Abstract
Tumour hypoxia is a major cause of treatment failure for a variety of malignancies. However, tumour hypoxia also offers treatment opportunities, exemplified by the development compounds that target hypoxic regions within tumours. TH-302 is a pro-drug created by the conjugation of 2-nitroimidazole to bromo-isophosphoramide (Br-IPM). When TH-302 is delivered to regions of hypoxia, Br-IPM, the DNA cross linking toxin, is released. In this study we assessed the cytotoxic activity of TH-302 against osteosarcoma cells in vitro and evaluated its anticancer efficacy as a single agent, and in combination with doxorubicin, in an orthotopic mouse model of human osteosarcoma (OS). In vitro, TH-302 was potently cytotoxic to osteosarcoma cells selectively under hypoxic conditions, whereas primary normal human osteoblasts were protected. Animals transplanted with OS cells directly into their tibiae and left untreated developed mixed osteolytic/osteosclerotic bone lesions and subsequently developed lung metastases. TH-302 reduced tumor burden in bone and cooperated with doxorubicin to protect bone from osteosarcoma induced bone destruction, while it also reduced lung metastases. TH-302 may therefore be an attractive therapeutic agent with strong activity as a single agent and in combination with chemotherapy against OS.
Keywords: Hypoxia, TH-302, osteosarcoma, metastasis, chemotherapy
1. Introduction
Osteosarcoma (OS) is the most frequent primary malignancy of the skeleton, developing in children and adolescent and accounts for 20% of all primary osseous neoplasms [3, 24]. Metastatic spread, preferentially to the lungs compared with other sites, is seen in 20% of presenting patients and is correlated with poor survival [20, 25]. Treatment of OS has undergone considerable changes over the past 20 years, with the efficacy of chemotherapy significantly improving long-term survival. Response to chemotherapy depends on the type and combination of drugs used, the doses given and the sensitivity/resistance of the tumour cells. However, despite recent advances, the development of drug resistance to chemotherapeutic agents remains a problem [5].
Bone lesions caused by OS are characterised on the basis of their radiologic appearance and present as either osteolytic, osteoblastic (osteosclerotic), or mixed [23]. Osteolysis is a common appearance associated with OS, even within predominantly osteoblastic lesions, and is mediated primarily by the osteoclasts and their bone resorbing activity [10, 28]. Factors released from the bone stimulate tumour growth and in turn tumour cells produce factors that stimulate osteoclast differentiation and activity, resulting in the establishment of a mutually beneficial relationship, often termed “the vicious cycle” due to its progressively bone destructive nature [6]. Conversely, tumour cells associated with osteoblastic lesions stimulate osteogenesis [9, 11].
Tumor Hypoxia in OS and in most solid tumours is a major cause of treatment failure and poor outcomes. Within most solid tumours, there are significant areas of hypoxia, which contain cancer cells that are resistant to chemotherapy and radiotherapy and this predisposes to tumour recurrence and metastasis. Low oxygen levels found in these tumor sub-regions are rarely observed in normal tissues. Therefore, tumour hypoxia offers treatment opportunities, exemplified by the development of highly active compounds that selectively target hypoxic regions within solid tumours and can serve as the basis for selective cancer therapy. This is exemplified by the development of highly active compounds that selectively target hypoxic regions within solid tumours [12].
TH-302 is a newly developed hypoxia-activated DNA cross-linking pro-drug that displays potent hypoxia-dependent cytotoxicity both in vitro and in preclinical cancer models [14, 16, 21]. TH-302 is currently being evaluated in phase I/II clinical trials for the treatment of solid tumours as a single agent and in combination with conventional therapies [7]. TH-302 is an inactive pro-drug created by the covalent conjugation of 2-nitroimidazole as an oxygen sensor to bromo-isophosphoramide (Br-IPM). While inactive under aerobic conditions, when TH-302 is delivered to hypoxic regions the imidazole sensor moiety undergoes irreversible reduction and the Br-IPM moiety, which is the basis of the DNA cross linking toxin in the pro-drug, is released. TH-302 overcomes some of the limitations of the earlier HAPs, including the ability to be activated in severe hypoxia unlikely to be present in non-pathologic tissues in the body. Importantly, due to the stability of the Br-IPM cytotoxin, the drug exerts “bystander” effects, killing not only hypoxic cells but diffusing into the surrounding normoxic zones, thus treating both the hypoxic and normoxic components of the tumour [26].
In this study we investigated the cytotoxic activity of TH-302 against human OS cells in vitro and evaluated its anticancer efficacy as a single agent, and in combination with the chemotherapeutic agent doxorubicin, using clinically relevant orthotopic mouse models of osteolytic and osteosclerotic OS and evaluating its effect on the development of subsequent pulmonary metastases.
2. Materials and Methods
2.1 Cells
The human OS cell lines, K-HOS, MG-63, SAOS, SJSA-1 BTK-143, were obtained from ATCC (Manassas, VA, USA) and were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM), supplemented with 2 mM glutamine, 100 IU/ml penicillin, 160 μg/ml gentamicin and 10% fetal bovine serum (Life Technologies, Carlsbad, CA, USA), in a 5% CO2-containing humidified atmosphere.
Normal human osteoblasts (NHB) were obtained from trabecular bone of osteoarthritic patients at joint replacement surgery or from needle aspirates from the iliac crest of normal healthy donors and grown in αMEM (SIGMA, Saint Louis, Missouri, USA) containing 10% fetal bovine serum (Life Technologies, Carlsbad, CA, USA) and L-ascorbic acid 2-phosphate (NovaChem, Victoria, Australia). Medium was replaced at 4 day intervals. Cells were then subcultured by treatment with a (0.1%) (w/v) mixture of collagenase and dispase. Cells from the first passage were used in all experiments. The use of all normal human donor-derived one tissue was approved by the human ethics committee of the Royal Adelaide Hospital/University of Adelaide (Approval No. RAH 090101).
2.2 Drugs
TH-302 was provided by Threshold Pharmaceuticals and dissolved in sterile saline at a concentration of 13.2mM. Doxorubicin (Dox) was purchased from Ebewe Pharma (A-4866 Unterach, Austria).
2.3 Retroviral infection of OS cells with the triple reporter gene construct SFG-NES-TGL
Luciferase expressing BTK-143 and K-HOS cells were generated using the retroviral expression vector SFG-NES-TGL, which gives rise to a single fusion protein encoding herpes simplex virus thymidine kinase (TK), green fluorescence protein (GFP) and firefly luciferase (Luc)[32, 33]. Virus particle-containing supernatants were generated and filtered to remove any cellular debris and then used to infect cells [31]. The retrovirally transduced cells were grown as bulk cultures for 48 h and subsequently sorted for positive GFP expression, using fluorescence-activated sorting (FACS) (Aria BD Biosciences). The cells were allowed to proliferate and the 10% of cells expressing GFP most strongly were obtained by FACS to generate the sub-lines K-HOS-TGL and BTK-143-TGL.
