Abstract
Aim
We investigated whether moxifloxacin-induced QTc prolongations in Japanese and Caucasian healthy male volunteers were significantly different.
Methods
A two period, randomized, crossover, ICH-E14-compliant thorough QT (TQT) study compared placebo-corrected changes in QTc interval from baseline (ΔΔQTcF) and concentration–effect relationships following administration of placebo and 400 mg moxifloxacin to 40 healthy male volunteers from each ethnic population. The point estimates of ΔΔQTcF for each population, and the difference between the two, were calculated at a geometric mean Cmax of moxifloxacin using a linear mixed effects model. The concentration–effect slopes of the two populations were also compared. Equivalence was concluded if the two-sided 90% confidence interval of the difference in ΔΔQTcF was contained within −5 ms to +5 ms limits and the ratio of the slopes was between 0.5 and 2.
Results
There were no statistically significant differences between the two populations studied, Japanese vs. Caucasians, respectively, for moxifloxacin Cmax (3.27 ± 0.6 vs. 2.98 ± 0.7 µg ml–1), ΔΔQTcF (9.63 ± 1.15 vs. 11.46 ± 1.19 ms at Cmax of 3.07 µg ml–1) and concentration–response slopes (2.58 ± 0.62 vs. 2.34 ± 0.64 ms per µg ml–1). The difference in the two ΔΔQTcF of −1.8 (90% CI −4.6, 0.9) and the ratio of the two slopes (1.1; 90% CI 0.63, 1.82) were within pre-specified equivalence limits.
Conclusions
Moxifloxacin-induced QTc prolongations did not differ significantly between the Japanese and Caucasian subjects. However, before our findings are more widely generalized, further studies in other populations and with other QT-prolonging drugs are needed to clarify whether inter-ethnic differences in QT sensitivity exist and whether ethnicity of the study population may affect the outcome of a TQT study.
Keywords: Caucasians, ethnicity, Japanese, moxifloxacin, QTc interval, thorough QT studies
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Regulatory guidelines recommend subgroup analysis of drug response by ethnicity.
Studies with quinidine and levofloxacin suggest inter-ethnic differences in QT sensitivity which, if applicable to moxifloxacin, may adversely affect establishing assay sensitivity in some thorough QT studies.
An ethnicity-based direct comparison, evaluating moxifloxacin as a universal positive control, was required.
WHAT THIS STUDY ADDS
Healthy Japanese and Caucasian adult males display similar QT sensitivity to moxifloxacin.
Moxifloxacin appears to be suitable as a universal positive control for thorough QT studies.
Further studies in other populations and with other drugs are needed to clarify whether ethnicity may affect the overall outcomes of thorough QT studies.
Introduction
Drug-induced prolongation of the QT interval of the surface electrocardiogram (ECG), and the associated potential for lethal ventricular arrhythmias, is one of the major reasons for removal of approved drugs from the market and for discontinuation of development of some new chemical entities [1]. It is also responsible for the delay in approval or restricted prescribing of a large number of drugs [2]. Therefore, characterizing the QT effects of a new drug, and of marketed drugs when appropriate, has become an important component of modern pharmaceutical development programmes.
The International Conference on Harmonisation (ICH) guideline, ICH E14 [3] adopted in May 2005, calls for all new drugs with systemic bioavailability, and already marketed drugs if their post-marketing safety experience warrants, to be carefully studied for their effect on QT interval. This guideline calls for a specifically dedicated study, popularly known as a thorough QT (TQT) study or thorough ECG trial (TET), aimed at definitive pre-approval characterization of the drug for this effect. The requirements for, and the design of, a TQT study are discussed in the ICH E14 guidance and some aspects of it have been updated in the subsequent Q&A documents which have been released by the ICH E14 Implementation Working Group [4,5]. A typical TQT study involves four treatment arms: the investigational drug at a therapeutic and a supratherapeutic dose, placebo and an active positive control with a known QT-prolonging effect to establish assay sensitivity. In terms of the study population, ICH E14 notes that ‘Although data are limited, it is not expected that the results of the ‘thorough QT/QTc study’ would be affected by ethnic factors’.
However, there is abundant published literature documenting inter-ethnic differences in drug response, which has been reviewed earlier [6]. A particularly striking example of drugs with ethnic sensitivity is BiDil, approved by the US FDA in June 2005, which is restricted for use in heart failure in self-identified Black patients. There are inter-ethnic differences in the frequencies of variants of drug metabolizing enzymes and transporters [7–10] and of pharmacological targets, including cardiac sodium and potassium channels [11,12]. First degree relatives of patients with acquired long QT syndrome have greater drug-induced prolongation compared with control relatives, supporting a genetic predisposition to acquired long QT syndrome [13]. Consequently, the possibility of differences in ethnic sensitivities to drug-induced QT interval prolongation cannot be excluded. Indeed, isolated studies have already reported this possibility following investigations comparing Caucasians with Black Nigerians, Koreans and Japanese [14–16]. There are also data that are suggestive of inter-ethnic differences in QT sensitivity to moxifloxacin but the ethnicity of the populations studied has ranged widely and includes Asians (which may include Japanese and non-Japanese) and Caucasians from different geographical regions [17]. Current regulatory guidance, recommending sponsors of drugs to address ethnicity of the study population (and therefore, by inference, potential inter-ethnic differences in drug response including QT interval prolongation), has been reviewed elsewhere [6,18]. However, hitherto, the issue of potential differences in ethnic sensitivities to QT prolongation by drugs has not been satisfactorily resolved in an ICH-E14-compliant TQT study.
This prospective study was, therefore, designed specifically to provide a direct comparison of two ethnically distinct populations of healthy male volunteers, Caucasians in the US and Japanese in Japan, with regard to their QT sensitivity to moxifloxacin. This particular drug was selected for investigation not only because a single 400 mg oral dose of moxifloxacin is widely used as an active control in TQT studies [19] but also because it has predictable absorption and pharmacokinetics and a small but well characterized QTc effect with a predictable time course (time-matched placebo-corrected increase in QTc interval from baseline (ΔΔQTc) of the order of 10–15 ms following a single 400 mg oral dose) [17,20]. Given this modest effect on QTc interval, the ethnicity of a TQT study population might adversely impact significantly on establishing assay sensitivity and therefore, the regulatory validity of the study, if there were inter-ethnic differences in QT sensitivity to moxifloxacin.
Methods
Objective
The primary objective of this study was to assess the effects of moxifloxacin, relative to placebo, on Fridericia-corrected QTc interval (ΔΔQTcF) in healthy adult male Japanese and Caucasian subjects following a single 400 mg oral dose to determine if there were any differences between their QTcF interval responses. For the purpose of assessing equivalency, the primary parameter was the difference in ΔΔQTcF at a geometric mean (gMean) Cmax of moxifloxacin, and the secondary parameter was the PK–PD model-based difference in the observed concentration–response slopes, between the two populations. The gMean Cmax of moxifloxacin to be used was a single common value relevant to both the populations.
Study design
A two period, randomized, crossover study design, compliant of the ICH E14 recommendations and best practices commonly employed in a TQT study, was selected since each subject acted as his own control. It was conducted as identically as possible at two separate sites (one in Japan and the other in the United States) in healthy male subjects housed in phase I units. Moxifloxacin was administered as a single 400 mg oral dose. Since there is no standard placebo that matches the exact appearance of a moxifloxacin tablet and the majority of TQT studies have administered moxifloxacin without blinding, no attempt was made to match the appearance of the placebo and moxifloxacin tablets in this study. Each site used its own placebo tablets. To identify treatment-induced changes in the QTcF interval in the presence of high spontaneous variability in the duration of this interval, we carefully controlled for any residual source of variability. The study used strict procedural controls including admission to the study site 2 days prior to dosing. Subjects were controlled for posture, meal intake and activity throughout the study period. Three sequential ECGs were collected at each time point to control for the normal biologic or spontaneous variation. The study was conducted in compliance with the standards of Good Clinical Practice and Declaration of Helsinki, 2008 and the protocol was approved by the sites’ institutional review boards (SeaView Ethics Committee, approval number 201403096 and Kitasato University Medical Ethics Committee, approval number 13–836).
