Abstract
Aims
The long-term disposition of tacrolimus following kidney transplantation is characterized by a gradual decrease in dose requirements and increase in dose-corrected exposure. This phenomenon has been attributed to a progressive decline in cytochrome P450 3A4 (CYP3A4) activity, although this has never been demonstrated in vivo.
Methods
Sixty-five tacrolimus- and 10 cyclosporine-treated renal transplant recipients underwent pharmacokinetic testing at day 7 and months 1, 3, 6 and 12 after transplantation, including 8-h area under the concentration-time curve (AUC) for tacrolimus or cyclosporine and assessment of CYP3A4 activity using oral and intravenous midazolam (MDZ) drug probes.
Results
Tacrolimus clearance decreased gradually throughout the entire first year but only in CYP3A5*3/*3 homozygous recipients (25.6 ± 11.1 l h–1 at day 7; 17 ± 9.1 l h–1 at month 12; P < 0.001). In mixed model analysis, decreasing CYP3A4 activity, measured by apparent oral MDZ clearance (924 ± 443 ml min–1 at day 7 vs. 730 ± 344 ml min–1 at month 1; P < 0.001), explained 55.4% of the decline in tacrolimus clearance in the first month. CYP3A4 activity decreased by 18.9 ml min–1 for every milligram of methylprednisolone dose tapering within the first month; beyond this point it remained stable. A gradual rise in haematocrit throughout the entire first year explained 31.7% of the decrease in tacrolimus clearance in the first month and 23.6% of the decrease between months 1 and 12. Cyclosporine clearance did not change over time.
Conclusions
The maturation of tacrolimus disposition in the first year after renal transplantation observed in CYP3A5*3/*3 homozygous patients can partly be explained by a (steroid tapering-related) decline in CYP3A4 activity and a progressive increase in haematocrit.
Keywords: cyclosporine, CYP3A4, CYP3A5, CYP3A5*1, kidney transplantation, tacrolimus
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Tacrolimus clearance decreases continuously after kidney transplantation.
Tacrolimus is extensively metabolized by Cyp3A4 and Cyp3A5.
MDZ is a suitable drug probe for in vivo combined intestinal and hepatic CYP3A4 activity, through calculation of apparent oral MDZ clearance.
WHAT THIS STUDY ADDS
CYP3A4 activity decreases in CYP3A5 non-expressers in the first month after kidney transplantation, but not thereafter.
This decline in CYP3A4 activity and a rising haematocrit explain the initial decline in tacrolimus clearance.
The subsequent decline in tacrolimus clearance is partly explained by a continued rise in haematocrit.
Introduction
The calcineurin inhibitors (CNIs) tacrolimus and cyclosporine are characterized by highly variable pharmacokinetics and a narrow therapeutic index, and display a wide range of potentially severe drug-related toxicities [1,2]. Despite these unfavourable characteristics, they have become cornerstones of immunosuppressive therapy in solid organ transplantation [3].
In vitro studies have demonstrated that both cyclosporine and tacrolimus are metabolized by CYP3A4 and CYP3A5, and that both drugs are substrates of the drug transporter p-glycoprotein [p-GP, also known as ATP-binding cassette subfamily B member 1 (ABCB1), encoded by the multidrug resistance 1 (MDR1) gene] [2,4–8]. As CYP3A4 and CYP3A5, as well as ABCB1, are expressed in the gastrointestinal tract and the liver, and because of the important transporter–enzyme interplay occurring at both sites of drug metabolism, CYP3A isoenzymes and ABCB1 are expected to be major determinants of tacrolimus and cyclosporine pharmacokinetics in vivo [2,7,9–11].
Long-term tacrolimus disposition in renal transplant recipients is characterized by a progressive decline in apparent oral steady-state clearance (CLss), which continues up to at least 5 years post-transplantation [12,13]. As a consequence, tacrolimus dose requirements gradually decrease, while dose-corrected exposure [i.e. initial concentration (C0) and area under the plasma concentration–time curve from 0 to 12 h [AUC0–12]) increases, as time after transplantation elapses [12–15]. Importantly, the recipient's CYP3A5 genotype, which is known to have a major impact on tacrolimus CL and dose requirements [13,16,17], not only explains interindividual variability, but also affects these time-related changes in tacrolimus disposition. It has, indeed, been shown that the progressive decline in tacrolimus CL is only present in CYP3A5*3/*3 homozygous patients (i.e. patients not expressing CYP3A5), whereas it is absent in CYP3A5*1-allele carriers (i.e. patients expressing CYP3A5) [13,14]. Moreover, for cyclosporine, in contrast to tacrolimus, no time-related changes in its disposition have been documented.
Currently, it is unclear how to explain the time-related decrease in tacrolimus CL following transplantation. It has been hypothesized that it might be attributed to a progressive decline in in vivo CYP3A4 activity, caused by steroid tapering and other (unidentified) factors [12–15]. However, this has never been investigated in vivo in a clinically relevant setting. Furthermore, it is unclear why this phenomenon is only apparent in tacrolimus-treated patients not expressing CYP3A5 and seems to be absent in tacrolimus-treated patients expressing CYP3A5 and cyclosporine-treated patients. Therefore, we performed a longitudinal follow-up study in 65 tacrolimus- and ten cyclosporine-treated renal transplant recipients, in whom we investigated the evolution of in vivo CYP3A4 activity, using midazolam (MDZ) as a drug probe [18–23], and tacrolimus/cyclosporine pharmacokinetics in the first year after transplantation.
Methods
Study population
De novo renal transplant recipients were considered for participation in the present study. The minimum age for inclusion was 18 years. Exclusion criteria included those with combined organ transplants; women with child-bearing potential not using an acceptable method of birth control, or pregnant or breastfeeding women; patients with medical or surgical gastrointestinal or hepatic disorders or with significant comorbidity (severe chronic lung disease or heart failure with or without respiratory insufficiency); those who had experienced an acute rejection in the first week after transplantation; severe anaemia (Hb < 7 g dl–1) and hypoalbuminaemia (<25 g l–1); documented noncompliance; addiction to any known drug, nicotine or alcohol (>7 units week–1); the use of opioid or antipsychotic drugs; and known allergy or intolerance to MDZ. The use of drugs and substances that are known to either induce or inhibit CYP3A isoenzymes or to interfere with the absorption, distribution, metabolism or excretion of the CNIs, other than corticosteroids and the CNIs themselves, was prohibited. All participating patients were treated with either tacrolimus (Astellas Pharma Europe Ltd., Staines, UK) or cyclosporine (Novartis, Basel, Switzerland), combined with mycophenolic acid, either administered as its prodrug mycophenolate mofetil (Roche, Basel, Switzerland) or as enteric-coated mycophenolate sodium (Novartis, Basel, Switzerland), and methylprednisolone (Pfizer, New York, NY, USA). Tacrolimus dosing was adjusted to achieve target C0 levels of 12–15 ng ml–1 in the first 3 months after grafting and 10–12 ng ml–1 for the remainder of the first year. For cyclosporine, C0 levels of 130–220 ng ml–1 were targeted in the first 2 months, followed by 120–180 ng ml–1 in month 3 and 100–150 ng ml–1 thereafter. All patients received 500 mg intravenous methylprednisolone perioperatively, 40 mg intravenously (IV), on the first postoperative day and were started on oral methylprednisolone from the second day onwards, which was gradually tapered from 16 to 4 mg day–1 over the first 3 months. At that time point, methylprednisolone was either discontinued or a low dose (2–4 mg day–1) was maintained.
The present study was performed according to the Declaration of Helsinki and was approved by the ethics committee of the University Hospitals Leuven; the Faculty of Medicine, Catholic University Leuven, Belgium and the Belgian Federal Agency for Medicines and Health Products (EudraCT 2007-004069-16, https://eudract.ema.europa.eu). All study participants provided written informed consent, and ethical approval was granted by the University Hospitals Leuven ethics committee (study number S51157).
Study design
Longitudinal follow-up study in 65 tacrolimus- and 10 cyclosporine-treated adult renal transplant recipients. Patients were tested 7 days, and 1, 3, 6 and 12 months after transplantation. At each of these time points systemic and apparent oral MDZ CL, reflecting hepatic and first-pass in vivo CYP3A4 activity [18–23], respectively, and CNI pharmacokinetic parameters (i.e. dose requirements; dose-corrected C0 and AUC0–12; and CL) were assessed.
