Abstract
Aim
The aim was to investigate the pharmacokinetics and pharmacodynamics of an extrafine pressurized metered-dose inhaler (pMDI) fixed combination of beclometasone dipropionate (BDP)/formoterol fumarate (FF) in adolescent and adult asthma.
Methods
This was a three-way crossover study, on 30 asthmatic adolescents receiving BDP/FF pMDI with or without a valved holding chamber (VHC) or a free licenced combination of BDP pMDI and FF pMDI plus a parallel arm of 30 asthmatic adults receiving BDP/FF pMDI. All patients received a single dose of BDP and FF of 400 µg and 24 µg, for each treatment, respectively. Assessments were performed over 8 hours.
Results
In adolescents, the 90% confidence intervals (CIs) for the systemic exposure (AUC(0,t)) geometric mean ratio of the fixed combination with or without VHC vs. the free combination were within the bioequivalence range 0.80–1.25, both for beclometasone-17-monopropionate (B17MP, the active metabolite of BDP) and formoterol. Pharmacodynamic variables for plasma potassium and glucose, pulse rate and pulmonary function in adolescents were equivalent between treatments, 95% CI within 0.9, 1.09. The upper level of 90% CIs for AUC(0,t) geometric mean ratio adolescents : adults of B17MP and formoterol after treatment with BDP/FF pMDI was lower than 1.25, 90% CI 0.78, 1.04 and 0.86, 1.17, respectively.
Conclusions
In adolescents the pharmacodynamics and the overall systemic exposure to the active ingredients of an extrafine fixed combination of BDP/FF pMDI with or without a VHC was equivalent to that of a free licenced combination of pMDIs of established safety and efficacy profiles. The systemic exposure in adolescents was not higher than in adults. These results support the indication for use of inhaled corticosteroid/long acting β2-adrenoceptor agonist pMDIs in adolescents at the same dosage as in adults.
Keywords: adolescents, asthma, beclometasone, formoterol, pMDI, valved holding chamber
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
An extrafine fixed pressurized metered-dose inhaler (pMDI) combination of beclometasone dipropionate (BDP)/formoterol fumarate (FF) 100/6μg per actuation is currently licenced for use in adult asthma with or without a valved holding chamber (VHC) device.
Asthmatic adolescents are generally recommended to be dosed like adults. However, this population is unique in many ways and limited pharmacokinetic and pharmacodynamic data are available on fixed combinations of inhaled corticosteroids (ICS) and long acting β2-adrenoceptor agonists (LABA). In addition, the influence of age on the systemic exposure of drugs to be administered via pMDI with or without a VHC is still not fully elucidated.
WHAT THIS STUDY ADDS
In adolescents, the overall systemic exposure to the active ingredients of an extrafine fixed pMDI combination of BDP/FF with or without VHC was equivalent to that of a free licenced combination of pMDIs of established safety and efficacy profile. The pharmacodynamics was equivalent between treatments.
The systemic exposure in adolescents was not higher than in adults.
These results support the indication for use of a fixed ICS/LABA pMDI combination with or without a VHC in adolescents at the same dosage as in adults.
Introduction
International guidelines indicate that the goal of asthma treatment is to achieve and maintain clinical control of the disease. In this respect, inhaled corticosteroids (ICS) and long acting β2-adrenoceptor agonists (LABA) are classified as controllers since they are medications taken daily on a long term basis to keep asthma under clinical control [1]. ICS have been shown to reduce asthma symptoms [2], improve quality of life and lung function [2], decrease airway hyper-responsiveness [3], control airway inflammation [3], reduce frequency and severity of exacerbations [4] and asthma mortality [5]. When a medium dose of ICS alone fails to achieve control of asthma, a combination with a LABA becomes the preferred option of treatment [6]. Indeed, the addition of LABA to a daily regimen of ICS improves symptom scores, decreases nocturnal asthma, improves lung function, decreases the use of short-acting inhaled β2-adrenoceptor agonists, reduces the number of exacerbations, and achieves clinical control of asthma in a greater number of patients, more rapidly, and at a lower dose of inhaled corticosteroids than ICS given alone [7]. Moreover, controlled studies have shown that delivering this therapy in a combination inhaler is as effective as giving each drug separately, but fixed combinations are more convenient for asthmatic patients, increasing compliance and ensuring that the LABA is always accompanied by an ICS [8,9].
Asthmatic adolescents (12 to 17 years of age) are generally recommended to be treated with the same dosage as adults. However, this population is unique in many ways and limited efficacy, safety and pharmacokinetic (PK) data are available on fixed ICS/LABA combinations. Indeed, adolescents in comparison with adults have a lower body size and therefore a lower apparent volume in which the drug can be distributed (volume of distribution) after systemic absorption [10]. Thus, assuming a comparable elimination of the drug from the body, the same nominal dose could potentially lead to higher plasma concentrations in adolescents with a consequent increased risk of systemic side effects. Therefore, the optimal dosage for adolescents may be difficult to assess and a careful monitoring of the systemic exposure is critical to assure an appropriate safety profile [11].
A pressurized metered dose inhaler (pMDI) fixed ICS/LABA combination containing extrafine beclometasone dipropionate (BDP)/formoterol fumarate (FF) 100/6μg (Foster®, Chiesi Farmaceutici, Italy) [12] is currently licenced for use in adult asthma with or without the valved holding chamber (VHC) device (AeroChamber Plus™). The aim of the present study was to investigate, in adolescents, the PK and pharmacodynamic (PD) profile of a BDP/FF pMDI with or without a VHC in comparison with a free licenced combination of pMDIs of established safety and efficacy profile. The effect of age was also investigated by a comparison with adults.
Methods
Study design, patients and treatments
This was an open, randomized, three way crossover, single dose study in adolescent asthmatics (≥12 and < 18 years) with an open parallel arm for adult asthmatics (≥18 and ≤ 65 years) as control group. Patients already treated with ICS or ICS/LABA or using short-acting inhaled β2-adrenoceptor agonists as a reliever to control asthma symptoms and with a documented clinical history of asthma diagnosed by the responsible physician of the trial according to the GINA guidelines [1] were considered for inclusion in the study. Eligible patients were all those able to use properly a pMDI with or without a VHC and with a pre-bronchodilator forced expiratory volume in 1 s (FEV1) > 70% of predicted values (% pred).
Main exclusion criteria were exacerbation of asthma symptoms or lower respiratory tract infection within the previous 4 weeks, past or present diagnoses of cardiovascular, renal or liver disease and a diagnosis of chronic obstructive pulmonary disease (COPD) in the adult patients.
Treatments were the following:
Extrafine fixed combination (BDP/FF pMDI) in adolescents consisting of four inhalations of BDP/FF 100/6 µg pMDI for a total single dose of 400 µg BDP and 24 µg FF.
Extrafine fixed combination (BDP/FF pMDI) with VHC in adolescents consisting of four inhalations of BDP/FF 100/6 µg pMDI using VHC for a total single dose of 400 µg BDP and 24 µg FF.
