Abstract
Objectives
Proton pump inhibitor-responsive esophageal eosinophilia (PPI-REE) is a newly recognized entity that must be differentiated from eosinophilic esophagitis (EoE). Little is known about this condition. We aimed to determine the prevalence of PPI-REE and EoE in patients undergoing upper endoscopy, and determine features that distinguish the two groups.
Methods
This prospective study conducted at University of North Carolina from 2009–2011 enrolled consecutive adult patients undergoing outpatient upper endoscopy. Subjects had esophageal biopsies to quantify the maximum eosinophil count per high-powered field (eos/hpf; hpf = 0.24mm2). If biopsies revealed ≥15 eos/hpf, subjects were treated with twice daily PPI for 8 weeks and endoscopy was repeated. If ≥15 eos/hpf persisted despite PPI therapy, EoE was diagnosed. If there were <15 eos/hpf, PPI-REE was diagnosed. The proportion of patients in each group was calculated, and patients with EoE and PPI-REE were compared.
Results
Of the 223 subjects enrolled, 173 had dysphagia and 50 did not. Of those with dysphagia, 66 (38%) had ≥15 eos/hpf. After the PPI trial, 40 (23%) were confirmed to have EoE, and 24 (14%) had PPI-REE. Of those without dysphagia, 2 (4%) had ≥15 eos/hpf and after the PPI trial, 1 (2%) had EoE. Compared with EoE, PPI-REE patients were more likely to be older and male, and less likely to have typical endoscopic findings of EoE. However, none of the individual factors was independently predictive of PPI-REE status on multivariable analysis. Similarly, while some endoscopic findings were differentially distributed between PPI-REE and EoE, none were significantly associated with disease status on multivariable analysis.
Conclusions
Esophageal eosinophilia is common among patients undergoing EGD for dysphagia. While EoE was seen in nearly a quarter of patients with dysphagia, PPI-REE was almost as common, and accounted for over one-third of those with ≥15 eos/hpf. No clinical or endoscopic features independently distinguished PPI-REE from EoE prior to the PPI trial.
Keywords: proton pump inhibitor-responsive esophageal eosinophilia, eosinophilic esophagitis, prevalence, endoscopy, dysphagia
Introduction
Eosinophilic esophagitis (EoE) is an immune-mediated clinicopathologic condition characterized by symptoms of esophageal dysfunction in the setting of eosinophilic inflammation on esophageal biopsy (1, 2). Over the past decade, this condition has become increasingly recognized, and it is now frequently encountered in patients undergoing upper endoscopy (3–5). However, the finding of esophageal eosinophilia is not specific for EoE. The differential diagnosis is relatively broad and can include infections, drug hypersensitivity, autoimmune and connective tissue disorders, and hypereosinophilic syndrome (1, 2, 6, 7). From a practical standpoint, however, gastroesophageal reflux disease (GERD) and proton pump-inhibitor esophageal eosinophilia (PPI-REE) are the most commonly encountered conditions that must be distinguished from EoE (1, 2, 8).
In particular, the recent recognition of PPI-REE has complicated the diagnostic algorithm for EoE. PPI-REE is the term used to describe patients with esophageal eosinophilia on biopsy who respond to a course of PPI therapy. It was first observed in a series of pediatric patients (9), and now accounts for at least one third of children and adults with esophageal eosinophilia (10–15). While recent guidelines require the exclusion of PPI-REE with a PPI trial before a formal diagnosis of EoE can be made (1, 2), it is currently unclear if PPI-REE is a subtype of GERD, an EoE phenotype, or an independent condition. In addition, the prevalence of PPI-REE has not been prospectively determined in a cohort in the United States. PPI-REE is poorly understood, and predictors that might distinguish EoE from PPI-REE are unknown.
The aim of this study was to determine the prevalence of PPI-REE and EoE in patients with and without dysphagia undergoing upper endoscopy, and to determine if clinical, endoscopic, or histologic features could distinguish the two groups. Based on our experience, we hypothesized that no such factors would distinguish PPI-REE from those with EoE.
Methods
Study design and patients
This was a prospective cohort study conducted at the University of North Carolina (UNC) between 2009 and 2011. Consecutive adult patients (ages 18–80 years) referred for routine outpatient esophagogastroduodenoscopy (EGD) were recruited from the two UNC GI procedure units. Patients were stratified by indication (dysphagia vs other indications), and enrolled in an approximately 3:1 ratio of dysphagia vs non-dysphagia indications, in order to enrich the study pool for patients with dysphagia. Subjects were excluded if they had a known (prevalent) diagnosis of EoE or a different eosinophilic gastrointestinal disorder (EGID), GI bleeding, active anticoagulation, known esophageal cancer, prior esophageal surgery, known esophageal varices, medical instability or multiple comorbidities precluding enrollment in the clinical opinion of the endoscopist, or inability to read or understand the consent form. Subjects provided informed consent and were enrolled prior to the endoscopy. This study was approved by the UNC Institutional Review Board.
