Abstract
Migraine is a neurological disorder that is far more than just a bad headache. A hallmark of migraine is altered sensory perception. A likely contributor to this altered perception is the neuropeptide calcitonin gene-related peptide (CGRP). Over the past decade, CGRP has become firmly established as a key player in migraine. Although the mechanisms and sites of action by which CGRP might trigger migraine remain speculative, recent advances with mouse models provide some hints. This brief review focuses on how CGRP might act as both a central and peripheral neuromodulator to contribute to the migraine-like symptom of light aversive behaviour in mice.
Keywords: calcitonin gene-related peptide, CGRP, migraine, mouse model, neuropeptide, photophobia
What is a migraine?
To appreciate the actions of the neuropeptide calcitonin gene-related peptide (CGRP) in migraine, it is essential to recognize that migraine is more than just a headache. Migraine is a complex neurological disorder that involves altered sensory perception and processing. This has been documented by functional imaging studies and patient reports that point to altered brain function, even between attacks [1,2]. Migraine appears to involve a number of brain structures, including the cortex, hypothalamus, brainstem, trigeminal nerve and meninges [3,4]. Despite this complexity, an emerging feature shared by many, if not all, of these structures is communication with the trigeminovascular pathway [5] and involvement of CGRP [6–8].
Mouse models
Given the complexity of migraine, there is a need for animal models to understand the pathology and develop new therapeutics. Many of the models for studying migraine have been recently reviewed [9–12]. Perhaps the most widely used model is meningeal stimulation by direct application of inflammatory compounds. Inflammatory soup administered onto the dura activates nociceptive trigeminal ganglia neurones, leading to peripheral and central sensitization, as shown by electrophysiological and nociceptive assays [13]. In recent years, this model has been refined to allow repeated stimulation of the dura coupled to behavioural assays [14,15]. The model has also been adapted to conditioned place preference assays that go beyond nociceptive reflexes [16]. These types of operant-based preference and aversion assays hold great promise for future studies.
More recent animal models often use established human triggers of migraine [17] or genetic mutations identified in rare familial forms of migraine [18,19]. The most widely used rodent models based on human triggers involve infusion of nitroglycerine, which generates nitric oxide (NO). While reservations have been raised about this model [20], nitroglycerine induces allodynic responses to fine touch [21], and has been shown to induce light aversive behaviour [22]. A promising new model is based on medication overuse headaches experienced by people who overuse triptan migraine drugs [23]. The model that is the focus of the present review is based on the ability of CGRP injections to cause a delayed migraine-like headache in migraineurs. This model has overexpressed CGRP receptor activity in the nervous system, as described below. The converse knockout models lacking CGRP or receptor subunits have some interesting pain and vascular phenotypes, but have either not yet been fully tested or been very informative with respect to migraine [24–26].
CGRP and migraine
CGRP is a multifunctional neuropeptide that is widely recognized as a regulator of the cardiovascular system, a mediator of neurogenic inflammation and a modulator of nociceptive input [8,24,27]. These neurovascular and nociceptive activities made it a logical candidate to be involved in migraine. Clinical studies have fully established the importance of CGRP in migraine pathogenesis [6–8]. Briefly, there are three lines of evidence that support this conclusion. First, CGRP levels have been reported to be elevated during spontaneous and nitroglycerine-induced migraine and reduced by triptans, coincident with pain relief. This elevation was not seen in one well-controlled study, which may reflect the heterogeneity of migraine pathology or differences in techniques [28]. Elevated CGRP levels have also been reported between attacks in people with chronic migraine [29].
Second, intravenous injection of CGRP caused delayed headaches, which for some subjects met the criteria for experimentally induced migraine [30–32]. Notably, the delayed onset of migraine-like headaches was seen only in migraineurs. Nonmigraineurs experienced only an initial mild headache or fullness-of-head sensation [31,33]. This suggests that migraineurs are unusually sensitive to CGRP actions, and provided the rationale for designing a CGRP-sensitized mouse model, discussed below [34,35]. CGRP-induced migraines were also reversed by a triptan [32]. Although two other vasodilators, nitroglycerine and pituitary adenylate cyclase-activating polypeptide (PACAP), can also induce delayed migraine-like headaches similarly to CGRP, this is not a general property of vasodilators [36]. Vasoactive intestinal peptide can induce significant cerebral vasodilation, but does not cause migraine [37]. Results with the vasodilatory peptide adrenomedullin were somewhat more complex as, although it did not cause migraine yet dilated the extracranial temporal artery, it apparently did not dilate intracranial arteries [38]. Along this line, extracranial blood vessels are not dilated and intracranial arteries are only slightly dilated during spontaneous migraine [39], although technical limitations preclude analysis of smaller intracranial dural vessels [40]. Hence, although a direct connection between migraine and vasodilation cannot yet be ruled out or in, the ability of CGRP to induce migraine apparently involves more than just its vasodilatory actions.
Third, a total of five small-molecule CGRP receptor antagonists have proven effective in phase II and III clinical trials [41–46]. These drugs relieved both the pain and associated symptoms of migraine, including photophobia. Although one of these drugs, telcagepant, was successful in six phase III trials, further development was halted following reports of liver toxicity after repeated usage [47]. The site(s) of action of these drugs remain unknown [6,7,48–50]. As they are not very central nervous system (CNS)-penetrant, it is possible that peripheral inhibition of CGRP is sufficient to treat migraine. However, other evidence suggests a central site of action and it is possible that sufficient amounts can enter the CNS [48]. In addition to small molecule antagonists, a promising complementary strategy is to block CGRP actions using monoclonal antibodies (mAbs) that have been designed as biological drugs against either CGRP or its receptor [51]. Because of their prolonged half-life, humanized mAbs have tremendous potential as prophylactic drugs to prevent migraine. Currently, four mAbs are under development for preventing migraine, and initial reports indicate that they are successful [52,53]. The safety profile of CGRP-targeting drugs to date is consistent with CGRP being primarily a compensatory and modulatory peptide. It should be noted, however, that CGRP has long-term protective effects in prolonged hypertension in mouse models [54,55]. Nonetheless, to date, the clinical trials have supported the safety of CGRP antibodies in humans. Thus, although long-term safety remains to be demonstrated, there is no evidence from human studies to date that suggests that the antibodies should be contraindicated in hypertensive individuals or patients with cardiovascular disease.
Strategy to sensitize mice to CGRP
The rationale to generate a CGRP-sensitized mouse was based on reports that injection of CGRP caused migraine-like headaches in migraineurs, but not nonmigraineurs [30,33]. This suggested that migraineurs are more sensitive to CGRP. One mechanism that could account for this increased sensitivity might be elevated CGRP receptors (Figure1A). We therefore reasoned that increasing CGRP receptor expression might sensitize a mouse to CGRP actions. However, CGRP acts at an unusual G protein-coupled receptor called calcitonin-like receptor (CLR) which has an obligate requirement for a subunit called receptor activity-modifying protein 1 (RAMP1). CLR also binds an intracellular protein, receptor component protein (RCP) which increases G protein coupling. RAMP1 is necessary for trafficking CLR to the cell surface and CGRP binding specificity [56]. Interaction of CLR with the related RAMP 2 and 3 subunits generates adrenomedullin receptors that have lower affinity for CGRP, but can still bind CGRP. Importantly, RAMP1 can also bind other G protein-coupled receptors. Most notably, RAMP1 converts a calcitonin receptor (CTR) to an amylin receptor [57]. Further studies found that the CTR/RAMP1 complex can also bind CGRP with relatively high affinity and is present in trigeminal nerves [58]. Our studies with adenoviral vectors encoding human RAMP1 (hRAMP1) in cultured trigeminal ganglia neurones and vascular smooth muscle revealed that RAMP1 is functionally rate limiting [59,60]. These observations provided the rationale for engineering CGRP-sensitized mice by transgenic overexpression of hRAMP1. Use of the human RAMP1 gene had two advantages. First, it allowed detection of an untagged RAMP1 transgene (at the time, there was controversy over whether tagged RAMP1 functioned correctly, but it is now known to be fine). Second, the human gene would potentially generate mice more sensitive to the CGRP receptor antagonists olcegepant and telcagepant, which had surprising human selectivity.
Figure 1.