2.4 Cell Viability Assay
To determine the cytotoxic effects of TH-302 and Dox on cell growth, 1×104 cells per well were seeded in 96-well microtitre plates and allowed to adhere overnight. Cells were then treated with increasing concentrations of TH-302 (1–100μM) for 24 hours under normoxic and hypoxic (0, 0.5, 1 and 5% O2) conditions. Cell viability was determined by Crystal Violet staining and optical density was measured at 570 nm wavelength (OD570). Experiments were performed in triplicate and repeated at least 3 times. Results of representative experiments are presented as the mean +/− SD.
2.5 Apoptosis Analysis
2.5.1 DAPI staining of nuclei
Cells were seeded on plastic chamber slides and treated at 1% O2 with TH-302 at 50μM for 24 hours. After two washes with PBS, cells were fixed in ethanol:acetic acid (6:1) for 10 minutes, washed again with PBS, and incubated with 0.8 mg/ml of 4′, 6-diamidine-2′-phenylindole dihydrochloride (DAPI; Roche Diagnostics, Mannheim, Germany) in methanol for 15 min. After several washes in PBS, the coverslips were mounted on ProLong®Gold antifade (Life Technologies, Carlsbad, CA, USA). DAPI staining was visualised by fluorescence microscopy.
2.5.2 Measurement of DEVD-caspase activity
DEVD-caspase activity was assayed by cleavage of zDEVD-AFC (z-asp-glu-val-asp-7-amino-4-trifluoro-methyl-coumarin), a fluorogenic substrate based on the peptide sequence at the caspase-3 cleavage site of poly (ADP-ribose) polymerase (Kamiya Biomedical Company, Seattle, WA, USA). KHOS and BTK-143 cells were seeded at 1 × 104 per well in triplicate in 96-well plates and were treated with TH-302 in normoxic and hypoxic (1% O2) conditions at a concentration of 50μM for 24 hours. Cells were washed once in PBS and lysed in 50μL of NP-40 lysis buffer containing 1 mM Tris-HCl, 1 mM EDTA and 10% NP40, at pH 7.6, for 15–20 minutes on ice. Insoluble material was pelleted at 15,000g and an aliquot of the lysate analyzed for caspase-3 activity. For each 20μl of cell lysate, 8 μM of the fluorogenic substrate (zDEVD-AFC) was added in 1 mL of fluorometric protease buffer (50 mM HEPES, 10 % sucrose, 10 mM DTT and 1% CHAPS, pH 7.4) and incubated for 4h at RT in darkness. Fluorescence activity was quantified using a BMG LABTECH FLUOstar OPTIMA microplate reader at 400 nm excitation and 505 nm emission wavelengths. Results were expressed relative to the protein concentration of the sample, determined using a commercial BCA protein assay reagent from Thermo Fisher Scientific (Waltham, MA, USA). All experiments were conducted in triplicate and repeated at least 3 times. Representative experiments are presented as the mean +/− SD.
2.6 Western Blot analysis
K-HOS, BTK-143 and NHB cells were treated with TH-302 at 50μM in a time dependent manner (0, 6, 12, 24, 48 hours) under normoxic (21%O2) and hypoxic (1% O2) conditions and lysed in buffer containing 10 mM Tris HCl, pH 7.6, 150 mM NaCl, 1% Triton X-100, 0.1% sodium dodecyl sulphate (SDS), 2 mM sodium vanadate and a protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany) and stored at −70°C until analysis. The amount of protein in each sample was quantified using the BCA™ Protein Assay Reagent from Thermo Fisher Scientific (Waltham, MA, USA), according to the manufacturer’s instructions. Prior to loading, protein extracts were mixed with 4x Nupage LDS Sample Buffer, 1M DTT, and MQ in amount according to protein estimation. Protein samples were then heated at 70°C for 10 minutes and loaded into 4–12% polyacrylamide gels for electrophoresis under reducing conditions. Separated proteins were transferred to PVDF membranes via electrophoresis (GE Healthcare, Buckinghamshire, UK) and blocked in PBS containing 5% blocking reagent (GE Healthcare, Buckinghamshire, UK) for 1 hour at room temperature.
Immunodetection was performed overnight at 4°C in PBS/blocking reagent containing 0.1% Tween 20, using the following primary antibodies at the dilutions suggested by the manufacturer. mAb anti-caspase-8, pAb anti-caspase-9 and pAb anti-bid were purchased from Cell Signaling Technology (Beverly, MA, USA), pAb anti-cIAP1, pAb anti-cIAP2, pAb anti-XIAP from R&D systems, pAb anti-BAX and mAb anti-Bcl-2 were purchased from Santa Cruz Biotechnology (Texas, USA) and pAb anti-PARP from Roche Diagnostics (Mannheim, Germany). Anti-actin mAb (SIGMA, Saint Louis, Missouri, USA) was used as a loading control.
Membranes were then rinsed several times with PBS containing 0.1% Tween-20 and incubated with 1:5,000 dilution of anti-mouse, anti-goat or anti-rabbit alkaline phosphatase-conjugated secondary antibodies (Thermo Fisher Scientific, Waltham, MA, USA) for 1 hr. Visualization and quantification of protein bands was performed using the ECF substrate reagent kit (GE Healthcare, Buckinghamshire, UK) on a FluorImager (Molecular Dynamics Inc., Sunnyvale, CA, USA).
2.7 Animals
Female athymic nude mice at 4 weeks old (Institute of Medical and Veterinary Services Division, Gilles Plains, SA, Australia) were acclimatized to the animal housing facility for a minimum period of 1 week prior to the commencement of experimentation. The general physical well-being and weight of animals were monitored continuously throughout the experiments. All of the experimental procedures on animals were carried out with strict adherence to the rules and guidelines for the ethical use of animals in research and were approved by the Animal Ethics Committees of the University of Adelaide and the Institute of Medical and Veterinary Science, Adelaide, SA, Australia.
2.8 Intra-tibial injections of osteosarcoma cells
Four week old female Balb/c Nu/Nu mice were housed under pathogen free conditions, in accordance with the guidelines approved by the Institute of Medical and Veterinary Science animal research committee. The OS cell lines K-HOS-TGL and BTK-143-TGL were cultured as described above until they reached 70–80% confluency. Cells were removed from flasks with 2mM EDTA and resuspended in 1 × PBS at 1×105 cells/10μl and kept on ice in an eppendorf tube. The left tibia was wiped with 70% ethanol and a 27 gauge needle coupled to a Hamilton syringe was inserted through the tibial plateau with the knee flexed and 1×105 K-HOS-TGL or BTK-143-TGL cells resuspended in 10μl of PBS were injected in the marrow space. All animals were injected with PBS in the contralateral tibia as the control. Mice were assigned randomly into groups of 8 animals and drug dosing started 7 days after cancer cell transplantation. TH-302 was administered at 50mg/kg body weight via i.p injection once a day for 5 days followed by 2 days of rest, whereas Dox was administered at 4mg/kg i.v once weekly until the end of the experiment.
