Abstract
Aims
Low-dose aspirin (LDA) and non-steroidal-anti-inflammatory drugs (NSAIDs) both increase the risk of upper gastrointestinal events (UGIEs). In the Netherlands, recommendations regarding the prescription of gastroprotective agents (GPAs) in LDA users were first issued in 2009 in the HARM-Wrestling consensus. National guidelines on gastroprotective strategies (GPSs) in NSAID users were issued in the first part of the preceding. The aim of the present study was to examine time-trends in GPSs in patients initiating LDA and those initiating NSAIDs between 2000 and 2012.
Methods
Within a large electronic primary healthcare database, two cohorts were selected: (i) patients newly prescribed LDA and (ii) patients newly prescribed NSAIDs between 2000 and 2012. Patients who had been prescribed a GPA in the previous six months were excluded. For both cohorts, patients’ risk of a UGIE was classified as low, moderate or high, based on the HARM-Wrestling consensus, and the presence of an adequate GPSwas determined.
Results
A total of 37 578 patients were included in the LDA cohort and 352 025 patients in the NSAID cohort. In both cohorts, an increase in GPSs was observed over time, but prescription of GPAs was lower in the LDA cohort. By 2012, an adequate GPS was present in 31.8% of high-risk LDA initiators, vs. 48.0% of high-risk NSAID initiators.
Conclusions
Despite a comparable risk of UGIEs, GPSs are prescribed less in high-risk LDA initiators than in high-risk NSAID initiators. For both groups of patients, there is still room for improvement in guideline adherence.
Keywords: gastrointestinal hemorrhages, gastroprotective agents, low-dose aspirin, NSAIDs, pharmacoepidemiology
WHAT IS KNOWN ABOUT THIS SUBJECT
Low-dose aspirin and NSAIDs both increase the risk of serious upper gastrointestinal events. This risk can be reduced by concomitant prescription of a gastroprotective agent.
Various national and international guidelines have been issued regarding gastroprotective strategies in high-risk NSAID users, but recommendations on the prescription of gastroprotective agents in low-dose aspirin users were not issued until more recently.
WHAT THIS STUDY ADDS
This study shows that the prescription of gastroprotective agents is far lower in high-risk low-dose aspirin users than in high-risk NSAID users, despite a comparable risk of upper gastrointestinal events in these high-risk groups. Less familiarity with the recommendations for low-dose aspirin users may play a role. However, even for NSAID users there is room for improvement in guideline adherence.
Of the environmental factors investigated, a change in the reimbursement policy of proton pump inhibitors had the clearest visible effect on prescription of gastroprotective agents in both low-dose aspirin and NSAID users, demonstrating the influence of such policy measures on guideline adherence.
Introduction
Low-dose aspirin (LDA) and non-steroidal anti-inflammatory drugs (NSAIDs) are both widely used in primary care [1,2]. They are associated with an increased risk of serious upper gastrointestinal events (UGIEs), such as ulceration, bleeding and perforation [3–7]. The risk of the occurrence of a UGIE is influenced by various factors, including age, comorbidity and concomitant use of other ulcerogenic medications [3,4,8,9]. The risk can be reduced by concomitant prescription of a gastroprotective agent (GPA) [10–12]. For NSAIDs, prescription of a cyclo-oxygenase-2 (COX-2) selective NSAID (coxib) rather than a non-selective NSAID also helps to lower the risk of a UGIE [11].
In the last decade, several national and international guidelines have been developed, aimed at reducing the number of NSAID-related UGIEs [11,13,14]. In the Netherlands, a multidisciplinary guideline for the prevention of UGIEs in NSAID users was first issued in 2003 [13]. For the prevention of UGIEs in LDA users, however, national recommendations were issued until 2009, when the HARM-Wrestling report was published (initially in Dutch, with a subsequent, definitive version in English) [15,16]. This consensus report was issued by a multidisciplinary task force, and contained revised recommendations on the prescription of GPAs in high-risk NSAID users and new recommendations on the prescription of GPAs in high-risk LDA users. As LDA is less ulcerogenic than NSAIDs, risk groups for LDA users were defined separately, to equate the risk of a UGIE in high-risk LDA users and high-risk NSAID users. The risk of UGIEs is thus comparable in high-risk LDA users and high-risk NSAID users, and both high-risk groups have an equally strong indication for prescription of a GPA [16].
The implementation of gastroprotective strategies (GPSs) in high-risk NSAID users has been previously studied and was found to be around 40–60% in the years following publication of the first guideline on this topic in 2003 [17,18]. However, much less is known about the prescription of GPAs in high-risk LDA users. As the HARM-Wrestling consensus defined high-risk users of LDA in such a way that their risk corresponded as closely as possible to the risk in high-risk NSAID users, one might expect the prescription patterns of GPAs to be similar in both populations.
Many factors are known to play a role in the level of adherence to guidelines. Firstly, factors relating to healthcare professionals, such as awareness of a guideline and familiarity with its content, are known to play a role [19]. As guidelines for LDA users are relatively new, adherence may therefore currently be lower than for NSAID users.
Secondly, environmental factors such as electronic decision systems, economic and policy-related factors have also been demonstrated to affect adherence to guidelines [20,21]. With regard to LDA and NSAID users, various environmental factors may have influenced the prescription of GPAs over the last decade: (i) medication surveillance for drug–drug interactions, which were introduced at various time points, depending on the electronic prescribing systems used; (ii) the introduction of two inspectorate summative indicators for community pharmacists: one in 2008, measuring the percentage of NSAID users aged over 70 years receiving a GPS, and one in 2011, measuring the percentage of high-risk LDA users receiving a GPA; (iii) the availability of cheaper generic proton pump inhibitors (PPIs) since March 2002 [22]; (iv) the introduction of a policy in July 2008 which allowed health insurance companies only to reimburse a specific selection of medications, including PPIs, of their choosing [23]; and (v) an alteration in the national reimbursement policy for PPIs in January 2012: incidental prescriptions of PPIs were no longer reimbursed, but chronic use was still reimbursed, with the exception of the first prescription issued [24]. Concerns regarding both the cardiovascular safety of coxibs and a possible negative effect of PPIs on the efficacy of clopidogrel may also have affected the prescription of these medications [16,17,25].
