Abstract
Current mixer and applicator devices on the market are not able to properly and efficiently mix two-component surgical adhesives in small volumes necessary to achieve economic viability. Furthermore, in these devices a significant amount of adhesive is wasted during the application process, as material within the dead space of the mixing chamber must be discarded. We have designed and demonstrated a new active mixer and applicator system capable of rapidly and efficiently mixing two components of an adhesive and applying it to the surgical site. Recently, Messersmith et al. have developed a tissue adhesive inspired by the mussel byssus and have shown that it is effective as a surgical sealant, and is especially suited for wet environments such as in fetal surgery. Like some other tissue sealants, this one requires that two components of differing viscosities be thoroughly mixed within a specified and short time period. Through a combination of compression and shear testing, we demonstrated that our device could effectively mix the adhesive developed by Messersmith et al. and improve its shear strength to significantly higher values than what has been reported for vortex mixing. Overall, our mixer and applicator system not only has potential applications in mixing and applying various adhesives in multiple surgical fields but also makes this particular adhesive viable for clinical use.
1. Background
Surgical adhesives and sealants have become important tools in a number of surgical procedures [1]. Most approved aqueous medical adhesives have two components which need to be mixed prior to application at the surgical site, thus creating a need for appropriate mixer and applicator devices [2]. Despite the wide variety of mixing devices available in other industries with approaches ranging from large-scale vat mixing [3] to nanoscale lab-on-a-chip methods [4], current surgical adhesive mixers remain primitive and employ a simple double-barreled syringe design to pass the components through a mixing chamber [5]. The double-barreled syringe design is not ideal for many adhesives because of the inability of this passive mixing method to efficiently mix adhesive components with significantly different viscosities [6] and because of the inevitable waste of potentially expensive material that becomes trapped in the dead space of the mixing chamber. Our purpose is to develop a two-component surgical adhesive mixer and applicator to improve the mixing efficacy and reduce the dead space common in many currently available mixers. To demonstrate the efficacy of our mixer, we tested the device using a tissue glue developed by Messersmith et al. [7] that has been shown in animal model testing to effectively seal the womb in fetal surgery [8], yet is nonviable for a surgical setting with current mixing methods. Double-barreled syringe mixers are not able to effectively mix this adhesive, in part due to the differing viscosities of the two components. Our design takes into account these opposing viscosities and even takes advantage of the viscosity difference by promoting diffusion through viscous fingering. According to the relatively low Reynolds number we estimated, the primary mixing method employed by our design is chaotic advection, as opposed to turbulent mixing. We demonstrated that our device not only mixes the tissue adhesive more effectively than products currently on the market but also with virtually no dead space, and therefore minimal waste of the expensive, custom-made adhesive components.
2. Methods
2.1. Design Overview.
The schematic in Fig. 1 shows the concept of our design. First, the activator component is injected via the syringe needle into the Eppendorf tube containing the polymer component. Next, a motor rotates the Eppendorf tube relative to the needle to induce mixing. Finally, the mixed adhesive is drawn up into the syringe and is ready for application.
Fig. 1.
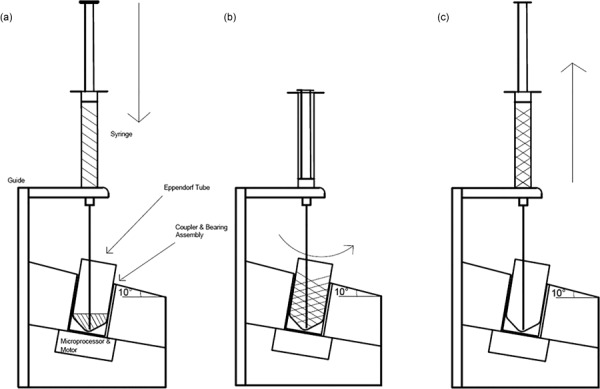
Schematic of design concept. Steps: (1) Injection of activator component into polymer, (2) Eppendorf tube containing mixture rotates relative to needle, and (3) mixed adhesive is pulled up into syringe and is ready for application.
2.2. Design Criteria.
We determined that an adhesive set time of 1 min would be favorable, as a representative model of the set times of currently available surgical adhesives, which range from 30 s to 2 min [9,10]. Because the set time of the adhesive developed by Messersmith et al. was sensitive to pH, we were able to achieve the 1 min set time by adjusting the pH of the formulation to 6.6, which was used in all subsequent tests.
Similarly, we chose to test our device using a total volume of 1 ml of the tissue glue per use, as this volume is typical of surgical adhesives currently on the market [11,12]. The volume also created a mechanical limitation by bounding the maximum rotational speed of the motor at 2200 rpm to avoid displacement of the adhesive components out of the tube during mixing.
