Abstract
Deep tissue injuries (DTI) are serious lesions which may develop in deep tissue layers as a result of sustained tissue loading or ischemic injury. These lesions may not become visible on the skin surface until the injury reaches an advanced stage making their early detection a challenging task. Early diagnosis leading to early treatment mitigates the progression of lesion and remains one of the priorities in management. The aim of this study is to examine skin surface temperature distributions of damaged tissue and develop criteria for the detection of incipient DTI. A multilayer quantitative heat transfer model of the skin tissue was developed using finite element based software COMSOL Multiphysics. Thermal response of the skin surface was computed during deep tissue inflammation and deep tissue ischemia and then compared with that of healthy tissue. In the presence of a DTI, an increase of about 0.5°C in skin surface temperatures was noticed during initial phase of deep tissue inflammation, which was followed by a surface temperature decrease of about 0.2°C corresponding to persistent deep tissue ischemia. These temperature differences are large enough to be detected by thermographic imaging. This study, therefore, also enhances the understanding of the previously detected thermographic quantitative changes associated with DTI.
1. INTRODUCTION
Deep tissue injuries (DTI) are serious lesions which may develop in deep tissue layers as a result of sustained tissue loading or ischemic injury. The mechanics of these injuries have only recently been investigated; formal recognition of this injury pattern was made in 2002 by the National Pressure Ulcer Advisory Panel [1]. Since that time, DTI have been recognized as a major health care concern for high risk patients, including those patients who experience repetitive motion or sustained pressure. High risk patients also include those with chronic neurological or musculoskeletal impairments as well as residents of acute and long term health care facilities. Unlike superficial ulcers, DTI begin to occur in muscle layers near bony prominences, and may not become visible on the skin surface until the lesion reaches an advanced stage. This makes early detection of incipient deep ulcers a challenging task, and remains one of the priorities in management.
One class of deep tissue injuries is pressure ulcer that develops in the soft tissue when sustained pressure on tissue results in compromised blood flow leading to ischemia and ultimately to tissue infarction. These soft tissue injuries have a significant impact on individual’s health. In addition to the functional effect these damaging injuries can have, their cost to the US health care system exceeds 4 billion dollars annually. Treatment of DTI is difficult because by the time they are recognized, irrevocable tissue damage has already occurred. Ideally, management of DTI should be focused on early detection and prevention, before tissue death occurs.
Skin surface temperature has been the subject of inspection when detecting tissue injuries that are caused by sustained tissue loading or ischemia. A number of studies have reported an increase in skin surface temperatures upon pressure relief, suggesting reactive hyperemia [2–5]. A decrease in skin surface temperature on the site of injury, however, has also been reported in the literature [6, 7]. Sato et al [6] measured the temperature at a deteriorating erythemic site to be lower than that of the surrounding healthy tissue. They suspected that the decrease was caused by reduced blood perfusion in the region. Benbow et al [7] associated the decrease in the mean tissue temperature of a neuropathic foot with a risk of ischemic injury. Computational thermal models of the injury would be very useful in order to better understand the variations in skin surface temperature associated with DTIs. The models can help clinicians to better understand the results to expect of thermographic imaging measurements and to identify the patients with tissue at risk at an early stage of the disease.
In this work, we present a computational model for heel tissue as a representative of deep tissue injury. The objective of the analysis is to aid early thermographic interpretation of inflammatory and ischemic changes associated with impending infarction. The surface temperature distributions computed using this model will support the analysis and interpretation of thermographic images. The heel is one of the most common sites for DTI and is the source of significant morbidity especially among hospitalized patients. This model could thus be used by clinicians to provide early detection of heel DTI and avoid the more serious complications associated with pressure ulcers.
2. HEEL ANATOMY AND VASCULARITY
Tissue viability is determined by a number of factors including, most importantly, tissue perfusion which is necessary to provide much needed oxygen delivery and removal of the products of metabolism. Perfusion is mediated through the central nervous system which adjusts blood flow to meet the metabolic demands of the tissue. Several factors, such as temperature, pain, external pressure, blood volume, arterial and venous function, can all affect tissue perfusion. If tissue perfusion is inadequate, cell death can occur, resulting in deep tissue injury. The heel is the most common site for the occurrence of deep tissue injuries [8–10]. Although the heel is equipped to handle the pressure of walking, running or standing, prolonged immobility, as what happens in chronically ill patients, can result in external pressures of long duration to parts of the heel that normally are not subject to prolonged pressure. When an individual is recumbent for extended periods during an extended hospitalization or a profound disability, it's the posterior aspect of the heel that is primarily subject to prolonged compression. Interestingly, the posterior aspect of the heel has the least amount of soft tissue and also has the least vascularity.