The objective of the present study was to examine time trends in GPSs both in LDA users and NSAID users, by performing a population-based cohort study among incident LDA users and incident NSAID users between 2000 and 2012. We also explored temporal relationships with various environmental factors that may have played a role.
Methods
Study design
A cohort study was conducted among incident LDA users and incident NSAID users. Only incident users were included as prescribers tend to adhere to pharmacotherapeutic guidelines more stringently in new users than in prevalent users [26].
Setting
Data for this study were retrieved from the Integrated Primary Care Information (IPCI) database. This longitudinal primary healthcare database contains the electronic patient records of over 1.5 million patients registered with general practitioners (GPs) throughout the Netherlands. In the Netherlands, GPs form the first point of care and act as gatekeepers to secondary care. The medical records can therefore be assumed to contain all relevant medical information. They contain all journal entries by the GP, coded diagnoses using the International Classification for Primary Care (ICPC) [27], and referrals, clinical findings by specialists, laboratory findings and hospitalizations. In addition, there is a complete record of all drug prescriptions, their dosage regimen and the Anatomical Therapeutic Chemical (ATC) classification code [28]. More extensive details on the database have been reported elsewhere [29,30].
Study cohorts
The source population comprised all patients contributing data to the IPCI database between 2000 and 2012, with at least 12 months of valid database history before the date of study entry. From this source population, two cohorts of patients were identified and included in the present study. The first cohort consisted of all patients newly prescribed LDA (defined as acetylsalicylic acid (ASA) ≤ 80 mg day–1 or carbasalate calcium ≤ 100 mg day–1). A new prescription was defined as the drug not being used in the previous six months. Only the first LDA prescription for each patient was included, and the date this was issued was defined as the index date. In this LDA cohort, patients were excluded if they were using an NSAID on the index date, because recommendations on gastroprotection are more stringent for patients using NSAIDs [16]. The second cohort consisted of all patients newly prescribed a non-selective NSAID or a coxib. Again, only the first NSAID prescription for each patient was included and the date this was issued was defined as the index date. In both cohorts, patients who had received a GPA in the six months prior to the index date were excluded, to avoid overestimation of adherence, as these patients may have had other indications for using GPAs. As the same source population was used for each cohort, patients could be included in both. All prescriptions were identified based on the ATC code (for specification see Appendix 1).
Risk of a UGIE
In order to determine if patients were at an increased risk of developing a UGIE, each patient's age, medical history and use of co-medication were recorded. Concomitant use of medication was defined as overlapping duration of use on the index date. Definitions of UGIE risk were based on the HARM-Wrestling consensus for both cohorts and are described in Table 1 [16]. The HARM-Wrestling consensus also defined concomitant use of therapeutic doses of low-molecular-weight heparin (LMWH) as a risk factor. This risk factor was not included in our definition of UGIE risk as LMWH is mostly used for bridging while starting anticoagulants, so including LMWH as a risk factor would have led to false-positive high-risk cases.
Table 1.
Definition of low, moderate and high risk of a UGIE in each cohort
| UGIE risk group | Definition in LDA cohort | Definition in NSAID cohort |
|---|---|---|
| High risk | At least one of the following high risk factors: | At least one of the following high risk factors: |
| History of UGIE | History of UGIE | |
| Age ≥ 80 | Age ≥ 70 | |
| Age 70–79 and ≥ one other moderate risk factor | ≥ Two moderate risk factors | |
| Age 60–69 and ≥ two moderate risk factors | ||
| Moderate risk | No high risk and at least one of the following moderate risk factors: | No high risk and at least one of the following moderate risk factors: |
| Age 70–79 | Age 60–69 years | |
| Use of VKA | History of DM | |
| Use of clopidogrel | History of HF | |
| Use of a corticosteroid | History of severe RA | |
| Use of SSRI | Use of VKA | |
| Use of spironolactone | Use of LDA | |
| Use of clopidogrel | ||
| Use of a corticosteroid | ||
| Use of an SSRI | ||
| Use of spironolactone | ||
| High-dose NSAID | ||
| Low risk | No moderate or high risk | No moderate or high risk |
DM, diabetes mellitus; HF, heart failure; LDA, low-dose aspirin; NSAID, non-steroidal anti-inflammatory drug; RA, rheumatoid arthritis; SSRI, serotonin-reuptake inhibitor; UGIE, upper gastrointestinal event; VKA, vitamin K antagonist.
The history of the diseases and conditions described in Table 1 was assessed based on ICPC coding and free-text search strings (see Appendix 1 for specifications). In the case of diabetes and severe rheumatoid arthritis, the use of specific types of medication, identified based on the ATC classification code, was also taken into account, in addition to ICPC coding as a proxy for the identification of these comorbidities (see Appendix 1 for specifications).
GPS
For all included patients, we subsequently assessed whether a GPS was implemented. In line with the definitive HARM-Wrestling consensus [16], a GPS was defined as follows:
For the LDA cohort: concomitant prescription of a PPI or double-dose histamine-2 receptor antagonist (H2RA).
For the NSAID cohort: (i) prescription of a non-selective NSAID or coxib with concomitant prescription of a PPI or double-dose H2RA or (ii) prescription of a coxib alone, provided that there was no concomitant use of LDA.
According to the HARM-Wrestling consensus [16], such a GPS should only be implemented in high-risk users. We also determined whether a GPS was prescribed in other risk groups, to examine whether GPs take this into account. Double-dose H2RA was defined as H2RA in a prescribed daily dosage of at least twice the defined daily dosage. Concomitant prescription was defined as overlapping duration of use on the index date or within two days after the index date. In line with the HARM-Wrestling consensus, we did not consider diclofenac–misoprostol to be an adequate GPS [15], but we did determine the frequency of prescription of this combination, as misoprostol has been suggested for gastroprotection in previous literature and guidelines [11,13].
Statistical analysis
For each cohort, a comparison was made between prescription of a GPS in patients at a high or moderate risk, and a low risk of a UGIE, and odds ratios (ORs) and their 95% confidence intervals (CIs) were determined using univariate logistic regression. Univariate logistic regression was also used to compare the odds of a GPS in high-risk LDA users with those in high-risk NSAID users. For 2012, potential predictors of high UGIE risk patients receiving a GPS were examined, to evaluate which risk factors influence the GP's decision to implement a GPS. Crude ORs and their 95% CIs were calculated by performing univariate logistic regression analyses. In addition, multivariate logistic regression was performed to calculate ORs adjusted for age (ORadj). We chose not to adjust for other UGIE risk factors because of the potential interactions between these factors. All analyses were performed using SPSS version 20 (SPSS, Chicago, IL, USA).