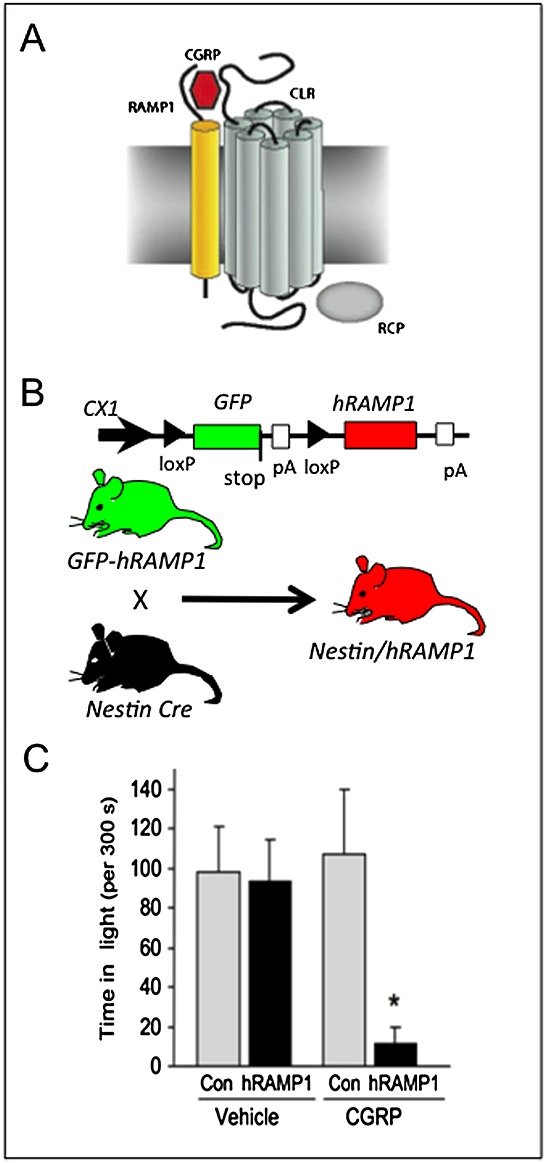
Calcitonin gene-related peptide (CGRP)-induced light-aversive behaviour in nestin/human receptor activity-modifying protein 1 (hRAMP1) mice. (A) A schematic of the CGRP receptor complex consisting of calcitonin-like receptor (CLR), receptor activity-modifying protein 1 (RAMP1), and receptor component protein (RCP) is shown (reproduced from Russo [8]). (B) The conditional hRAMP1 expression strategy is outlined (modified from Zhang et al. [60]). The parental green fluorescent protein (GFP)–hRAMP1 mouse transgene contains a GFP stop sequence that prevents the expression of hRAMP1 in the absence of Cre recombinase action at loxP sites. After crossing GFP–hRAMP1 mice with nestin–cre mice, the GFP stop sequence is removed and hRAMP1 is expressed in the nervous system of double transgenic nestin/hRAMP1 mice. (C) CGRP administration by intracerebroventricular injection caused nestin/hRAMP1 mice to spend less time in the light compared with control mice or mice injected with vehicle. *P < 0.05). Data obtained from Recober et al. [34]
We chose a conditional expression strategy that relies on Cre recombinase to activate the RAMP1 transgene (Figure1B). The first studies were done using RAMP1 expressed throughout the peripheral and CNS both in glia and neurones. These mice, referred to as nestin/hRAMP1, are double transgenics that express hRAMP1 only after removal of an upstream stop sequence in neurones and glia by Cre recombinase under control of the nestin promoter [60]. Nestin/hRAMP1 mice have 1.5–2.0-fold greater levels of total mouse and human RAMP1 in peripheral ganglia and the CNS and increased CGRP-induced neurogenic inflammation [60]. We have subsequently overexpressed hRAMP1 in all tissues, referred to as global hRAMP1 mice. These mice are sensitized to CGRP actions on the vasculature, with improved baroreceptor sensitivity and resistance to angiotensin II-induced hypertension [61,62].
The nestin/hRAMP1 mice have additional properties that are probably not relevant to migraine. Of particular note, they have an unexpectedly lean phenotype, which is most likely caused by increased sympathetic activation of brown fat metabolism due to enhanced amylin activity in combination with CGRP actions [63,64]. Although this metabolic phenotype is interesting, increased metabolism is not a symptom of migraine.
How do you tell if a mouse has a migraine?
Having established the transgenic mouse, we faced the question of how migraine can be measured in a mouse. Of course, we will never fully know if a mouse has migraine. Instead, we reasoned that the associated nonheadache symptoms could be measurable parameters. The primary migraine-like symptom that we tested was photophobia. Photophobia is a subjective experience in which normal levels of light are perceived as unpleasant or painful [65]. It is one of the diagnostic criteria of migraine [66] and is one of the most common migraine symptoms, affecting 66–88% of migraineurs [67]. Sensitivity to light is also reported between attacks, albeit to a lesser degree [67]. As a secondary indicator, we also measured movement as aggravation of the headache by movement is one of the diagnostic criteria of migraine. Although not further discussed in the present review, we also found that the nestin/hRAMP1 mice developed CGRP-induced cutaneous allodynia owing to central sensitization [68]. Mechanical allodynia is reported by over half of migraineurs [69].
Light-aversive behaviour in mice
The strategy to measure photophobia in mice was to use light-aversive behaviour as a surrogate. To do this, we used the classic light/dark box developed to study anxiety behaviour in rodents [70,71]. This assay has been further developed with variations to address anxiety issues by Matynia and colleagues [72]. When CGRP was administered by intracerebroventricular injection, the transgenic hRAMP1 mice spent significantly less time in the light compared with either vehicle- or CGRP-treated control mice (Figure1C) [34,73]. Although there have been no significant differences based on gender, female mice generally show a trend towards greater light aversion. Further studies that monitor the oestrus cycle and/or test hormone replacements may possibly reveal a gender bias. The receptor specificity of CGRP-induced light aversion was confirmed by coinjecting olcegepant, which was effective in migraine clinical trials [43] and has greater affinity for CLR/hRAMP1 than for CTR/hRAMP1 [57]. Although this suggests that the CTR/hRAMP1 receptor has only minimal contributions in this mouse model, a caveat is that the drug concentrations at the relevant sites are not known. Thus, we cannot exclude a combination of multiple receptor actions contributing to the light-aversive phenotype. In this regard, the ability of the CTR/hRAMP1 amylin receptor to also act as a CGRP receptor in cultured trigeminal ganglia neurones raises the prospect of parallel pathways activated by CLR/RAMP1 and CTR/RAMP1 receptors in migraine [74]. Further studies with amylin and the amylin antagonist AC187 in mouse models should prove interesting.
The motility measurements in the light and dark chambers were also very informative. In general, our findings with control and nestin/hRAMP1 mice agreed with previous reports that CGRP decreases motor activity [34,73]. Unexpectedly, we found that CGRP-treated nestin/hRAMP1 and control mice had similar behaviour in the light zone, whereas in the dark zone, nestin/hRAMP1 moved less than control mice [73]. We interpret this light-dependent difference to indicate that when the mice have reached the non-aversive dark environment, they prefer not to move as much. This may reflect pain being aggravated upon movement and is consistent with the preference of people to lie down in a dark room during a migraine.
As mentioned earlier, the light/dark assay was originally developed to test drugs and mutations on anxiety. As such, CGRP-induced light aversion could be due to increased anxiety or fear behaviour. We ruled out a major contribution from anxiety using open-field and predator-odour behavioural tests [34]. However, it is important to realize that this does not rule out an anxiety component to light-aversive behaviour. Indeed, we have speculated that the presence of CGRP in amygdalo–thalamic fibres suggests that the amygdala might modulate photophobia [65]. In addition, although CGRP was centrally administered, these experiments do not rule out a peripheral site of action, especially as some extracranial leakage occurred during injection [75]. Future experiments with targeted activation of the hRAMP1 transgene and/or injection of CGRP should elucidate the pathways underlying light-aversive behaviour.
We then reasoned that, given a sufficiently strong stimulus, even wild-type mice might show CGRP-induced light aversion. With the light increased from 55 lux (a dim room) to 27 000 lux (equivalent to a bright sunny day), there was an apparent trend, although not significant, for wild-type mice to prefer the dark zone following CGRP injection. In this test, we noticed that the trend appeared to be greater the longer that the mice were in the testing chamber. We reasoned that prior habituation to the chamber might reduce the drive to explore the adverse lit zone. When the mice were habituated to the chamber and in the presence of very bright light, CGRP treatment drove them into the dark [75]. This wild-type phenotype demonstrated that endogenous CGRP receptors are sufficient to induce light-aversive behaviour, which indicates that the nestin/hRAMP1 phenotype was due to an elevated number of CGRP receptors, not ectopic transgene receptors. Importantly, the antimigraine drug rizatriptan attenuated the CGRP-induced behaviour in wild-type mice [75], as well as in hRAMP1 transgenic mice [76].
In summary, the efficacy of clinically proven migraine drugs on CGRP-induced light aversion in mice validates this behaviour as a surrogate for migraine-associated photophobia. Furthermore, as with migraineurs, the CGRP-sensitized mice prefer the dark, even at low light levels, whereas wild-type mice only respond in very bright light. Interestingly, the behaviour coincides with reduced locomotor activity in the dark, but not in the light.
Where might CGRP be acting?
Given the ability of CGRP to induce light-aversive behaviour, where might it be acting? Over the past decade, several neural networks have been implicated in the enhanced light sensitivity and pain of photophobia [77,78]. These paths include primarily central locations, but also peripheral sites. A target shared by many of the central and peripheral mechanisms is the trigeminal nerve [79]. The trigeminal nerve is ideally poised at the interface of the CNS and periphery, with central efferent terminals and peripheral afferent fibres. Indeed, it seems likely that CGRP has actions in both the CNS and the periphery that ultimately contribute to migraine pathophysiology. The mechanisms by which CGRP could potentially act at both central and peripheral sites are briefly discussed below.
