Abstract
Epithelial cells are closely connected to each other and to the extracellular matrix by a set of adhesive contacts that provide tissues with unique barrier properties and play a prominent role in cell morphology, tissue physiology, and cell signaling. This review highlights advances made in understanding the contributions of galectin-3, a carbohydrate-binding protein with affinity toward β-galactosides, as a modulator of epithelial junction assembly and function. The interactions of galectin-3 within adhesive structures are discussed in relation to wound healing and tumor progression.
Keywords: cancer, epithelial junction, galectin-3, matrix metalloproteinases, wound healing
Introduction
The presence of specialized cell-cell and cell-extracellular matrix junctions within a polarized epithelium provides tissues with unique barrier functions, and is critical for cell morphology, tissue physiology, and cell signaling.1,2 Adhesive structures connected to intermediate filaments (desmosomes) and to microfilaments (adherens junctions and tight junctions) ensure the appropriate stability and protection of epithelial sheets.3 Desmosomes and adherens junctions provide the mechanical strength to the epithelial cell contacts, whereas tight junctions (zonula occludens) form a selective permeable barrier to ions and small molecules.4 Other types of junctions include the gap junction, which provides direct chemical communication between adjacent cells, and the hemidesmosomes, specialized junctional complexes that contribute to the attachment of epithelial cells to the underlying basement membrane. Loss of cell polarity and reorganization of adhesion junctions are hallmarks of wound repair and cancer,5-7 and are intimately associated with the transdifferentiation of epithelial cells into motile mesenchymal cells, a process known as epithelial–mesenchymal transition.8
It has become increasingly clear that members of the galectin class of β-galactoside-binding proteins play a critical role as modulators of wound re-epithelialization and tumor progression.9,10 Based on molecular characterization, galectins (previously known as S-type lectins) are defined by the presence of a canonical carbohydrate recognition domain (CRD) of approximately 130 amino acids containing a conserved sequence motif known to make contacts with β-galactoside ligands.11 They are named and numbered chronologically in order of discovery, and so far, 15 have been found in mammals. These have been classified into 3 groups according to their structure: prototypical, chimeric, and tandem repeat. The prototypical galectins (galectin-1, -2, -5, -7, -10, -11, -13, -14 and -15) contain a single CRD that may associate to form homodimers; chimeric galectins, of which galectin-3 is the only known species found in vertebrates, contain a single CRD and a large amino-terminal domain that contributes to self-aggregation; and tandem-repeat galectins (galectin-4, -6, -8, -9 and -12) contain 2 CRDs within a single polypeptide. Each galectin recognizes different types of glycans in a complex manner that depends on the multivalency and oligomeric state of the galectins, the multivalency of their glycoconjugate ligands, and the mode of presentation of the glycans.11
Galectins can be found in numerous cell types and tissues, within intracellular (nucleus, mitochondria and cytoplasm) and extracellular (cell surface and extracellular matrix) compartments, where they modulate a variety of biological functions. Functions related to their intracellular localization have now been documented, and include modulation of cell growth and apoptosis, and pre-mRNA splicing.12 Galectins also mediate signal transduction events on the cell surface as well as a variety of extracellular processes such as cell adhesion and migration.13,14 It is well known that epithelial cells display dynamic behaviors important to the rearrangement, movement and shape of the cell, during regenerative processes such as wound healing or during pathogenetic processes such as cancer invasion.7 As evidenced by the number of comprehensive reviews of the field, significant progress has been made in the identification of the roles of galectins in epithelia.15-19 In this review, we summarize recent progress made in establishing the contribution of galectin-3 as a regulator of the epithelial junction.
Galectin-3: Structure and biosynthesis
Galectin-3, also known as Mac-2, L-29 or CBP35, is a 28- to 35-kD protein identified in the early 1980s on the surface of murine macrophages.20 It has a single CRD domain of approximately 130 amino acids in the C-terminus, and a 110–130 amino acid N-terminus with 7–14 repeats of a 9-amino acid sequence rich in proline, glycine, alanine and tyrosine—sometimes referred to as the collagen-like domain.21 The crystal structure of the galectin-3 CRD has been reported when in association with lactose and N-acetyllactosamine. Similarly to galectins-1 and -2, the galectin-3 CRD consists of a β-sandwich structure made of 6-stranded (named S1−S6) and 5-stranded (F1−F5) β-sheets, with the carbohydrate binding site located within a shallow groove over β-strands S4−S6.22 A significant rearrangement of the protein backbone near the binding site occurs in the absence of ligand, with the S4−S5 and the S5−S6 loops of the binding site moving inward to close up the binding cleft.23
Galectin-3, like other members of the galectin family, lacks both a membrane-anchoring domain and a signal sequence. Instead of being transferred into the endoplasmic reticulum and Golgi compartments for classical secretion, galectin-3 is synthesized on clusters of free ribosomes in the cytoplasm of the cell as an non-glycosylated protein before secretion.24 The N-terminal domain of galectin-3 has been implicated in the non-classical secretion of the protein.25 Interestingly, whether galectin-3 is secreted via basolateral or apical membranes in polarized epithelial cells depends on the cell type and the experimental conditions used. For instance, galectin-3 is primarily secreted from the apical domain in monolayer cultures of Madin-Darby canine kidney (MDCK) cells grown in Transwell inserts,26 but is directed to basolateral surfaces in MDCK cells grown as polarized cysts in Matrigel.27 The signals that drive galectin-3 secretion into different polarity compartments are so far unknown.