Free combination (BDP pMDI + FF pMDI) in adolescents consisting of four inhalations of BDP 100 µg pMDI plus four inhalations of FF 6 µg pMDI for a total single dose of 400 µg BDP and 24 µg FF.
Extrafine fixed combination BDP/FF pMDI in adults consisting of four inhalations of BDP/FF 100/6 µg pMDI for a total single dose of 400 µg BDP and 24 µg FF.
All study drugs contained hydrofluoroalkane (HFA-134a) as excipient. BDP pMDI (Qvar®, 100 µg metered dose per actuation, Novartis, Switzerland) and FF pMDI (Atimos®, 6 µg metered dose per actuation, Chiesi Farmaceutici, Italy) are both licenced for use in adolescents [13] while BDP/FF pMDI (Foster®, 100/6 µg extrafine metered dose per actuation, Chiesi Farmaceutici, Italy) with or without a VHC (AeroChamber Plus™,Trudell Medical International, Canada), is only licenced for use in adults [12]. The dosage in each treatment corresponded to the allowed total daily dose in order to assess properly the plasma profile of the analytes also during the elimination phase, taking into account that formoterol plasma concentrations in particular are very low even at the maximum daily dosage of 24 µg.
A screening visit, evaluating medical history, vital signs, physical examination and lung function took place within 9 days before the first drug administration and a medical discharge procedure was performed within 10 days after the last drug administration. Each treatment period in the crossover design was separated by a washout period of at least 7 days. At the screening visit and prior to the study drug administration, patients were trained on the inhalation technique using an Aerosol Inhalation Monitor (AIM, Vitalograph®) and placebo pMDIs alone or in combination with the VHC (where applicable) until the investigator judged the technique to be optimal. The patients were instructed to hold their breath for at least 10 s following each long inhalation and to wait about 30 s before taking the next inhalation. All pMDIs were primed prior to administration. The VHC was cleaned in accordance with the instructions in the manufacturer's leaflet. Intake of short and long acting β2-adrenoceptor agonists had to be avoided for at least 6 and 24 h, respectively, prior to screening and each study drug administration. Intake of inhaled BDP and other ICS had to be avoided for at least 2 and 1 day, respectively, prior to study drug administration. PK and PD parameters, described later in the methods section, were assessed for beclometasone-17-monopropionate (B17MP, active metabolite of BDP) and formoterol for each treatment over 8 h post-dose. Safety was assessed by documenting all adverse events that occurred during the study. The study was carried out in accordance with the Declaration of Helsinki, the ICH Harmonized Tripartite Guideline for Good Clinical Practice and with applicable regulatory requirements. All subjects were required to give written informed consent. The study was published on http://Clinicaltrials.gov (NCT01803087) and was approved by the Office for Registration of Medicinal Products, Medical Devices and Biocidal Products 41 zabkowska str. 03-736 Warsaw; approval: ur.dbl.ble.4500.430.2011 dated 30 Jan 2012 and by the Bioethics Committee at the Medical University in Lodz 4 kosciuszki av., 90-419 Lodz approval: rnn/221/11/ke dated 13 December 2011.
Pharmacokinetic and pharmacodynamic assessments
Patients attended the clinic in the morning of the administration day after fasting overnight and until 2 h post-dosing. At each administration period, the patients were not allowed to lie down or sleep for 2 h after administration, except when undergoing clinical assessments. They remained seated as much as possible avoiding strenuous activities under constant surveillance of the nursing staff. No alcohol or xanthine (tea, chocolate, cola, etc.) containing beverages or foods, or grapefruit were taken from 48 h before each drug administration until 24 h after administration. No food or drink, except water, was allowed for 2 h after drug administration. Blood samples were collected during each study treatment day for B17MP, FF, plasma potassium and glucose determination. Pulse rate and lung function parameters (forced expiratory volume in 1 s, FEV1) were also evaluated during each study treatment day. Time 0 was defined as the moment when the first inhalation of the study treatment took place (i.e. at the moment of the first puff).
Evaluable patients were all those receiving the study treatment excluding subjects without any valid PK/PD measurement or with major protocol deviations significantly affecting the PK, e.g. incorrect inhalation, change in patient condition (worsening of asthma, cold), failure in delivery of the device, use of non-permitted medications. Patients were not excluded based on statistical analysis or for PK reasons except in the cases defined in the European Medicines Agency (EMA) ‘Guideline on the Investigation of Bioequivalence’ [14].
Plasma B17MP and formoterol assessment Blood samples for B17MP and formoterol determination in plasma were collected into vacuum tubes containing EDTA and lithium heparin, respectively, at pre-dose and at 5, 10, 30 min, 1, 2, 4, 6 and 8 h post-dose. All samples were immediately chilled (ice bath) and plasma preparation was done within 15 min after blood collection. The plasma was separated in a refrigerated centrifuge at +4°C and at 2500 rev min–1 for 15 min and transferred into pre-labelled polypropylene tubes. For stabilizing the formoterol compound, the polypropylene tubes were pre-filled with 50 µl of citric acid and centrifuged before use in order to ensure that the citric acid was at the bottom of each tube. Samples for B17MP and formoterol analysis were stored below –20°C and –65°C, respectively, before shipment on cold dry ice to the laboratory (SGS Life Sciences Services, Belgium). The pharmacokinetic assays were performed using validated liquid chromatography-mass spectrometry (LC-MS/MS) methods with a lower limit of quantification of 2 pg ml–1 for formoterol and 50 pg ml–1 for B17MP [15]. The following PK parameters were calculated from the individual plasma drug concentration vs. time profiles: maximum plasma concentration (Cmax), time to maximum plasma concentration (tmax), area under the plasma concentration–time curve observed from 0 to last measurable point (AUC(0,t)), area under the plasma concentration–time curve observed from 0 to 30 min post-dose (AUC(0,0.5h)) and terminal half-life (t1/2) calculated as 0.693/λz, where λz is the first order terminal rate constant.
Plasma potassium and glucose assessment Blood samples for potassium and glucose determination in plasma were collected into vacuum tubes containing lithium heparin and fluoride, respectively, at pre-dose and at 30 min, 1, 2, 4, 6 and 8 h post dose. All samples were immediately chilled (ice bath) and plasma preparation was done within 15 min after blood collection. The plasma was separated in a refrigerated centrifuge at +4°C and at 2500 rev min–1 for 15 min and transferred into pre-labelled polypropylene tubes. Plasma samples were stored below –20°C before shipment on cold dry ice to the laboratory (SGS Life Sciences Services, Belgium). The assays for potassium and glucose were performed using a validated potentiometric and colorimetric method, respectively. The following parameters were calculated from the individual plasma drug concentration vs. time profiles: the area under the plasma potassium and glucose concentration–time curve observed from 0 to last measurable point (AUC(0,t)), the area under the plasma glucose concentration–time curve observed from 0 to 2 h post dose (AUC(0,2 h)), the value of maximum glucose concentration (Cmax) and the value of minimum potassium concentration (Cmin).
