Abstract
Intrahepatic sarcomatoid cholangiocarcinomais is a very rare disease with a poor prognosis due to its biologically aggressive tumor behavior. We report a patient who presented with subcapsular hemorrhage and a rapidly growing liver mass. A 57 year-old man was admitted with severe abdominal pain. CT and MRI images showed the presence of a 10 cm-sized subcapsular hemorrhage connected with a multi-lobulated mass with hemorrhage and necrotic foci in the right liver. The patients underwent right hemihepatectomy with caudate lobectomy and lymphadenectomy. The operation findings revealed metastatic nodules to the diaphragm and omentum. Detailed histopathological analysis through immunohistochemistry confirmed the diagnosis of sarcomatoid cholangiocarcinoma with a poorly undifferentiated sarcomatous component. The patient underwent chemotherapy. To date, the patient is doing well for 8 months after initial diagnosis.
Keywords: Intrahepatic cholangicarcinoma, Sarcomatous change, Subcapsular hematoma, Chemotherapy
INTRODUCTION
Sarcomatous change of the epithelial neoplasm is a rare condition with a poor clinical course. This condition has been observed in renal cell carcinoma and squamous cell carcinoma and originated from the esophagus, skin and lungs.1 In the liver, sarcomatous transformation has been reported in about 3.9 to 9.4% of hepatocellular carcinoma in autopsy and in about 4.5% of cholangiocarcinoma.2,3 Sarcomatous changes of cholangiocarcinoma are defined as "sarcomatous intrahepatic cholangiocarcinoma" in the World Health Organization (WHO) classification of tumor. To the best of our knowledge, intrahepatic cholangiocarcinoma associated with intraparenchymal hemorrhage had been rarely found. We report a patient with a large subcapsular hematoma caused by intrahepatic sarcomatoid cholangicarcinoma who underwent a hepatic resection, through literature review.
CASE
A 59 year-old man admitted to our emergency center complaining of pain in the right upper quadrant area which had abruptly started 1 day before. During the assessment, he felt dizziness and had mild fever. He had been treated for hypertension for 5 years. There was no past history of liver or biliary diseases. On physical examination, mild tenderness in right upper abdomen was felt. The laboratory results were as follows: hemoglobin, 13.1 g/dl (reference range: 13-18 g/dl), white blood cell, 12.44×103/µl (reference range: 4-10×103/µl) and platelet, 281×103/µl (reference range: 150-450×103/µl). The results of serum chemistry and electrolyte were as follows: AST, 205 IU/L (reference range: 5-35 IU/L) and ALT 325 IU/L (reference range: 5-40 IU/L). Other results were within the normal limits. Plain chest and abdomen X-rays showed no abnormal findings. Serum carbohydrate antigen (CA) 19-9 was 34.9 IU/ml (reference range: 0-33 IU/ml) and serum alpha-fetoprotein (AFP) and chorioembryonic antigen (CEA) were 2.58 IU/ml (reference range: 0.-5.5 IU/ml) and 7.32 IU/ml (reference range 0-4.6 IU/ml), respectively.