Study populations
Healthy male volunteers from each of the two ethnic regions (Caucasians from US and the Japanese from Japan) were selected as the study population in line with the recommendations in ICH E14. Subjects in the USA were categorized as Caucasians if they were White and of European descent whereas in Japan, the categorization of a subject as Japanese was based on ancestry and local self-identification. Each subject gave his fully informed written consent prior to participation. During the recruitment, screening and baseline visit, the subjects complied with the following restrictions:
No strenuous physical exercise for 3 days before dosing until after study completion evaluation.
No alcohol from 48 h before dosing until after study completion evaluation.
Meals were served at the same time on the baseline and on the treatment days. Meals were similar in caloric content and distribution for all subjects on both days of dosing.
The same inclusion and exclusion criteria were applied at each site and required the healthy volunteers to be aged 18–45 years and have a body mass index (BMI) within 18–28 kg m–2 at screening. Exclusion criteria included a family history of QTc prolongation or of unexplainable sudden death at <50 years of age, and at screening, resting supine heart rate less than 50 beats min–1 or greater than 100 beats min–1 (resting vital signs could be repeated once at the discretion of the investigator), resting supine systolic blood pressure less than 90 mmHg or greater than 140 mmHg; resting supine diastolic blood pressure less than 50 mmHg or greater than 90 mmHg. An abnormal 12-lead ECG at screening, defined as showing the presence QTc >450 ms, QRS> 110 ms, PR > 200 ms and/or second or third degree heart block, was also an exclusion criterion.
Sample size
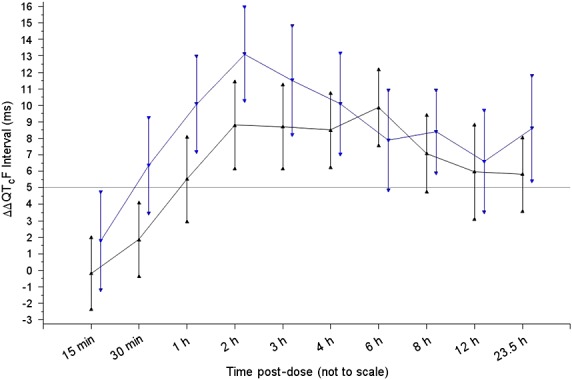
The hypotheses to be tested for the primary efficacy variable are formulated as follows:
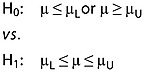 |
where μ is the ΔΔQTcF between the two ethnic groups at a common moxifloxacin concentration of 3 µg ml–1 and μU and μL are the upper and lower margins of equivalence. The sample size was calculated based on a boostrap simulation approach. A historical study with 124 subjects was used as the population to sample from randomly. The subjects were ranked based on their body weights within each gender. The lower 50% female subjects were combined with the lower 50% male subjects to approximate the Japanese population and the rest was used to approximate the Caucasian population because the major difference between the two ethnic groups was considered to be the body weight. Body weight is known to affect moxifloxacin concentration given the same dose (higher body weight is associated with lower moxifloxacin concentration). This historical study had μ = 0.95 at 3 µg ml–1 of moxifloxacin concentration and 10.5 ms as the standard deviation (SD) for ΔΔQTcF. The equivalence margin is set as 5 ms. As a result, μL is –5 ms and μU is 5 ms. Under these conditions, 80 subjects (40 at each site) were expected to provide approximately 80% power for the equivalence test (90% CI within the equivalence margins).
Study drugs and randomization
Following a day (day 0) for baseline evaluations, subjects randomized to each treatment period were confined to the phase I unit and received the assigned study drug (placebo or 400 mg moxifloxacin tablet) as a single oral dose in the fasted state in the morning of study day 1 of each treatment period. The randomized administration of study drugs was double-blind with half of the subjects being assigned to each of the two treatment sequences (half of the subjects receiving placebo followed by moxifloxacin and vice versa for the other half) with a minimum 3-days washout period between the treatments. All subjects were fasted (no food and liquid except water) for at least 10 h prior to administration of study drug and at least 4 h thereafter. The type of the meal provided following this restriction was comparable at both the sites. No fluid intake apart from the fluid given at the time of drug intake was allowed from 2 h before until 2 h after dosing. Besides these restrictions, subjects could drink water ad libitum. Similar restrictions were employed on the baseline day.
Assessments
ECG data
A full day of baseline (day 0) ECG collections was implemented to allow for time-matched analyses. Baseline ECGs on day 0 and post-dose ECGs on day 1 of each treatment period used the same time points, namely, 0, 0.25, 0.5, 1, 2, 3, 4, 6, 8, 12 and 23.5 h. At each ECG time point, the subjects were required to rest in a supine position for a 10 min period. ECGs were obtained as 10 12-lead ECGs at each time point within a 5 min window. For this study, the first three ECGs were used in the analysis since the use of three ECGs at each time point and the values from triplicate ECGs at any single time point were averaged to produce a single nominal value for each ECG interval for that time point. All ECGs were assessed by a central ECG laboratory (ERT Inc., Philadelphia, PA, USA) where ECGs of any individual patient were assessed by the same analyst who was blinded to treatment.
ECGs were obtained digitally using Mortara Instrument H-12+ ECG continuous 12-lead digital recorders (Milwaukee, WI, USA), which obtained ECGs on day 0 and day 1 of each period of the crossover treatment. ECGs were recorded and stored continuously on flash cards and were not available for review until the cards were received by ERT and analyzed. The predetermined time points for ECGs to be used for analysis of treatment effect on day 1 were time-matched to day 0 (baseline). On each treatment day, the H-12+ recordings were started approximately 0.5 h prior to the dosing and continued through approximately 23.5 h post-dosing. Digital ECGs were transmitted to ERT's validated data management system, EXPERT®.
Trained analysts reviewed all ECGs for correct lead placement and the ECG analysis was conducted using lead II or lead V5 if lead II could not be analyzed. If lead V5 was not analyzable either, then lead V2 was used, followed by any other lead that was the most appropriate for the purpose. All ECGs were read centrally using a high resolution manual on-screen calliper semiautomatic method with annotations. Trained analysts adjudicated the pre-placed algorithm callipers as necessary using the proprietary validated electronic calliper system applied on a computer screen. ECG readers were blinded to subject identifiers, treatment and time point. Each fiduciary point (onset of P wave, onset of Q wave, offset of S wave and offset of T wave) was electronically marked. The original ECG waveform and such annotations were saved separately in XML format for independent review. A cardiologist then verified the interval durations and performed the morphology analysis, noting any T-U wave complexes that were compatible with an effect on cardiac repolarization. On-screen measurements of the RR, PR, QRS, and QT interval durations were performed and ECG variables of QTcF, QTcB, PR and QRS intervals and heart rate were computed. A full day of baseline ECGs on day 0 allowed for computation of an individualized QT correction (QTcI).
Pharmacokinetic data
Blood samples for pharmacokinetic analysis of moxifloxacin concentration were obtained in all subjects on day 1 of each treatment period of this study. The time points on day 1, used for this sampling were exactly the same as those used for recording the ECGs. A pre-dose (trough concentration) sample was also taken on day 1 of each treatment period. In order to avoid changes in autonomic tone from venesection, blood samples were drawn at each time point within 5 min after the ECG at that time point. Concentrations of moxifloxacin in plasma were determined by a validated LCMS/MS method by Northeast Bioanalytical Laboratories (Hamden, CT, USA). The lower limit of quantification (LLOQ) for concentration of moxifloxacin in plasma was 0.001 µg ml–1. All concentrations below the LLOQ were excluded from PK/PD analyses and missing data were labelled as such.