Studies on the carbohydrate-binding activity and specificity of galectin-3 have shown that N-acetyllactosamine (Galβ1,4(3)GlcNAc) is the preferential ligand, although it can also accommodate other galactose-containing structures.28 Affinity of galectin-3 toward glycosylated receptors is dependent on the metabolic flux within the target cell. Data collected during the past few years support an important function of the hexosamine pathway in modulating the strength of the association of individual receptors within a cell-surface galectin lattice.29,30 Quantitative precipitation studies also indicate that galectin-3 promptly precipitates as a pentamer in the presence of multivalent N-acetyllactosamine ligands in a process mediated through the N-terminal domain.31 In cell culture, oligomerization of galectin-3 occurs on the cell surface at physiological concentrations of the lectin, resulting in galectin-3 lattices that are robust and resistant to lateral movement of cell surface components.32 Increasing concentrations of multivalent glycoprotein ligands promote the formation of these galectin-3 lattices, now known to be involved in the regulation of receptor clustering, endocytosis and signaling.30 In the absence of saccharide ligands, galectin-3 self-associates in a manner that is dependent on the C-terminal domain.33
Post-translational processing of galectin-3
The major structural modifications of galectin-3 are phosphorylation and proteolysis, which occur outside the CRD. Phosphorylation of galectin-3 was initially observed in confluent 3T3 fibroblasts in both the cytosol and nucleus.34,35 Huflejt et al. later determined the site of phosphorylation of galectin-3 in confluent polarized MDCK cells, taking advantage of the abundant levels of galectin-3 (about 1% of total soluble cell protein) in these cells.36 Their analyses revealed phosphate incorporation only at serine, primarily at serine 6, with lower amounts at serine 12. Although it has been suggested that phosphorylation may modulate the translocation of galectin-3 between different cellular compartments, mutational studies in BT-549 human breast carcinoma cells show no obvious difference in cellular localization between wild-type and serine 6 mutated transfectants.37 In contrast, it has been proposed that phosphorylation can modulate protein-carbohydrate interactions serving as an “on/off” switch for the glycan binding capabilities of galectin-3.38 This hypothesis is supported by data using synthetic peptides derived from the rather flexible human galectin-3 N-terminal domain, which can interact with the CRD in a serine and tyrosine phosphorylation-dependent manner.39
The N-terminal domain of galectin-3 has been referred to as the collagen-like domain, based on the presence of a motif susceptible to collagenase degradation.21 It is now clear that the Ala62-Tyr63 bond within the galectin-3 N-terminus contains a cleavage site for the matrix metalloproteinases MMP2 (gelatinase A) and MMP9 (gelatinase B).40 The biological implications of the proteolytic degradation of galectin-3 by metalloproteinases are multiple. For example, proteolysis can modulate the biological activities of galectin-3 by reducing the expression of the protein on the cell-surface.40,41 Also, the enzymatic cleavage of the galectin-3 by metalloproteinases occurs within the proline- and glycine-rich N-terminal domain and, therefore, may interfere with its oligomerization properties and the clustering of its receptors.42 Finally, cleavage of galectin-3 by metalloproteinases alters the structure of the CRD to keep this domain in a high affinity conformation.43
Similarly to other galectins, the N-terminus of galectin-3 can also be post-translationally modified by cleavage of the N-terminal methionine followed by acetylation at alanine 2—common post-translational modifications found in cytoplasmic proteins.44
Contributions of galectin-3 to the epithelial junction
Upon secretion, galectins can bind glycosylated receptors on cell surfaces and extracellular matrices to regulate cell-cell and cell-matrix adhesion.19,45 Following binding to receptors (e.g., matrix components such as laminin and integrins on the cell surface), galectins can either inhibit or enhance adhesion through multiple mechanisms that include ligation of glycans and recruitment of signaling molecules.19,46 The role of galectins as agonists or antagonists of adhesion depends on the level of expression and location of the lectin, the glycosylation pattern of the receptors, the binding valency of the interaction, and the repertoire of receptors clustered by the lectin.19 In epithelial tissues, galectins are also thought to have a dual positive or negative regulatory function during the assembly and stabilization of intercellular junctions. As indicated below, contributions of chimeric galectin-3 to the epithelial junction have been described in desmosomes, adherens junctions and tight junctions.
Desmosomes
Desmosomes are intercellular junctions that anchor intermediate filaments to the plasma membrane, forming a network of adhesive bonds that provide mechanical strength to tissues. Recent data indicates that galectin-3 enhances epithelial intercellular adhesion through association with the ectodomain of desmoglein-2, a calcium-binding transmembrane glycoprotein component of desmosomes in vertebrate epithelial cells. In experiments using intestinal epithelial cells, Jiang et al. demonstrated that galectin-3 binds to N-linked β-galactosides on the extracellular domain of desmoglein-2.47 Moreover, in the absence of galectin-3, desmoglein-2 is internalized from the plasma membrane and undergoes proteasomal degradation. These events resulted in decreased intercellular adhesion, supporting a role for galectin-3 in controlling desmosome structure and function. In addition to intestinal cells, it is noteworthy to mention that galectin-3-reactive binding sites have also been found to co-localize with desmoglein by immunohistochemical analysis in human corneal and conjunctival epithelia, suggesting that galectin-3 also contributes to desmosome formation in stratified epithelia.48
Adherens junctions
One of the most important and ubiquitous families of adhesive proteins required for the maintenance of solid tissues are the cadherins, a group of transmembrane calcium-dependent proteins that serve as the major adhesion molecules located within adherens junctions.49 The ectodomain of cadherins mediates intercellular adhesion while the cytosolic region binds to adaptor proteins such as the catenins that connect to the actin cytoskeleton. N-cadherin is found in many different types of intercellular junctions and often replaces E-cadherin at cell-cell junctions of tumor cells that have undergone epithelial-to-mesenchymal transition. Recent evidence by Boscher et al. indicates that galectin-3 lattices are critical regulators of N-cadherin dynamics and cell-cell junction stability in mammary carcinoma cells.50 Using a combination of crosslinking and immunoprecipitation approaches, the authors demonstrated that galectin-3 interacts directly with N-cadherin. Further, they showed that galectin-3 and N-cadherin accumulate at the junctions with the raft marker ganglioside GM1 to promote destabilization of cell-cell junctions. These negative regulatory processes appear to be controlled by N-glycans present on N-cadherin, predominantly by branched N-glycans generated by N-acetylglucosaminyltransferase V (Mgat5) activity. Abrogation of N-cadherin glycosylation by targeting Mgat5 in human fibrosarcoma HT1080 cells resulted in promotion of N-cadherin-mediated cell-cell adhesion and decrease of cell migration and invasion.51,52 Similarly, disruption of the galectin-3 lattice by deletion of Mgat5 in mammary carcinoma cells also resulted in the stabilization of cell-cell junctions, suggesting that interaction of galectin-3 with N-glycans present on N-cadherin enhances the turnover of N-cadherin and negatively regulates intercellular adhesion.50
Tight junctions
Tight junctions are intramembrane multiprotein complexes composed of integral plasma membrane linker proteins, such as occludin and claudins, and cytoplasmic adaptor proteins, such as zonula occludens. Occludin, a 65-kDa tetraspan membrane protein, is crucial for maintaining the stability of tight junctions and for regulating actin organization and epithelial migration.53 Studies in our laboratory using the cornea as a model have recently shown that galectin-3 promotes cell–cell detachment and redistribution of occludin through its N-terminal polymerizing domain.54 In these experiments, galectin-3 initiates cell–cell disassembly by inducing matrix metalloproteinase expression in a manner that is dependent on the interaction with and clustering of the matrix metalloproteinase inducer CD147 on the cell surface. The requirement of galectin-3 for matrix metalloproteinase induction suggests that galectin-3 is critical to mitigating the physical constraint to cell movement.