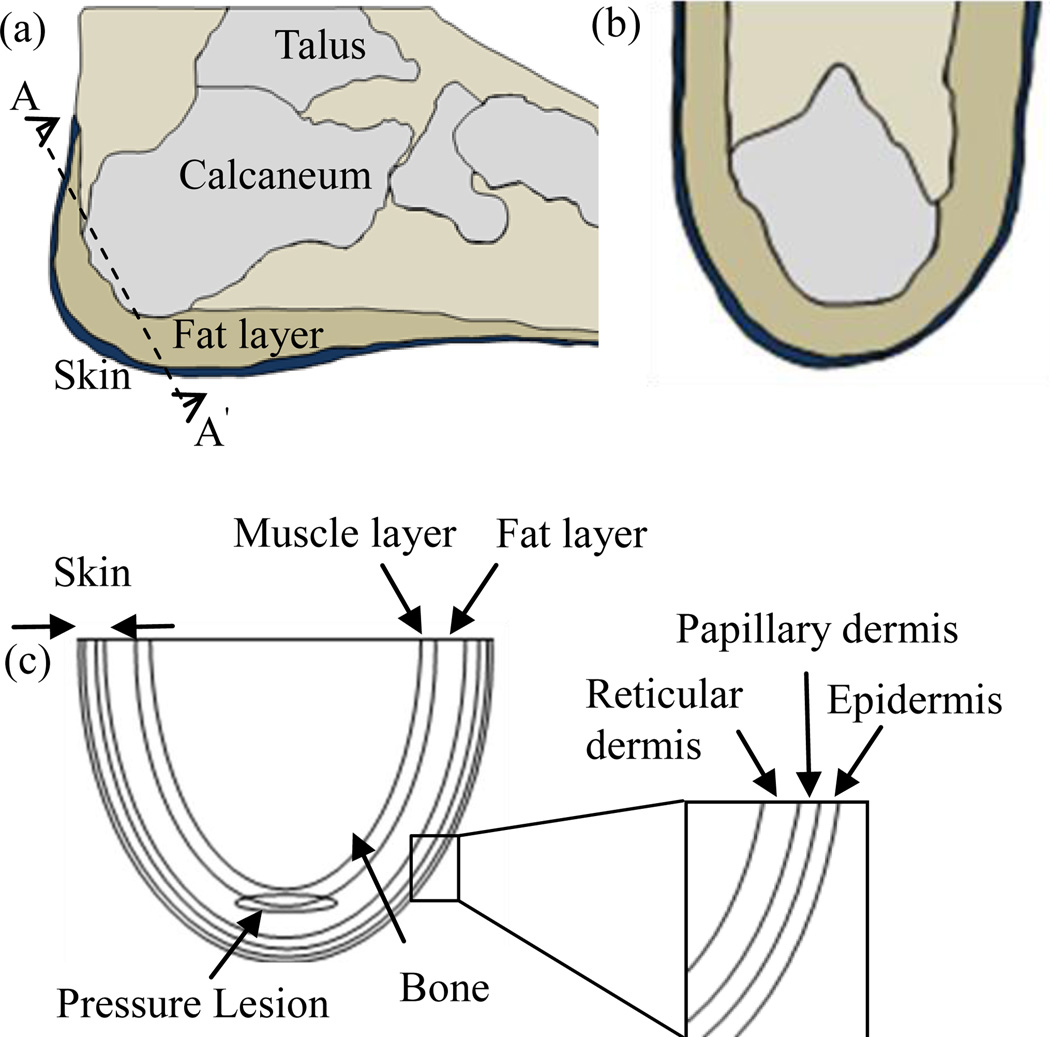
The heel is composed of the talus bone, a thick and large bone that comprises the posterior aspect of the foot (Fig. 1(a), (b)). The inferior portion of the heel is covered by thick skin and is well padded by a well supported structure, the cup ligament. However, the posterior aspect of the heel lacks this thick padding and the rich vascularity that characterizes the inferior portion. In the recumbent position, the tissue of the posterior portion of the heel is compressed against the talus. If the compression continues for any extended period, this low vascularized tissue is at risk for ischemia resulting in deep tissue injury.
FIGURE 1.
Heel Anatomy: (A) Lateral View of the Heel, (B) View Across The Cut Section AA’, (C) Schematic of the Heel Model Used for Computations. The Heel Has A Pressure Lesion in the Deep Tissue.
3. MATHEMATICAL MODEL AND COMPUTATIONAL METHOD
Thermographic imaging of pressure ulcers was attempted as a means of early diagnosis, however the results appeared inconclusive. Some studies indicated temperature increase whereas others showed lower temperatures. Investigators therefore concluded that IR imaging was not suitable for the diagnosis of DTI. These seemingly contradictory findings are explained by our study and by introducing the concepts of ischemia and inflammation into the thermal model as stages of DTI. DTI can be manifested as either ischemia or inflammation and thermal models of deep tissue ischemia and deep tissue inflammation will be introduced in the next sections.