Statistical plan
The physiologically inverse relationship between heart rate and the measured QT interval duration requires an adjustment process to ‘correct’ or ‘normalize’ the measured QT interval to a standard heart rate. Therefore, the corrected QT interval (QTc) allows comparisons of QTc intervals across a range of heart rates. QTcB is the duration of the QT interval corrected for heart rate by Bazett's formula (exponent is 0.50) and QTcF is the duration of the QT interval corrected for heart rate by Fridericia's formula (exponent is 0.333). For calculation of individually-corrected QTc (QTcI) intervals, baseline ECGs were used to calculate a parabolic log–log QTc correction by using a log QT vs. log RR regression in each subject and using the slope parameter as the coefficient.
Descriptive statistics (e.g. frequency, percentage and mean as well as standard deviation (SD), median, maximum and minimum) were used to summarize the ECG variables and the corresponding changes from the mean baseline (day 0) to on treatment (day 1) of each treatment period.
Descriptive analysis on the time point means, which resulted from comparing the time-matched baseline findings to the on-treatment findings for the ECG interval parameters (i.e. heart rate, PR, QRS, QT, QTc (QTcI, QTcF and QTcB) were also computed.
The primary endpoint for the QT/QTc data in this study was the time-matched ΔΔQTcF, the placebo-corrected change from baseline in the QTcF interval. Specifically, for each individual subject, the baseline value from day 0 was subtracted from the ‘time-matched’ value on day 1. Baseline was defined separately for each period in each subject. The primary analysis is based on the relationship between ΔΔQTcF and plasma concentration of moxifloxacin. To demonstrate the equivalence of moxifloxacin-induced QTc prolongation between the two populations, two questions that require answers are (a) is the relationship between concentration and response (ΔΔQTcF) the same in both races (by looking at both the country effect and the interaction term in the model) and (b) is the effect as seen the same, taking into account any pharmacokinetic differences. Therefore, the point estimate and its two sided 90% confidence interval (CI) for the difference in ΔΔQTcF between Japanese and Caucasian were calculated at the estimated gMean Cmax of moxifloxacin using a linear mixed effects model that included terms for ethnicity (country), drug concentration and ethnicity by concentration interaction as shown in Equation 1:
 |
(1) |
where the parameter ρ is the country effect, parameters α and β are the population mean intercept and slope for the Japanese (country = 0), α + ρ and β + γ are the population mean intercept and slope for Caucasians (country = 1), sij is the random effect of subject ij (i.e. subject j from country i) on the intercept and di is the random effect of subject ij on the slope. The random effects sij and di are assumed to be independent and identically distributed bivariate normal BVN(0,Σ), where Σ is a 2 × 2 covariance matrix. The error term eij is assumed to be independent and identically distributed normal N(0,σ2). If this model did not converge, then plasma concentration was to be included as a fixed effect with intercept and subject included as random effects. The model as described in equation 1, however, did converge.
Equivalence was to be concluded if the two sided 90% confidence interval of the difference in ΔΔQTcF at the estimated gMean Cmax of moxifloxacin was entirely contained within the −5 ms to +5 ms limits. Plots of ΔΔQTcF vs. moxifloxacin plasma concentrations at the corresponding time points were planned from individual subjects and mean data for each treatment group. The ratio of the estimated slopes was calculated and the CIs of the slopes were calculated using an empirical non-parametric bootstrap (percentile interval) method using 1000 replicates [21]. For moxifloxacin, the resulting parameters (β, SE β, P value, predicted ΔΔQTc at average Cmax, two-sided 90% CI of predicted ΔΔQTc and overall model fit) in the Japanese and Caucasians were summarized for QTcF interval.
The typical analysis (comparison between placebo and moxifloxacin at each time point) based on the inter-section union test was also conducted within each ethnic group without considering the difference in moxifloxacin concentration. This analysis also was presented in a graphical manner. All confidence intervals (corresponding to the number of post-baseline time points) were presented in a graph showing the moxifloxacin effect (placebo-corrected). All analyses were separately done for QTcI and QTcF comparing Japanese and Caucasian subjects.
An exploratory outlier or categorical analysis (number and percentage of subjects meeting pre-defined responses) supplemented the central tendency analysis to determine if there were subjects who had an exaggerated effect on any ECG parameter that would not be revealed in a mean change from baseline central tendency analysis. Since the outlier summary tables include counts of subjects, a subject displaying a particular response more than once is counted only once for that response. A subject was considered to have an outlier value if a value at any of the post-dose time points met the following pre-specified change from baseline value where baseline value is a time-averaged mean of all baseline time points on day 0.
Bradycardia: heart rate <50 beats min–1 and at least a 25% decrease from baseline mean heart rate
Tachycardia: heart rate >100 beats min–1 and at least a 25% increase from the baseline mean heart rate.
PR increase: >200 ms and at least a 25% increase from baseline mean PR interval.
QRS increase: >100 ms and at least a 25% increase from the baseline mean QRS interval.
QTc increase: QTcI and QTcF intervals of > 450 ms, > 480 ms and > 500 ms if the baseline mean values were ≤450 ms, ≤480 ms and ≤ 500 ms, respectively.
QTc change (ΔQTc): ΔQTcI and ΔQTcF intervals of >30–60 ms and >60 ms from the baseline mean QTc interval.
Morphological changes: New onset findings within each treatment period not present on any baseline ECG and appearing on at least one on-treatment ECG. New onset findings of interest were atrial flutter or fibrillation, any degree or type of heart block, ST-segment changes, T wave abnormalities, new U waves and myocardial infarction pattern.
Results
Study population and exposure
Forty subjects completed both periods of treatment at each site. The mean (SD) ages were 33.8 ± 7.9 and 30.9 ± 7.2 years in the Japanese and Caucasian study groups, respectively. The weight of the Japanese subjects was lower (65.9 ± 8.9 kg) compared with the Caucasian subjects (76.6 ± 8.3 kg).
The lowest observed concentration of moxifloxacin measured in any subject was 0.0112 µg ml–1. Following a single oral dose of 400 mg moxifloxacin, the Japanese experienced an overall higher concentration (3.27 ± 0.6 vs. 2.98 ± 0.7 µg ml–1), exposure (38.3 ± 6.0 vs. 33.4 ± 4.8 µg ml–1 h) and overall half-life (11.7 ± 1.2 vs. 10.0 ± 1.3 h) compared with the Caucasians (Table 1 and Figure1). These differences were not statistically significant. The median time to peak concentration was 2 h in both groups. However, the concentrations and exposure were higher in the Caucasians during the first 4 h post-dose. Therefore, for the purpose of comparing the two populations, it was considered more appropriate to compute and use a common gMean not a separate gMean for each ethnicity.
Table 1.
Pharmacokinetic parameters of moxifloxacin in Japanese and Caucasian populations
| tmax | t1/2 | Cmax | AUC(0,tlast) | AUC(0,∞) | |
|---|---|---|---|---|---|
| (h)* | (h) | (µg ml–1) | (µg ml–1 h) | (µg ml–1 h) | |
| Arithmetic mean ± SD | |||||
| Japanese | 2 (0.25, 6) | 11.7 ± 1.2 | 3.27 ± 0.6 | 38.3 ± 6.0 | 52.1 ± 9.6 |
| Caucasian | 2 (0.5, 3) | 10.0 ± 1.3 | 2.98 ± 0.7 | 33.4 ± 4.8 | 42.4 ± 7.0 |
| Geometric mean (CV %) | |||||
| Japanese | 11.6 (10.6%) | 3.22 (17.7%) | 37.8 (15.8%) | 51.2 (18.4%) | |
| Caucasian | 9.9 (12.7%) | 2.92 (21.9%) | 33.0 (14.5%) | 41.8 (16.4%) | |
For tmax, the values are median (minimum, maximum) values
Figure 1.