Galectin-3 and the epithelial junction in wound repair
Encouraging progress has been made in the last decade identifying the contribution of galectins to wound repair.10 Galectin-3 has been shown to promote re-epithelialization of wounds in epithelia from the cornea,55 colon,56 and skin.57 The molecular mechanisms, by which galectin-3 modulates wound healing in epithelial cells, involve interaction with Mgat5-modified complex N-glycans on α3β1 integrin and promotion of lamellipodia formation.58 To promote cell migration, galectin-3 activates both focal adhesion kinase, a key regulator of integrin-dependent intracellular signaling, and Rac1 GTPase, a member of the family of Rho GTPases crucial to the reorganization of the actin cytoskeleton and formation of lamellipodial extensions.58
Despite findings supporting a role for galectin-3 in wound repair, the direct contribution of this lectin to the adhesive structures in migrating epithelia remains understudied. Epithelial cells at the leading edge of wounds require significant fluidity to change their shape.59 Abrogation of galectin-3 has been shown to impair matrix metalloproteinase expression in an in vivo model of wound healing, suggesting that galectin-3-mediated matrix metalloproteinase expression is necessary to rearrange the position of the epithelial cell in order to facilitate re-epithelialization.54,55 As mentioned above, this process appears to be mediated by CD147. Both affinity chromatography and confocal microscopy studies in human keratinocytes have revealed that galectin-3 binds to, and promotes cell surface redistribution of CD147, concomitant with cell–cell detachment and occludin redistribution.54
The role of galectin-3 in wound repair does not appear to be associated uniquely to the cell-cell junction, as epithelial cells lacking galectin-3 expression are also known to interact poorly with their extracellular matrices.15 A potential mechanism by which galectin-3 modulates these interactions seem to involve the glycosylation status of integrins and components of the extracellular matrix. This seems to be the case for laminin-332, a basement membrane protein that promotes cell migration and is over-expressed at sites of epidermal wounds.60 Elegant studies from Kariya et al. have shown that galectin-3 significantly enhances the cell motility of keratinocytes through recognition of poly-N-acetyllactosamine-modified laminin-332, but not laminin-332 containing carbohydrates with low galectin-3 affinity.61 The authors further demonstrated that galectin-3 promotes the association of laminin-332 with α6β4 integrin, which is part of the hemidesmosomal adhesion complex,62,63 and epidermal growth factor receptor, to form clusters of cell surface proteins important to the regulation of cellular signaling and cell migration.61 More recently, galectin-3 has been identified as a factor that enhances corneal epithelial wound healing by promoting adhesion to collagen IV, a major structural component of basement membranes.64
Galectin-3 and the epithelial junction in cancer
Changes in galectin-3 expression and localization are commonly seen in tumors and have been associated with cancer cell growth, transformation, adhesion, invasion and metastasis.65 Overexpression of galectin-3 has been shown to enhance tumor cell adhesion to the extracellular matrix and promote cancer dissemination. Such an effect of galectin-3 is attributed partly through association with a range of the extracellular matrix glycoproteins, such as fibronectin,66,67 collagen IV,67 elastin,68 and laminin.69
Crucial to the understanding of cancer cell invasion and metastasis is the elucidation of the dynamic roles of focal adhesion proteins during cell migration. During the past decade, researchers have provided evidence that supports the involvement of galectin-3 in the stimulation of focal adhesion turnover and tumor cell motility.70 Mgat5-modified N-glycans on integrins were initially proposed as potential candidates in promoting focal adhesion turnover, cell migration and tumor growth.71 It is now established that, at least in mammary tumor cells, galectin-3 interacts with Mgat5-branched N-glycans on integrin α5β1 to promote focal adhesion turnover, phosphatidylinositol 3-kinase signaling and cell spreading.66,72 Interestingly, in vivo studies have also shown that Mgat5−/− cells have stable focal adhesions and express reduced levels of caveolin-1.71,73 Recruitment of tyrosine-phosphorylated caveolin-1 following galectin-3–mediated activation of integrins is now considered a central mechanism to promote focal adhesion disassembly and tumor cell migration.74
In addition to focal adhesions, loss of intercellular cell-cell contacts also contributes to tumor invasion and metastasis, as it allows malignant cells to dissociate from the primary tumor mass. Localization of galectin-3 within intercellular junctional complexes has been reported in tumor cells. As mentioned before, galectin-3 destabilizes cell-cell junctions by enhancing junctional mobility of N-cadherin and other glycoconjugates critical to cell migration in mammary carcinoma cells.50 Further, galectin-3-reactive sites have been found to co-localize with the desmosomal proteins desmoplakin-1 and desmoglein in primary squamous carcinomas, pointing to a potential role of galectin-3 in mediating intercellular contacts in these tumors.75 Similarly, galectin-3 has been shown to co-immunoprecipitate in a carbohydrate-dependent manner with liver-intestine cadherin in ductal adenocarcinoma of the pancreas,76 although the contribution of this association to the junctional complex in tumor cells remains unclear.
