Abstract
COPD is a long-term condition associated with considerable disability with a clinical course characterized by episodes of worsening respiratory signs and symptoms associated with exacerbations. Gastroesophageal reflux disease (GERD) is one of the most common gastrointestinal conditions in the general population and has emerged as a comorbidity of COPD. GERD may be diagnosed by both symptomatic approaches (including both typical and atypical symptoms) and objective measurements. Based on a mix of diagnostic approaches, the prevalence of GERD in COPD ranges from 17% to 78%. Although GERD is usually confined to the lower esophagus in some individuals, it may be associated with pulmonary microaspiration of gastric contents. Possible mechanisms that may contribute to GERD in COPD originate from gastroesophageal dysfunction, including altered pressure in the lower esophageal sphincter (which normally protect against GERD) and changes in esophageal motility. Proposed respiratory contributions to the development of GERD include respiratory medications that may alter esophageal sphincter tone and changes in respiratory mechanics, with increased lung hyperinflation compromising the antireflux barrier. Although the specific cause and effect relationship between GERD and COPD has not been fully elucidated, GERD may influence lung disease severity and has been identified as a significant predictor of acute exacerbations of COPD. Further clinical effects could include a poorer health-related quality of life and an increased cost in health care, although these factors require further clarification. There are both medical and surgical options available for the treatment of GERD in COPD and while extensive studies in this population have not been undertaken, this comorbidity may be amenable to treatment.
Keywords: COPD, GERD, pulmonary aspiration, treatment
Introduction
COPD is a chronic, progressive condition, characterized by an increased inflammatory response within the airways and airflow limitation that is not fully reversible.1 The clinical profile is frequently punctuated by acute exacerbations,2 which increase the risk of morbidity and mortality of COPD3 and are linked to worsening quality of life and accelerated decline in lung function.4 The prevalence of COPD is continually rising,5 particularly in those aged 65 years and older. Accompanying the clinical profile of COPD is a range of comorbidities, which have the potential to complicate the clinical presentation of this condition and may influence morbidity and mortality.
Gastroesophageal reflux disease (GERD) develops when the reflux of gastric contents results in troublesome symptoms or complications.6 It is a common upper gastrointestinal condition, affecting up to 33% of the general population7 and may be associated with either esophageal or extra-esophageal syndromes.6 Refluxate may be acidic or nonacidic (alkaline), liquid, or gaseous.8 The frequency and duration of episodes of reflux as well as the destination of the gastroesophageal refluxate affect the impact of GERD.
As both GERD and COPD are highly prevalent conditions, the possibility of an interaction has long been recognized.9–12 With the potential for GERD to aggravate the clinical status of COPD and of the mechanical changes associated with COPD to exacerbate GERD, it is important to understand the relationship and possible consequences of the two conditions co-occurring. This review will explore the underlying pathophysiology of GERD, the commonly applied diagnostic tools, its prevalence and clinical presentation as well as risk factors, and current management strategies.
Gastroesophageal reflux disease
Pathophysiology
Gastroesophageal reflux (GER) is a normal physiological occurrence, and the integrity of the gastroesophageal junction influences the occurrence and frequency of GER events. Physiologically, there are four causes of GER of gastrointestinal origin. The most common trigger is transient, spontaneous relaxation of the lower esophageal sphincter (LES),13 which may occur in both an upright or recumbent position14 and promotes reflux. GER may also occur due to diminished basal LES pressure,15 as a result of straining or free reflux. Strain-induced reflux occurs when a hypotensive LES is overcome by an abrupt increase in intra-abdominal pressure (eg, during bending).16 Free reflux occurs when the basal LES pressure is within 1–4 mmHg of the intragastric pressure; this small pressure gradient heightens the likelihood of GER.15 A hiatus hernia is displacement of the gastroesophageal junction above the diaphragm.17 The pressure gradient between the thorax and the abdomen promotes the movement of gastric contents into the esophagus.18 Transient LES relaxations are more likely to be followed by GER episodes in the presence of a hiatus hernia. Normally, esophageal peristalsis facilitates esophageal clearance following reflux episodes.19 Peristaltic dysfunction, with absent or low-amplitude contractions in the distal esophagus, which can be detected through manometry studies, contributes to prolonged esophageal clearance, which increases the chance of reflux.20 The diagnosis of GERD should be considered when symptoms associated with these physiological changes are reported by the patient.6
Changes in LES tone are often triggered by lifestyle factors such as stress or by the consumption of specific foods, including products high in fat (delayed gastric emptying) or those that lower the LES pressure (chocolate, peppermint, onion, garlic, caffeine, and alcohol).21 Eating or drinking acidic foods (tomato products, citrus, and carbonated beverages) may trigger symptoms.21 Other lifestyle factors include sleeping in a supine position or consumption of a meal immediately before sleeping; both may be linked to nocturnal awakening from symptoms.21
Clinical presentation
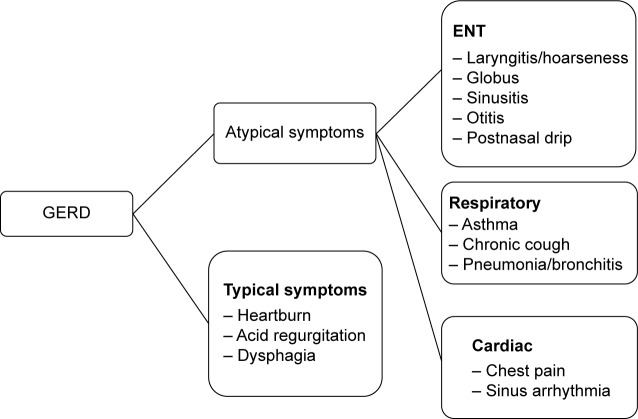
Typical symptoms of GERD include heartburn, acid regurgitation,22 chest pain,23 epigastric pain, or sleep disturbances.6 These clinical features together with esophageal complications, including reflux esophagitis, Barrett’s esophagus, and adenocarcinoma are collectively referred to as esophageal syndromes.24 Symptoms such as chronic cough or laryngitis that occur secondary to reflux are classed as extra-esophageal syndromes. An outline of typical and atypical clinical presentations of GERD is presented in Figure 1. Either may be present in COPD.
Figure 1.
Typical and atypical clinical presentations of GERD.
Abbreviations: GERD, gastroesophageal reflux disease; ENT, ears-nose-throat.
Diagnostic tools
Diagnosis of GERD
The most common approach to the diagnosis of GERD is through an accurate medical history, enquiring about typical GERD symptoms and their relationship to food, posture, and stress.21 It is important to be aware that symptoms of GERD may be similar to some symptoms of COPD. Therefore, it is necessary to enquire as to the timing of the GERD symptoms and their association with awakening from sleep, the use of respiratory inhalers in association with GERD symptoms, or the presence of respiratory symptoms after meals. Further evaluation may include symptomatic assessment through validated questionnaires, which ideally incorporate both typical and atypical symptoms.25,26 In the presence of symptoms, an empirical trial of acid suppression therapy is often undertaken, with resolution of symptoms considered clinically indicative of GERD, provided the patient has been symptomatic.27 If symptoms are present, objective tools such as esophageal endoscopy may be used to identify secondary complications of mucosal injury and esophagitis.28,29
If asymptomatic reflux is suspected, alternative options for diagnosing GERD include ambulatory 24-hour esophageal pH monitoring. This is the current “gold standard” for diagnosing GERD.30–33 Dual-channel monitoring measures proximal and distal esophageal pH, giving a comprehensive profile of GERD34 using well-defined criteria.31,35 Via a small catheter positioned in the esophagus, this technique measures the esophageal luminal pH. The frequency and duration of reflux episodes and the proximal spread of the refluxate over a complete circadian cycle are quantified.35 For distal GERD, sensitivity ranges from 81% to 96% with specificity from 85% to 100%.30–33 For proximal GERD, the sensitivity ranges from 55% to 86%, although the specificity is slightly higher (80%–91%).36,37 A variation on this is telemetry capsule pH monitoring, which offers increased patient tolerability and the option to extend the monitoring period to 48 hours or 96 hours.38 With the identification of both acid and nonacid reflux, together with the mixture of gas and liquid reflux, combined multichannel intraluminal impedance and pH monitoring records GERD at all pH levels.39 It quantifies volume and gas reflux and the air–liquid composition of the refluxate, giving an exact assessment of the proximal extent of refluxed material and a detailed characterization of each reflux episode.8,39,40
Diagnosis of pulmonary microaspiration of gastric contents
Pulmonary microaspiration of gastric contents can be detected through various methods. Proximal esophageal pH monitoring has been considered a surrogate marker.10,41,42 One of the more novel measures of pulmonary microaspiration is the measurement of pepsin in airway samples. Pepsin is secreted by cells unique to the gastric mucosa as pepsinogen I or II43,44 and requires acidic conditions to be converted to active pepsin. The detection of pepsin in pulmonary secretions is suggested to indicate pulmonary microaspiration of gastric contents.45 Pepsin has been detected in bronchoalveolar lavage of lung transplant recipients who demonstrated GERD on esophageal pH monitoring or impedance monitoring45–48 and more recently in sputum44,49 and exhaled breath condensate (EBC)50 in individuals with COPD. EBC is a sample of breath water vapor containing pulmonary epithelial lining fluid. Acidification of the hypopharynx can occur when gastric contents reach beyond the upper esophageal sphincter (UES), which can be reflected by the presence of pepsin or lower pH levels in EBC.51
Prevalence of GERD in COPD
The prevalence of GERD in individuals with COPD has been explored in a number of studies.11,41,42,49,52–57 A range of diagnostic tools have been used, including symptom questionnaires and objective measurements, outlined in Table 1. Based on self-reported symptoms and questionnaires, the prevalence of GERD ranges from 17% to 54%.12,52–55,57–61 Variation is partially attributable to the heterogeneity of questionnaire content. However, while typical GERD symptoms exhibit a sensitivity of 90%, the specificity is as low as 47%,10 which may limit their diagnostic value.