2.9 In vivo bioluminescent imaging (BLI)
Non-invasive, whole body imaging to monitor luciferase-expressing OS cell lines K-HOS-TGL and BTK-143-TGL in mice was performed weekly using the IVIS 100 Imaging system (Xenogen, Alameda, CA). Mice were injected i.p. with 100 μl of the D-Luciferin solution at final dose of 3mg/20g mouse body weight (Xenogen Alameda, CA) and then gas-anaesthetized with Isoflurane (Faulding Pharmaceuticals, Salisbury, SA, Australia). Images were acquired for 0.5–30 seconds (representative images are shown at 1 second) from the side angle and the photon emission transmitted from mice was captured and quantitated in photons/sec/cm2 using Xenogen Living image (Igor Pro version 2.5) software.
2.10 Micro-computed tomography ex vivo imaging analysis
Limbs for μCT analysis were surgically resected and scanned using the SkyScan-1174 high-resolution μCT Scanner (Skyscan, Belgium. The Scanner was operated at 80kV, 120 μA, rotation step 0.675, 0.5 mm Al filter and scan resolution of 5.2 μm/pixel. The cross sections were reconstructed using a cone-beam algorithm (software Cone rec, Skyscan). Using the 2D images obtained from the μCT scan, the growth plate was identified and 400 sections were selected starting from the growth plate/tibial interface and moving down the tibia. For quantification, 3D evaluation was performed on all data sets acquired by selecting total bone of the proximal tibia, to determine 3D bone morphometric parameters (software CTAn, Skyscan). The cross sections were reconstructed using a cone-beam algorithm (software Cone_rec, Skyscan). Files were then imported into CTAn software (Skyscan) for 3D analysis and 3D image generation. All images were viewed and edited using ANT visualisation software.
2.11 Data Analysis and Statistics
Experiments were performed in triplicate, and data presented as mean ± SE. All statistical analysis was performed using SigmaStat for Windows version 3.0 (Systat Software, Inc., Port Richmond, CA) using the unpaired Students’ T test. Measures of association between two variables were assessed using the Spearman Rank correlation coefficient. Comparisons between groups were assessed using a one way ANOVA test. In all cases, p < 0.05 was considered statistically significant.
3. Results
3.1 TH-302 exhibits potent hypoxia-selective cytotoxicity in human OS cell lines
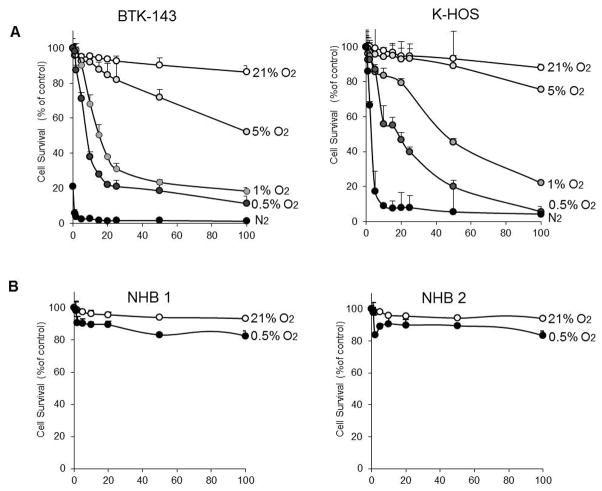
As a prelude to testing the anticancer efficacy of TH-302 in animal models of osteosarcoma, we first examined the cytotoxicity of TH-302 against BTK-143 and K-HOS human osteosarcoma cells in vitro under normoxic (air atmosphere) and hypoxic (N2 atmosphere) conditions. Under normoxic conditions (21% O2) TH-302 exhibited only minimal cytotoxicity, whereas the cytotoxicity of TH-302, measured as a ratio of IC50 values under varying conditions of hypoxia (0%, 0.5%, 1.0%, 5.0% oxygen), increased significantly and was greater than 200-fold during extreme hypoxia of <1.0% after 24 hours of continuous drug exposure (Fig 1A). Similar hypoxia selectivity in toxicity was also observed in three additional human osteosarcoma cell lines (SJSA-1, MG-63, and SAOS2) tested (Supplementary Fig 1). An important attribute of TH-302 is the apparent selectivity in toxicity for tumor cells over normal cells. We observed that primary normal human osteoblasts, cultured from patients undergoing hip replacement surgery, were refractory to TH-302 treatment even under extreme hypoxia of 0.5% O2 (Fig 1B), an effect that is likely related to the proliferative capacity of tumour cells when compared to normal cells.
Figure 1. Activity of TH-302 against OS cells in vitro.
A. OS Cell lines BTK-143 and K-HOS were seeded in 96 well plates at 1×104 cells per well and treated with increasing doses of TH-302 in normoxic (21% O2) under a range of hypoxic conditions from 0% to 5% O2, for 24 hours. B. Normal human bone cells (osteoblasts) from two donors, were seeded into 96 well plates and treated with increasing doses of TH-302 under normoxic (21% O2) and hypoxic conditions (0.5%), as indicated. Cell viability was assessed by crystal violet staining 24 hours after treatment. Data points show means of quadruplicate results from a representative experiment, repeated at least twice. Data are presented as the mean ±SD of quadruplicate wells, and are expressed as a percentage of the number of control cells.
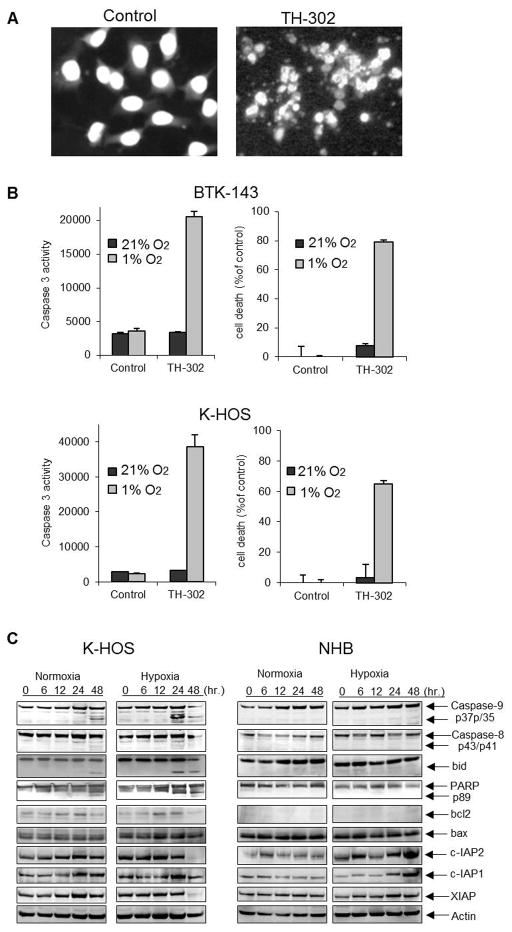
3.2 TH-302 induces apoptosis of OS cells
TH-302-mediated cytotoxicity in both OS cell lines under hypoxic conditions was associated with morphological changes characteristic of apoptosis, including chromatin condensation, nuclear fragmentation as assessed by DAPI staining of nuclei (Fig 2A) and a concomitant activation of the effector caspase 3 assessed by the fluorometric caspase 3 assay (Fig 2B). We next used the K-HOS cell line as a model system to dissect further the apoptotic signaling mediated by TH-302. We assessed some of the molecular determinants involved in both the extrinsic and intrinsic apoptotic signaling pathways and compared the results with those seen in normal primary human osteoblast for which no cytotoxicity was observed even under hypoxic conditions. In addition to the observed activation of caspase-3, TH-302 treatment resulted in the robust activation of caspase-9, which was clearly evident as early as 24 hours post treatment of K-HOS cells under hypoxic conditions (Fig 2C). Activation of the initiator caspase-8 was not observed under these conditions, whereas the mitochondrial anti-apoptotic Bcl-2 family protein BID was cleaved and this was concomitant with processing of poly ADP-ribose polymerase (PARP) protein. The levels of Bcl-2, cIAP1, cIAP2 and XIAP under hypoxic conditions were significantly reduced with TH-302 treatment but this occurred only at the later time point of 48hours indicating that these changes were an effect rather than a cause of TH-302 –induced apoptosis. The levels of BAX remained unchanged (Fig 2C). TH-302 mediated downregulation of cIAP1 and cIAP2 was not restricted to K-HOS cells but was also observed when BTK-143 cells were treated with TH-302 under the same conditions (Supplementary Fig 2). In contrast, TH-302 treatment of normal human bone cells was without effect on the caspase cascade. On the contrary, the levels of cIAP1, cIAP2 and XIAP proteins were significantly up-regulated in normal human bone cells with TH-302 treatment (Fig 2C), suggesting a compensatory and protective response of normal cells to TH-302 death-inducing signals.