The device is intended to be disposable to avoid the problems of cleaning and maintenance; therefore, low cost was an important design requirement. While the ultimate costs of this mixer and applicator system are difficult to estimate from our prototype, the components of the device are expected to cost less than $30 in practice.
2.3. Mixing Method.
As the tissue glue used in this study is comprised of two components with different viscosities, chaotic advection, as opposed to turbulent mixing, was deemed to be the appropriate mixing method. The success of our mixing method relies on incorporating known physical phenomena that induce mixing into our design. For example, the mixing step of injecting the water-based activation solution into the viscous catechol-functionalized polyethylene glycol (cPEG) component takes advantage of miscible viscous fingering, which is a hydrodynamic instability caused by a less viscous fluid displacing a more viscous fluid that can enhance mixing due to velocity disorder and the creation of increased interfacial area between the fluids [13]. We found that this step causes an immediate color change from clear to burnt orange throughout the solution, indicating that viscous fingering may be aiding the foremost mixing of the components by initializing diffusion.
Another important mixing mechanism that our device takes advantage of is the mixing that occurs from the motion induced by the action of drawing fluid into the shaft of the needle. This is a common method used to mix reagents in the laboratory that we mimic with the motion of the syringe. A study conducted by Amornthammarong et al. showed that a pipette tip, when used in conjunction with a syringe pump, was able to achieve superior mixing within a few number of mixing cycles [14]. While this mixing action is likely to be insufficient by itself to mix the tissue glue completely, the up-and-down motion of the syringe likely plays a role in the mixing process.
Finally, our predominant mixing method is similar to that of a well-studied batch stirring device [15], with the single, eccentric, angled needle acting as the stirrer. The Reynolds number was an important value to estimate for this aspect of our design, as it determines our mixing process and gives us insight into how our device mixes the two adhesive components at the molecular level. Low Reynolds numbers are frequently observed in mixing components with different viscosities, such as the components used in this study. Since the fluid flow of our mixer is in the laminar regime, as evidenced by the subcritical Reynolds number, chaos must be introduced in order to bring the fluid layers of the separate components together to promote crosslinking [16]. Chaotic advection relies on stretching and folding of material to promote diffusion to drive mixing, as opposed to using co-existing eddies in turbulent mixing [17]. Finn et al. [15,18] claim that although it is necessary to have three or more stir bars in order to attain topological chaos, generating chaotic flow is possible with as little as one stir bar. Even without stir bars, Ward and Metchik have shown that the rotation of a tilted tank can induce effective mixing through periodic shear [19]. By optimizing the parameters of the mixer to maximize the chaotic effect of the stir bar and the periodic shear of the tilted tank, we were able to significantly increase the mixing efficiency of our device.
2.4. Mechanical Design.
The mechanical components of our design consist of a 1 ml luer-slip plastic syringe (National Scientific Company, Part #S7510-1), a 14 gage, 4-in. needle with luer lock hub (McMaster-Carr, Part #6710A42), a 5 V 400 rpm DC Motor (Jameco Reliapro. Part #2158397), and a 3D-printed, custom-designed acrylonitrile butadiene styrene (ABS) casing, as shown in Fig. 2, to integrate all the components. A 1.5 ml Eppendorf tube (Eppendorf, Part #0030120.086) serves as the mixing chamber and is temporarily attached to the motor during use via an aluminum coupler. The motor–coupler component is installed onto the casing at 10 deg with respect to the horizontal. The nylon bearing (McMaster-Carr, Part #6389K623) provides a fluid-tight barrier between the interior of the casing and atmosphere, thus maintaining sterility inside the box which is crucial for the device's intended use in the operating room.
Fig. 2.
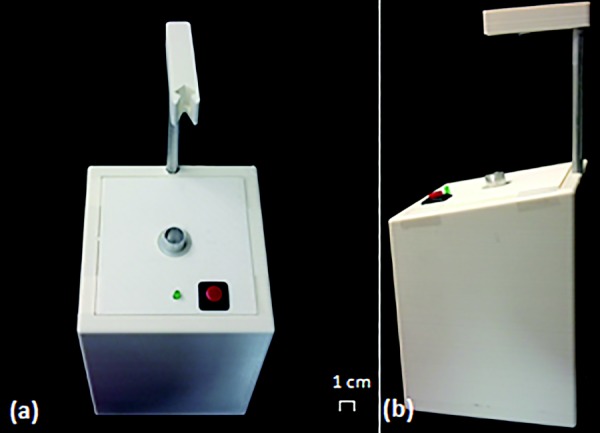
(a) Top view of device and (b) side view of device
To confirm that the needle is correctly positioned, so as to avoid impeding the withdrawal of the adhesive if the needle were too deep or causing ineffective mixing if the needle were too shallow, we included a mechanical guide customized to the shape of the needle that would fix the location of the needle with respect to the device. The syringe guide allows for vertical extension of the syringe needle tip into the Eppendorf tube, establishing a 10 deg interface between the needle and the mixing chamber. The guide reduces user-induced variability in the mixing from improper positioning of the syringe/needle. The guide itself is divided into two parts: a support rod providing the necessary height to accommodate the syringe/needle length, and the syringe holder that ensures that the needle extends to the optimal position in the Eppendorf tube. The length of the support rod, as well as the syringe holder, may be adjusted to account for various surgical applications that require different syringe/needle dimensions.
