 10.1113/jphysiol.2003.058255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levick JR, Michel CC. Microvascular fluid exchange and the revised Starling principle. Cardiovasc Res. 2010;87:198–210. doi: 10.1093/cvr/cvq062. [DOI] [PubMed] [Google Scholar]
- 14.Mulivor AW, Lipowsky HH. Role of glycocalyx in leukocyte-endothelial cell adhesion. Am J Physiol Heart Circ Physiol. 2002;283:H1282–91. doi: 10.1152/ajpheart.00117.2002. [DOI] [PubMed] [Google Scholar]
- 15.Vink H, Constantinescu AA, Spaan JA. Oxidized lipoproteins degrade the endothelial surface layer: implications for platelet-endothelial cell adhesion. Circulation. 2000;101:1500–2. doi: 10.1161/01.cir.101.13.1500. [DOI] [PubMed] [Google Scholar]
- 16.Chappell D, Dorfler N, Jacob M, Rehm M, Welsch U, Conzen P, Becker BF. Glycocalyx protection reduces leukocyte adhesion after ischemia/reperfusion. Shock. 2010;34:133–9. doi: 10.1097/SHK.0b013e3181cdc363. [DOI] [PubMed] [Google Scholar]
- 17.Chappell D, Heindl B, Jacob M, Annecke T, Chen C, Rehm M, Conzen P, Becker BF. Sevoflurane reduces leukocyte and platelet adhesion after ischemia-reperfusion by protecting the endothelial glycocalyx. Anesthesiology. 2011;115:483–91. doi: 10.1097/ALN.0b013e3182289988. [DOI] [PubMed] [Google Scholar]
- 18.Chappell D, Brettner F, Doerfler N, Jacob M, Rehm M, Bruegger D, Conzen P, Jacob B, Becker BF. Protection of glycocalyx decreases platelet adhesion after ischaemia/reperfusion: an animal study. Eur J Anaesth. 2014;31:474–81. doi: 10.1097/EJA.0000000000000085. [DOI] [PubMed] [Google Scholar]
- 19.Reitsma S, Oude Egbrink MG, Heijnen VV, Megens RT, Engels W, Vink H, Slaaf DW, van Zandvoort MA. Endothelial glycocalyx thickness and platelet-vessel wall interactions during atherogenesis. Thromb Haemost. 2011;106:939–46. doi: 10.1160/TH11-02-0133. [DOI] [PubMed] [Google Scholar]
- 20.Alexander CM, Reichsman F, Hinkes MT, Lincecum J, Becker KA, Cumberledge S, Bernfield M. Syndecan-1 is required for Wnt-1-induced mammary tumorigenesis in mice. Nat Gen. 2000;25:329–32. doi: 10.1038/77108. [DOI] [PubMed] [Google Scholar]
- 21.Gotte M. Syndecans in inflammation. FASEB J. 2003;17:575–91. doi: 10.1096/fj.02-0739rev. [DOI] [PubMed] [Google Scholar]
- 22.Li S, Pettersson US, Hoorelbeke B, Kolaczkowska E, Schelfhout K, Martens E, Kubes P, Van Damme J, Phillipson M, Opdenakker G. Interference with glycosaminoglycan-chemokine interactions with a probe to alter leukocyte recruitment and inflammation in vivo. PLoS One. 2014;9:e104107. doi: 10.1371/journal.pone.0104107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu WH, Woessner JF., Jr Heparan sulfate proteoglycans as extracellular docking molecules for matrilysin (matrix metalloproteinase 7) J Biol Chem. 2000;275:4183–91. doi: 10.1074/jbc.275.6.4183. [DOI] [PubMed] [Google Scholar]
- 24.Yu WH, Yu S, Meng Q, Brew K, Woessner JF., Jr TIMP-3 binds to sulfated glycosaminoglycans of the extracellular matrix. J Biol Chem. 2000;275:31226–32. doi: 10.1074/jbc.M000907200. [DOI] [PubMed] [Google Scholar]
- 25.Gotte M, Echtermeyer F. Syndecan-1 as a regulator of chemokine function. Scie World J. 2003;3:1327–31. doi: 10.1100/tsw.2003.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rehm M, Bruegger D, Christ F, Conzen P, Thiel M, Jacob M, Chappell D, Stoeckelhuber M, Welsch U, Reichart B, Peter K, Becker BF. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation. 2007;116:1896–906. doi: 10.1161/CIRCULATIONAHA.106.684852. [DOI] [PubMed] [Google Scholar]
- 27.Becker BF, Chappell D, Jacob M. Endothelial glycocalyx and coronary vascular permeability: the fringe benefit. Basic Res Cardiol. 2010;105:687–701. doi: 10.1007/s00395-010-0118-z. [DOI] [PubMed] [Google Scholar]
- 28.Chappell D, Hofmann-Kiefer K, Jacob M, Rehm M, Briegel J, Welsch U, Conzen P, Becker BF. TNF-alpha induced shedding of the endothelial glycocalyx is prevented by hydrocortisone and antithrombin. Basic Res Cardiol. 2009;104:78–89. doi: 10.1007/s00395-008-0749-5. [DOI] [PubMed] [Google Scholar]
- 29.Johansson PI, Stensballe J, Rasmussen LS, Ostrowski SR. A high admission syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, protein C depletion, fibrinolysis, and increased mortality in trauma patients. Ann Surg. 2011;254:194–200. doi: 10.1097/SLA.0b013e318226113d. [DOI] [PubMed] [Google Scholar]
- 30.Ostrowski SR, Berg RM, Windelov NA, Meyer MA, Plovsing RR, Moller K, Johansson PI. Coagulopathy, catecholamines, and biomarkers of endothelial damage in experimental human endotoxemia and in patients with severe sepsis: a prospective study. J Crit Care. 2013;28:586–96. doi: 10.1016/j.jcrc.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 31.Mulivor AW, Lipowsky HH. Inflammation- and ischemia-induced shedding of venular glycocalyx. Am J Physiol Heart Circ Physiol. 2004;286:H1672–80. doi: 10.1152/ajpheart.00832.2003. [DOI] [PubMed] [Google Scholar]
