Abstract
BACKGROUND
Understanding gut development may illuminate the adaptive response to massive small-bowel resection and facilitate enteral nutrition. We reported that Schlafen-3 (Slfn3) mediates differentiation in vitro in rat intestinal epithelial. We hypothesized that Slfn3 is involved in intestinal development in vivo.
METHODS
We removed fetal intestines, liver, and lungs on day 20 of gestation, at birth, and on postnatal days 1 and 5. Expression of Slfn3, markers of intestinal differentiation, and Slfn5, to address specificity, were determined by quantitative reverse-transcription polymerase chain reaction.
RESULTS
Villin expression increased on days 1 and 5 (8.7 ± .6 and 5.4 ± .4, respectively; P < .01). Intestinal Slfn3 expression was increased substantially after birth (2.1- ± .5-fold) and on days 1 and 5 (P < .02). Slfn3 was higher after birth in liver and lung but decreased sharply thereafter. Slfn5 expression was mostly unchanged.
CONCLUSIONS
The data suggest that the developmental/maturation effects we observed correlate with Slfn3 but not Slfn5 and are more relevant to the intestines. A better understanding of how Slfn3 promotes intestinal differentiation could help promote intestinal maturation, improving outcomes in children or adults with short-gut syndrome.
Keywords: Intestinal, development, Schlafen, Short-gut syndrome, Adaptation
The mucosa atrophies and intestinal absorptive capacity decreases in fasting or starving patients despite total parental nutrition (TPN). This occurs after massive bowel resection, in severe mucosal disease, or in congenital abnormalities such as intestinal atresias.1–3 Many short-bowel syndrome (SBS) patients eventually experience enough mucosal adaptation for enteral nutrition but some require chronic TPN or small-bowel transplantation, each with complications.4,5 Chronic TPN mortality rates range from 15% to 47%.6 Attempts to compensate for these changes using exogenous hormones or growth factors have yielded variable results. Growth hormone is Food and Drug Administration– approved to treat SBS, but is of limited efficacy.7 Glucagon-like peptide-2 (GLP-2) analogs are on the near horizon in the United States as of this writing. GLP-2 may have disparate effects in the young and adults.8 Growth hormone and GLP-2 primarily stimulate adaptation via intestinal epithelial proliferation. However, although such mitogenic agents increase the number of intestinal epithelial cells, the average enterocytic maturity may decrease.9 A fully functional gut mucosa is likely to require not only more cells but mature fully differentiated cells.
The intracellular signals that influence mucosal differentiation in atrophic or adapting intestinal mucosa are poorly understood. Although adaptation and development are different, understanding the signals that regulate perinatal intestinal epithelial development may suggest targets for the study of adaptation.
The Schlafen (Slfn) protein family10 has been studied mostly in rodent hematopoietic cells, macrophages, and fibroblasts, where various Slfns may influence myelopoiesis, activation, or differentiation.11–13 Slfns also widely are expressed in lung, kidney, and gut mucosa. Schlafen 3 (Slfn3) is required for rat intestinal epithelial (IEC-6) cell differentiation,14 and decreased in aging gut in vivo.15 We thus hypothesized that if Slfn3 influences intestinal epithelial differentiation, then it might change in perinatal intestinal epithelial development.
The small intestine undergoes significant growth and functional changes during the immediate prenatal and postnatal periods. We assayed intestinal expression of Slfn3 and several differentiation markers on embryonic day 20, at birth, and on postnatal days 1 and 5 to gain a better understanding of intestinal epithelial cell differentiation. We measured Slfn3 expression in lung and liver and the related Slfn5 for comparison.
Methods
Animals
Timed-pregnant Sprague–Dawley rats (Charles River, Wilmington, MA) were killed by CO2 inhalation on embryonic day 20. Fetal intestines were removed and snap-frozen in liquid N2 as embryonic day 20 (E20) samples. Other rats were monitored for delivery, and their pups were euthanized by CO2 inhalation at birth (postnatal day 0 [P0]), and on days 1 (P1) and 5 (P5). Jejunal segments, and liver and lung for comparison, were snap-frozen for future extraction. All procedures were approved by the Michigan State Institutional Animal Care and Use Committee.