CGRP as a central modulator
CGRP is widely distributed across the CNS [80]. The predominant CGRP-immunoreactive cell bodies are in the thalamus (especially in the posterior thalamic nuclear group), hypothalamus, ventral tegmental area, periaqueductal gray (PAG) and brainstem nuclei, including the spinal trigeminal nucleus [81–83]. Fibres containing CGRP project to the frontal cortex, amygdala and nucleus accumbens [84]. CGRP receptor binding sites have been mapped to many central structures, including the cortex, limbic system (amygdala, nucleus accumbens, hypothalamus) and brainstem (PAG, medulla, pons) [80,85,86] and are enriched in the PAG and amygdala [80]. The PAG receives ascending pain signals and is part of the descending pain inhibitory system [87]. It also cooperates with the amygdala in processing fear and anxiety [88]. The amygdala is a principal site for processing these behaviours and also relays nociceptive information [89]. In the rat, CGRP is involved in the processing of fear-related sensory information [90], fear conditioning [91,92] and the neuroendocrine fear response [93]. The amygdala is of particular interest, given its high levels of both CGRP and CGRP receptors [80].
With respect to light aversion, perhaps the most relevant sites of CGRP receptors are in the spinal trigeminal nucleus (also called the trigeminal nucleus caudalis), posterior thalamic nuclei, PAG, amygdala nuclei, selected nuclei in the hypothalamus, and the visual and somatosensory cortices (Figure2). Particular interest has been focused on the posterior (Po), lateral posterior (LP) and ventroposteromedial (VPM) thalamus, where Burstein and colleagues [77,94,95] showed convergence of signals initiated by dural trigeminal afferents and melanopsin retinal ganglion cells. Moreover, the VPM nucleus is known to contain CGRP receptors, and CGRP receptor antagonists can inhibit nociceptive trigeminovascular activation of this nucleus [96]. However, a CGRP modulatory role in this region remains to be tested. A recent report described a number of potential modulators in fibres from relevant hypothalamic nuclei, although, surprisingly, there was a lack of CGRP fibres adjacent to thalamic trigeminovascular neurones [97]. Nonetheless, it remains possible that CGRP could diffuse from nearby neurones. In the thalamus, CGRP-immunopositive neurones are located in the peripeduncular nucleus, subparafascicular nucleus and posterior thalamic nuclear group as well as areas ventromedial to this group [82]. Interestingly, somatosensory and nociceptive activity is integrated and relayed from ascending pathways to higher cortical areas via CGRP-containing neurones of the subparafascicular thalamus and the caudal part of the posterior thalamic group [98,99]. These CGRP-containing fibres project to the secondary somatosensory cortex, amygdala, insula and hypothalamus [98–100], indicating roles in nociception, stress, autonomic responses, anxiety and auditory perception. It should prove interesting to see if light perception is also modulated by these neurones.
Figure 2.
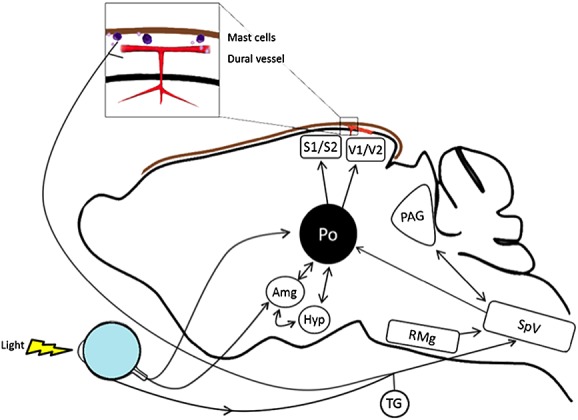
Potential sites of CGRP action in light-aversive behaviour. The rodent brain is shown schematically, with arrows indicating pathways between relevant nuclei and input from the trigeminovascular system and light detected by the eye. These nuclei have calcitonin gene-related peptide (CGRP) receptors and respond to CGRP to modulate either affective behaviour or spinal trigeminal nucleus activity. Not all pathways are shown in this simplified presentation. Abbreviations are as follows: Amg, amygdala; Hyp, hypothalamus (which refers to the A11 nucleus and paraventricular nucleus); TG, trigeminal ganglion (which includes neurones and satellite glia); PAG, periaqueductal gray; Po, posterior thalamic nuclei (which include the posterior, ventroposteromedial and lateral posterior thalamus); Rmg, raphe magnus nucleus; SpV, spinal trigeminal nucleus; S1, S2, somatosensory cortex; V1, V2, visual cortex. Dural mast cells and blood vessels with associated trigeminal fibres and Schwann cells (not shown) are also indicated (brown line represents the dura)
CGRP is a recognized neuromodulator in the CNS that can enhance synaptic transmission mediated by glutamatergic signalling and contribute to central sensitization [6,27,50]. Central sensitization in migraine has been extensively studied and reviewed by Burstein and Burstein [13]. CGRP actions in the central terminals of sensory neurones can increase the responsiveness of glutamate receptors [79,101,102]. CGRP may also promote heat hyperalgesia through additional mechanisms [103]. Recently, CGRP in the ventrolateral PAG has been shown to influence nociceptive transmission in the trigeminal nucleus from dural afferents [104]. Higher in the brain, CGRP can modulate synaptic transmissions to the amygdala and nearby bed nucleus of the stria terminalis, which cause fear and anxiety-like responses [105], and between the lateral parabrachial nucleus and the central nucleus of the amygdala, which is associated with central sensitization and pain-related behaviour [106,107]. Likewise, CGRP has been shown to act on the paraventricular nucleus (PVN) of the hypothalamus to increase corticotrophin-releasing hormone release in the stress response [108], and the PVN has recently been shown to regulate trigeminovascular-evoked activity in the spinal trigeminal nucleus, although a direct CGRP connection has not yet been made [109]. CGRP is also involved in descending efferent pathways from the nucleus raphe magnus and A11 neurones in the posterior hypothalamus that modulate nociception in the spinal trigeminal nucleus [110,111]. The interaction of pain and limbic pathways is especially intriguing. These pathways could potentially be involved in the affective aspects of photophobia [65]. Thus, CGRP is involved in nociceptive central sensitization at multiple levels within the CNS. Whether any of these are involved in light-aversive behaviour can now be tested with mouse models.
CGRP as a peripheral modulator
The ability of peripherally administered CGRP to cause a migraine in people and the prophylactic efficacy of CGRP mAbs strongly suggest (but do not prove) peripheral CGRP actions in migraine. Although the blood–brain barrier prevents 99.9% of most antibodies from reaching the CNS, it is not an absolute barrier [112]. So, it could always be argued that the small amounts of antibodies that do cross the barrier are clinically effective. Such an argument seems unlikely to hold up, but a similar debate has raged with respect to peripherally administered small-molecule CGRP receptor antagonists, which are also not very CNS penetrant [49]. In this regard, a recent positron emission tomography study using telcagepant indicated that central CGRP receptor-mediated effects are not responsible for clinical efficacy [113], which supports (but does not prove) a peripheral site of action.
In the periphery, there are CGRP receptors in the trigeminovasculature on dural mast cells, Schwann cells, trigeminal ganglia neurones and satellite glia, and, of course, blood vessels (Figure2). CGRP actions on vessel tone cannot be ignored. However, although the role of the vasculature in migraine remains a possibility [114], most evidence suggests that vascular changes are an epiphenomenon [115]. Could CGRP actions on the vasculature play a causal role in migraine, even if the vasodilation is an epiphenomenon? As discussed below, one possible mechanism may involve CGRP-induced vascular and nonvascular synthesis and release of NO, which can affect nerves. Indeed, peripheral action does not only involve the vasculature. It is generally accepted that migraine involves the activation and sensitization of the trigeminal nerves that innervate meningeal blood vessels, and the major peptide of these nerves is CGRP [5,6,8]. Indeed, given that migraine is a neurovascular disorder, a peptide such as CGRP, which sits at the interface of trigeminal neural and vascular systems, is an ideal modulator.
In the trigeminovasculature, CGRP contributes to both neurogenic inflammation and peripheral sensitization of nociceptive neurones. There is good evidence for peripheral sensitization in migraine [116], and a likely mechanism involves neurogenic inflammation [13,50]. Although the role of CGRP in neurogenic inflammation is commonly thought to be only as a vasodilator, it can also play an indirect role in plasma extravasation by increasing substance P release [60], and by triggering the release of inflammatory signals from mast cells and glia [117,118]. A direct role for CGRP in degranulation is supported by the presence of receptors on rodent mast cells [119], but this has been cast into doubt by a recent study that did not find receptors on human mast cells [120]. Both rodent and human glia of the trigeminal ganglia and nerve contain CGRP receptors [119,121], which can lead to sensitization of sensory neurones [122–124]. However, despite many animal studies, it remains controversial whether neurogenic inflammation plays a role in human migraine. Moreover, importantly, CGRP administration fails to cause nociceptor activation, and dural administration of a receptor antagonist does not prevent the activation of meningeal nociceptors [125,126].
What, then, might be the mechanism of action of CGRP in the periphery? One possibility is that CGRP may play more of a long-term modulatory role by increasing the expression of nociceptive genes involved in feedback loops that lead to neural sensitization. Three examples of potential CGRP-triggered feedback loops are: NO synthesis and release, purine receptor signalling, and CGRP synthesis and release.