Time course of moxifloxacin plasma concentrations (µg ml–1) (mean ± 2SE).  Japan,
Japan,  USA
USA
ECG results
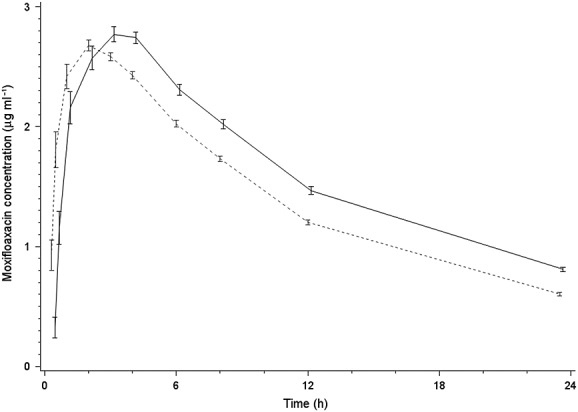
Figure2 describes the time-matched difference in heart rates between moxifloxacin and placebo in the two populations and there is no significant difference. The inter-subject variability for QTcF interval, the primary endpoint, was similar between the two populations (5.4 ms for the Japanese and 6.5 ms for the Caucasians). While QTcF was a priori chosen as the primary endpoint, QTcI and QTcB intervals were also computed and analyzed. The RR interval regression of the on-placebo data on QTc intervals using the three corrected methods demonstrated that the Fridericia correction was the most appropriate in this study (slope closest to zero) followed very closely by the slope for QTcI, while the Bazett correction had the worst fit. Therefore, data for only the QTcF are detailed in this report, an approach consistent with the recommendations from the FDA [17].
Figure 2.
Time course of placebo-corrected change in heart rate (beats min–1) from baseline (mean ± 90% CI).  Japan moxifloxacin,
Japan moxifloxacin,  USA moxifloxacin
USA moxifloxacin
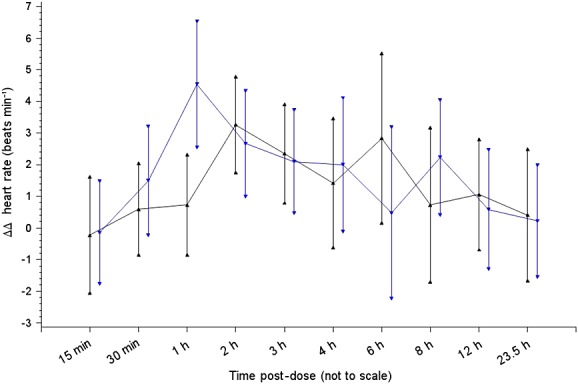
Since ΔQTcF on placebo can affect ΔΔQTcF due to moxifloxacin, Table 2 summarizes changes from baseline in QTcF interval (ΔQTcF) at each time point following moxifloxacin as well as placebo administration to the two populations to display clearly how the two treatments performed in each group. Figure3 shows moxifloxacin-induced placebo-corrected changes in QTcF interval from baseline (ΔΔQTcF) (mean and 90% CI) in the Japanese and Caucasian adult healthy male volunteers. Statistical analysis of these data did not demonstrate any significant difference between the two populations (and therefore P values are not shown). The apparent differences between the two, observed in ΔΔQTcF during the first 4 h post-dose, correspond to the differences in exposure to moxifloxacin. Neither were there significant differences between the two populations in terms of the effect of moxifloxacin on other ECG intervals (PR or QRS durations), morphological changes or outlier analyses (Table 3).
Table 2.
Time-matched changes in QTcF interval from baseline (ms) on moxifloxacin and placebo
| Japanese (n = 40) | Caucasians (n = 40) ‡ | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Moxifloxacin | Placebo | Moxifloxacin | Placebo | |||||||||
| Time | Estimate * | Lower bound † | Upper bound † | Estimate* | Lower bound † | Upper bound † | Estimate * | Lower bound † | Upper bound † | Estimate * | Lower bound † | Upper bound † |
| 15 min | −4.6 | −6.4 | −2.7 | −4.4 | −5.8 | −3.0 | −2.2 | −4.3 | −0.1 | −4.0 | −6.1 | −1.9 |
| 30 min | −4.2 | −5.9 | −2.5 | −6.0 | −8.0 | −4.1 | −2.4 | −5.3 | 0.5 | −8.8 | −11.1 | −6.5 |
| 1 h | 0.8 | −1.2 | 2.7 | −4.7 | −6.6 | −2.8 | 5.0 | 2.6 | 7.4 | −5.1 | −7.5 | −2.7 |
| 2 h | 3.6 | 1.6 | 5.5 | −5.2 | −7.2 | −3.3 | 7.5 | 5.5 | 9.5 | −5.6 | −8.2 | −3.0 |
| 3 h | 4.9 | 3.4 | 6.4 | −3.8 | −6.1 | −1.6 | 8.1 | 5.8 | 10.3 | −3.5 | −5.7 | −1.2 |
| 4 h | 4.9 | 3.2 | 6.7 | −3.6 | −5.3 | −1.8 | 8.7 | 6.1 | 11.3 | −1.4 | −3.6 | 0.8 |
| 6 h | 7.2 | 5.5 | 8.9 | −2.7 | −4.2 | −1.2 | 5.3 | 2.7 | 7.9 | −2.6 | −4.6 | −0.6 |
| 8 h | 7.4 | 5.7 | 9.2 | 0.3 | −1.1 | 1.7 | 7.0 | 4.9 | 9.0 | −1.4 | −3.5 | 0.6 |
| 12 h | 6.0 | 4.3 | 7.7 | 0.0 | −2.1 | 2.1 | 4.9 | 2.7 | 7.2 | −1.7 | −3.7 | 0.3 |
| 23.5 h | 3.9 | 2.2 | 5.5 | −2.0 | −3.6 | −0.4 | 4.3 ‡ | 2.0 | 6.6 | −4.3 | −6.1 | −2.5 |
The mean estimate and upper and lower confidence intervals
Lower or upper bound = lower or upper two-sided 90% data-based confidence limit.
n = ΔQTcF based on 39 Caucasians for moxifloxacin at 23.5 h
Figure 3.
Time course of placebo-corrected change in QTcF interval (ms) from baseline (ΔΔQTcF) (mean ± 90% CI).  Japan moxifloxacin,
Japan moxifloxacin,  USA moxifloxacin
USA moxifloxacin
Table 3.
Effect of moxifloxacin (not placebo-corrected) on ECG intervals and wave morphology and outlier analysis
| Japanese | Caucasians | |
|---|---|---|
| Mean (SD) change from baseline in time-averaged | ||
| PR interval duration (ms) | − 2.7 (3.5) | − 1.0 (3.3) |
| 90% CI | −3.7, −1.8 | −1.9, −0.2 |
| QRS interval duration (ms) | − 0.4 (1.5) | 0.0 (1.3) |
| 90% CI | −0.8, 0.0 | −0.4, 0.3 |
| QTcF interval (ms) | 3.0 (3.0) | 4.6 (4.7) |
| 90% CI | 2.2, 3.8 | 3.3, 5.9 |
| Outliers | ||
| Significant morphological changes (n and %) | 0 | 0 |
| Bradycardia (n and %) | 0 | 0 |
| Tachycardia (n and %) | 0 | 0 |
| PR interval increased (n and %) | 0 | 0 |
| QRS interval increased (n and %) | 0 | 0 |
| QTcI or QTcF interval increased (n and %) | ||
| >450 ms | 1 (2.5) | 2 (5.0) |
| >480 ms | 0 | 0 |
| >500 ms | 0 | 0 |
| ΔQTcI or ΔQTcF from baseline (n and %) | ||
| >30–60 ms | 0 | 1 (2.5) |
| >60 ms | 0 | 0 |
Concentration–ΔΔQTcF relationship
Figure4 shows the relationship between exposure (plasma concentration) to moxifloxacin and the effect (placebo-corrected change in QTcF from baseline) for Japanese vs. Caucasian subjects. It also shows the ΔΔQTcF as predicted by a mixed effects linear model at a gMean concentration of 3.07 µg ml–1. These values were 9.63 ± 1.15 ms in the Japanese vs. 11.46 ± 1.19 ms in the Caucasians. The results showed that the difference in ΔΔQTcF at this concentration between Japanese and Caucasian subjects was −1.8 ms (90% CI −4.6, 0.9), which is within the pre-specified limits of −5 ms to +5 ms.