- 32.Salmon AH, Satchell SC. Endothelial glycocalyx dysfunction in disease: albuminuria and increased microvascular permeability. J Path. 2012;226:562–74. doi: 10.1002/path.3964. [DOI] [PubMed] [Google Scholar]
- 33.Salmon AH, Ferguson JK, Burford JL, Gevorgyan H, Nakano D, Harper SJ, Bates DO, Peti-Peterdi J. Loss of the endothelial glycocalyx links albuminuria and vascular dysfunction. J Am Soc Nephrol. 2012;23:1339–50. doi: 10.1681/ASN.2012010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henry CB, Duling BR. TNF-alpha increases entry of macromolecules into luminal endothelial cell glycocalyx. Am J Physiol Heart Circ Physiol. 2000;279:H2815–23. doi: 10.1152/ajpheart.2000.279.6.H2815. [DOI] [PubMed] [Google Scholar]
- 35.Padberg JS, Wiesinger A, di Marco GS, Reuter S, Grabner A, Kentrup D, Lukasz A, Oberleithner H, Pavenstadt H, Brand M, Kumpers P. Damage of the endothelial glycocalyx in chronic kidney disease. Atherosclerosis. 2014;234:335–43. doi: 10.1016/j.atherosclerosis.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 36.Nieuwdorp M, Mooij HL, Kroon J, Atasever B, Spaan JA, Ince C, Holleman F, Diamant M, Heine RJ, Hoekstra JB, Kastelein JJ, Stroes ES, Vink H. Endothelial glycocalyx damage coincides with microalbuminuria in type 1 diabetes. Diabetes. 2006;55:1127–32. doi: 10.2337/diabetes.55.04.06.db05-1619. [DOI] [PubMed] [Google Scholar]
- 37.Nieuwdorp M, van Haeften TW, Gouverneur MC, Mooij HL, van Lieshout MH, Levi M, Meijers JC, Holleman F, Hoekstra JB, Vink H, Kastelein JJ, Stroes ES. Loss of endothelial glycocalyx during acute hyperglycemia coincides with endothelial dysfunction and coagulation activation in vivo. Diabetes. 2006;55:480–6. doi: 10.2337/diabetes.55.02.06.db05-1103. [DOI] [PubMed] [Google Scholar]
- 38.Zuurbier CJ, Demirci C, Koeman A, Vink H, Ince C. Short-term hyperglycemia increases endothelial glycocalyx permeability and acutely decreases lineal density of capillaries with flowing red blood cells. J Appl Physiol. 2005;99:1471–6. doi: 10.1152/japplphysiol.00436.2005. [DOI] [PubMed] [Google Scholar]
- 39.Nieuwdorp M, Holleman F, de Groot E, Vink H, Gort J, Kontush A, Chapman MJ, Hutten BA, Brouwer CB, Hoekstra JB, Kastelein JJ, Stroes ES. Perturbation of hyaluronan metabolism predisposes patients with type 1 diabetes mellitus to atherosclerosis. Diabetologia. 2007;50:1288–93. doi: 10.1007/s00125-007-0666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van den Berg BM, Nieuwdorp M, Stroes ES, Vink H. Glycocalyx and endothelial (dys) function: from mice to men. Pharmacol Rep. 2006;58(Suppl):75–80. [PubMed] [Google Scholar]
- 41.Boels MG, Lee DH, van den Berg BM, Dane MJ, van der Vlag J, Rabelink TJ. The endothelial glycocalyx as a potential modifier of the hemolytic uremic syndrome. Eur J Int Med. 2013;24:503–9. doi: 10.1016/j.ejim.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 42.Savery MD, Jang JX, Park PW, Damiano ER. The endothelial glycocalyx in syndecan-1 deficient mice. Microvasc Res. 2013;87:83–91. doi: 10.1016/j.mvr.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Voyvodic PL, Min D, Liu R, Williams E, Chitalia V, Dunn AK, Baker AB. Loss of syndecan-1 induces a pro-inflammatory phenotype in endothelial cells with a dysregulated response to atheroprotective flow. J Biol Chem. 2014;289:9547–59. doi: 10.1074/jbc.M113.541573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hofmann-Kiefer KF, Chappell D, Knabl J, Frank HG, Martinoff N, Conzen P, Becker BF, Rehm M. Placental syncytiotrophoblast maintains a specific type of glycocalyx at the fetomaternal border: the glycocalyx at the fetomaternal interface in healthy women and patients with HELLP syndrome. Reprod Sci. 2013;20:1237–45. doi: 10.1177/1933719113483011. [DOI] [PubMed] [Google Scholar]
- 45.Hofmann-Kiefer KF, Knabl J, Martinoff N, Schiessl B, Conzen P, Rehm M, Becker BF, Chappell D. Increased serum concentrations of circulating glycocalyx components in HELLP syndrome compared to healthy pregnancy: an observational study. Reprod Sci. 2013;20:318–25. doi: 10.1177/1933719112453508. [DOI] [PubMed] [Google Scholar]
- 46.Osmers RG, Schutz E, Diedrich F, Wehry B, Krauss T, Oellerich M, Kuhn W. Increased serum levels of hyaluronic acid in pregnancies complicated by preeclampsia or hemolysis, elevated liver enzymes, and low platelets syndrome. Am J Obstet Gynecol. 1998;178:341–5. doi: 10.1016/s0002-9378(98)80023-9. [DOI] [PubMed] [Google Scholar]
- 47.Berg S, Engman A, Holmgren S, Lundahl T, Laurent TC. Increased plasma hyaluronan in severe pre-eclampsia and eclampsia. Scand J Clin Lab Invest. 2001;61:131–7. doi: 10.1080/00365510151097647. [DOI] [PubMed] [Google Scholar]
- 48.Stafford-Smith M, Lefrak EA, Qazi AG, Welsby IJ, Barber L, Hoeft A, Dorenbaum A, Mathias J, Rochon JJ, Newman MF. Members of the Global Perioperative Research Group. Efficacy and safety of heparinase I versus protamine in patients undergoing coronary artery bypass grafting with and without cardiopulmonary bypass. Anesthesiology. 2005;103:229–40. doi: 10.1097/00000542-200508000-00005. [DOI] [PubMed] [Google Scholar]