Future perspectives
Evidence from different model systems has accumulated in recent years implicating galectin-3 in the dynamic regulation of cell-cell and cell-extracellular matrix junctions (Fig. 1). Functions assigned to galectin-3 in adhesion complexes are diverse and include control of cell surface expression, mobility, and integrity, of adhesion proteins. Research on the role of galectin-3 in the epithelial junction is still in its infancy but, based on the multifunctional roles ascribed to galectin-3, it is entirely possible that there are yet more important galectin-3 partners and regulatory functions within the epithelial junction to be discovered. A thorough characterization of these associations and processes is warranted. In addition, further research of the role of galectin-3 on the epithelial junction might provide novel opportunities to a better understanding of the pathophysiology of epithelial diseases. Galectin-3 has been ascribed a plethora of functions during wound healing and cancer, and there is sufficient evidence to suggest that targeting galectins may have a significant effect on pathological processes.77 Through a greater understanding of the role of galectin-3 at the epithelial junction, there is no doubt that novel therapeutic targets will come to light.
Figure 1.
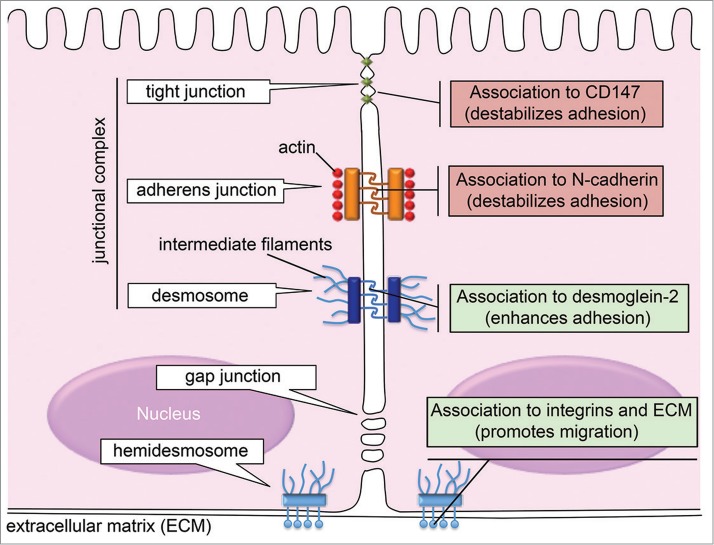
Roles attributed to galectin-3 in epithelial junctions.
An important gap in our knowledge also relates to the role of phosphorylation and proteolysis as modulators of the biological activities of galectin-3 at the epithelial junction. It is tempting to speculate that galectin-3 proteolysis influences re-epithelialization in wound healing and cell migration in cancer progression. This hypothesis is supported by data showing increased MMP7 expression in epithelial cells bordering intestinal ulcerations78 and elegant studies from Puthenedam et al. demonstrating that cleavage of the N-terminal region of galectin-3 by MMP7 impairs galectin-3-mediated wound healing.56 Moreover, galectin-3 cleavage is known to be an active process during tumor progression.79 Considering that phosphorylation and proteolysis can affect the glycan binding capabilities of galectin-3 and the clustering of galectin-3-binding partners on cell surfaces and extracellular matrices, analyses of the post-translational processing of this lectin will become crucial to understanding the dynamics of epithelial adhesion, motility, and invasion.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by a grant from the National Eye Institute of the National Institutes of Health (R01EY024031 to P. Argüeso).