Figure 2. TH-302 induced apoptosis of OS cells.
A. DAPI nuclear fluorescence stain of untreated K-HOS cells showing nuclei homogenously stained. Cells treated with 50μM TH-302 under hypoxic conditions for 24 hours exhibit changes in the nuclei consistent with the induction of apoptosis. B. BTK-143 and K-HOS cells were treated with 50μM TH-302 for 24 hours under normoxic (21% O2) and hypoxic (1% O2) conditions and lysates were obtained to determine caspase activity using the caspase-3-specific Fluorogenic substrate, zDEVD-AFC. The increase in caspase 3 activity is correlated with an increase in cell death by TH-302 under hypoxia. C. K-HOS and NHB cells were seeded at 2 × 106 per T25 flask and were treated with TH-302 at 50μM under normoxic and hypoxic (1% O2) conditions. Protein lysates were collected at 0, 6, 12, 24 and 48 hours after treatment and immunoblotted with various Ab, as shown.
3.3 TH-302 inhibits tumour growth and protects the bone from osteosarcoma-induced bone destruction
To evaluate the efficacy of TH-302 against OS growth in bone, its effects on cancer-induced bone destruction and on the subsequent lung metastases, luciferase tagged BTK-143 and K-HOS OS cells were injected directly into the tibial marrow cavity of athymic nude mice. These cells were chosen because when transplanted intratibially in mice they replicate the bone cancer lesions commonly seen in OS patients [18]. BTK-143 cells give rise to predominantly osteolytic lesions and when compared to the contralateral non-tumour injected tibia, exhibit a net loss of bone volume (BV). In contrast, K-HOS OS cells when transplanted intratibially give rise to mixed osteolytic/osteosclerotic lesions and while they are destructive in nature because of osteoclastic bone resorption, they also exhibit a characteristic but extensive spicular new bone formation extending from the periosteum resulting in an overall net gain of BV when compared to the contralateral non-tumour bearing tibiae [18]. Importantly, as in the human disease, both cell lines develop lung metastases three to four weeks after cancer cell transplantation, allowing assessment of the anti-metastatic activity of TH-302.
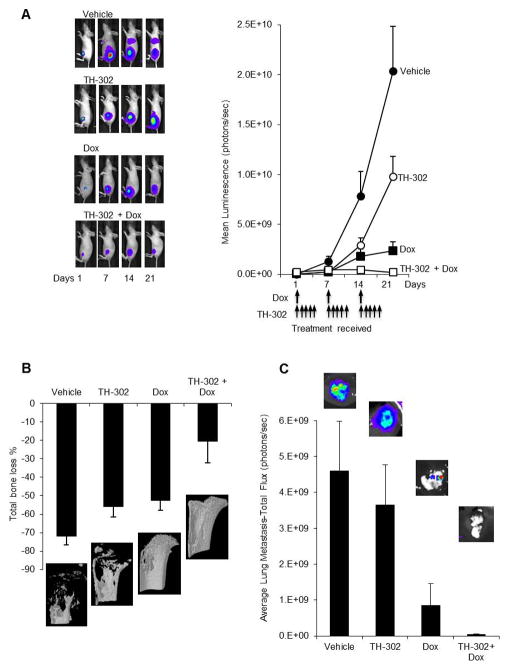
In the first animal model, the highly osteolytic BTK-143 cells expressing luciferase were injected directly into the tibial marrow cavity. Treatment with TH-302 was initiated 7 days post cancer cell transplantation after confirming that they established growth in bone. All vehicle treated animals showed an exponential increase of mean photon emission associated with an increase in tumour burden, which was clearly evident from day 7 onwards. By day 21, all vehicle treated mice were humanely killed because they developed large intratibial tumours that penetrated the cortical bone and the tumour mass extended into the surrounding soft tissue (Fig 3A). In contrast, when mice were treated with TH-302 as a single agent, tumour growth was significantly delayed and tumour burden measured as a function of bioluminescence signal in bone was significantly reduced. Treatment with Dox as a single agent also resulted in a significant inhibition in tumour growth. Combination therapy with TH-302 and Dox was far superior in inhibiting tumour growth when compared to that seen with each agent alone. In fact, combination therapy completely prevented BTK-143 tumour progression in the bone marrow (Fig 3A).
Figure 3. TH-302 co-operates with Dox for increased anticancer efficacy against the highly osteolytic BTK-143 tumours.
A. BTK-143-TGL cells were injected directly into the tibial marrow cavity of 4 week female athymic mice (1×105/10μl injection), as described in the methods, and mice were imaged weekly using the Xenogen IVIS 100 bioluminescence imaging system. Representative whole body bioluminescent images (BLI) of a single mouse from each group during the course of the experiment are shown. The line graph represents average tumor signal over time, measured as photon counts per second. All vehicle-treated animals were humanely killed on day 21 due to high tumour load. TH-302 and Dox as single agents reduced tumour growth and in addition, the combination of TH-302 and Dox resulted in an even greater inhibition of tumour growth. B. Quantitative assessment of Total bone loss (%) comparing the tumour bearing tibiae of each group to the contralateral tibiae. The qualitative 3-D micro CT images showing the osteolytic nature of the BTK-143-TGL cell line, which was greatly reduced by TH-302 and Dox as single agents and by the combination of both agents. C. Average lung tumor growth was assessed via bioluminescence showing Dox and the combination of both agents caused a reduction in lung tumor growth of the BTK-143-TGL cell line. TH-302 did not significantly affect tumour burden in the lungs when compared to the vehicle group.