Quantitative reverse-transcription polymerase chain reaction
RNA was extracted using the RNeasy kit (Qiagen, Valencia, CA). Complementary DNA generated were amplified using SYBR Green Real-Time PCR Master Mix on an Applied Biosystems 7500 Real-Time PCR System (Invitrogen, Grand Island, NY). The Slfn3 primers ugcaaaugaagaaacaguuugagggag and cccucaaacuguuucauuugca specifically amplified Slfn3 and not the related Slfn4.14,16 Other specific primers amplified Slfn5 (f-agccgctgactgcaaaggactgc and r-tgagggaaactgaaaagctctgggt), villin (f-caacttctatgagggagactgctac and r-tagtcatccagctgtgtggtataga), dipeptidyl peptidase-4 (DPPIV) (f-tctccgcgctgacttct and r-agttctcgcgctatcagcggc), and sucrase isomaltase (SI) (f-ggcacagcgacagaggggaca and r-tgcccgtgggcaaaggttgaa).
Statistics
Data are presented as X ± standard error. 18S and hypoxanthine phosphoryltransferase internal standards yielded similar results; we show expression relative to 18S here. Analysis of variance compared P0, P1, and P5 expression, excluding E20 samples from the analysis because they required the entire small bowel and were not strictly comparable with jejunal P0, P1, and P5 samples.
Results
Villin and DPPIV expression increase after birth
Although differentiation marker expression was lower at birth than in fetal intestine, villin, and DPPIV expression increased thereafter (Table 1). Villin increased 9-fold 1 day after birth and remained high at P5 (n = 5; P < .01). SI did not change over P0 to P5, as expected.
Table 1.
Perinatal intestinal differentiation marker expression
| E20 | P0 | P1 | P5 | |
|---|---|---|---|---|
| Villin | .4 ± .08 | .11 ± .02 | .9 ± .07* | .6 ± .04* |
| DPPIV | 17.0 ± 4.4 | 1.0 ± .4 | 3.9 ± .96† | 2.4 ± .6 |
| SI | 1.3 ± .3 | .3 ± .16 | .2 ± .1 | .25 ± .24 |
P < .05 by one-way analysis of variance; P1 and P5 compared with P0.
P < .05, P1 vs P0 by one-way analysis of variance.
Slfn3 expression increases during perinatal development and parallels villin and DPPIV after birth
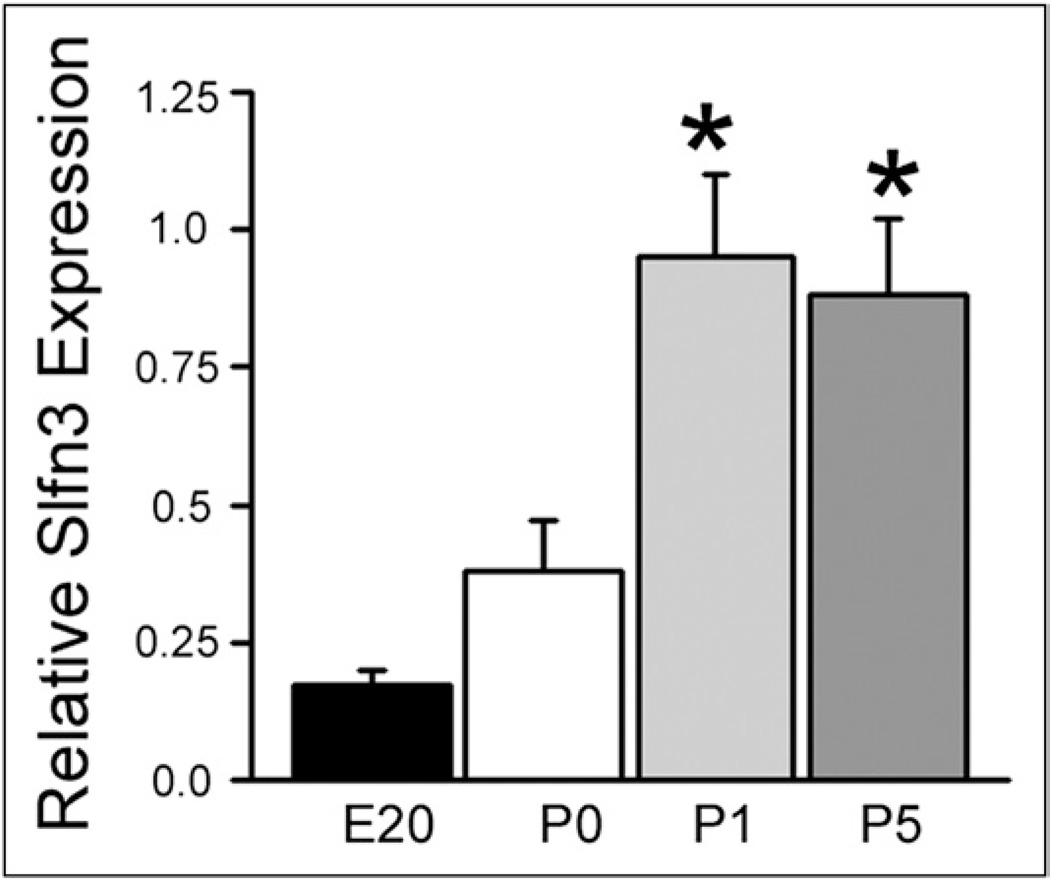
Slfn3 expression was low in embryonic intestine but 2-fold higher at birth (P0) (Fig. 1). Slfn3 more than doubled again on P1, and remained high at P5 (n = 5; P < .02), mimicking villin and DPPIV on P1 and P5. These increases were not related to feeding because milk was present in the stomach and duodenum of all pups at death, even on P0.