Subjects with a new (incident) diagnosis of EoE met consensus guidelines (1). Specifically, cases were required to have at least one typical symptom of esophageal dysfunction (for example dysphagia, food impaction, heartburn, or feeding intolerance); at least 15 eosinophils per high-power field (eos/hpf) on esophageal biopsy persisting after an 8 week PPI trial (20–40 mg twice daily of any of the available agents, prescribed at the discretion of the clinician); and other causes of esophageal eosinophilia excluded. While the majority of the EoE group included subjects who were PPI-naïve on their index endoscopy (n = 24), there were patients who were on high-dose PPI for at least 8 weeks at the time of their index endoscopy (n = 17) who did not have pre-PPI endoscopy or histology data available.
Subjects with PPI-REE were required to have at least one typical symptom of esophageal dysfunction (for example dysphagia, food impaction, heartburn, or feeding intolerance); at least 15 eosinophils per high-power field (eos/hpf) on esophageal biopsy; improvement of esophageal eosinophilia to < 15 eos/hpf after an 8 week PPI trial (20–40 mg twice daily of any of the available agents, prescribed at the discretion of the patient’s clinician); and improvement of symptoms by self-report at the time of the repeat endoscopy. By definition, the PPI-REE group included only subjects who were PPI-naïve at the time of their index endoscopy and required follow-up endoscopy after the PPI trial.
Clinical and histologic data
Clinical data including demographics, symptoms, and the indications for endoscopy were recorded. At the time of enrollment, a blood sample was drawn for the peripheral eosinophil count (cells x 109/L) and total IgE level (kU/L). During endoscopy, all endoscopic findings were recorded using a standardized case report form. A total of 5 research protocol esophageal biopsies were obtained (2 from the proximal, 1 from the mid, and 2 from the distal esophagus) to maximize the sensitivity of EoE diagnosis (16). Additional clinical biopsies were taken as needed at the discretion of the endoscopist. Esophageal biopsies were reviewed by the study pathologists to determine eosinophil counts according to our previously validated protocol (17). In brief, the slides were masked to the clinical case status and digitized. Using Aperio ImageScope (Aperio Technologies, Vista, CA), the maximum eosinophil density (eosinophils/mm2 [eos/mm2]) was determined after examination of five microscopy fields from each of the five biopsies. For purposes of comparison to previous studies, eosinophil density was then converted to eosinophil counts (eos/hpf) for an assumed hpf size of 0.24 mm2, the size of an average field as reported in the literature (18). The eosinophil infiltration was further examined to determine whether it was patchy (localized eosinophilia ≥15 eos/hpf in only one hpf in the biopsy) or diffuse (eosinophilic inflammation seen in multiple hpfs) throughout the entire biopsy sample, as well as whether the eosinophil distribution throughout the mucosa was superficial only, basal only, or diffuse (throughout the epithelium). Of note, gastric and duodenal biopsies were also collected and examined to exclude co-existing eosinophilic gastritis or gastroenteritis.
Statistical analysis
Characteristics of the study groups were summarized using descriptive statistics, and the proportion of EoE cases and PPI-REE subjects were calculated. Bivariate comparisons were made between patients with and without esophageal eosinophilia ≥ 15 eos/hpf, and between EoE cases and PPI-REE subjects. Chi-square was used for categorical variables and t-tests were used for continuous variables. For the comparison between EoE and PPI-REE, baseline data from the PPI-naïve visit were used. Thus, patients diagnosed with EoE on an index endoscopy while on high-dose PPI were excluded from these comparisons. Multivariable logistic regression was used to assess for factors that would independently distinguish PPI-REE and EoE. Based on the final sample size, different models were constructed with no more than 4 covariates.
Results
Patient flow and characteristics of subjects with esophageal eosinophilia
There were 565 patients screened for this study (Figure 1). Of these, 205 were ineligible (26 were out of the age range, 22 had GI bleeding, 6 had esophageal cancer, 6 had prior esophageal resection, 9 had esophageal varices, 2 were actively anticoagulated, 26 had prior EoE or EGID, 89 had medical instability/comorbidities, 19 did not speak English and were unable to provide consent), 48 were screened but cancelled their endoscopy appointment, 36 were screened but were unable to be recruited at the time of endoscopy, and 53 refused participation.
Figure 1.
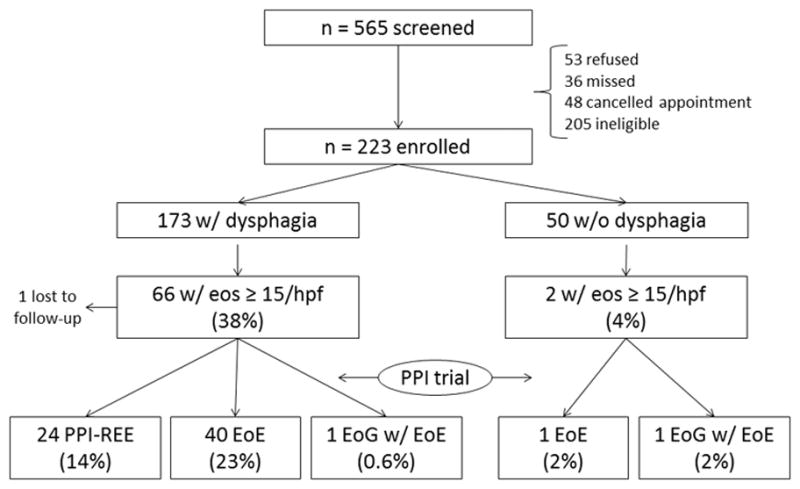
Patient flow through the study.