Pharmacokinetic study
Following an overnight fast, patients were evaluated either on our ward or at our outpatient clinic. A full physical examination was performed and an intravenous citrate-locked nonpolyurethane catheter was placed in an antecubital vein for blood sampling. Blood samples were drawn for a full biochemical analysis, including haematology, serum creatinine, serum albumin, liver tests, electrolytes and lipids. Estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease (MDRD) formula [24]. All concomitant medication was registered. Prior to testing, participants had to abstain from consuming alcohol and grapefruit-containing products for at least 7 days. In addition, they were not allowed to take any herbal products or over-the-counter medication. On day 1 of the study, 2 mg of MDZ [2 ml of a 1 mg ml–1 MDZ solution (Roche, Basel, Switzerland)] mixed in 30 ml of a glucose 5% solution was administered orally from a glass container followed by 100 ml of water to rinse the glass. Immediately thereafter, patients took their usual morning dose of tacrolimus and their other immunosuppressive medication. Two 4 ml blood samples were collected in ethylenediaminetetraacetic acid tubes before and at 0.25, 0.5, 1, 1.5, 2, 3, 4, 5, 6 and 8 h after MDZ administration. For each time point, one sample was centrifuged for 10 min at 1860 g, 4°C and plasma was stored at −80°C pending analysis of MDZ plasma concentrations. The other sample was stored as whole blood at −80°C pending analysis of CNI whole blood concentrations. On the second day, 1 mg of MDZ (1 ml of a 1 mg ml–1 MDZ solution) diluted in 4 ml of a 0.9% sodium chloride solution was injected slowly over 15–30 s through a second intravenous access. Plasma was obtained from blood samples drawn at the same 11 time points as on day 1. On both days, the subject's blood pressure, pulse and oxygen saturation were monitored throughout the first hour after MDZ administration. Patients were kept fasting until 2 h after the tests were initiated, but were allowed to drink water. At that time, a standard breakfast was provided and patients were allowed to take their concomitant non-immunosuppressive medication. Patients were not allowed to perform any exercise during the entire duration of the test.
Identification of selected CYP3A5, CYP3A4 and MDR-1 single nucleotide polymorphisms (SNPs)
Genomic DNA was isolated from whole blood samples using a salting-out procedure [25]. Participants were genotyped for the CYP3A5*1/*3 SNP using a previously published polymerase chain reaction–restriction fragment length polymorphism method [13]. In addition, all participants were genotyped for CYP3A4*1/*1b, CYP3A4*22, MDR1 –129 T>C, MDR1 1236C>T, MDR1 2677G>T/A and MDR1 3435C>T.
Quantification of MDZ plasma concentrations
MDZ plasma concentrations were measured using a recently published high-performance liquid chromatography–tandem mass spectrometry method [26]. Analytes were quantified by use of peak area ratios of analyte over internal standard using nonweighted linear regression. The calibration curves were linear over the range 0.10–50.0 ng ml–1 (R2 > 0.999). The lower limit of quantification was 0.10 ng ml–1. Imprecision was assessed according to the National Committee on Clinical Laboratory Standards (NCCLS) EP5-T guideline. The within-run precision was 1.6%, 1.7% and 1.7%, the between-run precision was 1.3%, 0.9% and 0.7% and the between-day precision was 3.5%, 3.6% and 2.9%, at low, intermediate and high MDZ concentrations, respectively. The accuracy was also acceptable, as mean recoveries were 95.2%, 101.2% and 101.1% at low, intermediate and high MDZ concentrations, respectively.
Quantification of tacrolimus whole blood concentrations
Tacrolimus whole blood concentrations were measured using a commercially available and, according to NCCLS and Food and Drug Administration guidelines, validated liquid chromatography–tandem mass spectrometry kit designed specifically for tacrolimus therapeutic drug monitoring in transplant recipients (MassTrak Immunosuppressants Kit, Waters, Zellik, Belgium) [27]. Tacrolimus was quantified by use of peak area ratios of analyte over internal standard using nonweighted linear regression. Imprecision was assessed according to the NCCLS EP5-T guideline (available at http://www.clsi.org). The within-run precision was 5.7%, 3.5% and 2.4% and between-run precision was 5.3%, 2.0% and 1.4%, at low, intermediate and high tacrolimus concentrations, respectively. Accuracy was also acceptable, as mean recoveries were 98.8%, 102.4% and 105.2% at low, intermediate and high tacrolimus concentrations, respectively. The analytical performance of the kit was validated by successful participation of our laboratory in the International Tacrolimus Proficiency Testing Scheme provided by Analytical Services International Ltd. (London, UK).
Determination of pharmacokinetic parameters
The concentration–time data were evaluated by standard noncompartmental methods (WinNonlin 5.2.1, Pharsight, Mountain View, CA, USA). The maximum concentration (Cmax) and time to reach maximum concentration (Tmax) after oral MDZ and CNI administration were determined by visual inspection of the data. The terminal elimination rate constant (λZ) was determined by linear regression of the log concentration vs. time data. The AUC was calculated by a combination of linear and logarithmic trapezoidal methods (‘linear up/log down’). The MDZ AUC0–8 was calculated from the time of drug administration to the last sampling time (8 h) and was then extrapolated to infinity (AUC0→∞). The systemic MDZ clearance (CL) of IV administered MDZ (MDZ IV CL) = DoseIV/AUC0→∞IV and the apparent oral CL of orally administered MDZ (MDZ PO CL/F) = DosePO/AUC0→∞PO (with F denoting the fraction of the drug that is absorbed). The CNI AUC0–8 and CNI AUC0–12 were calculated from the time of drug administration to the last sampling time (8 h) and assuming that C12 = C0, respectively. Estimates of CNI CLss)were obtained assuming C12 = C0 as well.
Statistical analysis
Data are expressed as mean ± standard deviation (SD), except when stated otherwise. The distribution of continuous data was evaluated according to the Shapiro–Wilk test, and parametric and nonparametric tests were applied when appropriate. Linear mixed models with random intercepts and random slopes were used to estimate the effect of covariates on differences in MDZ, tacrolimus and cyclosporine CL over time. A scaled identity covariance structure was used as variance of outcome parameters did not change significantly over time. Calculation of estimates was based on restricted maximum likelihoods. Fixed effects included (when appropriate) CYP3A5 and CYP3A4*22 genotypes, type of CNI (tacrolimus vs. cyclosporine), dose of methylprednisolone, age, gender, weight, body mass index, eGFR, serum albumin, haematocrit, oral and intravenous MDZ CL (absolute and weight-corrected). In the final mixed model, we only included those terms which were statistically significant using the F test and improved the model according to Akaike's information criterion. A two-sided P value < 0.05 was considered statistically significant. For collinearity diagnostics, a variance inflation factor of >5 was considered indicative of multicollinearity. IBM SPSS Statistics version 22 was used for all statistical analyses.
Results
Patient characteristics, biochemistry and corticosteroids over time
Seventy-five adult renal transplant recipients were enrolled in the current study. Sixty-five patients were treated with tacrolimus and ten patients were treated with cyclosporine. In the tacrolimus-treated subgroup, 13 patients carried a CYP3A5*1 allele (i.e. were CYP3A5 expressers) and 52 did not (i.e. were CYP3A5*3/*3 homozygotes and thus CYP3A5 non-expressers). In the cyclosporine-treated subgroup, no CYP3A5*1 allele carriers were identified (i.e. they were all CYP3A5 non-expressers). MDZ and CNI pharmacokinetics were assessed in 36, 29, 67, 22 and 50 renal transplant recipients at day 7 and months 1, 3, 6 and 12, respectively. Nineteen patients were tested at all time points, whereas 20, 25, five and six patients were tested once, twice, three times and four times, respectively, during the first year following transplantation.
Patient demographics, haemoglobin/haematocrit, serum creatinine/eGFR, serum albumin and corticosteroid dose are summarized in Table 1. All patients were of Caucasian ancestry. It should be noted that methylprednisolone was systematically tapered from 16 mg day–1 at day 2 to 4 mg day–1 at month 3. At that time point, it was discontinued in 14% of patients. Acute rejection treated with high-dose steroids occurred in three patients: one on day 8 and two during month 4.
Table 1.