References
- 1. Nelson WJ. Remodeling epithelial cell organization: transitions between front-rear and apical-basal polarity. Cold Spring Harb Perspect Biol 2009; 1:a000513; PMID:20066074; http://dx.doi.org/ 10.1101/cshperspect.a000513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rodriguez-Boulan E, Macara IG. Organization and execution of the epithelial polarity programme. Nat Rev Mol Cell Biol 2014; 15:225-42; PMID:24651541; http://dx.doi.org/ 10.1038/nrm3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Braga VM. Cell-cell adhesion and signalling. Curr Opin Cell Biol 2002; 14:546-56; PMID:12231348; http://dx.doi.org/ 10.1016/S0955-0674(02)00373-3 [DOI] [PubMed] [Google Scholar]
- 4. Van Aelst L, Symons M. Role of Rho family GTPases in epithelial morphogenesis. Genes Dev 2002; 16:1032-54; PMID:12000787; http://dx.doi.org/ 10.1101/gad.978802 [DOI] [PubMed] [Google Scholar]
- 5. Lee M, Vasioukhin V. Cell polarity and cancer–cell and tissue polarity as a non-canonical tumor suppressor. J Cell Sci 2008; 121:1141-50; PMID:18388309; http://dx.doi.org/ 10.1242/jcs.016634 [DOI] [PubMed] [Google Scholar]
- 6. Du D, Xu F, Yu L, Zhang C, Lu X, Yuan H, Huang Q, Zhang F, Bao H, Jia L, et al. The tight junction protein, occludin, regulates the directional migration of epithelial cells. Dev Cell 2010; 18:52-63; PMID:20152177; http://dx.doi.org/ 10.1016/j.devcel.2009.12.008 [DOI] [PubMed] [Google Scholar]
- 7. Takeichi M. Dynamic contacts: rearranging adherens junctions to drive epithelial remodelling. Nat Rev Mol Cell Biol 2014; 15:397-410; PMID:24824068; http://dx.doi.org/ 10.1038/nrm3802 [DOI] [PubMed] [Google Scholar]
- 8. Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 2014; 15:178-96; PMID:24556840; http://dx.doi.org/ 10.1038/nrm3758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer 2005; 5:29-41; PMID:15630413; http://dx.doi.org/ 10.1038/nrc1527 [DOI] [PubMed] [Google Scholar]
- 10. Panjwani N. Role of galectins in re-epithelialization of wounds. Ann Transl Med 2014; 2:89; PMID:25405164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cummings RD, Liu FT. Galectins. Essentials of Glycobiology. Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, et al. eds. Cold Spring Harbor (NY); 2009 [PubMed] [Google Scholar]
- 12. Liu FT, Patterson RJ, Wang JL. Intracellular functions of galectins. Biochim Biophys Acta 2002; 1572:263-73; PMID:12223274; http://dx.doi.org/ 10.1016/S0304-4165(02)00313-6 [DOI] [PubMed] [Google Scholar]
- 13. Garner OB, Baum LG. Galectin-glycan lattices regulate cell-surface glycoprotein organization and signalling. Biochem Soc Trans 2008; 36:1472-7; PMID:19021578; http://dx.doi.org/ 10.1042/BST0361472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. He J, Baum LG. Galectin interactions with extracellular matrix and effects on cellular function. Methods Enzymol 2006; 417:247-56; PMID:17132509; http://dx.doi.org/ 10.1016/S0076-6879(06)17017-2 [DOI] [PubMed] [Google Scholar]
- 15. Ochieng J, Furtak V, Lukyanov P. Extracellular functions of galectin-3. Glycoconj J 2004; 19:527-35; PMID:14758076; http://dx.doi.org/ 10.1023/B:GLYC.0000014082.99675.2f [DOI] [PubMed] [Google Scholar]
- 16. Viguier M, Advedissian T, Delacour D, Poirier F, Deshayes F. Galectins in epithelial functions. Tissue Barriers 2014; 2:e29103; PMID:25097826; http://dx.doi.org/ 10.4161/tisb.29103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rhodes JM, Campbell BJ, Yu LG. Lectin-epithelial interactions in the human colon. Biochem Soc Trans 2008; 36:1482-6; PMID:19021580; http://dx.doi.org/ 10.1042/BST0361482 [DOI] [PubMed] [Google Scholar]
- 18. Saussez S, Kiss R. Galectin-7. Cell Mol Life Sci 2006; 63:686-97; PMID:16429325; http://dx.doi.org/ 10.1007/s00018-005-5458-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hughes RC. Galectins as modulators of cell adhesion. Biochimie 2001; 83:667-76; PMID:11522396; http://dx.doi.org/ 10.1016/S0300-9084(01)01289-5 [DOI] [PubMed] [Google Scholar]
- 20. Ho MK, Springer TA. Mac-2, a novel 32,000 Mr mouse macrophage subpopulation-specific antigen defined by monoclonal antibodies. J Immunol 1982; 128:1221-8; PMID:6173426 [PubMed] [Google Scholar]
- 21. Raz A, Pazerini G, Carmi P. Identification of the metastasis-associated, galactoside-binding lectin as a chimeric gene product with homology to an IgE-binding protein. Cancer Res 1989; 49:3489-93; PMID:2525069 [PubMed] [Google Scholar]
- 22. Seetharaman J, Kanigsberg A, Slaaby R, Leffler H, Barondes SH, Rini JM. X-ray crystal structure of the human galectin-3 carbohydrate recognition domain at 2.1-A resolution. J Biol Chem 1998; 273:13047-52; PMID:9582341; http://dx.doi.org/ 10.1074/jbc.273.21.13047 [DOI] [PubMed] [Google Scholar]
- 23. Umemoto K, Leffler H, Venot A, Valafar H, Prestegard JH. Conformational differences in liganded and unliganded states of Galectin-3. Biochemistry 2003; 42:3688-95; PMID:12667058; http://dx.doi.org/ 10.1021/bi026671m [DOI] [PubMed] [Google Scholar]
- 24. Hughes RC. Secretion of the galectin family of mammalian carbohydrate-binding proteins. Biochim Biophys Acta 1999; 1473:172-85; PMID:10580137; http://dx.doi.org/ 10.1016/S0304-4165(99)00177-4 [DOI] [PubMed] [Google Scholar]