3.1 THE BIOHEAT EQUATION
The Pennes bioheat equation is used to model tissue heat transfer processes [29] as
| (1) |
where i represents the layer of the tissue and varies in this study vary from 1 to 7(six tissue layers and one lesion), Ti is the local tissue temperature, ρi is the tissue density, ci is the tissue specific heat, ki is the tissue thermal conductivity, Wbi is the tissue blood perfusion rate, and qmeti is the tissue volumetric metabolic heat generation rate in the ith tissue layer respectively. Similarly, Tai, ρbi and cbi represent arterial blood temperature, blood density, and blood specific heat in the ith tissue layer respectively. The bioheat transfer equation is based on the law of conservation of energy. The rate of change of thermal energy stored in a unit control volume of the tissue, according to the equation, is equal to the rate of net amount of heat that enters/leaves the control volume by conduction, blood perfusion and metabolic heat generation respectively. If equation for each tissue layer is simultaneously solved, the solution gives the spatial and temporal temperature distribution in the entire tissue. In the next section, a computational model is presented in which this equation has been used to calculate temperature profile in the heel tissue in the presence of a pressure lesion.
3.2 COMPUTATIONAL MODEL
An axial cross section of the heel tissue is represented by the 2D computational domain as shown in Fig. 1(c). A simplified semi-elliptical shape is assumed to represent the heel geometry, the tissue layers were assumed to have constant thickness, and typical values for tissue layer thicknesses were used in the computations. The heel has a semi-elliptical geometry and consists of the underlying calcaneum along with five tissue layers of uniform thicknesses: epidermis (0.46 mm), papillary dermis (1.67 mm), reticular dermis (1.67 mm), fat layer (5 mm), and the muscle (2.5 mm). The pressure lesion is represented by a regular ellipse (with minor axis and major axis of length 0.25 cm and 1.5 cm) occupying regions in both muscle and fat layers [11, 12]. The depth of the lesion from the skin surface is approximately 9 mm. The thickness of the fat layer along with other layers is assumed to be uniform for simplicity. Each sub-domain is homogenous, and has constant thermo-physical properties [13]. Dermal and muscle layer arterial blood temperature (Tai is assumed to be equal to 37°C, the core body temperature [14]. In addition, the bone-muscle interface can be maintained at a constant temperature if changes in deep tissue temperature are neglected during cooling and subsequent recovery stages in the simulations. Conduction within tissue layers and lesion, and convection between the skin surface and the surroundings are the dominant heat transfer processes in this study. Evaporative and radiative processes responsible for the loss of heat from the tissue are assumed to be small and are thus neglected.
The main aim of the study is to quantify temperature distributions and the differences in the skin surface thermal response to a cooling excitation in the presence and absence of a deep tissue lesion. Before applying a cooling load on the skin, calculation of steady state temperature distribution is essential. This is important, since a steady state temperature profile not only predicts the thermal state of the tissue before the cooling begins, but also describes a state that it gradually attains during thermal recovery process [14]. Our effort is thus divided into three steps: computing the steady state temperature distribution, application of cooling load on the skin surface, and computing the recovery state temperature distribution as a function of time.
In each step, bioheat transfer equation is simultaneously solved for the lesion, for the five tissue layers and for the bone, by using the continuity of heat flux and temperature at each interface and appropriate thermal conditions at each domain boundaries. Equation (2) and (3) give the interfacial conditions of temperature and heat flux continuity at each tissue interface respectively, where values of i represent the tissue layer.
| (2) |
| (3) |
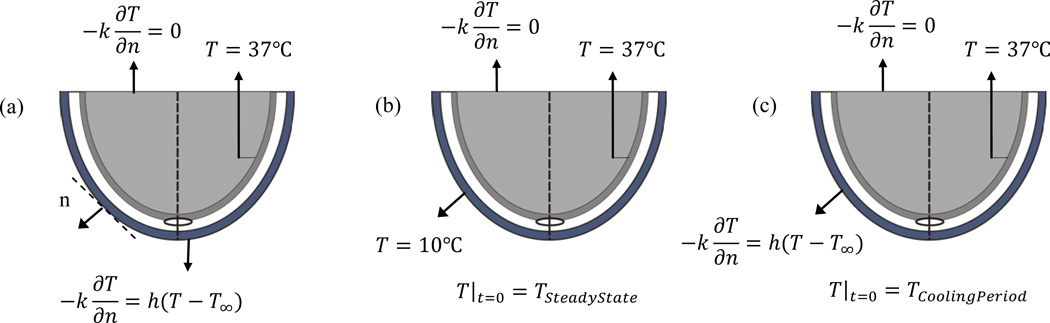
Figure 2. shows the thermal boundary conditions for the three steps of the analysis. In all three stages, the interface between bone and muscle layer is maintained at a core body temperature of 37°C and the boundary condition at the deep tissue (top horizontal surface in the schematic) is approximated by zero heat flux. During steady state, heat transfer occurs at the outermost boundary, i.e. at the interface between the skin and the surroundings, due to convection. For a surrounding temperature of 22°C the heat transfer coefficient h is assumed to be equal to 10W/m2K. After steady state equilibrium is reached, the skin surface is cooled for 1 min at a temperature of 10°C (constant temperature boundary condition that can be approximated by applying a cold gel pack) and the transient temperature distribution is calculated. The steady state temperature distribution computed previously is used as the initial condition for this calculation. After 1 min, the cooling is removed and skin surface is again exposed to the surroundings. It takes about 20–25 min for the skin surface to return to its steady state conditions, however, the first few minutes of the thermal recovery process are of interest for practical diagnostic applications [13]. The analysis is done by using a finite element analysis and solver based software package COMSOL Multiphysics v4.2a.