Figure 4.
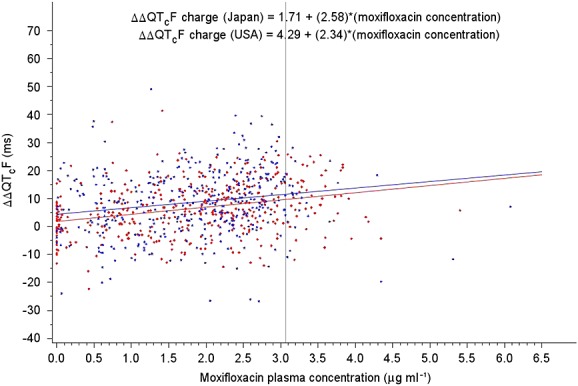
Placebo-corrected change in QTcF interval from baseline (ΔΔQTcF) vs. moxifloxacin plasma concentrations from the mixed effects linear model.  Japan,
Japan,  USA
USA
As Figure4 shows, the key difference between the Japanese and the Caucasians appears to be the intercept of the concentration–ΔΔQTcF analysis. The difference between the concentration–ΔΔQTcF analysis and the typical statistical analysis is that concentration–ΔΔQTcF analysis adjusted for the different moxifloxacin concentrations between the Japanese and the Caucasian subjects at the same time points. The current standard concentration–ΔΔQTcF analysis at FDA is to always include intercept in this linear regression to account for potential model mis-specification because the actual moxifloxacin concentration–ΔΔQTcF relationship is non-linear (Emax shape) in a wider range of moxifloxacin concentration. However, following a single 400 mg oral dose of moxifloxacin, the concentration range is narrow enough to use a linear relationship to approximate the underlying non-linear shape.
It has been suggested that a significant similarity of the exposure–response relationship between two ethnic populations reliably excludes the effect of ethnicity on drug-induced QTc interval prolongation since this analysis adjusts for any difference in drug exposure [18]. Table 4 summarizes the results of a linear mixed effect model, including the slopes of the relationships between ΔΔQTcF and plasma concentration of moxifloxacin for the two populations. The mean (±SE) concentration–response slopes in the two populations in this study were 2.58 ± 0.62 vs. 2.34 ± 0.64 ms per µg ml–1, respectively, and the ratio of the slopes of the Japanese vs. Caucasians was 1.1. As shown in Table 4, at a common value of the gMean Cmax, the two ethnic groups were not significantly different in terms of their response to moxifloxacin.
Table 4.
Results of the pharmacokinetic–pharmacodynamic evaluation of moxifloxacin in Japanese and Caucasian populations
| Model parameters | |||||
|---|---|---|---|---|---|
| Parameter | Estimate | Standard error | P value | ||
| Intercept | 1.71 | 1.29 | 0.1876 | ||
| Moxifloxacin plasma concentration | 2.58 | 0.62 | <0.0001 | ||
| Country | 2.58 | 1.82 | 0.1606 | ||
| Concentration-by-country interaction | −0.24 | 0.89 | 0.7853 | ||
| Estimates of prediction lines by ethnicity | |||||
| Japan | USA | Japan/USA | |||
| Intercept | Slope vs. plasma concentration | Intercept | Slope vs. plasma concentration | Ratio of slopes | 90% CI |
| 1.71 | 2.58 | 4.29 | 2.34 | 1.10 | (0.63, 1.82)*** |
| Predicted ΔΔQTcF (ms) at the geometric mean of 3.07 ng ml–1 | |||||
| Country | Predicted ΔΔQTcF * | Standard error of predicted ΔΔQTcF | 90% CI | P value | |
| Japan | 9.63 | 1.15 | <0.0001 | ||
| USA | 11.46 | 1.19 | <0.0001 | ||
| Difference USA-Japan ** | 1.83 | 1.65 | (−0.92, 4.58) | 0.2722 | |
CI confidence intervals;
The model is ΔΔQTcF as a function of plasma concentration, country and the interaction of plasma concentration and country. The alpha level is 0.10.
Equivalence margins are set at –5 ms to +5 ms.
Equivalence margins are set at 0.50 to 2.00 with Caucasians as the reference group
Thus, the comparison of the Japanese and Caucasian populations studied by us by either the difference in ΔΔQTcF (Figure3) or the ratio of slopes (Table 4) did not demonstrate any significant differences between the two populations.
Discussion
Comparison with other moxifloxacin studies
Our study did not demonstrate any significant differences between healthy male Japanese and Caucasian volunteers in terms of moxifloxacin-induced QTc interval prolongation. If the positive control does not have its usual amplitude or time course of effect on ΔΔQTcF, concentration–response analysis of the data can provide reassurance that the effects seen are similar to those reported in other studies, after correcting for any confounding factors [5]. The slope of the concentration–ΔΔQTcF effect, an important indicator of QT sensitivity, was similar between the two populations in our study and both the pre-defined criteria of equivalence were met. Thus, this TQT study (designed to ICH E14 standards) revealed no statistically significant difference between the Japanese and the Caucasian subjects with regard to the QTc effect of moxifloxacin, as determined by either the placebo-corrected changes in QTcF from baseline (ΔΔQTcF) or the concentration–effect modelling.
The results of our study compare well with the effects of moxifloxacin reported by Florian et al. from a retrospective analysis of pooled data from 20 TQT studies with moxifloxacin given as a single 400 mg dose [17]. A closer scrutiny of the data reported by them showed a trend towards a slightly blunted response in non-Caucasians [18] and the maximum mean (90% CI) placebo and baseline-corrected change in QTcF (ΔΔQTcF) was 9.1 ms (8.1, 10.1) in males. The moxifloxacin concentration–QTc relationship in the pooled data was best described by a linear model with a mean (90% CI) slope of 3.1 (2.8, 3.3) ms per µg ml–1. The mean slope for individual studies ranged from 1.6 to 4.8 ms per µg ml–1. In our study, the slopes observed were slightly lower (2.58 ± 0.62 ms per µg ml–1 in the Japanese and 2.34 ± 0.64 ms per µg ml–1 in the Caucasians), with the slope being slightly steeper in the Japanese. We note that Florian et al. [17] reported only a modest hysteresis between moxifloxacin plasma concentrations and QTc in the pooled data, and incorporating hysteresis did not materially alter the slope (3.3 ms per µg ml–1). We confirmed lack of hysteresis in our study by displaying and examining the data graphically.
Taubel et al. have previously reported what is the first direct comparison of the Caucasians and the Japanese for their QTc response to moxifloxacin [22]. However, their small sample study investigated a number of other variables including the effect of food and gender. They found that moxifloxacin Cmax concentrations observed in the Japanese subjects were approximately 18% higher than in the Caucasian subjects. An increase in plasma moxifloxacin concentration was associated with QTcF prolongation in both the Japanese and Caucasian subjects. This relationship was similar for both ethnicities. More specifically, the slopes differed by less than 10% and this difference was not statistically significant. However, their report did not include any data on Cmax or QTc effect specifically in a single gender from the two populations during the fasting state. Our study observed numerical trends suggestive of a slightly greater ΔΔQT effect in the Caucasians compared with the Japanese at earlier time points after dosing (Figure3). This numerical trend, however, was consistent with the higher moxifloxacin concentrations at early time points for Caucasians compared with Japanese (Figure1).