Pulse rate assessmen Pulse rate was recorded with a calibrated pulse-oximeter at pre-dose and 5, 10, 15, 30 min, 1, 2, 4, 6 and 8 h post-dose in a sitting position and rest in this position for at least 10 min before each reading. The time averaged pulse rate value calculated as the area under the curve–time interval (AUC(0,8 h)/8 h) was calculated from the individual pulse rate values vs. time profiles.
FEV1 assessment Lung function assessments and daily calibration of the spirometer were done according to the recommendation of the Official Statement of the European Respiratory Society and American Thoracic Society [16]. Measurements were done at pre-dose and at 30 min, 1, 2, 4, 6 and 8 h post-dose with patients in a sitting position with the nose clipped after at least 10 min rest. Calibration of the spirometer was performed by the same investigator at each visit prior to any spirometry manoeuvres. The highest value from three technically satisfactory attempts was recorded and if the difference between two consecutive measurements exceeded 200 ml, up to eight measurements were made and the largest value was reported. The following parameter were calculated from the individual FEV1 values vs. time profiles: time averaged FEV1 value calculated as the area under the curve time interval (AUC(0,8 h)/8 h) and the maximum FEV1 value (peak FEV1).
Data analyses
PK variables were calculated according to a non-compartmental kinetic model using WinNonlin version 5.2 (Pharsight Corporation, Palo Alto, CA, USA). PD variables and statistical analyses were performed using SAS® version 9.1.3 (SAS Institute Inc., Cary, NC, USA). AUCs were calculated using the linear trapezoidal rule. In adolescents log-transformed values for the PK variables of B17MP and formoterol (Cmax, AUC(0,0.5 h) and AUC(0,t)) and PD variables (potassium: Cmin and AUC(0,t), glucose: Cmax, AUC(0,2 h) and AUC(0,t), pulse rate: AUC(0,8 h)/8 h and FEV1: AUC(0,8 h)/8 h and peak FEV1) were analyzed using an anova model with treatment, sequence, period and subject-within-sequence as fixed effects for treatment comparisons. The ratios of adjusted geometric means between each treatment comparison were calculated with their 90% two-sided confidence intervals (CIs) for PK variables and with 95% CIs for PD variables. To be able to demonstrate equivalence for the different PK/PD variables between treatments in adolescents, the CIs for the ratios of the adjusted geometric means had to be entirely within the 0.8–1.25 acceptance region [14].
The PK and PD variables were compared between adolescents and adults in a descriptive manner by calculation of summary statistics (geometric means and CV%). Log-transformed PK and PD parameters were analyzed using one way analysis of variance. Geometric mean ratios with their 90% confidence intervals (95% for the PD variables) were calculated and P values reported. PK variables in adolescents were considered not higher than in adults if the upper level of 90% CI of their geometric mean ratios was lower than 1.25.
Statistical comparisons of demography data were performed by unpaired t-test using GraphPad Prism® version 6.0 (GraphPad Software, La Jolla, USA). Differences between subgroups for PK variables were performed by means of Kruskal–Wallis one-way analysis of variance followed by post hoc Dunn's test for multiple comparisons using GraphPad Prism® version 6.0. Differences were considered significant at P < 0.05.
Results
Study population
Thirty adolescents and thirty adults were screened and all of them were randomized. All adolescents and adults completed the study, except for one adolescent who discontinued after receiving drugs in one period due to a protocol violation (insufficient PK and PD blood sampling data). This resulted in 29 adolescents and 30 adults evaluable for the PK and PD analyses. Among the 29 evaluable adolescents, one patient was excluded from the PK analysis for the BDP/FF pMDI treatment due to quantifiable concentrations of B17MP and formoterol at pre-dose > 5% Cmax [14].
Demographic data of the PK/PD population are summarized in Table 1. All patients were White and approximately half were male (48.3% and 53.3% for adolescents and adults, respectively). Median (range) age of patients was 16.0 (12–17) years for adolescents and 40 (18–64) years for adults. No relevant differences in demographic data were seen between the treatment sequences. The mean body surface area (BSA), calculated according to the Mosteller formula [17], increased significantly from adolescents to adults (P = 0.007). The mean BSA was 1.69 m2 (range 1.24–2.10) for adolescents and 1.85 m2 (range 1.53–2.55) for adults.
Table 1.
Patient's demography
| Adolescents (n = 29) | Adults (n = 30) | |
|---|---|---|
| Age (years) | 14.9 (1.7; 12–17) | 40.1 (12.1; 18–64) |
| Weight (kg) | 62 (12; 39–91) | 73 (15; 53–120) |
| Height (cm) | 168 (12; 139–191) | 172 (12; 155–196) |
| BMI (kg/m2) | 21.8 (2.8; 18.5–30.1) | 24.6 (3.6; 18.9–31.6) |
| BSA (m2) | 1.69 (0.21; 1.24-2.10) | 1.85 (0.23; 1.53–2.55) |
| FEV1 (L) | 3.22 (0.82; 1.79–4.79) | 3.06 (0.88; 1.70–4.69) |
| FEV1 %pred (%) | 99 (17; 74–140) | 88 (11; 72–117) |
Results are presented as the mean (SD; range). BMI, Body Mass Index; BSA, Body Surface Area calculated according to the Mosteller formula; FEV1, Pre-bronchodilator forced expiratory volume in 1 second; FEV1 % pred, FEV1 % of predicted normal value.
Pharmacokinetics and pharmacodynamics
The overall summary of PK parameters in adolescents is represented in Table 2 for B17MP and Table 4 for formoterol. Statistics for PK and PD parameters in adolescents are reported in Table 3 for B17MP, Table 5 for formoterol and Table 6 for plasma glucose, plasma potassium, pulse rate and FEV1. Comparison between adolescents and adults is reported in Table 7.
Table 2.
Pharmacokinetic parameters in adolescents - beclometasone 17-monopropionate (B17MP)
| B17MP | BDP/FF pMDI(n =28)* | BDP/FF pMDI with VHC(n =29) | BDP pMDI+FF pMDI(n =29) |
|---|---|---|---|
| Cmax (pg ml–1) | 1056 (1137) | 1044 (349) | 1116 (508) |
| tmax (h) | 0.50 (0.08–2.00) | 0.25 (0.08–1.00) | 0.48 (0.08–2.00) |
| t1/2 (h) | 2.90 (0.83) | 2.94 (0.73) | 3.05 (0.82) |
| AUC(0,0.5 h) (pg ml–1 h) | 337 (237) | 406 (167) | 409 (197) |
| AUC(0,t) (pg ml–1 h) | 2798 (846) | 2724 ( 957) | 3028 (965) |
Values are arithmetic mean (SD), except median (range) for tmax.
One patient was excluded from the PK analysis due to quantifiable concentrations of B17MP at pre-dose > 5% Cmax [11]
Table 4.