Study approval
This study was approved by the Board of Directors of the IPCI database.
Results
Baseline characteristics
In total, 37 578 patients were newly prescribed LDA between 2000 and 2012 and were included in the LDA cohort (Table 2). The mean age at prescription was 66.2 (± 14.1) years and 55.2% of the cohort was male. In this cohort, 24.8% of patients were found to have a high risk and 28.5% a moderate risk of a UGIE. Overall, 14.1% of patients in this cohort received a GPS.
Table 2.
Baseline characteristics of the two study cohorts
| Cohort | ||
|---|---|---|
| LDA | NSAID | |
| n = 37 578 | n = 352 025 | |
| n (%) | n (%) | |
| Age, years (mean ± sd) | 66.2 (± 14.1) | 46.1 (± 17.9) |
| Gender (% male) | 20 758 (55.2) | 156 122 (44.3) |
| Type of index-prescription | ||
| Non-selective NSAID | NA | 312 179 (88.7) |
| Diclofenac–misoprostol | NA | 25 288 (7.2) |
| Coxib | NA | 14 558 (4.1) |
| Individual UGIE risk factors | ||
| Age 60–69 years | 10 163( 27.0) | 44 988 (12.8) |
| Age 70–79 years | 9107 (24.2) | 24 914 (7.1) |
| Age ≥ 80 years | 6529 (17.4) | 10 691 (3.0) |
| History of UGIE | 1757 (4.7) | 7750 (2.2) |
| History of DM | 7575 (20.2) | 25 378 (7.2) |
| History of HF | 7763 (20.7) | 21 746 (6.2) |
| History of severe RA | 134 (0.4) | 923 (0.3) |
| Use of VKA | 1361 (3.6) | 3106 (0.9) |
| Use of clopidogrel | 2815 (7.5) | 557 (0.2) |
| Use of corticosteroids | 558 (1.5) | 1324 (0.4) |
| Use of an SSRI | 1220 (3.2) | 9322 (2.6) |
| Use of spironolactone | 802 (2.1) | 687 (0.2) |
| Use of LDA | NA | 14 916 (4.2) |
| High-dose NSAID | NA | 190 662 (54.2) |
| UGIE risk group* | ||
| Low risk | 17 565 (46.7) | 111 462 (31.7) |
| Moderate risk | 10 709 (28.5) | 151 570 (43.1) |
| High risk | 9304 (24.8) | 88 993 (25.3) |
| Gastroprotective agent | ||
| PPI | 5309 (14.1) | 61 733 (17.5) |
| H2RA double dose | 7 (0.0) | 26 (0.0) |
| Gastroprotective strategy† | 5316 (14.1) | 72 240 (20.5) |
DM, diabetes mellitus; HF, heart failure; H2RA, histamine-2 receptor antagonist; LDA, low-dose aspirin; NA, not applicable; NSAID, non-steroidal anti-inflammatory drug; PPI, proton pump inhibitor; RA, rheumatoid arthritis; SSRI, selective serotonin-reuptake inhibitor; UGIE, upper gastrointestinal event; VKA, vitamin K antagonist.
As defined separately for each cohort.
Gastroprotective strategy: concomitant PPI or double-dose H2RA; in NSAID cohort also coxib (provided that concomitant LDA was not prescribed).
In the NSAID cohort, 352 025 patients were included (Table 2). The mean age at prescription was 46.1 (± 17.9) years and 44.3% of the cohort was male. In this cohort, 25.3% of patients had a high risk and 43.1% a moderate risk of a UGIE. Overall, 20.5% of the cohort received a GPS.
GPS for each UGIE risk group
In both cohorts, over the entire period, patients were more likely to receive an adequate GPS if they had a moderate or high UGIE risk (Table 3). In the LDA cohort, a GPS was prescribed in 7.7% of low-risk patients, 16.4% of moderate-risk patients and 23.7% of high-risk patients [OR 2.3 (2.2–2.5) and 3.7 (3.5–4.0) for moderate-risk vs. low-risk patients, and high-risk vs. low-risk patients, respectively]. In the NSAID cohort, these percentages were 10.7% for low-risk, 15.6% for moderate-risk and 41.2% for high-risk patients [OR 1.6 (1.6–1.7) and 6.2 (6.0–6.3) for moderate-risk vs. low-risk and high-risk vs. low-risk patients, respectively]. This meant that within all high-risk patients, the odds of LDA users receiving a GPS were half that of NSAID users [OR 0.5 (0.4–0.5) for high-risk LDA users versus high-risk NSAID users].
Table 3.
Gastroprotective strategy in each cohort for each GI risk group
| Gastroprotective strategy† | OR | |||
|---|---|---|---|---|
| UGIE risk group* | No | Yes | ||
| n (%‡) | n (%‡) | (95% CI) | P-value | |
| LDA cohort | n = 32 262 | n = 5316 | ||
| Low risk | 16 210 (92.3) | 1355 (7.7) | 1 (ref) | |
| Moderate risk | 8954 (83.6) | 1755 (16.4) | 2.3 (2.2–2.5) | < 0.001 |
| High risk | 7098 (76.3) | 2206 (23.7) | 3.7 (3.5–4.0) | < 0.001 |
| NSAID cohort | n = 279 785 | n = 72 240 | ||
| Low risk | 99 482 (89.3) | 11 980 (10.7) | 1 (ref) | |
| Moderate risk | 127 955 (84.4) | 23 615 (15.6) | 1.5 (1.5–1.6) | < 0.001 |
| High risk | 52 348 (58.8) | 36 645 (41.2) | 5.8 (5.7–5.9) | < 0.001 |
CI, confidence interval; H2RA: histamine-2 receptor antagonist; LDA: low-dose aspirin; NSAID: non-steroidal anti-inflammatory drug; OR, odds ratio; PPI, proton pump inhibitor; UGIE, upper gastrointestinal event.
As defined separately for each cohort.
Gastroprotective strategy: concomitant PPI or double-dose H2RA; in NSAID cohort also coxib (if no concomitant LDA).
Row percentage.
GPS for each UGIE risk group over time
Figure1 shows the percentage of incident users prescribed an adequate GPS over time for each UGIE risk group, for each cohort.