- 25. Menon RP, Hughes RC. Determinants in the N-terminal domains of galectin-3 for secretion by a novel pathway circumventing the endoplasmic reticulum-Golgi complex. Eur J Biochem 1999; 264:569-76; PMID:10491105; http://dx.doi.org/ 10.1046/j.1432-1327.1999.00671.x [DOI] [PubMed] [Google Scholar]
- 26. Lindstedt R, Apodaca G, Barondes SH, Mostov KE, Leffler H. Apical secretion of a cytosolic protein by Madin-Darby canine kidney cells. Evidence for polarized release of an endogenous lectin by a nonclassical secretory pathway. J Biol Chem 1993; 268:11750-7; PMID:8505302 [PubMed] [Google Scholar]
- 27. Bao Q, Hughes RC. Galectin-3 expression and effects on cyst enlargement and tubulogenesis in kidney epithelial MDCK cells cultured in three-dimensional matrices in vitro. J Cell Sci 1995; 108(Pt 8):2791-800; PMID:7593320 [DOI] [PubMed] [Google Scholar]
- 28. Agrwal N, Sun Q, Wang SY, Wang JL. Carbohydrate-binding protein 35. I. Properties of the recombinant polypeptide and the individuality of the domains. J Biol Chem 1993; 268:14932-9; PMID:8325870 [PubMed] [Google Scholar]
- 29. Lau KS, Partridge EA, Grigorian A, Silvescu CI, Reinhold VN, Demetriou M, Dennis JW. Complex N-glycan number and degree of branching cooperate to regulate cell proliferation and differentiation. Cell 2007; 129:123-34; PMID:17418791; http://dx.doi.org/ 10.1016/j.cell.2007.01.049 [DOI] [PubMed] [Google Scholar]
- 30. Dennis JW, Nabi IR, Demetriou M. Metabolism, cell surface organization, and disease. Cell 2009; 139:1229-41; PMID:20064370; http://dx.doi.org/ 10.1016/j.cell.2009.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ahmad N, Gabius HJ, Andre S, Kaltner H, Sabesan S, Roy R, Liu B, Macaluso F, Brewer CF. Galectin-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous cross-linked complexes. J Biol Chem 2004; 279:10841-7; PMID:14672941; http://dx.doi.org/ 10.1074/jbc.M312834200 [DOI] [PubMed] [Google Scholar]
- 32. Nieminen J, Kuno A, Hirabayashi J, Sato S. Visualization of galectin-3 oligomerization on the surface of neutrophils and endothelial cells using fluorescence resonance energy transfer. J Biol Chem 2007; 282:1374-83; PMID:17082191; http://dx.doi.org/ 10.1074/jbc.M604506200 [DOI] [PubMed] [Google Scholar]
- 33. Yang RY, Hill PN, Hsu DK, Liu FT. Role of the carboxyl-terminal lectin domain in self-association of galectin-3. Biochemistry 1998; 37:4086-92; PMID:9521730; http://dx.doi.org/ 10.1021/bi971409c [DOI] [PubMed] [Google Scholar]
- 34. Cowles EA, Agrwal N, Anderson RL, Wang JL. Carbohydrate-binding protein 35. Isoelectric points of the polypeptide and a phosphorylated derivative. J Biol Chem 1990; 265:17706-12; PMID:2170392 [PubMed] [Google Scholar]
- 35. Hamann KK, Cowles EA, Wang JL, Anderson RL. Expression of carbohydrate binding protein 35 in human fibroblasts: variations in the levels of mRNA, protein, and isoelectric species as a function of replicative competence. Exp Cell Res 1991; 196:82-91; PMID:1879474; http://dx.doi.org/ 10.1016/0014-4827(91)90458-7 [DOI] [PubMed] [Google Scholar]
- 36. Huflejt ME, Turck CW, Lindstedt R, Barondes SH, Leffler H. L-29, a soluble lactose-binding lectin, is phosphorylated on serine 6 and serine 12 in vivo and by casein kinase I. J Biol Chem 1993; 268:26712-8; PMID:8253806 [PubMed] [Google Scholar]
- 37. Gong HC, Honjo Y, Nangia-Makker P, Hogan V, Mazurak N, Bresalier RS, Raz A. The NH2 terminus of galectin-3 governs cellular compartmentalization and functions in cancer cells. Cancer Res 1999; 59:6239-45; PMID:10626818 [PubMed] [Google Scholar]
- 38. Mazurek N, Conklin J, Byrd JC, Raz A, Bresalier RS. Phosphorylation of the beta-galactoside-binding protein galectin-3 modulates binding to its ligands. J Biol Chem 2000; 275:36311-5; PMID:10961987; http://dx.doi.org/ 10.1074/jbc.M003831200 [DOI] [PubMed] [Google Scholar]
- 39. Berbis MA, Andre S, Canada FJ, Pipkorn R, Ippel H, Mayo KH, Kübler D, Gabius HJ, Jiménez-Barbero J. Peptides derived from human galectin-3 N-terminal tail interact with its carbohydrate recognition domain in a phosphorylation-dependent manner. Biochem Biophys Res Commun 2014; 443:126-31; PMID:24269589; http://dx.doi.org/ 10.1016/j.bbrc.2013.11.063 [DOI] [PubMed] [Google Scholar]
- 40. Ochieng J, Fridman R, Nangia-Makker P, Kleiner DE, Liotta LA, Stetler-Stevenson WG, Raz A. Galectin-3 is a novel substrate for human matrix metalloproteinases-2 and -9. Biochemistry 1994; 33:14109-14; PMID:7947821; http://dx.doi.org/ 10.1021/bi00251a020 [DOI] [PubMed] [Google Scholar]
- 41. Ochieng J, Platt D, Tait L, Hogan V, Raz T, Carmi P, Raz A. Structure-function relationship of a recombinant human galactoside-binding protein. Biochemistry 1993; 32:4455-60; PMID:8476870; http://dx.doi.org/ 10.1021/bi00067a038 [DOI] [PubMed] [Google Scholar]
- 42. Hsu DK, Zuberi RI, Liu FT. Biochemical and biophysical characterization of human recombinant IgE-binding protein, an S-type animal lectin. J Biol Chem 1992; 267:14167-74; PMID:1629216 [PubMed] [Google Scholar]
