Abstract
The purpose of this review is to summarize the available information regarding salt sensitivity particularly as it relates to non-Hispanic blacks and Hispanics and to clarify possible etiologies, especially those that might shed light on potential treatment options. In non-Hispanic blacks, there is evidence that endothelial dysfunction, reduced potassium intake, decreased urinary kallikrein excretion, upregulation of sodium channel activity, dysfunction in atrial natriuretic peptide (ANP) production, and APOL1 gene nephropathy risk variants may cause or contribute to salt sensitivity. Supported treatment avenues include diets high in potassium and soybean protein, the components of which stimulate nitric oxide production. Racial heterogeneity complicates the study of salt sensitivity in Hispanic populations. Caribbean Hispanics, who have a higher proportion of African ancestry, may respond to commonly prescribed anti-hypertensive agents in a way that is characteristic of non-Hispanic black hypertensives. The low-renin hypertensive phenotype commonly seen in non-Hispanic blacks has been linked to salt sensitivity and may indicate an increased risk for salt sensitivity in a portion of the Hispanic population. In conclusion, increased morbidity and mortality associated with salt sensitivity mandates further studies evaluating the efficacy of tailored dietary and pharmacologic treatment in non-Hispanic blacks and determining the prevalence of low renin hypertension and salt sensitivity within the various subgroups of Hispanic Americans.
Keywords: Atrial natriuretic peptide, Hispanic, hypertension, kallikrein, nitric oxide, non-Hispanic black, potassium salt sensitivity, renal sodium channel
Introduction
Blood pressure (BP), a measure of the force exerted by circulating blood on arterial vessel walls, is used as an important indicator of cardiovascular health. Higher BPs are associated with an increased risk of myocardial infarction, heart failure, stroke, and kidney disease.1 Beginning at 115/75 mm Hg, an individual’s risk of developing cardiovascular disease doubles with every additional rise of 20/10 mm Hg. There is some controversy as to the short-and long-term effects of high sodium diets on BP. The BP of most healthy normotensive individuals does not adversely respond to changes in sodium intake.2 Dietary changes in sodium are normally compensated for by renal sodium excretion. Those individuals whose BP does fluctuate with changes in dietary sodium have been labeled as “salt-sensitive.”
Strictly speaking, the term “salt-sensitive” is used to describe persons with acute BP responses to changes in salt intake over days to weeks. One of the earliest studies to examine this defined salt-sensitive subjects as those whose mean arterial pressure (MAP) decreased by 10% when oral salt intake was decreased from 248 mmol/day to 9 mmol/day for 1 week, focusing on the magnitude of BP reduction after the low sodium period.3 Most studies since have used similar approaches (ie, requiring a 10 mm Hg absolute increase or a 10% relative increase between MAP on low- versus high-salt diets) to define salt sensitivity. However, some studies have sodium loaded subjects with intravenous normal saline infusions rather than oral salt intake.3–8 (Table 1)
Table 1.
Previous study methods and thresholds for establishing salt sensitivity
| Study | Method (Sodium/Day) | Salt Sensitivity Definition | Results (% of Subjects Salt-sensitive) |
|---|---|---|---|
| Morris RC6 (Normotensives) | 15 mmol/70 kg (2 weeks) → 250 mmol/70 kg (1 week) |
⊿ MAP ≥3 mm Hg | Blacks – 79% Whites – 36% |
| Kawasaki T3 (Hypertensives) | 9 mmol (1 week) → 249 mmol (1 week) | ⊿ MAP >10% | 47% |
| Weinberger MH4 | 2L NS (308 mmol) (1 day) → 10 mmol and furosemide (1 day) |
⊿ MAP ≥10 mm Hg | Normotensive – 26% Hypertensive – 51% Black normotensive – 36% Black hypertensive – 73% |
| Obarzanek E5 | 140 mmol (1 month) → 62 mmol (1 month) AND 104 mmol (1 month) → 62 mmol (1 month) |
⊿ SBP >6.4 mm Hg AND ⊿ SBP >3.4 mm Hg |
Normotensive – 20% Hypertensive – 41% |
| Wright JT8 (Women) | 20 mmol → 200 mmol (1 week) | ⊿ MAP ≥10 mm Hg | Black normotensive – 43% White normotensive – 50% Black hypertensive – 64% White hypertensive – 52% |
MAP, mean arterial pressure.
A few noteworthy reviews of salt sensitivity, including Franco, Rodriguez-Iturbe, and Katori, further discuss some of the foundation work regarding salt sensitivity.9–11 Despite the different methods of determining salt sensitivity, studies have consistently found approximately one-quarter to one-third of all normotensives, 50% of all hypertensives, and up to 75% of non-Hispanic black hypertensives to be salt-sensitive. Additionally, studies have consistently found salt sensitivity to be positively correlated with age and more common in subjects who are overweight and/or have renal insufficiency.12 The consistency of these findings makes a strong argument for the existence of the salt-sensitive phenomenon. The increased prevalence of salt sensitivity in populations with increased rates of hypertension, such as non-Hispanic blacks, suggests that it may play an important role in the pathogenesis of this dangerous disease.
Further underscoring the importance of salt sensitivity and its potentially harmful impact is data regarding the associated morbidity and mortality. Salt sensitivity has been associated with a greater propensity to develop target organ damage, such as renal failure and left ventricular hypertrophy,13 along with significantly higher urinary albumin excretion.7 Weinberger et al conducted long-term follow up of normotensive and hypertensive subjects after salt sensitivity studies. Normotensive salt-sensitive patients had the same cumulative mortality as hypertensive subjects; while salt-resistant normotensive subjects had improved survival.14 A study of hypertensive Japanese adults corroborated these results, finding salt sensitivity to be an independent risk factor for incident cardiovascular events.15 The rate of cardiovascular events, both fatal and non-fatal, was significantly higher in the salt-sensitive group (Figure 1).
Figure 1.
