Abstract
Splicing is a predominantly co-transcriptional process that has been shown to be tightly coupled to transcription. Chromatin structure is a key factor that mediates this functional coupling. In light of recent evidence that shows the importance of higher order chromatin organization in the coordination and regulation of gene expression, we discuss here the possible roles of long-range chromatin organization in splicing and alternative splicing regulation.
Keywords: alternative splicing, coupling between transcription and splicing, chromatin, long-range chromatin interactions
Recent evidence indicates that pre-mRNA splicing occurs co-transcriptionally, i.e., before RNA polymerase II (RNAP II) has reached the end of the gene and while the transcript is associated to chromatin.1,2 Co-transcriptional splicing seems to be prevalent for most introns and in those introns whose excision was demonstrated to take place post-transcriptionally, splice site commitment and spliceosome assembly on the pre-mRNA were shown to occur mostly co-transcriptionally.1,3 Multiple levels of alternative splicing regulation have been described.4 On the one hand, the presence and relative position of regulatory elements on the pre-mRNA determine the recruitment of splicing factors and spliceosome components,5 whose abundance, activity and intracellular localization are subjected to regulation.6 On the other hand, as a consequence of the spatio-temporal coordination, both transcription and splicing were shown to be functionally coupled.4 In one of the mechanisms, known as “recruitment coupling,” splicing factors can be recruited to splice sites by the transcription machinery,7-9 which may also affect alternative splicing outcomes through the differential recruitment of these factors to promoters and enhancers as well as by alternative promoter usage.10,11 Alternatively, in the mechanism known as “kinetic coupling,” RNAP II elongation rate was shown to regulate alternative splicing by modulating the relative timing by which splice sites and regulatory sequences are transcribed and thus exposed to splicing factor binding and spliceosome component assembly.4,12,13 RNAP II elongation rate can be regulated both by factors recruited at gene promoters or along gene bodies and by the chromatin configuration of the DNA template. This introduces chromatin structure as another regulatory layer of alternative splicing.4,14 Chromatin is a highly dynamic structure that serves as an active platform for protein recruitment through specific histone marks and DNA methylation. Similar to the behavior of several protein complexes involved in transcriptional activation or repression, splicing factors can also be recruited to chromatin by direct or indirect binding to specific histone marks.15,16 The elongation control of alternative splicing is also dependent on how tightly DNA is wrapped around nucleosomes together with the capacity of the transcribing machinery to overcome these nucleosomal physical barriers.17,18 DNA packed into chromatin is also specified by histone marks that elicit the recruitment of nonhistone proteins with enzymatic activity that further modify chromatin structure.19 All together, nucleosome positioning,20,21 histone marks that determine a more loosened or compact chromatin,22-26 and DNA methylation27 regulate RNAP II elongation through the body of genes and modulate alternative splicing choices.