- 49.Battelli MG, Bolognesi A, Polito L. Pathophysiology of circulating xanthine oxidoreductase: new emerging roles for a multi-tasking enzyme. Biochim Biophys Acta. 1843;2014:1502–17. doi: 10.1016/j.bbadis.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 50.Lupu C, Poulsen E, Roquefeuil S, Westmuckett AD, Kakkar VV, Lupu F. Cellular effects of heparin on the production and release of tissue factor pathway inhibitor in human endothelial cells in culture. Arterioscler Thromb Vasc Biol. 1999;19:2251–62. doi: 10.1161/01.atv.19.9.2251. [DOI] [PubMed] [Google Scholar]
- 51.Wood JP, Bunce MW, Maroney SA, Tracy PB, Camire RM, Mast AE. Tissue factor pathway inhibitor-alpha inhibits prothrombinase during the initiation of blood coagulation. Proc Natl Acad Sci U S A. 2013;110:17838–43. doi: 10.1073/pnas.1310444110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dallinga-Thie GM, Franssen R, Mooij HL, Visser ME, Hassing HC, Peelman F, Kastelein JJ, Péterfy M, Nieuwdorp M. The metabolism of triglyceride-rich lipoproteins revisited: new players, new insight. Atherosclerosis. 2010;211:1–8. doi: 10.1016/j.atherosclerosis.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kersten S. Physiological regulation of lipoprotein lipase. Biochim Biophys Acta. 2014;184:919–33. doi: 10.1016/j.bbalip.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 54.Sarrazin S, Lamanna WC, Esko JD. Heparan sulphate proteoglycans. Cold Spring Harb Perspect Biol. 2011;3:a004952. doi: 10.1101/cshperspect.a004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Becker BF. Towards the physiological function of uric acid. Free Radic Biol Med. 1993;14:615–31. doi: 10.1016/0891-5849(93)90143-i. [DOI] [PubMed] [Google Scholar]
- 56.Bruegger D, Brettner F, Rossberg I, Nussbaum C, Kowalski C, Januszewska K, Becker BF, Chappell D. Acute degradation of the endothelial glycocoalyx in infants undergoing cardiac surgical procedures. Ann Thorac Surg. 2015;99:926–31. doi: 10.1016/j.athoracsur.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 57.Grundmann S, Fink K, Rabadzhieva L, Bourgeois N, Schwab T, Moser M, Bode C, Busch HJ. Perturbation of the endothelial glycocalyx in post cardiac arrest syndrome. Resuscitation. 2012;83:715–20. doi: 10.1016/j.resuscitation.2012.01.028. [DOI] [PubMed] [Google Scholar]
- 58.Massoudy P, Zahler S, Barankay A, Becker BF, Richter JA, Meisner H. Sodium nitroprusside during coronary artery bypass grafting: evidence for an antiinflammatory action. Ann Thorac Surg. 1999;67:1059–64. doi: 10.1016/s0003-4975(99)00157-5. [DOI] [PubMed] [Google Scholar]
- 59.Massoudy P, Zahler S, Freyholdt T, Henze R, Barankay A, Becker BF, Braun SL, Meisner H. Sodium nitroprusside in patients with compromised left ventricular function undergoing coronary bypass: reduction of cardiac proinflammatory substances. J Thorac Cardiovasc Surg. 2000;119:566–74. doi: 10.1016/s0022-5223(00)70138-3. [DOI] [PubMed] [Google Scholar]
- 60.Massoudy P, Zahler S, Becker BF, Braun SL, Barankay A, Meisner H. Evidence for inflammatory responses of the lungs during coronary artery bypass grafting with cardiopulmonary bypass. Chest. 2001;119:31–6. doi: 10.1378/chest.119.1.31. [DOI] [PubMed] [Google Scholar]
- 61.Zahler S, Massoudy P, Hartl H, Hahnel C, Meisner H, Becker BF. Acute cardiac inflammatory responses to postischemic reperfusion during cardiopulmonary bypass. Cardiovasc Res. 1999;41:722–30. doi: 10.1016/s0008-6363(98)00229-6. [DOI] [PubMed] [Google Scholar]
- 62.Annecke T, Fischer J, Hartmann H, Tschoep J, Rehm M, Conzen P, Sommerhoff CP, Becker BF. Shedding of the coronary endothelial glycocalyx: effects of hypoxia/reoxygenation vs ischaemia/reperfusion. Br J Anaesth. 2011;107:679–86. doi: 10.1093/bja/aer269. [DOI] [PubMed] [Google Scholar]
- 63.Becker BF, Fischer J, Hartmann H, Chen CC, Sommerhoff CP, Tschoep J, Conzen PC, Annecke T. Inosine, not adenosine, initiates endothelial glycocalyx degradation in cardiac ischemia and hypoxia. Nucleosides Nucleotides Nucleic Acids. 2011;30:1161–7. doi: 10.1080/15257770.2011.605089. [DOI] [PubMed] [Google Scholar]
- 64.Becker BF. All because of the mast cell: blocking the angiotensin receptor-1 should be better than inhibiting ACE (theoretically) Cardiovasc Res. 2011;92:7–9. doi: 10.1093/cvr/cvr214. [DOI] [PubMed] [Google Scholar]
- 65.Chappell D, Jacob M, Rehm M, Stoeckelhuber M, Welsch U, Conzen P, Becker BF. Heparinase selectively sheds heparan sulphate from the endothelial glycocalyx. Biol Chem. 2008;389:79–82. doi: 10.1515/BC.2008.005. [DOI] [PubMed] [Google Scholar]