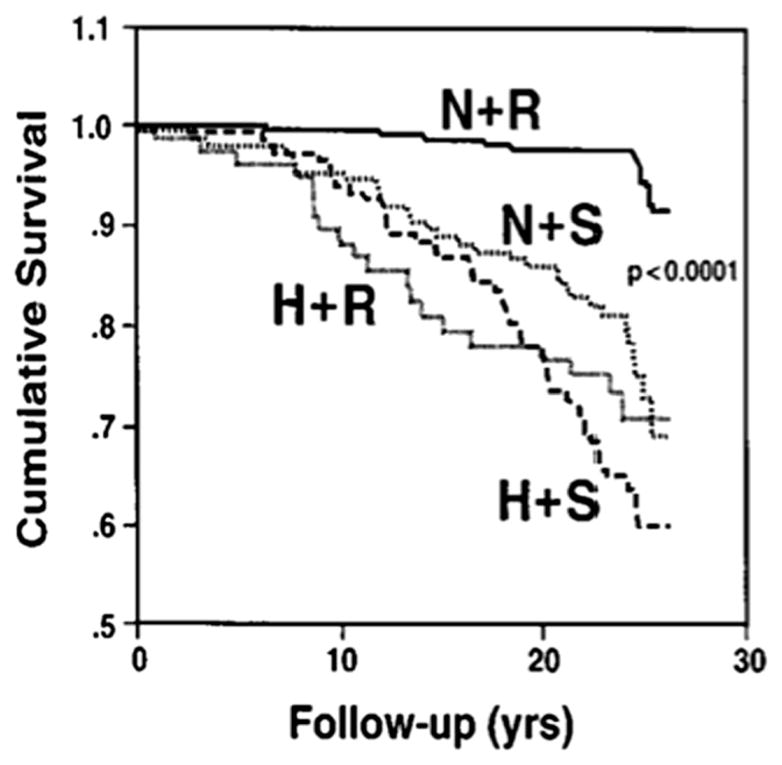
Survival curves for salt sensitive and salt resistant subjects. Kaplan-Meier survival curves for normotensive salt-resistant subjects (N+R), normotensive salt-sensitive subjects (N+S), hypertensive salt-resistant subjects (H+R), and hypertensive salt-sensitive subjects (N+S) over the follow-up period. As noted, only the N+R group had an increased survival. Adapted from Weinberger et al.14
Hypertension and salt sensitivity in non-Hispanic blacks†
Studies dating from as early as the 1930s have reported an increased prevalence of hypertension in non-Hispanic blacks.16 According to the National Health and Nutrition Examination Survey, census prevalence data reported 42.0% of non-Hispanic black adults as being hypertensive versus 29.9% of all adult Americans.17 A variety of social, behavioral, and biological theories have been proposed; however, salt sensitivity remains an underappreciated contributing factor to explain the epidemiology of hypertension among non-Hispanic blacks.
As early as the late 1960s, in one of the first studies using the newly developed quantitative essay for the measurement of plasma renin activity (PRA), black hypertensives were found to have a greater frequency of low PRA (52%) compared with white hypertensives (31%).18 More recent studies have found lower PRA in both normotensive and hypertensive blacks compared with whites.19 Interestingly, angiotensin-converting enzyme (ACE) levels have been shown to be inversely associated with BP in blacks but positively associated with BP in whites. These studies, and many others like them, point to a distinct hypertensive phenotype in blacks.
Studies have found increased peripheral sympathetic nerve activity in non-Hispanic blacks.20 However, increased sympathetic tone among non-Hispanic blacks may be more of a determinant of BP than a direct mechanism by which salt sensitivity raises blood pressure. Additionally, the finding of increased sympathetic tone in non-Hispanic blacks may be confounded by social and behavioral factors that are beyond the scope of this review.21 Regardless, the findings of both lower PRA and lower ACE levels suggest that the renin-angiotensin-aldosterone-system (RAAS) does not play a significant causative role in hypertension in non-Hispanic blacks. Over activity of the RAAS system is believed to play a significant role in hypertension in the general population. However, in non-Hispanic blacks it may be high salt intake, salt retention, and/or volume overload that both lowers RAAS activity and cause hypertension.
The existence of a distinct low-renin, volume-overloaded hypertensive phenotype is supported by evidence of increased sodium retention in non-Hispanic blacks that does not appear to be directly secondary to increased RAAS activity as noted by increased production of aldosterone, deoxycorticosterone, cortisol, or 18-hydrocortisol.22 Further support comes from the treatment response of non-Hispanic blacks to commonly prescribed anti-hypertensives. Diuretics and calcium channel blockers are more effective than anti-hypertensives that target the RAAS.23,24 Diuretics, salt restriction, and calcium channel blockers would all be expected to lower BP caused by volume overload and salt retention. The phenomenon of salt sensitivity, again defined as BP that responds acutely to changes in salt intake, is likely related to salt retention and the low-renin, volume-overloaded hypertensive phenotype. It was directly linked in a study that found that racial differences in response to RAAS active drugs are lessened when salt sensitivity is controlled for.25 Further exploration of the mechanisms and potential therapies for salt sensitivity might serve to shed some light on the low-renin hypertensive phenotype seen more commonly in non-Hispanic blacks. Further examining the pathophysiology of salt sensitivity might provide some explanation for and solution to the increased prevalence of hypertension seen in non-Hispanic blacks.
Potential mediators and mechanisms of salt sensitivity
Nitric oxide
Endothelial cells generate the potent vasodilator nitric oxide (NO) from L-arginine using the endothelial NO synthase (eNOS). Endothelial dysfunction is marked by inadequate endothelium-dependent vasodilation. Researchers often assess this by measuring flow-mediated dilation (FMD). A standard BP cuff is used to occlude the brachial artery. The diameter is measured at this point and after the cuff is deflated to find the relative increase in size.
A small study of well-matched healthy non-Hispanic black and non-Hispanic white men showed reduced FMD in non-Hispanic blacks.26 Notably, there was no difference in endothelium-independent dilation, which is measured as a response to orally administered nitrates. The authors found that circulating asymmetric dimethylarginine (ADMA) levels, a competitive endogenous inhibitor of NOS, were nearly one-third higher in non-Hispanic blacks compared with non-Hispanic whites. ADMA level was found to be the only independent determinant of FMD. This study suggests that non-Hispanic blacks exhibit higher levels of endothelial dysfunction compared with non-Hispanic whites and suggests that higher plasma ADMA levels may contribute to this.
Endothelial dysfunction in non-Hispanic blacks has been linked to salt sensitivity. In a separate study, normotensive and hypertensive middle-aged non-Hispanic black subjects were first classified as either salt-sensitive or salt-resistant.27 L-arginine infusion caused a fall in BP in all groups; however, MAP was lowered by 11.5 ± 2.5 mm Hg versus 3.7 ± 1.5 mm Hg in salt-sensitive versus salt-resistant subjects, respectively. Although the number of subjects was small, the study suggests that salt-sensitive individuals may have a defect in or dysregulation of NO production that may play a role in hypertension among non-Hispanic blacks. Endothelial dysfunction may contribute to the salt sensitivity phenotype by impeding normal compensatory vasodilation after increased salt intake and thus facilitating volume overload and causing augmented increases in BPs.