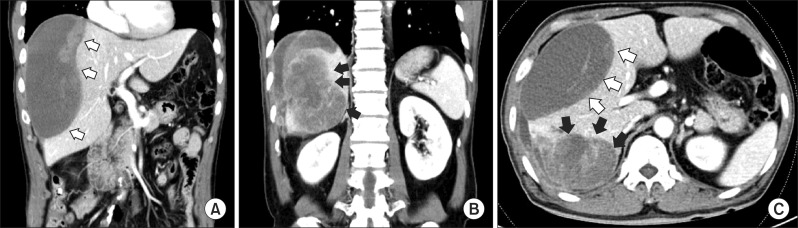
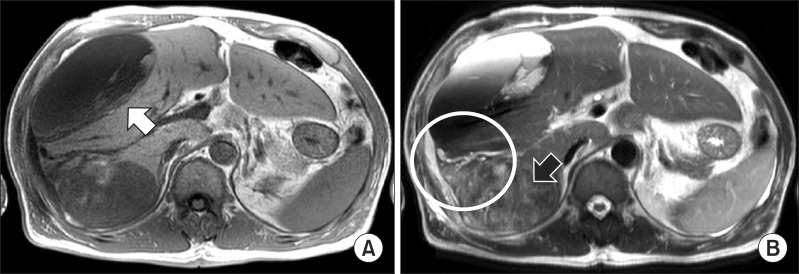
An abdominal computed tomography (CT) showed a homogenous low density lesion in the anterior section of the liver and a heterogeneous enhanced mass lesion in the delayed phase with the enhanced peripheral bile duct in the posterior section (Fig. 1). In magnetic resonance imaging (MRI) study, a large tumor of the right posterior section in the liver showed heterogeneous lower signal intensity in T1-weighted image and heterogeneous high signal intensity with necrosis and hemorrhage on mass in T2-weighted image. It was associated with focal dilation around the bile duct and hepatic capsular retraction of the posterior section in the liver. The large hematoma of the right anterior section was connected with the heterogenous tumor of the posterior section by high signal intensity in the right perihepatic space that was suspected as hemorrhage (Fig. 2). Intrahepatic cholangiocarcinoma was highly suspected through MRI and CT findings. We concluded that the large subcapsular hematoma was caused by ruptured cholangiocarcinoma.
Fig. 1. Computed tomography (CT) images show (A) a homogenous low density lesion in the anterior section of the liver (white arrows); (B) heterogeneous enhanced mass lesion in the delayed phase with enhanced peripheral bile duct in the posterior section (black arrows); and (C) transverse CT image showing a homogenous anterior mass and heterogenous enhanced of posterior section.
Fig. 2. Magnetic resonance imaging study shows (A) the large tumor of posterior lobe in the liver show heterogeneous lower signal intensity in T1 weighted image (white arrow) and (B) heterogeneous high signal intensity with necrosis and hemorrhage on mass in T2 weighted image (black arrow). It was associated with focal dilation around the bile duct and hepatic capsular retraction of the posterior section in the liver. The large hematoma of the right anterior section was connected with the heterogenous tumor of the right posterior section by high signal intensity in the right perihepatic space that was suspected as hemorrhage (white circle).
After 1 week of conservative treatment, a follow-up CT scan showed no internal bleeding but increase in hematoma size. The patient was discharged from the hospital for personal reasons and re-admitted after 1 month. The tumor was further increased in size compared with the previous CT scan findings.
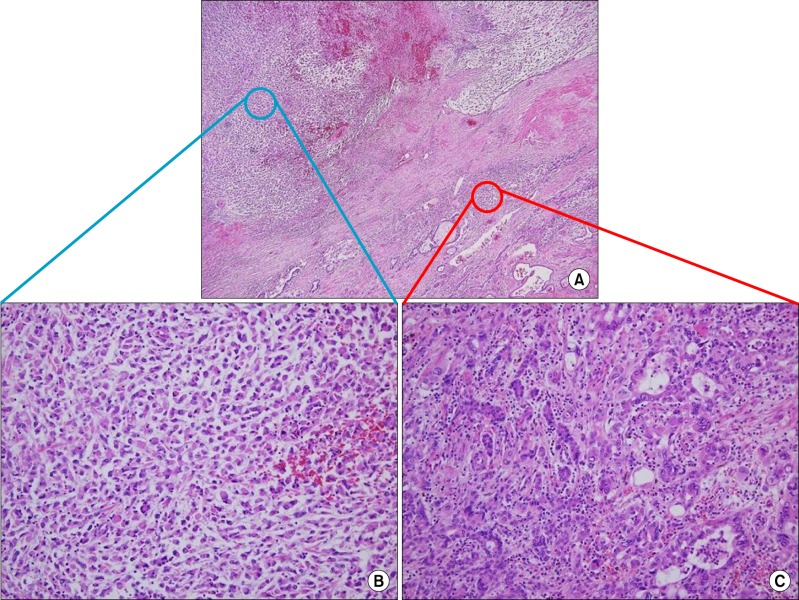