At the end of the experiment, the tibiae of all mice were dissected and assessed, using high resolution μCT for both quantitative and qualitative analysis (Fig 3B). Extensive osteolysis was clearly evident in the vehicle treated animals such that the net loss in bone volume (BV) was 71% in the left tumour-bearing tibiae when compared to the contralateral non-tumour bearing tibiae of the same animals (Fig 3B). The extent of osteolysis was significantly reduced following treatment with TH-302 as a single agent, such that the net loss of BV decreased from 71% to 56%. Treatment with Dox alone was also effective, decreasing bone loss to 52%. Combination therapy with TH-302 and Dox demonstrated additional protection of the bone architecture such that the net loss of BV was only 20%. The inhibition of tumour growth observed in the bone marrow of animals treated with TH-302 alone or Dox alone translated to a 31% (4 of 9) and 25% (4 of 8) reduction in the number of mice that subsequently developed lung metastases respectively, as assessed by ex vivo bioluminescence imaging. However, the number of animals that developed lung metastases in the combination treatment group was not reduced further. While there was a reduction in the number of animals with lung metastases in the TH-302 alone treated group the tumour burden measured as a function of bioluminescence signal in the lungs of the remaining mice with metastases was not significantly different from that of the vehicle treated animals, indicating that cancer cells that escaped from the bone marrow and lodged in the lungs were refractory to TH-302 treatment in this highly oxygenated environment. In contrast, Dox maintained cytotoxicity in the lungs, leading to a significant reduction in tumour burden in the Dox alone group and also in the combination treatment group (Fig 3C).
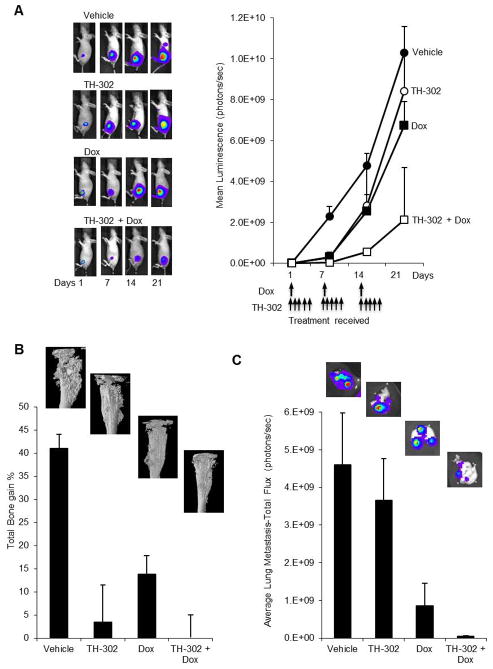
In the second OS model where K-HOS cells were injected intratibially, treatment with TH-302 as a single agent resulted in a significant delay in tumour growth within the first 7 to 14 days (Fig 4A). However tumour growth persisted exponentially thereafter so that tumour burden was not statistically different from vehicle treated tumours by day 21. Treatment with Dox alone followed the same pattern seen with TH-302 alone. In contrast, combination therapy with TH-302 and Dox resulted in a longer delay in tumour growth, which translated to a significant reduction in the overall tumour burden within the bone marrow at each time, when compared to that seen with each agent alone (Fig 4A). High resolution micro CT analysis of tibiae from the vehicle treated animals demonstrated extensive bone remodeling associated with cortical and trabecular bone destruction, representing the osteolytic component of K-HOS lesions. In addition, extensive areas of spicular new bone formation extending from the cortical surface representing the osteosclerotic component of these lesions were also present in the tumour bearing tibiae (Fig 4B).
Figure 4. TH-302 co-operates with Dox for increased anticancer efficacy against the mixed osteolytic/osteosclerotic K-HOS tumours.
A. Tibial tumours were allowed to establish for 7 days and mice were randomized into 4 groups, which received PBS (vehicle), TH-302, Dox or the combination of both TH-302 and Dox as described in the methods. Shown are representative whole body bioluminescent images, with the graph representing the average tumour signal over time, measured as mean photon counts per second. B. Representative CT images of the tumor affected left tibia showing the osteosclerotic bone remodeling caused by K-HOS-TGL and the protection of the tibia with TH-302 and Dox as single agents and in combination. In addition, quantitative assessment of Total bone volume (%) comparing the left untreated tibia to the cancer affected right tibia, exhibited a reduction in osteosclerotic bone destruction in the TH-302, Dox and combination groups. C. The intensity of the lung metastases were quantified by BLI after lungs were removed. TH-302 had no significant effect on tumour burden when compared to the vehicle group. However Dox and the combination of both TH-302 and Dox significantly reduced metastatic lung tumour burden Data shown in each case are the average BLI from all animals in that group: points are means ±SEM.
The bone forming activity in the K-HOS cells was greater than the osteolytic activity since overall there was a net gain in BV of 41% in the left tumour-bearing tibiae when compared to the contralateral non-tumour bearing tibiae of the same animals (Fig 4B). Comparison of the relative BV of tumour bearing tibiae and contralateral tibiae after TH-302 treatment or Dox treatment showed that the OS induced increase in BV was reduced from 41% to 3.5% and 13.9% respectively (Fig 4B). In contrast, combination therapy with TH-302 and Dox showed significant preservation of the tibiae, with no difference in BV measurements between the tumour and non-tumour bearing tibiae. In this K-HOS OS model, 100% of the animals in the vehicle treated group developed lung metastases four weeks post cancer cell transplantation. In contrast, the number of animals with lung metastases in the TH-302 alone, Dox alone and in the combination group decreased by 50% (4 of 8), 25% (6 of 8) and 50% (4 of 8) respectively. Similarly, to what had been observed with BTK-143 tumours, metastatic tumour burden in the lungs of the remaining 50% of mice treated with TH-302 alone was no different when compared to the vehicle treated animals. In contrast lung tumour burden in the Dox alone treated group and in the combination group was significantly reduced (Fig 4C).
3.4 Effect of TH-302 on normal bone metabolism
While prior clinical studies in patients with solid tumours have shown hematological toxicity with TH-302 therapy to be minimal [8, 29], there is no data on the effects of TH-302, or indeed any other HAP, on normal bone metabolism. The intratibial injection model described in this study provided an opportunity to evaluate the consequence of TH-302 treatment on normal bone parameters. After three weeks of therapy, comparisons of the contralateral non-tumour bearing tibiae of TH-302-treated and untreated animals using high resolution micro-CT analysis demonstrated no significant changes in any of the micro architectural bone morphometric parameters, including total bone volume (BV), bone surface (BS), trabecular number (Tb.N), thickness (Tb.Th) or spacing Tb.Sp) (Table 1).
Table 1. Bone morphometric parameters of contralateral non–tumor-injected tibiae of vehicle and TH-302 treated animals.