Increased soybean consumption may be one possible solution to stimulate NO production, improve endothelial function, and potentially lower BP among individuals. Soy protein contains significant amounts of isoflavones, which have been shown to improve vascular reactivity through enhanced NO synthesis. Soy proteins also have significant amounts of L-arginine, which is the substrate for eNOS, and whose increased consumption has been associated with higher plasma concentrations of NO metabolites.28 One study found a greater decrease in systolic and diastolic BP in Chinese subjects whose diets were supplemented with soybean protein.29 Soy nuts and soy milk have also been shown to lower BP with a more intense effect in hypertensive individuals.30,31 Lastly, genistein, one of several known isoflavones found in soy protein, has been found to have direct effects on eNOS activity, leading to eNOS activation and increased NO synthesis.32 Thus, soy intake may be a factor that helps with BP regulation among salt-sensitive individuals and in particular non-Hispanic blacks, but soy intake has never been tested in this regard.
Potassium
Many studies have found potassium intake to have a BP lowering effect,33 and some have examined potassium’s role in salt sensitivity. In one study of normotensive non-Hispanic white and non-Hispanic black men, participants were placed on a low sodium diet for 2 weeks, and then sodium-loaded for the following 4 weeks, during which potassium was added to their diets at incremental dose increases of 40, 70, and 120 mmol/day.6 On low sodium diets, BP, serum, and urinary electrolytes were not different between non-Hispanic blacks and non-Hispanic whites, although PRAwas predictably lower in non-Hispanic blacks. Sodium loading caused a significant BP rise in non-Hispanic blacks but not non-Hispanic whites. Seventy-nine percent of non-Hispanic blacks compared with 36% of non-Hispanic whites were found to be salt-sensitive. Potassium supplementation at 70 mmol/day reduced the percentage of salt-sensitive non-Hispanic black and non-Hispanic white participants. Supplementation to a level of 120 mmol/day was performed only in non-Hispanic black subjects and reduced the frequency of salt sensitivity in non-Hispanic black men to the levels found in non-Hispanic white men on an average potassium diet. Thus, salt sensitivity in non-Hispanic blacks may be worsened by dietary deficiencies in potassium or a need for increased potassium requirements compared with non-Hispanic whites.
The connection between salt sensitivity, dietary potassium, and ambulatory BP non-dipping has also been studied. Non-dipping is defined as a less than 10% decrease from awake to nocturnal systolic BP. Non-dipping is associated with end-organ disease such as stroke and left ventricular hypertrophy.34 In 58 normotensive healthy non-Hispanic blacks,35 non-dipping was almost six-fold more common in the salt-sensitive versus the non-salt-sensitive group (33% vs. 6%) and of the salt-sensitive non-dippers, 100% became normal dippers after a dietary intervention with supplemental potassium.
Hyperaldosteronism
Since aldosterone is involved in sodium reabsorption, it is not surprising that mineralocorticoid excess in the form of hyperaldosteronism may play a significant role in low renin hypertension. Adults with hyperaldosteronism are known to have BP that is salt-sensitive.36 Furthermore, there is the observation that low renin hypertension patients derive greater BP reduction with mineralocorticoid antagonism than with other antihypertensive drugs.37 Aldosterone excess causes inappropriate fluid retention and suppression of renin-angiotensin pathway, thus producing a picture of high aldosterone levels in setting of a low PRA despite increased sodium retention. Non-Hispanic blacks retain more sodium, have more salt sensitivity, lower plasma renin activity, and higher aldosterone levels; thus, may have a variant of hyperaldosteronism contributing to salt-sensitive hypertension.9,10,38 However, the role of aldosterone in salt sensitivity in non-Hispanic blacks has not been directly evaluated.
Renal blood flow
Renal blood flow may be important to the development of salt-sensitive hypertension. Indirectly, bilateral renal artery stenosis causes low renin hypertension39 (synonymous with the volume dependent hypertension that is usually seen in salt-sensitive individuals). Impaired renal vasodilation during high salt intake may contribute to salt sensitivity. In normotensive controls and salt-resistant hypertensive patients, a high sodium intake induces an increase in renal blood flow and glomerular filtration rate. In contrast, the renal hemodynamic response to salt loading in salt-sensitive patients or subjects is characterized by a decrease in renal blood flow.40 Salt loading may decrease renal blood flow, thus stimulating the sympathetic nervous system and resulting in further sodium retention among salt-sensitive individuals. It is not known if differential levels of NO or ADMA in salt-sensitive individuals contribute to the findings of differences in renal blood flow and vascular function in response to alteration in sodium balance.
Kallikreins and kinin generation
A potential pathophysiologic mechanism underlying salt sensitivity involves abnormalities in urinary kallikrein excretion in hypertensive and salt-sensitive subjects.11 The renal kallikrein-kinin system is a tissue-based system separate from the plasma system. Renal kallikreins generate kinins from kininogens, which stimulate the bra-dykinin receptor 2 located on the luminal membranes of the epithelial cells along the collecting duct and inhibit re-absorption of sodium and chloride. When sodium concentrations exceed the reabsorption capacity in the proximal tubule, excess sodium can be reabsorbed along the collecting duct. (Figure 2) Insufficient renal kallikrein generation by tubule cells would enhance distal sodium retention, producing a salt-sensitive phenotype.
Figure 2.

Flow diagram detailing the role of kallikrein in promoting diuresis. Kallikrein secretion, triggered by excess sodium in the connecting tubule, generates renal kinins which act on the brady-kinin receptor 2 (BK B2 R) to promote diuresis.
An association between urinary kallikrein excretion and hypertension has been observed since 1934.41 Hypertensive patients have significantly lower urinary kallikrein levels than normotensives, and decreased urinary kallikrein excretion in hypertensive subjects might mark an inability to prevent reabsorption of excess sodium. Interestingly, mean urinary kallikrein levels in non-Hispanic black normotensives are not different from mean levels in hypertensive non-Hispanic whites.42 Thus, the dysregulation in sodium reabsorption may be more pronounced among non-Hispanic blacks.