2.5. Circuit Design.
The electromechanical component is designed with the main purpose of improving the efficacy, reliability, and simplicity of the medical device. The circuit diagram shown in Fig. 3 illustrates the electromechanical parts.
Fig. 3.
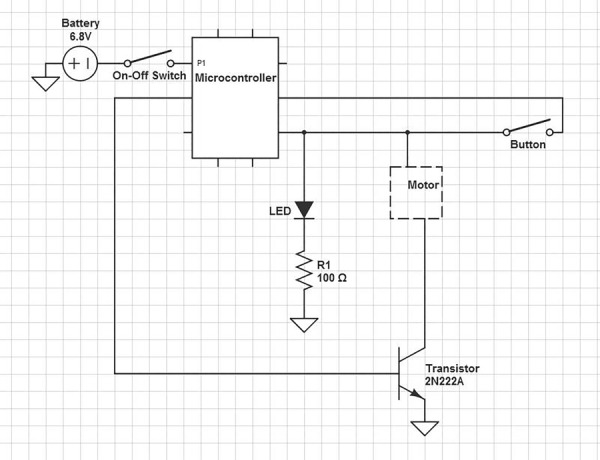
Circuit diagram of electromechanical components
The device is battery powered for easy positioning in the operating room. The circuit is powered by four AA batteries and allows the device to be portable with no requirement of an outlet or external power source. The AA batteries power the Adafruit Trinket—Mini Microcontroller (Adafruit Industries, New York), which then outputs a constant 5 V to the 12 V DC motor (Jameco Electronics, Belmont, CA).
In our design, we also considered the amount of time the user spends mixing and the position of the needle in the mixing tube as the two primary sources of error that needed to be addressed. To ensure that the user spends the minimal time required during the mixing process in order to apply the adhesive before the 1-min set time, we added two features: (1) a built-in timer that automatically terminates the rotation of the motor after 5 s and (2) a light-emitting diode (LED) to indicate device functionality. The microcontroller is programed by the arduino software to send a 5-s signal to the motor when the start button is pressed. The signal causes the motor to spin at ∼2200 rpm then automatically shut off. The automatic timer is used to prevent variability between trials and remove as much user error as possible. An LED is also incorporated in the circuit to indicate that the battery life is sufficient for the device to work properly. It acts as another safety countermeasure to ensure that the device is properly functioning before being used in the operating room.
2.6. Preparation of Adhesive.
In order to synthesize 4cPEGa from 4PEG-OH, 25 g of 4PEG-OH was dissolved in 600 ml of H2O, mixed with 200 mg of TEMPO, 200 mg of NaBr, and 50 ml of NaClO, and stirred for 30 min at a pH between 10 and 11. 50 ml of ethanol was added to halt the reaction and the pH was decreased to < 2 with HCl. The solution was extracted with four portions of 150–200 ml DCM before being recombined and extracted with 600–800 ml NanoPure H2O. The DCM solution was dried with MgSO4, filtered, and rotovapped. After being dissolved in methanol and frozen overnight, the solution was centrifuged, decanted, and placed under vacuum to obtain 4PEG-COOH. Ten grams of the 4PEG-COOH was then dissolved in 75 ml of 2:1 DMF:CH2Cl2 solution and 1.2 molar equivalents of both dopamine and 2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU) were added to the mixture. 2.2 molar equivalents of triethylamine was then added to start the reaction, which proceeded for 2 hrs before being rotovapped and precipitated in −20 °C acidified ether. The mixture was centrifuged and the precipitate was dissolved in acidified methanol and incubated at −20 °C for 2 hrs. After being centrifuged and the liquid decanted again, the precipitate was frozen at −80 °C for 1 hr and place under vacuum overnight. The precipitate was then dissolved in HCl solution and dialyzed in a 3500 MWCO cassette against acidified MilliQ water at a pH of 3.5 for 24 hrs. A second dialysis was then performed against unadjusted MilliQ water for 2–4 hrs to remove excess acid before the solution was frozen at −80 °C and lyophilized for three days to obtain the 4cPEGa product. All of the 4cPEGa products used for our experiments were from the same batch. The polymer component is prepared within 1 hr of use by dissolving it at room temperature in room-temperature 2× phosphate-buffered saline (PBS) at a pH of 6.6 and concentration of 300 mg/ml. The polymer solution is then aliquoted into Eppendorf tubes and centrifuged for 15 s at approximately 1500 rpm in order to remove bubbles. The cPEG is not prepared too far in advance to prevent natural oxidation due to exposure to air. One can determine if the dissolved cPEG has become oxidized, and therefore unusable, as it turns a light pink color when oxidation has begun to take place. The activator component can be prepared up to weeks in advance, as it is simply an aqueous solution of NaIO4 in water at a concentration of 12 mg/ml at room temperature. In order to form the adhesive, the two components are mixed in equal volumes.