Table 1.
Diagnostic approaches and prevalence of GERD in COPD
| Study | N, sex, age mean (SD) | Disease severity (FEV1% pd) | Questionnaire/objective measure | Prevalence of GERD and symptom descriptions |
|---|---|---|---|---|
| Symptom-based diagnosis | ||||
| Mokhlesi et al12 | COPD: n= 100, Sex: NR Age: 70 (8) years Control: n=55, Sex: NR Age: 66 (13) years |
NR | Modified Mayo Clinic GER questionnaire: common symptoms (frequency and severity), effect on respiratory symptoms, and medications. | Prevalence in COPD: 26%. Prevalence in controls: 25%. Increase in respiratory symptoms (cough, SOB, wheezing associated with heartburn in 26% of those with GERD). |
| Phulpoto et al61 | COPD: n= 100, Sex: NR Age: 57 (8) years Control: n= 150 Sex: NR Age: 50 (5) years |
NR | Modified Mayo clinic questionnaire: frequency and characterization of reflux or heartburn symptoms over past year. | Prevalence in COPD: 25%. Prevalence in controls: 9%. Heartburn, acid regurgitation more frequent in COPD to controls. More frequent symptoms in those with COPD with FEV1 <50% pd compared to those with FEV1 >50% pd. |
| Rascon-Aguilar et al57 | COPD: n=91, Sex: 55% male Age: 67 (8) years |
NR | Mayo clinic GER questionnaire: frequency and characterization of reflux or heartburn symptoms over past year. Weekly symptoms classed as positive GERD. | Prevalence in COPD: 32%. |
| Terada et al52 | COPD: n=82, Sex: 94% male Age: 73 (8) years |
COPD: 57 (20)% pd | Self-reported FSSG: typical and dysmotility symptoms. | FSSG: Prevalence in COPD: 27%, Prevalence in control: 13%. |
| Control: n=40, Sex: 48% male Age: 71 (9) years |
Control: 101 (16)% pd | QUEST | QUEST: Prevalence in COPD: 24%, Prevalence in control: 10%. | |
| Rogha et al55 | COPD: n= 110 Sex: 87% male Age: 68 (8) years |
NR | Mayo clinic questionnaire: frequency and characterization of reflux or heartburn symptoms over past year. Weekly symptoms classed as positive GERD. | Prevalence in COPD: 54%. 66% with weekly and 34% with daily symptoms. |
| Bor et al58 | COPD: n= 133 Sex: 26% female Age: NR |
NR | Reflux questionnaire: effect of GERD symptoms on respiratory disease; medications, typical reflux symptoms. | Prevalence in COPD: 16.5%. Prevalence in controls: 19.4%. In COPD: occasional (symptoms less than once weekly in last year) heartburn in 11%, occasional regurgitation in 1 1.3%. |
| Takada et al60 | COPD: n=221 Sex: 95% male Age: 72 (8) years |
NR | FSSG: typical and dysmotility symptoms. | Prevalence in COPD: 27%. |
| Shimizu et al59 | COPD: n=40 Sex: 95% male Age: 70 (10) years Control: n=40 Sex: 55% male Age: 65 (10) years |
COPD: 1.4 (0.9) L Control: NR |
FSSG: typical and dysmotility symptoms. | Prevalence in COPD: 33%. Prevalence in controls: 28%. Higher proportion of dysmotility symptoms in COPD compared to controls. |
| Ambulatory esophageal pH monitoring | ||||
| Casanova et al11 | COPD: n=42 Sex: 100% male Age: 68 (47–78)* years Control: n= 15 Sex: 88% male Age: 67 (47–78)* years |
COPD: 35 (20–49)*% pd Control: 106 (81–130)*% pd |
Single-channel (distal), 24-hour pH monitoring. Antireflux medication ceased 7 days prior to study. | Prevalence in COPD: 62%. Prevalence in controls: 19%. Combined reflux pattern (supine and upright) in 73% of those with COPD. Symptomatic (heartburn and acid regurgitation) GERD in 23% of those with COPD. |
| Andersen and Jensen63 | COPD: n=264 Sex: NR Age: 58 (45)* years Control: n=809 Sex: NR Age: 61 (51)* years |
COPD: NR Control: NR |
12-hour esophageal pH monitoring. Symptom questionnaire. Status of antireflux medication at the time of study NR. |
Prevalence in COPD: 8%. Esophageal symptoms in 14% of COPD compared to 4% control group. |
| D’Ovidio et al41 | COPD: n=21 Sex: NR Age: 55 (38)* years |
20 (23)% pd | Dual-channel, 24-hour pH monitoring. Antireflux medication ceased 2–5 days prior to study. |
Prevalence in COPD: 19%, with asymptomatic GERD in 52%. Proximal reflux present in 29%. |
| Sweet et al42 | COPD: n=21 Sex: NR Age: 55 (46)* years |
NR | Dual-channel, 24-hour pH monitoring. Antireflux medication ceased 3–14 days prior to study. |
Prevalence in COPD: 43%. Proximal reflux was 25%. |
| Kempainen et al56 | COPD: n=42 Sex: 43% male Age: 56 (30–65)* years |
24 (9)% pd | Dual-channel, 24-hour pH monitoring. Antireflux medication cased 7 days prior to study. |
Prevalence in COPD: 52%. Proximal and distal reflux present in 46% and 41 % of patients. 15% had elevated proximal reflux only Asymptomatic reflux in 74%. |
| Gadel et al64 | COPD: n=40 Sex: 100% male Age: NR Control: n=10 Sex: NR Age: NR |
NR | Single-channel, 24-hour pH monitoring. Status of antireflux medication NR. |
Prevalence in COPD: 35%. Symptoms of GERD in 55%. |
| Kamble et al62 | COPD: n=50 Sex: 82% male Age: 56 (5) years |
59 (5)% pd | Dual-channel, 24-hour pH monitoring. Questionnaire-based symptom score. Status of antireflux medication NR. |
Prevalence in COPD: 78% based on pH monitoring, with asymptomatic GERD in 21%. Those with symptomatic GERD: distal and proximal GERD in 53%, distal only in 29%. Those with asymptomatic GERD: 50% had distal reflux only and 17% had distal and proximal reflux. |
| Lee et al49 | COPD: n=27 Sex: 78% male Age: 67 (8) years Control: n= 17, Sex: 41% male Age: 37 (15) years |
COPD: 47 (17)% pd Control: 96 (9)% pd |
Dual-channel, 24-hour pH monitoring. Structured symptom questionnaire. Antireflux medication ceased 7 days prior to study. |
Prevalence in COPD: 37%, with asymptomatic reflux in 20%. In COPD, distal reflux only in 80%, proximal and distal reflux in 20%. Prevalence in controls: 18%. |
Note:
Median (interquartile range).
Abbreviations: GERD, gastroesophageal reflux disease; SD, standard deviation; FEV1 pd, predicted; forced expiratory volume in 1 second; NR, not reported; GER, gastroesophageal reflux; SOB, shortness of breath; FSSG, frequency scale for the symptoms of GERD; QUEST, Questionnaire for the Diagnosis of Reflux Disease.
By comparison, according to esophageal pH monitoring, the prevalence ranges from 19% to as high as 78%.11,41,42,49,56,62,64 Such a wide spread is related to several factors, including the differing GERD criteria applied31,34,65,66 and whether the test was undertaken on or off antireflux medication. Mixed patterns of reflux are evident, with distal reflux only, proximal reflux only, and a mix of both demonstrated.11,49,56,62 In those with COPD, the prevalence is five times greater than the non-COPD population for proximal and distal reflux.7,67 GERD can affect patients with moderate to very severe COPD.41,42,49,56,62,68 Although a detailed clinical history of symptom presentation is recommended,69 this method of diagnosis is reliant upon the provocation of symptoms by reflux events, which in the event of asymptomatic (clinically silent) reflux is not a reliable indicator. The presence of asymptomatic reflux (20%–74%) in COPD11,41,49,56,62 emphasizes the importance of objective confirmation of GERD in some individuals.
The cause and effect relationship between COPD and GERD has been reported through case–control and cohort studies. El-Serag and Sonnenberg70 in a case–control study found that a higher risk of esophageal disease was evident in those with COPD compared to controls (odds ratio [OR] 1.43, 95% confidence interval [CI] 1.33–1.53).70 A recent longitudinal cohort study followed two groups of patients, those diagnosed with GERD with no previous history of COPD and those diagnosed with COPD with no history of GERD over 5 years.71 In those with GERD, the incidence of COPD was with a risk ratio of 1.17 (95% CI 0.91–1.49). In those with COPD, the incidence of GERD was 14.9 cases per 1,000 (95% CI 13.9–16.0), with a relative risk of 1.49 (95% CI 1.19–1.78). While this suggests that a diagnosis of COPD may predispose patients to developing symptoms of GERD, the reasons require further clarification.