A two-way connection between CGRP and NO that could result in a self-sustaining positive feedback loop has been suggested by rodent and cell culture studies. The evidence for this regulation has been reviewed by Messlinger and colleagues [127]. For example, CGRP can increase NO synthesis and release from trigeminal glia [128,129] and NO release from the vascular endothelium [130]. Conversely, NO can increase CGRP synthesis and release from trigeminal neurones [131,132]. A recent study has extended this observation by demonstrating that NO can form an NO-like molecule, nitroxyl (HNO) with hydrogen sulfide, to cause CGRP release from trigeminal fibres in the dura via a transient receptor potential cation channel A1 (TRPA1)-dependent mechanism [133]. The link between CGRP and NO is further emphasized by the ability of olcegepant to block nitroglycerine-induced activation and sensitization of neurones in the spinal trigeminal nucleus [134]. It is interesting that Tvedskov et al. found that olcegepant was unable to block nitroglycerine-induced migraine [135]. One interpretation that the authors suggest is that CGRP acts upstream of NO release. Thus, CGRP actions on glia and the vascular endothelium could contribute to migraine independently of vasodilation by a positive feedback mechanism involving CGRP and NO.
A second candidate nociceptive molecule regulated by CGRP is the purine receptor P2X3. CGRP is known to increase P2X3 gene expression in nociceptive trigeminal ganglia neurones by a direct autocrine mechanism and by a paracrine mechanism involving brain-derived neurotrophic factor (BDNF) [136]. The P2X3 receptor is an ATP-gated ion channel involved in inflammatory pain transmission [137] and BDNF is also involved in nociception [138] and is elevated during migraine [139]. Although it is not known if BDNF or P2X3 signalling feedback increases CGRP synthesis, it seems likely, given their signalling activities and colocalization with CGRP.
Finally, CGRP can induce its own synthesis in trigeminal ganglia neurones by paracrine and autocrine mechanisms. Neuronal release of CGRP induces the release of tumour necrosis factor-α from satellite glia [122], which feeds back onto the neurones to activate CGRP transcription [140]. Direct autocrine regulation of the CGRP gene in trigeminal ganglia was demonstrated in primary cultures [60] and supported by elevated CGRP levels in the cerebrospinal fluid of the nestin/hRAMP1 transgenic mice [34]. Although the possibility of autocrine regulation in the trigeminal ganglia under normal conditions is unlikely as the CGRP receptor subunits were only rarely colocalized with CGRP in situ [119,121], NO treatment leads to an increase in the number of cell bodies expressing the RAMP1 receptor subunit [141], and CGRP receptor subunit expression can also be induced by other migraine-relevant stimuli (e.g. stress and hypoxia) [57]. Thus, we propose that CGRP can initiate direct and indirect positive feedback loops that lead to a sustained peripheral sensitization and eventually central sensitization of nociceptive neural pathways.
Conclusion
Migraine is a debilitating headache with sensory disturbances that may in part be attributable to the neuropeptide CGRP. Animal studies have demonstrated that CGRP can induce several symptoms consistent with a migraine state. Although the sites of action are still not known, CGRP actions in both the periphery and CNS are well positioned to contribute to these symptoms. Future studies with mouse models should continue to provide clues to the mechanisms played by CGRP in migraine.
Acknowledgments
The author would like to thank members of his laboratory and colleagues for stimulating discussions and acknowledge grant support from NIH grant NS075599.
Competing Interests
The author has completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declares: AFR had support from the NIH for the submitted work; AFR served as a consultant with Alder Biopharma and Pharmovo in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.de Tommaso M, Ambrosini A, Brighina F, Coppola G, Perrotta A, Pierelli F, Sandrini G, Valeriani M, Marinazzo D, Stramaglia S, Schoenen J. Altered processing of sensory stimuli in patients with migraine. Nat Rev Neurol. 2014;10:144–55. doi: 10.1038/nrneurol.2014.14. [DOI] [PubMed] [Google Scholar]
- 2.Charles A. Migraine: a brain state. Curr Opin Neurol. 2013;26:235–9. doi: 10.1097/WCO.0b013e32836085f4. [DOI] [PubMed] [Google Scholar]
- 3.Goadsby PJ, Lipton RB, Ferrari MD. Migraine – current understanding and treatment. N Engl J Med. 2002;346:257–70. doi: 10.1056/NEJMra010917. [DOI] [PubMed] [Google Scholar]
- 4.Pietrobon D, Moskowitz MA. Pathophysiology of migraine. Annu Rev Physiol. 2013;75:365–91. doi: 10.1146/annurev-physiol-030212-183717. [DOI] [PubMed] [Google Scholar]
- 5.Noseda R, Burstein R. Migraine pathophysiology: anatomy of the trigeminovascular pathway and associated neurological symptoms, cortical spreading depression, sensitization, and modulation of pain. Pain. 2013;154:S44–53. doi: 10.1016/j.pain.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 6.Ho TW, Edvinsson L, Goadsby PJ. CGRP and its receptors provide new insights into migraine pathophysiology. Nat Rev Neurol. 2010;6:573–82. doi: 10.1038/nrneurol.2010.127. [DOI] [PubMed] [Google Scholar]
- 7.Villalon CM, Olesen J. The role of CGRP in the pathophysiology of migraine and efficacy of CGRP receptor antagonists as acute antimigraine drugs. Pharmacol Ther. 2009;124:309–23. doi: 10.1016/j.pharmthera.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Russo AF. Calcitonin gene-related peptide (CGRP): a new target for migraine. Annu Rev Pharmacol Toxicol. 2015;55:533–52. doi: 10.1146/annurev-pharmtox-010814-124701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jansen-Olesen I, Tfelt-Hansen P, Olesen J. Animal migraine models for drug development: status and future perspectives. CNS Drugs. 2013;27:1049–68. doi: 10.1007/s40263-013-0121-7. [DOI] [PubMed] [Google Scholar]
- 10.Erdener SE, Dalkara T. Modelling headache and migraine and its pharmacological manipulation. Br J Pharmacol. 2014;171:4575–94. doi: 10.1111/bph.12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romero-Reyes M, Ye Y. Pearls and pitfalls in experimental in vivo models of headache: conscious behavioral research. Cephalalgia. 2013;33:566–76. doi: 10.1177/0333102412472557. [DOI] [PubMed] [Google Scholar]
- 12.Ferrari MD, Klever RR, Terwindt GM, Ayata C, van den Maagdenberg AM. Migraine pathophysiology: lessons from mouse models and human genetics. Lancet Neurol. 2015;14:65–80. doi: 10.1016/S1474-4422(14)70220-0. [DOI] [PubMed] [Google Scholar]
- 13.Bernstein C, Burstein R. Sensitization of the trigeminovascular pathway: perspective and implications to migraine pathophysiology. J Clin Neurol. 2012;8:89–99. doi: 10.3988/jcn.2012.8.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oshinsky ML, Gomonchareonsiri S. Episodic dural stimulation in awake rats: a model for recurrent headache. Headache. 2007;47:1026–36. doi: 10.1111/j.1526-4610.2007.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stucky NL, Gregory E, Winter MK, He YY, Hamilton ES, McCarson KE, Berman NE. Sex differences in behavior and expression of CGRP-related genes in a rodent model of chronic migraine. Headache. 2011;51:674–92. doi: 10.1111/j.1526-4610.2011.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Felice M, Eyde N, Dodick D, Dussor GO, Ossipov MH, Fields HL, Porreca F. Capturing the aversive state of cephalic pain preclinically. Ann Neurol. 2013;74:257–65. doi: 10.1002/ana.23922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashina M, Hansen JM, Olesen J. Pearls and pitfalls in human pharmacological models of migraine: 30 years’ experience. Cephalalgia. 2013;33:540–53. doi: 10.1177/0333102412475234. [DOI] [PubMed] [Google Scholar]
- 18.Chanda ML, Tuttle AH, Baran I, Atlin C, Guindi D, Hathaway G, Israelian N, Levenstadt J, Low D, Macrae L, O'Shea L, Silver A, Zendegui E, Mariette Lenselink A, Spijker S, Ferrari MD, van den Maagdenberg AM, Mogil JS. Behavioral evidence for photophobia and stress-related ipsilateral head pain in transgenic Cacna1a mutant mice. Pain. 2013;154:1254–62. doi: 10.1016/j.pain.2013.03.038. [DOI] [PubMed] [Google Scholar]