Pharmacokinetic parameters in adolescents - formoterol
| Formoterol | BDP/FF pMDI(n =28)* | BDP/FF pMDI with VHC(n =29) | BDP pMDI+FF pMDI(n =29) |
|---|---|---|---|
| Cmax (pg ml–1) | 31.2 (15.4) | 49.5 (17.9) | 33.9 (26.6) |
| tmax (h) | 0.25 (0.07–2.00) | 0.08 (0.07–0.27) | 0.10 (0.08–2.00) |
| t1/2 (h) | 4.05 (1.44) | 4.29 (1.47) | 5.56 (3.86) |
| AUC(0,0.5 h) (pg ml–1 h) | 10.3 (4.7) | 16.0 (4.4) | 10.8 (7.9) |
| AUC(0,t) (pg ml–1 h) | 76.1 (20.1) | 81.4 (23.4) | 79.8 (23.5) |
Values are arithmetic mean (SD), except median (range) for tmax.
One patient was excluded from the PK analysis due to quantifiable concentrations of FF at pre-dose > 5% Cmax [11]
Table 3.
Statistical analysis of pharmacokinetic parameters in adolescents - beclometasone 17-monopropionate (B17MP)
| B17MP | BDP/FF pMDI vs.BDP pMDI+FF pMDI | BDP/FF pMDI with VHC vs.BDP pMDI+FF pMDI | BDP/FF pMDI with VHC vs.BDP/FF pMDI |
|---|---|---|---|
| PE (90% CI) | PE (90% CI) | PE (90% CI) | |
| Cmax | 0.84 (0.70, 1.01) | 0.97 (0.81, 1.16) | 1.15 (0.96, 1.38) |
| AUC(0,0.5 h) | 0.82 (0.64, 1.05) | 1.15 (0.90, 1.46) | 1.39 (1.09, 1.78) |
| AUC(0,t) | 0.92 (0.82, 1.03) | 0.90 (0.80, 1.01) | 0.98 (0.87, 1.10) |
PE, Point estimate calculated as ratio of the adjusted geometric means for the different PK parameters between treatments
Table 5.
Statistical analysis of pharmacokinetic parameters in adolescents - formoterol
| Formoterol | BDP/FF pMDI vs.BDP pMDI+FF pMDI | BDP/FF pMDI with VHC vs.BDP pMDI+FF pMDI | BDP/FF pMDI with VHC vs.BDP/FF pMDI |
|---|---|---|---|
| PE (90% CI) | PE (90% CI) | PE (90% CI) | |
| Cmax | 1.00 (0.82, 1.22) | 1.68 (1.38, 2.05) | 1.68 (1.38, 2.06) |
| AUC(0,0.5 h) | 0.97 (0.74, 1.20) | 1.71 (1.39, 2.10) | 1.76 (1.43, 2.15) |
| AUC(0,t) | 0.95 (0.86, 1.05) | 1.02 (0.93, 1.13) | 1.08 (0.97, 1.19) |
PE, Point estimate calculated as ratio of the adjusted geometric means for the different PK parameters between treatments
Table 6.
Statistical analysis of pharmacodynamic parameters in adolescents - plasma potassium, plasma glucose, pulse rate and forced expiratory volume in 1 s (FEV1)
| BDP/FF pMDI vs.BDP pMDI+FF pMDI | BDP/FF pMDI with VHC vs.BDP pMDI+FF pMDI | BDP/FF pMDI with VHC vs.BDP/FF pMDI | |
|---|---|---|---|
| Plasma potassium | PE (95% CI) | PE (95% CI) | PE (95% CI) |
| Cmin | 1.00 (0.98, 1.02) | 0.99 (0.97, 1.02) | 0.99 (0.97, 1.02) |
| AUC(0,t) | 1.00 (0.97, 1.03) | 1.00 (0.97, 1.02) | 1.00 (0.97, 1.02) |
| Plasma glucose | PE (95% CI) | PE (95% CI) | PE (95% CI) |
| Cmax | 1.00 (0.94, 1.07) | 1.02 (0.95, 1.09) | 1.02 (0.95, 1.09) |
| AUC(0,t) | 0.99 (0.95, 1.03) | 1.01 (0.98, 1.05) | 1.02 (0.99, 1.07) |
| AUC(0,2 h) | 0.99 (0.96, 1.03) | 1.00 (0.97, 1.03) | 1.01 (0.98, 1.04) |
| Pulse rate | PE (95% CI) | PE (95% CI) | PE (95% CI) |
| AUC(0,t)/8 h | 1.01 (0.97, 1.06) | 1.02 (0.98, 1.07) | 1.01 (0.97, 1.05) |
| FEV1 | PE (95% CI) | PE (95% CI) | PE (95% CI) |
| AUC(0,t)/8 h | 0.99 (0.97, 1.02) | 0.98 (0.96, 1.01) | 0.99 (0.96, 1.02) |
| Peak FEV1 | 0.98 (0.95, 1.01) | 0.98 (0.95, 1.01) | 1.00 (0.97, 1.03) |
PE, Point estimate calculated as ratio of the adjusted geometric means for the different PD parameters between treatments
Table 7.
Pharmacokinetic and pharmacodynamic comparison between adolescents and adults
| BDP/FF pMDI in adolescents | BDP/FF pMDI in adults | ||
|---|---|---|---|
| PK variable | Geometric means(CV%) | Geometric mean ratio PE(90%CI); P | |
| B17MP | |||
| Cmax (pg ml–1) | 859 (108) | 946 (44.2) | 0.91 (0.72, 1.14); P = 0.479 |
| AUC(0,0.5 h) (pg ml–1 h) | 277 (70.2) | 328 (51.3) | 0.84 (0.63, 1.13); P = 0.338 |
| AUC(0,t) (pg ml–1 h) | 2671 (30.2) | 2963 (31.6) | 0.90 (0.78, 1.04); P = 0.219 |
| Formoterol | |||
| Cmax (pg ml–1) | 28.0 (49.3) | 34.3 (56.1) | 0.82 (0.64, 1.04); P = 0.164 |
| AUC(0,0.5 h) (pg ml–1 h) | 9.0 (45.4) | 10.6 (53.7) | 0.85 (0.63, 1.13); P = 0.344 |
| AUC(0,t) (pg ml–1 h) | 73.4 (26.5) | 73.0 (38.8) | 1.00 (0.86, 1.17); P = 0.958 |
| PD variable | Geometric means(CV%) | Geometric mean ratio PE(95%CI); P | |
|---|---|---|---|
| Plasma potassium | |||
| Cmin (mEq l–1) | 3.77 (5.33) | 3.86 (7.66) | 0.98 (0.94, 1.02); P = 0.172 |
| AUC(0,t) (mEq l–1 h) | 32.0 (6.58) | 33.2 (10.1) | 0.96 (0.92, 1.01); P = 0.103 |
| Plasma glucose | |||
| Cmax (mg dl–1) | 105 (13.3) | 105 (17) | 1.00 (0.92, 1.09); P = 0.979 |
| AUC(0,t) (mg dl–1 h) | 683 (8.23) | 707 (12.6) | 0.97 (0.91, 1.02); P = 0.233 |
| AUC(0,2 h) (mg dl–1 h) | 151 (8.25) | 166 (11.6) | 0.91 (0.86, 0.96); P = 0.001 |
| Pulse rate | |||
| AUC(0,t)/8 h (beats min–1) | 81.6 (10.1) | 76 (10.7) | 1.07 (1.02, 1.13); P = 0.011 |
| FEV1 | |||
| AUC(0,t)/8 h (l) | 3.28 (23.6) | 3.17 (28.8) | 1.04 (0.90, 1.20); P = 0.624 |
| Peak FEV1 (l) | 3.41 (23.5) | 3.29 (28.0) | 1.04 (0.90, 1.19); P = 0.609 |
PE, Point estimate calculated as ratio of the geometric means for the different PK and PD parameters between treatments
Extrafine fixed (BDP/FF pMDI) vs. free combination (BDP pMDI + FF pMDI) in adolescents (Figure1)
Figure 1.