Patient demographics, biochemistry and concomitant corticosteroid therapy over time
| Variable | D7(n = 36) | M1(n = 29) | M3(n = 67) | M6(n = 22) | M12(n = 50) |
|---|---|---|---|---|---|
| Time after transplantation (months) | 0.25 ± 0.06 | 1.06 ± 0.07 | 3.13 ± 0.21 | 6.12 ± 0.31 | 12.19 ± 0.43 |
| Age (years) | 53.6 ± 8.1 | 53.3 ± 8.1 | 53.7 ± 10.8 | 55.5 ± 7.3 | 56.5 ± 9.4 |
| Gender: female/male (%) | 33.3/66.7 | 34.5/65.5 | 31.3/68.7 | 27.3/72.7 | 33.3/66.7 |
| Ethnicity: Caucasian/other (%) | 100/0 | 100/0 | 100/0 | 100/0 | 100/0 |
| Weight (kg) | 72.4 ± 15.8 | 69.8 ± 16.0 | 71.8 ± 13.8 | 72.2 ± 16.0 | 73.0 ± 13.9 |
| Height (m) | 1.71 ± 0.10 | 1.70 ± 0.10 | 1.72 ± 0.14 | 1.72 ± 0.09 | 1.71 ± 0.10 |
| Body mass index (kg m–2) | 24.5 ± 4.0 | 23.9 ± 3.7 | 24.3 ± 4.0 | 24.1 ± 3.7 | 25.0 ± 3.9 |
| Haemoglobin (g dl–1) | 10.1 ± 1.3 | 11.2 ± 1.4 | 11.3 ± 1.6 | 12.2 ± 1.1 | 12.8 ± 1.3 |
| Haematocrit | 0.32 ± 0.04 | 0.35 ± 0.04 | 0.36 ± 0.05 | 0.38 ± 0.03 | 0.38 ± 0.04 |
| Creatinine (mg dl–1) | 1.64 ± 0.58 | 1.52 ± 0.37 | 1.49 ± 0.42 | 1.39 ± 0.37 | 1.42 ± 0.45 |
| Estimated glomerular filtration rate (ml min–1/1.73 m–2) | 46.8 ± 20.7 | 47.0 ± 14.1 | 49.7 ± 17.1 | 53.6 ± 18.1 | 51.4 ± 14.7 |
| Albumin (g l–1) | 35.0 ± 3.3 | 42.2 ± 2.3 | 44.1 ± 2.7 | 43.7 ± 2.6 | 43.8 ± 2.3 |
| Methylprednisolone: yes/no (%) | 100/0 | 100/0 | 100/0 | 86.4/13.6 | 86.3/13.7 |
| Methylprednisolone dose (mg d–1) | 16.4 ± 1.3 | 10.7 ± 1.9 | 4.1 ± 0.7 | 3.3 ± 0.8 | 3.2 ± 1.3 |
Evolution of MDZ pharmacokinetics over time
Figure1 shows the evolution of systemic (Figure1A) and apparent oral (Figure1B) MDZ CL in the first year after transplantation in all patients and within the subgroups of tacrolimus- and cyclosporine-treated patients, respectively. In the entire cohort (weight-corrected), systemic and apparent oral MDZ CL decreased significantly over time (P < 0.0001) (Figure1, Table 2). This decrease was largely driven by a marked difference in MDZ CLs between day 7 and the other time points (P < 0.005), whereas MDZ CLs on months 1, 3, 6 and 12 did not differ significantly. Of note, limiting the analysis to the 19 patients who were tested at all time points did not alter the results (Friedman test, data not shown).
Figure 1.
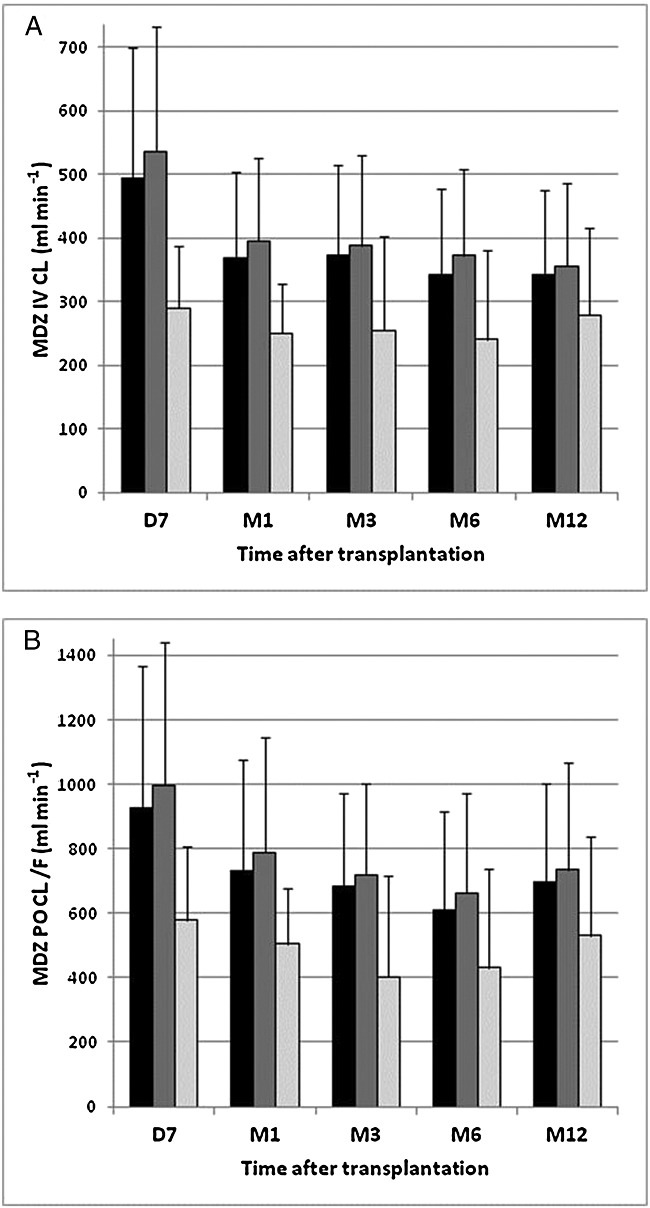
Systemic (A) and apparent oral midazolam (MDZ) (B) clearance over time. Evolution of systemic and apparent oral midazolam clearance over time in all patients (black bars) and in tacrolimus (Tac)- (dark grey bars) and cyclosporine (CsA)- (light gray bars) treated patients, respectively. Systemic and apparent oral midazolam clearance are expressed as mean ± standard deviation. D, day; M, month
Table 2.
(Weight-corrected) midazolam systemic and apparent oral clearance, (weight-corrected) tacrolimus dose requirements, dose-corrected tacrolimus initial concentration (C0) and area under the plasma concentration–time curve from 0 to 12 h (AUC0–12), and (weight-corrected) tacrolimus steady-state clearance (CL) over time
| D7 | M1 | M3 | M6 | M12 | Pvalue | |
|---|---|---|---|---|---|---|
| MDZ IV CL (ml min–1) | ||||||
| All | 494 ± 204 (n = 35)a | 369 ± 135 (n = 28)b | 373 ± 141 (n = 64)b | 341 ± 134 (n = 21)b | 341 ± 125 (n = 46)b | <0.001 |
| CsA† | 290 ± 98 (n = 6)a | 250 ± 77 (n = 5)a | 255 ± 58 (n = 7)a | 241 ± 83 (n = 5)a | 279 ± 87 (n = 9)a | 0.843 |
| Tac† | 535 ± 196 (n = 29)a | 395 ± 131 (n = 23)b | 388 ± 142 (n = 57)b | 373 ± 133 (n = 16)b | 356 ± 129 (n = 37)b | <0.001 |
| Tac CYP3A5*1/*3 | 460 ± 258 (n = 5)a | 274 ± 125 (n = 3)a | 389 ± 190 (n = 0)a | 262 ± 39 (n = 2)a | 368 ± 108 (n = 9)a | 0.120 |
| Tac CYP3A5*3/*3† | 552 ± 184 (n = 24)a | 413 ± 125 (n = 20)b | 388 ± 132 (n = 47)b | 388 ± 135 (n = 14)b | 352 ± 137 (n = 28)b | <0.001 |
| MDZ IV Cl/weight (ml min–1 kg–1) | ||||||
| All | 7.1 ± 3.2 (n = 35)a | 5.5 ± 2.5 (n = 28)b | 5.3 ± 2.1 (n = 64)b | 4.9 ± 2.1 (n = 21)b | 4.7 ± 1.6 (n = 46)b | <0.001 |
| CsA† | 4.1 ± 1.7 (n = 6)a | 3.4 ± 1.0 (n = 5)a | 3.5 ± 1.2 (n = 7)a | 3.2 ± 1.3 (n = 5)a | 4.2 ± 2.0 (n = 9)a | 0.863 |
| Tac† | 7.7 ± 3.1 (n = 29)a | 5.9 ± 2.2 (n = 23)b | 5.5 ± 2.1 (n = 57)b | 5.5 ± 2.0 (n = 16)b | 4.7 ± 1.5 (n = 37)b | <0.001 |
| Tac CYP3A5*1/*3 | 6.7 ± 4.4 (n = 5)a | 4.6 ± 2.7 (n = 3)a | 5.6 ± 2.8 (n = 10)a | 4.5 ± 1.4 (n = 2)a | 5.4 ± 1.5 (n = 9)a | 0.127 |
| Tac CYP3A5*3/*3† | 7.9 ± 2.9 (n = 24)a | 6.1 ± 2.1 (n = 20)b | 5.5 ± 1.9 (n = 47)b | 5.6 ± 2.1 (n = 14)b | 4.7 ± 1.1 (n = 28)b | <0.001 |
| MDZ PO Cl/F (ml min–1) | ||||||
| All | 924 ± 443 (n=35)a | 730 ± 344 (n=29)b | 685 ± 285 (n=66)b | 611 ± 324 (n=22)b | 694 ± 323 (n=50)b | <0.001 |
| CsA† | 579 ± 227 (n = 6)a | 505 ±171 (n = 6)a | 404 ± 89 (n = 7)a | 431 ± 151 (n = 5)a | 531 ± 240 (n = 10)a | 0.605 |
| Tac All† | 996 ± 445 (n = 29)a | 789 ± 356 (n = 23)b | 719 ± 282 (n = 59)b | 664 ± 345 (n = 17)b | 735 ± 331 (n = 40)b | <0.001 |