Figure 1.
Rat intestinal Slfn3 expression increases with postnatal age. RNA from fetal small intestine (E20) and jejunum from newly born (P0) and early postnatal rat pups (P1 and P5) was extracted for quantitative reverse-transcription polymerase chain reaction assessment of Slfn3 expression as compared with the 18S control. Day of birth (P0) Slfn3 expression is twice that of the fetus and doubles again on the first day after birth (P1). This expression level is maintained on postnatal day 5 (P5). *P < .02 by one-way analysis of variance comparing P0 with P1 and P5 (n = 5).
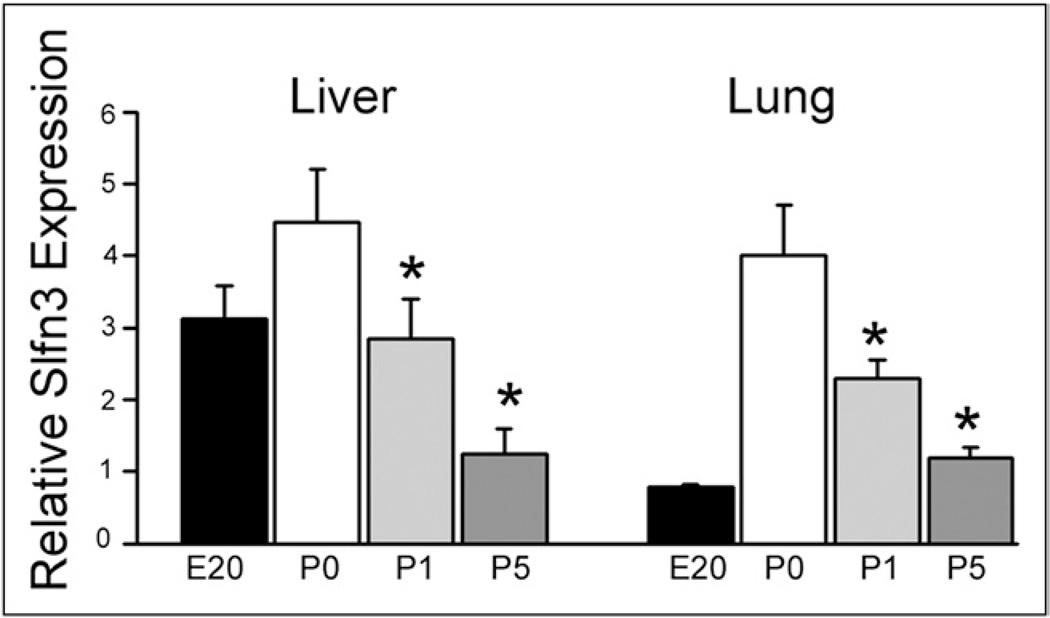
Slfn3 decreases with postnatal age in lung and liver
Embryonic liver Slfn3 is higher than lung Slfn3. Slfn3 is higher in both at birth than at the end of gestation (Fig. 2). However, in contrast to the increased intestinal Slfn3 after birth, Slfn3 expression decreases by approximately 50% on each day after birth in both liver and lung (n = 5; P < .05).
Figure 2.
Slfn3 expression levels decrease with postnatal age in liver and lung. RNA similarly was extracted from liver and lung tissue samples obtained at the same time (E20, P0, P1, and P5). Relative (to 18S) Slfn3 expression was higher on day of birth than in the fetal sample but decreased by approximately 50% each day thereafter in both organs, suggesting that this Slfn is not involved in maturation/differentiation of the liver or the lung. *P < .05 by one-way analysis of variance comparing P0 with P1 and P5 (n = 5).