- 66.Gilles S, Zahler S, Welsch U, Sommerhoff CP, Becker BF. Release of TNF-alpha during myocardial reperfusion depends on oxidative stress and is prevented by mast cell stabilizers. Cardiovasc Res. 2003;60:608–16. doi: 10.1016/j.cardiores.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 67.Ross AM, Gibbons RJ, Stone GW, Kloner RA, Alexander RW, Investigators A-I. A randomized, double-blinded, placebo-controlled multicenter trial of adenosine as an adjunct to reperfusion in the treatment of acute myocardial infarction (AMISTAD-II) J Am Coll Cardiol. 2005;45:1775–80. doi: 10.1016/j.jacc.2005.02.061. [DOI] [PubMed] [Google Scholar]
- 68.Miura T, Miki T. Limitation of myocardial infarct size in the clinical setting: current status and challenges in translating animal experiments into clinical therapy. Basic Res Cardiol. 2008;103:501–13. doi: 10.1007/s00395-008-0743-y. [DOI] [PubMed] [Google Scholar]
- 69.Bruegger D, Rehm M, Abicht J, Paul JO, Stoeckelhuber M, Pfirrmann M, Reichart B, Becker BF, Christ F. Shedding of the endothelial glycocalyx during cardiac surgery: on-pump versus off-pump coronary artery bypass graft surgery. J Thorac Cardiovasc Surg. 2009;138:1445–7. doi: 10.1016/j.jtcvs.2008.07.063. [DOI] [PubMed] [Google Scholar]
- 70.Bruegger D, Schwartz L, Chappell D, Jacob M, Rehm M, Vogeser M, Christ F, Reichart B, Becker BF. Release of atrial natriuretic peptide precedes shedding of the endothelial glycocalyx equally in patients undergoing on- and off-pump coronary artery bypass surgery. Basic Res Cardiol. 2011;106:1111–21. doi: 10.1007/s00395-011-0203-y. [DOI] [PubMed] [Google Scholar]
- 71.Svennevig K, Hoel T, Thiara A, Kolset S, Castelheim A, Mollnes T, Brosstad F, Fosse E, Svennevig J. Syndecan-1 plasma levels during coronary artery bypass surgery with and without cardiopulmonary bypass. Perfusion. 2008;23:165–71. doi: 10.1177/0267659108098215. [DOI] [PubMed] [Google Scholar]
- 72.Maksimenko AV, Turashev AD. No-reflow phenomenon and endothelial glycocalyx of microcirculation. Biochem Res Int. 2012;2012:859231. doi: 10.1155/2012/859231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bruegger D, Jacob M, Rehm M, Loetsch M, Welsch U, Conzen P, Becker BF. Atrial natriuretic peptide induces shedding of endothelial glycocalyx in coronary vascular bed of guinea pig hearts. Am J Physiol Hear Circ Physiol. 2005;289:H1993–9. doi: 10.1152/ajpheart.00218.2005. [DOI] [PubMed] [Google Scholar]
- 74.Jacob M, Saller T, Chappell D, Rehm M, Welsch U, Becker BF. Physiological levels of A- B- and C-type natriuretic peptide shed the endothelial glycocalyx and enhance vascular permeability. Basic Res Cardiol. 2013;108:347. doi: 10.1007/s00395-013-0347-z. [DOI] [PubMed] [Google Scholar]
- 75.Murray DB, Gardner JD, Levick SP, Brower GL, Morgan LG, Janicki JS. Response of cardiac mast cells to atrial natriuretic peptide. Am J Physiol Hear Circ Physiol. 2007;293:H1216–22. doi: 10.1152/ajpheart.01388.2006. [DOI] [PubMed] [Google Scholar]
- 76.Robinson JW, Lou X, Potter LR. The indolocarbazole, Go6976, inhibits guanylyl cyclase-A and -B. Br J Pharmacol. 2011;164:499–506. doi: 10.1111/j.1476-5381.2011.01291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kitakaze M, Asakura M, Kim J, Shintani Y, Asanuma H, Hamasaki T, Seguchi O, Myoishi M, Minamino T, Ohara T, Nagai Y, Nanto S, Watanabe K, Fukuzawa S, Hirayama A, Nakamura N, Kimura K, Fujii K, Ishihara M, Saito Y, Tomoike H, Kitamura S investigators JW. Human atrial natriuretic peptide and nicorandil as adjuncts to reperfusion treatment for acute myocardial infarction (J-WIND): two randomised trials. Lancet. 2007;370:1483–93. doi: 10.1016/S0140-6736(07)61634-1. [DOI] [PubMed] [Google Scholar]
- 78.Mentzer RM, Jr, Oz MC, Sladen RN, Graeve AH, Hebeler RF, Jr, Luber JM, Jr, Smedira NG, Investigators N. Effects of perioperative nesiritide in patients with left ventricular dysfunction undergoing cardiac surgery:the NAPA Trial. J Am Coll Cardiol. 2007;49:716–26. doi: 10.1016/j.jacc.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 79.Sezai A, Wakui S, Akiyama K, Hata M, Yoshitake I, Unosawa S, Shiono M, Hirayama A. Myocardial protective effect of human atrial natriuretic peptide in cardiac surgery. ‘hANP shot’ in clinical safety trial. Circ J. 2011;75:2144–50. doi: 10.1253/circj.cj-11-0185. [DOI] [PubMed] [Google Scholar]
- 80.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K. Zile MR; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapil in heart failure. N Engl J Med. 2014;37:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 81.Rehm M, Haller M, Orth V, Kreimeier U, Jacob M, Dressel H, Mayer S, Brechtelsbauer H, Finsterer U. Changes in blood volume and hematocrit during acute preoperative volume loading with 5% albumin or 6% hetastarch solutions in patients before radical hysterectomy. Anesthesiology. 2001;95:849–56. doi: 10.1097/00000542-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 82.Rehm M, Orth V, Kreimeier U, Thiel M, Haller M, Brechtelsbauer H, Finsterer U. Changes in intravascular volume during acute normovolemic hemodilution and intraoperative retransfusion in patients with radical hysterectomy. Anesthesiology. 2000;92:657–64. doi: 10.1097/00000542-200003000-00008. [DOI] [PubMed] [Google Scholar]