| Tac CYP3A5*1/*3 | 833 ± 494 (n = 5)a | 473 ± 305 (n = 3)a | 707 ± 332 (n = 10)a | 344 ± 35 (n = 2)a | 797 ± 266 (n = 10)a | 0.183 |
| Tac CYP3A5*3/*3† | 1031 ± 438 (n = 24)a | 836 ± 344 (n = 20)b | 721 ± 274 (n = 49)b | 708 ± 344 (n = 15)b | 715 ± 351 (n = 30)b | 0.001 |
| MDZ PO Cl/F/weight (ml min–1 kg–1) | ||||||
| All | 13.2 ± 6.5 (n = 35)a | 10.7 ± 5.2 (n =2 9)b | 9.8 ± 4.4 (n = 66)b | 8.6 ± 4.4 (n = 22)b | 9.7 ± 4.3 (n = 50)b | <0.001 |
| CsA† | 8.5 ± 4.2 (n = 6)a | 7.0 ± 2.2 (n = 6)a | 5.6 ± 1.9 (n = 7)a | 5.8 ± 2.3 (n = 5)a | 8.1 ± 5.3 (n = 10)a | 0.584 |
| Tac All‡ | 14.1 ± 6.6 (n = 29)a | 11.7 ± 5.3 (n = 23)b | 10.3 ± 4.4 (n = 59)b | 9.5 ± 4.5 (n = 17)b | 10.1 ± 3.9 (n = 40)b | 0.001 |
| Tac CYP3A5*1/*3 | 12.3 ± 8.6 (n = 5)a | 8.0 ± 6.2 (n = 3)a | 10.1 ± 5.1 (n = 10)a | 5.8 ± 2.0 (n = 2)a | 11.6 ± 3.7 (n = 10)a | 0.314 |
| Tac CYP3A5*3/*3‡ | 14.5 ± 6.2 (n = 24)a | 12.3 ± 5.1 (n = 20)b | 10.4 ± 4.3 (n = 49)b | 10.0 ± 5.0 (n = 15)b | 9.6 ± 3.9 (n = 30)b | <0.001 |
| Tac C0 (ng ml–1) | ||||||
| Tac All | 12.5 ± 3.7 (n = 29)a | 13.0 ± 3.3 (n = 23)a | 12.5 ± 2.9 (n = 59)a | 11.9 ± 3.3 (n= 1 7)a | 11.3 ± 3.1 (n = 40)a | 0.223 |
| Tac CYP3A5*1/*3§ | 10.9 ± 3.2 (n = 5 )a | 14.2 ± 7.5 (n = 3)a | 11.6 ± 2.3 (n = 10)a | 13.8 ± 4.4 (n = 2)a | 11.4 ± 2.7 (n = 10)a | 0.657 |
| Tac CYP3A5*3/*3§ | 12.9 ± 3.7 (n = 24)a | 12.8 ± 2.6 (n = 20)a | 12.6 ± 2.6 (n = 49)a | 11.6 ± 3.1 (n = 15)a | 11.2 ± 3.2 (n = 30)a | 0.152 |
| Tac AUC0–12 (ng h ml–1) | ||||||
| Tac All | 248 ± 70 (n = 29)a | 259 ± 59 (n = 23)a | 250 ± 53 (n = 59)a | 234 ± 116 (n = 17)a | 225 ± 54 (n = 40)a | 0.105 |
| Tac CYP3A5*1/*3§ | 252 ± 76 (n = 5)a | 255 ± 54 (n = 3)a | 248 ± 55 (n = 10)a | 230 ± 59 (n = 2)a | 222 ± 57 (n = 10)a | 0.561 |
| Tac CYP3A5*3/*3§ | 231 ± 35 (n = 24)a | 289 ± 96 (n = 2 0)a | 259 ± 47 (n = 49)a | 266 ± 97 (n = 15)a | 232 ± 43 (n = 30)a | 0.169 |
| Tac dose (mg day–1) | ||||||
| Tac All | 12.5 ± 5.5 (n = 29)a | 10.9 ± 4.4 (n = 23)b | 9.8 ± 4.8 (n = 59)c | 7.6 ± 2.5 (n = 17)de | 7.3 ± 4.2 (n = 40)e | <0.001 |
| Tac CY¨3A5*1/*3§ | 17.0 ± 5.3 (n = 5)a | 11.5 ± 2.2 (n = 3)ab | 15.8 ± 3.8 (n = 10)ab | 10.5 ± 0.7 (n = 2)ab | 13.2 ± 3.7 (n = 10)b | 0.031 |
| Tac CYP3A5*3/*3§ | 11.6 ± 5.2 (n = 24)a | 10.8 ± 4.7 (n = 20)a | 8.6 ± 4.1 (n = 49)b | 7.3 ± 2.4 (n = 15)c | 5.4 ± 2.0 (n = 30)c | <0.001 |
| Tac dose/weight (mg kg–1 day–1) | ||||||
| Tac All | 0.18 ± 0.07 (n = 29)a | 0.16 ± 0.06 (n = 23)ab | 0.14 ± 0.08 (n = 59)b | 0.11 ± 0.05 (n = 17)c | 0.10 ± 0.06 (n = 40)d | <0.001 |
| Tac CYP3A5*1/*3§ | 0.24 ± 0.06 (n = 5)a | 0.19 ± 0.07 (n = 3)a | 0.23 ± 0.08 (n = 10)a | 0.19 ± 0.10 (n = 2)a | 0.19 ± 0.05 (n = 10)a | 0.052 |
| Tac CYP3A5*3/*3§ | 0.16 ± 0.07 (n = 24)a | 0.15 ± 0.06 (n = 20)a | 0.12 ± 0.06 (n = 49)b | 0.10 ± 0.03 (n = 15)c | 0.07 ± 0.02 (n = 30)c | <0.001 |
| Tac C0/dose (ng ml–1 mg–1) | ||||||
| Tac All | 1.30 ± 0.88 (n = 29)a | 1.44 ± 0.89 (n = 23)a | 1.65 ± 1.07 (n = 59)a | 1.71 ± 0.82 (n = 17)ab | 1.95 ± 1.06 (n = 40)b | 0.003 |
| Tac CYP3A5*1/*3§ | 0.72 ± 0.31 (n = 5)a | 1.14 ± 0.56 (n = 3)a | 0.77 ± 0.24 (n = 10)a | 1.24 ± 0.24 (n = 2)a | 0.90 ± 0.23 (n = 10)a | 0.145 |
| Tac CYP3A5*3/*3§ | 1.42 ± 0.92 (n = 24)a | 1.49 ± 0.94 (n = 20)a | 1.83 ± 1.10 (n = 49)a | 1.77 ± 0.85 (n = 15)a | 2.30 ± 1.00 (n = 30)b | 0.001 |
| Tac AUC0–12/dose (ng h ml–1 mg–1) | ||||||
| Tac | 47.0 ± 23.9 (n = 29)a | 54.7 ± 30.9 (n = 23)ab | 61.3 ± 33.3 (n = 59)b | 64.1 ± 18.7 (n = 17)b | 75.5 ± 37.2 (n = 40)c | <0.001 |
| Tac CYP3A5*1/*3§ | 29.6 ± 7.8 (n = 5)a | 43.9 ± 10.5 (n = 3)ab | 34.0 ± 9.3 (n = 10)ab | 47.6 ± 11.6 (n = 2)b | 37.4 ± 10.8 (n = 10)ab | 0.043 |
| Tac CYP3A5*3/*3§ | 50.6 ± 24.6 (n = 24)a | 56.4 ± 32.9 (n = 20)ab | 67.0 ± 33.7 (n = 49)bc | 66.3 ± 18.7 (n = 15)bc | 88.2 ± 34.1 (n = 30)c | <0.001 |
Abbreviations are as follows: All, all patients; CsA, cyclosporine-treated patients; MDZ IV CL. systemic midazolam clearance; MDZ IV CL/weight, weight-corrected systemic midazolam clearance; MDZ PO CL/F, apparent oral midazolam clearance; MDZ PO CL/F/weight, weight-corrected apparent oral midazolam clearance; Tac All, all tacrolimus-treated patients; Tac AUC0–12, tacrolimus dose-interval area under the concentration–time curve; Tac AUC0–12/dose, dose-corrected tacrolimus dose-interval area under the concentration–time curve; Tac C0, tacrolimus predose trough level; Tac CLss, tacrolimus steady-state clearance; Tac CLss/weight, weight-corrected tacrolimus steady-state clearance; Tac dose, tacrolimus daily dose-requirements; Tac dose/weight, weight-corrected tacrolimus daily dose requirements; Tac C0/dose, dose-corrected tacrolimus predose trough level; Tac CYP3A5*1/*3, tacrolimus-treated patients expressing CYP3A5; Tac CYP3A5*3/*3, tacrolimus-treated patients not expressing CYP3A5;
P < 0.01;
P < 0.05 for CsA vs. Tac all and for CsA vs. Tac CYP3A5*3/*3.