FIGURE 2.
Initial and Boundary Conditions Used for Heat Transfer Analysis in the Heel Tissue (A) During Steady State, (B) During Cooling Period for 0 ≤ t ≤ tc, (C) DURING RECOVERY STATE for t ≥ tc, n is the Direction Normal to the Surface of the Skin.
3.2.1 THERMOPHYSICAL PROPERTIES OF THE LESION
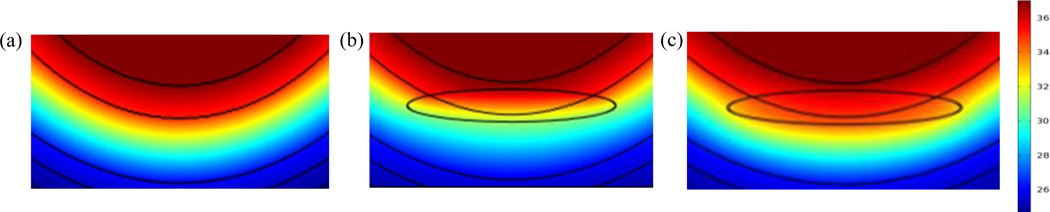
Table 1. summarizes the thermophysical properties of various tissue layers and of the lesion. Tissue damage in a deep tissue injury is caused during ischemia and reperfusion processes by sequences of biochemical reactions. Details of such processes may be found in [15, 17, 18]. We are interested in observing tissue temperature changes that may accompany such processes. For this purpose we need to identify the thermophysical properties that can influence the temperature changes. Pirtini Cetingul and Herman C. [13], in their thermal model of near surface lesions, showed that changes in tissue blood perfusion and metabolic heat generation rates influence the skin surface temperature, the latter, however, having a weaker influence than the former. Ruschkewitz Y. and Gefen A. [16], in studying cell level temperature changes associated with DTI in spinal cord injury patients, considered changes in tissue conductivity, tissue density, and arterial blood temperature in their model. Based upon these considerations, three thermal models are described in the next section. The first model is for a healthy heel tissue when no lesion is present. In the second model, changes in thermo-physical properties that may characterize an ischemic phase are associated with the lesion. In the third model thermo-physical properties that may be associated with inflammation and reperfusion processes are co nsidered. Figure 3. shows the recovery state temperature distributions in these three models.
TABLE 1.
Thermophysical Properties of the Tissue Layers and the Pressure Lesion (All data is from [13] unless indicated otherwise in the table)
| Specific heat (J/kg.K) |
Density (kg/m3) |
Thermal conductivity (W/m.K) |
Perfusion rate (10–3) (1/s) |
Metabolic heat rate (W/m3) |
Arterial blood temperature (°C) |
Blood density (kg/m3) |
Blood specific heat (J/kg.K) |
|
|---|---|---|---|---|---|---|---|---|
| Epidermis | 3589 | 1200 | 0.235 | 0 | 0 | - | 1060 | - |
| Papillary dermis | 3300 | 1200 | 0.445 | 0.18 | 368.1 | 37 | 1060 | 3700 |
| Reticular dermis | 3300 | 1200 | 0.445 | 1.26 | 368.1 | 37 | 1060 | 3700 |
| Fat | 2674 | 1000 | 0.185 | 0.08 | 368.3 | 37 | 1060 | 3700 |
| Muscle | 3600[16] | 1085 | 0.51 | 2.7 | 684.2 | 37 | 1060 | 3700 |
| Bone | 1300[26] | 2000[25] | 0.4[25],[26] | 0[27] | 0[27] | - | - | - |
| Lesion (Ischemia) | 2450[16],[13] | 1037[16] | 0.1[16] | 0.262[19–21] | 342.1 | 35[16] | 1060 | 3700 |
| Lesion(Inflammation) | 2450 | 1037 | 0.558 | 6.95 | 5262.5 | 37 | 1060 | 3700 |
FIGURE 3.
Temperature Distributions Inside the Heel Tissue Near the Location of the Lesion During Thermal Recovery for (A) No Lesion Present, (B) Lesion With Ischemia, (C) Lesion With Inflammation. This Distribution is Computed At 3.5 Min of Thermal Recovery Where Maximum Temperature Difference Can Be Observed.
3.2.2 HEALTHY TISSUE MODEL
The healthy tissue model represents the case when heel is free of any lesions. This model is necessary as baseline to be able to compare the thermal response of a healthy heel tissue with a heel tissue which contains a lesion. Figure 3(a). shows the computed temperature distribution when no lesion is present in the heel tissue.