The QTc effect of moxifloxacin observed in our study is also consistent with available data from other well-designed QT studies in selected ethnic groups, which also suggest lack of significant ethnic sensitivity to the QT-prolonging effect of moxifloxacin [22–26]. In a TQT study of telbivudine in 53 subjects, all of whom were Hispanics, the ΔΔQTcF for moxifloxacin was lower at 10.0 (90% CI 6.9, 13.1) ms [23]. A prospective study of two doses (400 mg and 800 mg) of moxifloxacin effect in only the Korean population also reported that its QT effect in that population was comparable with its known effect in Caucasians with concentration vs. ΔΔQTcF response slope of 5.35 ms per µg ml–1 [25]. Sugiyama et al. have recently reported what is probably the first TQT study conducted in the Japanese subjects [26]. In this study investigating the QT-liability of topiroxostat, the mean QTcF interval was prolonged by moxifloxacin, of which the largest time-matched difference from placebo administration was 13.6 (90% CI 11.2, 15.9) ms at 4 h post-dose. The upper limit value of 90% CI of maximum mean ΔΔQTcF for moxifloxacin was 15.8 ms in males at 1 h and 18.5 ms in females at 4 h. The gMean Cmax value after the administration of moxifloxacin was 3.6 µg ml–1 (3.2 µg ml–1 in males and 4.1 µg ml–1 in females) and the concentration–response slope for moxifloxacin with linear regression analysis was 3.03 ms per µg ml–1.
Overall, therefore, review of available data on ΔΔQTc effect of moxifloxacin in different ethnic groups and the data from our study comparing Japanese and Caucasians reliably excludes ethnic sensitivity to the QTc effect of moxifloxacin. Our findings, therefore, also suggest that when conducting a TQT study, the Japanese or the Caucasian ethnicity of the study population may not be an issue, at least in establishing assay sensitivity through the use of moxifloxacin as a positive control.
Our study has a number of limitations. First, the two populations were studied at two different study sites. We are unable to quantify a site bias, if any, in the study conclusions. Neither were we able to determine any effect of each site formulating its own placebo to match moxifloxacin. Another limitation of our study is that it enrolled only male volunteers. It is generally accepted that independent of lower body mass and the resulting higher exposure to a drug, females have a greater sensitivity to drug-induced QT interval prolongation [27–30], thus further limiting the wider generalization of our findings.
Importantly, however, our study has identified a number of interesting issues for discussion. These concern (a) the regulatory guidance to address issues of ethnicity in clinical trial populations, (b) the choice of criteria to establish equivalency of drug response between two populations, (c) rigid application of regulatory threshold of QT-liability for development, approval and labelling of drugs, (d) post-dose time points for studying moxifloxacin effect in a TQT study, (e) the potential for extrapolation of our QTc data to other populations and other drugs and (f) the choice of correction formula to compute a heart rate-corrected QTc interval.
Ethnicity and drug regulation
Inter-ethnic differences in clinical response to a number of drugs are well documented and not surprisingly, regulatory authorities have promulgated a number of guidelines on the need to address the issue of ethnicity of the study population [6,18]. In 1995, the ICH adopted a guideline (ICH E5) entitled ‘Ethnic factors in the acceptability of foreign clinical data’ [31]. The guidance is based on the premise that it is not necessary to repeat the entire clinical drug development programme in the new region and is intended to recommend strategies for accepting foreign clinical data in full or partial support for approval of an application in a new region. The FDA has also issued a number of documents concerning the need to collect ethnicity data and provide a subgroup analysis by ethnicity [reviewed in 6]. Section 907 of the 2012 Food and Drug Administration Safety and Innovation Act directs the FDA to take a closer look at the inclusion and analysis of demographic subgroups - including by gender, race and ethnicity, and age - in applications for drugs, biologics and devices. As recently as August 2014, the FDA announced an ‘Action Plan to Enhance the Collection and Availability of Demographic Subgroup Data’ [32]. Against this background of the need to collect ethnicity data, ICH E14 guidance speculated that although data are limited, it is not expected that the results of the ‘thorough QT/QTc study’ would be affected by ethnic factors [3]. This would suggest that the ethnicity of the study population, and hence the QTc response to the study drug, need not be considered. The subsequent Q&A documents [4,5] that followed the initial adoption of ICH E14 in May 2005 have also not re-visited the issue of ethnicity of a TQT study population. Neither does ICH E14 express any preference for the gender of the study population. Therefore, given the challenges of studying a new drug in young females, many TQT studies enrol only the male population.
Criteria for QTc equivalency
The primary aim of our study was to address this core issue of potential inter-ethnic differences in QT sensitivity with regard to the outcome of a TQT study. Therefore, a key question was to set up criteria of equivalency between the two populations (in a sense, equivalency margins such as those used in bioequivalence studies) and justification thereof. Since the estimated ΔΔQTcF at a typical moxifloxacin concentration of 2.9 µg ml–1 has been reported to be 10.9 ms from analysis of pooled data from 20 TQT studies [17], we determined that the equivalency margin should be −5 ms to +5 ms at the gMean Cmax of moxifloxacin by allowing a difference of approximately 50% of 10.9 ms. In addition, the ratio of the PK–PD slopes of the two populations should be within ± 50% as well. Our justification for this equivalency margin is that 50% difference is commonly used in determining the non-inferiority margin for a non-inferiority trial [33] and the magnitude of QT prolongation for moxifloxacin is dependent on its concentration. Since the estimated ΔΔQTcF at 2.9 µg ml–1 moxifloxacin concentration was 10.9 ms [17] for Caucasian subjects, we determined that the equivalency margin between the two populations should be −5 to 5 ms at a common moxifloxacin concentration (approximately 3.0 µg ml–1) by allowing a difference of approximately 50% of 10.9 ms. In addition, the ratio of slopes should be within 50% to 200% to demonstrate a similar overall concentration–ΔΔQTcF relationship (Caucasian as the reference group).
Ethnicity and thorough QT studies
A TQT study is required to include a positive control to test the ability of the study (its ‘assay sensitivity’) to detect the study endpoint of interest, in this case QT prolongation by about 5 ms and moxifloxacin is the most widely used active control to establish the assay sensitivity as recommended in ICH E14. Data suggesting an underestimation of QTc effect of the positive control might question the assay sensitivity, thus jeopardizing the interpretability of the study results. Therefore, despite the trend towards globalization of clinical trials, the possibility of an inter-ethnic difference in QT susceptibility has given rise to anxieties in conducting a TQT study in a non-Caucasian population, lest the non-Caucasian population is less sensitive than Caucasians to QT effects of a drug and therefore, assay sensitivity cannot be established in the TQT study. Not surprisingly, therefore, the majority of these studies have hitherto been performed in white Caucasian populations, an approach which also raises uncertainties regarding the extrapolation of the resulting data to non-Caucasians.
The observed difference during 0–4 h in QTc response of the two populations studied by us was consistent with the difference in moxifloxacin concentrations between the two populations. However, our findings would caution against limiting the analysis of assay sensitivity in a TQT study to the first 4 h following administration of moxifloxacin.
Ethnicity of a TQT study and wider implications for its interpretation
With the assay sensitivity established, the investigational drug that is the focus of a TQT study is declared a QT prolonger if the upper bound of 90% CI of the mean placebo-corrected increase from baseline breaches a 10 ms margin, the regulatory threshold of concern. A drug determined to be a QT prolonger is either dropped from further development by the sponsor or may be subject to restrictive labelling by regulators. This leads us to discuss whether the above conclusions concerning the lack of ethnic sensitivity to QT effects of moxifloxacin can be generalized to other populations or to other QT prolonging drugs.
At present, it would be premature to generalize this lack of ethnic sensitivity to the QT effect of moxifloxacin to other ethnic populations. Furthermore, the potential difference between any two populations may amplify with increasing QT prolonging potency of a drug [14,15,27] and therefore, the findings on moxifloxacin cannot be generalized to other drugs either.
As discussed below, statistically non-significant but small numerical differences in ethnic sensitivity to drug-induced QT prolongation could have consequences for drugs with an effect close to the regulatory threshold (which has been arbitrarily set in ICH E14 at around 5 ms as evidenced by an upper bound of the 95% CI around the mean effect on QTc of 10 ms). It seems reasonable to suggest that a drug that has either none to minimal QT liability or a strong QT effect will invariably declare itself to be so in a TQT study regardless of the small numerical differences in QT sensitivity that may exist between any two populations or the ethnic mix of the study population [14,15,23,26,34,35]. Therefore, the issue of ethnicity of the study population is only relevant to those drugs with an effect close to the regulatory threshold of concern.