Trabecular bone volume (BV), bone surface (B.S.), intersection surface (i.S.), trabecular space (Tb.Sp.), trabecular number (Tb.N.), trabecular thickness (Tb.Th.), trabecular pattern factor (Tb.Pf.) and structure model index (SMI) were measured by three dimensional analysis of μCT images of trabecular bone only. Results are expressed as mean ± SE. Significance of results is with respect to untreated animals obtained using Student’s t test.
| Parameters | BTK-143 | K-HOS | ||||||
|---|---|---|---|---|---|---|---|---|
| Vehicle control | TH-302 | Vehicle control | TH-302 | |||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| BV(mm3) | 2.87 | 0.10 | 2.82 | 0.07 | 2.87 | 0.10 | 2.82 | 0.07 |
| B.S. (mm2) | 76.21 | 4.07 | 74.46 | 4.89 | 76.21 | 4.07 | 74.46 | 4.89 |
| i.S. (mm2) | 2.16 | 0.66 | 2.58 | 0.66 | 2.16 | 0.66 | 2.58 | 0.66 |
| Tb.Sp. (mm) | 26.63 | 1.66 | 26.24 | 1.53 | 0.39 | 0.02 | 0.49 | 0.02 |
| Tb.N. (1/mm) | 2.08 | 0.14 | 1.72 | 0.17 | 2.08 | 0.14 | 1.72 | 0.17 |
| Tb.Th. (mm) | 0.06 | 0.00 | 0.06 | 0.00 | 0.06 | 0.00 | 0.06 | 0.00 |
| Tb.Pf. (1/mm) | 15.35 | 2.93 | 13.57 | 3.16 | 0.22 | 1.18 | 0.21 | 2.32 |
| SMI | 2.02 | 0.08 | 2.09 | 0.07 | 2.02 | 0.08 | 2.09 | 0.07 |
4. Discussion
Osteosarcoma (OS) is the most common primary malignant tumour of bone in children and adolescents and accounts for 20% of all primary osseous neoplasms. It is a highly aggressive neoplasm, typically composed of spindle cells producing osteoid. The outcome for patients with OS is poor without the use of effective chemotherapy, with 2-year overall survival rates of 15%–20% following surgical resection and/or radiotherapy. Although OS can occur in any bone, it is most common in the metaphysis of long bones. The most common primary sites are the proximal humerus, distal femur, and proximal tibia, with approximately 50% of cases originating around the knee area. In addition to surgical intervention, the choice of treatment for OS is chemotherapy and multiple anticancer drugs such as Dox, cisplatin, etoposide and cyclophosphamide, are commonly used, either alone, or in combination. However, despite significant improvements in treatment of the primary tumour and consequent patient survival, a significant proportion of these patients eventually develop pulmonary metastases and succumb to their disease even after conventional multi-stage chemotherapy and surgical excision. Therefore, there is a need to develop new and safe approaches to the treatment of OS [1, 17, 30].
In considering the use of HAPs in OS therapy, it is important to note that the bone marrow, and particularly the haematopoietic niche proximal to the endosteal surface, is hypoxic and this is a prerequisite for normal haemopoiesis. In humans, the average oxygen tension (pO2) in the bone marrow is between 1–7% (cf atmospheric oxygen=21%)[4]. Cancer cells normally home to hypoxic endosteal niches where the oxygen tension is estimated to be <1.3%. Unlike soft tissue tumours, cancer cells in bone are adapted to survive and grow in a microenvironment, which is already hypoxic [2]. Therefore tumour hypoxia is a major contributor to the incurability of bone cancer. The strategy of hypoxia-targeted treatment was recently tested in two separate mouse models of multiple myeloma [13, 15]. In these studies, TH-302 treatment as monotherapy, and in combination with bortezomib, demonstrated efficacy against multiple myeloma (MM) both in vitro and in vivo and provided supporting evidence that targeting the hypoxic bone microenvironment niche is a useful and novel strategy for the treatment of cancer in bone.
Consistent with previously published data for other cancer cell types, TH-302 resulted in a dose-dependent increase in cytotoxicity of OS cells selectively under hypoxic conditions, whereas primary normal human osteoblasts under the same conditions were relatively resistant to treatment, highlighting not only the hypoxic selectivity but also the tumour selectivity of the drug, which is likely related to the proliferative capacity of tumour cells when compared to normal cells. Mechanistically, we found that TH-302-mediated cytotoxicity was associated with caspase activation involving mitochondrial events since apoptosis induction by TH-302 was associated with caspase-9 activation and cleavage of the Bcl-2 family protein Bid. In addition, we found that the protein levels of anti-apoptotic Bcl-2, and inhibitor of apoptosis proteins, cIAP1, cIAP2 and XIAP were significantly decreased by TH-302 treatment under hypoxic conditions. However, these changes occurred 48 hours post TH-302 treatment, well after apoptosis induction and therefore likely constitute events of the cell death process rather than being causatively involved in TH-302-induced apoptosis.
IAP proteins represent one line of defence against apoptosis induction of cancer cells by inhibiting caspase activity, which is essential for tumour survival and the maintenance of therapeutic resistance. IAP proteins are expressed at elevated levels in many tumour types, including osteosarcoma, and as such makes IAP proteins attractive targets for anticancer therapy [19, 22, 27]. In contrast, the levels of IAPs were strongly upregulated in NHB donors treated with TH-302 under the same hypoxic condition, indicating the activation of a compensatory mechanism where normal cells may be protected from TH-302 death-inducing signals.
OS have a variable bone-forming ability, but are destructive by virtue of their ability to expand in bone by inducing osteoclast-mediated bone resorption. The K-HOS and BTK-143 cell lines used in these studies were chosen on the basis that when injected intratibially in mice they replicate well the bone cancer lesions commonly seen in OS patients. K-HOS OS cells give rise to mixed osteolytic/osteosclerotic lesions but are destructive by virtue of their ability to induce osteoclast-mediated bone resorption, as we have shown previously [18]. In contrast, BTK-143 cells give rise to predominantly osteolytic lesions. Importantly, as in the human disease, both cell lines develop lung metastases three to four weeks post cancer cell transplantation. A limitation in measuring tumour burden in bone is that it is not possible to accurately assess the progression of tumour growth by palpation, as in soft tissue tumours, before they break through the cortical bone. However, non-invasive bioluminescence imaging used in these studies provided sensitive real time in vivo qualitative and quantitative tracking of OS growth in bone and the subsequent metastasis to the lung. OS induced bone destruction and the consequence of drug treatment on bone architecture was assessed using high resolution micro-CT analysis.
Our results show that TH-302 monotherapy delayed tumour growth in bone and cooperated with Dox for increased anticancer efficacy. This effect was concomitant with significant protection from OS induced bone destruction and was observed in two distinct orthotopic mouse models of human OS giving rise to different bone lesions. TH-302 monotherapy reduced the incidence of lung metastases by 40%–50% in both models. However, the tumour burden within the lungs of the remaining animals with metastases were no different to those seen in the vehicle treated groups, suggesting that OS cells that escaped from the bone marrow and lodged into the lungs were refractory to TH-302 treatment in this highly oxygenated environment, highlighting the hypoxia selectivity of this drug. In contrast, Dox maintained its tumour suppressive activity in the lung.
Overall, the results presented in this study, together with the clinical activity observed in early phase I and II clinical trials of TH-302, and the lack of toxicity to normal bone metabolism, suggest that patients diagnosed with primary OS may benefit from TH-302 therapy when used either alone or in combination with conventional chemotherapy.