3.2.3 ISCHEMIA MODEL
This model describes the heel temperature distribution when ischemic conditions are present in the lesion. During ischemia there is partial or complete occlusion of the blood vessels in the tissue as the pressure levels in the tissue exceed the capillary closing pressure of 32 mm Hg [9]. Many experimental studies have predicted low levels of blood perfusion during ischemic period [19–21]. Based upon these studies, we associate a reduction in blood perfusion rate in the lesion. We have also assumed a decrease in the metabolic heat generation within the tissue in ischemic conditions. In addition to these properties, we also characterize the lesion as a region of low thermal conductivity [16] and low density [16, 12]. Figure 3(b) shows the computed temperature profile inside an ischemic lesion. Clearly, the temperature distribution is influenced by the presence of a lesion.
3.2.4 INFLAMMATION MODEL
This model describes the heel temperature distribution when inflammation is present in the lesion. Inflammation may occur during an early phase of the injury in the form of repair mechanisms initiated by the adjacent tissue or during the advanced stage of ischemia-reperfusion, when increased blood perfusion in the ischemic region results in the generation of inflammatory mediators. Changes in tissue blood perfusion before and after a prolonged epidermal loading have been studied in a trochanteric rat model by Mark et al. [22]. They noted that blood perfusion increased twice during the first loading-unloading cycle. The first increase was detected before prolonged loading, which we assume was due to the initial response of the tissue to the injury, and the second was observed after tissue unloading, which was found as a result of tissue reperfusion. The presence of inflammatory mediators during ischemia-reperfusion injury has been reported in other studies as well [23, 24].
In the inflammation model, we thus characterize the lesion as a region of increased blood perfusion. Based on the study by Pirtini Cetingul and Herman C. [13], we also associate an increased metabolic heat generation rate with the lesion. The numerical values for these properties have been taken from the same study. Figure 3(c). shows the computed temperature profile inside a lesion when inflammation characteristics are present.
4. RESULTS
Figure 3. displays the temperature distribution in and around the lesion for the three models at 3.5 minutes into the thermal recovery. The presence of a lesion changes the temperature distribution in the deep tissue when compared to the baseline case without lesion. This difference exists because of different thermal characteristics of lesion. When ischemic conditions are present, a temperature decrease is observed in the lesion and the surrounding tissue. On the other hand, when inflammation is present in the lesion, temperature increase is observed in the lesion, and the surrounding tissue.
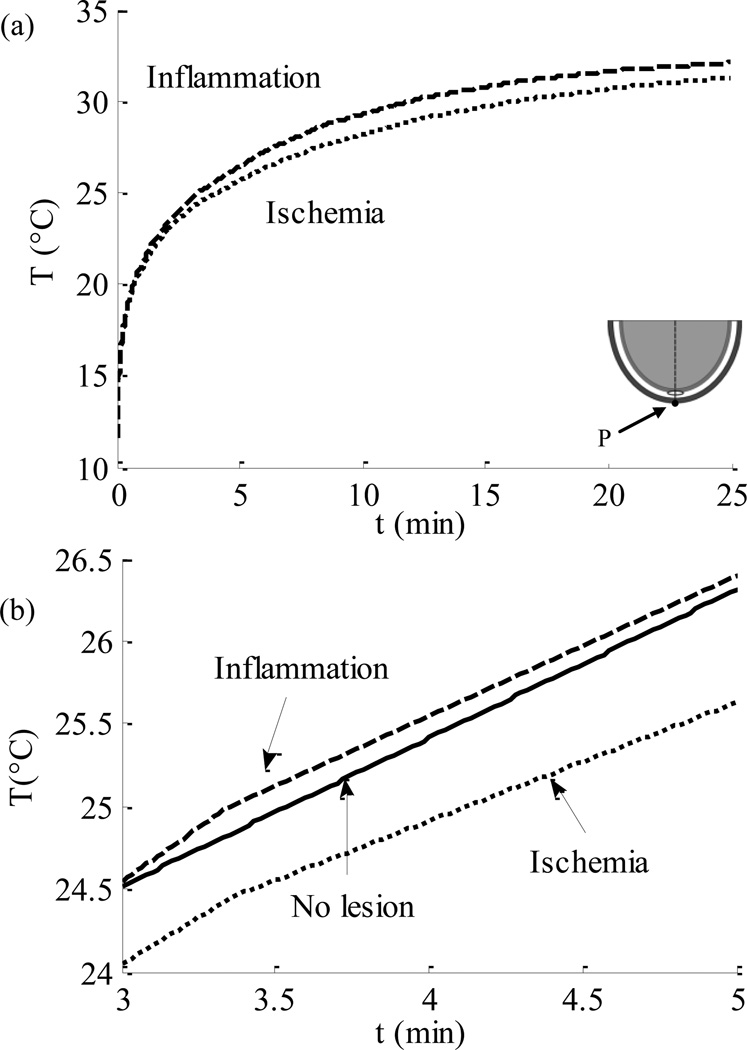
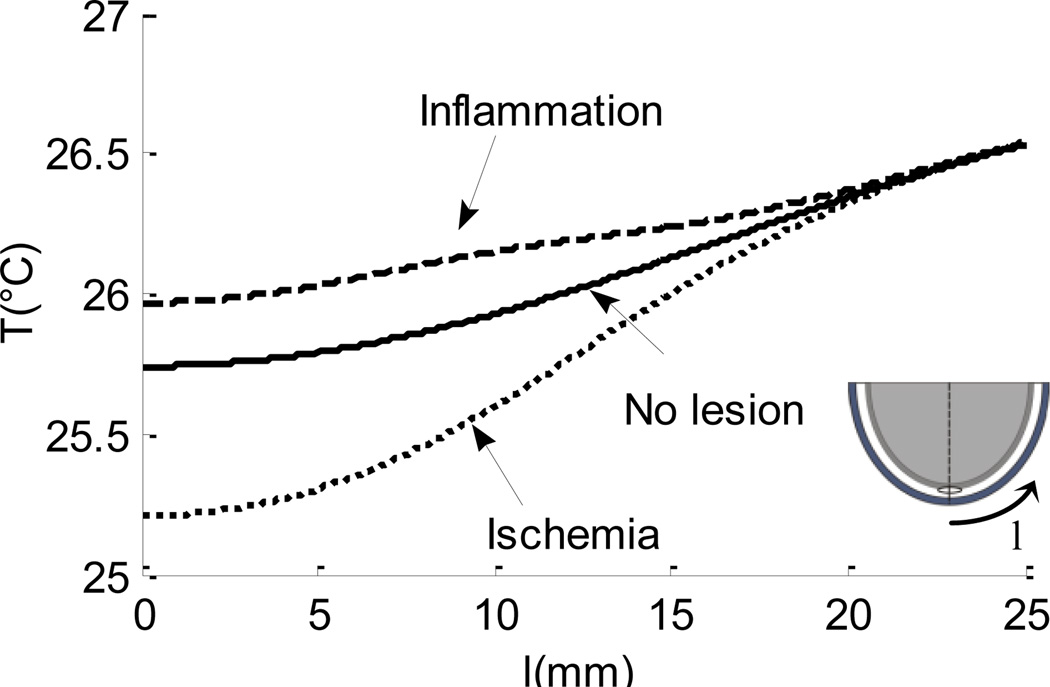