- 19.Brennan KC, Bates EA, Shapiro RE, Zyuzin J, Hallows WC, Huang Y, Lee HY, Jones CR, Fu YH, Charles AC, Ptacek LJ. Casein kinase idelta mutations in familial migraine and advanced sleep phase. Sci Transl Med. 2013;5:1–11. doi: 10.1126/scitranslmed.3005784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olesen J, Jansen-Olesen I. Towards a reliable animal model of migraine. Cephalalgia. 2012;32:578–80. doi: 10.1177/0333102412441719. [DOI] [PubMed] [Google Scholar]
- 21.Bates EA, Nikai T, Brennan KC, Fu YH, Charles AC, Basbaum AI, Ptacek LJ, Ahn AH. Sumatriptan alleviates nitroglycerin-induced mechanical and thermal allodynia in mice. Cephalalgia. 2010;30:170–8. doi: 10.1111/j.1468-2982.2009.01864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Markovics A, Kormos V, Gaszner B, Lashgarara A, Szoke E, Sandor K, Szabadfi K, Tuka B, Tajti J, Szolcsanyi J, Pinter E, Hashimoto H, Kun J, Reglodi D, Helyes Z. Pituitary adenylate cyclase-activating polypeptide plays a key role in nitroglycerol-induced trigeminovascular activation in mice. Neurobiol Dis. 2012;45:633–44. doi: 10.1016/j.nbd.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 23.De Felice M, Ossipov MH, Wang R, Lai J, Chichorro J, Meng I, Dodick DW, Vanderah TW, Dussor G, Porreca F. Triptan-induced latent sensitization: a possible basis for medication overuse headache. Ann Neurol. 2010;67:325–37. doi: 10.1002/ana.21897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russell FA, King R, Smillie SJ, Kodji X, Brain SD. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev. 2014;94:1099–142. doi: 10.1152/physrev.00034.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salmon AM, Damaj MI, Marubio LM, Epping-Jordan MP, Merlo-Pich E, Changeux JP. Altered neuroadaptation in opiate dependence and neurogenic inflammatory nociception in alpha CGRP-deficient mice. Nat Neurosci. 2001;4:357–8. doi: 10.1038/86001. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L, Hoff AO, Wimalawansa SJ, Cote GJ, Gagel RF, Westlund KN. Arthritic calcitonin/alpha calcitonin gene-related peptide knockout mice have reduced nociceptive hypersensitivity. Pain. 2001;89:265–73. doi: 10.1016/s0304-3959(00)00378-x. [DOI] [PubMed] [Google Scholar]
- 27.Benarroch EE. CGRP: sensory neuropeptide with multiple neurologic implications. Neurology. 2011;77:281–7. doi: 10.1212/WNL.0b013e31822550e2. [DOI] [PubMed] [Google Scholar]
- 28.Tfelt-Hansen P, Le H. Calcitonin gene-related peptide in blood: is it increased in the external jugular vein during migraine and cluster headache? A review. J Headache Pain. 2009;10:137–43. doi: 10.1007/s10194-009-0112-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cernuda-Morollon E, Larrosa D, Ramon C, Vega J, Martinez-Camblor P, Pascual J. Interictal increase of CGRP levels in peripheral blood as a biomarker for chronic migraine. Neurology. 2013;81:1191–6. doi: 10.1212/WNL.0b013e3182a6cb72. [DOI] [PubMed] [Google Scholar]
- 30.Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J. CGRP may play a causative role in migraine. Cephalalgia. 2002;22:54–61. doi: 10.1046/j.1468-2982.2002.00310.x. [DOI] [PubMed] [Google Scholar]
- 31.Hansen JM, Hauge AW, Olesen J, Ashina M. Calcitonin gene-related peptide triggers migraine-like attacks in patients with migraine with aura. Cephalalgia. 2010;30:1179–86. doi: 10.1177/0333102410368444. [DOI] [PubMed] [Google Scholar]
- 32.Asghar MS, Hansen AE, Amin FM, van der Geest RJ, Koning P, Larsson HB, Olesen J, Ashina M. Evidence for a vascular factor in migraine. Ann Neurol. 2011;69:635–45. doi: 10.1002/ana.22292. [DOI] [PubMed] [Google Scholar]
- 33.Petersen KA, Lassen LH, Birk S, Lesko L, Olesen J. BIBN4096BS antagonizes human alpha-calcitonin gene related peptide-induced headache and extracerebral artery dilatation. Clin Pharmacol Ther. 2005;77:202–13. doi: 10.1016/j.clpt.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 34.Recober A, Kuburas A, Zhang Z, Wemmie JA, Anderson MG, Russo AF. Role of calcitonin gene-related peptide in light-aversive behavior: implications for migraine. J Neurosci. 2009;29:8798–804. doi: 10.1523/JNEUROSCI.1727-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Russo AF, Kuburas A, Kaiser EA, Raddant AC, Recober A. A potential preclinical migraine model: CGRP-sensitized mice. Mol Cell Pharmacol. 2009;1:264–70. [PMC free article] [PubMed] [Google Scholar]
- 36.Schytz HW, Schoonman GG, Ashina M. What have we learnt from triggering migraine? Curr Opin Neurol. 2010;23:259–65. doi: 10.1097/WCO.0b013e328337b884. [DOI] [PubMed] [Google Scholar]
- 37.Rahmann A, Wienecke T, Hansen JM, Fahrenkrug J, Olesen J, Ashina M. Vasoactive intestinal peptide causes marked cephalic vasodilation, but does not induce migraine. Cephalalgia. 2008;28:226–36. doi: 10.1111/j.1468-2982.2007.01497.x. [DOI] [PubMed] [Google Scholar]
- 38.Petersen KA, Birk S, Kitamura K, Olesen J. Effect of adrenomedullin on the cerebral circulation: relevance to primary headache disorders. Cephalalgia. 2009;29:23–30. doi: 10.1111/j.1468-2982.2008.01695.x. [DOI] [PubMed] [Google Scholar]
- 39.Anttila V, Winsvold BS, Gormley P, Kurth T, Bettella F, McMahon G, Kallela M, Malik R, de Vries B, Terwindt G, Medland SE, Todt U, McArdle WL, Quaye L, Koiranen M, Ikram MA, Lehtimaki T, Stam AH, Ligthart L, Wedenoja J, Dunham I, Neale BM, Palta P, Hamalainen E, Schurks M, Rose LM, Buring JE, Ridker PM, Steinberg S, Stefansson H, Jakobsson F, Lawlor DA, Evans DM, Ring SM, Farkkila M, Artto V, Kaunisto MA, Freilinger T, Schoenen J, Frants RR, Pelzer N, Weller CM, Zielman R, Heath AC, Madden PA, Montgomery GW, Martin NG, Borck G, Gobel H, Heinze A, Heinze-Kuhn K, Williams FM, Hartikainen AL, Pouta A, van den Ende J, Uitterlinden AG, Hofman A, Amin N, Hottenga JJ, Vink JM, Heikkila K, Alexander M, Muller-Myhsok B, Schreiber S, Meitinger T, Wichmann HE, Aromaa A, Eriksson JG, Traynor BJ, Trabzuni D, Rossin E, Lage K, Jacobs SB, Gibbs JR, Birney E, Kaprio J, Penninx BW, Boomsma DI, van Duijn C, Raitakari O, Jarvelin MR, Zwart JA, Cherkas L, Strachan DP, Kubisch C, Ferrari MD, van den Maagdenberg AM, Dichgans M, Wessman M, Smith GD, Stefansson K, Daly MJ, Nyholt DR, Chasman DI, Palotie A. North American Brain Expression Consortium, UK Brain Expression Consortium, International Headache Genetics Consortium. Genome-wide meta-analysis identifies new susceptibility loci for migraine. Nat Genet. 2013;45:912–7. doi: 10.1038/ng.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MaassenVanDenBrink A, Ibrahimi K, Edvinsson L. Intracranial and extracranial arteries in migraine. Lancet Neurol. 2013;12:847–8. doi: 10.1016/S1474-4422(13)70198-4. [DOI] [PubMed] [Google Scholar]
- 41.Connor KM, Shapiro RE, Diener HC, Lucas S, Kost J, Fan X, Fei K, Assaid C, Lines C, Ho TW. Randomized, controlled trial of telcagepant for the acute treatment of migraine. Neurology. 2009;73:970–7. doi: 10.1212/WNL.0b013e3181b87942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ho TW, Ferrari MD, Dodick DW, Galet V, Kost J, Fan X, Leibensperger H, Froman S, Assaid C, Lines C, Koppen H, Winner PK. Efficacy and tolerability of MK-0974 (telcagepant), a new oral antagonist of calcitonin gene-related peptide receptor, compared with zolmitriptan for acute migraine: a randomised, placebo-controlled, parallel-treatment trial. Lancet. 2008;372:2115–23. doi: 10.1016/S0140-6736(08)61626-8. [DOI] [PubMed] [Google Scholar]
- 43.Olesen J, Diener HC, Husstedt IW, Goadsby PJ, Hall D, Meier U, Pollentier S, Lesko LM. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med. 2004;350:1104–10. doi: 10.1056/NEJMoa030505. [DOI] [PubMed] [Google Scholar]
- 44.Hewitt DJ, Aurora SK, Dodick DW, Goadsby PJ, Ge YJ, Bachman R, Taraborelli D, Fan X, Assaid C, Lines C, Ho TW. Randomized controlled trial of the CGRP receptor antagonist MK-3207 in the acute treatment of migraine. Cephalalgia. 2011;31:712–22. doi: 10.1177/0333102411398399. [DOI] [PubMed] [Google Scholar]
- 45.Diener HC, Barbanti P, Dahlof C, Reuter U, Habeck J, Podhorna J. BI 44370 TA, an oral CGRP antagonist for the treatment of acute migraine attacks: results from a phase II study. Cephalalgia. 2011;31:573–84. doi: 10.1177/0333102410388435. [DOI] [PubMed] [Google Scholar]