One possible explanation is the effect of smoking, and in particular, nicotine on esophageal sphincter tone and esophageal clearance. Smoking has been associated with a reduction in LES tone, believed to be secondary to nicotine-induced relaxation of the circular muscle of the LES72 and reflected by the increased acid exposure in the upright position and an increased frequency of reflux events >5 minutes in duration.73 Prolonged acid clearance secondary to diminished salivation, which may persists for >6 hours after smoking, has also been reported to result in reduced neutralization of esophageal reflux by swallowed saliva.72 With nicotine levels persisting for at least 6 hours after smoking, the implication is that the drug effect may last for a similar duration.72 A higher severity of GERD has been demonstrated in those with COPD who have a high smoking index,64 and pack-years has been found to be an independent risk factor for GERD (OR 1.015 [95% CI 1.004–1.025]).74 Smoking is a risk factor for GERD in the general population,75 and this together with smoking being a leading cause of COPD76 suggests that smoking and the associated effects of nicotine may contribute to GERD in COPD.
Presence of pulmonary microaspiration in GERD
Surrogate indicators of pulmonary microaspiration of gastric contents have been examined in COPD. Pepsin in sputum samples was detected in 33% of individuals with moderate-to-severe COPD.49 Although no significant association between esophageal pH monitoring indices and pepsin concentrations in sputum was evident, this has been previously observed in individuals with other types of lung disease.44,46,77,78 Briefly, isolated reflux events that could be aspirated may be insufficiently frequent to contribute to the criteria defining GERD. Laryngopharyngeal reflux, based on laryngeal examination and symptom questionnaires, has also been detected in 44% of individuals with COPD.79 A pilot study of EBC in ten individuals with COPD found pepsin in 70%, irrespective of a diagnosis of GERD based on esophageal pH monitoring.80 Lower EBC pH has been related to severe GERD symptoms in COPD,52 although there was no significant correlation between EBC pH and sputum inflammatory indices (tumor necrosis factor-α and interleukin-8). This suggests that EBC pH may reflect acid reflux rather than tracheobronchial inflammation. Greater clarity is required to determine the optimal frequency and timing of EBC collection necessary for it to be included as an index of acid reflux.
Possible contributing factors to GERD in COPD
Gastroesophageal mechanisms
A number of possible mechanisms originating from a gastrointestinal or respiratory perspective may increase the vulnerability to GERD in those with COPD. Although esophageal motility studies have not been extensively applied, reduced daytime and nocturnal esophageal peristalsis40,56,81 and a decrease in UES82,83 and LES pressure has been demonstrated in those with severe COPD.11,40,64,82 Change in LES pressure may be partially attributed to smoking and the effects of nicotine.72
Other possible explanations for pulmonary aspiration secondary to GERD are related to swallowing dysfunction in COPD. Precise coordination between swallowing and respiration is necessary, with the swallowing reflex an important defense against airway infection and aspiration.83 Compared to healthy controls, the swallowing reflex can be impaired in COPD,84 with a lack of coordination of the pharyngeal musculature and disruption of the breathing–swallowing coordination.85–87 Patients are more likely to swallow during inhalation or inhale directly after swallowing, as respiratory requirements take precedence over swallowing.85 Low subglottic air pressure occurs during early inhalation, late exhalation, or at the transition point between exhalation and inhalation. If swallowing takes place during times of subglottic air pressure, the physiology of swallowing can also be altered. If the preferred pattern of exhale–swallow–exhale is altered, the risk of aspiration increases.86 This may be a contributing factor to exacerbations of COPD, illustrated by a greater frequency of annual exacerbations (OR 4.86 [95% CI 1.45–18.43]) in individuals with an abnormal swallowing reflex.83 In turn, exacerbations of COPD, with altered respiratory demands, may increase the risk of further aspiration.86
Respiratory mechanisms
Both alterations in respiratory mechanics and side effects of respiratory medications could contribute to GERD. Severe hyperinflation requires increased respiratory muscle inspiratory effort to overcome the increased inspiratory load at high lung volume. The resulting increase in negative pressure amplifies the pressure gradient between the thorax and abdomen, which impacts on LES tone and predisposes to reflux.88,89 This may be especially present during COPD exacerbations when reductions in airflow together with increased coughing impact on this pressure gradient. Airflow obstruction significantly increases the frequency of transient LES relaxation, a mechanism documented in asthma.90 In stable COPD, although differences in lung mechanics between those with and without GERD were not apparent,11 a negative correlation between LES and UES pressure and indices of hyperinflation has been described.64 To date, the association between airway obstruction and LES relaxation requires further clarity.90 The reduction in LES tone secondary to smoking together with coughing, a common symptom of COPD, may predispose some individuals with COPD to strain-induced acid reflux.72 Heightened anxiety is known to aggravate GERD symptoms by increasing acid production.91 As increased anxiety is common in COPD,1 this may be an additional contributory factor to GERD.
Respiratory medications
Respiratory medications, including beta agonists, anticholinergics, corticosteroids, and theophylline preparations have been proposed as possible factors that may be related to GERD.53,92–98 While these medications may alter esophageal function by reducing LES pressure or esophageal motility,92–94 their specific contribution to the risk of GERD is variable. Some studies observed that a greater proportion of individuals with COPD (stable or those at risk of an exacerbation) and GERD were prescribed inhaled corticosteroids, short-and long-acting beta2 agonists, and combination therapy (inhaled corticosteroids/long-acting beta2 agonists);53,59 others found no difference in the prescription of these respiratory medication classes and the presence/absence of GERD.12,54,55,57,58,60,74 Although it has been hypothesized that these classes of medications may contribute to GERD, the nature of this relationship in COPD has not been fully determined. An increased use of anticholinergics in those with COPD and GERD has been reported by Garcia Rodriguez et al71 while another study found no difference.94 Although central and peripherally acting anticholinergics can reduce LES pressure, their antitussive effect can encourage cough suppression and may minimize the occurrence of changes in intra-abdominal pressure, which may predispose GERD.94 It has been suggested that those with GERD may require more intense bronchodilator therapy secondary to increased severity of respiratory symptoms and exacerbations.53 The increased use of bronchodilator therapy when reflux symptoms are experienced lends support to a possible association between reflux events and worsening symptoms.12 The association between GERD and respiratory medications may also be a reflection of the severity of lung disease rather than the specific physiological effects of these medications on esophageal function. Further exploration of the cause and effect relationship between respiratory medications and GERD in COPD is warranted.
Non-COPD specific factors
A mix of demographic factors may increase the risk of GERD in COPD. Older age (>60 years) is often a factor,53,58,64,95,99 with an increased risk (OR 3.7 [95% CI 2.4–5.9]) reported in those over 70 years.71 Given the high proportion of COPD patients aged over 65 years, this finding is not surprising. The contribution of sex is variable, with some studies finding females at greater risk,53 others demonstrating that GERD is more common in males71 and some finding no difference.54,58 This is consistent with studies of GERD among the general population100,101 and leaves open the influence of sex as an independent risk factor for GERD.
A larger body mass index (BMI; >25 kg/m2 – classed as overweight) has been identified as a risk for GERD in COPD,53–56,58,64 a risk which increases as BMI increases.53 For those with severe COPD, a higher BMI was a predictor for GERD (OR 1.2 [95% CI 1.0–1.6]).56 While the BMI of participants in these studies did not meet the criteria for obesity (>30 kg/m2), a greater BMI impacts on the contour of the diaphragm and will influence the elastic work of breathing.21 When combined with respiratory-related risk factors, this may increase the contribution of a higher BMI to GERD in COPD. The prediction of a higher BMI being a contributing factor is not unexpected, given that it is identified as a common contributing factor in the general population.102
Other comorbidities, including cardiac disease and obstructive sleep apnea, have also been associated with a heightened risk of GERD.53 In those with obstructive sleep apnea, increased intrathoracic pressure during apneic episodes is accompanied by increased transdiaphragmatic pressure, which encourages migration of gastric contents up the esophagus.103 The repetitive pressure changes also contribute to LES insufficiency.103 Whether they are independent variables or common consequences of poor diet and obesity remains to be established.104
Influence of GERD on COPD severity
Two of the possible mechanisms by which GERD may impact on the severity of COPD are vagally mediated reflex bronchoconstriction and pulmonary microaspiration.105 Vagally mediated reflex bronchoconstriction originates from the shared autonomic innervation between the tracheobronchial tree and the esophagus. The presence of esophageal acid in the distal esophagus stimulates airway irritation and an inflammatory response, with the release of potent mediators of bronchoconstriction.106 The second mechanism by which GERD may impact on respiratory disease is pulmonary microaspiration. During microaspiration, refluxed gastric material extends proximally to the esophagus and then enters the hypopharynx, directly triggering a laryngeal or tracheal response, which may manifest as coughing, wheezing, or a sensation of dyspnea.105
The relationship between the severity of COPD based on measures of lung function and GERD is controversial, with studies demonstrating mixed results. Some studies observed no significant relationship between GERD and pulmonary function, based on dynamic and static lung volume measurements or pulmonary resistance,9,11,12,49,56,57,96,107 whereas other studies found poorer lung function in those with GERD symptoms who had more severe lung disease.12,55 The correlation between oxygen desaturation and nocturnal episodes of distal reflux suggests that GERD may influence nocturnal respiratory status in some patients.11 A single dimension of disease severity may be insufficient to accurately reflect the relationship between GERD and COPD, which may require serial measures of lung function over time.