2.7. Adhesive Material Property Characterization.
The two components of the adhesive are a prepolymer derivative of polyethylene glycol (PEG)-20,000 and sodium periodate, the latter of which has a viscosity similar to that of water, 1 cSt, as it is 99% water. We then used a falling-sphere viscometer and Stokes' law to calculate the viscosity of the four-arm PEG 20,000 MW in order to approximate the viscosity of the cPEG. We used the force balance equation V sphere ρ fluid g + 6πμv d = m sphere g, where V sphere is the volume of the sphere, ρ fluid is assumed to be that of water, 997.05 kg/m3 at 25 °C, and 991.27 kg/m3 at 37 °C, g is 9.8 m/s2, μ is kinematic viscosity, v d is the measured velocity of the sphere, from the measured distance divided by the measured time it took to fall that distance, and m sphere is the measured mass of the sphere.
2.8. Compression Testing.
Compression testing was conducted using a Sintech 20 g and serves as an indication of polymer crosslinking and mixing efficiency. For the vortex control group, 250 μl of NaIO4 was injected into an Eppendorf tube containing 250 μl of dissolved cPEG, and the solution was mixed on the vortex at high speed for 5 s. The adhesive was then pulled up into the syringe with the 8 cm stainless steel needle tip, and injected into the teflon mold. For the samples prepared using our device, 250 μl of NaIO4 was injected into the Eppendorf tube containing 250 μl of dissolved cPEG, and the solution was mixed using our device for 5 s. The adhesive was then pulled up and injected into the polytetrafluoroethylene (PTFE) mold in the same manner as the control. The teflon mold produces disks with a height of ∼6.1 mm and a diameter of ∼7.6 mm.
The polymer, as shown in Fig. 4, was then placed in the Sintech 20 g machine and compressed. Compression was loaded to 90% of the strain of the adhesive or stopped when there was a significant drop in the force graph which indicated that the polymer mold had broken under the stress and the resulting maximum compressive strength was recorded. Testing was conducted to evaluate the control mixing method of vortexing as well as mixing with our device at a 0 and 10 deg tilt.
Fig. 4.

Formed adhesive gel for compression testing
2.9. Shear Testing.
The samples were prepared according to ASTM standard F2255-05, “Standard Test Method for Strength Properties of Tissue Adhesives in Lap-Shear by Tension Loading [20].” Porcine pericardium (Animal Technologies, Tyler, TX) was used for testing, and was secured to the acrylic fixtures with cyanoacrylate glue. The cyanoacrylate glue was allowed to set for 30 min before further sample preparation. The adhesive was prepared in the same way as for compression testing for the control and our mixing method groups, and approximately 100 μl of the prepared volume of 500 μl was applied to the fixtures, which were overlapped by approximately 1 cm. The design of our device, with the 10 deg angle between the tube and needle, was the only mixing method used in shear testing. A 1 N weight was applied to the overlapped fixtures for 15 min, removed, and the adhesive was subsequently allowed to set for 45 min. The tissue and fixtures were kept wet with PBS throughout the entire sample preparation period, until they were placed in the Sintech 20 g machine for load testing. The overlapped samples were loaded to failure at a constant cross-head speed of 5 mm/min. The type of failure was noted for each specimen, and all were cohesive or adhesive failures. The apparent shear strength was determined by dividing the recorded maximum load by the measured bond area.
3. Results
3.1. Viscosity Measurement and Reynolds Number Calculations.
At 25 °C, the kinematic viscosity is 25 cSt, or 25 times higher than the sodium periodate solution, and at 37 °C the viscosity is 19 cSt. Although the literature describes a kinematic viscosity of about 150 cSt for linear PEG at 20,000 MW [21], the lower value that we calculated from our measurements was not unexpected since the PEG-20,000 we measured was four-arm branched, which has been shown to results in lower viscosities [22]. However, since the branches on our actual polymer are terminated by L-3,4-dihydroxyphenylalanine (DOPA) groups that cause cross-linking of the PEG chains and our polymer itself is mixed with sodium periodate solution, which is 99% water, to form the adhesive, we expect the viscosity of our adhesive to be less than or equal to 25 cSt at room temperature [23].