Supplementary Material
Activity of TH-302 against OS cells in vitro: A. OS Cell lines MG-63, SAOS and SJSA-1 were seeded in 96 well plates at 1×104 cells per well and treated with TH-302 in normoxia (21% O2) and of hypoxia (1% O2) for 24 hours. TH-302 exhibited dose dependent cytotoxicity in hypoxic conditions to all 3 OS cell lines. Data points represent means of quadruplicate results from a representative experiment, repeated at least twice. Data are presented as the mean ±SD of quadruplicate wells, and are expressed as a percentage of the number of control cells.
TH-302 downregulates c-IAP1 and c-IAP2 in the OS BTK-143
BTK-143 cells were seeded and treated in a similar manner to the K-HOS and NHB cells. Protein lysates were then collected and immunoblotted with Abs against c-IAP1, c-IAP2, XIAP and Actin, as shown.
Highlights.
TH-302 is the most advanced and promising hypoxia activated pro-drug (HAP) in clinical development with demonstrated anticancer efficacy against a variety of solid and haematological malignancies.
Osteosarcoma (OS) is the most frequent primary malignancy of bone, effecting children and adolescent. OS initiates in the bone marrow microenvironment which is intrinsically hypoxic thus making OS highly susceptible to therapeutic intervention with HAPs.
In vitro, TH-302 induces hypoxia-dependent cytotoxicity to a variety human OS cell lines but exerts limited or no toxicity to normal human osteoblast under the same conditions.
Using two-independent orthotopic mouse models of osteosarcoma that recapitulate the OS lesions commonly seen in human patients namely osteolytic and/or osteosclerotic we have shown for the first time that TH-302 administration as a single agent reduces tumour burden in bone, and cooperates with doxorubicin to protect the bone from OS-induced bone destruction while also limiting pulmonary metastases.
Importantly, we have shown for the first time that TH-302 treatment is without effect on normal bone metabolism as assessed by high resolution micro CT.
Our data support the clinical development of TH-302 as monotherapy and in combination with chemotherapy in patients with osteosarcoma
Acknowledgments
“This work was supported by grants from the National Health and Medical Research Council (NHMRC) of Australia ID# 627015, the Hospital Research Foundation, Adelaide, South Australia, the National Breast Cancer Foundation and the Australian Breast Cancer Research. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript”. We wish to thank Adelaide microscopy Adelaide Microscopy at the University of Adelaide for technical assistance in bioluminescence imaging and Micro-CT.
Footnotes
Conflict of interest statement
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Botter SM, Neri D, Fuchs B. Recent advances in osteosarcoma. Current opinion in pharmacology. 2014;16:15–23. doi: 10.1016/j.coph.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Brahimi-Horn MC, Chiche J, Pouyssegur J. Hypoxia and cancer. Journal of Molecular Medicine-Jmm. 2007;85:1301–1307. doi: 10.1007/s00109-007-0281-3. [DOI] [PubMed] [Google Scholar]
- 3.Campanacci M. Bone and Soft Tissue Tumors. 2. Piccin Nuova Libraria; Padova: 1999. [Google Scholar]
- 4.Carreau A, El Hafny-Rahbi B, Matejuk A, Grillon C, Kieda C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J Cell Mol Med. 2011;15:1239–1253. doi: 10.1111/j.1582-4934.2011.01258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan HS, Grogan TM, Haddad G, DeBoer G, Ling V. P-glycoprotein expression: critical determinant in the response to osteosarcoma chemotherapy. J Natl Cancer Inst. 1997;89:1706–1715. doi: 10.1093/jnci/89.22.1706. [DOI] [PubMed] [Google Scholar]
- 6.Chirgwin JM, Guise TA. Molecular mechanisms of tumor-bone interactions in osteolytic metastases. Crit Rev Eukaryot Gene Expr. 2000;10:159–178. [PubMed] [Google Scholar]
- 7.Duan J, Jiao H, Kaizerman J, Stanton T, Evans J, Lan L, Lorente G, Banica M, Jung D, Wang J, Ma H, Li X, Yang Z, Hoffman R, Ammons W, Hart C, Matteucci M. Potent and highly selective hypoxia-activated achiral phosphoramidate mustards as anticancer drugs. J Med Chem. 2008;51:2412–2420. doi: 10.1021/jm701028q. [DOI] [PubMed] [Google Scholar]
- 8.Ganjoo KN, Cranmer LD, Butrynski JE, Rushing D, Adkins D, Okuno SH, Lorente G, Kroll S, Langmuir VK, Chawla SP. A Phase I Study of the Safety and Pharmacokinetics of the Hypoxia-Activated Prodrug TH-302 in Combination with Doxorubicin in Patients with Advanced Soft Tissue Sarcoma. Oncology. 2011;80:50–56. doi: 10.1159/000327739. [DOI] [PubMed] [Google Scholar]
- 9.Goltzman D. Mechanisms of the development of osteoblastic metastases. Cancer. 1997;80:1581–1587. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1581::aid-cncr8>3.3.co;2-8. [DOI] [PubMed] [Google Scholar]
- 10.Goltzman D. Osteolysis and cancer. J Clin Invest. 2001;107:1219–1220. doi: 10.1172/JCI13073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goltzman D, Karaplis AC, Kremer R, Rabbani SA. Molecular basis of the spectrum of skeletal complications of neoplasia. Cancer. 2000;88:2903–2908. doi: 10.1002/1097-0142(20000615)88:12+<2903::aid-cncr4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 12.Guise CP, Mowday AM, Ashoorzadeh A, Yuan R, Lin WH, Wu DH, Smaill JB, Patterson AV, Ding K. Bioreductive prodrugs as cancer therapeutics: targeting tumor hypoxia. Chinese journal of cancer. 2013 doi: 10.5732/cjc.012.10285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu J, Handisides DR, Van Valckenborgh E, De Raeve H, Menu E, Vande Broek I, Liu Q, Sun JD, Van Camp B, Hart CP, Vanderkerken K. Targeting the multiple myeloma hypoxic niche with TH-302, a hypoxia-activated prodrug. Blood. 2010;116:1524–1527. doi: 10.1182/blood-2010-02-269126. [DOI] [PubMed] [Google Scholar]
- 14.Hu J, Van Valckenborgh E, Xu D, Menu E, De Raeve H, De Bryune E, Xu S, Van Camp B, Handisides D, Hart CP, Vanderkerken K. Synergistic induction of apoptosis in multiple myeloma cells by bortezomib and hypoxia-activated prodrug TH-302, in vivo and in vitro. Molecular cancer therapeutics. 2013;12:1763–1773. doi: 10.1158/1535-7163.MCT-13-0123. [DOI] [PubMed] [Google Scholar]