Interaction between GERD and acute exacerbations of COPD
A large proportion of health care expenditure is related to hospital costs for those admitted with an acute exacerbation of COPD (AECOPD),3,108 and prompt intervention is critical in preventing hospital admissions.4 A systematic review and meta-analyses of seven observational studies over varying durations of follow-up (12–18 months) found the presence of GERD to be associated with a greater risk of experiencing an AECOPD (risk ratio 7.57 [95% CI 3.84–14.94]).109 More recent studies outlined in Table 2 have consistently demonstrated this positive relationship and have noted a higher rate of hospitalization or emergency room visits52,54,55,57,59,60,79,83,110–113 among the GERD population. This is consistent with a defined phenotype for patients with COPD who experience frequent AECOPD (two per year), with GERD as an independent predictor.113 Studies with a 5-year follow-up found that those who experience both nocturnal and daytime symptoms experienced more exacerbations, with a higher risk in those who did not use regular acid inhibitory treatment (HR 2.7 [95% CI 1.3–5.4]).113
Table 2.
Studies exploring the effect of GERD on AECOPD
| Study | N, age | Disease severity (FEV1% pd) | Definition of GERD | Definition of AECOPD | Key findings |
|---|---|---|---|---|---|
| Rascon-Aguilar et al57 | COPD: n=91 Age: 67 years |
NR | Mayo Clinic GERD | Worsening dyspnea, increasing sputum volume or purulent sputum in conjunction with corticosteroids or antibiotics, hospitalization or emergency department visits in previous 12 months. | Greater number of AECOPD in those with GERD symptoms (3.2 vs 1.6, P=0.02). Average of 1.56 more AECOPD (95% CI 0.44–2.69) in those with weekly GERD symptoms. Those with GERD had increased rate of hospitalizations. |
| Terada et al52 | COPD: n=82 Age: 73 years Control: n=40 Age: 71 years |
COPD: 60% pd Control: 101% pd |
Self-reported FSSG questionnaire | Modified Anthonisen’s criteria (two or more of three major symptoms (increase in dyspnea, sputum purulence, or quantity) or any major symptoms with one minor symptom (wheeze, sore throat, cough, fever, and nasal discharge) for at least 2 consecutive days. | Higher incidence of exacerbations in those with GERD symptoms (RR 1.93 [95% CI 1.32–2.84]). FSSG score correlated with number of exacerbations (r=0.24). |
| Eryuksel et al79 | COPD: n=29 Age: NR |
NR | Laryngeal reflux: LPR criteria (reflux symptoms index, reflux finding score and laryngoscope) | Worsening of dyspnea, sputum volume, or color change in last year. | Increased risk of AECOPD in those with LPR (3.67 [95% CI 0.17–79.61]). No difference in number of AECOPD. |
| Hurst et al112 | n=2, 138 Age: 63 years |
NR | Self-reported symptoms and history of heartburn | Physician prescription of systemic corticosteroids or antibiotics, alone or in combination. | Risk of AECOPD in those with GERD (OR 1.69 [95% CI 1.38–2.06]). |
| Rogha et al55 | COPD: n=110 Age: 68 years |
NR | Mayo GERQ | Increase in cough frequency, severity, increase in dyspnea or change in volume, and/or sputum purulence. | Increased number of exacerbations in those with GERD (2.1 vs 1.4, P<0.001) and increased rate of hospitalizations (1.7 vs 1.1, P<0.005). Correlations between frequency of GERD symptoms and exacerbation frequency (r=0.32), frequency of hospitalization (r=0.26). |
| Terada et al83 | COPD: n=67 Age: 74 (72–75)* years Control: n=19 Age: 70 (67–74)* years |
COPD: 56 (52–61)*% pd Control: 105 (96–114)*% pd | FSSG questionnaire | Modified Anthonisen’s criteria. | Increased risk of AECOPD in those with GERD (RR 6.24 [95% CI 0.90–3.34]). Abnormal swallowing reflex associated with >3 AECOPD/year. |
| Takada et al60 | COPD: n=221 Age: 72 years |
68 (27)% pd | FSSG questionnaire | Modified Anthonisen’s criteria and prescription of additional systemic corticosteroids or antibiotics. | GERD increased frequency of AECOPD (RR 3.42 [95% CI 2.06–5.69]). GERD increased frequency of hospitalization due to AECOPD with RR 3.66 (95% 1.62–8.24). Significant correlation between FSSG acid reflux score and number of AECOPD (r=0.46) and number of hospitalizations due to AECOPD (r=0.45). |
| Liang and Feng54 | COPD: n=428 Age: 57–74# years |
47–71#% pd | RDQ | CAT score: increase in 5 points considered to have an exacerbation. | Those with exacerbations had higher RDG scores (high risk with score > 12). High GERD risk increased risk of AECOPD (OR 3.02 [95% CI 1.76–4.31]). |
| Ozyilmaz et al110 | COPD: n=107 Age: 66 years |
51 (18)% pd | Modified simple questionnaire of symptoms (heartburn, regurgitation, dyspepsia, dysphagia, nausea, vomiting, abdominal pain, dry cough, chest pain). Those with at least two symptoms weekly with antireflux therapy classed as GERD | AECOPD requiring oral steroids and/or antibiotics resulting in emergency room admission or hospitalization defined as severe. | Higher prevalence of GERD symptoms in frequent exacerbators (58% vs 22%, P<0.00l). GERD symptoms were increased risk of AECOPD (OR 3.59 [95% CI 1.18–10.90]). |
| Ingebrigtsen et al113 | COPD: n=1,259 Age: NR |
NR | Question relating to the experience of heartburn (daytime or nocturnal) | Prescription of oral corticosteroids with/without antibiotics dispensed <4 weeks apart. | Coexisting nocturnal and daytime GERD increased risk of AECOPD in those not using acid inhibitory treatment (HR 2.1 [95% CI 1.1–4.1]). Attributable risk of AECOPD was 31% in those with daytime and nocturnal GERD due to lack of regular acid inhibitory treatment. |
Notes:
Mean (range).
Range.
Abbreviations: GERD, gastroesophageal reflux disease; AECOPD, acute exacerbation of COPD; FEV1, forced expiratory volume in 1 second; NR, not reported; CI, confidence interval; FSSG, frequency scale for the symptoms of GERD; RR, risk ratio; LPR, laryngopharyngeal reflux; OR, odds ratio; GERQ, gastroesophageal reflux questionnaire; RDQ, reflux diagnostic questionnaire; CAT, COPD Assessment Test; RDG, Reflux Disease Questionnaire; HR, hazard ratio.
Establishing the precise nature of the relationship between AECOPD and GERD is challenging. Individuals with COPD often demonstrate lower airway bacterial colonization, which may increase their susceptibility to inflammation and infection.114 GERD may increase this bacterial load in the lower airways and thereby increase the risk of exacerbations.83 With increased pneumonia and wheezing in those with GERD symptoms,53 it might be that recurrent aspiration contributes to pneumonia. If GERD is an independent predictor of AECOPD (independent of respiratory infection, degree of airway obstruction, heart failure, cardiac medications, poor adherence to medical therapy, and older age),54,109,112 then it may represent a modifiable risk factor.
Impact on quality of life
Comorbidities in COPD may exert influence on health-related quality of life (HRQOL). When GERD was defined by esophageal pH monitoring, it had only a minimal impact on disease-specific HRQOL among those with moderate to severe COPD,11,115 an observation confirmed using GERD-specific questionnaires.115 However, some studies with a greater sample size have reported a poorer HRQOL reflected in disease-specific and generic questionnaires53,57 as well as greater levels of anxiety and depression.96 In those aged over 65 years, GERD was associated with a poorer perception of physical health and higher rates of depression and anxiety.116
Cost consequences of GERD in COPD
A substantial proportion of the economic burden associated with COPD is from hospitalization secondary to an acute exacerbation.108,117 According to a retrospective cost study of 2,461 individuals aged >65 years,116 in the 29% who had coexisting COPD and GERD, the annual Medicare cost was $US36,793 per patient per year compared to US$24,722 for those without GERD.116 This 36% increase in costs was attributed to hospitalization for AECOPD. Although specific direct and indirect costs are not yet available, the economic burden appears to be heightened for those with COPD in whom GERD is a comorbidity.
Treatment of GERD
Lifestyle modification and medical and surgical management have all been used to treat GERD. Suggestions for minimizing the risk of GERD include weight loss, avoidance of late-night meals, and specific food and drink that might aggravate reflux by relaxing the LES.21 Altered posture, including adapting a semirecumbent posture when sleeping and avoiding sleeping on the left side21 have also been suggested.38 Stress reduction has also been associated with symptom improvement.21 These broad recommendations also apply to individuals without COPD38 and are generally recommended as first-line management.
Pharmacologic management includes antacids, histamine2-receptor antagonists (H2-RA), and proton pump inhibitor (PPI) therapy,21 as determined by the severity of GERD. There have been few studies of antireflux therapy specifically for those with COPD (Table 3). In a 12-month trial of 100 older patients with GERD, PPI therapy reduced the frequency of AECOPD and common colds compared to usual care.118 Improvement in symptoms of laryngopharyngeal reflux, GERD, and respiratory symptoms in individuals with COPD has been found with a combined approach of H2-RA and PPI therapy in some studies.12,79 Although several studies reported on the prescription of antireflux medication in COPD, they did not report on the impact of therapy on lung function.12,56,57 Therefore, the effects of pharmacological management of GERD on lung function, the co-occurrence of respiratory and GERD symptoms, and the use of respiratory medications remain to be clarified. The persistence of symptoms despite antireflux therapy suggests that acid reflux may not always be the primary cause;119 this pharmacological approach does not target nonacid or weakly acidic reflux. Surgical management, with a Nissen Fundoplication, has been successfully applied to patients with severe lung disease, including COPD, awaiting transplantation,120–122 with reductions in symptoms of GERD as well as of lung disease123 and improved lung function in the small group of individuals with COPD.120–122 Antireflux surgery is not widely used in COPD but should be considered when medical management fails, especially when GERD remains severe in individuals with COPD at risk of respiratory deterioration.