Of the 223 subjects who were enrolled, 173 had dysphagia and 50 did not have dysphagia. Of the 173 patients with dysphagia, 66 (38%) had esophageal eosinophilia with ≥ 15 eos/hpf. After the PPI-trial, 40 (23%) were confirmed to have EoE, 24 (14%) had PPI-REE, and 1 (0.6%) had eosinophilic gastroenteritis. Of the 50 patients without dysphagia, 2 (4%) had esophageal eosinophilia with ≥ 15 eos/hpf. After the PPI trial, 1 (2%) had EoE and 1 (2%) had eosinophilic gastroenteritis.
There were multiple clinical, endoscopic, and histologic differences between those with and without esophageal eosinophilia (Table 1). When compared to subjects without esophageal eosinophilia, those with eosinophil counts ≥ 15 eos/hpf were younger (41 vs 54 years; p < 0.001), more likely to be male (73% vs 37%; p < 0.001), and more likely to be white (92% vs 77%; p = 0.006). They were less likely to have a normal endoscopic exam (2% vs 23%; p < 0.001), and more likely to have the typical findings of EoE, including esophageal rings, strictures, narrowing, linear furrows, crêpe-paper mucosa, and decreased vascularity.
Table 1.
Characteristics of the study population
| Subjects without esophageal eosinophilia (n = 155) | Subjects with esophageal eosinophilia* (n = 66) | p | |
|---|---|---|---|
| Age at diagnosis (mean ± SD) | 53.7 ± 15.1 | 40.5 ± 12.3 | < 0.001 |
| Male (n, %) | 58 (37) | 48 (73) | < 0.001 |
| White (n, %) | 118 (77) | 61 (92) | 0.006 |
| Symptoms/EGD indication (n, %) | |||
| Dysphagia | 109 (70) | 64 (97) | < 0.001 |
| Heartburn | 25 (16) | 5 (8) | 0.09 |
| Abdominal pain | 23 (15) | 1 (2) | 0.004 |
| Nausea/vomiting | 6 (4) | 1 (2) | 0.36 |
| EGD findings (n, %) | |||
| Normal | 36 (23) | 1 (2) | < 0.001 |
| Rings | 23 (15) | 52 (79) | < 0.001 |
| Stricture | 30 (19) | 20 (30) | 0.08 |
| Narrowing | 8 (5) | 19 (29) | < 0.001 |
| Furrows | 10 (6) | 51 (77) | < 0.001 |
| Crêpe-paper | 0 (0) | 4 (6) | 0.002 |
| White plaques/exudates | 9 (6) | 26 (39) | < 0.001 |
| Decreased vascularity | 4 (3) | 13 (20) | < 0.001 |
| Erosive esophagitis | 28 (18) | 7 (11) | 0.17 |
| Schatzki’s ring | 12 (8) | 6 (9) | 0.75 |
| Hiatal hernia | 47 (31) | 14 (21) | 0.16 |
| Dilation performed | 52 (34) | 20 (30) | 0.64 |
| PPI use on baseline exam | 103 (71) | 0 (0) | < 0.001 |
| Maximum eosinophil count (mean ± SD)† | 3.2 ± 11.2 | 60.3 ± 41.9 | < 0.001 |
| Peripheral eosinophils (mean cells x 109/L ± SD) | 0.18 ± 0.14 | 0.29 ± 0.21 | < 0.001 |
| IgE levels (mean kU/L ± SD) | 141 ± 319 | 231 ± 414 | 0.10 |
Patients with EoE overlapping with eosinophilic gastroenteritis (n = 2) are excluded from esophageal eosinophilia group for analysis.
Eosinophil counts presented in eosinophils per high-power field (eos/hpf), for a hpf size of 0.24 mm2. For the esophageal eosinophil group, this value it the baseline count for the 48 subjects who had a PPI-naïve baseline endoscopy.
When examining the post-PPI treatment eosinophil counts, there were several histologically borderline cases. In the EoE group, there were 3 subjects with an eosinophil count of 16; all of the other subjects had counts > 20 eos/hpf. In the PPI-REE group there were 4 patients with an eosinophil count of 14 eos/hpf and 4 patients with an eosinophil count of 13 eos/hpf; the remainder of the subjects had counts < 10 eos/hpf. In this group, however, these counts were substantially decreased from a mean of 90 after the PPI therapy (the pre-PPI individual counts were 256, 100, 40, 120, 25, 53, 57, and 49 eos/hpf).
EoE cases and PPI-REE subjects
When the 24 PPI-REE subjects and 41 EoE cases were compared, those with PPI-REE were more likely to be older (48 vs 36 years; p < 0.001), male (88% vs 63%; p = 0.04), and have a Schatzki’s ring (21% vs 4 %; p = 0.01) (Table 2). They were less likely to have esophageal rings (63% vs 100%), narrowing (8% vs 33%; p = 0.03), linear furrows (58% vs 92%; p = 0.008), and decreased vascularity (0% vs 17%; p = 0.04). There was no difference in the maximum pre-PPI-trial eosinophil count between the PPI-REE and EoE groups (58 vs 63 eos/hpf; p = 0.74). There were also no significant differences in eosinophil distributions. The eosinophil infiltration was patchy throughout the biopsy specimen in 61% of the EoE subjects as compared with 83% of those with PPI-REE (p = 0.16). In the EoE group, the eosinophil mucosal distribution was superficial, basal, and diffuse, in 7%, 24%, and 69%, respectively, and in the PPI-REE group it was 27%, 9%, and 63%, respectively (p = 0.17). While the peripheral blood eosinophil count was statistically lower in the PPI-REE group, levels for both groups were still within the normal range (0.21 vs 0.35 cells x 109/L; p = 0.01); there were no differences for the total IgE level. After the PPI-trial, the mean eosinophil count was 9 eos/hpf in the PPI-REE group (range 0–14 eos/hpf; IQR 4–13 eos/hpf).