DNA packed into chromatin is not linearly nor randomly organized in the nucleus. Instead, a network exists where distant regions of the genome interact in a functional manner to elicit complex coordination and regulation of gene expression.28,29 Based on chromosome conformation capture (3C) technologies, genome-wide analyses of long range genome interactions performed in various species, developmental stages and cell types upon various stimuli, are increasingly showing the importance of topology in transcriptional regulation.28,30-36 Enhancers interact with promoters of distant genes to activate transcription.37 Pioneer work on the β globin gene cluster revealed for the first time a loop formation between an enhancer element of the locus control region (LCR) and the active β-globin gene, located 50 kbp downstream.38 Most importantly, the loop is necessary for the transcriptional activation of the β globin gene.39-41 Genome-wide studies on the estradiol-mediated transcriptional activation in mammary human cells revealed that genes that have the estrogen receptor bound to their promoters without being involved in interactions with other regions of the genome display less activation upon estradiol treatment than those in which the estrogen receptor is engaged in long range interactions.30 The human genome contains more enhancers than genes and more than 50% of these enhancers are located intragenically.42,43 Many of these intragenic enhancers were reported to act as alternative promoters of their host genes.42 Additionally, it was demonstrated that the distribution of enhancer-specific histone mark H3K4me1 is more specific of the cell type than that of the promoter-specific mark H4K4me3.43 Chromatin interactions are not only restricted to promoters and enhancers. Promoters and the tails of active genes interact and this interaction seems to enhance transcriptional directionality.44 Long range interactions were also described in the establishment of transcriptional repression domains by the Polycomb group proteins.45
In view of the reported functional association between transcription and splicing, we wish to hypothesize that genome three-dimensional architecture can also have a role in alternative splicing regulation. Favoring this hypothesis, Mercer et al.46 reported that a considerable fraction of exons significantly overlaps with DNase hypersensitivity sites (DHS-exons).47 ENCODE data analysis revealed that several transcription factors and histone marks are enriched in DHS-exons, in comparison to non-DHS-exons.46 Based on the specific transcription regulatory factors and histone marks that overlap with DHS-exons, these were grouped into promoter-like, enhancer-like and cohesin-like.46 The latter refers to sites enriched in the CCCTC-binding factor (CTCF) and cohesins, proteins known to be involved in chromatin distant interactions, in particular of promoters with enhancers.47 Promoter-like DHS exons show no evidence of acting as real promoters and native ChIP-seq of H3K4me3 revealed a decrease of the enrichment of this histone mark at DHS exons, supporting the notion that the observed transcription factor and promoter specific histone mark enrichment is most probably due to the interaction of DHS exons with promoters.46 In fact, an enrichment of DHS-exons was observed in genomic regions involved in long-range chromatin interactions where the RNAP II pre-initiation complex is present.46 Interactions between DHS-exons and promoters were also found to be cell-specific.46 Similarly to promoter-like DHS-exons, the presence of enhancer specific histone marks and factors on enhancer like DHS exons can also be explained, at least partially, by interactions between these exons and enhancers.46 In the same way, cohesin and CTCF associated DHS exons were found to be enriched in regions involved in interactions centered in CTCF.46 It cannot be completely ruled out, however, that some enhancer- and promoter-like DHS exons actually function as alternative promoters or regulatory elements. Favoring the hypothesis that interactions of exons with promoters and enhancers might favor co-transcriptional exon recognition, it was found that Ser2-phosphorylated RNAP II accumulates more in DHS exons than in total exons.46 Most interestingly, DHS exons were found to be enriched in alternative splicing events.46
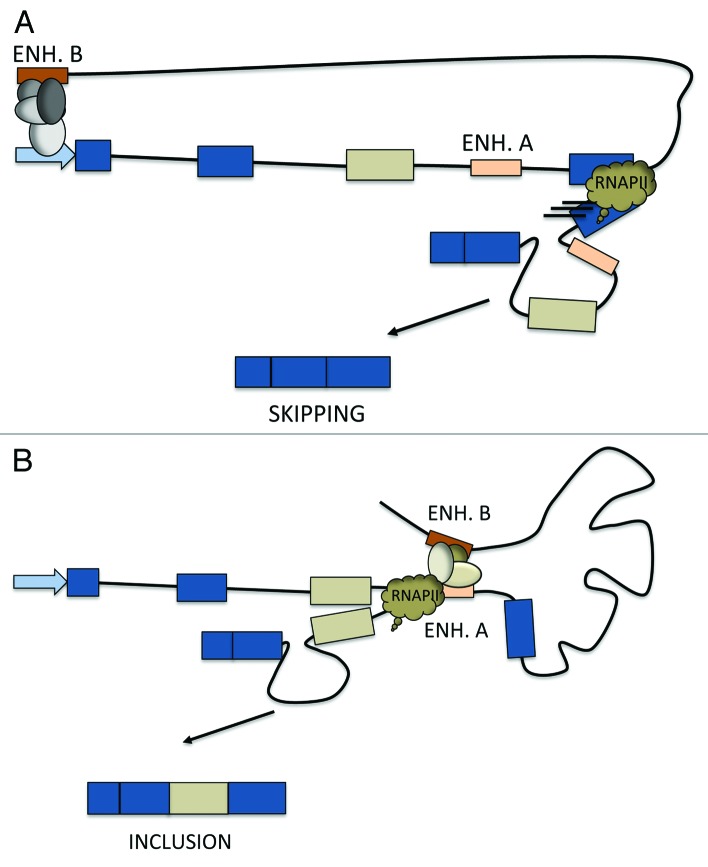