Figure 1.
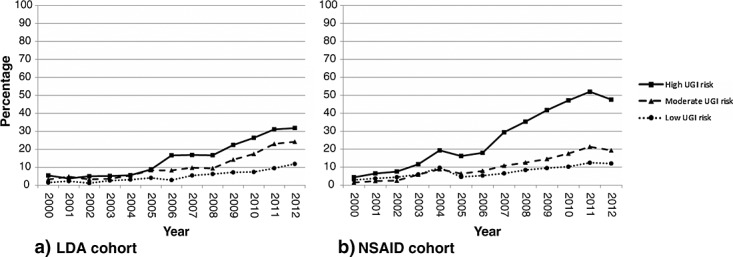
Percentage of patients prescribed a gastroprotective strategy per year for each upper gastrointestinal event risk group (as defined separately for each cohort). LDA, low-dose aspirin; NSAID, UGIE, upper gastrointestinal event
In the LDA cohort (Figure1a), prescription of a GPS was fairly stable in all risk groups in the first part of the decade. In the second part of the decade, an increase was observed in all risk groups, with the strongest increase occurring in the high UGIE risk group. By 2012, a GPS was present in 31.8%, 24.2% and 11.8% of patients with a high, moderate and low risk of UGIE, respectively.
In the NSAID cohort (Figure1b), a slight increase in gastroprotection in patients with a high UGIE risk had been observed before publication of the first national guideline on this topic in 2003. A temporary decrease was observed in 2005, and from 2006 onwards a further increase over time was observed in all risk groups. In 2012, a GPS was prescribed in 48.0% of incident users with a high UGIE risk, 19.4% of those with moderate risk and 12.6% of those with low risk.
Types of GPSs
Figure2 shows the types of GPS over time in high-risk patients in each cohort. In both cohorts, double-dose H2RA is rarely prescribed. An increase in PPI prescription was present in the second part of the decade in both cohorts, but this trend did not continue into 2012, with a decrease occurring, particularly in the NSAID cohort. In the NSAID cohort, there was a sudden drop in coxib prescription in 2005. The combination of diclofenac–misoprostol, which was recommended in the early guidelines but not in the HARM-Wrestling consensus in 2009, was still being prescribed in 9.7% of high-risk NSAID patients in 2012.
Figure 2.
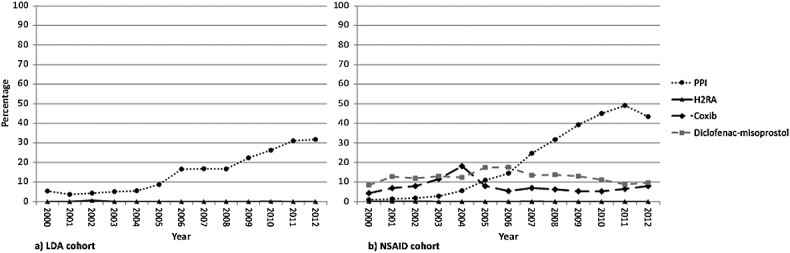
Type of gastroprotective strategy in high-risk patients per year. H2RA, histamine-2 receptor antagonist; LDA, low-dose aspirin; NSAID, non-steroidal anti-inflammatory drug; PPI, proton pump inhibitor
Predictors of adequate GPS in patients at high UGIE risk in 2012
Table 4 shows the predictors of prescription of a GPS in patients at high UGIE risk within each cohort in 2012. In the LDA cohort, a history of UGIE was not significantly associated with a GPS prescription [ORadj 1.2 (95% CI 0.9–1.5), P = 0.237]. When compared with patients aged < 60 years, those aged 70–79 with at least one moderate risk factor were significantly more likely to receive a GPS [ORadj 2.5 (95% CI 1.5–3.0), P < 0.001], as were those aged 60–69 with at least two other moderate risk factors [ORadj 2.6 (95% CI 1.1–6.4), P = 0.032], but for those aged ≥ 80 years no statistically significant association was found [ORadj 1.3 (95% CI 0.8–2.1), P = 0.216]. Of the moderate risk factors, concomitant use of an SSRI and corticosteroids were the strongest predictors of a GPS [ORadj 4.2 (95% CI 2.9–6.1), P < 0.001 and 3.4 (95% CI 2.2–5.3), P < 0.001, respectively].
Table 4.
Predictors of prescription of gastroprotective strategy in patients at high risk of a UGIE in 2012 for each cohort
| Gastroprotective strategy* | |||||
|---|---|---|---|---|---|
| No | Yes | OR crude (95% CI) | OR adjusted† (95% CI) | ||
| n (%‡) | n (%‡) | P-value | |||
| LDA cohort | 1479 | 689 | |||
| Gender | |||||
| Male | 689 (69.3) | 305 (30.7) | 1 (ref) | 1 (ref) | |
| Female | 790 (67.3) | 384 (32.7) | 1.1 (0.9–1.3) | 1.1 (0.9–1.3) | 0.428 |
| High-risk factors | |||||
| History of UGIE | 322 (67.8) | 153 (32.2) | 1.0 (0.8–1.3) | 1.2 (0.9–1.5) | 0.237 |
| Age ≥ 80 years | 1031 (69.9) | 445 (30.1) | 0.8 (0.7–1.0) | 1.3 (0.8–2.1)§ | 0.216 |
| Age 70–79 years and ≥ 1 other MRF | 175 (55.6) | 140 (44.4) | 1.9 (1.5–2.4) | 2.5 (1.5–4.0)§ | < 0.001 |
| Age 60–69 years and ≥ 2 other MRF | 14 (53.8) | 12 (46.2) | 1.9 (0.9–4.0) | 2.6 (1.1–6.4)§ | 0.032 |
| Moderate risk factors | |||||
| Age 60–69 years | 107 (67.3) | 52 (32.7) | 1.0 (0.7–1.5) | 1.5 (0.9–2.6)§ | 0.150 |
| Age 70–79 years | 258 (61.0) | 165 (39.0) | 1.5 (1.2–1.9) | 2.0 (1.2–3.2)§ | 0.005 |
| Use of VKA | 119 (68.4) | 55 (31.6) | 1.0 (0.7–1.4) | 1.0 (0.7–1.4) | 0.963 |
| Use of clopidogrel | 110 (49.3) | 113 (50.7) | 2.4 (1.8–3.2) | 2.6 (1.9–3.4) | < 0.001 |
| Use of corticosteroids | 34 (40.0) | 51 (60.0) | 3.4 (2.2–5.3) | 3.4 (2.2–5.3) | < 0.001 |
| Use of an SSRI | 49 (36.6) | 85 (63.4) | 4.1 (2.9–5.9) | 4.2 (2.9–6.1) | < 0.001 |
| Use of spironolactone | 73 (54.9) | 60 (45.1) | 1.8 (1.3–2.6) | 1.9 (1.3–2.7) | 0.001 |