In all three models, after calculation of steady state temperature distribution, the skin surface was cooled down to 10°C for a period of 1 min. Figure 4. compares the thermal recovery of the skin surface in different models. The abscissa in the diagram represents the recovery time t in minutes, and the ordinate displays the temperature T in °C of a point P on the skin surface (the point lies above the center of the lesion, as shown in the figure). It was found that the skin surface takes about 20–25 min to reach steady state in both inflammation and ischemia models, however, the recovery rate is faster in the presence of lesion inflammation (Fig. 4(a)). Figure 4(b). shows the recovery curve for all three models for the early stages of thermal recovery, from 3 to 5 min. This result suggests that the skin surface temperature recovers faster in the presence of lesion inflammation than the healthy skin. On the other hand, in the presence of lesion ischemia, the rate of recovery of the skin surface temperature is slower when compared to the healthy skin. A temperature difference of 0.5–0.55°C is observed between the healthy tissue model and inflammation model, and a difference of 0.2–0.23°C is observed between healthy tissue and ischemia model. This difference is large enough to be measured by the infrared imaging technique described in the previous study of the group [28]. Figure 5. shows the recovery state skin surface temperature 3.5 min into the thermal recovery for three cases: tissue inflammation, healthy tissue and ischemic tissue. The abscissa in the figure represents the distance l in mm, on the heel periphery measured from the bottommost point of the computational domain. The ordinate represents the tissue temperature T in °C. The results suggest that inflammation causes a rise in the temperature at the skin surface, while a temperature decrease is observed in the case of an ischemic lesion. The magnitude of temperature difference decreases as we move away from the lowermost point of the domain. The maximum temperature difference from the baseline temperature observed during ischemia and inflammation is between 0.5–0.55°C and 0.2–0.23°C respectively.
FIGURE 4.
Thermal Recovery Curves of Point P On The Skin Surface (A) Comparison Between Ischemic Tissue and Tissue With Inflammation During Thermal Recovery, (B) Comparison Between Ischemic Tissue, Tissue With Inflammation and Tissue Without Any Lesion (Recovery is Shown for the Time Interval of 3–5 Minutes).
FIGURE 5.
Skin Surface Temperature Plotted Along the Heel Periphery, X-Axis Represents The Distance Measured From The Lowermost Point as Shown in the Figure (Temperature is Computed At 3.5 Min of Thermal Recovery).
5. CONCLUSION
The thermal response of the skin surface was found to be different under ischemic conditions and inflammation. The lesion is at a temperature lower than the surrounding tissue under ischemia, and the temperature is higher than the surrounding tissue during inflammation. Skin surface temperature during the recovery period also exhibits a similar pattern. Its temperature is higher during lesion inflammation than its temperature during lesion ischemia. On the skin surface, we can expect a temperature 0.5–0.55°C higher than the healthy tissue during lesion inflammation and a temperature 0.2–0.23°C lower than the healthy tissue during lesion ischemia. The healthy tissue is described by the case when no lesion is present in the deep tissue. Temperature was also observed along the heel periphery to find out a location for the maximum temperature difference on the skin surface. It was found that this location coincides with a point that lies on the skin surface directly above the lesion center.