As hypothesized by Mercer et al.,46 chromatin loops could bring in close proximity splicing and transcription machineries so that splicing factors recruited to gene promoters are efficiently delivered to splice sites. More than ten years ago, our lab showed, using splicing reporter minigenes, that the usage of an SV40 enhancer element located upstream of the minigene promoter induces exon skipping.48 It was hypothesized that the enhancer element increased RNAP II elongation at the body of the minigene, with this increase being responsible for the observed exon skipping (Fig. 1A).48 In view of the more recent evidence discussed, we can now also speculate that the enhancer might directly interact with the alternative exon region and favor the delivery of factors that inhibit exon inclusion.48 Consistently, in recent report a Mediator subunit was shown to be involved in alternative splicing regulation by interacting and recruiting the splicing inhibitor hnRNPL to alternative exons.10 Mediator is a protein complex known to interact with several transcription factors involved in the preinitiation complex and with cohesin that facilitates Mediator interaction with enhancers and promoters of active genes.49 Suggestively, in the proximity of the alternative exon 4 of the SLC2A2 gene, used to describe the Mediator effect on hnRNPL-regulated alternative splicing, an enhancer can be mapped according to the analysis of several histone marks (H3K4me3, H3K4me2, H3K4me2 and H3K27ac) and transcription factor binding in several cell lines.50 This brings forward another intriguing possibility by which intragenic enhancer activity and loop formation might influence alternative splicing of neighboring exons (Alló, Gómez Acuña, Kornblihtt et al., in preparation). Considering the mentioned prevalence in the intragenic location of enhancers,42,43 we can speculate that tissue specific enhancer usage might determine tissue specific alternative splicing outcomes.
Figure 1. Speculative models by which enhancer usage might influence alternative splicing choices through changes in RNAP II elongation rates. (A) Enhancer B interacts with the gene promoter and determines the recruitment of elongation factors that enhance RNAP II elongation rate through the body of the gene, thus promoting alternative exon skipping. (B) The intragenic enhancer A, located in the intron downstream of the alternative exon, interacts enhancer B. The resulting loop formation and protein complex recruitment to the region induces RNAP II stalling upregulating alternative exon inclusion.
Another matter that emerges when considering the possibility of constitutive and alternative spicing regulation by three dimensional chromatin organization is how RNAP II elongation is affected when transcribing through regions looped to distant regulatory elements by long range interactions. It was reported that DNA methylation antagonizes with CTCF recruitment to exon 5 of the CD45 gene, which regulates splicing of the alternative exons 4–5 by modulating RNAP II elongation rate.27 It was shown that CTCF binding induces a slowing down of transcription and thus favors exon inclusion. Further analysis revealed that the CTCF binding site is engaged in a chromatin loop with another distant genomic region.46 Figure 1B depicts how chromatin interactions may favor exon recognition by slowing transcription immediately downstream of an alternative exon. As discussed above, many intragenic enhancers are involved in interactions with the promoter of the host gene or other regions in a tissue-dependent manner, so their formation may also lead to alternative splicing regulation of neighboring exons. The dynamics of chromatin organization is currently being studied both by genome-wide and imaging technologies.29 It is known that chromatin architecture undergoes changes during cell differentiation and proliferation.40,51 Hi-C experiments performed in human embryonic stem (ES) cells and fibroblasts show that chromatin is organized in discrete modules, named topological domains, separated by boundary regions enriched in CTCF. The position of the boundary regions and lower resolution domains are largely conserved between the cell lines, suggesting that the gross domain structure remains unchanged along differentiation.28 However, interactions within each topological domain are very variable and depend on cell type-specific gene expression.28,32 It has been shown that looping does occur before gene activation but that effective transcriptional activation is associated with additional loop formation.31Deeper genome-wide analysis confirmed that promoter-enhancer interactions pre-exist to gene activation in various cell types and upon different stimuli, being loop formation a strong predictor of gene activation.32 In view of these findings, the hypothesis that high order chromatin organization might determine cell-specific alternative splicing becomes of particular relevance and deserves further investigation.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed
Acknowledgments
This work was supported by grants to A.R.K. from the Agencia Nacional de Promoción de Ciencia y Tecnología of Argentina (ANPCYT), the University of Buenos Aires and the Department of Environmental Sciences, Faculty of Meteorology, Environment and Arid Land Agriculture, King Abdulaziz University, Saudi Arabia. L.G.A. recipients of a fellowship and A.R.K. is a career investigator from the Consejo Nacional de Investigaciones Científicas y Técnicas of Argentina (CONICET). A.R.K. is a senior international research scholar of the Howard Hughes Medical Institute.