We calculated a Reynolds number using Couette flow and the formula below to estimate the Reynolds number, where the dynamic viscosity ν = 25 cSt, the approximate distance between the outside of the needle and the tube wall D = 0.4 cm, and rotation speed of the 0.5 cm Eppendorf tube V = 2200 rpm or 424.34 cm/s, we calculated a Reynolds number of 679, which is within the laminar flow regime
| (1) |
3.2. Compression Testing.
Compression testing was used to optimize the mixing process. Although the desired characteristics of the adhesive gel is mostly controlled by altering the chemical properties of the reagents, such as creating a 1-min set time by setting the pH of the 2 × PBS at 6.6, mechanical testing was used to measure the compressive strength as an indication of the contribution of mixing protocol to material performance. While high compressive strength does not directly correlate with high shear strength, the standard mechanical measure for adhesives, low compressive strength would suggest poor cross-linking as a result of poor mixing. Since compression tests are more easily performed, the compression testing results serve to rule out design elements that provide inadequate mixing.
One such design element is a 10-deg tilt between the axis of rotation of the motor and the needle stir bar. Common practice in industrial mixing is to introduce a relative angle of tilt to a stir rod to increase chaotic mixing [16]. We found that the incorporation of the 10-deg tilt produced adhesive disks that had significantly higher compressive strengths of 0.94 ± 0.33 MPa compared to the 0.43 ± 0.10 MPa produced by the vortex control (p < 0.05), which was not reflected in the compressive strength of 0.39 ± 0.21 MPa for our device without the 10-deg tilt (n = 4 for each group) (Fig. 5). Based on the results of the compression testing, it was decided that the angular tilt would be a critical element of our design.
Fig. 5.
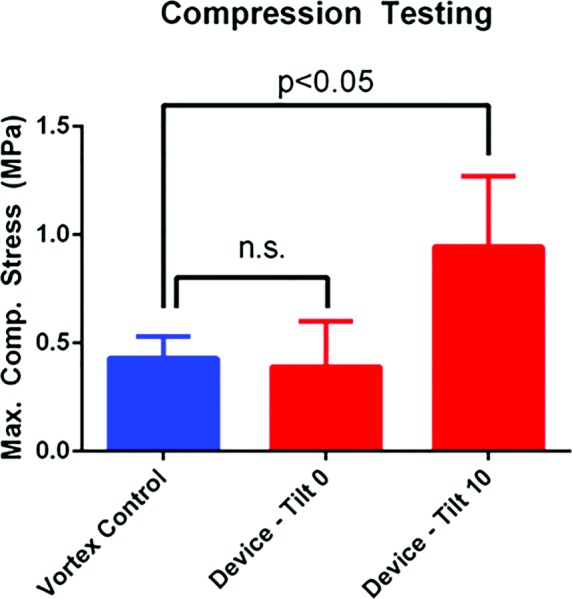
Comparison of maximum compression stress between vortex mixing (control), device mixing with 0 deg tilt angle, and device mixing with 10 deg tilt angle. Values are (mean±SD).
3.3. Shear Testing.
The ASTM shear test was performed on porcine pericardium in order to directly measure the effectiveness of our device. The current mixing method used by the Messersmith lab consisted of vortexing the adhesive components for 5 s for which they reported an ultimate shear strength value of 30 kPa. We found that adhesives mixed with our device exhibited significantly higher maximum shear strength of 38.9 ± 4.0 kPa compared to a shear strength of 29.0 ± 7.6 kPa for adhesives mixed using the vortexing method (n = 4 for each group, p < 0.05) (Fig. 6). We also found that the standard deviation for the shear values was smaller for our device when compared with the vortex control suggesting greater consistency and reliability of mixing.
Fig. 6.
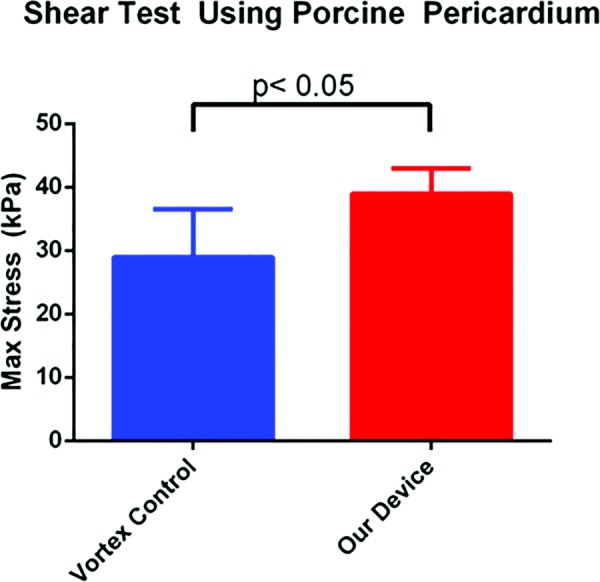
Comparison of maximum shear stress between vortex mixing (control) and device mixing with 10 deg tilt angle. Values are (mean±SD).
