Abstract
Backgrounds/Aims
Although recent advances in surgical techniques and alternative treatment, the long-term survival >5 years after liver resection for hepatocellular carcinoma (HCC) is still unsatisfactory due to the high recurrence rate compared with other solid organ cancers. This study was conducted to analyze long-term survival after HCC resection and to develop an optimal strategy to achieve long-term survival.
Methods
A retrospective review was performed for HCC patients who underwent liver resection between 1996 and 2006. The survival rates and prognostic factors were assessed. The clinical and pathological factors of patients who survived more than 5 years were compared with those of patients whose survival was less than 5 years. The clinicopathological features characterizing long-term survivors were also reviewed.
Results
The overall and disease-free 5-year survival rates of 87 cases were 38.5% and 29.4%, respectively. Twenty-seven of 87 patients survived longer than 5 years after liver resection. The univariate analysis revealed that hepatitis C, the serum aspartate sminotransferase (AST) level, liver cirrhosis, Edmondson-Steiner grade, AJCC stage, and vascular invasion were significant factors for overall survival, and serum AST level, liver cirrhosis, Edmondson-Steiner grade, AJCC stage, and vascular invasion were the affecting factors for disease-free survival. In multivariate analysis, serum AST level, hepatitis C and vascular invasion were related with the overall survival, liver cirrhosis and vascular invasion which were associated with disease-free survival. Vascular invasion, AJCC stage, and the Edmondson-Steiner grade were significant factors in long-term survivors.
Conclusions
Patients without liver cirrhosis, vascular invasion and normal liver function, good differentiation and an early stage may be expected to have a long-term survival.
Keywords: Liver resection, Hepatocellular carcinoma, Prognostic factors
INTRODUCTION
As the initial clinical symptom of hepatocellular carcinoma (HCC) is not apparent, only a periodic examination makes it possible to be detected in the early stage. When it is detected with symptoms, intensive and extensive treatments are not feasible because of the regression of liver function accompanied by chronic liver disease or liver cirrhosis,1,2 and even resection is possible, the 5-year survival rate is low owing to recurrence as compared with other solid cancers.3,4
Since the long-term survival rate is the most important index when estimating a treatment strategy for malignant cancer, many studies focused on treatment methods to increase the survival rate after treatments are in progress multilaterally. A single operation, such as a liver transplantation or liver resection which can eliminate all tumor cells leading to a non-tumorous state, and an operation with non-operative adjuvant therapy such as percutaneous ethanol injection therapy, radiofrequency ablation, and transcatheter arterial chemoembolization have been attempted. But a high recurrence rate resulted in a low long-term survival rate.5 Such a high rate of recurrence was related to mortality, so that there was a trial to increase the long-term survival rate by analyzing the factors associated with recurrence, but it has not yet been established.6,7
We had followed up the patients who had undergone liver resection, and evaluated the survival rate and prognostic factors. We tried to figure out the condition to derive the long-term survival rate by analyzing clinical and pathologic characteristics of two groups, the group surviving more than 5 years and the other group with short-term survival.
METHODS
Of the patients who were diagnosed with HCC and underwent liver resection between January 1996 and August 2006, 87 cases had an elapse of more than 5 years from the first day of September 2011 were studied retrospectively by their medical records.
To evaluate preoperative liver function, liver function tests and indocyanin green 15 minute retention rate (ICG R15) were performed, and abdominal sonography, computed tomography (CT) and angiography were checked. Couinaud's classification was used for resection surgery, and massive liver resection was defined as resection of more than 3 segments and minimal as less than 3 segments. The range of resection was determined by ICG R15. The positive resection margin was defined as when microscopic finding was positive even though it was negative for gross finding.
Prognostic factors were analyzed as followed; sex, age, hepatitis B, hepatitis C, alcohol consumption, aspartate aminotransferase (AST), alanine aminotransferase (ALT), ICG R15, serum alpha-fetoprotein (AFP), a history of preoperative treatment, the range of the resection, blood transfusion, number of tumors, the maximal size of the tumor, differentiation of the tumor (Edmondson-Steiner grade), invasiveness into the resection margin, AJCC TNM stage,8 and vascular invasion.
A follow-up study was performed in the out-patient department, which included a routine physical examination, liver function test, AFP, simple chest radiograph, abdominal ultrasonography or CT at an interval of 3 to 4 months. A recurrence was checked out with serum AFP, abdominal ultrasonography or CT, and hepatic artery angiography, and the time of recurrence was determined on the date of the final confirmation for recurrence.