In contrast to studies on moxifloxacin, studies investigating the QT effect of levofloxacin revealed a numerically, but statistically non-significant, steeper slope of the QTcF effect in Caucasians compared with the Japanese [16]. Given these inconsistencies, great caution must be exercised in the interpretation of these statistically non-significant differences. Notwithstanding, it is conceivable that as a result of these small differences, a drug with an effect close to regulatory threshold may be declared free from QT liability in a TQT study in one population and a QT prolonger in another, with all the consequences that such a determination may incur for the conduct of larger phase III clinical trials. Thus, significant issues resulting from these small differences may arise only if the regulatory threshold is applied rigidly and in a binary manner to determine whether a TQT study is positive or negative for the investigational drug. Given that a TQT study is cost-ineffective in terms of identifying the clinical risk of pro-arrhythmia, it would also seem paradoxical to have to undertake a TQT study with the same drug in two or more ethnically diverse populations. The inevitable conclusion is that considerable pragmatism is required in interpretation of TQT studies on part of both the regulators and the sponsors and the aim should be to identify drugs with QT effects large enough to induce pro-arrhythmia. Available evidence suggests that a mean placebo-corrected increase greater than 15 ms from baseline may identify such drugs [2,36].
QTcF or QTcI correction
Given the vast experience with the use of moxifloxacin as the active control, our study also supports an earlier recommendation that a single oral dose of 400 mg moxifloxacin should be the standard positive control unless there are reasons not to use it [37]. Furthermore, our study found that correction of the measured QT interval for heart rate by Fridericia's formula was superior to an individually-derived correction formula. For precision in interpretation of drug effect on cardiac repolarization, it has often been suggested that the measured QT interval be corrected for heart rate by applying subject-specific (individual) correction formula to compute QTcI interval, especially when changes in heart rate are substantial. This approach, however, adds further to the cost of the study with no information on potential benefits as a result. A previous analysis of 75 TQT studies concluded that a vast majority of drugs do not affect the heart rate substantially (±6 beats min–1 or more) and correction by Fridericia's formula may be adequate and that a default correction method should be QTcF correction [38]. In the present study, we found that QTcF interval was superior to the QTcI interval in terms of removing the effect of heart rate on the corrected QT interval. In fact, the FDA has now recommended that the QTcF be employed as the default method in all TQT studies unless otherwise justified.
In conclusion our study did not find any significant differences between healthy male Japanese and Caucasian volunteers in terms of moxifloxacin-induced QTc interval prolongation as assessed by two key parameters. However, given the limitations of our study, known inter-ethnic differences in pharmacogenetic determinants of QT sensitivity and previous reports of inter-ethnic differences in drug-induced QTc interval prolongation, further studies in other populations and with other QT prolonging drugs are needed before our findings are more widely generalized and to clarify whether inter-ethnic differences in QT sensitivity exist and whether ethnicity of the study population may affect the outcome of a TQT study. More importantly, our study suggests that when extrapolating data from one ethnic region to another, data should be interpreted pragmatically bearing in mind that drug-induced changes in QTc interval are only an imperfect surrogate of the clinical risk that really matters, namely drug-induced pro-arrhythmia.
Competing Interests
All authors have completed the Unified Competing Interest Form (http://www.icmje.org/coi_disclosure.pdf) which is available on request from the corresponding author, and declare no support from any organization for the work submitted. None of the authors declare any financial relationships with any outside organization with an interest in the work submitted. However, JM, MT, RS, SH and RK provide consultancy services to pharmaceutical companies in fields related to cardiac safety and design, analysis and interpretation of thorough QT studies and other trials. All the authors declare no other relationships or activities that could appear to have influenced the submitted work.
Author contributions
Professor Kumagai and Dr Harris were the principal investigators for the two clinical sites. All the authors contributed equally to study design, data analysis and interpretation, drafting and commenting on the manuscript and approving the final version.
There is no funding to be acknowledged. The authors thank Robert Brown, Brian Rafter and Micelle Ladyansky from ERT for their invaluable assistance in the conception and conduct of this study.
References
- 1.Stockbridge N, Morganroth J, Shah RR, Garnett C. Dealing with global safety issues: was the response to QT-liability of non-cardiac drugs well coordinated? Drug Saf. 2013;36:167–82. doi: 10.1007/s40264-013-0016-z. [DOI] [PubMed] [Google Scholar]
- 2.Shah RR. The significance of QT interval in drug development. Br J Clin Pharmacol. 2002;54:188–202. doi: 10.1046/j.1365-2125.2002.01627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Committee for Medicinal Products for Human Use (CHMP) 2005. ICH E14 Note for Guidance on: The Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-antiarrhythmic Drugs (CHMP/ICH/2/04). European Medicines Agency, London, November. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002879.pdf (last accessed 12 September 2014)
- 4.Committee for Medicinal Products for Human Use (CHMP) 2012. ICH Topic E14: The Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs Questions and Answers (EMA/CHMP/ICH/310133/2008). European Medicines Agency, London, May. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002878.pdf (last accessed 12 September 2014)
- 5.ICH E14 Implementation Working Group. 2014. ICH E14 Guideline: The Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs. Questions & Answers (R2) International Conference on Harmonisation, Geneva, March. Available at: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E14/E14_QAs_R2_Step4.pdf (last accessed 12 September 2014)
- 6.Shah RR. Pharmacogenetics, ethnic differences in drug response and drug regulation. In: Saurez-Kurtz G, editor. Pharmacogenomics in Admixed Populations. First Edition. Austin (Texas): Landes Bioscience; 2007. pp. 180–97. [Google Scholar]
- 7.Mizutani T. PM frequencies of major CYPs in Asians and Caucasians. Drug Metab Rev. 2003;35:99–106. doi: 10.1081/dmr-120023681. [DOI] [PubMed] [Google Scholar]
- 8.Ozawa S, Soyama A, Saeki M, Fukushima-Uesaka H, Itoda M, Koyano S, Sai K, Ohno Y, Saito Y, Sawada J. Ethnic differences in genetic polymorphisms of CYP2D6, CYP2C19, CYP3As and MDR1/ABCB1. Drug Metab Pharmacokinet. 2004;19:83–95. doi: 10.2133/dmpk.19.83. [DOI] [PubMed] [Google Scholar]
- 9.Sistonen J, Sajantila A, Lao O, Corande J, Barbujani G, Fuselli S. CYP2D6 worldwide genetic variation shows high frequency of altered activity variants and no continental structure. Pharmacogenet Genomics. 2007;17:93–101. doi: 10.1097/01.fpc.0000239974.69464.f2. [DOI] [PubMed] [Google Scholar]
- 10.Ono C, Kikkawa H, Suzuki A, Suzuki M, Yamamoto Y, Ichikawa K, Fukae M, Ieiri I. Clinical impact of genetic variants of drug transporters in different ethnic groups within and across regions. Pharmacogenomics. 2013;14:1745–64. doi: 10.2217/pgs.13.171. [DOI] [PubMed] [Google Scholar]
- 11.Ackerman MJ, Tester DJ, Jones GS, Will ML, Burrow CR, Curran ME. Ethnic differences in cardiac potassium channel variants: implications for genetic susceptibility to sudden cardiac death and genetic testing for congenital long QT syndrome. Mayo Clin Proc. 2003;78:1479–87. doi: 10.4065/78.12.1479. [DOI] [PubMed] [Google Scholar]
- 12.Ackerman MJ, Splawski I, Makielski JC, Tester DJ, Will ML, Timothy KW, Keating MT, Jones G, Chadha M, Burrow CR, Stephens JC, Xu C, Judson R, Curran ME. Spectrum and prevalence of cardiac sodium channel variants among black, white, Asian, and Hispanic individuals: implications for arrhythmogenic susceptibility and Brugada/long QT syndrome genetic testing. Heart Rhythm. 2004;1:600–7. doi: 10.1016/j.hrthm.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Kannankeril PJ, Roden DM, Norris KJ, Whalen SP, George AL, Jr, Murray KT. Genetic susceptibility to acquired long QT syndrome: pharmacologic challenge in first-degree relatives. Heart Rhythm. 2005;2:134–40. doi: 10.1016/j.hrthm.2004.10.039. [DOI] [PubMed] [Google Scholar]
- 14.Olatunde A, Evans DAP. Blood quinidine levels and cardiac effects in white British and Nigerian subjects. Br J Clin Pharmacol. 1982;14:513–8. doi: 10.1111/j.1365-2125.1982.tb02022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shin JG, Kang WK, Shon JH, Arefayene M, Yoon YR, Kim KA, Kim DI, Kim DS, Cho KH, Woosley RL, Flockhart DA. Possible interethnic differences in quinidine-induced QT prolongation between healthy Caucasian and Korean subjects. Br J Clin Pharmacol. 2007;63:206–15. doi: 10.1111/j.1365-2125.2006.02793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugiyama A, Nakamura Y, Nishimura S, Adachi-Akahane S, Kumagai Y, Gayed J, Naseem A, Ferber G, Taubel J, Camm J. Comparison of the effects of levofloxacin on QT/QTc interval assessed in both healthy Japanese and Caucasian subjects. Br J Clin Pharmacol. 2012;73:455–9. doi: 10.1111/j.1365-2125.2011.04110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Florian JA, Tornøe CW, Brundage R, Parekh A, Garnett CE. Population pharmacokinetic and concentration--QTc models for moxifloxacin: pooled analysis of 20 thorough QT studies. J Clin Pharmacol. 2011;51:1152–62. doi: 10.1177/0091270010381498. [DOI] [PubMed] [Google Scholar]
- 18.Shah RR. Drug-induced QT interval prolongation: does ethnicity of the thorough QT study population matter? Br J Clin Pharmacol. 2013;75:347–58. doi: 10.1111/j.1365-2125.2012.04415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J. 2012. ‘ Novel approaches to TQT study design and analysis ’. A presentation at the 3rd DIA Cardiac Safety Workshop in Japan. Tower Hall Funabori, Tokyo, 28–9 May.