4. Interpretation
Based on the results of our experiments, we were able to determine the engineering parameters to incorporate in our mixing design. We incorporated into our design a tilt of 10 deg between the needle and rotation axis that would still allow the needle to touch the bottom of the tube. We showed that this tilt significantly improved mixing compared to either the vortex control or the device without the tilt, as determined by compression testing (Fig. 5). Parameters such as adhesive pH and total volume were predetermined based on knowledge of current surgical adhesives on the market, and the maximum rotational speed was limited by the volume of material in the tube.
The ultimate determinant of the device's success is the overall efficacy of our mixing. Based on lap shear tests conducted with the entire device as a whole, we were able to achieve significantly higher maximum shear strength than the vortex control (Fig. 6). The shear strength of the vortex control matched the previously reported values of the Messersmith lab. The superior maximum shear stress values we obtained indicate that we were able to improve the strength of the adhesive beyond its reported value. Vortex mixing introduces air bubbles in order to attain efficient mixing, and the trajectory of these bubbles in vortex mixing has been investigated by Sotiropoulos et al. [24] We hypothesize that the increase in shear strength is likely due to fewer microbubbles being embedded in the polymer during the mixing process, increasing the strength of the crosslinking bonds and consequently the overall shear strength of the adhesive.
Beyond the matter of microbubbles, our design has several advantages over conventional passive mixers. Active mixing tends to be more effective in fluids that are difficult to mix, such as fluids with very different viscosities [6]. Many of the passive mixing devices also work on the micro- and nanoscales, often incorporated as components of lab-on-a-chip designs, making them unsuitable for a fluid volume of ∼1 ml that is more typically observed in surgical situations [25]. By using external sources such as motors and stir bars, we are also able to exert more control over the mixing process by modifying design features to take into account requirements of fast mixing time and minimal waste of adhesive. In our design, virtually no volume is wasted. All materials that are pulled into the syringe exit the syringe upon application, and minimal residue of the adhesive is left behind in the needle. In many passive adhesive mixing devices, large volumes of material become trapped within the mixing chamber of the device, therefore creating waste. In addition, the simplicity of the design means that very inexpensive (even disposable) devices can be manufactured without adding undue cost to the end user.
According to our data described above, our device is thus able to mix together an adhesive that has maximum shear strength of ∼40 kPa, which is an improvement upon the ∼30 kPa shear strength previously reported in literature. This is much stronger than fibrin glue when applied to tissues, which is a common alternative adhesive that has a shear strength of approximately 10 kPa [26]. The significant improvement in shear strength should improve the adhesive's clinical performance and beyond what can be achieved from conventional passive mixers. This device is intended to be versatile and can therefore be used to mix other adhesives that cannot be effectively mixed using traditional means.
5. Conclusions
Existing mixers and applicators fail to satisfy several requirements shared by two-component adhesives, and thus, an active mixing design was developed. The efficacy of our device was demonstrated using the adhesive developed by Messersmith et al. The active mixer design uses a DC motor at 2200 rpm to mix two components of the mussel adhesive for 5 s at a 10 deg tilt angle. These parameters have shown to significantly increase maximum shear strength of the adhesive compared to the passive microfluidic mixer previously used. In addition, a seal between the electrical components and the atmosphere has been achieved, which will prevent damage to functionality of the device during sterilization. Further design considerations such as a battery life indicator light and syringe guide increase the user-friendliness of the device, while mitigating the risks for variability and error.
Acknowledgment
The authors thank Nicholas Marchuk for help with electronic design, Devin Barrett for adhesive formulation advice, Mark Sinew for help with materials testing, and Michael Beltran for help with device fabrication.
Support was provided by the National Institutes of Health, NIH R25 EB013060 and R37 DE014193.
Contributor Information
Kevin Go, Department of Biomedical Engineering, , McCormick School of Engineering and Applied Science, , Northwestern University, , 2145 Sheridan Road, , Evanston, IL 60208 , e-mail: KevinGo2014@u.northwestern.edu.
Yeong Kim, Department of Biomedical Engineering, , McCormick School of Engineering and Applied Science, , Northwestern University, , 2145 Sheridan Road, , Evanston, IL 60208 , e-mail: YeongKim2011@u.northwestern.edu.
Andy H. Lee, Department of Biomedical Engineering, , McCormick School of Engineering and Applied Science, , Northwestern University, , 2145 Sheridan Road, , Evanston, IL 60208 , e-mail: AndyLee2015@u.northwestern.edu.
Kelly Staricha, Department of Biomedical Engineering, , McCormick School of Engineering and Applied Science, , Northwestern University, , 2145 Sheridan Road, , Evanston, IL 60208 , e-mail: Kelly@northwestern.edu.