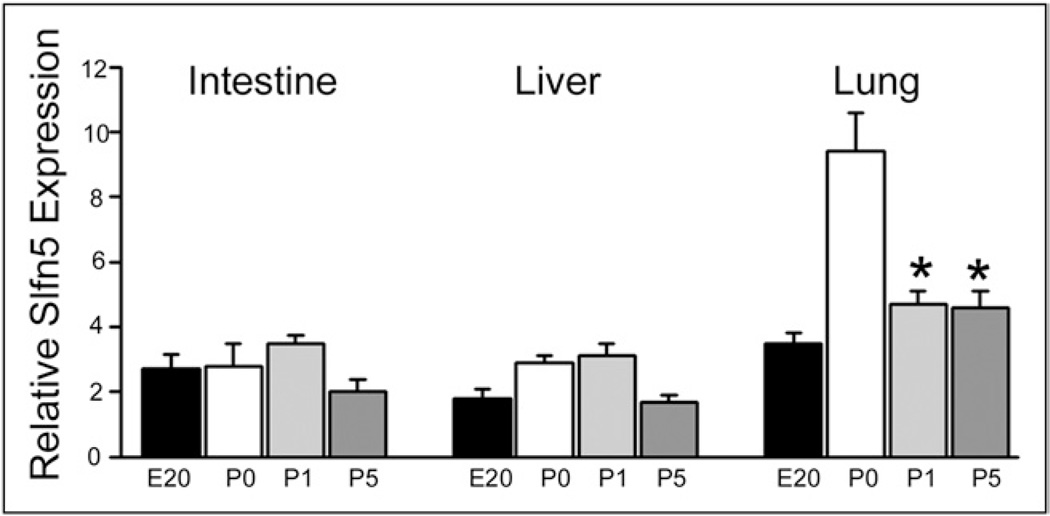
Intestinal Slfn5 does not vary with Slfn3, villin, or DPPIV
Slfn5 messenger RNA (Fig. 3) was constant from E20 to P5 in intestine (left) and liver (middle). The neonatal expression spike in lung Slfn5 (right) was not sustained on postnatal days 1 and 5.
Figure 3.
Slfn5 expression does not differ appreciably with postnatal age in the intestine, liver, or lung. Relative Slfn5 expression, as determined by quantitative reverse-transcription polymerase chain reaction, is essentially the same in the fetal (E20) and postnatal (P0, P1, P5) samples in the intestine and the liver. The P0 spike observed in the lung is not maintained on subsequent days. The data support a specific role for Slfn3 in postnatal intestinal epithelial development. *P < .05 by one-way analysis of variance comparing P0 with P1 and P5 (n = 5).
Comments
Three disparate stimuli to differentiation induce Slfn3 expression in rat intestinal epithelial IEC-6 cells and reducing Slfn3 blocks such differentiation, assessed by villin expression and DPPIV activity.14 We now report that intestinal Slfn3 expression in the immediate postnatal period increases in parallel with villin and DPPIV while Slfn5 remains constant. The effect on differentiation appears to be intestine-specific because hepatic and pulmonary Slfn3 decreases at this time. The results suggest that Slfn3, but not Slfn5, is associated closely with postnatal rat intestinal mucosal differentiation.
The small intestine develops by morphogenesis and proliferation, differentiation, and then functional maturation. Developmental activation of intestine-specific genes is controlled cooperatively by several transcription families while complex processes involving many growth factors coordinately regulate late intrauterine and early postnatal development. Brush-border and crypt development are postnatal in rodents but prenatal in human beings, but the processes are otherwise similar between species.17
The actin-binding cytoskeletal protein villin is a marker for differentiated intestinal epithelia, increasing expression from crypt to villus.18 DPPIV is most prevalent in renal and intestinal brush borders. We previously used these markers to document intestinal epithelial differentiation.19 The higher intestinal villin and DPPIV expression on days 1 and 5 confirms enterocytic differentiation during the early postnatal period.
It is believed that early intestinal epithelial development is regulated by an autonomous genetic program.20 Villin is expressed as early as 9 days post-coitum in mice. A transient villin increase also occurs around day 17, just before microvillus length and density increase.21 Other brush-border hydrolases also are expressed late in fetal development.22 This may explain the villin and DPPIV increases in our E20 samples. SI is suppressed until just before weaning, also consistent with our results.