The patients were grouped into two groups as the long-term and short-term survival groups: an L-group who survived 5 or more years after liver resection and an S-group survived less than 5 years. The clinical and pathologic characteristics, treatment methods, and recurrence were analyzed. The SPSS Windows version 15.0 was used for statistical analysis; the continuous variables for a clinical pathologic analysis were analyzed by Student t test, chi-square test and fisher's exact test for categorical variables, the Kaplan-Meier for survival rate, and log-rank test to compare the survival rate of the two groups were used. To analyze the factors which influenced survival rate, a univariate analysis was performed first and a multivariate analysis using Cox-proportional hazards model method was performed for meaningful factors. A p-value<0.05 was considered significant.
RESULTS
The clinicopathologic characteristics
The clinical, pathological, and surgical data were summarized at Table 1. There were 65 males (74.7%) in our study, the mean age was 52.7 (range: 21-73) years. Most patients (n=84, 96.6%) were classified as having Child-Pugh class A, and 67 patients (77.1%) were diagnosed with liver cirrhosis in a histological examination. Before surgery, 30 patients (34.5%) were treated with transarterial embolization. Ten percent or less ICG R15 was found in 51 patients (58.6%). The serum AFP, a mean level of 5,156.9 IU/ml, was 400 IU/ml or more in 35 patients (40.2%). The mean size of the tumor was 5.4 cm, the greater diameter was 5 cm or beyond 5 cm in 44 patients (50.6%) of all cases. Seventy-three (83.9%) patients had solitary lesions, and vascular invasion was confirmed in 46 (52.9%) specimens. A radical resection was possible for 78 patients (89.7%), 9 patients were tumor-negative grossly in the resection margin, but tumor cells were detected microscopically. According to the TNM stage classification, 34 patients (39.1%) in stage I, 27 patients (31.0%) in stage II, and 26 patients (29.9%) in stage III. Also grade I and II in 60 patients (69.0%) by the Edmonson-Steiner grade were more than grade III and IV (Table 1).
Table 1. The characteristics of 87 patients who received hepatic resections.

HBV, hepatitis B virus; HCV, hepatitis C virus; ICG, indocyanin green; AST, aspartate aminotransferase; ALT, alanine aminotransferase
Operative treatments
Massive liver resection was performed in 38 patients (43.7%), and blood transfusions were necessary for 17 patients (19.5%). Postoperative morbidity occurred in 32 patients (36.8%), and mortality occurred in one patient (1.1%) as a result of bleeding.
Survival results and prognostic factors
The overall survival rate for all patients was 78.0% in the first year, 53.4% at 3 years, and 38.5% at 5 years, with a median survival of 43 months. Disease-free survival was 60.1% at 1 year, 46.1% at 3 years, and 29.4% at 5 years, with a median disease-free survival of 26 months. Twenty-seven of 87 patients survived longer than 5 years after a liver resection. The univariate analysis revealed those that were hepatitis C negative, serum AST level, of 50 U/L or less without liver cirrhosis, good differentiation (Edmondson-Steiner grade 1 to 2), AJCC early stage) (stage 1), and no vascular invasion were the significant factors for overall survival. Also, serum AST level of 50 U/L or less, without liver cirrhosis, good differentiation (Edmondson-Steiner grade 1 to 2), early AJCC stage) (stage 1), and no vascular invasion were the affecting factors for disease-free survival (Table 2). After detecting the impact of the abovementioned significant factors on the survival of patients by Cox regression, those that were hepatitis C negative, serum AST level of 50 U/L or less, and no vascular invasion were independent predictors for the overall survival. Meanwhile, liver cirrhosis and vascular invasion independently affected disease-free survival (Table 3).
Table 2. Univariate analysis of prognostic factors, overall survival and disease-free survival after a hepatic resection (n=87).

AST, aspartate aminotransferase; ALT, alanine aminotransferase; AFP, alpha-fetoprotein; ICG, indocyanin green
Table 3. Multivariate analysis of the overall and disease-free survival after hepatic resection (n=87).