Upon readmission, the patient underwent a laparotomy. The intraoperative examination revealed a small amount of ascites in the peritoneal cavity. The tumor had strongly adhered to the diaphragm and a 3 cm-sized metastatic nodule was found in the diaphragm adjacent to the live dome without peritoneal seeding or lympadenopathy. Right hemihepatectomy with cholecystectomy and radical lymph node dissection was performed. The tumor was measured 18×15×10.5 cm. On the cut section, the surface showed massive necrosis with hemorrhage and 6.5 cm of cystic change (Fig. 3). In histological examination, the tumor showed a malignant neoplasm with a carcinomatous component and a sarcomatous component. The former consisted of moderately differentiated adenocarcinoma, while the latter consisted of a poorly differentiated sarcoma (Fig. 4). Immunohistochemical examination of the carcinomatous component revealed positive staining for epithelial membrane antigen (EMA), pan-cytokeratin (CK) stain and CEA stain and negative staining for vimentin. This immunoprofile was in keeping with cholangiocarcinoma. The sarcomatous component was positive for vimentin but negative for pan-CK and S-100 proteins (Fig. 5). Based on the histological and immunohistochemical findings, a diagnosis of sarcomatoid cholangiocarcinoma was made and the patient was referred to the department of Hemato-oncology for consideration of adjuvant chemotherapy.
Fig. 3. The gross finding of a cut surface showing a cystic change of the anterior section that was defined as a subcapsular hematoma (red circle) and severe necrosis and hemorrhage in the tumor with well-developed fibrous capsule (yellow circle).
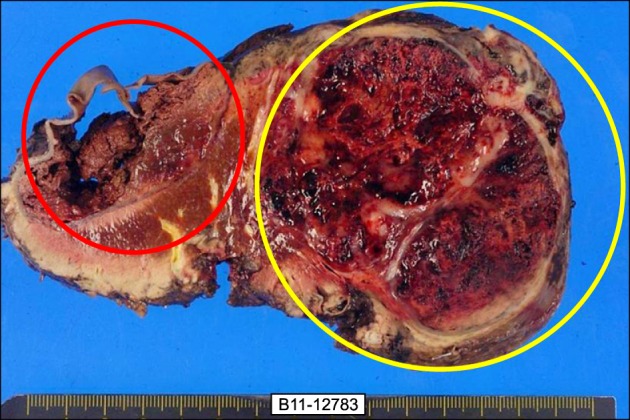
Fig. 4. Microphotographs show (A) microscopic findings of the resected specimen revealing carcinomatous and sarcomatous component (H&E, ×100). (B) Sarcomatous areas are composed of poorly differentiated cells that were showed a bizarre fashion with scanty cytoplasm and without the spindle bundle (H&E, ×200). (C) There were no features of hepatocytes and carcinomatous area shows moderately differentiated adenocarcinoma (H&E, ×200).
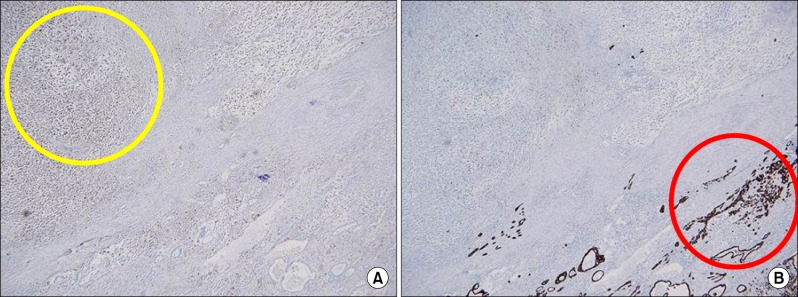
Fig. 5. Immunohistochemical examination showing the sarcomatous component being positive for vimentin but negative for pan-cytokeratin (A), and the carcinomatous component revealing positive staining for pan-cytokeratin stain and negative staining for vimentin (B).