Urinary kallikrein excretion in humans is a determinant of salt sensitivity. Salt-sensitive hypertensives have decreased urinary excretion of kallikrein compared with non-salt-sensitive hypertensives.43 Those with low levels have significant increases in systolic BP with sodium loading.44 In animal models, mutant rats with reduced urinary kininogen excretion are salt-sensitive and develop higher systolic BPs with a 2% sodium diet, a diet that does not impact BP in normal rats. Verifying a causal relationship, exogenous kininogen infusion alleviated the rise in systolic BP associated with salt loading in this strain of rats.45
High-potassium diets may exert hypotensive effects by impacting the kallikrein-kinin system. An electron-microscopic study revealed that exposure to potassium caused hypertrophy and hyperplasia of the kallikrein-containing renal cells.46 In animal models, rat urinary kallikrein excretion rapidly increased by 49% within 60 minutes after potassium infusion.47 Potassium infusion also caused an immediate diuresis and natriuresis that was nearly completely inhibited by an antagonist of the kallikrein-kinin system. This indicates that the effects of potassium intake are at least in part attributable to renal kallikrein release.
These studies indicate that reduction of function in the kallikrein-kinin system may not only lead to hypertension, but also directly cause salt sensitivity. Because even normotensive non-Hispanic blacks have decreased levels of urinary kallikrein secretion, it is plausible that they would be more susceptible to inappropriately absorbing excess sodium along the collecting duct and exhibiting a salt-sensitive phenotype.
Thiazide-sensitive Na-Cl co-transporter (NCC)
Just as non-Hispanic blacks are more sensitive to salt, they are also more sensitive to thiazide diuretics.48 In animals49–51 and humans,52,53 thiazide sensitivity is related to NCC activity. Individuals without functional NCC (Gitelman syndrome) exhibit little response to thiazides, whereas individuals with strongly activated NCC (individuals with pseudohypoaldosteronism type II52) are extraordinarily responsive. In experimental work, thiazide sensitivity is widely accepted as a surrogate for NCC activity.51 This suggests that NCC activity is enhanced in non-Hispanic blacks.
In addition to genetic factors, dietary differences may contribute to this effect. Suboptimal dietary potassium intake leads to sodium chloride retention,54 in part, by increasing NCC activity. In animals, increasing dietary potassium intake reduces the abundance of the phosphorylated (activated) form of NCC, and reducing dietary potassium intake increases it.55 Conversely, activation of NCC was shown to be essential for maintenance of salt-sensitive hypertension.56 Thus, suboptimal consumption of potassium may be one factor contributing to enhanced NCC activity in non-Hispanic blacks.
Atrial natriuretic peptide
Abnormalities in the production and function of atrial natriuretic peptide (ANP) have also been linked to salt sensitivity. ANP is synthesized in the heart, released in periods of high blood volume or pressure, and promotes natriuresis, dieresis, and vasodilation.
Under conditions of high sodium intake, salt-sensitive non-Hispanic blacks manifest a deficiency in ANP secretion.57 Under low-salt conditions, plasma ANP levels were not different in normotensive controls versus salt-sensitive or salt-resistant hypertensive non-Hispanic blacks. During high salt intake, ANP levels did not change among normotensive or salt-resistant subjects but were significantly decreased in salt-sensitive subjects. These results suggest that deficient ANP production under conditions of high salt intake acts as a potential contributor to salt sensitivity.
Animal and genetic studies have implicated a defect in ANP production as a contributor to salt-sensitive hypertension. Corin is a transmembrane serine protease that converts pro-ANP to active ANP. Knockout mice lacking corin develop hypertension and salt sensitivity.58 Epidemiologic study has demonstrated that the minor corin allele I555/P568 is more common in non-Hispanic blacks than non-Hispanic whites (~12% vs <0.2%) and is associated with an increased risk for hypertension.59 This corin genotypic variant may be associated with abnormal loss of function causing dysregulated reduced ANP generation and contributing to the decreased natriuretic response to a sodium load and the salt-sensitive phenotype in non-Hispanic blacks.
Other genetic determinants of renal sodium excretion
Increased epithelial sodium channel (ENaC) activity leads to increased reabsorption of sodium being filtered by the kidney, which can produce a low-renin, volume-overloaded hypertensive phenotype. Common genetic variants of ENaC in non-Hispanic blacks exist in the kidney sites of sodium reabsorption.60 Seven single nucleotide polymorphisms in the gene responsible for the ENaC have been found, one of which, the T594M allele, may be associated with hypertension. This allele was found in ~6.1% of normo- and hypertensive non-Hispanic blacks, but not in non-Hispanic whites.61 In this study and others,60 genetic ENaC variants were found to be associated with higher ENaC activity and more sodium reabsorption in response to stimulation.
Apolipoprotein L1 gene (APOL1) nephropathy risk variants are present in 50% of non-Hispanic blacks.62,63 These allele variants protected from trypanosomal infection in sub-Saharan Africa; however, two copies markedly increase the risk of non-diabetic end stage renal disease. APOL1-associated subclinical nephropathy could lead to an inability to rapidly excrete salt loads, potentially contributing to the salt sensitivity phenotype and to the risk of progressive nephropathy in non-Hispanic blacks.
Hypertension and salt sensivity in Hispanics
According to 2010 Census data, Hispanics make up over 16% of the United States population.64 This ethnic group is composed of several subgroups, including those with origins in Mexico, the Caribbean, and Central and South America. Mexicans and Caribbean Hispanics make up the largest proportion of United State Hispanics at about 65% and 19%, respectively.64 Hispanics are a racially and genetically heterogeneous group, due to differing proportions of European, African, and Native American ancestral influence. For example, in general, Mexican-origin Hispanics are of primarily Native American ancestry, whereas Caribbean Hispanics have a greater admixture of African ancestry compared with Mexicans.65
Understanding some of the genetic diversity within the Hispanic population can help to make sense of the limited and sometimes seemingly contradictory data on hypertension and salt sensitivity in this group. The 2005–2008 National Health and Nutrition Examination Surveys reports an age-adjusted hypertension prevalence of 42.0% among non-Hispanic blacks, 28.8% among non-Hispanic whites, and 28.8% among Mexicans. The Northern Manhattan Study found hypertension to be more common in non-Hispanic blacks and in a population of Hispanics that is largely Caribbean, compared with non-Hispanic whites, with rates of 62%, 58%, and 43%, respectively.66 The Multi-Ethnic Study of Atherosceloris also revealed that, when looking at Hispanic subgroups, the prevalence of hypertension among Hispanics of Caribbean origin approximated that of non-Hispanic blacks, while the prevalence in Mexican-origin Hispanics approximated that of non-Hispanic whites.67 Additionally, in a study of 438 non-Hispanic black, non-Hispanic white, and Caribbean Hispanic hypertensives, 24-hour average BP was similar in all racial groups; however, the absence of nocturnal dipping was more common in non-Hispanic black and Caribbean Hispanic men.68
In the following sections, we will present what limited information is currently available about salt sensitivity in Hispanics. There is no data estimating the prevalence of salt sensitivity in this large ethnic group. As for determining etiology, unfortunately, in many cases circumstantial evidence is all there is to draw conclusions from. Although speculative, it may that when it comes to salt sensitivity and hypertension, Caribbean-origin Hispanics may have some similarities to non-Hispanic blacks that have been underappreciated and deserve further study.