These results can explain the reasons for the presence of warmer and colder regions on the skin surface as suggested by National Pressure Ulcer Advisory Panel (NPUAP) in the definition of suspected deep tissue injury (sDTI). Our model suggests that the lesion possessed inflammatory characteristics when a warmer region was noticed, whereas ischemic conditions may be the reason for the occurrence of a colder region on the skin surface. Ischemic conditions are believed to prevail in the tissue in early stages of the injury. During lesion ischemia, our results suggest a temperature drop on the skin surface. A criterion to detect incipient pressure lesions may be therefore developed based upon the thermal signatures on the skin surface. A sensitivity analysis of skin surface temperature distribution to parameters such as thermophysical properties, blood perfusion rate, metabolic heat generation rate, and tissue layer thicknesses is ongoing. The results also suggest that the lesion is present in the tissue directly below the location of maximum temperature difference on the skin surface. Knowledge of the location of the pressure lesion on the pressure lesion site would be important for its efficient management by healthcare providers.
ACKNOWLEDGMENTS
The authors appreciate the financial support provided for this research by NIH (Grant No. 1R01CA161265-01).
Contributor Information
Arjun Chanmugam, Department of Emergency Medicine, Johns Hopkins School of Medicine, Baltimore, MD, U.S.A.
Akanksha Bhargava, Department of Mechanical Engineering, Johns Hopkins University, Baltimore, MD, U.S.A.
Cila Herman, Department of Mechanical Engineering, Johns Hopkins University, Baltimore, MD, U.S.A.
REFERENCES
- 1.Ankrom MA, Bennett RG, Sprigle S. Pressure-Related Deep Tissue Injury Under Intact Skin and the Current Pressure Ulcer Staging Systems. Advances in Skin & Wound Care. 2005;18(1):35–42. doi: 10.1097/00129334-200501000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Mahanty SD, Roemer RB. Thermal Response of Skin to Application of Localized Pressure. Archives of Physical Medicine and Rehabilitation. 1979;60(12):584–590. [PubMed] [Google Scholar]
- 3.Schubert V, Fagrell B. Evaluation of the Dynamic Cutaneous Post-Ischaemic Hyperaemia and Thermal Response in Elderly Subjects and in an Area at Risk for Pressure Sores. Clinical Physiology (Oxford, England) 1991;11(2):169–182. doi: 10.1111/j.1475-097x.1991.tb00110.x. [DOI] [PubMed] [Google Scholar]
- 4.Sprigle S, Linden M, McKenna D. Clinical Skin Temperature Measurement to Predict Incipient Pressure Ulcers. Advances in Skin & Wound Care. 2001;14(3):133–137. doi: 10.1097/00129334-200105000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Angelidis I, Lidman D, Sjöberg F. Decubitus Ulcer Development. Pressure Alone Increases Tissue Temperature. 2009;32(5):241–244. [Google Scholar]
- 6.Sato M, Sanada H, Konya C. Prognosis of Stage I Pressure Ulcers and Related Factors. International Wound Journal. 2006;3(4):355–362. doi: 10.1111/j.1742-481X.2006.00267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benbow SJ, Chan AW, Bowsher DR. The Prediction of Diabetic Neuropathic Plantar Foot Ulceration by Liquid-Crystal Contact Thermography. Diabetes Care. 1994;17(8):835–839. doi: 10.2337/diacare.17.8.835. [DOI] [PubMed] [Google Scholar]
- 8.VanGilder C, MacFarlane GD, Harrison P. The Demographics of Suspected Deep Tissue Injury in the United States: An Analysis of the International Pressure Ulcer Prevalence Survey 2006–2009. Advances in Skin & Wound Care. 2010;23(6):254–261. doi: 10.1097/01.ASW.0000363550.82058.7f. [DOI] [PubMed] [Google Scholar]
- 9.Wong VK, Stotts NA. Physiology and Prevention of Heel Ulcers: The State of Science. Journal of Wound, Ostomy, and Continence Nursing: Official Publication of the Wound, Ostomy and Continence Nurses Society / WOCN. 2003;30(4):191–198. doi: 10.1067/mjw.2003.133. [DOI] [PubMed] [Google Scholar]
- 10.Kottner J, Dassen T, Lahmann N. Prevalence of Deep Tissue Injuries in Hospitals and Nursing Homes: Two Cross-Sectional Studies. International Journal of Nursing Studies. 2010;47(6):665–670. doi: 10.1016/j.ijnurstu.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Quintavalle PR, Lyder CH, Mertz PJ. Use of High-Resolution, High-Frequency Diagnostic Ultrasound to Investigate the Pathogenesis of Pressure Ulcer Development. Advances in Skin & Wound Care. 2006;19(9):498–505. doi: 10.1097/00129334-200611000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Aoi N, Yoshimura K, Kadono T. Ultrasound Assessment of Deep Tissue Injury in Pressure Ulcers: Possible Prediction of Pressure Ulcer Progression. Plastic and Reconstructive Surgery. 2009;124(2):540–550. doi: 10.1097/PRS.0b013e3181addb33. [DOI] [PubMed] [Google Scholar]