Glossary
Abbreviations:
- RNAP II
RNA polymerase II
- FISH
Fluorescence in Situ Hybridization
- DHS
DNase hypersensitivity sites
- 3C
Chromosome Conformation Capture
- LCR
Locus control region
- CTCF
CCCTC-binding factor
References
- 1.Listerman I, Sapra AK, Neugebauer KM. Cotranscriptional coupling of splicing factor recruitment and precursor messenger RNA splicing in mammalian cells. Nat Struct Mol Biol. 2006;13:815–22. doi: 10.1038/nsmb1135. [DOI] [PubMed] [Google Scholar]
- 2.Tilgner H, Knowles DG, Johnson R, Davis CA, Chakrabortty S, Djebali S, Curado J, Snyder M, Gingeras TR, Guigó R. Deep sequencing of subcellular RNA fractions shows splicing to be predominantly co-transcriptional in the human genome but inefficient for lncRNAs. Genome Res. 2012;22:1616–25. doi: 10.1101/gr.134445.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kotovic KM, Lockshon D, Boric L, Neugebauer KM. Cotranscriptional recruitment of the U1 snRNP to intron-containing genes in yeast. Mol Cell Biol. 2003;23:5768–79. doi: 10.1128/MCB.23.16.5768-5779.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kornblihtt AR, Schor IE, Alló M, Dujardin G, Petrillo E, Muñoz MJ. Alternative splicing: a pivotal step between eukaryotic transcription and translation. Nat Rev Mol Cell Biol. 2013;14:153–65. doi: 10.1038/nrm3525. [DOI] [PubMed] [Google Scholar]
- 5.Barash Y, Calarco JA, Gao W, Pan Q, Wang X, Shai O, Blencowe BJ, Frey BJ. Deciphering the splicing code. Nature. 2010;465:53–9. doi: 10.1038/nature09000. [DOI] [PubMed] [Google Scholar]
- 6.Risso G, Pelisch F, Quaglino A, Pozzi B, Srebrow A. Regulating the regulators: serine/arginine-rich proteins under scrutiny. IUBMB Life. 2012;64:809–16. doi: 10.1002/iub.1075. [DOI] [PubMed] [Google Scholar]
- 7.Das R, Yu J, Zhang Z, Gygi MP, Krainer AR, Gygi SP, Reed R. SR proteins function in coupling RNAP II transcription to pre-mRNA splicing. Mol Cell. 2007;26:867–81. doi: 10.1016/j.molcel.2007.05.036. [DOI] [PubMed] [Google Scholar]
- 8.Muñoz MJ, de la Mata M, Kornblihtt AR. The carboxy terminal domain of RNA polymerase II and alternative splicing. Trends Biochem Sci. 2010;35:497–504. doi: 10.1016/j.tibs.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 9.de la Mata M, Muñoz MJ, Alló M, Fededa JP, Schor IE, Kornblihtt AR. RNA polymerase II elongation at the crossroads of transcription and alternative aplicing. Genet Res Int. 2011;2011:309865. doi: 10.4061/2011/309865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang Y, Li W, Yao X, Lin QJ, Yin JW, Liang Y, Heiner M, Tian B, Hui J, Wang G. Mediator complex regulates alternative mRNA processing via the MED23 subunit. Mol Cell. 2012;45:459–69. doi: 10.1016/j.molcel.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Auboeuf D, Dowhan DH, Kang YK, Larkin K, Lee JW, Berget SM, O’Malley BW. Differential recruitment of nuclear receptor coactivators may determine alternative RNA splice site choice in target genes. Proc Natl Acad Sci U S A. 2004;101:2270–4. doi: 10.1073/pnas.0308133100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de la Mata M, Alonso CR, Kadener S, Fededa JP, Blaustein M, Pelisch F, Cramer P, Bentley D, Kornblihtt AR. A slow RNA polymerase II affects alternative splicing in vivo. Mol Cell. 2003;12:525–32. doi: 10.1016/j.molcel.