- 83.Curry FR. Atrial natriuretic peptide: an essential physiological regulator of transvascular fluid, protein transport, and plasma volume. J Clin Invest. 2005;115:1458–61. doi: 10.1172/JCI25417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chappell D, Bruegger D, Potzel J, Jacob M, Vogeser M, Conzen P, Becker BF, Rehm M. Hypervolemia increases release of atrial natriuretic peptide and shedding of the endothelial glycocalyx. Crit Care. 2014;18:538. doi: 10.1186/s13054-014-0538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chappell D, Jacob M, Hofmann-Kiefer K, Conzen P, Rehm M. A rational approach to perioperative fluid management. Anesthesiology. 2008;109:723–40. doi: 10.1097/ALN.0b013e3181863117. [DOI] [PubMed] [Google Scholar]
- 86.Jacob M, Chappell D, Hollmann MW. Current aspects of perioperative fluid handling in vascular surgery. Curr Opin Anaesthesiol. 2009;22:100–8. doi: 10.1097/ACO.0b013e32831f1c65. [DOI] [PubMed] [Google Scholar]
- 87.McCarthy KJ, Wassenhove-McCarthy DJ. The glomerular basement membrane as a model system to study the bioactivity of heparan sulphate glycosaminoglycans. Microsc Microanal. 2012;18:3–21. doi: 10.1017/S1431927611012682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Arkill KP, Knupp C, Michel CC, Neal CR, Qvortrup K, Rostgaard J, Squire JM. Similar endothelial glycocalyx structures in microvessels from a range of mammalian tissues: evidence for a common filtering mechanism? Biophys J. 2011;101:1046–56. doi: 10.1016/j.bpj.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Haraldsson B, Nystrom J, Deen WM. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev. 2008;88:451–487. doi: 10.1152/physrev.00055.2006. [DOI] [PubMed] [Google Scholar]
- 90.Jeansson M, Haraldsson B. Morphological and functional evidence for an important role of the endothelial cell glycocalyx in the glomerular barrier. Am J Physiol Renal Physiol. 2006;290:F111–F116. doi: 10.1152/ajprenal.00173.2005. [DOI] [PubMed] [Google Scholar]
- 91.Friden V, Oveland E, Tenstad O. Ebefors K, Nyström J, Nilsson UA, Haraldsson B. The glomerular endothelial cell coat is essential for glomerular filtration. Kidney Int. 2011;79:1322–1330. doi: 10.1038/ki.2011.58. [DOI] [PubMed] [Google Scholar]
- 92.Adembri C, Sgambati E, Vitali L. Selmi V, Margheri M, Tani A, Bonaccini L, Nosi D, Caldini AL, Formigli L, De Gaudio AR. Sepsis induces albuminuria and alterations in the glomerular filtration barrier: a morphofunctional study in the rat. Crit Care. 2011;15:R277. doi: 10.1186/cc10559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Singh A, Satchell SC, Neal CR, McKenzie EA, Tooke JE, Mathieson PW. Glomerular endothelial glycocalyx constitutes a barrier to protein permeability. J Am Soc Nephrol. 2007;18:2885–93. doi: 10.1681/ASN.2007010119. [DOI] [PubMed] [Google Scholar]
- 94.Singh A, Friden V, Dasgupta I, Foster RR, Welsh GI, Tooke JE, Haraldsson B, Mathieson PW, Satchell SC. High glucose causes dysfunction of the human glomerular endothelial glycocalyx. Am J Physiol Ren Physiol. 2011;300:F40–8. doi: 10.1152/ajprenal.00103.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Friden V, Oveland E, Tenstad O, Ebefors K, Nystrom J, Nilsson UA, Haraldsson B. The glomerular endothelial cell coat is essential for glomerular filtration. Kid Int. 2011;79:1322–30. doi: 10.1038/ki.2011.58. [DOI] [PubMed] [Google Scholar]
- 96.Vlahu CA, Lemkes BA, Struik DG, Koopman MG, Krediet RT, Vink H. Damage of the endothelial glycocalyx in dialysis patients. J Am Soc Nephrol. 2012;23:1900–8. doi: 10.1681/ASN.2011121181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dane MJ, Khairoun M, Lee DH, van den Berg BM, Eskens BJ, Boels MG, van Teeffelen JW, Rops AL, van der Vlag J, van Zonneveld AJ, Reinders ME, Vink H, Rabelink TJ. Association of kidney function with changes in the endothelial surface layer. Clin J Am Soc Nephrol. 2014;9:698–704. doi: 10.2215/CJN.08160813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Korte S, Wiesinger A, Straeter AS, Peters W, Oberleithner H, Kusche-Vihrog K. Firewall function of the endothelial glycocalyx in the regulation of sodium homeostasis. Pflugers Archiv: Eur J Physiol. 2012;463:269–78. doi: 10.1007/s00424-011-1038-y. [DOI] [PubMed] [Google Scholar]
- 99.Johansson PI, Sorensen AM, Perner A, Welling KL, Wanscher M, Larsen CF, Ostrowski SR. High sCD40L levels early after trauma are associated with enhanced shock, sympathoadrenal activation, tissue and endothelial damage, coagulopathy and mortality. J Thromb Haemost. 2012;10:207–16. doi: 10.1111/j.1538-7836.2011.04589.x. [DOI] [PubMed] [Google Scholar]