Table 3.
Effects of medical and surgical treatment on GERD in COPD
| Study | N | Treatment approach | Effects of treatment |
|---|---|---|---|
| Medical therapy | |||
| Mokhlesi et al12 | 100 | Antireflux therapy (duration not specified) Antacids (43% of participants) PPI (28% of participants) H2-RA (6% of participants) |
Significant respiratory and GER symptoms in 9% of patients, despite H2-RA and PPI therapy. Resolution of GER symptoms and chronic cough in 2% of patients, without change in PFTs. |
| Kempainen et al56 | 42 | Antireflux therapy (duration not specified) PPI (29% of participants) H2-RA (2% of participants) |
NR. |
| Rascon-Aguilar57 | 91 | Antireflux therapy (duration not specified) Antacids (51% of participants) H2-RA (22% of participants) PPI (38% of participants) 34% receiving a combination of at least two types of medication |
NR. |
| Sasaki et al118 | 100 | Antireflux therapy (12 months), Comparison of treatment (PPI therapy) vs usual care (bronchodilator therapy, smoking cessation) PPI therapy | Fewer exacerbations with PPI over 12 months compared to control (0.34 vs 1.18, P=0.03); fewer patients in the PPI group experienced COPD exacerbations more than once (24% vs 52%; P<0.004). Trend toward fewer common colds (1.22 vs 2.04) and less frequent common colds (>3 per year) with PPI therapy compared to control. PPI therapy independently reduced risk of exacerbation of COPD (OR 0.23 [95% CI 0.08–0.62]). |
| Eryuksel et al79 | 30 | Antireflux therapy (2 months) PPI therapy |
Reduced COPD symptoms (P<0.01), reduction in laryngopharyngeal reflux symptoms (P<0.01), and improved laryngeal examinations (P<0.001). |
| Ingebrigtsen et al113 | 1,259 | Regular use of acid inhibitory therapy (59%) in those with nighttime and/or daytime GERD | NR. |
| Surgical treatment | |||
| Hartwig et al120 | 20 | Following bilateral lung transplantation, Nissen fundoplication (<365 days post-transplant) undertaken in selected patients | FEV1 greater at 1-year with fundoplication compared to no fundoplication (8.8% difference). |
| Hoppo et al122 | 11 | Pretransplant Nissen fundoplication | Improved FEV1 and FVC% predicted in overall group (separate outcomes for COPD not reported). |
Abbreviations: GER, gastroesophageal reflux; H2-RA, H2 receptor antagonist; PPI, proton pump inhibitor; PFTs, pulmonary function tests; NR, not reported; OR, odds ratio; CI, confidence interval; GERD, gastroesophageal reflux disease; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity.
Conclusion
GERD is a common comorbidity in those with COPD and has a variety of clinical presentations. The index of clinical suspicion should remain high, and objective measures should be used for diagnostic confirmation. The best way to identify pulmonary microaspiration of gastric contents in COPD remains to be established. The presence of GERD appears to increase the risk of an AECOPD and may affect disease progression. Although the co-occurrence of these two common conditions may be associated with increased health care utilization, treatment approaches that have been successfully applied in individuals with GERD without COPD also appear to be effective in the presence of COPD.
Footnotes
Disclosure
The authors report no disclosures or conflicts of interest in this work.
References
- 1.Global Initiative for Chronic Obstructive Pulmonary Disease [homepage on the Internet] Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. 2014. [Accessed 24 February, 2015]. Available from: http://www.goldcopd.
- 2.Seemungal T, Donaldson G, Paul E, Bestall J, Jeffries D, Wedzicha J. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1418–1422. doi: 10.1164/ajrccm.157.5.9709032. [DOI] [PubMed] [Google Scholar]
- 3.Vos T, Flaxman A, Naghavi M. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seemungal T, Donaldson G, Bhowmik A, Jeffries DJ, Wedzicha JA. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161:1608–1613. doi: 10.1164/ajrccm.161.5.9908022. [DOI] [PubMed] [Google Scholar]
- 5.Rycroft C, Heyes A, Lanza L, Becker K. Epidemiology of chronic obstructive pulmonary disease: a literature review. Int J Chron Obstruct Pulmon Dis. 2012;7:457–494. doi: 10.2147/COPD.S32330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vakil N, Van Zanten S, Kahrilas PJ, Dent J, Jones R. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900–1920. doi: 10.1111/j.1572-0241.2006.00630.x. [DOI] [PubMed] [Google Scholar]
- 7.El-Serag H, Sweet S, Winchester C, Dent J. Update on the epidemiology of gastro-oesophageal disease: a systematic review. Gut. 2014;63(6):871–880. doi: 10.1136/gutjnl-2012-304269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sifrim D, Holloway R, Silny J. Acid, nonacid, and gas reflux in patients with gastroesophageal reflux disease during ambulatory 24-hour pH-impedance recordings. Gastroenterology. 2001;120(7):1588–1598. doi: 10.1053/gast.2001.24841. [DOI] [PubMed] [Google Scholar]
- 9.Ducoloné A, Vandevenne A, Jouin H, et al. Gastroesophageal reflux in patients with asthma and chronic bronchitis. Am Rev Respir Dis. 1987;135:327–332. doi: 10.1164/arrd.1987.135.2.327. [DOI] [PubMed] [Google Scholar]
- 10.Sweet M, Patti M, Hoopes C, Hayes SR, Golden JA. Gastro-oesophageal reflux and aspiration in patients with advanced lung disease. Thorax. 2009;64:167–173. doi: 10.1136/thx.2007.082719. [DOI] [PubMed] [Google Scholar]
- 11.Casanova C, Baudet JS, del Valle Velasco M, et al. Increased gastro-oesophageal reflux disease in patients with severe COPD. Eur Respir J. 2004;23:841–845. doi: 10.1183/09031936.04.00107004. [DOI] [PubMed] [Google Scholar]
- 12.Mokhlesi B, Morris A, Huang C-F, Curcio A, Barrett T, Kamp D. Increased prevalence of gastroesophageal reflux symptoms in patients with COPD. Chest. 2001;119(4):1043–1048. doi: 10.1378/chest.119.4.1043. [DOI] [PubMed] [Google Scholar]
- 13.Mittal R, Holloway R, Penagini R, Blackshaw L, Dent J. Transient lower esophageal sphincter relaxation. Gastroenterology. 1995;109:601–610. doi: 10.1016/0016-5085(95)90351-8. [DOI] [PubMed] [Google Scholar]
- 14.Schoeman MN, Tippett MD, Akkermans LM, Dent J, Holloway RH. Mechanisms of gastroesophageal reflux in ambulant healthy human subjects. Gastroenterology. 1995;108(1):83–91. doi: 10.1016/0016-5085(95)90011-x. [DOI] [PubMed] [Google Scholar]
- 15.Mittal R, McCallum R. Characteristics and frequency of transient relaxations of the lower esophageal sphincter in patients with reflux esophagitis. Gastroenterology. 1988;95:593–599. doi: 10.1016/s0016-5085(88)80003-9. [DOI] [PubMed] [Google Scholar]
- 16.Dent J, Holloway R, Toouli J, Dodds W. Mechanisms of lower oesophageal sphincter incompetence in patients with symptomatic gastro-oesophageal reflux. Gut. 1988;29:1020–1028. doi: 10.1136/gut.29.8.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chandrasoma P, DeMeester TR. GERD: Reflux to Esophageal Adenocarcinoma. San Diego: Elsevier; 2006. [Google Scholar]
- 18.Vandenplas Y, Hassall E. Mechanisms of gastroesophageal reflux and gastroesophageal reflux disease. J Pediatr Gastroenterol Nutr. 2002;35(2):119–136. doi: 10.1097/00005176-200208000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Richter J. Gastroesophageal reflux disease. In: Yamada T, editor. Textbook of Gastroenterology. Philadelphia: Lippincott Williams and Williams; 2003. pp. 1196–1224. [Google Scholar]
- 20.Kahrilas P, Dodds WJ, Hogan W. Effect of peristaltic dysfunction on esophageal volume clearance. Gastroenterology. 1988;94:73–80. doi: 10.1016/0016-5085(88)90612-9. [DOI] [PubMed] [Google Scholar]
- 21.Katz PO, Gerson LB, Vela MF. Corrigendum: guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108:308–328. doi: 10.1038/ajg.2012.444. [DOI] [PubMed] [Google Scholar]
- 22.Klauser A, Schindlbeck N, Muller-Lissner S. Symptoms in gastro-oesophageal reflux disease. Lancet. 1990;335:205–208. doi: 10.1016/0140-6736(90)90287-f. [DOI] [PubMed] [Google Scholar]
- 23.Locke GI, Talley N, Fett S. Prevalence and clinical spectrum of gas-troesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology. 1997;112:1448–1456. doi: 10.1016/s0016-5085(97)70025-8. [DOI] [PubMed] [Google Scholar]