P < 0.0001 for Tac CYP3A5*1/*3 vs. Tac CYP3A5*3/*3.
Common superscripts ‘valueabc…’ indicate the absence of a statistical significant difference between values at different time points, whereas values without common superscripts are statistically significantly different, with an adjusted P value < 0.05 (Tukey–Kramer). P, P value for evolution over time.
In a linear mixed model, predictors of apparent oral MDZ CL were type of CNI, methylprednisolone dose and presence of the CYP3A4*22 allele (Table 3). MDZ CL was significantly higher in patients treated with tacrolimus as opposed to cyclosporine; it also increased by 18.9 ml min–1 per 1 mg methylprednisolone dose increase. It should be noted, however, that this mixed model does not include time as a fixed effect (i.e. no random slope) because methylprednisolone dose and time were highly correlated (Pearson coefficient −0.841; P < 0.001). Collinearity diagnostics for time and steroid dose revealed a variance inflation factor (VIF) of 3.421 and, when limiting this analysis to the first 3 months (when standardized steroid tapering takes place), the VIF was 19.978. When time was included as a fixed effect, neither methylprednisolone dose nor time remained significant predictors. Five patients carried one CYP3A4*22 allele, all of whom were treated with tacrolimus and were CYP3A5*/*3 homozygous. Over the first year, two of these patients underwent one pharmacokinetic assessment and the others two, three and five assessments, respectively. Mixed model estimates (correcting for methylprednisolone dose and type of CNI) for apparent oral MDZ CL were 677.4 ml min–1 in CYP3A4*1/*1 wild-type patients vs. 346.4 ml min–1 in CYP3A4*22 heterozygote recipients (P < 0.001). The estimated decrease in MDZ CL between day 7 and month 12 was 238.1 ml min–1 in CYP3A4*22 carriers and 272.4 ml min–1 in CYP3A4*1/*1 patients.
Table 3.
Mixed model estimates for fixed-effect predictors of apparent oral midazolam clearance (ml min–1)
| 95% Confidence interval | |||||
|---|---|---|---|---|---|
| Parameter | Estimate | Standard error | Lower bound | Upper bound | P value |
| Intercept | 78.951 | 150.011 | −219.742 | 377.644 | 0.6 |
| CYP3A4*1/*1† | 321.074 | 124.670 | 72.851 | 569.296 | 0.012 |
| Tacrolimus‡ | 274.770 | 87.536 | 100.001 | 449.531 | <0.001 |
| Methylprednisolone dose (mg) | 18.897 | 3.282 | 12.413 | 25.381 | 0.003 |
Vs. CYP3A4*22 carriers;
Vs. cyclosporine.
Subgroup analysis revealed that MDZ CL only decreased in CYP3A5*3/*3 homozygous patients treated with tacrolimus (P < 0.001), but not in CYP3A5*1 allele carriers or cyclosporine-treated patients (Figure1, Table 2). Again, significant differences were noted between day 7 and the other time points, but not between months 1, 3, 6 and 12. Of note, comparing the cyclosporine-treated patients, who were all CYP3A5*3/*3 homozygous, with the subgroup of CYP3A5*3/*3 homozygous tacrolimus-treated patients did not alter the result (data not shown).
Evolution of tacrolimus pharmacokinetics over time
Figure2 shows the evolution of (weight-corrected) tacrolimus dose requirements (Figure2A,B), dose-corrected tacrolimus C0 and AUC0–12 (Figure2C,D) and (weight-corrected) tacrolimus CLss (Figure2E,F) in all tacrolimus-treated patients in the first 12 months following transplantation. In contrast to MDZ CLs, (weight-corrected) tacrolimus CL showed a more gradual decline throughout the first year after transplantation (P < 0.0001). This resulted in a progressive decline in tacrolimus (weight-corrected) dose-requirements (P < 0.0001) and an increase in tacrolimus dose-corrected exposure (P < 0.005) (Figure2, Table 2). Limiting the analysis to the 13 tacrolimus-treated patients who were tested at all time points did not alter the results (data not shown).
Figure 2.
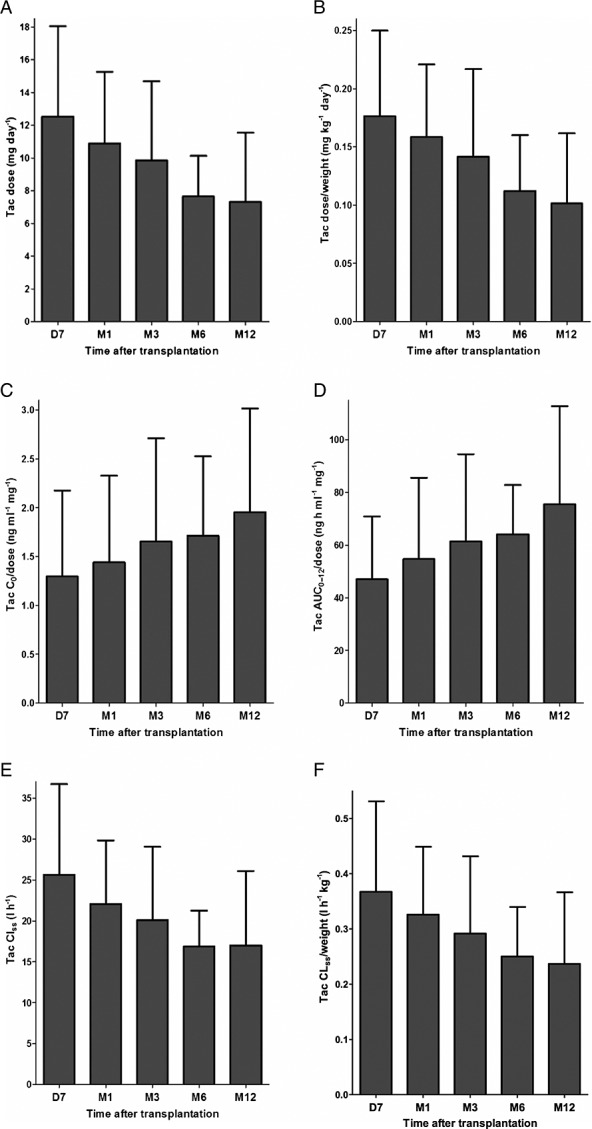
The first three panels show (weight-corrected) tacrolimus (Tac) dose requirements (A), dose-corrected Tac tacrolimus predose trough level (C0) and dose-interval area under the concentration–time curve (AUC0–12) (B) and (weight-corrected) Tac steady-state clearance (CLss) over time (C). The final three panels show the evolution of (weight-corrected) Tac dose requirements (D), dose-corrected Tac C0 and AUC0–12 (E) and (weight-corrected) Tac CLss over time (F) in all Tac-treated patients. The various Tac pharmacokinetic parameters are expressed as mean ± standard deviation
In the subgroup of CYP3A5*3/*3 homozygous tacrolimus-treated patients, similar gradual changes in tacrolimus (weight-corrected) CL (P < 0.0001), (weight-corrected) dose requirements (P < 0.001) and dose-corrected exposure (P < 0.0001) over time were noted (Table 2).
In CYP3A5*1 allele carriers, however, the evolution in tacrolimus disposition over time was less clear owing to marked fluctuations in tacrolimus pharmacokinetic parameters (Table 2). For tacrolimus CL (P = 0.0358), dose-corrected AUC0–12 (P = 0.0431) and (weight-corrected) dose requirements (P = 0.0309 and P = 0.0517) (borderline), significant changes over time were noted, whereas this was not the case for dose-corrected C0 (P = 0.1447) and weight-corrected CL (P = 0.4708). As a result of these inconsistent data and of the low number of CYP3A5*1 allele carriers at months 1 and 6, we reanalyzed the data after omitting these two suboptimal time points. In the latter analysis, no significant changes in (weight-corrected) tacrolimus CL (P = 0.2536 and P = 0.4455) and dose-corrected C0 (P = 0.3280) and AUC0–12 (P = 0.3072) were noted. (Weight-corrected) tacrolimus dose requirements decreased over time (P = 0.0201 and P = 0.0268), which is consistent with the lower target tacrolimus predose trough levels after month 3 (i.e. 12–15 ng ml–1 in the first 3 months after transplantation, and 10–12 ng ml–1 thereafter).