Phillip Messersmith, Department of Biomedical Engineering, , McCormick School of Engineering and Applied Science, , Northwestern University, , 2145 Sheridan Road, , Evanston, IL 60208 , e-mail: PhilM@berkeley.edu.
Matthew Glucksberg, Department of Biomedical Engineering, , McCormick School of Engineering and Applied Science, , Northwestern University, , 2145 Sheridan Road, , Evanston, IL 60208 , e-mail: M-Glucksberg@northwestern.edu.
References
- [1]. Duarte, A. P. , Coelho, J. F. , Bordado, J. C. , Cidade, M. T. , and Gil, M. H. , 2012, “Surgical Adhesives: Systematic Review of the Main Types and Development Forecast,” Prog. Polym. Sci., 37(8), pp. 1031–1050. 10.1016/j.progpolymsci.2011.12.003 [DOI] [Google Scholar]
- [2]. Spotnitz, W. D. , and Burks, S. , 2008, “Hemostats, Sealants, and Adhesives: Components of the Surgical Toolbox,” Transfusion, 48(7), pp. 1502–1516. 10.1111/j.1537-2995.2008.01703.x [DOI] [PubMed] [Google Scholar]
- [3]. Vrabel, P. , van der Lans, R. G. J. M. , Luyben, K. C. A. M. , Boon, L. , and Nienow, A. W. , 2000, “Mixing in Large-Scale Vessels Stirred With Multiple Radial or Radial and Axial Up-Pumping Impellers: Modelling and Measurements,” Chem. Eng. Sci., 55(23), pp. 5881–5896. 10.1016/S0009-2509(00)00175-5 [DOI] [Google Scholar]
- [4]. Ahmed, D. , Mao, X. L. , Shi, J. J. , Juluri, B. K. , and Huang, T. J. , 2009, “A Millisecond Micromixer Via Single-Bubble-Based Acoustic Streaming,” Lab Chip, 9(18), pp. 2738–2741. 10.1039/b903687c [DOI] [PubMed] [Google Scholar]
- [5]. Jackson, M. R. , 2001, “Fibrin Sealants in Surgical Practice: An Overview,” Am. J. Surg., 182(2), pp. 1s–7s. 10.1016/S0002-9610(01)00770-X [DOI] [PubMed] [Google Scholar]
- [6]. Liu, Y. Z. , Kim, B. J. , and Sung, H. J. , 2004, “Two-Fluid Mixing in a Microchannel,” Int. J. Heat Fluid Flow, 25(6), pp. 986–995. 10.1016/j.ijheatfluidflow.2004.03.006 [DOI] [Google Scholar]
- [7]. Haller, C. M. , Buerzle, W. , Kivelio, A. , Perrini, M. , Brubaker, C. E. , Gubeli, R. J. , Mallik, A. S. , Weber, W. , Messersmith, P. B. , Mazza, E. , Ochsenbein-Koelble, N. , Zimmermann, R. , and Ehrbar, M. , 2012, “Mussel-Mimetic Tissue Adhesive for Fetal Membrane Repair: An Ex Vivo Evaluation,” Acta Biomater., 8(12), pp. 4365–4370. 10.1016/j.actbio.2012.07.047 [DOI] [PubMed] [Google Scholar]
- [8]. Kivelio, A. , Dekoninck, P. , Perrini, M. , Brubaker, C. E. , Messersmith, P. B. , Mazza, E. , Deprest, J. , Zimmermann, R. , Ehrbar, M. , and Ochsenbein-Koelble, N. , 2013, “Mussel Mimetic Tissue Adhesive for Fetal Membrane Repair: Initial In Vivo Investigation in Rabbits,” Eur. J. Obstet. Gynecol. Reprod. Biol., 171(2), pp. 240–245. 10.1016/j.ejogrb.2013.09.003 [DOI] [PubMed] [Google Scholar]
- [9]. Covidien, 2014, “SwiftSet™ Topical Skin Adhesive. Advanced Medical Solutions Technology,” Covidien LLC, Mansfield, MA, http://www.covidien.com/content/dam/covidien/library/global/english/product/wound-closure/topical-skin-adhesives/swiftset-topical-skin-adhesive-vac-product-information-kit.pdf
- [10]. CryoLife, 2001, “Proposed Summary of Safety and Effectiveness. CryoLife, Inc., BioGlue® Surgical Adhesive,” CryoLife, Inc., Kennesaw, GA, http://www.fda.gov/ohrms/dockets/ac/01/briefing/3790b2_01_cryolife.pdf
- [11]. Braun, Aesculap, Inc., 2007, “B. Braun Closure Technologies: Histoacryl® Topical Skin Adhesive Patient Instructions,” Aesculap, Inc., Center Valley, PA, https://www.aesculapusa.com/assets/base/doc/DOC786-Histoacryl_Patient_Instructions.pdf