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Ip JY, Schmidt D, Pan Q, Ramani AK, Fraser AG, Odom DT, Blencowe BJ. Global impact of RNA polymerase II elongation inhibition on alternative splicing regulation. Genome Res. 2011;21:390–401. doi: 10.1101/gr.111070.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luco RF, Allo M, Schor IE, Kornblihtt AR, Misteli T. Epigenetics in alternative pre-mRNA splicing. Cell. 2011;144:16–26. doi: 10.1016/j.cell.2010.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luco RF, Pan Q, Tominaga K, Blencowe BJ, Pereira-Smith OM, Misteli T. Regulation of alternative splicing by histone modifications. Science. 2010;327:996–1000. doi: 10.1126/science.1184208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sims RJ, 3rd, Millhouse S, Chen CF, Lewis BA, Erdjument-Bromage H, Tempst P, Manley JL, Reinberg D. Recognition of trimethylated histone H3 lysine 4 facilitates the recruitment of transcription postinitiation factors and pre-mRNA splicing. Mol Cell. 2007;28:665–76. doi: 10.1016/j.molcel.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hodges C, Bintu L, Lubkowska L, Kashlev M, Bustamante C. Nucleosomal fluctuations govern the transcription dynamics of RNA polymerase II. Science. 2009;325:626–8. doi: 10.1126/science.1172926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petesch SJ, Lis JT. Overcoming the nucleosome barrier during transcript elongation. Trends Genet. 2012;28:285–94. doi: 10.1016/j.tig.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz S, Meshorer E, Ast G. Chromatin organization marks exon-intron structure. Nat Struct Mol Biol. 2009;16:990–5. doi: 10.1038/nsmb.1659. [DOI] [PubMed] [Google Scholar]
- 21.Tilgner H, Nikolaou C, Althammer S, Sammeth M, Beato M, Valcárcel J, Guigó R. Nucleosome positioning as a determinant of exon recognition. Nat Struct Mol Biol. 2009;16:996–1001. doi: 10.1038/nsmb.1658. [DOI] [PubMed] [Google Scholar]
- 22.Alló M, Buggiano V, Fededa JP, Petrillo E, Schor I, de la Mata M, Agirre E, Plass M, Eyras E, Elela SA, et al. Control of alternative splicing through siRNA-mediated transcriptional gene silencing. Nat Struct Mol Biol. 2009;16:717–24. doi: 10.1038/nsmb.1620. [DOI] [PubMed] [Google Scholar]
- 23.Ameyar-Zazoua M, Rachez C, Souidi M, Robin P, Fritsch L, Young R, Morozova N, Fenouil R, Descostes N, Andrau JC, et al. Argonaute proteins couple chromatin silencing to alternative splicing. Nat Struct Mol Biol. 2012;19:998–1004. doi: 10.1038/nsmb.2373. [DOI] [PubMed] [Google Scholar]
- 24.Saint-André V, Batsché E, Rachez C, Muchardt C. Histone H3 lysine 9 trimethylation and HP1γ favor inclusion of alternative exons. Nat Struct Mol Biol. 2011;18:337–44. doi: 10.1038/nsmb.1995. [DOI] [PubMed] [Google Scholar]
- 25.Schor IE, Fiszbein A, Petrillo E, Kornblihtt AR. Intragenic epigenetic changes modulate NCAM alternative splicing in neuronal differentiation. EMBO J. 2013;32:2264–74. doi: 10.1038/emboj.2013.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schor IE, Rascovan N, Pelisch F, Alló M, Kornblihtt AR. Neuronal cell depolarization induces intragenic chromatin modifications affecting NCAM alternative splicing. Proc Natl Acad Sci U S A. 2009;106:4325–30. doi: 10.1073/pnas.0810666106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shukla S, Kavak E, Gregory M, Imashimizu M, Shutinoski B, Kashlev M, Oberdoerffer P, Sandberg R, Oberdoerffer S. CTCF-promoted RNA polymerase II pausing links DNA methylation to splicing. Nature. 