- 24.Flook N, Jones R, Vakil N. Approach to gastroesophageal reflux disease in primary care. Putting the Montreal definition into practice. Can Fam Physician. 2008;54:701–705. [PMC free article] [PubMed] [Google Scholar]
- 25.Stanghellini V, Armstrong D, Monnikes H, Bardhans K. Systematic review: do we need a new gastro-oesophageal reflux disease questionnaire? Aliment Pharmacol Ther. 2004;19:463–489. doi: 10.1046/j.1365-2036.2004.01861.x. [DOI] [PubMed] [Google Scholar]
- 26.Fraser A, Delaney B, Moayyedi P. Symptom-based outcome measures for dyspepsia and GERD trials: a systematic review. Am J Gastroenterol. 2005;100:442–452. doi: 10.1111/j.1572-0241.2005.40122.x. [DOI] [PubMed] [Google Scholar]
- 27.Juul-Hansen P, Rydning A, Jackobsen C, Hansen T. High dose proton-pump inhibitors as a diagnostic test of gastro-oesophageal reflux disease in endoscopic-negative patients. Scand J Gastroenterol. 2001;36:806–810. doi: 10.1080/003655201750313315. [DOI] [PubMed] [Google Scholar]
- 28.Fass R, Ofman J. Gastroesophageal reflux disease – should we adopt a new conceptual framework? Am J Gastroenterol. 2002;97(8):1901–1909. doi: 10.1111/j.1572-0241.2002.05912.x. [DOI] [PubMed] [Google Scholar]
- 29.Richter J. Severe reflux esophagitis. Gastroenterol Clin North Am. 1994;4:677. [PubMed] [Google Scholar]
- 30.DeMeester TR, Wang CI, Wernly JA, et al. Techniques, indications and clinical use of 24 hour oesophageal monitoring. J Thorac Cardiovasc Surg. 1980;79:656–670. [PubMed] [Google Scholar]
- 31.Jamieson JR, Stein HJ, DeMeester TR, et al. Ambulatory 24-pH esophageal pH monitoring: normal values, optimal thresholds, specificity, sensitivity and reproducibility. Am J Gastroenterol. 1992;87(9):1102–1111. [PubMed] [Google Scholar]
- 32.Streets C, DeMeester T. Ambulatory 24-hour esophageal pH monitoring: why, when and what to do. J Clin Gastroenterol. 2003;37(1):14–22. doi: 10.1097/00004836-200307000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Johnsson F, Joelsson B, Isberg P-E. Ambulatory 24 hour intraesophageal pH-monitoring in the diagnosis of gastroesophageal reflux disease. Gut. 1987;28:1145–1150. doi: 10.1136/gut.28.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dobhan R, Castell D. Normal and abnormal proximal esophageal acid exposure: results of ambulatory dual-probe pH monitoring. Am J Gastroenterol. 1993;88(1):25–29. [PubMed] [Google Scholar]
- 35.Johnson L, DeMeester T. Twenty-four pH monitoring of the distal esophagus: a quantitative measure of gastroesophageal reflux. Am J Gastroenterol. 1974;62:325–332. [PubMed] [Google Scholar]
- 36.Vaezi M, Schroeder P, Richter J. Reproducibility of proximal probe pH parameters in 24-hour ambulatory pH monitoring. Am J Gastroenterol. 1997;92:825–829. [PubMed] [Google Scholar]
- 37.Emerenziani S, Ribolsi M, Pasqualetti P, Cicala M. Proximal reflux sensitivity: measurement of acid exposure of proximal esophagus: a better tool for diagnosing non-erosive reflux disease. Neurogastroenterol Motil. 2011;23(711):e324. doi: 10.1111/j.1365-2982.2011.01731.x. [DOI] [PubMed] [Google Scholar]
- 38.Katz PO, Matheus T. Telemetry capsule for ambulatory pH monitoring: is it time for a change? Am J Gastroenterol. 2008;103:2986–2987. doi: 10.1111/j.1572-0241.2008.02179.x. [DOI] [PubMed] [Google Scholar]
- 39.Shay S, Bomeli S, Richter J. Multichannel intraluminal impedance accurately detects fasting, recumbent reflux events and their clearing. Am J Physiol Gastrointest Liver Physiol. 2002;283:376–383. doi: 10.1152/ajpgi.00470.2001. [DOI] [PubMed] [Google Scholar]
- 40.Sifrim D, Silny J, Holloway R, Janssens J. Patterns of gas and liquid reflux during transient lower esophageal sphincter relaxation. A study using intraluminal electrical impedance. Gut. 1999;44:47–54. doi: 10.1136/gut.44.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.D’Ovidio F, Singer LG, Hadjiliadis D, et al. Prevalence of gastroesophageal reflux in end-stage lung disease candidates for lung transplant. Ann Thorac Surg. 2005;80:1254–1261. doi: 10.1016/j.athoracsur.2005.03.106. [DOI] [PubMed] [Google Scholar]
- 42.Sweet MP, Herbella FA, Leard L, et al. The prevalence of distal and proximal gastroesophageal reflux in patients awaiting lung transplantation. Ann Surg. 2006;244(4):491–497. doi: 10.1097/01.sla.0000237757.49687.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sole ML, Conrad J, Bennett M, et al. Pepsin and amylase in oral and tracheal secretions. A pilot study. Am J Crit Care. 2014;23(4):334–338. doi: 10.4037/ajcc2014292. [DOI] [PubMed] [Google Scholar]
- 44.Potluri S, Friedenberg F, Parkman HP, et al. Comparison of a salivary/sputum pepsin assay with 24-hour esophageal pH monitoring for detection of gastric reflux into the proximal esophagus, oropharynx and lung. Dig Dis Sci. 2003;48(9):1813–1817. doi: 10.1023/a:1025467600662. [DOI] [PubMed] [Google Scholar]
- 45.Ward C, Forrest IA, Brownlee IA, et al. Pepsin like activity in bronchoalveolar lavage fluid is suggestive of gastric aspiration in lung allografts. Thorax. 2005;60:872–874. doi: 10.1136/thx.2004.036426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stovold R, Forrest IA, Corris PA, et al. Pepsin, a biomarker of gastric aspiration in lung allografts. A putative association with rejection. Am J Respir Crit Care Med. 2007;175:1298–1303. doi: 10.1164/rccm.200610-1485OC. [DOI] [PubMed] [Google Scholar]
- 47.Blondeau K, Mertens V, Vanaudenaerde BA, et al. Gastro-oesophageal reflux and gastric aspiration in lung transplant patients with or without chronic rejection. Eur Respir J. 2008;31:707–713. doi: 10.1183/09031936.00064807. [DOI] [PubMed] [Google Scholar]
- 48.Reder N, Davis C, Kovacs E, Fisichella P. The diagnostic value of gastroesophageal reflux disease (GERD) symptoms and detection of pepsin and bile acids in bronchoalveolar lavage fluid and exhaled breath condensate for identifying lung transplantation patients with GERD-induced aspiration. Surg Endosc. 2014;28(6):1794–1800. doi: 10.1007/s00464-013-3388-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee AL, Button B, Denehy L, et al. Proximal and distal gastro-oesophageal reflux in chronic obstructive pulmonary disease and bronchiectasis. Respirology. 2014;19(2):211–217. doi: 10.1111/resp.12182. [DOI] [PubMed] [Google Scholar]
- 50.Timms C, Thomas P, Yates D. Detection of gastro-oesophageal reflux disease (GORD) in patients with obstructive lung disease using exhaled breath profiling. J Breath Res. 2012;6:016003. doi: 10.1088/1752-7155/6/1/016003. [DOI] [PubMed] [Google Scholar]
- 51.Kostikas K, Papatheodorou G, Ganas K, Psathakis K, Panagou P, Loukides S. pH in expired breath condensate of patients with inflammatory airway diseases. Am J Respir Crit Care Med. 2002;165(10):1364–1370. doi: 10.1164/rccm.200111-068OC. [DOI] [PubMed] [Google Scholar]
- 52.Terada K, Muro S, Sato S, et al. Impact of gastro-oesophageal reflux disease symptoms on chronic obstructive pulmonary disease exacerbation. Thorax. 2008;63:951–955. doi: 10.1136/thx.2007.092858. [DOI] [PubMed] [Google Scholar]
- 53.Martinez CH, Okajima Y, Murray S, et al. COPDGene Investigators Impact of self-reported gastroesophageal reflux disease in subjects from COPD Gene cohort. Respir Res. 2014;15:62. doi: 10.1186/1465-9921-15-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liang B-M, Feng Y-L. Association of gastroesophageal reflux disease symptoms with stable chronic obstructive pulmonary disease. Lung. 2012;190:277–282. doi: 10.1007/s00408-011-9365-5. [DOI] [PubMed] [Google Scholar]
- 55.Rogha M, Behravesh B, Pourmoghaddas Z. Association of gastroesophageal reflux disease symptoms with exacerbations of chronic obstructive pulmonary disease. J Gastrointest Liver Dis. 2010;19(3):253–256. [PubMed] [Google Scholar]
- 56.Kempainen R, Savik K, Whelan T, Dunitz J, Herrington C, Billings J. High prevalence of proximal and distal gastroesophageal reflux disease in advanced COPD. Chest. 2007;131:1666–1671. doi: 10.1378/chest.06-2264. [DOI] [PubMed] [Google Scholar]