The patient was started on chemotherapy combined with 5-fluorouracil and cisplatin, which he tolerated well. A postoperative contrast CT at 3 months after adjuvant chemotherapy confirmed disease recurrence with 2 small nodular lesions that had grown in the right subphrenic space (1 cm) and the omentum (8 mm) that was suspicious of peritoneal seeded metastasis. Since the follow-up CT, he received gemcitabine (1,000 mg/m2) and cisplatin (30 mg/m2) chemotherapy in a 21-day cycle. The patient received a total of six cycles and a follow up CT scan showed a partial response. At present, he is doing well for 8 months post-initial presentation.
DISCUSSION
Sarcomatous change of primary cancer of the liver is most common in hepatocellular carcinoma, especially after anticancer chemotherapy or transarterial embolization therapy.2,4 However, intrahepatic cholangicarcinoma with sarcomatous changes is rarely observed and is reported only in 4.5% of surgical autopsy cases. 3 There have been no previous reports concerning the interrelationship between intrahepatic sarcomatoid cholangiocarcinoma and anticancer therapy. The pathogenesis of sarcomatous change with epithelial malignancy is poorly understood. Several theories have been suggested to explain the intermingling of epithelial and mesenchymal malignancies as follows: 1) mesenchymal reaction, 2) true sarcoma (including the collision neoplasm hypothesis), 3) malignancy proliferation of epithelial origin (including the stromal induction/metaplasia model), 4) an embryonic cell rest origin, and 5) the totipotential stem cell hypothesis.5,6 Recently, Yoo et al.7 reported that the genetic and gene expression alterations may underlie the sarcomatous change or epithelial mesenchymal transition in cholangiocarcinoma. Diagnosis of sarcomatous change in intrahepatic cholangiocarcinoma is very difficult before pathologic confirmation. It appears to be difficult to differentiate between two carcinomas because there are no detailed distinguishing findings of clinical symptoms. Also, CT scans showed that the hypo-attenuated mass with peripheral regional enhancement was likely reported in most cases. These features resemble those of ordinary cholagiocarcinoma. Shimada et al.8 reported the following characteristics of patients who had been diagnosed with cholangicarcinoma with sarcomatous change: 1) Often demonstrating pyrexia; 2) without always demonstrating remarkable abnormal findings in the laboratory data including tumor markers; 3) histologically poorly differentiated adenocarcinoma; and 4) a very poor prognosis.
In our case, the patient admitted with severe right upper quadrant abdominal pain with fever and chills and elevated serum levels of CEA and CA19-9 of were detected. Histologically, intrahepatic sarcomatoid cholangiocarcinoma showed two intermingled components, intrahepatic cholangiocarcinoma and sarcomatoid carcinoma, in variable proportions. Kaibori M. et al. reviewed 17 previously reported cases of sarcomatous cholangicarcinoma in literature. Most of the cholangiocarcinoma region was comprised of moderate to poorly differentiated adenocarcinoma and most of the sarcomatous region was composed of a mixture of spindle cells and pleomorphic cells (75%).9 The sarcomatous component of most intrahepatic sarcomatoid cholangiocarcinoma according to previous reports showed immunoreaction to both vimentin and cytokeratin. Vimentin that is expressed in mesenchymal cells may reflect a sarcomatous transformation process, and the cytokeratins suggested that transformed sarcomatous carcinoma cells still retain some phenotypes of carcinoma cells.10 However, our case did not show immunoreaction of cytokeratins in the sarcomatous region, and this may be related to more dedifferentiation of tumor cells during sarcomatous transformation. It was reported that the prognosis of intrahepatic sarcomatoid cholangiocarcinoma is worse than that of ordinary intrahepatic cholangicarcinoma. Although recurrence or metastasis of tumor rapidly occurred regardless of the resection procedure because the sarcomatous component in the tumor seems to have a high potential to metastasize to variety of regions, a review of previous reports proposed that radical surgery is the treatment of choice in those with limited disease. The survival rates of the patients with surgically resected disease were significantly higher than in patients without resection.10 The role of adjuvant chemotherapy and the survival benefit to systemic chemotherapy is not well known. Malhotra et al.11 reported that the patient with resected intrahepatic sarcomatoid cholangiocarcinoma achieved a partial response after chemotherapy of combination of gemcitabine and cisplatin. Our patient received this combination chemotherapy as a purpose of palliative treatment because of a microscopic positive margin (sarcomatous component) and direct invasion of the diaphragm. During chemotherapy, the patient did not show progression of the tumor until 6 months after the resection.