- 46.Marcus R, Goadsby PJ, Dodick D, Stock D, Manos G, Fischer TZ. BMS-927711 for the acute treatment of migraine: a double-blind, randomized, placebo controlled, dose-ranging trial. Cephalalgia. 2014;34:114–25. doi: 10.1177/0333102413500727. [DOI] [PubMed] [Google Scholar]
- 47.Ho TW, Connor KM, Zhang Y, Pearlman E, Koppenhaver J, Fan X, Lines C, Edvinsson L, Goadsby PJ, Michelson D. Randomized controlled trial of the CGRP receptor antagonist telcagepant for migraine prevention. Neurology. 2014;83:958–66. doi: 10.1212/WNL.0000000000000771. [DOI] [PubMed] [Google Scholar]
- 48.Edvinsson L. CGRP blockers in migraine therapy: where do they act? Br J Pharmacol. 2008;155:967–9. doi: 10.1038/bjp.2008.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tfelt-Hansen P, Olesen J. Possible site of action of CGRP antagonists in migraine. Cephalalgia. 2011;31:748–50. doi: 10.1177/0333102411398403. [DOI] [PubMed] [Google Scholar]
- 50.Raddant AC, Russo AF. Calcitonin gene-related peptide in migraine: intersection of peripheral inflammation and central modulation. Expert Rev Mol Med. 2011;13:e36. doi: 10.1017/S1462399411002067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bigal ME, Walter S, Rapoport AM. Therapeutic antibodies against CGRP or its receptor. Br J Clin Pharmacol. 2015;79:886–95. doi: 10.1111/bcp.12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dodick DW, Goadsby PJ, Silberstein SD, Lipton RB, Olesen J, Ashina M, Wilks K, Kudrow D, Kroll R, Kohrman B, Bargar R, Hirman J, Smith J. for the ALDsi. Safety and efficacy of ALD403, an antibody to calcitonin gene-related peptide, for the prevention of frequent episodic migraine: a randomised, double-blind, placebo-controlled, exploratory phase 2 trial. Lancet Neurol. 2014;13:1100–7. doi: 10.1016/S1474-4422(14)70209-1. [DOI] [PubMed] [Google Scholar]
- 53.Dodick DW, Goadsby PJ, Spierings EL, Scherer JC, Sweeney SP, Grayzel DS. Safety and efficacy of LY2951742, a monoclonal antibody to calcitonin gene-related peptide, for the prevention of migraine: a phase 2, randomised, double-blind, placebo-controlled study. Lancet Neurol. 2014;13:885–92. doi: 10.1016/S1474-4422(14)70128-0. [DOI] [PubMed] [Google Scholar]
- 54.Smillie SJ, King R, Kodji X, Outzen E, Pozsgai G, Fernandes E, Marshall N, de Winter P, Heads RJ, Dessapt-Baradez C, Gnudi L, Sams A, Shah AM, Siow RC, Brain SD. An ongoing role of alpha-calcitonin gene-related peptide as part of a protective network against hypertension, vascular hypertrophy, and oxidative stress. Hypertension. 2014;63:1056–62. doi: 10.1161/HYPERTENSIONAHA.113.02517. [DOI] [PubMed] [Google Scholar]
- 55.Wang Z, Martorell BC, Walchli T, Vogel O, Fischer J, Born W, Vogel J. Calcitonin gene-related peptide (CGRP) receptors are important to maintain cerebrovascular reactivity in chronic hypertension. PLoS One. 2015;10:e0123697. doi: 10.1371/journal.pone.0123697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, Foord SM. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–9. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- 57.Hay DL, Poyner DR, Sexton PM. GPCR modulation by RAMPs. Pharmacol Ther. 2006;109:173–97. doi: 10.1016/j.pharmthera.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 58.Walker CS, Hay DL. CGRP in the trigeminovascular system: a role for CGRP, adrenomedullin and amylin receptors? Br J Pharmacol. 2013;170:1293–307. doi: 10.1111/bph.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Z, Dickerson IM, Russo AF. Calcitonin gene-related peptide receptor activation by receptor activity-modifying protein-1 gene transfer to vascular smooth muscle cells. Endocrinology. 2006;147:1932–40. doi: 10.1210/en.2005-0918. [DOI] [PubMed] [Google Scholar]
- 60.Zhang Z, Winborn CS, Marquez de Prado B, Russo AF. Sensitization of calcitonin gene-related peptide receptors by receptor activity-modifying protein-1 in the trigeminal ganglion. J Neurosci. 2007;27:2693–703. doi: 10.1523/JNEUROSCI.4542-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sabharwal R, Zhang Z, Lu Y, Abboud FM, Russo AF, Chapleau MW. Receptor activity-modifying protein 1 increases baroreflex sensitivity and attenuates angiotensin-induced hypertension. Hypertension. 2010;55:627–35. doi: 10.1161/HYPERTENSIONAHA.109.148171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chrissobolis S, Zhang Z, Kinzenbaw DA, Lynch CM, Russo AF, Faraci FM. Receptor activity-modifying protein-1 augments cerebrovascular responses to calcitonin gene-related peptide and inhibits angiotensin II-induced vascular dysfunction. Stroke. 2010;41:2329–34. doi: 10.1161/STROKEAHA.110.589648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fernandes-Santos C, Zhang Z, Morgan DA, Guo DF, Russo AF, Rahmouni K. Amylin acts in the central nervous system to increase sympathetic nerve activity. Endocrinology. 2013;154:2481–8. doi: 10.1210/en.2012-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Z, Liu X, Morgan DA, Kuburas A, Thedens DR, Russo AF, Rahmouni K. Neuronal receptor activity-modifying protein 1 promotes energy expenditure in mice. Diabetes. 2011;60:1063–71. doi: 10.2337/db10-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Russo AF, Recober A. Unanswered questions in headache: so what is photophobia, anyway? Headache. 2013;53:1677–8. doi: 10.1111/head.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Olesen J, Steiner TJ. The international classification of headache disorders, 2nd edn (ICDH-II) J Neurol Neurosurg Psychiatry. 2004;75:808–11. doi: 10.1136/jnnp.2003.031286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mulleners WM, Aurora SK, Chronicle EP, Stewart R, Gopal S, Koehler PJ. Self-reported photophobic symptoms in migraineurs and controls are reliable and predict diagnostic category accurately. Headache. 2001;41:31–9. doi: 10.1046/j.1526-4610.2001.111006031.x. [DOI] [PubMed] [Google Scholar]
- 68.Marquez de Prado B, Hammond DL, Russo AF. Genetic enhancement of calcitonin gene-related peptide-induced central sensitization to mechanical stimuli in mice. J Pain. 2009;10:992–1000. doi: 10.1016/j.jpain.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burstein R, Yarnitsky D, Goor-Aryeh I, Ransil BJ, Bajwa ZH. An association between migraine and cutaneous allodynia. Ann Neurol. 2000;47:614–24. [PubMed] [Google Scholar]
- 70.Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 1980;13:167–70. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- 71.Bourin M, Hascoet M. The mouse light/dark box test. Eur J Pharmacol. 2003;463:55–65. doi: 10.1016/s0014-2999(03)01274-3. [DOI] [PubMed] [Google Scholar]
- 72.Matynia A, Parikh S, Chen B, Kim P, McNeill DS, Nusinowitz S, Evans C, Gorin MB. Intrinsically photosensitive retinal ganglion cells are the primary but not exclusive circuit for light aversion. Exp Eye Res. 2012;105:60–9. doi: 10.1016/j.exer.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 73.Recober A, Kaiser EA, Kuburas A, Russo AF. Induction of multiple photophobic behaviors in a transgenic mouse sensitized to CGRP. Neuropharmacology. 2010;58:156–65. doi: 10.1016/j.neuropharm.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Walker CS, Eftekhari S, Bower RL, Wilderman A, Insel PA, Edvinsson L, Waldvogel HJ, Jamaluddin MA, Russo AF, Hay DL. A second trigeminal CGRP receptor: function and expression of the AMY1 receptor. Ann Clin Transl Neurol. 2015;2:595–608. doi: 10.1002/acn3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kaiser EA, Kuburas A, Recober A, Russo AF. Modulation of CGRP-induced light aversion in wild-type mice by a 5-HT(1B/D) agonist. J Neurosci. 2012;32:15439–49. doi: 10.1523/JNEUROSCI.3265-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Recober A, Kaiser EA, Kuburas A, Wemmie JA, Anderson MG, Russo AF. CGRP-induced photophobia blocked by olcegepant and rizatriptan in a transgenic migraine model. Cephalalgia. 2009;29:1. [Google Scholar]