- 15.Hu J, Van Valckenborgh E, Xu D, Menu E, De Raeve H, De Bryune E, Xu S, Van Camp B, Handisides D, Hart CP, Vanderkerken K. Synergistic Induction of Apoptosis in Multiple Myeloma Cells by Bortezomib and Hypoxia-Activated Prodrug TH-302, In Vivo and In Vitro. Molecular cancer therapeutics. 2013;12:1763–1773. doi: 10.1158/1535-7163.MCT-13-0123. [DOI] [PubMed] [Google Scholar]
- 16.Jessica QL, Sun D, Wang Jingli, Ahluwalia Dharmendra, Ferraro Damien, Wang Yan, WSA, Duan Jian-Xin, Curd John G, Matteucci Mark D, Hart Charles P. Selective Tumor Hypoxia Targeting by Hypoxia-Activated Prodrug TH-302 Inhibits Tumor Growth in Preclinical Models of Cancer. Clin Cancer Res. 2012;18:758–770. doi: 10.1158/1078-0432.CCR-11-1980. [DOI] [PubMed] [Google Scholar]
- 17.Jin-Peng He YH, Wang Xiao-Lin, Yang Xiao-Jin, Shao Jing-Fan, Feng J-XF, Guo Jin. Review of the Molecular Pathogenesis of Osteosarcoma. Asian Pacific Journal of Cancer Prevention. 2014;15:5967–5976. doi: 10.7314/apjcp.2014.15.15.5967. [DOI] [PubMed] [Google Scholar]
- 18.Labrinidis A, Hay S, Liapis V, Findlay DM, Evdokiou A. Zoledronic acid inhibits both the osteolytic and osteoblastic components of osteosarcoma lesions in a mouse model. Clinical & Experimental Metastasis. 2009;26:872–873. doi: 10.1158/1078-0432.CCR-08-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LaCasse EC, Mahoney DJ, Cheung HH, Plenchette S, Baird S, Korneluk RG. IAP-targeted therapies for cancer. Oncogene. 2008;27:6252–6275. doi: 10.1038/onc.2008.302. [DOI] [PubMed] [Google Scholar]
- 20.Link MP, Goorin AM, Horowitz M, Meyer WH, Belasco J, Baker A, Ayala A, Shuster J. Adjuvant chemotherapy of high-grade osteosarcoma of the extremity. Updated results of the Multi-Institutional Osteosarcoma Study. Clin Orthop Relat Res. 1991:8–14. [PubMed] [Google Scholar]
- 21.Liu Q, Sun JD, Wang J, Ahluwalia D, Baker AF, Cranmer LD, Ferraro D, Wang Y, Duan JX, Ammons WS, Curd JG, Matteucci MD, Hart CP. TH-302, a hypoxia-activated prodrug with broad in vivo preclinical combination therapy efficacy: optimization of dosing regimens and schedules. Cancer chemotherapy and pharmacology. 2012;69:1487–1498. doi: 10.1007/s00280-012-1852-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mirandola P, Sponzilli I, Gobbi G, Marmiroli S, Rinaldi L, Binazzi R, Piccari GG, Ramazzotti G, Gaboardi GC, Cocco L, Vitale M. Anticancer agents sensitize osteosarcoma cells to TNF-related apoptosis-inducing ligand downmodulating IAP family proteins. International Journal of Oncology. 2006;28:127–133. [PubMed] [Google Scholar]
- 23.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 24.Pringle JAS. Bone-forming neoplasms arising within bone. In: Helliwell TR, editor. Pathology of bone and joint neoplasms. Saunders; Philadelphia: 1999. [Google Scholar]
- 25.Saeter G, Alvegard TA, Elommaa I, Wiebe T, Bjork O, Strander H, Solheim OP. Chemotherapy for osteosarcoma and Ewings sarcoma. Acta Orthop Scand Suppl. 1997;273:120–125. doi: 10.1080/17453674.1997.11744716. [DOI] [PubMed] [Google Scholar]
- 26.Saggar JK, Tannock IF. Activity of the hypoxia- activated pro- drug TH-302 in hypoxic and perivascular regions of solid tumors and its potential to enhance therapeutic effects of chemotherapy. International Journal of Cancer. 2014;134:2726–2734. doi: 10.1002/ijc.28595. [DOI] [PubMed] [Google Scholar]
- 27.Tamm I, Kornblau SM, Segall H, Krajewski S, Welsh K, Kitada S, Scudiero DA, Tudor G, Qui YH, Monks A, Andreeff M, Reed JC. Expression and prognostic significance of IAP-family genes in human cancers and myeloid leukemias. Clinical Cancer Research. 2000;6:1796–1803. [PubMed] [Google Scholar]
- 28.Taube T, Elomaa I, Blomqvist C, Beneton MN, Kanis JA. Histomorphometric evidence for osteoclast-mediated bone resorption in metastatic breast cancer. Bone. 1994;15:161–166. doi: 10.1016/8756-3282(94)90703-x. [DOI] [PubMed] [Google Scholar]
- 29.Weiss GJ, Infante JR, Chiorean EG, Borad MJ, Bendell JC, Molina JR, Tibes R, Ramanathan RK, Lewandowski K, Jones SF, Lacouture ME, Langmuir VK, Lee H, Kroll S, Burris HA., 3rd Phase 1 study of the safety, tolerability, and pharmacokinetics of TH-302, a hypoxia-activated prodrug, in patients with advanced solid malignancies. Clinical cancer research: an official journal of the American Association for Cancer Research. 2011;17:2997–3004. doi: 10.1158/1078-0432.CCR-10-3425. [DOI] [PubMed] [Google Scholar]
- 30.Yang J, Zhang W. New molecular insights into osteosarcoma targeted therapy. Current Opinion in Oncology. 2013;25:398–406. doi: 10.1097/CCO.0b013e3283622c1b. [DOI] [PubMed] [Google Scholar]
- 31.Zannettino AC, Rayner JR, Ashman LK, Gonda TJ, Simmons PJ. A powerful new technique for isolating genes encoding cell surface antigens using retroviral expression cloning. J Immunol. 1996;156:611–620. [PubMed] [Google Scholar]
- 32.Zinonos I, Labrinidis A, Lee M, Liapis V, Hay S, Ponomarev V, Diamond P, Findlay DM, Zannettino ACW, Evdokiou A. Anticancer Efficacy of Apo2L/TRAIL Is Retained in the Presence of High and Biologically Active Concentrations of Osteoprotegerin In Vivo. Journal of Bone and Mineral Research. 2011;26:630–643. doi: 10.1002/jbmr.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zinonos I, Labrinidis A, Lee M, Liapis V, Hay S, Ponomarev V, Diamond P, Zannettino ACW, Findlay DM, Evdokiou A. Apomab, a fully human agonistic antibody to DR5, exhibits potent antitumor activity against primary and metastatic breast cancer. Molecular cancer therapeutics. 2009;8:2969–2980. doi: 10.1158/1535-7163.MCT-09-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Activity of TH-302 against OS cells in vitro: A. OS Cell lines MG-63, SAOS and SJSA-1 were seeded in 96 well plates at 1×104 cells per well and treated with TH-302 in normoxia (21% O2) and of hypoxia (1% O2) for 24 hours. TH-302 exhibited dose dependent cytotoxicity in hypoxic conditions to all 3 OS cell lines. Data points represent means of quadruplicate results from a representative experiment, repeated at least twice. Data are presented as the mean ±SD of quadruplicate wells, and are expressed as a percentage of the number of control cells.
TH-302 downregulates c-IAP1 and c-IAP2 in the OS BTK-143
BTK-143 cells were seeded and treated in a similar manner to the K-HOS and NHB cells. Protein lysates were then collected and immunoblotted with Abs against c-IAP1, c-IAP2, XIAP and Actin, as shown.