Table 2.
Characteristics of subjects with PPI-REE vs EoE
| PPI-REE (n = 24) | EoE (n = 41) | p | |
|---|---|---|---|
| Age at diagnosis (mean ± SD) | 48.3 ± 10.9 | 35.9 ± 11.0 | < 0.001 |
| Male (n, %) | 21 (88) | 26 (63) | 0.04 |
| White (n, %) | 21 (88) | 39 (95) | 0.27 |
| Symptoms/EGD indication (n, %) | |||
| Dysphagia | 23 (96) | 40 (98) | 0.70 |
| Heartburn | 2 (8) | 3 (7) | 0.88 |
| Abdominal pain | 0 (0) | 1 (2) | 0.44 |
| Nausea/vomiting | 1 (4) | 0 (0) | 0.19 |
| EGD findings (n, %)* | |||
| Normal | 1 (4) | 0 (0) | 0.31 |
| Rings | 15 (63) | 24 (100) | 0.001 |
| Stricture | 6 (25) | 8 (33) | 0.56 |
| Narrowing | 2 (8) | 8 (33) | 0.03 |
| Furrows | 14 (58) | 22 (92) | 0.008 |
| Crêpe-paper | 1 (4) | 2 (8) | 0.55 |
| White plaques/exudates | 8 (33) | 10 (42) | 0.55 |
| Decreased vascularity | 0 (0) | 4 (17) | 0.04 |
| Erosive esophagitis | 5 (21) | 2 (8) | 0.22 |
| Schatzki’s ring | 5 (21) | 1 (4) | 0.01 |
| Hiatal hernia | 7 (29) | 7 (29) | 0.99 |
| Dilation performed | 9 (38) | 6 (25) | 0.35 |
| Maximum eosinophil counts (mean ± SD)† | |||
| Before PPI trial* | 58.3 ± 50.8 | 62.6 ± 29.8 | 0.74 |
| After PPI trial | 8.6 ± 4.9 | 108.0 ± 113.5 | < 0.001 |
| Peripheral eosinophils (mean cells x 109/L, ± SD) | 0.21 ± 0.11 | 0.35 ± 0.24 | 0.01 |
| IgE levels (mean kU/L ± SD) | 230 ± 541 | 238 ± 322 | 0.95 |
n = 24 for the EoE group for baseline endoscopic findings and the baseline eosinophilic counts; the remaining 17 EoE subjects had their index endoscopy after the PPI trial, so pre-PPI endoscopic findings and histology data are not available.
Eosinophil counts presented in eosinophils per high-power field (eos/hpf), for a hpf size of 0.24 mm2
On multivariate analysis, there were no independent clinical, endoscopic, or histologic predictors that reliably distinguished PPI-REE from EoE.
Discussion
With the increasing recognition and diagnosis of EoE, esophageal eosinophilia is being encountered more frequently (2). While the most common causes of esophageal eosinophilia were presumed to be GERD and EoE (7), the recent identification of PPI-REE has complicated the diagnostic algorithms and this condition is little understood (1, 2, 9, 12). The present study aimed to determine the prevalence of EoE and PPI-REE in patients undergoing endoscopy and assess whether clinical, endoscopic, or histologic features could distinguish the two groups. There are several key findings. First, esophageal eosinophilia is commonly encountered in the GI procedure unit, with nearly 40% of subjects with dysphagia and 4% of patients without dysphagia having ≥ 15 eos/hpf, the current threshold required to suspect a diagnosis of EoE. Second, after an 8 week PPI trial, almost one quarter of patients with dysphagia and 2% of those without dysphagia, were confirmed to have EoE by consensus guidelines. However, more than one third of patients with esophageal eosinophilia were found to have PPI-REE. Third, no clinical, endoscopic, or histologic feature independently distinguished EoE from PPI-REE prior to the PPI trial.
Several prior studies have assessed the prevalence of EoE in patients undergoing endoscopy. The rate has ranged from 6.5% in all patients undergoing endoscopy (4), to 12–15% in patients undergoing endoscopy with an indication of dysphagia (3, 5), to more than 50% in patients undergoing an endoscopy in the setting of an active food impaction (19, 20). However, these studies were conducted before the recognition of PPI-REE, and not all subjects diagnosed with EoE had a prior PPI-trial. Therefore, these estimates almost certainly include a mix of both EoE and PPI-REE patients. The prevalence of EoE in patients undergoing endoscopy for dysphagia in our study is higher than previously reported, and may reflect either continuing trends in the increasing incidence of EoE (21), referral bias from procedures performed at an academic center, selection bias given that less than half of patients screened were enrolled, or a combination of all three. However, even if we assume that all of the subjects who were screen failures had normal esophageal biopsies, esophageal eosinophilia and EoE would still be quite common in the dysphagia population.