- 57.Rascon-Aguilar IE, Pamer M, Wludyka P, et al. Role of gastroe-sophageal reflux symptoms in exacerbations of COPD. Chest. 2006;130:1096–1101. doi: 10.1378/chest.130.4.1096. [DOI] [PubMed] [Google Scholar]
- 58.Bor S, Kitapcioglu G, Solak Z, Ertilav M, Erdinc M. Prevalence of gastroesophageal reflux disease in patients with asthma and chronic obstructive pulmonary disease. J Gastroenterol Hepatol. 2010;25:309–313. doi: 10.1111/j.1440-1746.2009.06035.x. [DOI] [PubMed] [Google Scholar]
- 59.Shimizu Y, Dobashi K, Kusano M, Mori M. Different gastroesophageal reflux symptoms of middle-aged to elderly asthma and chronic objective pulmonary disease patients. J Clin Biochem Nutr. 2011;50(2):169–175. doi: 10.3164/jcbn.11-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takada K, Matsumoto S, Kojima E, et al. Prospective evaluation of the relationship between acute exacerbations of COPD and gastroesophageal reflux disease diagnosed by questionnaire. Respir Med. 2011;105:1531–1536. doi: 10.1016/j.rmed.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 61.Phulpoto M, Ozyyum S, Rizvi N, Khuhaware S. Proportion of gastroesophageal symptoms in patients with chronic obstructive pulmonary disease. J Pak Med Assoc. 2005;55:276–279. [PubMed] [Google Scholar]
- 62.Kamble N, Khan N, Kumar N, Nayak H, Daga M. Study of gastrooesophageal reflux disease in patients with mild-to-moderate chronic obstructive pulmonary disease in India. Respirology. 2013;18:463–467. doi: 10.1111/j.1440-1843.2012.02285.x. [DOI] [PubMed] [Google Scholar]
- 63.Andersen L, Jensen G. Prevalence of benign oesophageal disease in the Danish population with special reference to pulmonary disease. J Intern Med. 1989;225:393–401. doi: 10.1111/j.1365-2796.1989.tb00102.x. [DOI] [PubMed] [Google Scholar]
- 64.Gadel AA, Mostafa M, Younis A, Haleem M. Esophageal motility pattern and gastroesophageal reflux in chronic obstructive pulmonary disease. Hepatogastroenterology. 2012;59(120):2498–2502. doi: 10.5754/hge10433. [DOI] [PubMed] [Google Scholar]
- 65.Weusten B, Akkermans L, vanBerge-Henegouwen GP, Smout AJ. Spatiotemporal characteristics of physiological gastroesophageal reflux. Am J Physiol. 1994;266:G357–G362. doi: 10.1152/ajpgi.1994.266.3.G357. [DOI] [PubMed] [Google Scholar]
- 66.DeMeester T, Johnson L, Joseph G, Toscano M, Hall A, Skinner D. Patterns of gastroesophageal reflux in health and disease. Ann Surg. 1976;184(4):459–470. doi: 10.1097/00000658-197610000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dent J, El-Serag H, Wallander M, Johansson S. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2005;54:710–717. doi: 10.1136/gut.2004.051821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Areias V, Carreira S, Anciaes M, Pinto P, Barbara C. Co-morbidities in patients with gold stage 4 chronic obstructive pulmonary disease. Rev Port Pneumol. 2014;20(1):5–11. doi: 10.1016/j.rppneu.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 69.McColl E. Best practice in symptom assessment: a review. Gut. 2004;53(suppl 4):49–54. doi: 10.1136/gut.2003.034355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.El-Serag H, Sonnenberg A. Comorbid occurrence of laryngeal or pulmonary disease with esophagitis in United States Military Veterans. Gastroenterology. 1997;113:755–760. doi: 10.1016/s0016-5085(97)70168-9. [DOI] [PubMed] [Google Scholar]
- 71.Garcia Rodriguez L, Ruigomez A, Martin-merino E, Johansson S, Wallander M. Relationship between gastroesophageal reflux disease and COPD in UK primary care. Chest. 2008;134(6):1223–1230. doi: 10.1378/chest.08-0902. [DOI] [PubMed] [Google Scholar]
- 72.Pandolfino JE, Kahrilas PJ. Smoking and gastro-esophageal reflux disease. Eur J Gastroenterol Hepatol. 2000;12:837–842. doi: 10.1097/00042737-200012080-00002. [DOI] [PubMed] [Google Scholar]
- 73.Kadakia SC, Kikendall JW, Maydonovitch C, Johnson LF. Effect of cigarette smoking on gastroesophageal reflux measured by 24-h ambulatory esophageal pH monitoring. Am J Gastroenterol. 1995;90:1785–1790. [PubMed] [Google Scholar]
- 74.Kim S, Lee J, Sim Y, Ryu Y, Chang J. Prevalence and risk factors for reflux esophagitis in patients with chronic obstructive pulmonary disease. Korean J Intern Med. 2014;29:466–473. doi: 10.3904/kjim.2014.29.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hill C, Jones R. Systematic review: the epidemiology of gastrooesophageal reflux disease in primary care, using the UK general practice research database. Aliment Pharmacol Ther. 2009;29:470–480. doi: 10.1111/j.1365-2036.2008.03901.x. [DOI] [PubMed] [Google Scholar]
- 76.Rahman I. Oxidative stress in pathogenesis of chronic obstructive pulmonary disease: cellular and molecular mechanisms. Cell Biochem Biophys. 2005;43:167–188. doi: 10.1385/CBB:43:1:167. [DOI] [PubMed] [Google Scholar]
- 77.Farrell S, McMaster C, Gibson D, Shields M, McCallion W. Pepsin in bronchoalveolar lavage fluid: a specific and sensitive method of diagnosing gastrooesophageal reflux-related pulmonary aspiration. J Pediatr Surg. 2006;41:289–293. doi: 10.1016/j.jpedsurg.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 78.Pauwels A, Decraene A, Blondeau K, et al. Bile acids in sputum and increased airway inflammation in patients with cystic fibrosis. Chest. 2012;141(6):1568–1574. doi: 10.1378/chest.11-1573. [DOI] [PubMed] [Google Scholar]
- 79.Eryuksel E, Dogan M, Olgun S, Kocak I, Celikel T. Incidence and treatment results of laryngopharyngeal reflux in chronic obstructive pulmonary disease. Eur Arch Otorhinolaryngol. 2009;266(8):1267–1271. doi: 10.1007/s00405-009-0922-y. [DOI] [PubMed] [Google Scholar]
- 80.Lee A, Button B, Denehy L, et al. Exhaled breath condensate pepsin: potential noninvasive test for gastroesophageal reflux in COPD and bronchiectasis. Respir Care. 2015;60(2):244–250. doi: 10.4187/respcare.03570. [DOI] [PubMed] [Google Scholar]
- 81.Orr W, Elsenbruch S, Harnish M, Johnson L. Proximal migration of esophageal acid perfusions during waking and sleep. Am J Gastroenterol. 2000;95:37–42. doi: 10.1111/j.1572-0241.2000.01669.x. [DOI] [PubMed] [Google Scholar]
- 82.Fortunato G, Machado M, Andrade C, Felicetti J, Camargo J, Cardoso P. Prevalence of gastroesophageal reflux in lung transplant candidates with advanced lung disease. J Bras Pneumol. 2008;34(10):772–778. doi: 10.1590/s1806-37132008001000004. [DOI] [PubMed] [Google Scholar]
- 83.Terada K, Muro S, Ohara T, et al. Abnormal swallowing reflex and COPD exacerbations. Chest. 2010;137(2):326–332. doi: 10.1378/chest.09-0482. [DOI] [PubMed] [Google Scholar]
- 84.Teramoto S, Kume H, Ouchi Y. Altered swallowing physiology and aspiration in COPD. Chest. 2002;122(3):1104–1105. doi: 10.1378/chest.122.3.1104. [DOI] [PubMed] [Google Scholar]
- 85.Gross R, Atwood DJ, Ross S, Olszewski J, Eichhorn K. The coordination between breathing and swallowing in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;179(7):559–565. doi: 10.1164/rccm.200807-1139OC. [DOI] [PubMed] [Google Scholar]
- 86.Mokhlesi B, Logemann JA, Rademaker AW, Stangl CA, Corbridge TC. Oropharyngeal deglutition in stable COPD. Chest. 2002;121(2):361–369. doi: 10.1378/chest.121.2.361. [DOI] [PubMed] [Google Scholar]
- 87.Mokhlesi B. Clinical implications of gastroesophageal reflux disease and swallowing dysfunction in COPD. Am J Respir Med. 2003;2(2):117–121. doi: 10.1007/BF03256643. [DOI] [PubMed] [Google Scholar]
- 88.Pauwels A, Blondeau K, Dupont L, Sifrim D. Mechanisms of increased gastroesophageal reflux in patients with cystic fibrosis. Am J Gastroenterol. 2012;107(9):1346–1353. doi: 10.1038/ajg.2012.213. [DOI] [PubMed] [Google Scholar]
- 89.Turbyville J. Applying principles of physics to the airway to help explain the relationship between asthma and gastroesophageal reflux. Med Hypotheses. 2010;74:1075–1080. doi: 10.1016/j.mehy.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 90.Field S, Underwood M, Brant R, Cowie R. Prevalence of gastroesophageal reflux symptoms in asthma. Chest. 1996;109:316–322. doi: 10.1378/chest.109.2.316. [DOI] [PubMed] [Google Scholar]