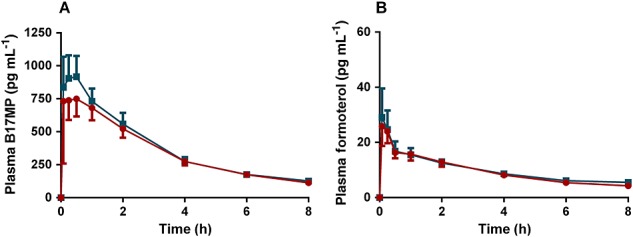
(A) B17MP and (B) formoterol mean plasma profiles (95% confidence intervals) following inhalation of the extrafine fixed (BDP/FF pMDI) combination in comparison with the free combination (BDP pMDI + FF pMDI) in asthmatic adolescents for a total single dose of 400 µg BDP and 24 µg FF.  , BDP pMDI+FF pMDI;
, BDP pMDI+FF pMDI;  , BDP/FF pMDI
, BDP/FF pMDI
In adolescents, the total systemic exposures to B17MP and formoterol assessed as AUC(0,t) were equivalent after treatment with the fixed and the free combination. The point estimates (PE) and 90% confidence intervals (CI) of the geometric mean ratio fixed/free were 0.92 (90% CI 0.82,1.03) and 0.95 (90% CI 0.86, 1.05) for B17MP and formoterol, respectively (Tables 3 and 5). The systemic exposures at early time points assessed as index of pulmonary absorption (AUC(0,0.5 h)) [18] for the fixed combination was 18% (90% CI 0.64, 1.05) and 3% (90% CI 0.74, 1.20) lower than for the free combination, for B17MP and formoterol, respectively (Tables 3 and 5). Peak concentration (Cmax) for the fixed combination was 16% (90% CI 0.70, 1.01) lower for B17MP while it was equivalent to the free combination for formoterol (90% CI 0.82, 1.22) (Tables 3 and 5).
All the pharmacodynamic parameters were equivalent after treatment with the fixed and the free combination. For plasma potassium, the PEs of the geometric mean ratio fixed/free for Cmin and AUC(0,t) were equal to 1 with 95% CI within the range 0.97, 1.03. For plasma glucose the PE for Cmax, AUC(0,t) and AUC(0,2 h) (area under the glucose concentration–time curve observed in fasting conditions) approximated 1 with 95% CIs within the range 0.94, 1.07 (Table 6). Finally, statistical equivalence was observed in mean pulse rate over time (AUC(0,t)/8h) and time-averaged FEV1 (AUC(0,t)/8h) or peak FEV1 between the two treatments, 95% CI within the range 0.95, 1.06 (Table 6).
Extrafine fixed combination (BDP/FF pMDI) with VHC vs. free combination (BDP pMDI + FF pMDI) in adolescents (Figure2)
Figure 2.
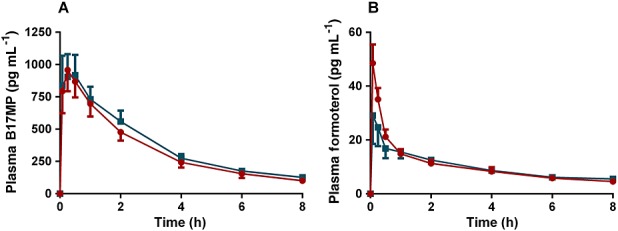
(A) B17MP and (B) formoterol mean plasma profiles (95% confidence intervals) following inhalation of the extrafine fixed (BDP/FF pMDI) combination with VHC in comparison with the free combination (BDP pMDI +FF pMDI) in asthmatic adolescents for a total single dose of 400 µg BDP and 24 µg FF.  , BDP pMDI+FF pMDI;
, BDP pMDI+FF pMDI;  , BDP/FF pMDI with VHC
, BDP/FF pMDI with VHC
In adolescents, when the fixed combination was used with the VHC, the total systemic exposures to B17MP and formoterol were equivalent to that of the free combination and 90% CIs for the AUC(0,t) ratio between treatments were within the range 0.80, 1.25. Differently from AUC(0,t), AUC(0,0.5 h) and Cmax tended to be higher for the fixed combination with VHC in comparison with the free combination, with the only exception for the Cmax of B17MP that was equivalent between the two treatments. AUC(0,0.5 h) was 15% (90% CI 0.90, 1.46) and 71% (90% CI 1.39, 2.10) higher for B17MP and formoterol, respectively, and Cmax was 68% (90% CI 1.38, 2.05) higher for formoterol (Tables 3 and 5).
Plasma potassium and glucose were equivalent between the two treatments. The 95% CIs of the geometric mean ratios for potassium Cmin and AUC(0,t) and for glucose Cmax, AUC(0,t) and AUC(0,2 h) were all within the range 0.95, 1.09 (Table 6). Statistical equivalence was observed in mean pulse rate over time (AUC(0,t)/8h) and time-averaged FEV1 (AUC(0,t)/8h) or peak FEV1 between the two treatments; 95% CI within the range 0.95, 1.07 (Table 6).
Extrafine fixed combination (BDP/FF pMDI) with VHC vs. extrafine fixed (BDP/FF pMDI) combination in adolescents (Figure3)
Figure 3.
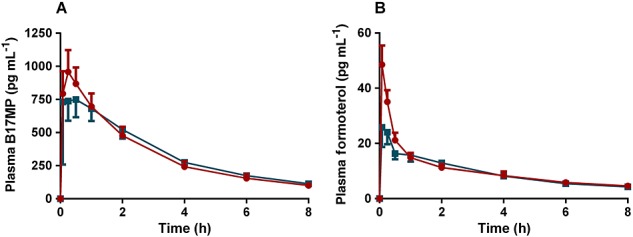
(A) B17MP and (B) formoterol mean plasma profiles (95% confidence intervals) following inhalation of the extrafine fixed (BDP/FF pMDI) combination with VHC in comparison with the extrafine fixed combination (BDP/FF pMDI) alone in asthmatic adolescents for a total single dose of 400 µg BDP and 24 µg FF.  , BDP/FF pMDI;
, BDP/FF pMDI;  , BDP/FF pMDI with VHC
, BDP/FF pMDI with VHC
Compared with the fixed combination alone, the use of the VHC increased both the Cmax and AUC(0,0.5 h) without changing the total systemic exposures for both B17MP and formoterol; Cmax and AUC(0,0.5 h) were 15% (90% CI 0.96, 1.38) and 39% (90% CI 1.09, 1.78) higher for B17MP and 68% (90% CI 1.38, 2.06) and 76% (90% CI 1.43, 2.15) higher for formoterol, respectively, with the use of the VHC while the 90% CIs of the geometric mean ratios between treatments was within the range 0.80, 1.25 for AUC(0,t) (Tables 3 and 5).