| NSAID cohort | n = 9 806 | n = 9 041 | |||
| Gender | |||||
| Male | 4672 (54.6) | 3880 (45.4) | 1 (ref) | 1 (ref) | |
| Female | 5134 (49.9) | 5161 (50.1) | 1.2 (1.1–1.3) | 1.2 (1.1–1.3) | < 0.001 |
| High-risk factors | |||||
| History of UGIE | 1017 (56.0) | 798 (44.0) | 0.8 (0.8–0.9) | 1.2 (1.1–1.3) | 0.001 |
| ≥ 70 years | 2782 (39.2) | 4315 (60.8) | 2.3 (2.2–2.4) | 2.8 (2.6–3.0)§ | < 0.001 |
| ≥ 2 moderate risk factors | 7395 (55.1) | 6033 (44.9) | 0.7 (0.6–0.7) | 1.4 (1.3–1.6)§ | < 0.001 |
| Age 60–69 years + risk medication | 617 (42.7) | 829 (57.3) | 1.5 (1.3–1.7) | 2.4 (2.2–2.8)§ | < 0.001 |
| Age 60–69 years + HF/DM/RA | 1548 (55.0) | 1266 (45.0) | 0.9 (0.8–0.9) | 1.5 (1.3-1.6)§ | < 0.001 |
| Age 60–69 years + high-dose NSAID | 2889 (57.0) | 2180 (43.0) | 0.8 (0.7–0.8) | 1.4 (1.3–1.5)§ | < 0.001 |
| Moderate risk factors | |||||
| Age 60–69 years | 3927 (56.6) | 3017 (43.4) | 0.8 (0.7–0.8) | 1.4 (1.3–1.5)§ | < 0.001 |
| History of DM | 3023 (56.3) | 2348 (43.7) | 0.8 (0.7–0.8) | 0.8 (0.8–0.9) | < 0.001 |
| History of HF | 3307 (54.8) | 2 725 (45.2) | 0.8 (0.8–0.9) | 0.8 (0.8–0.9) | < 0.001 |
| History of severe RA | 76 (51.0) | 73 (49.0) | 1.0 (0.8–1.4) | 1.1(0.8–1.6) | 0.431 |
| Use of VKA | 230 (35.3) | 421 (64.7) | 2.0 (1.7–2.4) | 1.7 (1.4–2.0) | < 0.001 |
| Use of clopidogrel | 54 (42.2) | 74 (57.8) | 1.5 (1.0–2.1) | 1.4 (1.0–2.0) | 0.080 |
| Use of corticosteroids | 95 (36.4) | 166 (63.6) | 1.9 (1.5–2.5) | 2.1 (1.6–2.8) | < 0.001 |
| Use of an SSRI | 596 (42.1) | 819 (57.9) | 1.5 (1.4–1.7) | 2.6 (2.3–2.9) | < 0.001 |
| Use of spironolactone | 49 (39.5) | 75 (60.5) | 1.7 (1.2–2.4) | 1.4 (1.0–2.0) | 0.078 |
| Use of LDA | 1121 (41.5) | 1577 (58.5) | 1.6 (1.5–1.8) | 1.4 (1.3–1.6) | < 0.001 |
| High-dose NSAID | 6469 (53.7) | 5569 (46.3) | 0.8 (0.8–0.9) | 1.0 (1.0–1.1) | 0.610 |
CI, confidence interval; DM, diabetes mellitus; HF, heart failure; H2RA, histamine-2 receptor antagonist; LDA, low-dose aspirin; MRF, moderate-risk factor; NSAID, non-steroidal anti-inflammatory drug, OR, odds ratio; PPI, proton pump inhibitor; RA, rheumatoid arthritis; SSRI, selective serotonin-reuptake inhibitor; UGIE, upper gastrointestinal event; VKA, vitamin K antagonist.
Gastroprotective strategy: concomitant PPI or double-dose H2RA; in NSAID cohort also coxib (if no concomitant LDA).
Adjusted for age.
Row percentage.
Reference group is age < 60 years.
In the NSAID cohort, all individual high-risk factors were associated with increased odds of being prescribed a GPS. For the high-risk factor ‘≥ 2 moderate risk factors’, the odds of a GPS varied depending on the combination of risk factors present (Table 4). Of the moderate-risk factors, most were significantly associated with the presence of a GPS, with the exception of ‘a history of severe RA’ [ORadj 1.1 (95% CI 0.8–1.6), P = 0.431] and ‘prescription of a high-dose NSAID’ [ORadj 1.1 (95% CI 1.0–1.1), P = 0.610]. For a history of diabetes mellitus and heart failure, negative associations were found [ORadj 0.8 (95% CI 0.8–0.9), P < 0.001 for both diabetes and heart failure].
Discussion
The present study shows that the prescription of a GPA is lower in high-risk LDA users than in high-risk NSAID users, despite a comparable risk of UGIEs in these high-risk groups. These results tend to confirm that there is a misconception that LDA is not gastrotoxic, despite evidence to the contrary. Although adherence to recommendations regarding gastroprotection improved over time in both cohorts, an adequate GPS was present in only 31.8% of high-risk LDA initiators in 2012, compared with 48.0% of high-risk NSAID initiators. Less familiarity with the recommendations for LDA users, which were not issued until 2009, may have played a role. However, environmental factors also appear to affect adherence.
Environmental factors potentially influencing prescribing behaviour
After the alteration in national reimbursement policy for PPIs in January 2012, a sudden decrease in PPI prescriptions was seen in high-risk NSAID initiators (Figure2). In the LDA cohort, no decrease was observed, but the prior increase in PPI prescriptions appeared to stabilize, despite the introduction in 2011 of an inspectorate indicator measuring adherence to LDA recommendations. The effect of the new reimbursement policy may be less strong in this cohort, because in contrast to NSAIDs, LDA tends to be prescribed chronically. In this case, patients only have to pay for the first PPI prescription.