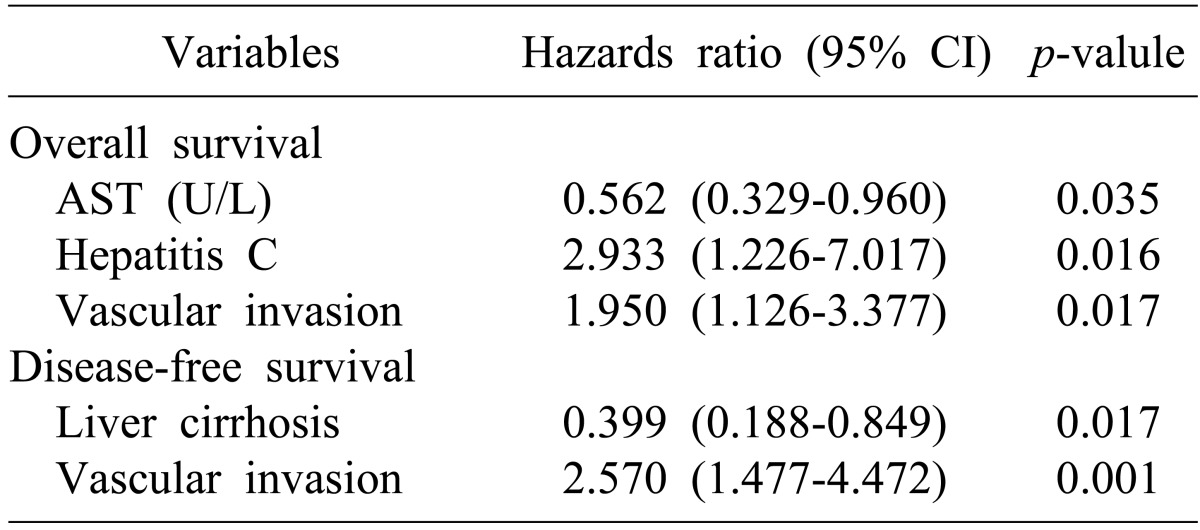
AST, aspartate aminotransferase
A total of 56 patients (64.4%) experienced tumor recurrence during the follow-up period, particularly in the liver (36 of 56) (Table 4). Among 56 patients with recurrence, 27 patients (48.2%) were treated mainly with transarterial chemoembolization, 10 patients did not receive any treatment (17.9%) (Table 5).
Table 4. Patterns of recurrence.

Table 5. Treatments of recurrence.

TACE, transarterial chemoembolization; RFA, radiofrequency ablation
Comparison of the long-term survival group (L-group) and the short-term survival group (S-group)
The S-group showed more vascular invasion than the L-group (p=0.014), and stage I of the TMN classification was overwhelmingly dominant as compared to stage 2 and 3 in L-group, while stage 2 was most dominant, which was similar distribution with stage 2 and 3 in S-group (p=0.037). According to Edmondson-Steiner grade, grade 1 and 2 were more than grade 3 and 4 in both of the two groups, which was much more remarkable in the L-group (p=0.011) (Table 6). Fourteen recurrences were found in the L-group and 42 in S-group. Intrahepatic tumor recurrence was found in 12 patients and two patients had extrahepatic recurrences in the L-group. Twenty-four in the intrahepatic, 11 in extrahepatic and 7 in both lobes were seen in the short-term group. Treatment for recurrence was performed in all of the patients of the L-group, while only 10 cases had a treatment in the S-group (Table 5). But treatment for recurrence was not statistically significant (p=0.898).
Table 6. Comparison of the clinicopathologic variables between the long-term (L-group) and short-term (S-group) survivors after surgical resection for hepatocellular carcinoma.