African ancestry and low-renin hypertensive phenotype
A study of anti-hypertensive medication efficacy in 117 subjects, 76% of whom were Caribbean-Hispanic, found this group to respond similarly to non-Hispanic blacks.69 Subjects were randomized to receive placebo, beta blocker, ACE inhibitor (ACEI), calcium channel blocker (CCB), hydrochlorothiazide (HCTZ) monotherapy, or a combination of HCTZ and ACEI for 8 to 12 weeks. Results showed that the BP reduction only by CCB, HCTZ, or the combination of ACEI and HCTZ to be significantly greater then placebo.
These results suggest that Caribbean Hispanics, who have a greater proportion of African ancestry than Mexican-origin Hispanics, may exhibit a similar hypertensive phenotype to non-Hispanic blacks. In non-Hispanic blacks, a stronger response to diuretics than to RAAS inhibiting drugs is thought to reflect an etiology of hypertension that implicates sodium retention and volume overload, which can be measured as salt sensitivity, as causes of the observed decrease in RAAS activity. This is in opposition to the high RAAS activity observed in non-Hispanic white hypertensives where ACEIs are generally felt to be more efficacious.
Obesity
An association between salt sensitivity and obesity was evaluated in a 12-month study of 20 otherwise healthy, salt-sensitive, obese Caribbean-Hispanics. After obtaining baseline values including weight and BP on low- and high-salt diets, subjects were put on a lifestyle program with a goal of weight reduction, and prescribed Metformin, titrated up to 500 mg three times a day.70 This intervention achieved a significant reduction in subject’s weight by 13%, reductions in systolic and diastolic BP, and most notably, reduced the sensitivity of the participant’s BP to salt intake by about 40%. Researchers also measured urinary excretion of NO metabolites. At baseline, excretion of NO metabolites (suggestive of NO production) decreased on the high sodium diet versus the low sodium diet. After the weight loss and Metformin intervention, NO metabolite excretion remained high regardless of whether the participant was on a low salt or high salt diet. This small study supports the notion that obesity acts as a contributor to the salt sensitivity phenotype via a mechanism that may involve blunted NO production in the obese state.
Discussion
The research discussed here points to endothelial dysfunction caused by decreased bioavailability of NO, reduced dietary potassium intake, decreased urinary kallikrein excretion, dysfunction in ANP production, and reduced sodium excretion as potential causes for salt sensitivity in non-Hispanic blacks. Each of these possible etiologies either alone or in combination with one another could cause the low-renin, volume-overloaded hypertensive phenotype seen more commonly in non-Hispanic blacks. (Table 2)
Table 2.
Potential mediators and mechanisms of salt sensitivity
| Mediator | Mechanism | Genetic or Acquired | Potential Treatment |
|---|---|---|---|
| Nitric oxide (NO) | Reduced NO production and/or increased plasma levels of ADMA (a competitive antagonist of NO) impedes normal compensatory vasodilation after increased salt intake | ? | Increased intake of soybean protein, the components of which stimulate NO production |
| Potassium | Inadequate potassium intake causes decreased generation of renal kinins, which inhibit reabsorption of sodium and chloride | Acquired | Increased potassium intake |
| Atrial Naturietic Peptide (ANP) | Minor corin allele causes dysfunctional conversation of pro-ANP to ANP, a peptide which promotes natriuresis, diuresis and vasodilation | Genetic | ? |
| Renal sodium channel | The T594M allele causes generalized upregulation of renal sodium channels and increased sodium reabsorption while APOL1 risk variant contribute to decreased sodium excretion | Genetic | Reduced sodium intake |
| Obesity | Obesity causes decreased NO production on high salt diets | Acquired | Healthy lifestyle behaviors |
Salt sensitivity in particular has not been studied extensively in Hispanics. Mexican-origin Hispanics seem to have a prevalence of hypertension that is lower than or comparable to the prevalence in the general population. However, studies suggests an increased prevalence of hypertension in Caribbean Hispanics compared with non-Hispanic whites and a response profile to anti-hypertensive agents similar to that seen in non-Hispanic blacks. Many of the factors described contributing to salt sensitivity among non-Hispanic blacks may also impact the prevalence of hypertension among Caribbean Hispanics who tend to have higher proportions of African ancestry. There is a clear need for further evaluation of the prevalence and etiology of the salt-sensitive phenotype within the various Hispanic subgroups. More information about the type and characteristics of salt-sensitive hypertension within Hispanics could lead to more efficient clinical and public health efforts to understanding and treating hypertension in this large segment of the United States population.
Salt sensitivity is an entity that has been validated in the aforementioned research. The role of salt sensitivity among non-Hispanic blacks is more defined and developed compared with that in Hispanics. The increased mortality and morbidity that has been associated with salt sensitivity places more importance on further research that might identify clinical markers and targeted treatment options. In addition to continued efforts at isolating pathophysiologic mechanisms, the effects of diets high in soybean protein merit evaluation in all salt-sensitive hypertensives, particularly non-Hispanic blacks. Lastly, large-scale examination of urinary kallikrein excretion may validate this as a potentially useful clinical marker of salt sensitivity.
Footnotes
For consistency, this review identifies race based on race AND ethnicity and allows for the terms non-Hispanic black and non-Hispanic white. It is acknowledged that several of the original source used for this review used other terms to identify race such as African-American or blacks.