- 100.Ostrowski SR, Sorensen AM, Windelov NA, Perner A, Welling KL, Wanscher M, Larsen CF, Johansson PI. High levels of soluble VEGF receptor 1 early after trauma are associated with shock, sympathoadrenal activation, glycocalyx degradation and inflammation in severely injured patients: a prospective study. Scand J Traum Resusc Emerg Med. 2012;20:27. doi: 10.1186/1757-7241-20-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ostrowski SR, Johansson PI. Endothelial glycocalyx degradation induces endogenous heparinization in patients with severe injury and early traumatic coagulopathy. J Traum Acute Care Surg. 2012;73:60–6. doi: 10.1097/TA.0b013e31825b5c10. [DOI] [PubMed] [Google Scholar]
- 102.Trung DT, Wills B. systemic vascular leakage associated with dengue infections – the clinical perspective. Curr Top Microbiol Immunol. 2010;338:57–66. doi: 10.1007/978-3-642-02215-9_5. [DOI] [PubMed] [Google Scholar]
- 103.Connolly-Andersen A-M, Thunberg T, Ahlm C. Open Forum Infectious Diseases. Vol. 1. Oxford University Press; 2014. Endothelial Activation and Repair During Hantavirus Infection: Association with Disease Outcome. 1 ofu027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ozturk B, Kuscu F, Tutuncu E, Sencan I, Gurbuz Y, Tuzun H. Evaluation of the association of serum levels of hyaluronic acid, sICAM-1, sVCAM-1, and VEGF-A with mortality and prognosis in patients with Crimean-Congo hemorrhagic fever. J Clin Virol. 2010;47:115–9. doi: 10.1016/j.jcv.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 105.Marsac D, Garcia S, Fournet A, Aguirre A, Pino K, Ferres M, Kalergis AM, Lopez-Lastra M, Veas F. Infection of human monocyte-derived dendritic cells by ANDES Hantavirus enhances pro-inflammatory state, the secretion of active MMP-9 and indirectly enhances endothelial permeability. Virol J. 2011;8:223. doi: 10.1186/1743-422X-8-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Luplertlop N, Misse D. MMP cellular responses to dengue virus infection-induced vascular leakage. Jap J Inf Dis. 2008;61:298–301. [PubMed] [Google Scholar]
- 107.Nelson A, Berkestedt I, Schmidtchen A, Ljunggren L, Bodelsson M. Increased levels of glycosaminoglycans during septic shock: relation to mortality and the antibacterial actions of plasma. Shock. 2008;30:623–7. doi: 10.1097/SHK.0b013e3181777da3. [DOI] [PubMed] [Google Scholar]
- 108.Steppan J, Hofer S, Funke B, Brenner T, Henrich M, Martin E, Weitz J, Hofmann U, Weigand MA. Sepsis and major abdominal surgery lead to flaking of the endothelial glycocalyx. J Surg Res. 2011;165:136–41. doi: 10.1016/j.jss.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 109.Sallisalmi M, Tenhunen J, Yang R, Oksala N, Pettila V. Vascular adhesion protein-1 and syndecan-1 in septic shock. Acta Anaesth Scand. 2012;56:316–22. doi: 10.1111/j.1399-6576.2011.02578.x. [DOI] [PubMed] [Google Scholar]
- 110.Yagmur E, Koch A, Haumann M, Kramann R, Trautwein C, Tacke F. Hyaluronan serum concentrations are elevated in critically ill patients and associated with disease severity. Clin Biochem. 2012;45:82–7. doi: 10.1016/j.clinbiochem.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 111.Nieuwdorp M, Meuwese MC, Mooij HL, van Lieshout MH, Hayden A, Levi M, Meijers JC, Ince C, Kastelein JJ, Vink H, Stroes ES. Tumor necrosis factor-alpha inhibition protects against endotoxin-induced endothelial glycocalyx perturbation. Atherosclerosis. 2009;202:296–303. doi: 10.1016/j.atherosclerosis.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 112.Delaney AP, Dan A, McCaffrey J, Finfer S. The role of albumin as a resuscitation fluid for patients with sepsis: a systematic review and meta-analysis. Crit Care Med. 2011;39:386–91. doi: 10.1097/CCM.0b013e3181ffe217. [DOI] [PubMed] [Google Scholar]
- 113.Jacob M, Bruegger D, Rehm M, Welsch U, Conzen P, Becker BF. Contrasting effects of colloid and crystalloid resuscitation fluids on cardiac vascular permeability. Anesthesiology. 2006;104:1223–31. doi: 10.1097/00000542-200606000-00018. [DOI] [PubMed] [Google Scholar]
- 114.Jacob M, Paul O, Mehringer L, Chappell D, Rehm M, Welsch U, Kaczmarek I, Conzen P, Becker BF. Albumin augmentation improves condition of guinea pig hearts after 4 hr of cold ischemia. Transplantation. 2009;87:956–65. doi: 10.1097/TP.0b013e31819c83b5. [DOI] [PubMed] [Google Scholar]
- 115.Zausig YA, Chappell D, Becker BF, Potschka D, Busse H, Nixdorf K, Bitzinger D, Jacob B, Jacob M. The impact of crystalloidal and colloidal infusion preparations on coronary vascular integrity, interstitial oedema and cardiac performance in isolated hearts. Crit Care. 2013;17:R203. doi: 10.1186/cc12898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Oberleitner H. Two barriers for sodium in vascular endothelium? Ann Med. 2012;44:S143–8. doi: 10.3109/07853890.2011.653397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. http://www.awmf.org/leitlinien/detail/ll/001-020.html.