2011;479:74–9. doi: 10.1038/nature10442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dixon JR, Selvaraj S, Yue F, Kim A, Li Y, Shen Y, Hu M, Liu JS, Ren B. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature. 2012;485:376–80. doi: 10.1038/nature11082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bickmore WA. The spatial organization of the human genome. Annu Rev Genomics Hum Genet. 2013;14:67–84. doi: 10.1146/annurev-genom-091212-153515. [DOI] [PubMed] [Google Scholar]
- 30.Fullwood MJ, Liu MH, Pan YF, Liu J, Xu H, Mohamed YB, Orlov YL, Velkov S, Ho A, Mei PH, et al. An oestrogen-receptor-alpha-bound human chromatin interactome. Nature. 2009;462:58–64. doi: 10.1038/nature08497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hakim O, Sung MH, Voss TC, Splinter E, John S, Sabo PJ, Thurman RE, Stamatoyannopoulos JA, de Laat W, Hager GL. Diverse gene reprogramming events occur in the same spatial clusters of distal regulatory elements. Genome Res. 2011;21:697–706. doi: 10.1101/gr.111153.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin F, Li Y, Dixon JR, Selvaraj S, Ye Z, Lee AY, Yen CA, Schmitt AD, Espinoza CA, Ren B. A high-resolution map of the three-dimensional chromatin interactome in human cells. Nature. 2013;503:290–4. doi: 10.1038/nature12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee BK, Iyer VR. Genome-wide studies of CCCTC-binding factor (CTCF) and cohesin provide insight into chromatin structure and regulation. J Biol Chem. 2012;287:30906–13. doi: 10.1074/jbc.R111.324962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li G, Ruan X, Auerbach RK, Sandhu KS, Zheng M, Wang P, Poh HM, Goh Y, Lim J, Zhang J, et al. Extensive promoter-centered chromatin interactions provide a topological basis for transcription regulation. Cell. 2012;148:84–98. doi: 10.1016/j.cell.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanyal A, Lajoie BR, Jain G, Dekker J. The long-range interaction landscape of gene promoters. Nature. 2012;489:109–13. doi: 10.1038/nature11279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li W, Notani D, Ma Q, Tanasa B, Nunez E, Chen AY, Merkurjev D, Zhang J, Ohgi K, Song X, et al. Functional roles of enhancer RNAs for oestrogen-dependent transcriptional activation. Nature. 2013;498:516–20. doi: 10.1038/nature12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stadhouders R, van den Heuvel A, Kolovos P, Jorna R, Leslie K, Grosveld F, Soler E. Transcription regulation by distal enhancers: who’s in the loop? Transcription. 2012;3:181–6. doi: 10.4161/trns.20720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tolhuis B, Palstra RJ, Splinter E, Grosveld F, de Laat W. Looping and interaction between hypersensitive sites in the active beta-globin locus. Mol Cell. 2002;10:1453–65. doi: 10.1016/S1097-2765(02)00781-5. [DOI] [PubMed] [Google Scholar]