- 43. Ochieng J, Green B, Evans S, James O, Warfield P. Modulation of the biological functions of galectin-3 by matrix metalloproteinases. Biochim Biophys Acta 1998; 1379:97-106; PMID:9468337; http://dx.doi.org/ 10.1016/S0304-4165(97)00086-X [DOI] [PubMed] [Google Scholar]
- 44. Herrmann J, Turck CW, Atchison RE, Huflejt ME, Poulter L, Gitt MA, Burlingame AL, Barondes SH, Leffler H. Primary structure of the soluble lactose binding lectin L-29 from rat and dog and interaction of its non-collagenous proline-, glycine-, tyrosine-rich sequence with bacterial and tissue collagenase. J Biol Chem 1993; 268:26704-11; PMID:8253805 [PubMed] [Google Scholar]
- 45. Perillo NL, Marcus ME, Baum LG. Galectins: versatile modulators of cell adhesion, cell proliferation, and cell death. J Mol Med (Berl) 1998; 76:402-12; PMID:9625297; http://dx.doi.org/ 10.1007/s001090050232 [DOI] [PubMed] [Google Scholar]
- 46. Boscher C, Dennis JW, Nabi IR. Glycosylation, galectins and cellular signaling. Curr Opin Cell Biol 2011; 23:383-92; PMID:21616652; http://dx.doi.org/ 10.1016/j.ceb.2011.05.001 [DOI] [PubMed] [Google Scholar]
- 47. Jiang K, Rankin CR, Nava P, Sumagin R, Kamekura R, Stowell SR, Feng M, Parkos CA, Nusrat A. Galectin-3 regulates desmoglein-2 and intestinal epithelial intercellular adhesion. J Biol Chem 2014; 289:10510-7; PMID:24567334; http://dx.doi.org/ 10.1074/jbc.M113.538538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hrdlickova-Cela E, Plzak J, Smetana K, Jr., Melkova Z, Kaltner H, Filipec M, Liu FT, Gabius HJ. Detection of galectin-3 in tear fluid at disease states and immunohistochemical and lectin histochemical analysis in human corneal and conjunctival epithelium. Br J Ophthalmol 2001; 85:1336-40; PMID:11673302; http://dx.doi.org/ 10.1136/bjo.85.11.1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell 1996; 84:345-57; PMID:8608588; http://dx.doi.org/ 10.1016/S0092-8674(00)81279-9 [DOI] [PubMed] [Google Scholar]
- 50. Boscher C, Zheng YZ, Lakshminarayan R, Johannes L, Dennis JW, Foster LJ, Nabi IR. Galectin-3 protein regulates mobility of N-cadherin and GM1 ganglioside at cell-cell junctions of mammary carcinoma cells. J Biol Chem 2012; 287:32940-52; PMID:22846995; http://dx.doi.org/ 10.1074/jbc.M112.353334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Guo HB, Johnson H, Randolph M, Pierce M. Regulation of homotypic cell-cell adhesion by branched N-glycosylation of N-cadherin extracellular EC2 and EC3 domains. J Biol Chem 2009; 284:34986-97; PMID:19846557; http://dx.doi.org/ 10.1074/jbc.M109.060806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Guo HB, Lee I, Kamar M, Pierce M. N-acetylglucosaminyltransferase V expression levels regulate cadherin-associated homotypic cell-cell adhesion and intracellular signaling pathways. J Biol Chem 2003; 278:52412-24; PMID:14561752; http://dx.doi.org/ 10.1074/jbc.M308837200 [DOI] [PubMed] [Google Scholar]
- 53. Cummins PM. Occludin: one protein, many forms. Mol Cell Biol 2012; 32:242-50; PMID:22083955; http://dx.doi.org/ 10.1128/MCB.06029-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mauris J, Woodward AM, Cao Z, Panjwani N, Argueso P. Molecular basis for MMP9 induction and disruption of epithelial cell-cell contacts by galectin-3. J Cell Sci 2014; 127:3141-8; PMID:24829150; http://dx.doi.org/ 10.1242/jcs.148510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cao Z, Said N, Amin S, Wu HK, Bruce A, Garate M, Hsu DK, Kuwabara I, Liu FT, Panjwani N. Galectins-3 and -7, but not galectin-1, play a role in re-epithelialization of wounds. J Biol Chem 2002; 277:42299-305; PMID:12194966; http://dx.doi.org/ 10.1074/jbc.M200981200 [DOI] [PubMed] [Google Scholar]
- 56. Puthenedam M, Wu F, Shetye A, Michaels A, Rhee KJ, Kwon JH. Matrilysin-1 (MMP7) cleaves galectin-3 and inhibits wound healing in intestinal epithelial cells. Inflamm Bowel Dis 2011; 17:260-7; PMID:20812334; http://dx.doi.org/ 10.1002/ibd.21443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Liu W, Hsu DK, Chen HY, Yang RY, Carraway KL, 3rd, Isseroff RR, Liu FT. Galectin-3 regulates intracellular trafficking of EGFR through Alix and promotes keratinocyte migration. J Invest Dermatol 2012; 132:2828-37; PMID:22785133; http://dx.doi.org/ 10.1038/jid.2012.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Saravanan C, Liu FT, Gipson IK, Panjwani N. Galectin-3 promotes lamellipodia formation in epithelial cells by interacting with complex N-glycans on alpha3beta1 integrin. J Cell Sci 2009; 122:3684-93; PMID:19755493; http://dx.doi.org/ 10.1242/jcs.045674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jacinto A, Martinez-Arias A, Martin P. Mechanisms of epithelial fusion and repair. Nat Cell Biol 2001; 3:E117-23; PMID:11331897; http://dx.doi.org/ 10.1038/35074643 [DOI] [PubMed] [Google Scholar]
- 60. Kariya Y, Miyazaki K. The basement membrane protein laminin-5 acts as a soluble cell motility factor. Exp Cell Res 2004; 297:508-20; PMID:15212952; http://dx.doi.org/ 10.1016/j.yexcr.2004.03.044 [DOI] [PubMed] [Google Scholar]