The PD parameters were equivalent after the fixed combination with or without the VHC. The 95% CIs of the geometric mean ratios for potassium Cmin and AUC(0,t) and for glucose Cmax, AUC(0,t) and AUC(0,2 h) were all within the range 0.95, 1.09 (Table 6). Statistical equivalence was observed in mean pulse rate over time (AUC(0,t)/8h) and time-averaged FEV1 (AUC(0,t)/8h) or peak FEV1 between the two treatments, 95% CI within the range 0.96, 1.05 (Table 6).
Extrafine fixed combination (BDP/FF pMDI) in adolescents vs. extrafine fixed combination (BDP/FF pMDI) in adults (Figure4)
Figure 4.
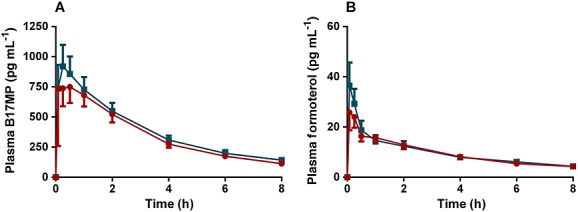
(A) B17MP and (B) formoterol mean plasma profiles (95% confidence intervals) following inhalation of the extrafine fixed (BDP/FF pMDI) combination in asthmatic adults in comparison with the extrafine fixed combination (BDP/FF pMDI) in asthmatic adolescents for a total single dose of 400 µg BDP and 24 µg FF.  , BDP/FF pMDI adults;
, BDP/FF pMDI adults;  , BDP/FF pMDI Adolescents
, BDP/FF pMDI Adolescents
PK parameters in adolescents after treatment with the fixed combination were not higher (upper level of 90% CI < 1.25) and not significantly statistically different from in adults. The geometric mean ratios (90% CI) and related P values for B17MP Cmax, AUC(0,0.5 h) and AUC(0,t) were 0.91 (0.72, 1.14), P = 0.48, 0.84 (0.63, 1.13), P = 0.34 and 0.90 (0.78, 1.04), P = 0.22, respectively (Table 7). The geometric mean ratio (90% CI) and P values for formoterol Cmax, AUC(0,0.5 h) and AUC(0,t) were 0.82 (0.64, 1.04), P = 0.16, 0.85 (0.63, 1.13), P = 0.34 and 1.00 (0.86, 1.17), P = 0.96, respectively (Table 7).
No clinically relevant difference between adolescents and adults was observed in plasma potassium and glucose concentrations, mean pulse rate over time (AUC(0,t)/8h) and time-averaged FEV1 (AUC(0,t)/8h) or peak FEV1 (Table 7).
Coordinated vs. non-coordinated patients, sub-group PK analysis
An additional analysis was performed by stratifying adolescents into two groups of patients based on their AUC(0,0.5 h) for B17MP and formoterol for the free and the fixed combination without VHC device. AUC(0,0.5 h) is an index of pulmonary absorption [18] and therefore patients having a low degree of coordination between actuation and inhalation are expected to have lower AUC(0,0.5 h) without the use of the VHC. Therefore, patients with AUC(0,0.5 h) below the 50th percentile were classified as ‘poor’ coordinators and those with AUC(0,0.5 h) above the 50th percentile were classified as ‘good’ coordinators. Without the use of the VHC, the AUC(0,0.5 h) for the ‘good’ coordinators in comparison with the ‘poor’ coordinators was increased by 113% (B17MP) and 170% (formoterol) for the free combination (P < 0.0001, data not shown) and by 134% (B17MP) and 99% (formoterol) for the fixed combination (P < 0.0001, Figure5A). Differently, with the use of the VHC, AUC(0,0.5 h) was not significantly affected between the two sub-groups of patients (P > 0.05) (Figure5B).
Figure 5.
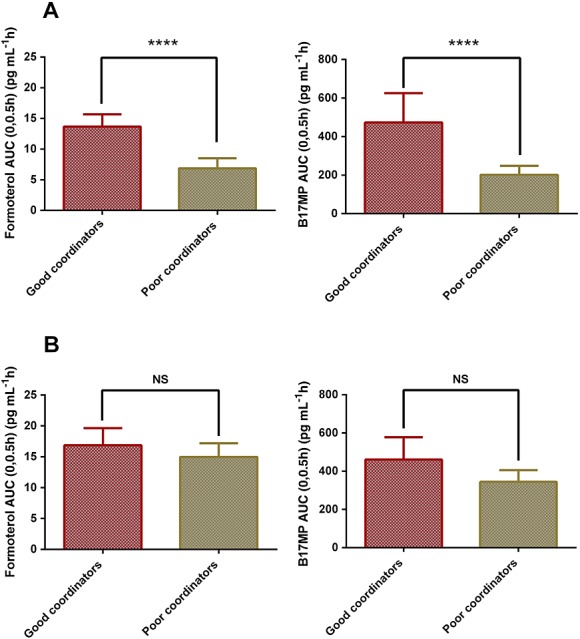
AUC(0,0.5 h) (index of pulmonary absorption) (95% confidence intervals) for formoterol and B17MP following inhalation of the extrafine fixed (BDP/FF pMDI) combination (A) and following inhalation of the extrafine fixed combination (BDP/FF pMDI) with VHC (B), in two groups of asthmatic adolescents: patients with poor inhalation technique (low50th percentile) and patients with good inhalation technique (high 50th percentile). Differences were considered significant at P < 0.05; ****, P < 0.0001; NS, not significant
General safety
No serious adverse events occurred during the study. Treatment-emergent adverse events were rare and all mild or moderate in intensity. Tremors were reported in two (6.9%) adolescents after inhalation of the free combination and considered treatment-related by the investigator.
Discussion
Asthmatic adolescents are generally recommended to be dosed like adults [13,19,20]. However limited PK/PD data are available on fixed ICS/LABA combinations. In addition, the influence of age on the systemic exposure of drugs to be administered via pMDI with or without a VHC is still not fully elucidated. In the present study, we showed that the PD and the overall systemic exposure to the active ingredients of an extrafine fixed pMDI combination of BDP/FF administered with or without a VHC in adolescents is equivalent to that of a free licenced combination of BDP pMDI and FF pMDI. In addition, we showed that the systemic exposure in adolescents was not higher than in adults.