References
- 1.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–13. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 2.Kotchen TA, McCarron DA. Dietary electrolytes and blood pressure: A statement for healthcare professionals from the American Heart Association Nutrition Committee. Circulation. 1998;98:613–7. doi: 10.1161/01.cir.98.6.613. [DOI] [PubMed] [Google Scholar]
- 3.Kawasaki T, Delea CS, Bartter FC, Smith H. The effect of high-sodium and low-sodium intakes on blood pressure and other related variables in human subjects with idiopathic hypertension. Am J Med. 1978;64:193–8. doi: 10.1016/0002-9343(78)90045-1. [DOI] [PubMed] [Google Scholar]
- 4.Weinberger M, Miller J, Luft F, Grim C, Fineberg N. Definitions and characteristics of sodium sensitivity and blood pressure resistance. Hypertension. 1986;8:II127. doi: 10.1161/01.hyp.8.6_pt_2.ii127. [DOI] [PubMed] [Google Scholar]
- 5.Obarzanek E, Proschan MA, Vollmer WM, Moore TJ, Sacks FM, Appel LJ, et al. Individual blood pressure responses to changes in salt intake. Hypertension. 2003;42:459–67. doi: 10.1161/01.HYP.0000091267.39066.72. [DOI] [PubMed] [Google Scholar]
- 6.Morris RC, Jr, Sebastian A, Forman A, Tanaka M, Schmidlin O. Normotensive salt sensitivity: effects of race and dietary potassium. Hypertension. 1999;33:18–23. doi: 10.1161/01.hyp.33.1.18. [DOI] [PubMed] [Google Scholar]
- 7.Bihorac A, Tezcan H, Özener Ç, Oktay A, Akoglu E. Association between salt sensitivity and target organ damage in essential hypertension. AM J Hypertens. 2000;13:864–72. doi: 10.1016/s0895-7061(00)00253-3. [DOI] [PubMed] [Google Scholar]
- 8.Wright JT, Jr, Rahman M, Scarpa A, Fatholahi M, Griffin V, Jean-Baptiste R, et al. Determinants of salt sensitivity in black and white normotensive and hypertensive women. Hypertension. 2003;42:1087–92. doi: 10.1161/01.HYP.0000101687.89160.19. [DOI] [PubMed] [Google Scholar]
- 9.Kotchen TA, Kotchen JM, Grim CE, Krishnaswami S, Kidambi S. Aldosterone and alterations of hypertension-related vascular function in African Americans. Am J Hypertens. 2009;22:319–24. doi: 10.1038/ajh.2008.327. [DOI] [PubMed] [Google Scholar]
- 10.Kidambi S, Kotchen JM, Krishnaswami S, Grim CE, Kotchen TA. Aldosterone contributes to blood pressure variance and to likelihood of hypertension in normal-weight and overweight African Americans. Am J Hypertens. 2009;22:1303–8. doi: 10.1038/ajh.2009.167. [DOI] [PubMed] [Google Scholar]
- 11.Katori M, Majima M. Are all individuals equally sensitive in the blood pressure to high salt intake? Acta Physiologica Hungarica. 2008;95:247–65. doi: 10.1556/APhysiol.95.2008.3.2. [DOI] [PubMed] [Google Scholar]
- 12.Savica V, Bellinghieri G, Kopple JD. The effect of nutrition on blood pressure. Annu Rev Nutr. 2010;30:365–401. doi: 10.1146/annurev-nutr-010510-103954. [DOI] [PubMed] [Google Scholar]
- 13.Campese VM. Salt sensitivity in hypertension. Renal and cardiovascular implications [clinical conference] Hypertension. 1994;23:531–50. doi: 10.1161/01.hyp.23.4.531. [DOI] [PubMed] [Google Scholar]
- 14.Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension. 2001;37:429–32. doi: 10.1161/01.hyp.37.2.429. [DOI] [PubMed] [Google Scholar]
- 15.Morimoto A, Uzu T, Fujii T, Nishimura M, Kuroda S, Nakamura S, et al. Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet. 1997;350:1734–7. doi: 10.1016/S0140-6736(97)05189-1. [DOI] [PubMed] [Google Scholar]
- 16.Adams JM. Some racial differences in blood pressures and morbidity in a group of white and colored workmen. Am J Med Sci. 1932;184:342. [Google Scholar]
- 17.Keenan NL, Rosendorf KA. Prevalence of hypertension and controlled hypertension — United States, 2005–2008. MMWR Morb Mortal Wkly Rep. 2011;60:94–7. [PubMed] [Google Scholar]
- 18.Helmer OM, Judson WE. Metabolic studies on hypertensive patients with suppressed plasma renin activity not due to hyperaldosteronism. Circulation. 1968;38:965–76. doi: 10.1161/01.cir.38.5.965. [DOI] [PubMed] [Google Scholar]
- 19.He J, Klag MJ, Appel LJ, Charleston J, Whelton PK. The renin-angiotensin system and blood pressure: Differences between blacks and whites. Am J Hypertens. 1999;12:555–62. doi: 10.1016/s0895-7061(99)00030-8. [DOI] [PubMed] [Google Scholar]
- 20.Fields LE, Burt VL, Cutler JA, Hughes J, Roccella EJ, Sorlie P. The burden of adult hypertension in the United States 1999 to 2000: A rising tide. Hypertension. 2004;44:398–404. doi: 10.1161/01.HYP.0000142248.54761.56. [DOI] [PubMed] [Google Scholar]
- 21.Anderson NB, Myers HF, Pickering T, Jackson JS. Hypertension in blacks: Psychosocial and biological perspectives. J Hypertens. 1989;7:161. [PubMed] [Google Scholar]
- 22.Pratt JH, Rebhun JF, Zhou L, Ambrosius WT, Newman SA, Gomez-Sanchez CE, et al. Levels of mineralocorticoids in whites and blacks. Hypertension. 1999;34:315–9. doi: 10.1161/01.hyp.34.2.315. [DOI] [PubMed] [Google Scholar]
- 23.Materson BJ, Reda DJ, Cushman WC, Massie BM, Freis ED, Kochar MS, et al. Single-drug therapy for hypertension in men–a comparison of six antihypertensive agents with placebo. NEJM. 1993;328:914–21. doi: 10.1056/NEJM199304013281303. [DOI] [PubMed] [Google Scholar]
- 24.Wright JT, Jr, Dunn JK, Cutler JA, Davis BR, Cushman WC, Ford CE, et al. Outcomes in hypertensive black and nonblack patients treated with chlorthalidone, amlodipine, and lisinopril. JAMA. 2005;293:1595–608. doi: 10.1001/jama.293.13.1595. [DOI] [PubMed] [Google Scholar]
- 25.Weir MR, Chrysant SG, McCarron DA, Canossa-Terris M, Cohen JD, Gunter PA, et al. Influence of race and dietary salt on the antihypertensive efficacy of an angiotensin-converting enzyme inhibitor or a calcium channel antagonist in salt-sensitive hypertensives. Hypertension. 1998;31:1088–96. doi: 10.1161/01.hyp.31.5.1088. [DOI] [PubMed] [Google Scholar]