- 91.Naliboff BD, Mayer M, Fass R, et al. The effect of life stress on symptoms of heartburn. Psychosom Med. 2004;66(3):426–434. doi: 10.1097/01.psy.0000124756.37520.84. [DOI] [PubMed] [Google Scholar]
- 92.Stein M, Towner T, Weber R. The effect of theophylline on the lower esophageal sphincter pressure. Ann Allergy. 1980;45:238. [PubMed] [Google Scholar]
- 93.Crowell M, Zayat E, Lacy B. The effects of an inhaled B2-adrenergic agonist on lower esophageal function: a dose-response study. Chest. 2001;121:1024–1027. doi: 10.1378/chest.120.4.1184. [DOI] [PubMed] [Google Scholar]
- 94.Kim J, Lee JH, Kim Y, et al. Association between chronic obstructive pulmonary disease and gastroesophageal reflux disease: a national cross-sectional cohort study. BMC Pulmon Med. 2013;13:51. doi: 10.1186/1471-2466-13-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ruzkowski C, Sanowski R, Austin J, Rohwedder JJ, Waring JP. The effects of inhaled albuterol and oral theophylline on gastroesophageal reflux in patients with gastroesophageal reflux disease and obstructive lung disease. Arch Intern Med. 1992;152(4):783–785. [PubMed] [Google Scholar]
- 96.Niklasson A, Strid H, Simren M, Engstrom C-P, Bjornsson E. Prevalence of gastrointestinal symptoms in patients with chronic obstructive pulmonary disease. Eur J Gastroenterol Hepatol. 2008;20(4):335–341. doi: 10.1097/MEG.0b013e3282f2d0ec. [DOI] [PubMed] [Google Scholar]
- 97.Sontag S, O’Connell S, Khandelwal S, Miller T, Nemchausky B, Schnell T. Most asthmatics have gastroesophageal reflux with or without bronchodilator therapy. Gastroenterology. 1990;99:613–620. doi: 10.1016/0016-5085(90)90945-w. [DOI] [PubMed] [Google Scholar]
- 98.Hubert D, Gaudric M, Guerre J, Lockhart A, Marsac J. Effect of theophylline on gastroesophageal reflux in patients with asthma. J Allergy Clin Immunol. 1988;81:1168–1174. doi: 10.1016/0091-6749(88)90886-x. [DOI] [PubMed] [Google Scholar]
- 99.Kulig M, Nocon M, Vieth M, et al. Risk factors of gastroesophageal reflux disease: methodology and first epidemiological results of the ProGERD study. J Clin Epidemiol. 2004;57:580–589. doi: 10.1016/j.jclinepi.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 100.Locke R, Talley N, Fett S, Zinsmeister A, Melton J. Risk factors associated with symptoms of gastroesophageal reflux. Am J Med. 1999;106:642–649. doi: 10.1016/s0002-9343(99)00121-7. [DOI] [PubMed] [Google Scholar]
- 101.Dore M, Maragkoudakis E, Fraley K, et al. Diet, lifestyle and gender in gastroesophageal reflux disease. Dig Dis Sci. 2008;53(8):2017–2032. doi: 10.1007/s10620-007-0108-7. [DOI] [PubMed] [Google Scholar]
- 102.Corley D, Kubo A. Body mass index and gastroesophageal reflux disease: a systematic review and meta-analysis. Am J Gastroenterol. 2006;108:2619–2628. doi: 10.1111/j.1572-0241.2006.00849.x. [DOI] [PubMed] [Google Scholar]
- 103.Demeter P, Pap A. The relationship between gastroesophageal reflux disease and obstructive sleep apnea. J Gastroenterol. 2004;39:815–820. doi: 10.1007/s00535-004-1416-8. [DOI] [PubMed] [Google Scholar]
- 104.Zagari RM, Fuccio L, Wallander MA, et al. Gastro-oesophageal reflux symptoms, oesophagitis and Barrett’s oesophagus in the general population: the Loiano-Monghidoro study. Gut. 2008;57(10):1354–1359. doi: 10.1136/gut.2007.145177. [DOI] [PubMed] [Google Scholar]
- 105.Harding S. GERD, airway disease and the mechanisms of interaction. In: Stein M, editor. Gastroesophageal Reflux Disease and Airway Disease. New York: Marcel Dekker Inc; 1999. pp. 162–163. [Google Scholar]
- 106.Canning B, Mazzone S. Reflex mechanisms in gastroesophageal reflux disease and asthma. Am J Med. 2003;115:45S–48S. doi: 10.1016/s0002-9343(03)00192-x. [DOI] [PubMed] [Google Scholar]
- 107.Orr W, Shamma-Othman Z, Allen M, Robinson M. Esophageal function and gastroesophageal reflux during sleep and waking in patients with chronic obstructive pulmonary disease. Chest. 1992;101:1521–1525. doi: 10.1378/chest.101.6.1521. [DOI] [PubMed] [Google Scholar]
- 108.Guarascio A, Ray S, Finch C, Self T. The clinical and economic burden of chronic obstructive pulmonary disease in the USA. Clinicoeconom Outcomes Res. 2013;5:235–245. doi: 10.2147/CEOR.S34321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sakae T, Pizzichini M, Teixeira P, de Silva R, Trevisol D, Pizzichini E. Exacerbations of COPD and symptoms of gastroesophageal reflux: a systematic review and meta-analysis. J Bras Pneumol. 2013;39(3):259–271. doi: 10.1590/S1806-37132013000300002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ozyilmaz E, Kokturk N, Teksut G, Tatlicioglu T. Unsuspected risk factors of frequent exacerbations requiring hospital admission in chronic obstructive pulmonary disease. Inter J Clin Pract. 2013;67:691–697. doi: 10.1111/ijcp.12150. [DOI] [PubMed] [Google Scholar]
- 111.Lin Y, Tsai C, Chien L, Chiou H, Jeng C. Newly diagnosed gastroesophageal reflux disease increased the risk of acute exacerbations of chronic obstructive pulmonary disease during the first year following diagnosis – a nationwide population-based cohort study. Inter J Clin Pract. 2015;69(3):350–357. doi: 10.1111/ijcp.12501. [DOI] [PubMed] [Google Scholar]
- 112.Hurst J, Vestbo J, Anzueto A. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 113.Ingebrigtsen T, Marott J, Vestbo J, Nordestgaard B, Hallas J, Lange P. Gastroesophageal reflux disease and exacerbations in chronic obstructive pulmonary disease. Respirology. 2015;20:101–107. doi: 10.1111/resp.12420. [DOI] [PubMed] [Google Scholar]
- 114.Pacheco-Galvan A, Hart S, Morice A. Relationship between gastrooesophageal reflux and airway diseases: the airway reflux paradigm. Arch Bronchoneumol. 2011;47:195–203. doi: 10.1016/j.arbres.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 115.Lee AL. PhD Thesis. Melbourne: The University of Melbourne; 2009. Gastro-oesophageal reflux in chronic obstructive pulmonary disease and bronchiectasis. [Google Scholar]
- 116.Ajmera M, Raval A, Shen C, Sambamoorthi U. Explaining the increased health care expenditures associated with gastroesophageal reflux disease among elderly Medicare beneficiaries with chronic obstructive pulmonary disease: a cost-decomposition analysis. Int J Chron Obstruct Pulmon Dis. 2014;9:339–348. doi: 10.2147/COPD.S59139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.National Institute for Health and Care Excellence Chronic Obstructive Pulmonary Disease. Management of Chronic Obstructive Pulmonary Disease in Adults in Primary and Secondary Care. 2010. [Accessed 24 February, 2015]. Available from: https://www.nice.org.uk/guidance/cg101/chapter/1-guidance.
- 118.Sasaki T, Nakayama K, Yasuda H, et al. A randomised, single-blind study of lansoprazole for the prevention of exacerbations of chronic obstructive pulmonary disease in older patients. J Am Geriatr Soc. 2009;57(8):1453–1457. doi: 10.1111/j.1532-5415.2009.02349.x. [DOI] [PubMed] [Google Scholar]
- 119.Storr MA. What is nonacid reflux disease? Can J Gastroenterol. 2011;25(1):35–38. doi: 10.1155/2011/626752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hartwig MG, Anderson DJ, Onaitis MW, et al. Fundoplication after lung transplantation prevents the allograft dysfunction associated with reflux. Ann Thorac Surg. 2011;92(2):462–468. doi: 10.1016/j.athoracsur.2011.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gasper WJ, Sweet MP, Hoopes C, et al. Antireflux surgery for patients with end-stage lung disease before and after lung transplantation. Surg Endosc. 2008;22:495–500. doi: 10.1007/s00464-007-9494-3. [DOI] [PubMed] [Google Scholar]
- 122.Hoppo T, Jarido V, Pennathur A, et al. Antireflux surgery preserves lung function in patients with gastroesophageal reflux disease and end-stage lung disease before and after lung transplantation. Arch Surg. 2011;146(9):1041–1047. doi: 10.1001/archsurg.2011.216. [DOI] [PubMed] [Google Scholar]
- 123.Ekstrom T, Johansson K. Effects of anti-reflux surgery on chronic cough and asthma in patients with gastro-oesophageal reflux disease. Respir Med. 2000;94(12):1166–1170. doi: 10.1053/rmed.2000.0944. [DOI] [PubMed] [Google Scholar]