In a linear mixed model, predictors of tacrolimus CL were CYP3A5 genotype (the estimated tacrolimus CL was 12.537 l h–1 higher in CYP3A5 expressers), MDZ CL (0.01 l h–1 increase in tacrolimus CL per ml min–1 increase in MDZ CL) and haematocrit (0.398 l h–1 decrease in tacrolimus CL per 1% increase) (Table 4). The tacrolimus CL was 2.513 l h–1 higher in men than in women, but this association was only borderline significant (P = 0.062). The mean decrease in apparent oral MDZ CL between day 7 and month 1 was 194 ml min–1, which corresponds with a 1.94 l h–1 decrease in tacrolimus CL. This means that 55.4% of the decline in mean tacrolimus CL over this period (3.5 l h–1) could be attributed to decreasing CYP3A4 activity. In tacrolimus-treated patients, haematocrit increased an average of 2.79% between day 7 and month 1, which corresponds with a decrease in tacrolimus CL of 1.11 l h–1, or an additional 31.7% of the decline in mean tacrolimus CL over this period. Between months 1 and 12, the haematocrit continued to increase by an average of 3.02%, which corresponds with a decrease in tacrolimus CL of 1.20 l h–1, or 23.6% of the total 5.1 l h–1 decrease in mean tacrolimus CL during this 11-month period.
Table 4.
Mixed model estimates for fixed-effect predictors of tacrolimus clearance (l h–1)
| 95%Confidence interval | |||||
|---|---|---|---|---|---|
| Parameter | Estimate | Standard error | Lower bound | Upper bound | Pvalue |
| Intercept | 39.499 | 5.054 | 29.515 | 49.482 | <0.001 |
| CYP3A5 expresser† | 12.537 | 1.660 | 9.209 | 15.868 | <0.001 |
| Haematocrit (%) | −0.398 | 0.096 | −0.588 | −0.208 | <0.001 |
| Age (years) | −0.090 | 0.053 | −0.197 | 0.015 | 0.091 |
| Male gender‡ | 2.513 | 1.323 | −0.135 | 5.161 | 0.062 |
| Apparent oral MDZ CL (ml min–1) | 0.010 | 0.002 | 0.007 | 0.013 | <0.001 |
| Time = day 7 | 5.518 | 1.539 | 2.472 | 8.564 | <0.001 |
| Time = month 1 | 5.718 | 1.461 | 2.825 | 8.611 | <0.001 |
| Time = month 3 | 3.789 | 1.078 | 1.652 | 5.926 | 0.001 |
| Time = month 6 | 3.472 | 1.588 | 0.326 | 6.617 | 0.031 |
| Time = month 12§ | 0 | N/A | N/A | N/A | N/A |
CL, clearance; MDZ, midazolam; NA, not applicable;
Vs. CYP3A5 non-expressers;
Vs. female gender;
Redundant parameter (reference category).
The presence of the CYP3A4*22 allele did not affect tacrolimus pharmacokinetics (P = 0.229) or its evolution over time when correcting for the other predictors in the model, and nor did MDR1 SNPs (MDR1 –129 T>C, 1236C>T, 2677G>T/A and 3435C>T) (data not shown).
Evolution of cyclosporine pharmacokinetics over time
In the cyclosporine-treated patients, no significant changes in (weight-corrected) cyclosporine CL (P = 0.1650 and P = 0.5011) and dose-corrected C0 (P = 0.3317) and AUC0–12 (P = 0.2390) were noted (data not shown). (Weight-corrected) cyclosporine dose requirements decreased over time (P = 0.0008 and P = 0.0033), which is consistent with the declining target pre-dose trough levels (data not shown).
Discussion
The present longitudinal follow-up study in renal transplant recipients is in line with previous studies that have demonstrated a progressive increase in dose-corrected tacrolimus exposure (i.e. C0 or AUC0–12) following kidney transplantation [12–15,28,29]. The present study therefore confirms the so-called maturation of tacrolimus disposition, which is explained by a progressive decline in tacrolimus CL as time after transplantation elapses [12,13]. It has been hypothesized that the latter might be attributed to a progressive decline in hepatic and intestinal CYP3A activity and that tapering and/or cessation of corticosteroids is probably one of the most important determinants of this phenomenon [12–15,17,30–32]. However, maturation of tacrolimus disposition also occurs in patients treated with a corticosteroid-free immunosuppressive regimen and has been shown to continue up to 5 years after transplantation, whereas steroid tapering and/or cessation generally takes place in the first few months [13,15]. The results of the present study support the hypothesis that steroid tapering is associated with lower in vivo CYP3A4 activity, with apparent oral MDZ CL being almost 19 ml min–1 lower per 1 mg methylprednisolone dose decrease. However, because steroid tapering was performed in a standardized fashion in the majority of patients, steroid dose and time were highly collinear. This can lead to strongly inflated variances and may explain why time and steroid dose, when entered in the same model, were no longer significant predictors of CYP3A4 activity, even though they were individually. This is a limitation that is a result of clinical practice and cannot be circumvented. We opted to exclude time as a predictor from this particular analysis, which warrants some caution in interpreting the results. Furthermore, the present observational trial does not prove that steroid tapering is causally related to diminishing CYP3A4 activity, but the strong association between steroid dose and CYP3A4 activity and biological plausibility lends support to this hypothesis. Our data confirm earlier observations of a strongly reduced in vivo CYP3A4 activity in patients carrying the recently described CYP3A4*22 allele, which has been linked with significantly reduced tacrolimus CL [33,34]. However, in our final model for tacrolimus CL which included apparent oral MDZ CL, the inclusion of CYP3A4*22 status did not improve predictive power. This suggests that the MDZ probe accurately reflects genetically determined differences in in vivo CYP3A4 activity, as expected. The low number of data points for these five patients limit the conclusions that can be drawn regarding the longitudinal evolution of CYP3A4 activity in this subgroup, but the absolute decrease in apparent oral MDZ CL seemed similar to that in CYP3A4*1/*1 wild-type patients. Given that the baseline value in CYP3A4*22 patients was significantly lower, this corresponds with a higher proportional reduction in CYP3A4 activity. As it has been demonstrated that patients with low baseline CYP3A4 activity (measured using the erythromycin breath test) are more susceptible to dexamethasone-related CYP3A4 induction [35], it is conceivable that CYP3A4 activity at day 7 in CYP3A4*22 patients was more strongly induced by steroids, before returning to very low values after steroid tapering.
Further, the current study demonstrates that in vivo CYP3A4 activity is predictive of tacrolimus CL, together with CYP3A5 genotype and haematocrit. This confirms previous cross-sectional data, where we showed that CYP3A4 activity, CYP3A5 genotype and haematocrit are the main determinants of tacrolimus CL and explain 60–72% of variability in tacrolimus pharmacokinetics [23]. However, CYP3A4 activity decreased predominantly between day 7 and month 1 and remained relatively stable thereafter, whereas tacrolimus CL decreased more gradually over the first 12 months after transplantation. A linear mixed model could explain 87.1% of decline in mean tacrolimus CL during the first month, with decreasing CYP3A4 activity accounting for 55.4% and increasing haematocrit for 31.7%. Between months 1 and 12, a continued increase in haematocrit explained 23.6% of the further decline in mean tacrolimus CL. Our model could not account for the residual variability beyond the first month, so other factors must play a role. As tacrolimus is metabolized by CYP3A4 and CYP3A5 and is a substrate of the drug transporter ABCB1, and in light of the important transporter–enzyme interplay occurring in both enterocytes and hepatocytes, one could hypothesize that time-related changes in ABCB1 expression and/or activity might explain the maturation of tacrolimus pharmacokinetics [2,5–11,36]. Decreasing ABCB1 expression/activity at the apical membrane of enterocytes could reduce tacrolimus efflux to the gut lumen, which might increase oral bioavailability either directly or indirectly by limiting the access of tacrolimus to the intestinal CYP3A isoenzymes [10,36]. Hence, the net effect of these intestinal processes could be a progressive increase in tacrolimus oral bioavailability, despite unchanged in vivo CYP3A activity. This hypothesis would also explain why the CL of MDZ, which is not a substrate for ABCB1, does not show further changes between months 1 and 12, whereas tacrolimus CL does. Currently, no in vivo data supporting this hypothesis are available and the present study did not assess in vivo ABCB1 activity.