- 26.Melikian N, Wheatcroft SB, Ogah OS, Murphy C, Chowienczyk PJ, Wierzbicki AS, et al. Asymmetric di-methylarginine and reduced nitric oxide bioavailability in young Black African men. Hypertension. 2007;49:873–7. doi: 10.1161/01.HYP.0000258405.25330.80. [DOI] [PubMed] [Google Scholar]
- 27.Campese V, Amar M, Anjali C, Medhat T, Wurgaft A. Effect of L-arginine on systemic and renal haemodynamics in salt-sensitive patients with essential hypertension. J Hum Hypertens. 1997;11:527. doi: 10.1038/sj.jhh.1000485. [DOI] [PubMed] [Google Scholar]
- 28.Hallund J, Bugel S, Tholstrup T, Ferrari M, Talbot D, Hall W, et al. Soya isoflavone-enriched cereal bars affect markers of endothelial function in postmenopausal women. Br J Nutr. 2006;95:1120–6. doi: 10.1079/bjn20061734. [DOI] [PubMed] [Google Scholar]
- 29.He J, Gu D, Wu X, Chen J, Duan X, Whelton PK. Effect of soybean protein on blood pressure: A randomized, controlled trial. Ann Intern Med. 2005;143:1–9. doi: 10.7326/0003-4819-143-1-200507050-00004. [DOI] [PubMed] [Google Scholar]
- 30.Welty FK, Lee KS, Lew NS, Zhou JR. Effect of soy nuts on blood pressure and lipid levels in hypertensive, prehypertensive, and normotensive postmenopausal women. Arch Intern Med. 2007;167:1060. doi: 10.1001/archinte.167.10.1060. [DOI] [PubMed] [Google Scholar]
- 31.Rivas M, Garay RP, Escanero JF, Cia P, Jr, Cia P, Alda JO. Soy milk lowers blood pressure in men and women with mild to moderate essential hypertension. J Nutr. 2002;132:1900–2. doi: 10.1093/jn/132.7.1900. [DOI] [PubMed] [Google Scholar]
- 32.Liu D, Homan LL, Dillon JS. Genistein acutely stimulates nitric oxide synthesis in vascular endothelial cells by a cyclic adenosine 5′-monophosphate-dependent mechanism. Endocrinology. 2004;145:5532–9. doi: 10.1210/en.2004-0102. [DOI] [PubMed] [Google Scholar]
- 33.Cappuccio FP, MacGregor GA. Does potassium supplementation lower blood pressure? A meta-analysis of published trials. J Hypertens. 1991;9:465. doi: 10.1097/00004872-199105000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Kobrin I, Oigman W, Kumar A, Ventura H, Messerli F, Frohlich E, et al. Diurnal variation of blood pressure in elderly patients with essential hypertension. J Am Geriatr Soc. 1984;32:896. doi: 10.1111/j.1532-5415.1984.tb00890.x. [DOI] [PubMed] [Google Scholar]
- 35.Wilson DK, Sica DA, Miller SB. Effects of potassium on blood pressure in salt-sensitive and salt-resistant black adolescents. Hypertension. 1999;34:181–6. doi: 10.1161/01.hyp.34.2.181. [DOI] [PubMed] [Google Scholar]
- 36.Kimura G, Brenner BM. A method for distinguishing salt-sensitive from non-salt-sensitive forms of human and experimental hypertension. Curr Opin Nephrol Hypertens. 1993;2:341–9. [PubMed] [Google Scholar]
- 37.Hood SJ, Taylor KP, Ashby MJ, Brown MJ. The spironolactone, amiloride, losartan, and thiazide (SALT) double-blind crossover trial in patients with low-renin hypertension and elevated aldosterone-renin ratio. Circulation. 2007;116:268–75. doi: 10.1161/CIRCULATIONAHA.107.690396. [DOI] [PubMed] [Google Scholar]
- 38.Shibata S, Fujita T. The kidneys and aldosterone/mineralocorticoid receptor system in salt-sensitive hypertension. Curr Hypertens Rep. 2011;13:109–15. doi: 10.1007/s11906-010-0175-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilcox CS. Use of angiotensin-converting-enzyme inhibitors for diagnosing renovascular hypertension. Kidney Int. 1993;44:1379–90. doi: 10.1038/ki.1993.392. [DOI] [PubMed] [Google Scholar]
- 40.Campese VM, Parise M, Karubian F, Bigazzi R. Abnormal renal hemodynamics in black salt-sensitive patients with hypertension. Hypertension. 1991;18:805–12. doi: 10.1161/01.hyp.18.6.805. [DOI] [PubMed] [Google Scholar]
- 41.Elliot A, Nuzum F. The urinary excretion of a depressor substance (kallikrein of Frey and Kraut) in arterial hypertension. Endocrinology. 1934;18:462–74. [Google Scholar]
- 42.Carretero OA, Scicli AG. The renal kallikrein-kinin system in human and in experimental hypertension. J Mol Med (Berl) 1978;56:113–25. doi: 10.1007/BF01477462. [DOI] [PubMed] [Google Scholar]
- 43.Ferri C, Bellini C, Carlomagno A, Perrone A, Santucci A. Urinary kallikrein and salt sensitivity in essential hypertensive males. Kidney Int. 1994;46:780–8. doi: 10.1038/ki.1994.333. [DOI] [PubMed] [Google Scholar]
- 44.Bönner G, Thieven B, Rütten H, Chrosch R, Krone W. Renal kallikrein is a determinant of salt sensitivity. J Hypertens. 1993;11:S210. [PubMed] [Google Scholar]
- 45.Majima M, Yoshida O, Mihara H, Muto T, Mizogami S, Kuribayashi Y, et al. High sensitivity to salt in kininogen-deficient Brown Norway Katholiek rats. Hypertension. 1993;22:705–14. doi: 10.1161/01.hyp.22.5.705. [DOI] [PubMed] [Google Scholar]
- 46.Vío CP, Figueroa CD. Evidence for a stimulatory effect of high potassium diet on renal kallikrein. Kidney Int. 1987;31:1327–34. doi: 10.1038/ki.1987.146. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki T, Katori M, Fujita T, Kumagai Y, Majima M. Involvement of the renal kallikrein-kinin system in K+-induced diuresis and natriuresis in anesthetized rats. Eur J Pharmacol. 2000;399:223–7. doi: 10.1016/s0014-2999(00)00382-4. [DOI] [PubMed] [Google Scholar]
- 48.Chapman AB, Schwartz GL, Boerwinkle E, Turner ST. Predictors of antihypertensive response to a standard dose of hydrochlorothiazide for essential hypertension. Kidney Int. 2002;61:1047–55. doi: 10.1046/j.1523-1755.2002.00200.x. [DOI] [PubMed] [Google Scholar]