Interestingly, our estimate of the prevalence of the proportion of patients with esophageal eosinophilia who responded to a PPI trial, is very similar to other estimates of PPI-REE in the literature (Table 3) (10–15, 22–26). The majority of studies on PPI-REE have been retrospective, and report that 39–71% of children and adults with esophageal eosinophilia have PPI-REE. In the first prospective study examining PPI-REE, Molina-Infante and colleagues enrolled 712 adults in Spain undergoing upper endoscopy for any indication, and 35 (4.9%) had ≥ 15 eos/hpf on esophageal biopsy (12). Subjects were treated with rabeprazole 20 mg twice daily for 2 months, and then underwent upper endoscopy. A total of 26 (74%) had resolution of esophageal eosinophilia and were classified as PPI-REE. Both in this study, and in one by Francis and colleagues where the frequency of PPI-REE was 61%, baseline reflux testing was not predictive of PPI-REE status (12, 14). Other prospective studies in which information can indirectly be inferred about PPI-REE prevalence are two clinical trials of fluticasone vs esomeprazole for patients with esophageal eosinophilia, where the rates of PPI response were remarkably similar at 33% and 35% (22, 23).
Table 3.
Reported prevalences of PPI-REE in the literature
| Author | Year | Population | Design | Esophageal eosinophilia (n treated with PPI) | PPI-REE (n, %) |
|---|---|---|---|---|---|
| Dranove | 2009 | Children | Retrospective | 43 | 17 (40) |
| Sayej | 2009 | Children | Retrospective | 36 | 14 (39) |
| Peterson | 2010 | Adults | RCT* | 12 | 4 (33) |
| Molina-Infante | 2011 | Adults | Prospective | 35 | 26 (74) |
| Abe | 2011 | Adults | Retrospective | 7 | 3 (43) |
| Francis | 2012 | Adults | Prospective | 18 | 11 (61) |
| Fujiwara | 2012 | Adults | Prospective | 5 | 3 (60) |
| Schroeder | 2013 | Children | Retrospective | 7 | 5 (71) |
| Moawad | 2013 | Adults | RCT* | 20 | 7 (35) |
| Vazquez-Elizondo† | 2013 | Adults | Retrospective | 54 | 29 (54) |
| Mangla† | 2013 | Adults | Retrospective | 146 | 80 (55) |
RCT = randomized clinical trial of esomeprazole vs fluticasone in patients with esophageal eosinophilia; data only presented for the PPI arms in these trials.
Abstract data only.
There are several mechanisms that might explain this response to PPI medications. It is possible that in some patients with PPI-REE, the esophageal eosinophilic infiltrate is due to GERD, and the mechanism is simply related to decreasing acid exposure (27, 28). Similarly, it is possible that PPIs heal damaged epithelial barriers in EoE, thus decreasing antigen exposure and reducing eosinophilia (27). It is also possible that PPIs have a direct anti-eosinophilic/anti-inflammatory effect (29, 30). Others suggest in that PPIs may decrease eosinophil degranulation (31).
Regardless of the mechanism, it is difficult to predict which patients with esophageal eosinophilia will respond to PPIs and which will have EoE. In our study, while those with PPI-REE were somewhat older and had fewer typical endoscopic findings of EoE, the groups were largely indistinguishable and clinical, endoscopic, and histologic factors were not predictive after multivariate analysis. These findings are similar to those of other investigators (12, 32), as well as recent abstract data showing that eotaxin-3 and Th2 cytokines were decreased in PPI-REE patients, in patterns similar to what is observed after EoE patients are treated with topical steroids (33). Currently, it remains unknown if some patients with PPI-REE will eventually be categorized as a subtype of EoE, particularly as PPI-REE may change over time; there has been one report of 4 children who developed EoE after initially having a PPI response (34).
We have previously reported that EoE and GERD could be distinguished using a multivariable analysis of clinical, endoscopic and histologic features (35). The contrast between those results and the present study, where EoE and PPI-REE could not be similarly differentiated, is notable. We believe that this is explained by differences in the comparator groups (GERD vs. PPI-REE). In the first study, GERD patients had to have at least one typical symptom of GERD (i.e. heartburn, regurgitation, etc) which was the main indication for EGD, consistent biopsy findings (inflammation which could, but did not have to contain, eosinophils), and a clinical evaluation which excluded other possible causes. There were no restrictions on esophageal eosinophil counts in the GERD patients, but while some in this group had eosinophil-predominant inflammation, many had mixed inflammatory infiltrates that would not be consistent with EoE, and therefore would not be eligible for the present study. In contrast, in the present study, all patients started with a purely eosinophilic infiltrate and a clinical picture that was suspicious for EoE. Subjects with GERD-predominant symptoms and with eosinophils and a mixed inflammatory infiltrate would not have been included in the esophageal eosinophilia group. Therefore, the conclusions of the studies are different – predictors do exist to separate EoE from GERD patients, but not EoE from PPI-REE patients.