The present study also confirms that the progressive decrease in tacrolimus CL and increase in dose-corrected tacrolimus exposure is only present in CYP3A5*3/*3 homozygous patients, but not in CYP3A5*1 allele carriers, which display a markedly higher tacrolimus CL and lower dose-corrected exposure throughout the first year following transplantation [13,14,37]. This cannot be attributed to differences in in vivo CYP3A4 activity, as MDZ CLs and the evolution of MDZ CL over time do not differ between CYP3A5*3/*3 homozygous patients and CYP3A5*1 allele carriers [22,23]. One could speculate that, if the maturation of tacrolimus disposition could be explained by time-related changes in ABCB1 expression and/or activity, CYP3A5*1 allele carriers would be less susceptible to these alterations. Indeed, the presence of both CYP3A4 and CYP3A5 in the enterocytes of these individuals makes it less likely that saturation of CYP3A-mediated tacrolimus metabolism would occur with increasing intracellular concentrations of tacrolimus because of a reduced ABCB1-mediated tacrolimus efflux to the gut lumen [10,36]. In other words, in CYP3A5*3/*3 homozygous patients, declining ABCB1 expression/activity could result in saturation of intestinal CYP3A-mediated tacrolimus metabolism, whereas this is not the case in CYP3A5*1 allele carriers because of the presence of both CYP3A4 and CYP3A5. Although our data are in accordance with this hypothesis, our study does not allow confirmation of this as in vivo ABCB1 expression and/or activity were not assessed.
Finally, our data confirm that in vivo CYP3A4 activity, reflected by systemic and apparent oral MDZ CL, is approximately 30–35% lower in cyclosporine- as compared with tacrolimus-treated renal allograft recipients, indicating that, in vivo, at clinically used doses, cyclosporine is a stronger CYP3A4 inhibitor than tacrolimus [38,39]. Moreover, our study shows that in cyclosporine-treated patients, in contrast to tacrolimus-treated patients, in vivo CYP3A4 activity does not change significantly in the first year following transplantation, probably because inhibition of in vivo CYP3A4 activity by cyclosporine prevents other factors from affecting in vivo CYP3A4 activity. Of note, this might partially explain why no time-related changes in cyclosporine disposition are observed.
In conclusion, the present longitudinal follow-up study in renal allograft recipients demonstrates that in vivo CYP3A4 activity, reflected by systemic and apparent oral MDZ CL, decreases significantly in the first month following transplantation but remains stable thereafter. By contrast, tacrolimus CL decreases gradually over the first 12 months, but only in CYP3A5*3/*3 homozygous patients. Our data indicate that decreasing CYP3A4 activity contributes significantly to the decline in tacrolimus CL during the first month and that steroid tapering could be the underlying cause. The continued decline in tacrolimus CL beyond month 1 cannot be attributed to changes in CYP3A4 activity, but is partly explained by a gradual rise in haematocrit throughout the entire first year after transplantation. This indicates that other, currently unidentified, processes play a role in the maturation of tacrolimus disposition. Further translational research, in a clinically relevant setting, is warranted in order to identify and elucidate the underlying mechanisms.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
We thank our trial nurses, C. Beerten, J. De Vis, M. Dubois, I. Laenen, A. Swinnen, H. Wielandt and A. Willems, for their great efforts in this study and A. Herelixka for managing the clinical database. We also thank M. Dekens, G. Lemmens, K. Vandormael, E. Vanhalewyck and K. Verstraete of the Laboratory of Nephrology, University Hospitals Leuven, for their excellent technical assistance. H. de Jonge and D. Kuypers received a grant from the Fund for Scientific Research Flanders (FWO Vlaanderen).
Contributors
HD, TV and DK wrote the manuscript; KV and DK revised the manuscript; HD and DK designed the study; HD performed the trial and HDL developed the analytical methods and analysed the samples.
References
- 1.Schiff J, Cole E, Cantarovich M. Therapeutic monitoring of calcineurin inhibitors for the nephrologist. Clin J Am Soc Nephrol. 2007;2:374–84. doi: 10.2215/CJN.03791106. [DOI] [PubMed] [Google Scholar]
- 2.De Jonge H, Naesens M, Kuypers DRJ. New insights into the pharmacokinetics and pharmacodynamics of the calcineurin inhibitors and mycophenolic acid: possible consequences for therapeutic drug monitoring in solid organ transplantation. Ther Drug Monit. 2009;31:416–35. doi: 10.1097/FTD.0b013e3181aa36cd. [DOI] [PubMed] [Google Scholar]
- 3.Meier-Kriesche HU, Li S, Gruessner RWG, Fung JJ, Bustami RT, Barr ML, Leichtman AB. Immunosuppression: evolution in practice and trends, 1994–2004. Am J Transplant. 2006;6:1111–31. doi: 10.1111/j.1600-6143.2006.01270.x. [DOI] [PubMed] [Google Scholar]
- 4.Aoyama T, Yamano S, Waxman DJ, Lapenson DP, Meyer UA, Fischer V, Tyndale R, Inaba T, Kalow W, Gelboin HV, Gonzalez FJ. Cytochrome P-450 hPCN3, a novel cytochrome P-450 IIIA gene product that is differentially expressed in adult human liver. cDNA and deduced amino acid sequence and distinct specificities of cDNA-expressed hPCN1 and hPCN3 for the metabolism of steroid hormones and cyclosporine. J Biol Chem. 1989;264:10388–95. [PubMed] [Google Scholar]
- 5.Dai Y, Hebert MF, Isoherranen N, Davis CL, Marsh C, Shen DD, Thummel KE. Effect of CYP3A5 polymorphism on tacrolimus metabolic clearance in vitro. Drug Metab Dispos. 2006;34:836–47. doi: 10.1124/dmd.105.008680. [DOI] [PubMed] [Google Scholar]
- 6.Kamdem LK, Streit F, Zanger UM, Brockmöller J, Oellerich M, Armstrong VW, Wojnowski L. Contribution of CYP3A5 to the in vitro hepatic clearance of tacrolimus. Clin Chem. 2005;51:1374–81. doi: 10.1373/clinchem.2005.050047. [DOI] [PubMed] [Google Scholar]
- 7.Hebert MF. Contributions of hepatic and intestinal metabolism and P-glycoprotein to cyclosporine and tacrolimus oral drug delivery. Adv Drug Deliv Rev. 1997;27:201–14. doi: 10.1016/s0169-409x(97)00043-4. [DOI] [PubMed] [Google Scholar]
- 8.Saeki T, Ueda K, Tanigawara Y, Hori R, Komano T. Human P-glycoprotein transports cyclosporin A and FK506. J Biol Chem. 1993;268:6077–80. [PubMed] [Google Scholar]
- 9.Cummins CL, Jacobsen W, Christians U, Benet LZ. CYP3A4-transfected Caco-2 cells as a tool for understanding biochemical absorption barriers: studies with sirolimus and midazolam. J Pharmacol Exp Ther. 2004;308:143–55. doi: 10.1124/jpet.103.058065. [DOI] [PubMed] [Google Scholar]
- 10.Benet LZ, Cummins CL, Wu CY. Unmasking the dynamic interplay between efflux transporters and metabolic enzymes. Int J Pharm. 2004;277:3–9. doi: 10.1016/j.ijpharm.2002.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Wu C-Y, Benet LZ. Predicting drug disposition via application of BCS: transport/absorption/ elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm Res. 2005;22:11–23. doi: 10.1007/s11095-004-9004-4. [DOI] [PubMed] [Google Scholar]
- 12.Kuypers DRJ, Claes K, Evenepoel P, Maes B, Coosemans W, Pirenne J, Vanrenterghem Y. Time-related clinical determinants of long-term tacrolimus pharmacokinetics in combination therapy with mycophenolic acid and corticosteroids: a prospective study in one hundred de novo renal transplant recipients. Clin Pharmacokinet. 2004;43:741–62. doi: 10.2165/00003088-200443110-00005. [DOI] [PubMed] [Google Scholar]