HBV, hepatitis B virus; HCV, hepatitis C virus; ICG, indocyanin green; AST, aspartate aminotransferase; ALT, alanine aminotransferase
DISCUSSION
It is difficult to perform aggressive treatment of HCC because of the deterioration of liver function caused by chronic hepatitis or liver cirrhosis. It has been generally recognized that HCC is a life-threatening malignancy, because of its high incidence of malignant biological behaviors, such as vascular invasion, infiltrating growth, strong potential of local recurrence and extrahepatic metastases. However, in the recent 10 years, the prominent improvement of safe surgical procedures are owed to the development of surgical techniques and anesthesia, the improvement of pre- and post-operative care, improved understanding of liver anatomy, and development of the ability to care in an intensive care unit reduces complications and mortality after liver resection.3 Moreover, early detection and treatment are practical owing to the application of computed tomography and ultrasound, and the development of diagnostic tools such as serum AFP. Various non-operative treatment methods, such as percutaneous ethanol injection therapy, radiofrequency ablation, and transarterial chemoembolization have been developed. Also it can be applied to patients who are not indicated to operation. Although this increases the overall survival rate,5 yet the survival rate is low as compared to other solitary tumors because of the high recurrence rate which is closely related to long-term survival.4,6,7
Examining previous studies on the factors that influence the recurrent rate showed that there were some agreed upon factors which had shown a significant effect, but some factors were controversial. Hepatitis B and C Infection is known to be directly related to hepatocellular carcinoma,4,9,10 but no significant relationship could be found in those studies due to the small number of samples similar to this study.6
The size and number of tumors, vascular invasions, existence of satellite nodules, range of resections and ruptures of membrane of tumors, suggested in previous studies7,11,12,13 may influence recurrence. Most studies7,12,13 mentioned that vascular invasion was a direct tumor forming factor through circulation. This had significant results for our study as well (p=0.001). For the size of tumor, many studies12,13,14 admitted that the significance that a tumor larger than 5 cm had a higher recurrence rate,6,12,13 but there was no statistical significance in our study (p=0.258). Lee and his collegues15 concluded that the tumor size did not have any statistical significance in their multivariate analysis, which was analyzed at every 1 cm of size from 2 cm to 7 cm. Although this study did not investigate the existence and rupture of the membrane, Laurent et al.16 found in their multivariate study that the recurrence rate was high when the membrane was ruptured. Another study17 reported the poor survival rate with the rupture of the tumor itself, on the other hand Han et al.18 found no difference in the survival rate according to the existence of a membrane, but they reported a difference in survival rate whether or not the tumor cell invaded the membrane. Many studies reported a poor result with multiple tumor masses, but this study found no statistical significance.7,11,18
Bleeding remains a major problem with liver resections. Blood transfusion of any amount, as shown some studies, is related to shortening the overall cumulative and disease-free survival periods.17,19,20,21 But it is considered the reflection of the severity of progressive cirrhosis which causes massive blood loss, not by blood transfusion. To reduce bleeding during mobilization of the tumor, adequate and wide exposure by a generous incision and adequate illumination of the operative field are essential. In our study, transfusion related to the operation had no significance.
Many institutions11,13,16 noted that a free margin of parenchyma has been regarded as a prognostic factor in terms of long-term survival and tumor recurrence, but this had no significance in our study. Shah et al.13 mentioned that cirrhosis was associated with recurrence, our study also found cirrhosis related to high recurrence rates.
HCC recurred in the intrahepatic region as reported by many institutions.22,23,24 In our study, there were 64.4% postoperative recurrences, and among these, intrahepatic recurrence was 64.3%. If a recurred hepatocellular carcinoma is detected early in the intrahepatic region, it is not only possible to resect all of lesion, but the non-operative modality, such as transarterial embolizaion or radiofrequency ablation, also this can be performed when an operation is not possible because of a poor general condition.
To improve the survival rate, it is important to prevent and detect recurrence, yet further study on prevention is needed. Closer follow-up in the out-patient department with more frequent radiologic study (USG or CT) and serologic tumor marker study (AFP) and perhaps with magnetic resonance imaging studies of the liver to detect an early recurrence should be considered. Additionally, patients with an extremely high surgical risk can be treated with other treatment modalities such as transarterial embolizaion or radiofrequency ablation.
ACKNOWLEDGEMENTS
This research was supported by grant of Inje University (2010).