This study has limitations that should be considered in interpreting the results. First, it was performed in an academic referral center, so the prevalences may not be generalizable to other practice settings. However, the features of subjects with esophageal eosinophilia, EoE, and PPI-REE are similar to those previously reported and the prevalence of PPI-REE is in the same range as other studies. Second, not every subject can be recruited in a PPI-naïve state, so some EoE patients had their index endoscopy on PPI. While this limits the sample size for baseline PPI-naïve comparisons, a post-hoc sensitivity analysis showed that there were no significant differences between EoE patients who had a PPI-naïve baseline endoscopy and those who did not. Furthermore, our population is similar to that which might be encountered by the practicing clinician, given the ubiquity of PPI use. Third, patients found to not have esophageal eosinophilia could be on a PPI at the time of their index endoscopy. It is theoretically possible that a proportion of these patients could have had unknown esophageal eosinophilia and were then misclassified as normal when they actually had PPI-REE. However, this is likely pertinent only for a small number, and if misclassification were present, it would bias the differences noted between the study groups towards the null and underestimate the prevalence of PPI-REE. Misclassification could also be possible, and would also bias the results towards the null, if many of the subjects had borderline post-PPI eosinophil counts that were close to the 15 eos/hpf cutoff. For instance, 3 EoE subjects had eosinophil counts of 16 eos/hpf, and would have been reclassified with just two cells fewer. Fourth, while a symptom response was included in the definition of PPI-REE, given that there were no validated symptom measures in EoE available when this study was designed, we relied on patient self-report, and treated it as a yes/no variable. Finally, given the size and structure of our trial, pH testing was not part of the study design. However, reflux testing has since been shown not to predict PPI-REE status (12, 14), and subjects in clinical practice are classified by PPI administration, not pH test results.
This study has multiple strengths. This is the first prospective trial in the United States to explicitly assess the prevalences of EoE and PPI-REE using a clinical PPI trial, as recommended by current guidelines for the diagnosis of EoE (1, 2). It is also among the largest cohorts of incident esophageal eosinophilia and PPI-REE reported in the literature. Because the study was prospective, it also allowed for careful exclusion of other competing causes of esophageal eosinophilia, and in fact 2 cases of eosinophilic gastroenteritis overlapping with EoE were discovered due to systematic gastric and duodenal research protocol biopsies that were performed. Follow-up was compulsive, with only a single patient lost to follow-up.
In conclusion, we found that esophageal eosinophilia is common among patients undergoing upper endoscopy, and that after a PPI trial, nearly a quarter of patients with dysphagia had EoE, and PPI-REE was almost as common, accounting for over one-third of those with ≥15 eos/hpf. Clinical, endoscopic, and histologic features could not distinguish the two groups at baseline prior to the PPI trial. This implies that novel methods are needed to distinguish these populations, with the ultimate goal of eliminating the requirement for a PPI trial from the diagnostic algorithm for EoE. However, given the current state of knowledge, these findings emphasize the necessity of a PPI trial as per current guidelines to accurately diagnose EoE. This is important clinically for patient care, but also crucial in research studies of epidemiology and treatment outcomes of patients with EoE to ensure interpretable results are generated from homogenous patient groups. Because there could be conceptual concerns with requiring a response to a pharmacologic agent in a disease definition, future investigations, potentially with molecular diagnostic methods, will be needed to clarify whether some patients with PPI-REE are a sub-phenotype of EoE, and whether EoE could eventually be diagnosed without a PPI trial.
Study highlights.
What is current knowledge?
Esophageal eosinophilia is increasingly recognized and gastroenterologists need to identify the underlying cause of inflammation.
Proton pump inhibitor-responsive esophageal eosinophilia (PPI-REE) is a newly recognized entity that must be differentiated from eosinophilic esophagitis (EoE), but little is known about this condition.
What is new here?
In this prospective cohort of patients undergoing upper endoscopy, esophageal eosinophilia was found commonly, particularly in patients with dysphagia where it was seen nearly 40% of the time.
23% of subjects with dysphagia were confirmed to have EoE after a PPI trial.
36% of subjects with esophageal eosinophilia were found to have PPI-REE.
It was difficult to distinguish PPI-REE and EoE prior to a PPI trial, and no feature independently predicted PPI-REE status.
Acknowledgments
Financial support:
Grant support: This research was conducted with support, in part, by NIH awards KL2RR025746 (ESD), K23DK090073 (ESD), and a Junior Faculty Development Award from the American College of Gastroenterology. It also utilized the Histology Core of the UNC Center for Gastrointestinal Biology and Disease which is funded by NIH P30DK034987, and the UNC Translational Pathology lab which is funded by NIH P30CA016086.
Abbreviations
- EGD
esophagogastroduodenoscopy
- EGID
eosinophilic gastrointestinal disorder
- EoE
eosinophilic esophagitis
- eos/hpf
eosinophils per high-power field
- GERD
gastroesophageal reflux disease
- mm2
square millimeters
- PPI
proton-pump inhibitor
- PPI-REE
PPI-responsive esophageal eosinophilia
- UNC
University of North Carolina
Footnotes
Specific author contributions (all authors approved the final draft):
Dellon: project conception/design; data acquisition/analysis/interpretation; drafting of the article; critical revision
Speck: data acquisition (slide review for eosinophil counts); critical revision
Woodward: data acquisition (slide review for eosinophil counts); critical revision
Gebhart: patient recruitment; data acquisition/management; critical revision
Madanick: performed procedures; data acquisition; critical revision
Levinson: performed procedures; data acquisition; critical revision
Fritchie: data acquisition (slide review for eosinophil counts); critical revision
Woosley: pathology supervision; eosinophil count review; and critical revision
Shaheen: project conception and design; supervision; data interpretation; critical revision
The study sponsors had no role in the study design, collection, analysis, or interpretation of the data.