- 49.Lalioti MD, Zhang J, Volkman HM, Kahle KT, Hoffmann KE, Toka HR, et al. Wnk4 controls blood pressure and potassium homeostasis via regulation of mass and activity of the distal convoluted tubule. Nat Genet. 2006;38:1124–32. doi: 10.1038/ng1877. [DOI] [PubMed] [Google Scholar]
- 50.Hoorn EJ, Walsh SB, McCormick JA, Fürstenberg A, Yang CL, Roeschel T, et al. The calcineurin inhibitor tacrolimus activates the renal sodium chloride cotrans-porter to cause hypertension. Nat Med. 2011;17:1304–9. doi: 10.1038/nm.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Madala Halagappa VK, Tiwari S, Riazi S, Hu X, Ecelbarger CM. Chronic candesartan alters expression and activity of NKCC2, NCC, and ENaC in the obese Zucker rat. Am J Physiol Renal Physiol. 2008;294:F1222–31. doi: 10.1152/ajprenal.00604.2007. [DOI] [PubMed] [Google Scholar]
- 52.Mayan H, Vered I, Mouallem M, Tzadok-Witkon M, Pauzner R, Farfel Z. Pseudohypoaldosteronism type II: Marked sensitivity to thiazides, hypercalciuria, normomagnesemia, and low bone mineral density. J Clin Endocrinol Metab. 2002;87:3248–54. doi: 10.1210/jcem.87.7.8449. [DOI] [PubMed] [Google Scholar]
- 53.Colussi G, Bettinelli A, Tedeschi S, De Ferrari ME, Syren ML, Borsa N, et al. A thiazide test for the diagnosis of renal tubular hypokalemic disorders. Clin J Am Soc Nephrol. 2007;2:454–60. doi: 10.2215/CJN.02950906. [DOI] [PubMed] [Google Scholar]
- 54.Krishna GG. Effect of potassium intake on blood pressure. J Am Soc Nephrol. 1990;1:43–52. doi: 10.1681/ASN.V1143. [DOI] [PubMed] [Google Scholar]
- 55.Vallon V, Schroth J, Lang F, Kuhl D, Uchida S. Expression and phosphorylation of the Na+-Cl− cotransporter NCC in vivo is regulated by dietary salt, potassium, and SGK1. Am J Physiol Renal Physiol. 2009;297:F704–12. doi: 10.1152/ajprenal.00030.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Capasso G, Rizzo M, Garavaglia ML, Trepiccione F, Zacchia M, Mugione A, et al. Upregulation of apical sodium-chloride cotransporter and basolateral chloride channels is responsible for the maintenance of salt-sensitive hypertension. Am J Physiol Renal Physiol. 2008;295:F556–67. doi: 10.1152/ajprenal.00340.2007. [DOI] [PubMed] [Google Scholar]
- 57.Campese VM, Tawadrous M, Bigazzi R, Bianchi S, Mann AS, Oparil S, et al. Salt intake and plasma atrial natriuretic peptide and nitric oxide in hypertension. Hypertension. 1996;28:335–40. doi: 10.1161/01.hyp.28.3.335. [DOI] [PubMed] [Google Scholar]
- 58.Chan JCY, Knudson O, Wu F, Morser J, Dole WP, Wu Q. Hypertension in mice lacking the proatrial natriuretic peptide convertase corin. Proc Natl Acad Sci U S A. 2005;102:785. doi: 10.1073/pnas.0407234102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dries DL, Victor RG, Rame JE, Cooper RS, Wu X, Zhu X, et al. Corin gene minor allele defined by 2 missense mutations is common in blacks and associated with high blood pressure and hypertension. Circulation. 2005;112:2403–10. doi: 10.1161/CIRCULATIONAHA.105.568881. [DOI] [PubMed] [Google Scholar]
- 60.Ambrosius WT, Bloem LJ, Zhou L, Rebhun JF, Snyder PM, Wagner MA, et al. Genetic variants in the epithelial sodium channel in relation to aldosterone and potassium excretion and risk for hypertension. Hypertension. 1999;34:631–7. doi: 10.1161/01.hyp.34.4.631. [DOI] [PubMed] [Google Scholar]
- 61.Su YR, Rutkowski MP, Klanke CA, Wu X, Cui Y, Pun R, et al. A novel variant of the beta-subunit of the amiloride-sensitive sodium channel in African Americans. J Am Soc Nephrol. 1996;7:2543–9. doi: 10.1681/ASN.V7122543. [DOI] [PubMed] [Google Scholar]
- 62.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329:841–5. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Freedman BI, Kopp JB, Langefeld CD, Genovese G, Friedman DJ, Nelson GW, et al. The apolipoprotein L1 (APOL1) gene and nondiabetic nephropathy in African Americans. J Am Soc Nephrol. 2010;21:1422–6. doi: 10.1681/ASN.2010070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ennis S, Ríos-Vargas M, Ng A. United States Census Briefs. 2011. The Hispanic Population: 2010. [Google Scholar]
- 65.Bertoni B, Budowle B, Sans M, Barton SA, Chakraborty R. Admixture in Hispanics: Distribution of ancestral population contributions in the Continental United States. Hum Biol. 2003;75:1. doi: 10.1353/hub.2003.0016. [DOI] [PubMed] [Google Scholar]
- 66.Sacco RL, Boden-Albala B, Abel G, Lin I, Elkind M, Hauser WA, et al. Race-ethnic disparities in the impact of stroke risk factors. Stroke. 2001;32:1725–31. doi: 10.1161/01.str.32.8.1725. [DOI] [PubMed] [Google Scholar]
- 67.Rodriguez CJ, Diez-Roux AV, Moran A, Jin Z, Kronmal RA, Lima J, et al. Left ventricular mass and ventricular remodeling among Hispanic subgroups compared with non-Hispanic blacks and whites: MESA (Multi-ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2010;55:234–42. doi: 10.1016/j.jacc.2009.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hyman DJ, Ogbonnaya K, Taylor AA, Ho K, Pavlik VN. Ethnic differences in nocturnal blood pressure decline in treated hypertensives. Am J Hypertens. 2000;13:884–91. doi: 10.1016/s0895-7061(00)00279-x. [DOI] [PubMed] [Google Scholar]
- 69.Laffer CL, Elijovich F. Essential hypertension of Caribbean Hispanics: Sodium, renin, and response to therapy. J Clin Hypertens (Greenwich) 2002;4:266–73. doi: 10.1111/j.1524-6175.2002.00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hoffmann I, Alfieri A, Cubeddu L. Effects of lifestyle changes and metformin on salt sensitivity and nitric oxide metabolism in obese salt-sensitive Hispanics. J Hum Hypertens. 2007;21:571–8. doi: 10.1038/sj.jhh.1002182. [DOI] [PubMed] [Google Scholar]