- 118.Oberleitner H. Vascular endothelium: a vulnerable transit zone for merciless sodium. Nephrol Dial Transplant. 2014;29:240–6. doi: 10.1093/ndt/gft461. [DOI] [PubMed] [Google Scholar]
- 119.Yang Y, Schmidt EP. The endothelial glycocalyx: an important regulator of the pulmonary vascular barrier. Tissue barriers. 2013;1 doi: 10.4161/tisb.23494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schmidt EP, Yang Y, Janssen WJ, Gandjeva A, Perez MJ, Barthel L, Zemans RL, Bowman JC, Koyanagi DE, Yunt ZX, Smith LP, Cheng SS, Overdier KH, Thompson KR, Geraci MW, Douglas IS, Pearse DB, Tuder RM. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med. 2012;18:1217–23. doi: 10.1038/nm.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schmidt EP, Li G, Li L, Fu L, Yang Y, Overdier KH, Douglas IS, Linhardt RJ. The circulating glycosaminoglycan signature of respiratory failure in critically ill adults. J Biol Chem. 2014;289:8194–202. doi: 10.1074/jbc.M113.539452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nandi A, Estess P, Siegelman MH. Hyaluronan anchoring and regulation on the surface of vascular endothelial cells is mediated through the functionally active form of CD44. J Biol Chem. 2000;275:14939–48. doi: 10.1074/jbc.275.20.14939. [DOI] [PubMed] [Google Scholar]
- 123.Donati A, Damiani E, Luchetti M, Domizi R, Scorcella C, Carsetti A, Gabbanelli V, Carletti P, Bencivenga R, Vink H, Adrario E, Piagnerelli M, Gabrielli A, Pelaia P, Ince C. Microcirculatory effects of the transfusion of leukodepleted or non-leukodepleted red blood cells in patients with sepsis: a pilot study. Crit Care. 2014;18:R33. doi: 10.1186/cc13730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Henry CB, Duling BR. Permeation of the luminal capillary glycocalyx is determined by hyaluronan. Am J Physiol. 1999;277:H508–14. doi: 10.1152/ajpheart.1999.277.2.H508. [DOI] [PubMed] [Google Scholar]
- 125.Fitzgerald ML, Wang Z, Park PW, Murphy G, Bernfield M. Shedding of syndecan-1 and -4 ectodomains is regulated by multiple signaling pathways and mediated by a TIMP-3-sensitive metalloproteinase. J Cell Biol. 2000;148:811–24. doi: 10.1083/jcb.148.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Barbouri D, Afratis N, Gialeli C, Vynios DH, Theocharis AD, Karamanos NK. Syndecans as modulators and potential pharmacological targets in cancer progression. Front Oncol. 2014;4:4. doi: 10.3389/fonc.2014.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mulivor AW, Lipowsky HH. Inhibition of glycan shedding and leukocyte-endothelial adhesion in postcapillary venules by suppression of matrixmetalloprotease activity with doxycycline. Microcirculation. 2009;16:657–66. doi: 10.3109/10739680903133714. [DOI] [PubMed] [Google Scholar]
- 128.Lipowsky HH, Sah R, Lescanic A. Relative roles of doxycycline and cation chelation in endothelial glycan shedding and adhesion of leukocytes. Am J Physiol Heart Circ Physiol. 2011;300:H415–22. doi: 10.1152/ajpheart.00923.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lipowsky HH, Lescanic A. The effect of doxycycline on shedding of the glycocalyx due to reactive oxygen species. Microvasc Res. 2013;90:80–5. doi: 10.1016/j.mvr.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ramnath R, Foster RR, Qiu Y, Cope G, Butler MJ, Salmon AH, Mathieson PW, Coward RJ, Welsh GI, Satchell SC. Matrix metalloproteinase 9-mediated shedding of syndecan 4 in response to tumor necrosis factor alpha: a contributor to endothelial cell glycocalyx dysfunction. FASEB J. 2014;3 doi: 10.1096/fj.14-252221. pii: fj.14-252221. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 131.Subramanian SV, Fitzgerald ML, Bernfield M. Regulated shedding of syndecan-1 and -4 ectodomains by thrombin and growth factor receptor activation. J Biol Chem. 1997;272:14713–20. doi: 10.1074/jbc.272.23.14713. [DOI] [PubMed] [Google Scholar]
- 132.Schmidt A, Echtermeyer F, Alozie A, Brands K, Buddecke E. Plasmin- and thrombin-accelerated shedding of syndecan-4 ectodomain generates cleavage sites at Lys(114)-Arg(115) and Lys(129)-Val(130) bonds. J Biol Chem. 2005;280:34441–6. doi: 10.1074/jbc.M501903200. [DOI] [PubMed] [Google Scholar]
- 133.Chung MC, Jorgensen SC, Popova TG, Bailey CL, Popov SG. Neutrophil elastase and syndecan shedding contribute to antithrombin depletion in murine anthrax. FEMS Immunol Med Microbiol. 2008;54:309–18. doi: 10.1111/j.1574-695X.2008.00480.x. [DOI] [PubMed] [Google Scholar]
- 134.Annecke T, Chappell D, Chen C, Jacob M, Welsch U, Sommerhoff CP, Rehm M, Conzen PF, Becker BF. Sevoflurane preserves the endothelial glycocalyx against ischaemia-reperfusion injury. Br J Anaesth. 2010;104:414–21. doi: 10.1093/bja/aeq019. [DOI] [PubMed] [Google Scholar]
- 135.Chappell D, Jacob M, Hofmann-Kiefer K, Bruegger D, Rehm M, Conzen P, Welsch U, Becker BF. Hydrocortisone preserves the vascular barrier by protecting the endothelial glycocalyx. Anesthesiology. 2007;107:776–84. doi: 10.1097/01.anes.0000286984.39328.96. [DOI] [PubMed] [Google Scholar]
- 136.Uhl B, Zuchtriegel G, Puhr-Westerheide D, Praetner M, Rehberg M, Fabritius M, Hessenauer M, Holzer M, Khandoga A, Furst R, Zahler S, Krombach F, Reichel CA. Tissue plasminogen activator promotes postischemic neutrophil recruitment via its proteolytic and nonproteolytic properties. Arterioscler Thromb Vas Biol. 2014;34:1495–504. doi: 10.1161/ATVBAHA.114.303721. [DOI] [PubMed] [Google Scholar]