In conclusion, we reported intrahepatic sarcomatoid cholangiocarcinoma with a large sized subcapsular hematoma in the liver. There were only a limited number of reports on adjuvant chemotherapy after resection in intrahepatic sarcomatoid cholangiocarcinoma. Our report showed a potential survival benefit of adjuvant gemcitabine and cisplatin chemotherapy in a patient with resected intrahepatic sarcomatoid cholangiocarcinoma, compared with previously reported cases.
ACKNOWLEDGEMENTS
This case report was supported by Wonkwang University in 2010.
References
- 1.Haratake J, Horie A. An immunohistochemical study of sarcomatoid liver carcinomas. Cancer. 1991;68:93–97. doi: 10.1002/1097-0142(19910701)68:1<93::aid-cncr2820680119>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 2.Kakizoe S, Kojiro M, Nakashima T. Hepatocellular carcinoma with sarcomatous change. Clinicopathologic and immunohistochemical studies of 14 autopsy cases. Cancer. 1987;59:310–316. doi: 10.1002/1097-0142(19870115)59:2<310::aid-cncr2820590224>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 3.Nakajima T, Tajima Y, Sugano I, et al. Intrahepatic cholangiocarcinoma with sarcomatous change. Clinicopathologic and immunohistochemical evaluation of seven cases. Cancer. 1993;72:1872–1877. doi: 10.1002/1097-0142(19930915)72:6<1872::aid-cncr2820720614>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 4.Kojiro M, Sugihara S, Kakizoe S, et al. Hepatocellular carcinoma with sarcomatous change: a special reference to the relationship with anticancer therapy. Cancer Chemother Pharmacol. 1989;23(Suppl):S4–S8. doi: 10.1007/BF00647229. [DOI] [PubMed] [Google Scholar]
- 5.Kubota K, Kakuta Y, Kawamura S, et al. Undifferentiated spindle-cell carcinoma of the gallbladder: an immunohistochemical study. J Hepatobiliary Pancreat Surg. 2006;13:468–471. doi: 10.1007/s00534-006-1100-x. [DOI] [PubMed] [Google Scholar]
- 6.Diebold-Berger S, Vaiton JC, Pache JC, et al. Undifferentiated carcinoma of the gallbladder. Report of a case with immunohistochemical findings. Arch Pathol Lab Med. 1995;119:279–282. [PubMed] [Google Scholar]
- 7.Yoo HJ, Yun BR, Kwon JH, et al. Genetic and expression alterations in association with the sarcomatous change of cholangiocarcinoma cells. Exp Mol Med. 2009;41:102–115. doi: 10.3858/emm.2009.41.2.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimada M, Takenaka K, Rikimaru T, et al. Characteristics of sarcomatous cholangiocarcinoma of the liver. Hepatogastroenterology. 2000;47:956–961. [PubMed] [Google Scholar]
- 9.Kaibori M, Kawaguchi Y, Yokoigawa N, et al. Intrahepatic sarcomatoid cholangiocarcinoma. J Gastroenterol. 2003;38:1097–1101. doi: 10.1007/s00535-003-1203-y. [DOI] [PubMed] [Google Scholar]
- 10.Tsou YK, Wu RC, Hung CF, et al. Intrahepatic sarcomatoid cholangiocarcinoma: clinical analysis of seven cases during a 15-year period. Chang Gung Med J. 2008;31:599–605. [PubMed] [Google Scholar]
- 11.Malhotra S, Wood J, Mansy T, et al. Intrahepatic sarcomatoid cholangiocarcinoma. J Oncol. 2010;70:701476. doi: 10.1155/2010/701476. [DOI] [PMC free article] [PubMed] [Google Scholar]