When BDP/FF pMDI is administered without a VHC, the total systemic exposure to B17MP and formoterol is characterized by both pulmonary and gastro-intestinal components [18]. Since the absorption by gastro-intestinal route is much slower than the absorption by the lung (tmax at approximately 4 h and 2 h for formoterol and B17MP, respectively [21,22]), it is reasonable to assume that the amount of drug reaching the blood in the first 30 min after inhalation is predominantly coming from the lungs. In fact in a study on healthy volunteers, the systemic exposures at early time points (AUC(0,0.5 h)) after BDP/FF pMDI administration were not affected by prevention of gastrointestinal absorption with charcoal block [18], demonstrating that this parameter is a valid indicator of pulmonary absorption and lung deposition. In view of these considerations, it was notable that BDP/FF pMDI in adolescents was not higher and not significantly different to BDP/FF pMDI in adults in terms of AUC(0,0.5 h) for both B17MP and formoterol (90% CI 0.63, 1.13). Indeed, assuming a monocompartmental system with the same elimination rate constant, adolescents have a lower volume in comparison with adults in which drugs distribute after lung absorption (reasonably proportional to the BSA [23]). Thus, the absolute amount of drug absorbed from the lung must necessarily be lower in adolescents as compared with adults. In line with these findings, two previous investigations demonstrated that when using a pMDI in combination with a VHC, the lung absorption for the same nominal dose of budesonide or BDP/FF, was lower in young patients but with equivalent systemic exposure in children, adolescents and adults [24,25] due to the lower body size of younger patients. Taken together, these results suggest that for the same nominal dose administered via pMDI with or without VHC, the amount of drug delivered to the lung increases relative to the body size of patients with no significant overall effect of age on the systemic exposure. Indeed, this could be explained by the different upper airway geometry [26] and/or the lower inspiratory capacity of young patients in comparison with adults [27] potentially leading to higher ingested amounts and lower pulmonary drug delivery in adolescents. Notably, the amount of drug delivered to the lung is also expected to increase relative to the pulmonary surface area of patients, thus maintaining comparable lung concentrations independently of the age. These findings support both the safety and efficacy of drugs administered via pMDI in adolescents at the same dosage as adults as also confirmed by the direct comparison of the fixed with a free combination of established safety and efficacy profile in which similar in vitro deposition data [28] and equivalent in vivo total systemic exposure to B17MP (PE 0.92, 90% CI 0.82, 1.03) and formoterol (PE 0.95, 90% CI 0.86, 1.05) were observed (Tables 3 and 5). The safety profile of the BDP/FF pMDI is further supported by the equivalent PD response to the free combination in terms of plasma glucose (AUC(0,t) PE 0.99), plasma potassium (AUC(0,t) PE 1.00) and pulse rate (PE 1.01) (Table 6) which are all potentially affected by ICS/LABA activity.
VHCs are generally used to optimize drug targeting to the airways in subjects with coordination difficulties. However, the increase in pulmonary deposition often observed with VHC devices [18], could potentially lead to an increase in overall systemic exposure. In our study, the use of the fixed combination with VHC in comparison with the fixed or the free combination alone did not increase the total systemic exposure to B17MP and formoterol measured by AUC(0,t) (upper level of 90% CI 1.19). However, there was a general increase in the systemic exposure at early time points measured as AUC(0,0.5 h) (Tables 3 and 5). This indicates that the increased lung deposition mediated by VHC is balanced by a decreased amount of drug swallowed and subsequently absorbed from the gut, leading to no change in overall systemic exposure. The equivalent (90% CI within 0.8, 1.25) total systemic exposure obtained with or without the use of the VHC device (Tables 3 and 5) was also reflected in equivalent plasma glucose, plasma potassium and pulse rate levels (Table 6).
In an attempt to demonstrate the improved drug delivery to the airways by using the VHC in subjects with reduced coordination, we divided the adolescent population in two sub-groups based on their AUC(0,0.5 h) after administration of the free or the fixed combination without the VHC device. Since this parameter is an index of pulmonary drug delivery, patients having a low degree of coordination between actuation and inhalation are expected to have lower AUC(0,0.5 h) values when inhaling without the VHC device. Therefore, subjects with low AUC(0,0.5 h) (below the 50th percentile) were considered ‘poor’ coordinators while those with values above the 50th percentile were considered ‘good’ coordinators. Interestingly, while without the use of the VHC there was a substantial difference between the ‘poor’ and ‘good’ coordinators in terms of AUC(0,0.5 h), with the use of the VHC, this parameter was not significantly different between the two sub-groups of patients. This analysis clearly demonstrated that the use of the VHC optimized the drug delivery to the airways in subjects having poorer coordination.
In conclusion, in the present study we showed that the PD and overall systemic exposure to the active ingredients of an extrafine fixed pMDI combination of BDP/FF with or without VHC in adolescents is equivalent to that of a free licenced combination of pMDIs of established safety and efficacy profile. The systemic exposure in adolescents was not higher than in adults. These results support the indication for use of a fixed ICS/LABA combination pMDI with or without a VHC in adolescents at the same dosage as adults.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare this study was financially supported by Chiesi Farmaceutici S.p.A and Mirco Govoni, Germano Lucci, Mario Scuri and Daniela Acerbi were employed at Chiesi Farmaceutici S.p.A. Piotr Kuna was the coordinating investigator of the trial and received lecture fees and cost of participation for international congresses from Chiesi. Iwona Stelmach was the principal investigator of the trial and had no competing interests. The relationship between the Sponsor and the authors’ affiliations was regulated by financial agreements. There are no other relationships or activities that could appear have influenced the submitted work.
Contributions
All authors contributed to study design conceptualization, analysis and interpretation, and manuscript preparation and/or critical revision.
References
- 1.Global Initiative for Asthma (GINA), National Institute of Health, National Heart Lung and Blood Institute (NHLBI) / World Health Organisation (WHO) Global strategy for asthma management and prevention. Workshop Report 2015. Available at http://www.ginasthma.org.