- 61. Kariya Y, Kawamura C, Tabei T, Gu J. Bisecting GlcNAc residues on laminin-332 down-regulate galectin-3-dependent keratinocyte motility. J Biol Chem 2010; 285:3330-40; PMID:19940114; http://dx.doi.org/ 10.1074/jbc.M109.038836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sonnenberg A, Calafat J, Janssen H, Daams H, van der Raaij-Helmer LM, Falcioni R, Kennel SJ, Aplin JD, Baker J, Loizidou M, et al. Integrin alpha 6/beta 4 complex is located in hemidesmosomes, suggesting a major role in epidermal cell-basement membrane adhesion. J Cell Biol 1991; 113:907-17; PMID:2026654; http://dx.doi.org/ 10.1083/jcb.113.4.907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Stepp MA, Spurr-Michaud S, Tisdale A, Elwell J, Gipson IK. Alpha 6 beta 4 integrin heterodimer is a component of hemidesmosomes. Proc Natl Acad Sci U S A 1990; 87:8970-4; PMID:2247472; http://dx.doi.org/ 10.1073/pnas.87.22.8970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yabuta C, Yano F, Fujii A, Shearer TR, Azuma M. Galectin-3 enhances epithelial cell adhesion and wound healing in rat cornea. Ophthalmic Res 2014; 51:96-103; PMID:24356704; http://dx.doi.org/ 10.1159/000355846 [DOI] [PubMed] [Google Scholar]
- 65. Newlaczyl AU, Yu LG. Galectin-3–a jack-of-all-trades in cancer. Cancer Lett 2011; 313:123-8; PMID:21974805; http://dx.doi.org/ 10.1016/j.canlet.2011.09.003 [DOI] [PubMed] [Google Scholar]
- 66. Lagana A, Goetz JG, Cheung P, Raz A, Dennis JW, Nabi IR. Galectin binding to Mgat5-modified N-glycans regulates fibronectin matrix remodeling in tumor cells. Mol Cell Biol 2006; 26:3181-93; PMID:16581792; http://dx.doi.org/ 10.1128/MCB.26.8.3181-3193.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sasaki T, Brakebusch C, Engel J, Timpl R. Mac-2 binding protein is a cell-adhesive protein of the extracellular matrix which self-assembles into ring-like structures and binds beta1 integrins, collagens and fibronectin. EMBO J 1998; 17:1606-13; PMID:9501082; http://dx.doi.org/ 10.1093/emboj/17.6.1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ochieng J, Warfield P, Green-Jarvis B, Fentie I. Galectin-3 regulates the adhesive interaction between breast carcinoma cells and elastin. J Cell Biochem 1999; 75:505-14; PMID:10536372; http://dx.doi.org/ 10.1002/(SICI)1097-4644(19991201)75:3%3c505::AID-JCB14%3e3.0.CO;2-I [DOI] [PubMed] [Google Scholar]
- 69. Woo HJ, Lotz MM, Jung JU, Mercurio AM. Carbohydrate-binding protein 35 (Mac-2), a laminin-binding lectin, forms functional dimers using cysteine 186. J Biol Chem 1991; 266:18419-22; PMID:1917966 [PubMed] [Google Scholar]
- 70. Goetz JG. Bidirectional control of the inner dynamics of focal adhesions promotes cell migration. Cell Adh Migr 2009; 3:185-90; PMID:19398887; http://dx.doi.org/ 10.4161/cam.3.2.7295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Granovsky M, Fata J, Pawling J, Muller WJ, Khokha R, Dennis JW. Suppression of tumor growth and metastasis in Mgat5-deficient mice. Nat Med 2000; 6:306-12; PMID:10700233; http://dx.doi.org/ 10.1038/73163 [DOI] [PubMed] [Google Scholar]
- 72. Dennis JW, Lau KS, Demetriou M, Nabi IR. Adaptive regulation at the cell surface by N-glycosylation. Traffic 2009; 10:1569-78; PMID:19761541; http://dx.doi.org/ 10.1111/j.1600-0854.2009.00981.x [DOI] [PubMed] [Google Scholar]
- 73. Lajoie P, Partridge EA, Guay G, Goetz JG, Pawling J, Lagana A, Joshi B, Dennis JW, Nabi IR. Plasma membrane domain organization regulates EGFR signaling in tumor cells. J Cell Biol 2007; 179:341-56; PMID:17938246; http://dx.doi.org/ 10.1083/jcb.200611106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Goetz JG, Joshi B, Lajoie P, Strugnell SS, Scudamore T, Kojic LD, Nabi IR. Concerted regulation of focal adhesion dynamics by galectin-3 and tyrosine-phosphorylated caveolin-1. J Cell Biol 2008; 180:1261-75; PMID:18347068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Plzak J, Smetana K, Jr., Hrdlickova E, Kodet R, Holikova Z, Liu FT, Dvoránkova B, Kaltner H, Betka J, Gabius HJ. Expression of galectin-3-reactive ligands in squamous cancer and normal epithelial cells as a marker of differentiation. Int J Oncol 2001; 19:59-64; PMID:11408923 [PubMed] [Google Scholar]
- 76. Takamura M, Sakamoto M, Ino Y, Shimamura T, Ichida T, Asakura H, Hirohashi S. Expression of liver-intestine cadherin and its possible interaction with galectin-3 in ductal adenocarcinoma of the pancreas. Cancer Sci 2003; 94:425-30; PMID:12824888; http://dx.doi.org/ 10.1111/j.1349-7006.2003.tb01459.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Klyosov AA, Traber PG. Galectins in disease and potential therapeutic approaches. Galect Dis Implicat Target 2012:3-43; http://dx.doi.org/ 10.1021/bk-2012-1115.ch001 [DOI] [Google Scholar]
- 78. Saarialho-Kere UK, Vaalamo M, Puolakkainen P, Airola K, Parks WC, Karjalainen-Lindsberg ML. Enhanced expression of matrilysin, collagenase, and stromelysin-1 in gastrointestinal ulcers. Am J Pathol 1996; 148:519-26; PMID:8579114 [PMC free article] [PubMed] [Google Scholar]
- 79. Nangia-Makker P, Raz T, Tait L, Hogan V, Fridman R, Raz A. Galectin-3 cleavage: a novel surrogate marker for matrix metalloproteinase activity in growing breast cancers. Cancer Res 2007; 67:11760-8; PMID:18089806; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-3233 [DOI] [PMC free article] [PubMed] [Google Scholar]