- 137.Chappell D, Jacob M, Hofmann-Kiefer K, Rehm M, Welsch U, Conzen P, Becker BF. Antithrombin reduces shedding of the endothelial glycocalyx following ischaemia/reperfusion. Cardiovas Res. 2009;83:388–96. doi: 10.1093/cvr/cvp097. [DOI] [PubMed] [Google Scholar]
- 138.Wang J, Wang Y, Wang J, Gao J, Tong C, Manithody C, Li J, Rezaie AR. Antithrombin is protective against myocardial ischemia and reperfusion injury. J Thromb Haeost. 2013;11:1020–8. doi: 10.1111/jth.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Meier HL, Heck LW, Schulman ES, MacGlashan DW., Jr Purified human mast cells and basophils release human elastase and cathepsin G by an IgE-mediated mechanism. Int Arch Allerg Appl Immun. 1985;77:179–83. doi: 10.1159/000233779. [DOI] [PubMed] [Google Scholar]
- 140.Winkler CW, Foster SC, Matsumoto SG, Preston MA, Xing R, Bebo BF, Banine F, Berny-Lang MA, Itakura A, McCarty OJ, Sherman LS. Hyaluronan anchored to activated CD44 on central nervous system vascular endothelial cells promotes lymphocyte extravasation in experimental autoimmune encephalomyelitis. J Biol Chem. 2012;287:33237–51. doi: 10.1074/jbc.M112.356287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Job KM, Dull RO, Hlady V. Use of reflectance interference contrast microscopy to characterize the endothelial glycocalyx stiffness. Am J Physiol Lung Cell Molec Physiol. 2012;302:L1242–9. doi: 10.1152/ajplung.00341.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mitchell MJ, King MR. Physical biology in cancer. 3. The role of cell glycocalyx in vascular transport of circulating tumor cells. Am J Physiol Cell Physiol. 2014;306:C89–97. doi: 10.1152/ajpcell.00285.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Stevens AP, Hlady V, Dull RO. Fluorescence correlation spectroscopy can probe albumin dynamics inside lung endothelial glycocalyx. Am J Physiol Lung Cell Molecul Physiol. 2007;293:L328–35. doi: 10.1152/ajplung.00390.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zeng Y, Adamson RH, Curry FR, Tarbell JM. Sphingosine-1-phosphate protects endothelial glycocalyx by inhibiting syndecan-1 shedding. Am J Physiol Hear Circ Physiol. 2014;306:H363–72. doi: 10.1152/ajpheart.00687.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Andreozzi GM. Sulodexide in the treatment of chronic venous disease. Am J Cardiovascular Drugs: Drugs, Devices, and other interventions. 2012;12:73–81. doi: 10.2165/11599360-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 146.Hoppensteadt DA, Fareed J. Pharmacological profile of sulodexide. Int Angiol. 2014;33:229–35. [PubMed] [Google Scholar]
- 147.Broekhuizen LN, Lemkes BA, Mooij HL, Meuwese MC, Verberne H, Holleman F, Schlingemann RO, Nieuwdorp M, Stroes ES, Vink H. Effect of sulodexide on endothelial glycocalyx and vascular permeability in patients with type 2 diabetes mellitus. Diabetologia. 2010;53:2646–55. doi: 10.1007/s00125-010-1910-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Weiss R, Niecestro R, Raz I. The role of sulodexide in the treatment of diabetic nephropathy. Drugs. 2007;67:2681–96. doi: 10.2165/00003495-200767180-00004. [DOI] [PubMed] [Google Scholar]
- 149.Gaddi AV, Cicero AF, Gambaro G. Nephroprotective action of glycosaminoglycans: why the pharmacological properties of sulodexide might be reconsidered. Int J Nephrol Renovasc Dis. 2010;3:99–105. doi: 10.2147/ijnrd.s5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Packham DK, Wolfe R, Reutens AT, Berl T, Heerspink HL, Rohde R, Ivory S, Lewis J, Raz I, Wiegmann TB, Chan JC, de Zeeuw D, Lewis EJ, Atkins RC, Collaborative StudyG. Sulodexide fails to demonstrate renoprotection in overt type 2 diabetic nephropathy. J Am Soc Nephrol. 2012;23:123–30. doi: 10.1681/ASN.2011040378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Coccheri S. Biological and clinical effects of sulodexide in arterial disorders and diseases. Int Angiol. 2014;33:263–74. [PubMed] [Google Scholar]
- 152.Coccheri S, Mannello F. Development and use of sulodexide in vascular diseases: implications for treatment. Drug Des Devel Ther. 2014;8:49–65. doi: 10.2147/DDDT.S6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Potter DR, Jiang J, Damiano ER. The recovery time course of the endothelial cell glycocalyx in vivo and its implications in vitro. Circ Res. 2009;104:1318–25. doi: 10.1161/CIRCRESAHA.108.191585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dane MJ, van den Berg BM, Avramut MC, Faas FG, van der Vlag J, Rops AL, Ravelli RB, Koster BJ, van Zonneveld AJ, Vink H, Rabelink TJ. Glomerular endothelial surface layer acts as a barrier against albumin filtration. Am J Pathol. 2013;182:1532–40. doi: 10.1016/j.ajpath.2013.01.049. [DOI] [PubMed] [Google Scholar]
- 155.Giantsos-Adams KM, Koo AJ-A, Song S, Sakai J, Sankaran J, Shin JH, Garcia-Cardena G, Dewey CF., Jr Heparan sulphate regrowth profiles under laminar shear flow following enzymatic degradation. Cell Mol Bioeng. 2013;6:160–74. doi: 10.1007/s12195-013-0273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