- 2.Fireman P, Prenner BM, Vincken W, Demedts M, Mol SJ, Cohen RM. Long-term safety and efficacy of a chlorofluorocarbon-free beclometasone dipropionate extrafine aerosol. Ann Allergy Asthma Immunol. 2001;86:557–65. doi: 10.1016/S1081-1206(10)62905-5. [DOI] [PubMed] [Google Scholar]
- 3.Fardon TC, Burns P, Barnes ML, Lipworth BJ. A comparison of 2 extrafine hydrofluoroalkane-134a-beclomethasone formulations on methacholine hyperresponsiveness. Ann Allergy Asthma Immunol. 2006;96:422–30. doi: 10.1016/S1081-1206(10)60909-X. [DOI] [PubMed] [Google Scholar]
- 4.Pauwels RA, Löfdahl CG, Postma DS, Tattersfield AE, O'Byrne P, Barnes PJ, Ullman A. Effect of inhaled formoterol and budesonide on exacerbations of asthma. Formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group. N Engl J Med. 1997;337:1405–11. doi: 10.1056/NEJM199711133372001. [DOI] [PubMed] [Google Scholar]
- 5.Suissa S, Ernst P, Benayoun S, Baltzan M, Cai B. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med. 2000;343:332–6. doi: 10.1056/NEJM200008033430504. [DOI] [PubMed] [Google Scholar]
- 6.Lazarus SC, Boushey HA, Fahy JV, Chinchilli VM, Lemanske RF, Sorkness CA, Kraft M, Fish JE, Peters SP, Craig T, Drazen JM, Ford JG, Israel E, Martin RJ, Mauger EA, Nachman SA, Spahn JD, Szefler SJ. Asthma Clinical Research Network for the National Heart Ln, and Blood Institute. Long-acting beta2-agonist monotherapy vs continued therapy with inhaled corticosteroids in patients with persistent asthma: a randomized controlled trial. JAMA. 2001;285:2583–93. doi: 10.1001/jama.285.20.2583. [DOI] [PubMed] [Google Scholar]
- 7.Bateman ED, Boushey HA, Bousquet J, Busse WW, Clark TJ, Pauwels RA, Pedersen SE, Group GI. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL study. Am J Respir Crit Care Med. 2004;170:836–44. doi: 10.1164/rccm.200401-033OC. [DOI] [PubMed] [Google Scholar]
- 8.Lalloo UG, Malolepszy J, Kozma D, Krofta K, Ankerst J, Johansen B, Thomson NC. Budesonide and formoterol in a single inhaler improves asthma control compared with increasing the dose of corticosteroid in adults with mild-to-moderate asthma. Chest. 2003;123:1480–7. doi: 10.1378/chest.123.5.1480. [DOI] [PubMed] [Google Scholar]
- 9.Kips JC, O'Connor BJ, Inman MD, Svensson K, Pauwels RA, O'Byrne PM. A long-term study of the antiinflammatory effect of low-dose budesonide plus formoterol versus high-dose budesonide in asthma. Am J Respir Crit Care Med. 2000;161:996–1001. doi: 10.1164/ajrccm.161.3.9812056. [DOI] [PubMed] [Google Scholar]
- 10.Chawes BL, Govoni M, Kreiner-Møller E, Vissing NH, Poorisrisak P, Mortensen L, Nilsson E, Bisgaard A, Dossing A, Deleuran M, Skytt NL, Samandari N, Piccinno A, Sergio F, Ciurlia G, Poli G, Acerbi D, Singh D, Bisgaard H. Systemic exposure to inhaled beclometasone/formoterol DPI is age and body size dependent. Respir Med. 2014;108:1108–16. doi: 10.1016/j.rmed.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Chawes BL, Govoni M, Piccinno A, Kreiner-Møller E, Vissing NH, Mortensen L, Nilsson E, Bisgaard A, Deleuran M, Skytt N, Samandari N, Acerbi D, Bisgaard H. A clinical pharmacology study of fixed vs. free combination of inhaled beclomethasone dipropionate and formoterol fumarate dry powder inhalers in asthmatic adolescents. Br J Clin Pharmacol. 2014;78:1169–71. doi: 10.1111/bcp.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fostair/Foster Summary of Product Characteristics. 2015. Available at http://www.medicines.org.uk/emc/
- 13.Atimos Summary of Product Characteristics 2014; Qvar Summary of Product Characteristics. 2013. Available at http://www.medicines.org.uk/emc/
- 14. Guideline on the Investigation of Bioequivalence. Doc. Ref.: CPMP/EWP/QWP/1401/98 Rev. 1/ Corr ** - EMA - 2010. 2012.
- 15.Chawes BL, Piccinno A, Kreiner-Møller E, Vissing NH, Poorisrisak P, Mortensen L, Nilson E, Bisgaard A, Dossing A, Deleuran M, Skytt NL, Samandari N, Sergio F, Ciurlia G, Poli G, Acerbi D, Bisgaard H. Pharmacokinetic comparison of inhaled fixed combination vs. the free combination of beclomethasone and formoterol pMDIs in asthmatic Children. Br J Clin Pharmacol. 2013;75:1081–8. doi: 10.1111/j.1365-2125.2012.04459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, Van Der Grinten CPM, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 17.Lam TK, Leung DT. More on simplified calculation of body-surface area. N Engl J Med. 1988;318:1130–3. doi: 10.1056/NEJM198804283181718. [DOI] [PubMed] [Google Scholar]
- 18.Singh D, Collarini S, Poli G, Acerbi D, Amadasi A, Rusca A. Effect of AeroChamber Plus™ on the lung and systemic bioavailability of beclomethasone dipropionate/formoterol pMDI. Br J Clin Pharmacol. 2011;72:932–9. doi: 10.1111/j.1365-2125.2011.04024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seretide Acuhaler Summary of Product Characteristics 2014; Symbicort Turbohaler Summary of Product Characteristics. 2013. Available at http://www.medicines.org.uk/emc/
- 20.Seretide Evohaler Summary of Product Characteristics. 2014. Available at http://www.medicines.org.uk/emc/
- 21.Daley-Yates PT, Price AC, Sisson JR, Pereira A, Dallow N. Beclomethasone dipropionate: absolute bioavailability, PK and metabolism following intravenous, oral, intranasal and inhaled administration in man. Br J Clin Pharmacol. 2001;51:400–9. doi: 10.1046/j.0306-5251.2001.01374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Derks MG, van den Berg BT, van der Zee JS, Braat MC, van Boxtel CJ. Biphasic effect-time courses in man after formoterol inhalation: eosinopenic and hypokalemic effects and inhibition of allergic skin reactions. J Pharmacol Exp Ther. 1997;283:824–32. [PubMed] [Google Scholar]
- 23.Rowland M, Tozer TN. Clinical Pharmacokinetics and Pharmacodynamics: Concepts and Applications. 4th edn. Lippincott Williams & Wilkins; 2010. Philadelphia, PA 19106. [Google Scholar]
- 24.Govoni M, Piccinno A, Lucci G, Poli G, Acerbi D, Baronio R, Singh D, Kuna P, Chawes BL, Bisgaard H. The systemic exposure to inhaled beclometasone/formoterol pMDI with valved holding chamber is independent of age and body size. Pulm Pharmacol Ther. 2015;30:102–9. doi: 10.1016/j.pupt.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Onhoj J, Thorsson L, Bisgaard H. Lung deposition of inhaled drugs increases with age. Am J Respir Crit Care Med. 2000;162:1819–22. doi: 10.1164/ajrccm.162.5.2002132. [DOI] [PubMed] [Google Scholar]
- 26.Berg E. In vitro properties of pressurized metered dose inhalers with and without spacer devices. J Aerosol Med. 1995;8:S3–10. doi: 10.1089/jam.1995.8.suppl_3.s-3. Suppl 3: discussion S1. [DOI] [PubMed] [Google Scholar]
- 27.Van Schayck CP, Donnell D. The efficacy and safety of QVAR (hydrofluoroalkane-beclomethasone diproprionate extrafine aerosol) in asthma (Part 2): Clinical experience in children. Int J Clin Pract. 2004;58:786–94. doi: 10.1111/j.1368-5031.2004.00274.x. [DOI] [PubMed] [Google Scholar]
- 28.Piccinno A, Poli G, Monno R, Goethals F, Nollevaux F, Acerbi D. Extrafine beclomethasone dipropionate and formoterol in single and separate inhalers. Clinic Pharmacol Biopharm. 2012;1:1–8. [Google Scholar]