- 13.Cetingul MP, Herman C. A Heat Transfer Model of Skin Tissue for the Detection of Lesions: Sensitivity Analysis. Physics in Medicine and Biology. 2010;55(19):5933–5951. doi: 10.1088/0031-9155/55/19/020. [DOI] [PubMed] [Google Scholar]
- 14.Wilson SB, Spence VA. A Tissue Heat Transfer Model for Relating Dynamic Skin Temperature Changes to Physiological Parameters. Physics in Medicine and Biology. 1988;33(8):895–912. doi: 10.1088/0031-9155/33/8/001. [DOI] [PubMed] [Google Scholar]
- 15.Carden DL, Granger DN. Pathophysiology of Ischaemia-Reperfusion Injury. The Journal of Pathology. 2000;190(3):255–266. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 16.Ruschkewitz Y, Gefen A. Cell-Level Temperature Distributions in Skeletal Muscle Post Spinal Cord Injury as Related to Deep Tissue Injury. Medical & Biological Engineering & Computing. 2010;48(2):113–122. doi: 10.1007/s11517-009-0566-5. [DOI] [PubMed] [Google Scholar]
- 17.Tsuji S, Ichioka S, Sekiya N. Analysis of Ischemia-Reperfusion Injury in a Microcirculatory Model of Pressure Ulcers. Wound Repair and Regeneration. 2005;13(2):209–215. doi: 10.1111/j.1067-1927.2005.130213.x. [DOI] [PubMed] [Google Scholar]
- 18.Liu P, Hock CE, Nagele R. Formation of Nitric Oxide, Superoxide, and Peroxynitrite in Myocardial Ischemia-Reperfusion Injury in Rats. The American Journal of Physiology. 1997;272(5Pt2):H2327–H2336. doi: 10.1152/ajpheart.1997.272.5.H2327. [DOI] [PubMed] [Google Scholar]
- 19.Herrman EC, Knapp CF, Donofrio JC. Skin Perfusion Responses to Surface Pressure-Induced Ischemia: Implication for the Developing Pressure Ulcer. Journal of Rehabilitation Research and Development. 1999;36(2):109–120. [PubMed] [Google Scholar]
- 20.Mayrovitz HN, Smith J. Heel-Skin Microvascular Blood Perfusion Responses to Sustained Pressure Loading and Unloading. Microcirculation. 1998;5(2–3):227–233. [PubMed] [Google Scholar]
- 21.Peirce SM, Skalak TC, Rodeheaver GT. Ischemia-Reperfusion Injury in Chronic Pressure Ulcer Formation: A Skin Model in the Rat. Wound Repair and Regeneration : Official Publication of the Wound Healing Society [and] the European Tissue Repair Society. 2000;8(1):68–76. doi: 10.1046/j.1524-475x.2000.00068.x. [DOI] [PubMed] [Google Scholar]
- 22.Mak AF, Zhang M, Tam EW. Biomechanics of Pressure Ulcer in Body Tissues Interacting with External Forces during Locomotion. Annual Review of Biomedical Engineering. 2010;12:29–53. doi: 10.1146/annurev-bioeng-070909-105223. [DOI] [PubMed] [Google Scholar]
- 23.Vinten-Johansen J, Jiang R, Reeves JG. Inflammation, Proinflammatory Mediators and Myocardial Ischemia-reperfusion Injury. Hematology/oncology Clinics of North America. 2007;21(1):123–145. doi: 10.1016/j.hoc.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Eltzschig HK, Collard CD. Vascular Ischaemia and Reperfusion Injury. British Medical Bulletin. 2004;70:71–86. doi: 10.1093/bmb/ldh025. [DOI] [PubMed] [Google Scholar]
- 25.Stanczyk M, van Rietbergen B. Thermal Analysis of Bone Cement Polymerisation at the Cement-Bone Interface. Journal of Biomechanics. 2004;37(12):1803–1810. doi: 10.1016/j.jbiomech.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Fukushima H, Hashimoto Y, Yoshiya S. Conduction Analysis of Cement Interface Temperature in Total Knee Arthroplasty. The Kobe Journal of Medical Sciences. 2002;48(1–2):63–72. [PubMed] [Google Scholar]
- 27.Emery AF, Sekins KM. The use of Heat Transfer Principles in Designing Optimal Diathermy and Cancer Treatment Modalities. International Journal of Heat and Mass Transfer. 1982;25(6):823–834. [Google Scholar]
- 28.Pirtini Çetingül M, Herman C. Quantification of the Thermal Signature of a Melanoma Lesion. International Journal of Thermal Sciences. 2011;50(4):421–431. [Google Scholar]
- 29.Pennes HH. Analysis of Tissue and Arterial Blood Temperature in the Resting Human Forearm. Journal of Applied Physiology. 1948;1:93–122. doi: 10.1152/jappl.1948.1.2.93. [DOI] [PubMed] [Google Scholar]