- [12]. CryoLife, 2015, “BioGlue® Surgical Adhesive,” CryoLife Inc., Kennesaw, GA, http://www.cryolife.com/international/products/bioglue-surgical-adhesive
- [13]. Jha, B. , Cueto-Felgueroso, L. , and Juanes, R. , 2013, “Synergetic Fluid Mixing From Viscous Fingering and Alternating Injection,” Phys. Rev. Lett., 111(14), p. 144501. 10.1103/PhysRevLett.111.144501 [DOI] [PubMed] [Google Scholar]
- [14]. Amornthammarong, N. , Ortner, P. B. , and Zhang, J. Z. , 2010, “A Simple, Effective Mixing Chamber Used in Conjunction With a Syringe Pump for Flow Analysis,” Talanta, 81(4–5), pp. 1472–1476. 10.1016/j.talanta.2010.02.054 [DOI] [PubMed] [Google Scholar]
- [15]. Finn, M. D. , Cox, S. M. , and Byrne, H. M. , 2003, “Topological Chaos in Inviscid and Viscous Mixers,” J. Fluid Mech., 493, pp. 345–361. 10.1017/S0022112003005858 [DOI] [Google Scholar]
- [16]. Gouillart, E. , Thiffeault, J. L. , and Finn, M. D. , 2006, “Topological Mixing With Ghost Rods,” Phys. Rev. E, 73(3), p. 036311. 10.1103/PhysRevE.73.036311 [DOI] [PubMed] [Google Scholar]
- [17]. Aref, H. , Blake, J. , Budišić, M. , Cartwright, J. , Clercx, H. , Feudel, U. , Golestanian, R. , Gouillart, E. , Le Guer, Y. , van Heijst, G. , Krasnopolskaya, T. , MacKay, R. , Meleshko, V. , Metcalfe, G. , Mezić, I. , de Moura, A. , El Omari, K. , Piro, O. , Speetjens, M. , Sturman, R. , Thiffeault, J. , and Tuval, I. , 2014, “Frontiers of Chaotic Advection,” e-print arXiv:1403.2953.http://arxiv.org/abs/1403.2953
- [18]. Finn, M. D. , and Cox, S. M. , 2001, “Stokes Flow in a Mixer With Changing Geometry,” J. Eng. Math., 41(1), pp. 75–99. 10.1023/A:1011840630170 [DOI] [Google Scholar]
- [19]. Ward, T. , and Metchik, A. , 2007, “Viscous Fluid Mixing in a Tilted Tank by Periodic Shear,” Chem. Eng. Sci., 62(22), pp. 6274–6284. 10.1016/j.ces.2007.07.037 [DOI] [Google Scholar]
- [20]. ASTM, 2010, “Standard Test Method for Strength Properties of Tissue Adhesives in Lap-Shear by Tension Loading,” ASTM International, West Conshohocken, PA, ASTM Standard No. F2255-05(2010). 10.1520/F2255-05R10 [DOI]
- [21]. Clariant, 2007, “Your Universally Applicable Polymer,” Functional Chemistry Division, Clariant International Ltd., Muttenz, Switzerland, http://www.essentialingredients.com/pdf/PolyglykolsforPersonalCare.pdf
- [22]. Sim, S. L. , He, T. , Tscheliessnig, A. , Mueller, M. , Tan, R. B. H. , and Jungbauer, A. , 2012, “Branched Polyethylene Glycol for Protein Precipitation,” Biotechnol. Bioeng., 109(3), pp. 736–746. 10.1002/bit.24343 [DOI] [PubMed] [Google Scholar]
- [23]. Landel, R. F. , and Lielson, L. E. , Mechanical Properties of Polymers and Composites, 2nd ed., CRC Press, Boca Raton, FL. [Google Scholar]
- [24]. Sotiropoulos, F. , Ventikos, Y. , and Lackey, T. C. , 2001, “Chaotic Advection in Three-Dimensional Stationary Vortex-Breakdown Bubbles: Sil'nikov's Chaos and the Devil's Staircase,” J. Fluid Mech., 444, pp. 257–297. 10.1017/S0022112001005286 [DOI] [Google Scholar]
- [25]. Capretto, L. , Cheng, W. , Hill, M. , and Zhang, X. L. , 2011, “Micromixing Within Microfluidic Devices,” Microfluidics, 304, pp. 27–68. 10.1007/128_2011_150 [DOI] [PubMed] [Google Scholar]
- [26]. Burke, S. A. , Ritter-Jones, M. , Lee, B. P. , and Messersmith, P. B. , 2007, “Thermal Gelation and Tissue Adhesion of Biomimetic Hydrogels,” Biomed. Mater., 2(4), pp. 203–210. 10.1088/1748-6041/2/4/001 [DOI] [PMC free article] [PubMed] [Google Scholar]