References
- 1.Nagasue N, Uchida M, Makino Y, et al. Incidence and factors associated with intrahepatic recurrence following resection of hepatocellular carcinoma. Gastroenterology. 1993;105:488–494. doi: 10.1016/0016-5085(93)90724-q. [DOI] [PubMed] [Google Scholar]
- 2.Roh MJ, Kim HJ, Yoon SS, et al. Longterm prognostic factors after hepatic resection for hepatocellular carcinoma. J Korean Surg Soc. 2009;76:225–230. [Google Scholar]
- 3.Ibrahim S, Roychowdhury A, Hean TK. Risk factors for intrahepatic recurrence after hepatectomy for hepatocellular carcinoma. Am J Surg. 2007;194:17–22. doi: 10.1016/j.amjsurg.2006.06.051. [DOI] [PubMed] [Google Scholar]
- 4.Nagasue N, Ono T, Yamanoi A, et al. Prognostic factors and survival after hepatic resection for hepatocellular carcinoma without cirrhosis. Br J Surg. 2001;88:515–522. doi: 10.1046/j.1365-2168.2001.01732.x. [DOI] [PubMed] [Google Scholar]
- 5.Marelli L, Stigliano R, Triantos C, et al. Treatment outcomes for hepatocellular carcinoma using chemoembolization in combination with other therapies. Cancer Treat Rev. 2006;32:594–606. doi: 10.1016/j.ctrv.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Cho HW, Lee JG, Lim CY, et al. Factors affecting the recurrence of hepatocellular carcinoma after surgical resection. J Korean Surg Soc. 2005;69:465–470. [Google Scholar]
- 7.Shah SA, Cleary SP, Wei AC, et al. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery. 2007;141:330–339. doi: 10.1016/j.surg.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 8.Edge SB, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual. 7th ed. Chicago: Springer; 2010. [Google Scholar]
- 9.Kalayci C, Johnson PJ, Davies SE, et al. Hepatitis B virus related hepatocellular carcinoma in the non-cirrhotic liver. J Hepatol. 1991;12:54–59. doi: 10.1016/0168-8278(91)90909-u. [DOI] [PubMed] [Google Scholar]
- 10.el-Refaie A, Savage K, Bhattacharya S, et al. HCV-associated hepatocellular carcinoma without cirrhosis. J Hepatol. 1996;24:277–285. doi: 10.1016/s0168-8278(96)80005-5. [DOI] [PubMed] [Google Scholar]
- 11.Chen MF, Tsai HP, Jeng LB, et al. Prognostic factors after resection for hepatocellular carcinoma in noncirrhotic livers: univariate and multivariate analysis. World J Surg. 2003;27:443–447. doi: 10.1007/s00268-002-6708-7. [DOI] [PubMed] [Google Scholar]
- 12.Fuster J, García-Valdecasas JC, Grande L, et al. Hepatocellular carcinoma and cirrhosis. Results of surgical treatment in a European series. Ann Surg. 1996;223:297–302. doi: 10.1097/00000658-199603000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah SA, Greig PD, Gallinger S, et al. Factors associated with early recurrence after resection for hepatocellular carcinoma and outcomes. J Am Coll Surg. 2006;202:275–283. doi: 10.1016/j.jamcollsurg.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Tsukuma H, Hiyama T, Tanaka S, et al. Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N Engl J Med. 1993;328:1797–1801. doi: 10.1056/NEJM199306243282501. [DOI] [PubMed] [Google Scholar]
- 15.Lee KU, Koh YT, Kim KH, et al. Prognostic factors of hepatocellular carcinoma after curative hepatic resection. Korean J Hepatobiliary Pancreat Surg. 1997;1:41–58. [Google Scholar]
- 16.Laurent C, Blanc JF, Nobili S, et al. Prognostic factors and longterm survival after hepatic resection for hepatocellular carcinoma originating from noncirrhotic liver. J Am Coll Surg. 2005;201:656–662. doi: 10.1016/j.jamcollsurg.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 17.Fan ST, Ng IO, Poon RT, et al. Hepatectomy for hepatocellular carcinoma: the surgeon's role in long-term survival. Arch Surg. 1999;134:1124–1130. doi: 10.1001/archsurg.134.10.1124. [DOI] [PubMed] [Google Scholar]
- 18.Han SH, Lee WJ, Noh SH, et al. Univariate and multivariate analysis of prognostic factors in survival after resection of primary hepatoma. J Korean Surg Soc. 1994;47:393–400. [Google Scholar]
- 19.Lee KW, Suh KS, Roh HR, et al. The effect of intraoperative transfusion on the prognosis in hepatectomized hepatocellular carcinoma patients. Korean J Hepatobiliary Pancreat Surg. 2000;4:77–83. [Google Scholar]
- 20.Yamamoto J, Kosuge T, Takayama T, et al. Perioperative blood transfusion promotes recurrence of hepatocellular carcinoma after hepatectomy. Surgery. 1994;115:303–309. [PubMed] [Google Scholar]
- 21.Matsumata T, Ikeda Y, Hayashi H, et al. The association between transfusion and cancer-free survival after curative resection for hepatocellular carcinoma. Cancer. 1993;72:1866–1871. doi: 10.1002/1097-0142(19930915)72:6<1866::aid-cncr2820720613>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 22.Cha C, Fong Y, Jarnagin WR, et al. Predictors and patterns of recurrence after resection of hepatocellular carcinoma. J Am Coll Surg. 2003;197:753–758. doi: 10.1016/j.jamcollsurg.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000;232:10–24. doi: 10.1097/00000658-200007000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poon RT, Fan ST, Lo CM, et al. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: long-term results of treatment and prognostic factors. Ann Surg. 1999;229:216–222. doi: 10.1097/00000658-199902000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]