Medication surveillance for drug–drug interactions may also play a role, as concomitant use of most types of ulcerogenic medication is predictive of a GPS in both cohorts (Table 4). While this may, of course, also be explained by GPs’ knowledge of the literature and guidelines, it is notable that factors such as a history of diabetes, heart failure and severe rheumatoid arthritis, which do not lead to warnings within these surveillance systems, were not associated with increased prescription of a GPS. One way of improving adherence in the future may thus be the implementation of more sophisticated decision support modules into GP electronic systems. These could provide a more in-depth risk profile of the patients, allowing for more differentiated warnings when a high-risk NSAID or LDA initiator should receive a GPA.
In the NSAID cohort, a decrease in coxib prescription was observed in 2005. This decrease has also been found in previous studies and appears to be in response to the removal of rofecoxib from the market in 2004, after evidence emerged that its use was associated with an increased incidence of ischaemic cardiovascular events [2,25,31]. Other environmental factors, such as the availability of cheaper generic PPIs since 2002, do not appear to have played a strong role in prescribing behaviour. A relatively strong increase in PPI prescriptions was seen in the LDA cohort in 2006. It remains unclear which factors caused this temporary additional increase, which was not seen in the NSAID cohort.
Comparison with the existing literature
Recently, a Dutch cohort study was published regarding predictors of PPI prescription in LDA users between 2008 and 2010 [32]. In this study, 46% of high-risk patients prescribed LDA were found to receive regular concomitant PPI prescriptions. In our cohort, concomitant prescription of a PPI was found to be much lower during this period (23%). This difference may be explained, in part, by differences in cohort definition, as the cohort described in the previous study consisted of all regular LDA users, rather than only LDA initiators, and patients who had used GPA prior to cohort entry were not excluded. In addition, LDA users with concomitant use of NSAIDs were not excluded. As the HARM-Wrestling recommendations for NSAID users are more stringent than those for LDA users, this may have increased the percentage of LDA users with concomitant PPI found. Indeed, concomitant NSAID use was found to be one of the strongest predictors of PPI prescription in this previous cohort of LDA users. The fact that we excluded patients with concomitant NSAID use from our LDA cohort allows for a better estimate of adherence to the HARM-Wrestling recommendations for LDA users.
Strengths and limitations
Strengths of the present study included the fact that it was conducted using a database containing a large number of patients, reflecting the general population of the Netherlands. By using consistent methods to include cohorts of LDA and NSAID initiators from this population, our study allowed for a comparison between adherence to guidelines in these two patient groups. There were, however, several limitations that should be considered when reviewing the results. First, the HARM-Wrestling recommendations not only contain measures which should be taken to decrease the risk of UGIEs if LDA or NSAIDs are prescribed, but also state that physicians should carefully weigh the risks and benefits of prescribing these medications in patients at risk [33]. In the present study, we did not examine the indications for NSAID and LDA prescriptions and we were therefore unable to assess whether GPs have become more cautious in prescribing these drugs in high-risk patients. Secondly, we only had access to prescriptions issued by GPs. Neither prescriptions issued by specialists in secondary care nor over-the-counter medications are captured in this database. This may have led to some underestimation of the number of patients with a high risk of a UGIE. Finally, we did not determine the duration of prescription. LDA is generally prescribed for continuous use, whereas NSAIDs are often prescribed for shorter periods. The HARM-Wrestling recommendations on GPA prescription in NSAID users do not make a distinction in the duration of NSAID treatment when determining whether a GPA should be prescribed. Nonetheless, GPs may take the expected duration of prescription into account when deciding whether to implement a GPS upon initial NSAID prescription, as they may feel it is less appropriate to prescribe a GPA for shorter treatment regimens. This may, in part, explain the lack of adherence to guidelines on GPSs in NSAID initiators.
Conclusion
Overall, the present study showed that GPSs are implemented less in high-risk LDA users than in NSAID users, despite a comparable risk of UGIEs. For both groups of patients, there is still room for improvement in guideline adherence. Of the various environmental factors that could have played a role in the level of adherence achieved, the change in the PPI reimbursement policy had the clearest visible effect.
Appendix 1
Specification of medications and comorbidities
| Medication | Specification |
|---|---|
| Clopidogrel | ATC code B01AC04 (clopidogrel), B01AC22 (prasugrel) or B01AC30 (platelet aggregation inhibitors, combinations) |
| Corticosteroid | ATC code H02AB (glucocorticoids), with the exception of topical or local application |
| Coxib | ATC code M01AH (coxibs) |
| Diclofenac-misoprostol | ATC code M01AB55 (Arthrotec) |
| H2RA | ATC code A02BA (H2RA) |
| High-dose NSAID | Prescribed daily dosage ≥ the recommended daily maximum |
| Low-dose aspirin | ATC code B01AC06 (acetylsalicylic acid), B01AC08 (carbasalate calcium), B01AC30 (platelet aggregation inhibitors, combinations), N02BA01 (acetylsalicylic acid) in dosage ≤ 80 mg, or N02BA15 (carbasalate calcium) in dosage ≤ 100 mg |