- 20.Yan LK, Zhang J, Ng MJ, Dang Q. Statistical characteristics of moxifloxacin-induced QTc effect. J Biopharm Stat. 2010;20:497–507. doi: 10.1080/10543400903581945. [DOI] [PubMed] [Google Scholar]
- 21.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. Boca Raton (Florida), USA: Chapman and Hall/CRC; 1998. [Google Scholar]
- 22.Taubel J, Ferber G, Lorch U, Batchvarov V, Savelieva I, Camm AJ. Thorough QT study of the effect of oral moxifloxacin on QTc interval in the fed and fasted state in healthy Japanese and Caucasian subjects. Br J Clin Pharmacol. 2014;77:170–9. doi: 10.1111/bcp.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poordad F, Zeldin G, Harris SI, Ke J, Xu L, Mayers D, Zhou XJ. Absence of effect of telbivudine on cardiac repolarization: results of a thorough QT/QTc study in healthy participants. J Clin Pharmacol. 2009;49:1436–46. doi: 10.1177/0091270009337943. [DOI] [PubMed] [Google Scholar]
- 24.Wheeler W, Olbertz J, Azzam S, DeGroot B, Reinbolt E, Clark K. Investigating ethnic differences in QTcF response to moxifloxacin in a randomized, double-blind study. Clin Pharmacol Ther. 2011;89(Suppl. 1):S42. (abstract PII-13) [Google Scholar]
- 25.Moon SJ, Lee J, An H, Yim DS, Chung JY, Yu KS, Cho JY, Lim KS. The effects of moxifloxacin on QTc interval in healthy Korean male subjects. Drugs R D. 2014;14:63–71. doi: 10.1007/s40268-014-0040-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sugiyama A, Hashimoto H, Nakamura Y, Fujita T, Kumagai Y. QT/QTc study conducted in Japanese adult healthy subjects: a novel xanthine oxidase inhibitor topiroxostat was not associated with QT prolongation. J Clin Pharmacol. 2014;54:446–52. doi: 10.1002/jcph.226. [DOI] [PubMed] [Google Scholar]
- 27.Somberg JC, Preston RA, Ranade V, Cvetanovic I, Molnar J. Gender differences in cardiac repolarization following intravenous sotalol administration. J Cardiovasc Pharmacol Ther. 2012;17:86–92. doi: 10.1177/1074248411406505. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez I, Kilborn MJ, Liu XK, Pezzullo JC, Woosley RL. Drug-induced QT prolongation in women during the menstrual cycle. JAMA. 2001;285:1322–6. doi: 10.1001/jama.285.10.1322. [DOI] [PubMed] [Google Scholar]
- 29.Dinh P, Sun J, Bai S, Kordzakhia G. Sex-related differences in QTc effects potential of drugs. Rev Recent Clin Trials. 2011;6:220–7. doi: 10.2174/157488711796575531. [DOI] [PubMed] [Google Scholar]
- 30.Lowe JS, Stroud DM, Yang T, Hall L, Atack TC, Roden DM. Increased late sodium current contributes to long QT-related arrhythmia susceptibility in female mice. Cardiovasc Res. 2012;95:300–7. doi: 10.1093/cvr/cvs160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.International Conference on Harmonisation. 1998. ICH E5 Guideline: Ethnic Factors in the Acceptability of Foreign Clinical Data (E5(R1)) International Conference on Harmonisation, Geneva, February. Available at: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E5_R1/Step4/E5_R1__Guideline.pdf (last accessed 12 September 2014)
- 32.FDA Report. 2014. FDA Action Plan to Enhance the Collection and Availability of Demographic Subgroup Data. Food and Drug Administration, Silver Spring, Maryland, USA, August. Available at: http://www.fda.gov/downloads/regulatoryinformation/legislation/federalfooddrugandcosmeticactfdcact/significantamendmentstothefdcact/fdasia/ucm410474.pdf (last accessed 12 September 2014)
- 33.Food and Drug Administration. 2010. Guidance for Industry: Non-inferiority Clinical Trials. Food and Drug Administration, Silver Spring, Maryland, USA, March. Available at: http://www.fda.gov/downloads/Drugs/Guidances/UCM202140.pdf (last accessed 12 September 2014)
- 34.Chen S, Min SS, Peppercorn A, Borland J, Lou Y, Song I, Fujiwara T, Piscitelli SC. Effect of a single supratherapeutic dose of dolutegravir on cardiac repolarization. Pharmacotherapy. 2012;32:333–9. doi: 10.1002/j.1875-9114.2012.01033.x. [DOI] [PubMed] [Google Scholar]
- 35.Zhang W, Smulders R, Abeyratne A, Dietz A, Krauwinkel W, Kadokura T, Keirns J. Ipragliflozin does not prolong QTc interval in healthy male and female subjects: a phase I study. Clin Ther. 2013;35:1150–61. doi: 10.1016/j.clinthera.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 36.Lin YL, Kung MF. Magnitude of QT prolongation associated with a higher risk of Torsades de Pointes. Pharmacoepidemiol Drug Saf. 2009;18:235–9. doi: 10.1002/pds.1707. [DOI] [PubMed] [Google Scholar]
- 37.Shah RR, Morganroth J, Kleiman RB. ICH E14 Q&A(R2) Document: Commentary on the further updated recommendations on thorough QT studies. Br J Clin Pharmacol. 2015;79:456–64. doi: 10.1111/bcp.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shah RR, Morganroth J. Early investigation of QTc liability: the role of multiple ascending dose (MAD) study. Drug Saf. 2012;35:695–709. doi: 10.1007/BF03261967. [DOI] [PubMed] [Google Scholar]