- 39.Vakoc CR, Letting DL, Gheldof N, Sawado T, Bender MA, Groudine M, Weiss MJ, Dekker J, Blobel GA. Proximity among distant regulatory elements at the beta-globin locus requires GATA-1 and FOG-1. Mol Cell. 2005;17:453–62. doi: 10.1016/j.molcel.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 40.Vernimmen D, De Gobbi M, Sloane-Stanley JA, Wood WG, Higgs DR. Long-range chromosomal interactions regulate the timing of the transition between poised and active gene expression. EMBO J. 2007;26:2041–51. doi: 10.1038/sj.emboj.7601654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deng W, Lee J, Wang H, Miller J, Reik A, Gregory PD, Dean A, Blobel GA. Controlling long-range genomic interactions at a native locus by targeted tethering of a looping factor. Cell. 2012;149:1233–44. doi: 10.1016/j.cell.2012.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kowalczyk MS, Hughes JR, Garrick D, Lynch MD, Sharpe JA, Sloane-Stanley JA, McGowan SJ, De Gobbi M, Hosseini M, Vernimmen D, et al. Intragenic enhancers act as alternative promoters. Mol Cell. 2012;45:447–58. doi: 10.1016/j.molcel.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 43.Heintzman ND, Hon GC, Hawkins RD, Kheradpour P, Stark A, Harp LF, Ye Z, Lee LK, Stuart RK, Ching CW, et al. Histone modifications at human enhancers reflect global cell-type-specific gene expression. Nature. 2009;459:108–12. doi: 10.1038/nature07829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tan-Wong SM, Zaugg JB, Camblong J, Xu Z, Zhang DW, Mischo HE, Ansari AZ, Luscombe NM, Steinmetz LM, Proudfoot NJ. Gene loops enhance transcriptional directionality. Science. 2012;338:671–5. doi: 10.1126/science.1224350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheutin T, Cavalli G. Polycomb silencing: from linear chromatin domains to 3D chromosome folding. Curr Opin Genet Dev. 2014;25C:30–7. doi: 10.1016/j.gde.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 46.Mercer TR, Edwards SL, Clark MB, Neph SJ, Wang H, Stergachis AB, John S, Sandstrom R, Li G, Sandhu KS, et al. DNase I-hypersensitive exons colocalize with promoters and distal regulatory elements. Nat Genet. 2013;45:852–9. doi: 10.1038/ng.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Merkenschlager M, Odom DT. CTCF and cohesin: linking gene regulatory elements with their targets. Cell. 2013;152:1285–97. doi: 10.1016/j.cell.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 48.Nogues G, Kadener S, Cramer P, Bentley D, Kornblihtt AR. Transcriptional activators differ in their abilities to control alternative splicing. J Biol Chem. 2002;277:43110–4. doi: 10.1074/jbc.M208418200. [DOI] [PubMed] [Google Scholar]
- 49.Kagey MH, Newman JJ, Bilodeau S, Zhan Y, Orlando DA, van Berkum NL, Ebmeier CC, Goossens J, Rahl PB, Levine SS, et al. Mediator and cohesin connect gene expression and chromatin architecture. Nature. 2010;467:430–5. doi: 10.1038/nature09380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ernst J, Kheradpour P, Mikkelsen TS, Shoresh N, Ward LD, Epstein CB, Zhang X, Wang L, Issner R, Coyne M, et al. Mapping and analysis of chromatin state dynamics in nine human cell types. Nature. 2011;473:43–9. doi: 10.1038/nature09906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Phillips-Cremins JE, Sauria ME, Sanyal A, Gerasimova TI, Lajoie BR, Bell JS, Ong CT, Hookway TA, Guo C, Sun Y, et al. Architectural protein subclasses shape 3D organization of genomes during lineage commitment. Cell. 2013;153:1281–95. doi: 10.1016/j.cell.2013.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]