| NSAID | ATC code M01A, with the exception of M01AX05 (glucosamine), M01AX12 (glucosaminoglycan polysulfate), M01AX21 (diacerein), M01AX24 (oxaceprol), M01AX25 (chondroitin sulfate) and M01AX26 (avocado and soyabean oil) |
| Non-selective NSAID | ATC code M01A, with the exception of M01AH (coxibs), M01AX05 (glucosamine), M01AX12 (glucosaminoglycan polysulfate), M01AX21 (diacerein), M01AX24 (oxaceprol), M01AX25 (chondroitin sulfate) and M01AX26 (avocado and soyabean oil) |
| PPI | ATC code A02BC (PPI) or M01AE52 (naproxen and esomeprazole) |
| Spironolactone | ATC code C03DA01 (spironolactone) |
| SSRI | ATC code N06AB (SSRI), N06AX21 (duloxetine) or N06AX16 (venlafaxine) |
| VKA | ATC code B01AA (vitamin K antagonists) |
| Comorbidity | Specification |
| History of UGIE | One of the following: |
| 1) History of UGIE according to journal text (search algorithm) | |
| 2) ICPC code D85 (duodenal ulcer) or D86 (other peptic ulcer) | |
| History of DM | One of the following: |
| 1) History of DM according to journal text (search algorithm); | |
| 2) ICPC code T90 (DM) | |
| 3) ATC code A10 (drugs used in diabetes) | |
| History of HF | One of the following: |
| 1) History of HF according to journal text (search algorithm) | |
| 2) ICPC code K77 (HF) | |
| History of severe RA | Both of the following: |
| 1) ICPC code L88 (RA) | |
| 2) prescription of at least one of the following in the year prior to index date: ATC code A07EC01, L01AA01, L04AA13, L04AA27, L04AB01, L04AB02, L04AB04, L04AB05, L04AB06, L04AC03, L04AC07, L04AD01, L04AX01, L04AX03, L01XC02, M01CB01, M01CC01, P01BA01 or P01BA02 (drugs used in severe RA) |
ATC, Anatomical Therapeutic Chemical; DM, diabetes mellitus; HF, heart failure; H2RA, histamine-2 receptor antagonist; ICPC, International Classification for Primary Care; LDA, low-dose aspirin; NSAID, non-steroidal anti-inflammatory drug; PPI, proton pump inhibitor; RA, rheumatoid arthritis; SSRI, selective serotonin-reuptake inhibitor; UGIE, upper gastrointestinal event; VKA, vitamin K antagonist.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: financial support from the Dutch Ministry of Health for the submitted work; MS guides a research group that sometimes conducts research for pharmaceutical companies, none related to the submitted work; no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Cardol M, van Dijk L, de Jong JD, de Bakker DH, Westert GP. 2004. The second Dutch National Survey of General Practice. GP care: what does the gatekeeper do? [Tweede Nationale Studie naar ziekten en verrichtingen in de huisartspraktijk. Huisartsenzorg: wat doet de poortwachter?]. NIVEL/RIVM: Utrecht/Bilthoven,. Available at http://www.nivel.nl/sites/default/files/bestanden/ns2_rapport2.pdf (last accessed 21 July 2014)
- 2.Koffeman AR, Valkhoff VE, Jong GW, Warlé-van Herwaarden MF, Bindels PJ, Sturkenboom MC, Luijsterburg PA, Bierma-Zeinstra SM. Ischaemic cardiovascular risk and prescription of non-steroidal anti-inflammatory drugs for musculoskeletal complaints. Scand J Prim Health Care. 2014;32:90–8. doi: 10.3109/02813432.2014.929810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gabriel SE, Jaakkimainen L, Bombardier C. Risk for serious gastrointestinal complications related to use of nonsteroidal anti-inflammatory drugs. A meta-analysis. Ann Intern Med. 1991;115:787–96. doi: 10.7326/0003-4819-115-10-787. [DOI] [PubMed] [Google Scholar]
- 4.Yeomans ND, Lanas AI, Talley NJ, Thomson AB, Daneshjoo R, Eriksson B, Appelman-Eszczuk S, Långström G, Naesdal J, Serrano P, Singh M, Skelly MM, Hawkey CJ. Prevalence and incidence of gastroduodenal ulcers during treatment with vascular protective doses of aspirin. Aliment Pharm Therap. 2005;22:795–801. doi: 10.1111/j.1365-2036.2005.02649.x. [DOI] [PubMed] [Google Scholar]
- 5.Sostres C, Gargallo CJ, Lanas A. Nonsteroidal anti-inflammatory drugs and upper and lower gastrointestinal mucosal damage. Arthritis Res Ther. 2013;15:S3. doi: 10.1186/ar4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valkhoff VE, Sturkenboom MC, Hill C, Veldhuyzen van Zanten S, Kuipers EJ. Low-dose acetylsalicylic acid use and the risk of upper gastrointestinal bleeding: a meta-analysis of randomized clinical trials and observational studies. Can J Gastroenterol. 2013;27:159–67. doi: 10.1155/2013/596015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lanas A, Wu P, Medin J, Mills EJ. Low doses of acetylsalicylic acid increase risk of gastrointestinal bleeding in a meta-analysis. Clin Gastroenterol Hepatol. 2011;9:762–8. doi: 10.1016/j.cgh.2011.05.020. e6. [DOI] [PubMed] [Google Scholar]
- 8.Fries JF, Williams CA, Bloch DA, Michel BA. Nonsteroidal antiinflammatory drug-associated gastropathy: incidence and risk factor models. Am J Med. 1991;91:213–22. doi: 10.1016/0002-9343(91)90118-h. [DOI] [PubMed] [Google Scholar]
- 9.McQuaid KR, Laine L. Systematic review and meta-analysis of adverse events of low-dose aspirin and clopidogrel in randomized controlled trials. Am J Med. 2006;119:624–38. doi: 10.1016/j.amjmed.2005.10.039. [DOI] [PubMed] [Google Scholar]
- 10.Hsiao FY, Tsai YW, Huang WF, Wen YW, Chen PF, Chang PY, Kuo KN. A comparison of aspirin and clopidogrel with or without proton pump inhibitors for the secondary prevention of cardiovascular events in patients at high risk for gastrointestinal bleeding. Clin Ther. 2009;31:2038–47. doi: 10.1016/j.clinthera.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Lanza FL, Chan FK, Quigley EM. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 2009;104:728–38. doi: 10.1038/ajg.2009.115. [DOI] [PubMed] [Google Scholar]