Schlafen functions are poorly understood. Whether Slfn1 and Slfn2 inhibit proliferation is controversial.23 Slfn4 engenders macrophage activation and myelopoiesis while Slfn3 may promote rodent T-cell differentiation and activation in vitro,12,13 although T cells are not altered in vivo by Slfn3 knockout.24 We confirmed that Slfn3 chiefly is expressed in the epithelial cells within the gut mucosa by immunohistochemistry (not shown). Slfn3 also has been reported to inhibit intestinal epithelial cell proliferation in vitro,16 but we14 did not observe this. Slfn3 may represent a final common pathway for enterocytic differentiation because it is induced by and required for the effects of 3 disparate differentiating stimuli: cyclic strain, butyrate, and transforming growth factor-β.14 The present study is consistent with a role for Slfn3 in developmental intestinal differentiation. That Slfn3 did not increase in lung or liver suggests that this action of Slfn3 is intestine-specific.
Even less is known about Slfn5. Slfn5 modulates T-cell activation25 and may promote growth and invasion in some melanoma cells.26 Slfn5 did not change in intestine, lung, or liver, so it may not influence their postnatal differentiation.
Reversal of mucosal atrophy or adaptation to SBS requires structural and functional changes that increase nutrient, electrolyte, and fluid absorption. These changes affect all intestinal layers but the mucosa is key. Although proliferation is important, intestinal epithelial maturation is required for full functional recovery. Growth hormone and glucagon-like peptide-2 or its degradation resistant analog, teduglutide, probably do not promote differentiation. The Slfn3-activated pathway could provide this stimulus.
References
- 1.Buchman AL, Moukarzel AA, Bhuta S, et al. Parenteral nutrition is associated with intestinal morphologic and functional changes in humans. JPEN J Parenter Enteral Nutr. 1995;19:453–460. doi: 10.1177/0148607195019006453. [DOI] [PubMed] [Google Scholar]
- 2.Schaart MW, de Bruijn AC, Bouwman DM, et al. Epithelial functions of the residual bowel after surgery for necrotising enterocolitis in human infants. J Pediatr Gastroenterol Nutr. 2009;49:31–41. doi: 10.1097/MPG.0b013e318186d341. [DOI] [PubMed] [Google Scholar]
- 3.Choudhry MS, Rahman N, Boyd P, et al. Duodenal atresia: associated anomalies, prenatal diagnosis and outcome. Pediatr Surg Int. 2009;25:727–730. doi: 10.1007/s00383-009-2406-y. [DOI] [PubMed] [Google Scholar]
- 4.Robinson MK, Ziegler TR, Wilmore DW. Overview of intestinal adaptation and its stimulation. Eur J Pediatr Surg. 1999;9:200–206. doi: 10.1055/s-2008-1072244. [DOI] [PubMed] [Google Scholar]
- 5.Sudan D. Long-term outcomes and quality of life after intestine transplantation. Curr Opin Organ Transplant. 2010;15:357–360. doi: 10.1097/MOT.0b013e3283398565. [DOI] [PubMed] [Google Scholar]
- 6.Schalamon J, Mayr JM, Höllwarth ME. Mortality and economics in short bowel syndrome. Best Pract Res Clin Gastroenterol. 2003;17:931–942. doi: 10.1016/s1521-6918(03)00079-9. [DOI] [PubMed] [Google Scholar]
- 7.Byrne TA, Wilmore DW, Iyer K, et al. Growth hormone, glutamine, and an optimal diet reduces parenteral nutrition in patients with short bowel syndrome: a prospective, randomized, placebo-controlled, double-blind clinical trial. Ann Surg. 2005;242:655–661. doi: 10.1097/01.sla.0000186479.53295.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pereira-Fantini PM, Nagy ES, Thomas SL, et al. GLP-2 administration results in increased proliferation but paradoxically an adverse outcome in a juvenile piglet model of short bowel syndrome. J Pediatr Gastroenterol Nutr. 2008;46:20–28. doi: 10.1097/01.mpg.0000304449.46434.06. [DOI] [PubMed] [Google Scholar]
- 9.Pereira-Fantini PM, Thomas SL, Wilson G, et al. Short- and long-term effects of small bowel resection: a unique histological study in a piglet model of short bowel syndrome. Histochem Cell Biol. 2011;135:195–202. doi: 10.1007/s00418-011-0778-2. [DOI] [PubMed] [Google Scholar]