Potential competing interests:
There are no potential conflicts of interest for any of the authors pertaining to this study.
References
- 1.Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: Updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128:3–20. e6. doi: 10.1016/j.jaci.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 2.Dellon ES, Gonsalves N, Hirano I, et al. ACG Clinical Guideline: Evidence based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis. Am J Gastroenterol. 2013;108:679–92. doi: 10.1038/ajg.2013.71. [DOI] [PubMed] [Google Scholar]
- 3.Prasad GA, Talley NJ, Romero Y, et al. Prevalence and Predictive Factors of Eosinophilic Esophagitis in Patients Presenting With Dysphagia: A Prospective Study. Am J Gastroenterol. 2007;102:2627–32. doi: 10.1111/j.1572-0241.2007.01512.x. [DOI] [PubMed] [Google Scholar]
- 4.Veerappan GR, Perry JL, Duncan TJ, et al. Prevalence of Eosinophilic Esophagitis in an Adult Population Undergoing Upper Endoscopy: A Prospective Study. Clin Gastroenterol Hepatol. 2009;7:420–426. doi: 10.1016/j.cgh.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Mackenzie SH, Go M, Chadwick B, et al. Prospective analysis of eosinophilic esophagitis in patients presenting with dysphagia. Am J Gastroenterol. 2006;101:S47. (A18) [Google Scholar]
- 6.Collins MH. Histopathologic features of eosinophilic esophagitis. Gastrointest Endosc Clin N Am. 2008;18:59–71. viii–ix. doi: 10.1016/j.giec.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Furuta GT, Liacouras CA, Collins MH, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133:1342–63. doi: 10.1053/j.gastro.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 8.Rodrigo S, Abboud G, Oh D, et al. High intraepithelial eosinophil counts in esophageal squamous epithelium are not specific for eosinophilic esophagitis in adults. Am J Gastroenterol. 2008;103:435–42. doi: 10.1111/j.1572-0241.2007.01594.x. [DOI] [PubMed] [Google Scholar]
- 9.Ngo P, Furuta GT, Antonioli DA, et al. Eosinophils in the esophagus--peptic or allergic eosinophilic esophagitis? Case series of three patients with esophageal eosinophilia. Am J Gastroenterol. 2006;101:1666–70. doi: 10.1111/j.1572-0241.2006.00562.x. [DOI] [PubMed] [Google Scholar]
- 10.Dranove JE, Horn DS, Davis MA, et al. Predictors of response to proton pump inhibitor therapy among children with significant esophageal eosinophilia. J Pediatr. 2009;154:96–100. doi: 10.1016/j.jpeds.2008.07.042. [DOI] [PubMed] [Google Scholar]
- 11.Sayej WN, Patel R, Baker RD, et al. Treatment With High-dose Proton Pump Inhibitors Helps Distinguish Eosinophilic Esophagitis From Noneosinophilic Esophagitis. J Pediatr Gastroenterol Nutr. 2009;49:393–9. doi: 10.1097/MPG.0b013e31819c4b3e. [DOI] [PubMed] [Google Scholar]
- 12.Molina-Infante J, Ferrando-Lamana L, Ripoll C, et al. Esophageal Eosinophilic Infiltration Responds to Proton Pump Inhibition in Most Adults. Clin Gastroenterol Hepatol. 2011;9:110–7. doi: 10.1016/j.cgh.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 13.Abe Y, Iijima K, Ohara S, et al. A Japanese case series of 12 patients with esophageal eosinophilia. J Gastroenterol. 2011;46:25–30. doi: 10.1007/s00535-010-0295-4. [DOI] [PubMed] [Google Scholar]
- 14.Francis DL, Foxx-Orenstein A, Arora AS, et al. Results of ambulatory pH monitoring do not reliably predict response to therapy in patients with eosinophilic oesophagitis. Aliment Pharmacol Ther. 2012;35:300–7. doi: 10.1111/j.1365-2036.2011.04922.x. [DOI] [PubMed] [Google Scholar]
- 15.Schroeder S, Capocelli KE, Masterson JC, et al. Effect of proton pump inhibitor on esophageal eosinophilia. J Pediatr Gastroenterol Nutr. 2013;56:166–72. doi: 10.1097/MPG.0b013e3182716b7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonsalves N, Policarpio-Nicolas M, Zhang Q, et al. Histopathologic variability and endoscopic correlates in adults with eosinophilic esophagitis. Gastrointest Endosc. 2006;64:313–9. doi: 10.1016/j.gie.2006.04.037. [DOI] [PubMed] [Google Scholar]
- 17.Dellon ES, Fritchie KJ, Rubinas TC, et al. Inter- and intraobserver reliability and validation of a new method for determination of eosinophil counts in patients with esophageal eosinophilia. Dig Dis Sci. 2010;55:1940–9. doi: 10.1007/s10620-009-1005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dellon ES, Aderoju A, Woosley JT, et al. Variability in diagnostic criteria for eosinophilic esophagitis: A systematic review. Am J Gastroenterol. 2007;102:2300–13. doi: 10.1111/j.1572-0241.2007.01396.x. [DOI] [PubMed] [Google Scholar]