- 12.Scheiman JM, Devereaux PJ, Herlitz J, Katelaris PH, Lanas A, Veldhuyzen van Zanten S, Nauclér E, Svedberg LE. Prevention of peptic ulcers with esomeprazole in patients at risk of ulcer development treated with low-dose acetylsalicylic acid: a randomised, controlled trial (OBERON) Heart. 2011;97:797–802. doi: 10.1136/hrt.2010.217547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Dutch Institute for Health Care improvement CBO. 2003. Guideline NSAID use and prevention of gastric damage. [Richtlijn NSAID-gebruik en preventie van maagschade]. The Dutch Institute for Health Care improvement CBO: Utrecht. Available at http://www.mdl.nl/uploads/240/117/NSAID-gebruik_en_preventie_van_maagschade_-_CBO_2003.pdf (last accessed 21 July 2014) [PubMed]
- 14.National Institute for Health and Care Excellence. 2013. NSAIDs – prescribing issues. Available at http://cks.nice.org.uk/nsaids-prescribing-issues#!scenariorecommendation:3 (last accessed 1 January 2014)
- 15.Warlé-Van Herwaarden MF, Kramers C, Sturkenboom MC, Van den Bemt PM, De Smet PA. 2009. Targeting outpatient drug safety: recommendations of the Dutch Harm-Wrestling Task Force. [HARM-WRESTLING: een voorstel van de Expergroep Medicatieveiligheid m.b.t. concrete interventies die de extramurale medicatieveiligheid op korte termijn kunnen verbeteren]. Ministry of Health, Welfare and Sport: Den Haag,. Available at http://www.knmp.nl/downloads/medicijnen-zorgverlening/medicatieveiligheid/harmwrestlingrapportdefnov2009.pdf (last accessed 21 July 2014)
- 16.Warlé-Van Herwaarden MF, Kramers C, Sturkenboom MC, Van den Bemt PM, De Smet PA. 2010. Targeting outpatient drug safety: recommendations of the Dutch Harm-Wrestling Task Force. Royal Dutch Pharmacists Association: Den Haag,. Available at http://www.knmp.nl/downloads/medicijnen-zorgverlening/medicatieveiligheid/rapport-harm-wrestling-english.pdf (last accessed 21 July 2014)
- 17.Valkhoff VE, van Soest EM, Sturkenboom MC, Kuipers EJ. Time-trends in gastroprotection with nonsteroidal anti-inflammatory drugs (NSAIDs) Aliment Pharmacol Ther. 2010;31:1218–28. doi: 10.1111/j.1365-2036.2010.04281.x. [DOI] [PubMed] [Google Scholar]
- 18.Helsper CW, Smeets HM, Numans ME, Knol MJ, Hoes AW, de Wit NJ. Trends and determinants of adequate gastroprotection in patients chronically using NSAIDs. Pharmacoepidemiol Drug Saf. 2009;18:800–6. doi: 10.1002/pds.1783. [DOI] [PubMed] [Google Scholar]
- 19.Francke AL, Smit MC, de Veer AJ, Mistiaen P. Factors influencing the implementation of clinical guidelines for health care professionals: a systematic meta-review. BMC Med Inf Decis Making. 2008;8:38. doi: 10.1186/1472-6947-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kooij FO, Klok T, Hollmann MW, Kal JE. Decision support increases guideline adherence for prescribing postoperative nausea and vomiting prophylaxis. Anesthesia Analgesia. 2008;106:893–8. doi: 10.1213/ane.0b013e31816194fb. [DOI] [PubMed] [Google Scholar]
- 21.Lavis JN, Rottingen JA, Bosch-Capblanch X, Atun R, El-Jardali F, Gilson L, Lewin S, Oliver S, Ongolo-Zogo P, Haines A. Guidance for evidence-informed policies about health systems: linking guidance development to policy development. PLoS Med. 2012;9:e1001186. doi: 10.1371/journal.pmed.1001186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foundation for Pharmaceutical Statistics. 2004. Facts and Figures 2004. Available at http://www.sfk.nl/english/Factsandfigures2004.pdf (last accessed 21 July 2014)
- 23.Foundation for Pharmaceutical Statistics. 2010. Facts and Figures 2010. Available at http://www.sfk.nl/english/2010fandf.pdf (last accessed 21 July 2014)
- 24.Foundation for Pharmaceutical Statistics. 2012. Decrease in gastroprotection in NSAID users. [Minder maagbescherming bij gebruik NSAID], Available at http://www.sfk.nl/nieuws-publicaties/PW/2012/minder-maagbescherming-bij-gebruik-nsaid (last accessed 21 July 2014)
- 25.Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, Lines C, Riddell R, Morton D, Lanas A, Konstam MA, Baron JA. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352:1092–102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- 26.Geijer RM, Rikken SA. Decrease in the number of prescriptions for third-generation contraceptive pills. [Afname van het aantal voorschriften van 3e-generatie-anticonceptiepillen] Ned Tijdschr Geneeskd. 2000;144:2069–70. [PubMed] [Google Scholar]
- 27.Lamberts H, Wood M, Hofmans-Okkens IM. International primary care classifications: the effect of fifteen years of evolution. Fam Pract. 1992;9:330–9. doi: 10.1093/fampra/9.3.330. [DOI] [PubMed] [Google Scholar]
- 28.WHO Collaborating Centre for Drug Statistics Methodology. 2013. Guidelines for ATC Classification and DDD Assignment. Oslo. Available at http://www.whocc.no/filearchive/publications/2014_guidelines.pdf (last accessed 21 July 2014)
- 29.van der Lei J, Duisterhout JS, Westerhof HP, van der Does E, Cromme PV, Boon WM, van Bemmel JH. The introduction of computer-based patient records in The Netherlands. Ann Intern Med. 1993;119:1036–41. doi: 10.7326/0003-4819-119-10-199311150-00011. [DOI] [PubMed] [Google Scholar]
- 30.Vlug AE, van der Lei J, Mosseveld BM, van Wijk MA, van der Linden PD, Sturkenboom MC, van Bemmel JH. Postmarketing surveillance based on electronic patient records: the IPCI project. Methods Inf Med. 1999;38:339–44. [PubMed] [Google Scholar]
- 31.Valkhoff VE, van Soest EM, Masclee GM, de Bie S, Mazzaglia G, Molokhia M, Kuipers EJ, Sturkenboom MC. Prescription of nonselective NSAIDs, coxibs and gastroprotective agents in the era of rofecoxib withdrawal – a 617 400-patient study. Aliment Pharmacol Ther. 2012;36:790–9. doi: 10.1111/apt.12028. [DOI] [PubMed] [Google Scholar]
- 32.de Jong HJ, Korevaar JC, van Dijk L, Voogd E, van Dijk CE, van Oijen MG. Suboptimal prescribing of proton-pump inhibitors in low-dose aspirin users: a cohort study in primary care. BMJ Open. 2013;3:7. doi: 10.1136/bmjopen-2013-003044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warlé-Van Herwaarden MF, Kramers C, Sturkenboom MC, Van den Bemt PM, De Smet PA. Targeting outpatient drug safety: recommendations of the Dutch Harm-Wrestling Task Force. Drug Saf. 2012;35:245–59. doi: 10.2165/11596000-000000000-00000. [DOI] [PubMed] [Google Scholar]