- 77.Noseda R, Burstein R. Advances in understanding the mechanisms of migraine-type photophobia. Curr Opin Neurol. 2011;24:197–202. doi: 10.1097/WCO.0b013e3283466c8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rossi HL, Recober A. Photophobia in primary headaches. Headache. 2015;55:600–4. doi: 10.1111/head.12532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Messlinger K. Migraine: where and how does the pain originate? Exp Brain Res. 2009;196:179–93. doi: 10.1007/s00221-009-1756-y. [DOI] [PubMed] [Google Scholar]
- 80.van Rossum D, Hanisch UK, Quirion R. Neuroanatomical localization, pharmacological characterization and functions of CGRP, related peptides and their receptors. Neurosci Biobehav Rev. 1997;21:649–78. doi: 10.1016/s0149-7634(96)00023-1. [DOI] [PubMed] [Google Scholar]
- 81.Kawai Y, Takami K, Shiosaka S, Emson PC, Hillyard CJ, Girgis S, MacIntyre I, Tohyama M. Topographic localization of calcitonin gene-related peptide in the rat brain: an immunohistochemical analysis. Neuroscience. 1985;15:747–63. doi: 10.1016/0306-4522(85)90076-4. [DOI] [PubMed] [Google Scholar]
- 82.Kresse A, Jacobowitz DM, Skofitsch G. Detailed mapping of CGRP mRNA expression in the rat central nervous system: comparison with previous immunocytochemical findings. Brain Res Bull. 1995;36:261–74. doi: 10.1016/0361-9230(94)00201-b. [DOI] [PubMed] [Google Scholar]
- 83.Ma W, Chabot JG, Powell KJ, Jhamandas K, Dickerson IM, Quirion R. Localization and modulation of calcitonin gene-related peptide-receptor component protein-immunoreactive cells in the rat central and peripheral nervous systems. Neuroscience. 2003;120:677–94. doi: 10.1016/s0306-4522(03)00159-3. [DOI] [PubMed] [Google Scholar]
- 84.Dobolyi A, Irwin S, Makara G, Usdin TB, Palkovits M. Calcitonin gene-related peptide-containing pathways in the rat forebrain. J Comp Neurol. 2005;489:92–119. doi: 10.1002/cne.20618. [DOI] [PubMed] [Google Scholar]
- 85.Skofitsch G, Jacobowitz DM. Autoradiographic distribution of 125I calcitonin gene-related peptide binding sites in the rat central nervous system. Peptides. 1985;6:975–86. doi: 10.1016/0196-9781(85)90331-6. [DOI] [PubMed] [Google Scholar]
- 86.Yashpal K, Kar S, Dennis T, Quirion R. Quantitative autoradiographic distribution of calcitonin gene-related peptide (hCGRP alpha) binding sites in the rat and monkey spinal cord. J Comp Neurol. 1992;322:224–32. doi: 10.1002/cne.903220208. [DOI] [PubMed] [Google Scholar]
- 87.Gebhart GF. Descending modulation of pain. Neurosci Biobehav Rev. 2004;27:729–37. doi: 10.1016/j.neubiorev.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 88.Behbehani MM. Functional characteristics of the midbrain periaqueductal gray. Prog Neurobiol. 1995;46:575–605. doi: 10.1016/0301-0082(95)00009-k. [DOI] [PubMed] [Google Scholar]
- 89.Calvino B, Grilo RM. Central pain control. Joint Bone Spine. 2006;73:10–6. doi: 10.1016/j.jbspin.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 90.Kocorowski LH, Helmstetter FJ. Calcitonin gene-related peptide released within the amygdala is involved in Pavlovian auditory fear conditioning. Neurobiol Learn Mem. 2001;75:149–63. doi: 10.1006/nlme.2000.3963. [DOI] [PubMed] [Google Scholar]
- 91.Kovacs A, Telegdy G. Effects of CGRP on active avoidance behavior in rats. Physiol Behav. 1995;58:429–35. doi: 10.1016/0031-9384(95)00066-r. [DOI] [PubMed] [Google Scholar]
- 92.Poore LH, Helmstetter FJ. The effects of central injections of calcitonin gene-related peptide on fear-related behavior. Neurobiol Learn Mem. 1996;66:241–5. doi: 10.1006/nlme.1996.0065. [DOI] [PubMed] [Google Scholar]
- 93.Brown MR, Gray TS. Peptide injections into the amygdala of conscious rats: effects on blood pressure, heart rate and plasma catecholamines. Regul Pept. 1988;21:95–106. doi: 10.1016/0167-0115(88)90094-8. [DOI] [PubMed] [Google Scholar]
- 94.Noseda R, Kainz V, Jakubowski M, Gooley JJ, Saper CB, Digre K, Burstein R. A neural mechanism for exacerbation of headache by light. Nat Neurosci. 2010;13:239–45. doi: 10.1038/nn.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Maleki N, Becerra L, Upadhyay J, Burstein R, Borsook D. Direct optic nerve pulvinar connections defined by diffusion MR tractography in humans: implications for photophobia. Hum Brain Mapp. 2012;33:75–88. doi: 10.1002/hbm.21194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Summ O, Charbit AR, Andreou AP, Goadsby PJ. Modulation of nocioceptive transmission with calcitonin gene-related peptide receptor antagonists in the thalamus. Brain. 2010;133:2540–8. doi: 10.1093/brain/awq224. [DOI] [PubMed] [Google Scholar]
- 97.Noseda R, Kainz V, Borsook D, Burstein R. Neurochemical pathways that converge on thalamic trigeminovascular neurons: potential substrate for modulation of migraine by sleep, food intake, stress and anxiety. PLoS One. 2014;9:e103929. doi: 10.1371/journal.pone.0103929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.de Lacalle S, Saper CB. Calcitonin gene-related peptide-like immunoreactivity marks putative visceral sensory pathways in human brain. Neuroscience. 2000;100:115–30. doi: 10.1016/s0306-4522(00)00245-1. [DOI] [PubMed] [Google Scholar]
- 99.Gauriau C, Bernard JF. Posterior triangular thalamic neurons convey nociceptive messages to the secondary somatosensory and insular cortices in the rat. J Neurosci. 2004;24:752–61. doi: 10.1523/JNEUROSCI.3272-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Campeau S, Watson SJ., Jr Connections of some auditory-responsive posterior thalamic nuclei putatively involved in activation of the hypothalamo–pituitary–adrenocortical axis in response to audiogenic stress in rats: an anterograde and retrograde tract tracing study combined with Fos expression. J Comp Neurol. 2000;423:474–91. [PubMed] [Google Scholar]
- 101.Seybold VS. The role of peptides in central sensitization. Handb Exp Pharmacol. 2009;194:451–91. doi: 10.1007/978-3-540-79090-7_13. [DOI] [PubMed] [Google Scholar]
- 102.Fischer MJ. Calcitonin gene-related peptide receptor antagonists for migraine. Expert Opin Investig Drugs. 2010;19:815–23. doi: 10.1517/13543784.2010.490829. [DOI] [PubMed] [Google Scholar]
- 103.Rogoz K, Andersen HH, Kullander K, Lagerstrom MC. Glutamate, substance P, and calcitonin gene-related peptide cooperate in inflammation-induced heat hyperalgesia. Mol Pharmacol. 2014;85:322–34. doi: 10.1124/mol.113.089532. [DOI] [PubMed] [Google Scholar]
- 104.Pozo-Rosich P, Storer RJ, Charbit AR, Goadsby PJ. Periaqueductal gray calcitonin gene-related peptide modulates trigeminovascular neurons. Cephalalgia. 2015 doi: 10.1177/0333102415576723. in press. [DOI] [PubMed] [Google Scholar]
- 105.Sink KS, Walker DL, Yang Y, Davis M. Calcitonin gene-related peptide in the bed nucleus of the stria terminalis produces an anxiety-like pattern of behavior and increases neural activation in anxiety-related structures. J Neurosci. 2011;31:1802–10. doi: 10.1523/JNEUROSCI.5274-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. Neuroscientist. 2004;10:221–34. doi: 10.1177/1073858403261077. [DOI] [PubMed] [Google Scholar]
- 107.Han JS, Adwanikar H, Li Z, Ji G, Neugebauer V. Facilitation of synaptic transmission and pain responses by CGRP in the amygdala of normal rats. Mol Pain. 2010;6:10. doi: 10.1186/1744-8069-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kovacs A, Biro E, Szeleczky I, Telegdy G. Role of endogenous CRF in the mediation of neuroendocrine and behavioral responses to calcitonin gene-related peptide in rats. Neuroendocrinology. 1995;62:418–24. doi: 10.1159/000127031. [DOI] [PubMed] [Google Scholar]
- 109.Robert C, Bourgeais L, Arreto CD, Condes-Lara M, Noseda R, Jay T, Villanueva L. Paraventricular hypothalamic regulation of trigeminovascular mechanisms involved in headaches. J Neurosci. 2013;33:8827–40. doi: 10.1523/JNEUROSCI.0439-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Huang Y, Brodda-Jansen G, Lundeberg T, Yu LC. Anti-nociceptive effects of calcitonin gene-related peptide in nucleus raphe magnus of rats: an effect attenuated by naloxone. Brain Res. 2000;873:54–9. doi: 10.1016/s0006-8993(00)02473-2. [DOI] [PubMed] [Google Scholar]