- 10.Schwarz DA, Katayama CD, Hedrick SM. Schlafen, a new family of growth regulatory genes that affect thymocyte development. Immunity. 1998;9:657–668. doi: 10.1016/s1074-7613(00)80663-9. [DOI] [PubMed] [Google Scholar]
- 11.Katsoulidis E, Carayol N, Woodard J, et al. Role of schlafen 2 (SLFN2) in the generation of interferon alpha-induced growth inhibitory responses. J Biol Chem. 2009;284:25051–25064. doi: 10.1074/jbc.M109.030445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Zuylen WJ, Garceau V, Idris A, et al. Macrophage activation and differentiation signals regulate schlafen-4 gene expression: evidence for schlafen-4 as a modulator of myelopoiesis. PLoS ONE. 2011;6:e15723. doi: 10.1371/journal.pone.0015723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Condamine T, Le Luduec JB, Chiffoleau E, et al. Characterization of schlafen-3 expression in effector and regulatory T cells. J Leukoc Biol. 2010;87:451–456. doi: 10.1189/jlb.0609410. [DOI] [PubMed] [Google Scholar]
- 14.Yuan L, Yu Y, Sanders MA, et al. Schlafen 3 induction by cyclic strain regulates intestinal epithelial differentiation. Am J Physiol Gastrointest Liver Physiol. 2010;298:G994–G1003. doi: 10.1152/ajpgi.00517.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel BB, Yu Y, Du J, et al. Schlafen 3, a novel gene, regulates colonic mucosal growth during aging. Am J Physiol Gastrointest Liver Physiol. 2009;296:G955–G962. doi: 10.1152/ajpgi.90726.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel VB, Yu Y, Das JK, et al. Schlafen-3: a novel regulator of intestinal differentiation. Biochem Biophys Res Commun. 2009;388:752–756. doi: 10.1016/j.bbrc.2009.08.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drozdowski LA, Clandinin T, Thomson AB. Ontogeny, growth and development of the small intestine: understanding pediatric gastroenterology. World J Gastroenterol. 2010;16:787–799. doi: 10.3748/wjg.v16.i7.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khurana S, George SP. Regulation of cell structure and function by actin-binding proteins: villin’s perspective. FEBS Lett. 2008;582:2128–2139. doi: 10.1016/j.febslet.2008.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basson MD, Turowski G, Emenaker NJ. Regulation of human (Caco-2) intestinal epithelial cell differentiation by extracellular matrix proteins. Exp Cell Res. 1996;225:301–305. doi: 10.1006/excr.1996.0180. [DOI] [PubMed] [Google Scholar]
- 20.Muncan V, Heijmans J, Krasinski SD, et al. Blimp1 regulates the transition of neonatal to adult intestinal epithelium. Nat Commun. 2011;2:452–461. doi: 10.1038/ncomms1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braunstein EM, Qiao XT, Madison B, et al. Villin: a marker for development of the epithelial pyloric border. Dev Dyn. 2002;224:90–102. doi: 10.1002/dvdy.10091. [DOI] [PubMed] [Google Scholar]
- 22.Bailey DS, Cook A, McAllister G, et al. Structural and biochemical differentiation of the mammalian small intestine during foetal development. J Cell Sci. 1984;72:195–212. doi: 10.1242/jcs.72.1.195. [DOI] [PubMed] [Google Scholar]
- 23.Zhao L, Neumann B, Murphy K, et al. Lack of reproducible growth inhibition by Schlafen1 and Schlafen2 in vitro. Blood Cells Mol Dis. 2008;41:188–193. doi: 10.1016/j.bcmd.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Berger M, Krebs P, Crozat K, et al. An Slfn2 mutation causes lymphoid and myeloid immunodeficiency due to loss of immune cell quiescence. Nat Immunol. 2010;11:336–343. doi: 10.1038/ni.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geserick P, Kaiser F, Klemm U, et al. Modulation of T cell development and activation by novel members of the schlafen (slfn) gene family harbouring an RNA helicase-like motif. Int Immunol. 2004;16:1535–1548. doi: 10.1093/intimm/dxh155. [DOI] [PubMed] [Google Scholar]
- 26.Katsoulidis E, Mavrommatis E, Woodard J, et al. Role of interferon {alpha} (IFN{alpha})-inducible Schlafen-5 in regulation of anchor-age-independent growth and invasion of malignant melanoma cells. J Biol Chem. 2010;285:40333–40341. doi: 10.1074/jbc.M110.151076. [DOI] [PMC free article] [PubMed] [Google Scholar]