- 19.Desai TK, Stecevic V, Chang CH, et al. Association of eosinophilic inflammation with esophageal food impaction in adults. Gastrointest Endosc. 2005;61:795–801. doi: 10.1016/s0016-5107(05)00313-5. [DOI] [PubMed] [Google Scholar]
- 20.Kerlin P, Jones D, Remedios M, et al. Prevalence of eosinophilic esophagitis in adults with food bolus obstruction of the esophagus. J Clin Gastroenterol. 2007;41:356–61. doi: 10.1097/01.mcg.0000225590.08825.77. [DOI] [PubMed] [Google Scholar]
- 21.Hruz P, Straumann A, Bussmann C, et al. Escalating incidence of eosinophilic esophagitis: A 20-year prospective, population-based study in Olten County, Switzerland. J Allergy Clin Immunol. 2011;128:1349–1350. e5. doi: 10.1016/j.jaci.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 22.Peterson KA, Thomas KL, Hilden K, et al. Comparison of esomeprazole to aerosolized, swallowed fluticasone for eosinophilic esophagitis. Dig Dis Sci. 2010;55:1313–9. doi: 10.1007/s10620-009-0859-4. [DOI] [PubMed] [Google Scholar]
- 23.Moawad FJ, Veerappan GR, Dias JA, et al. Randomized controlled trial comparing aerosolized swallowed fluticasone to esomeprazole for esophageal eosinophilia. Am J Gastroenterol. 2013;108:366–72. doi: 10.1038/ajg.2012.443. [DOI] [PubMed] [Google Scholar]
- 24.Vazquez-Elizondo G, Ngamruengphong S, DeVault KR, et al. The outcome of patients with esophageal eosinophilic infiltration after a PPI trial. Gastroenterology. 2013;144 (Suppl 1):S493. doi: 10.1111/apt.12513. (Su1857) [DOI] [PubMed] [Google Scholar]
- 25.Mangla S, Singal G, Hornick JL, et al. Clinical predictors of response to proton pump inhibitors in patients with esophageal eosinophilia. Gastroenterology. 2013;144 (Suppl 1):S495–6. (Su1866) [Google Scholar]
- 26.Fujiwara Y, Sugawa T, Tanaka F, et al. A multicenter study on the prevalence of Eosinophilic Esophagitis and PPI-responsive esophageal eosinophilic infiltration. Intern Med. 2012;51:3235–9. doi: 10.2169/internalmedicine.51.8670. [DOI] [PubMed] [Google Scholar]
- 27.Spechler SJ, Genta RM, Souza RF. Thoughts on the complex relationship between gastroesophageal reflux disease and eosinophilic esophagitis. Am J Gastroenterol. 2007;102:1301–6. doi: 10.1111/j.1572-0241.2007.01179.x. [DOI] [PubMed] [Google Scholar]
- 28.Merwat SN, Spechler SJ. Might the use of acid-suppressive medications predispose to the development of eosinophilic esophagitis? Am J Gastroenterol. 2009;104:1897–902. doi: 10.1038/ajg.2009.87. [DOI] [PubMed] [Google Scholar]
- 29.Cheng E, Zhang X, Huo X, et al. Omeprazole blocks eotaxin-3 expression by oesophageal squamous cells from patients with eosinophilic oesophagitis and GORD. Gut. 2013;62:824–32. doi: 10.1136/gutjnl-2012-302250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang X, Cheng E, Huo X, et al. Omeprazole blocks STAT6 binding to the eotaxin-3 promoter in eosinophilic esophagitis cells. PLoS One. 2012;7:e50037. doi: 10.1371/journal.pone.0050037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu Y, Levine JL, Edelman M, et al. Symptomatic improvement in children with eosinophilic esophagitis treated with proton pump inhibitors may be due to decreased eosinophil degranulation. Gastroenterology. 2013;144 (Suppl 1):S489. (Su1844) [Google Scholar]
- 32.Moawad FJ, Schoepfer A, Ally MR, et al. Eosinophilic esophagitis and proton pump inhibitor responsive esophageal eosinophilia: Are they one and the same? Gastroenterology. 2013;144 (Suppl 1):S489. (Su1845) [Google Scholar]
- 33.Molina-Infante J, Rivas MD, Rodriguez GV, et al. Remission in proton upmp inhibitors-responsive esophageal eosinophilia correlates with downregulation of eotaxin-3 and TH2 cytokines, similarly to eosinophilic esophagitis after steroids. Gastroenterology. 2013;144 (Suppl 1):S484. (Su1828) [Google Scholar]
- 34.Dohil R, Newbury RO, Aceves S. Transient PPI Responsive Esophageal Eosinophilia May Be a Clinical Sub-phenotype of Pediatric Eosinophilic Esophagitis. Dig Dis Sci. 2012;57:1413–9. doi: 10.1007/s10620-011-1991-5. [DOI] [PubMed] [Google Scholar]
- 35.Dellon ES, Gibbs WB, Fritchie KJ, et al. Clinical, endoscopic, and histologic findings distinguish eosinophilic esophagitis from gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2009;7:1305–1313. doi: 10.1016/j.cgh.2009.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]