- 111.Charbit AR, Akerman S, Holland PR, Goadsby PJ. Neurons of the dopaminergic/calcitonin gene-related peptide A11 cell group modulate neuronal firing in the trigeminocervical complex: an electrophysiological and immunohistochemical study. J Neurosci. 2009;29:12532–41. doi: 10.1523/JNEUROSCI.2887-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood–brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 113.Hostetler ED, Joshi AD, Sanabria-Bohorquez S, Fan H, Zeng Z, Purcell M, Gantert L, Riffel K, Williams M, O'Malley S, Miller P, Selnick HG, Gallicchio SN, Bell IM, Salvatore CA, Kane SA, Li CC, Hargreaves RJ, de Groot T, Bormans G, Van Hecken A, Derdelinckx I, de Hoon J, Reynders T, Declercq R, De Lepeleire I, Kennedy WP, Blanchard R, Marcantonio EE, Sur C, Cook JJ, Van Laere K, Evelhoch JL. In vivo quantification of calcitonin gene-related peptide receptor occupancy by telcagepant in rhesus monkey and human brain using the positron emission tomography tracer [11C]MK-4232. J Pharmacol Exp Ther. 2013;347:478–86. doi: 10.1124/jpet.113.206458. [DOI] [PubMed] [Google Scholar]
- 114.Ashina M. Vascular changes have a primary role in migraine. Cephalalgia. 2012;32:428–30. doi: 10.1177/0333102412438978. [DOI] [PubMed] [Google Scholar]
- 115.Charles A. Vasodilation out of the picture as a cause of migraine headache. Lancet Neurol. 2013;12:419–20. doi: 10.1016/S1474-4422(13)70051-6. [DOI] [PubMed] [Google Scholar]
- 116.Olesen J, Burstein R, Ashina M, Tfelt-Hansen P. Origin of pain in migraine: evidence for peripheral sensitisation. Lancet Neurol. 2009;8:679–90. doi: 10.1016/S1474-4422(09)70090-0. [DOI] [PubMed] [Google Scholar]
- 117.Ottosson A, Edvinsson L. Release of histamine from dural mast cells by substance P and calcitonin gene-related peptide. Cephalalgia. 1997;17:166–74. doi: 10.1046/j.1468-2982.1997.1703166.x. [DOI] [PubMed] [Google Scholar]
- 118.Theoharides TC, Donelan J, Kandere-Grzybowska K, Konstantinidou A. The role of mast cells in migraine pathophysiology. Brain Res Brain Res Rev. 2005;49:65–76. doi: 10.1016/j.brainresrev.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 119.Lennerz JK, Ruhle V, Ceppa EP, Neuhuber WL, Bunnett NW, Grady EF, Messlinger K. Calcitonin receptor-like receptor (CLR), receptor activity-modifying protein 1 (RAMP1), and calcitonin gene-related peptide (CGRP) immunoreactivity in the rat trigeminovascular system: differences between peripheral and central CGRP receptor distribution. J Comp Neurol. 2008;507:1277–99. doi: 10.1002/cne.21607. [DOI] [PubMed] [Google Scholar]
- 120.Eftekhari S, Warfvinge K, Blixt FW, Edvinsson L. Differentiation of nerve fibers storing CGRP and CGRP receptors in the peripheral trigeminovascular system. J Pain. 2013;14:1289–303. doi: 10.1016/j.jpain.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 121.Eftekhari S, Salvatore CA, Calamari A, Kane SA, Tajti J, Edvinsson L. Differential distribution of calcitonin gene-related peptide and its receptor components in the human trigeminal ganglion. Neuroscience. 2010;169:683–96. doi: 10.1016/j.neuroscience.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 122.Thalakoti S, Patil VV, Damodaram S, Vause CV, Langford LE, Freeman SE, Durham PL. Neuron-glia signaling in trigeminal ganglion: implications for migraine pathology. Headache. 2007;47:1008–23. doi: 10.1111/j.1526-4610.2007.00854.x. discussion 24–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Capuano A, De Corato A, Lisi L, Tringali G, Navarra P, Dello Russo C. Proinflammatory-activated trigeminal satellite cells promote neuronal sensitization: relevance for migraine pathology. Mol Pain. 2009;5:43. doi: 10.1186/1744-8069-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.De Corato A, Lisi L, Capuano A, Tringali G, Tramutola A, Navarra P, Dello Russo C. Trigeminal satellite cells express functional calcitonin gene-related peptide receptors, whose activation enhances interleukin-1beta pro-inflammatory effects. J Neuroimmunol. 2011;237:39–46. doi: 10.1016/j.jneuroim.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 125.Levy D, Burstein R, Strassman AM. Calcitonin gene-related peptide does not excite or sensitize meningeal nociceptors: implications for the pathophysiology of migraine. Ann Neurol. 2005;58:698–705. doi: 10.1002/ana.20619. [DOI] [PubMed] [Google Scholar]
- 126.Sixt ML, Messlinger K, Fischer MJ. Calcitonin gene-related peptide receptor antagonist olcegepant acts in the spinal trigeminal nucleus. Brain. 2009;132:3134–41. doi: 10.1093/brain/awp168. [DOI] [PubMed] [Google Scholar]
- 127.Messlinger K, Lennerz JK, Eberhardt M, Fischer MJ. CGRP and NO in the trigeminal system: mechanisms and role in headache generation. Headache. 2012;52:1411–27. doi: 10.1111/j.1526-4610.2012.02212.x. [DOI] [PubMed] [Google Scholar]
- 128.Li J, Vause CV, Durham PL. Calcitonin gene-related peptide stimulation of nitric oxide synthesis and release from trigeminal ganglion glial cells. Brain Res. 2008;1196:22–32. doi: 10.1016/j.brainres.2007.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cady RK, Vause CV, Ho TW, Bigal ME, Durham PL. Elevated saliva calcitonin gene-related peptide levels during acute migraine predict therapeutic response to rizatriptan. Headache. 2009;49:1258–66. doi: 10.1111/j.1526-4610.2009.01523.x. [DOI] [PubMed] [Google Scholar]
- 130.Brain SD, Grant AD. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev. 2004;84:903–34. doi: 10.1152/physrev.00037.2003. [DOI] [PubMed] [Google Scholar]
- 131.Bellamy J, Bowen EJ, Russo AF, Durham PL. Nitric oxide regulation of calcitonin gene-related peptide gene expression in rat trigeminal ganglia neurons. Eur J Neurosci. 2006;23:2057–66. doi: 10.1111/j.1460-9568.2006.04742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Eberhardt M, Hoffmann T, Sauer SK, Messlinger K, Reeh PW, Fischer MJ. Calcitonin gene-related peptide release from intact isolated dorsal root and trigeminal ganglia. Neuropeptides. 2008;42:311–7. doi: 10.1016/j.npep.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 133.Dux M, Will C, Vogler B, Filipovic MR, Messlinger K. Meningeal blood flow is controlled by H S-NO crosstalk activating HNO-TRPA1-CGRP signalling. Br J Pharmacol. 2015 doi: 10.1111/bph.13164. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Koulchitsky S, Fischer MJ, Messlinger K. Calcitonin gene-related peptide receptor inhibition reduces neuronal activity induced by prolonged increase in nitric oxide in the rat spinal trigeminal nucleus. Cephalalgia. 2009;29:408–17. doi: 10.1111/j.1468-2982.2008.01745.x. [DOI] [PubMed] [Google Scholar]
- 135.Tvedskov JF, Tfelt-Hansen P, Petersen KA, Jensen LT, Olesen J. CGRP receptor antagonist olcegepant (BIBN4096BS) does not prevent glyceryl trinitrate-induced migraine. Cephalalgia. 2010;30:1346–53. doi: 10.1177/0333102410363491. [DOI] [PubMed] [Google Scholar]
- 136.Simonetti M, Giniatullin R, Fabbretti E. Mechanisms mediating the enhanced gene transcription of P2X3 receptor by calcitonin gene-related peptide in trigeminal sensory neurons. J Biol Chem. 2008;283:18743–52. doi: 10.1074/jbc.M800296200. [DOI] [PubMed] [Google Scholar]
- 137.Souslova V, Cesare P, Ding Y, Akopian AN, Stanfa L, Suzuki R, Carpenter K, Dickenson A, Boyce S, Hill R, Nebenuis-Oosthuizen D, Smith AJ, Kidd EJ, Wood JN. Warm-coding deficits and aberrant inflammatory pain in mice lacking P2X3 receptors. Nature. 2000;407:1015–7. doi: 10.1038/35039526. [DOI] [PubMed] [Google Scholar]
- 138.Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–21. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 139.Fischer M, Wille G, Klien S, Shanib H, Holle D, Gaul C, Broessner G. Brain-derived neurotrophic factor in primary headaches. J Headache Pain. 2012;13:469–75. doi: 10.1007/s10194-012-0454-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bowen EJ, Schmidt TW, Firm CS, Russo AF, Durham PL. Tumor necrosis factor-alpha stimulation of calcitonin gene-related peptide expression and secretion from rat trigeminal ganglion neurons. J Neurochem. 2006;96:65–77. doi: 10.1111/j.1471-4159.2005.03524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Seiler K, Nusser JI, Lennerz JK, Neuhuber WL, Messlinger K. Changes in calcitonin gene-related peptide (CGRP) receptor component and nitric oxide receptor (sGC) immunoreactivity in rat trigeminal ganglion following glyceroltrinitrate pretreatment. J Headache Pain. 2013;14:74. doi: 10.1186/1129-2377-14-74. [DOI] [PMC free article] [PubMed] [Google Scholar]