- 13.Kuypers DRJ, de Jonge H, Naesens M, Lerut E, Verbeke K, Vanrenterghem Y. CYP3A5 and CYP3A4 but not MDR1 single-nucleotide polymorphisms determine long-term tacrolimus disposition and drug-related nephrotoxicity in renal recipients. Clin Pharmacol Ther. 2007;82:711–25. doi: 10.1038/sj.clpt.6100216. [DOI] [PubMed] [Google Scholar]
- 14.Hesselink DA, van Schaik RHN, van Agteren M, de Fijter JW, Hartmann A, Zeier M, Budde K, Kuypers DRJ, Pisarski P, Le Meur Y, Mamelok RD, van Gelder T. CYP3A5 genotype is not associated with a higher risk of acute rejection in tacrolimus-treated renal transplant recipients. Pharmacogenet Genomics. 2008;18:339–48. doi: 10.1097/FPC.0b013e3282f75f88. [DOI] [PubMed] [Google Scholar]
- 15.Naesens M, Salvatierra O, Li L, Kambham N, Concepcion W, Sarwal M. Maturation of dose-corrected tacrolimus predose trough levels in pediatric kidney allograft recipients. Transplantation. 2008;85:1139–45. doi: 10.1097/TP.0b013e31816b431a. [DOI] [PubMed] [Google Scholar]
- 16.Macphee IAM, Fredericks S, Tai T, Syrris P, Carter ND, Johnston A, Goldberg L, Holt DW. Tacrolimus pharmacogenetics: polymorphisms associated with expression of cytochrome p4503A5 and P-glycoprotein correlate with dose requirement. Transplantation. 2002;74:1486–9. doi: 10.1097/00007890-200212150-00002. [DOI] [PubMed] [Google Scholar]
- 17.Hesselink DA, Van Schaik RHN, Van Der Heiden IP, Van Der Werf M, Smak Gregoor PJH, Lindemans J, Weimar W, Van Gelder T. Genetic polymorphisms of the CΥP3A4CΥP3A5, and MDR-1 genes and pharmacokinetics of the calcineurin inhibitors cyclosporine and tacrolimus. Clin Pharmacol Ther. 2003;74:245–54. doi: 10.1016/S0009-9236(03)00168-1. [DOI] [PubMed] [Google Scholar]
- 18.Thummel KE, Shen DD, Podoll TD, Kunze KL, Trager WF, Bacchi CE, Marsh CL, McVicar JP, Barr DM, Perkins JD. Use of midazolam as a human cytochrome P450 3A probe: II. Characterization of inter- and intraindividual hepatic CYP3A variability after liver transplantation. J Pharmacol Exp Ther. 1994;271:557–66. [PubMed] [Google Scholar]
- 19.Thummel KE, O'Shea D, Paine MF, Shen DD, Kunze KL, Perkins JD, Wilkinson GR. Oral first-pass elimination of midazolam involves both gastrointestinal and hepatic CYP3A-mediated metabolism. Clin Pharmacol Ther. 1996;59:491–502. doi: 10.1016/S0009-9236(96)90177-0. [DOI] [PubMed] [Google Scholar]
- 20.Gorski JC, Jones DR, Haehner-Daniels BD, Hamman MA, O'Mara EM, Hall SD. The contribution of intestinal and hepatic CYP3A to the interaction between midazolam and clarithromycin. Clin Pharmacol Ther. 1998;64:133–43. doi: 10.1016/S0009-9236(98)90146-1. [DOI] [PubMed] [Google Scholar]
- 21.Tsunoda SM, Velez RL, von Moltke LL, Greenblatt DJ. Differentiation of intestinal and hepatic cytochrome P450 3A activity with use of midazolam as an in vivo probe: effect of ketoconazole. Clin Pharmacol Ther. 1999;66:461–71. doi: 10.1016/S0009-9236(99)70009-3. [DOI] [PubMed] [Google Scholar]
- 22.De Jonge H, de Loor H, Verbeke K, Vanrenterghem Y, Kuypers DRJ. Impact of CYP3A5 genotype on tacrolimus versus midazolam clearance in renal transplant recipients: new insights in CYP3A5-mediated drug metabolism. Pharmacogenomics. 2013;14:1467–80. doi: 10.2217/pgs.13.133. [DOI] [PubMed] [Google Scholar]
- 23.De Jonge H, de Loor H, Verbeke K, Vanrenterghem Y, Kuypers DR. In vivo CYP3A4 activity, CYP3A5 genotype, and hematocrit predict tacrolimus dose requirements and clearance in renal transplant patients. Clin Pharmacol Ther. 2012;92:366–75. doi: 10.1038/clpt.2012.109. [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–54. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 25.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Loor H, de Jonge H, Verbeke K, Vanrenterghem Y, Kuypers DR. A highly sensitive liquid chromatography tandem mass spectrometry method for simultaneous quantification of midazolam, 1′-hydroxymidazolam and 4-hydroxymidazolam in human plasma. Biomed Chromatogr. 2011;25:1091–8. doi: 10.1002/bmc.1576. [DOI] [PubMed] [Google Scholar]
- 27.Napoli KL, Hammett-Stabler C, Taylor PJ, Lowe W, Franklin ME, Morris MR, Cooper DP. Multi-center evaluation of a commercial Kit for tacrolimus determination by LC/MS/MS. Clin Biochem. 2010;43:910–20. doi: 10.1016/j.clinbiochem.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 28.Venkataramanan R, Swaminathan A, Prasad T, Jain A, Zuckerman S, Warty V, McMichael J, Lever J, Burckart G, Starzl T. Clinical pharmacokinetics of tacrolimus. Clin Pharmacokinet. 1995;29:404–30. doi: 10.2165/00003088-199529060-00003. [DOI] [PubMed] [Google Scholar]
- 29.Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2004;43:623–53. doi: 10.2165/00003088-200443100-00001. [DOI] [PubMed] [Google Scholar]
- 30.Shimada T, Terada A, Yokogawa K, Kaneko H, Nomura M, Kaji K, Kaneko S, Kobayashi K-I, Miyamoto K-I. Lowered blood concentration of tacrolimus and its recovery with changes in expression of CYP3A and P-glycoprotein after high-dose steroid therapy. Transplantation. 2002;74:1419–24. doi: 10.1097/00007890-200211270-00014. [DOI] [PubMed] [Google Scholar]
- 31.Van Duijnhoven EM, Boots JMM, Christiaans MHL, Stolk LML, Undre NA, van Hooff JP. Increase in tacrolimus trough levels after steroid withdrawal. Transpl Int. 2003;16:721–5. doi: 10.1007/s00147-003-0615-1. [DOI] [PubMed] [Google Scholar]
- 32.Anglicheau D, Flamant M, Schlageter MH, Martinez F, Cassinat B, Beaune P, Legendre C, Thervet E. Pharmacokinetic interaction between corticosteroids and tacrolimus after renal transplantation. Nephrol Dial Transplant. 2003;18:2409–14. doi: 10.1093/ndt/gfg381. [DOI] [PubMed] [Google Scholar]
- 33.Elens L, Bouamar R, Hesselink DA, Haufroid V, van der Heiden IP, van Gelder T, van Schaik RHN. A new functional CYP3A4 intron 6 polymorphism significantly affects tacrolimus pharmacokinetics in kidney transplant recipients. Clin Chem. 2011;57:1574–83. doi: 10.1373/clinchem.2011.165613. [DOI] [PubMed] [Google Scholar]
- 34.De Jonge H, Elens L, de Loor H, van Schaik RH, Kuypers DRJ. The CYP3A4*22 C>T single nucleotide polymorphism is associated with reduced midazolam and tacrolimus clearance in stable renal allograft recipients. Pharmacogenomics J. 2015;15:144–52. doi: 10.1038/tpj.2014.49. [DOI] [PubMed] [Google Scholar]
- 35.McCune JS, Hawke RL, LeCluyse EL, Gillenwater HH, Hamilton G, Ritchie J, Lindley C. In vivo and in vitro induction of human cytochrome P4503A4 by dexamethasone. Clin Pharmacol Ther. 2000;68:356–66. doi: 10.1067/mcp.2000.110215. United States. [DOI] [PubMed] [Google Scholar]
- 36.Benet LZ. The drug transporter-metabolism alliance: Uncovering and defining the interplay. Mol Pharm. 2009;6:1631–43. doi: 10.1021/mp900253n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferraresso M, Tirelli A, Ghio L, Grillo P, Martina V, Torresani E, Edefonti A. Influence of the CYP3A5 genotype on tacrolimus pharmacokinetics and pharmacodynamics in young kidney transplant recipients. Pediatr Transplant. 2007;11:296–300. doi: 10.1111/j.1399-3046.2006.00662.x. [DOI] [PubMed] [Google Scholar]
- 38.De Jonge H, de Loor H, Verbeke K, Vanrenterghem Y, Kuypers DRJ. In vivo CYP3A activity is significantly lower in cyclosporine-treated as compared with tacrolimus-treated renal allograft recipients. Clin Pharmacol Ther. 2011;90:414–22. doi: 10.1038/clpt.2011.130. [DOI] [PubMed] [Google Scholar]
- 39.Amundsen R, Åsberg A, Ohm IK, Christensen H. Cyclosporine A- and tacrolimus-mediated inhibition of CYP3A4 and CYP3A5 in vitro. Drug Metab Dispos. 2012;40:655–61. doi: 10.1124/dmd.111.043018. [DOI] [PubMed] [Google Scholar]


