Abstract
Although technical advances have been made in pancreaticoduodenectomy, the incidence of delayed gastric emptying (DGE) is reported as being high. Postoperative DGE is not fatal, but often results in prolonging the length of patients' stay in hospital, increasing their medical expenses, and further lowering their quality of life. DGE is a complex process caused by disorder and incoordination of various factors in charge of gastric mobility, such as smooth muscle cells (myogenic), enteric neuron (hormonal), and autonomic nervous system (neural). DGE often occurs after operative maneuvers that cause the loss of organs responsible for gastric motility and emptying or kinetic muscular or neuromuscular ischemia. To prevent DGE, it is most important to develop and universalize a standardized surgical technique in a way to reduce factors that are considered to cause DGE after pancreaticoduodenectomy. Moreover, if it is suspected that DGE occurred after pancreaticoduodenectomy, a differential diagnosis from diseases with similar symptoms via an accurate diagnostic approach should be implemented.
Keywords: Pancreaticoduodenectomy, Delayed gastric emptying, Therapy, Prevention
INTRODUCTION
In 1912, a standard pancreaticoduodenectomy was first introduced as a surgical treatment of periampullary cancer by Kausch and colleagues. Since then, the pylorus-preserving pancreaticoduodenectomy (PPPD) has been introduced and used as a universal surgical technique in many medical centers.1,2 With development of surgical techniques and improvement of perioperative patient management, the mortality rate after PPPD is reported as less than 1-5%, although the proportion varies depending on the center. However, the risk of developing serious complications, such as delayed gastric emptying (DGE), anastomotic leakage, and postoperative hemorrhage remains high, at approximately 30-65%.3,4 DGE after surgery refers to the condition that stomach cannot properly take food due to the symptoms of early satiety, nausea, and vomiting following upper gastrointestinal surgery without the mechanical obstruction of anastomosis or distal intestine. Postoperative DGE is not fatal, but often results in prolonging the length of patient stay in hospital, increasing their medical expenses, and further lowering their quality of life. Each researcher reported different results on the incidence of DGE after pylorus preserving pancreaticoduodenectomy, but the average was around 12-14%.5,6,7 Researchers suggested diverse causes on DGE and tried to find out the true causes, but many issues need to be sorted out. Therefore, this review is intended to offer clinical help by summarizing and explored the medical literature on the treatment and prevention of delayed gastric emptying after pancreaticoduodenectomy, including PPPD.
DEFINITION
The definition of DGE may differ according to the researchers, so it is difficult to simply compare the risk of postoperative complications and the assessment of treatment. Definitions proposed by the studies of Yeo and colleagues8 and van Berge Henegouwen and colleagues5 were most often adopted by many other literatures, but currently consensus definition proposed by the International Study Group of Pancreatic Surgery (ISGPS) in 2007 seems to be frequently quoted.9 According to this, delayed gastric emptying is divided into 3 grades (A, B and C) based on nasogastric intubation, type of diet patient is able to intake, and patient's general health condition, whether to use a prokinetic drug, and the need for further diagnostic tests. Grade A indicates the condition that the nasogastric intubation is removed within 7 days, dietary intake is possible, and self-limiting recovery is achieved without medications or surgical intervention. On the other hand, Grade B or C is the condition that medication or dietary control is required (Table 1).
Table 1. Summarized definition of delayed gastric emptying (DGE) according to the International Study Group of Pancreatic Surgery (ISGPS).
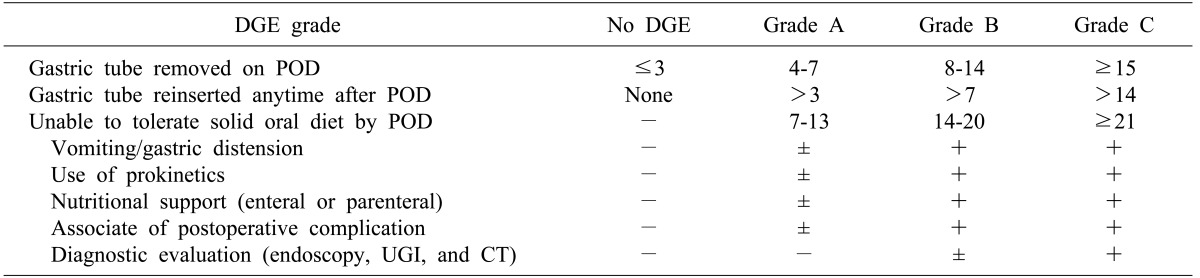
POD, postoperative days; UGI, upper gastrointestinal series; CT, computed tomography.
DIAGNOSIS OF DELAYED GASTRIC EMPTYING AFTER SURGERY
The first step in diagnostic evaluation is to see whether mechanical obstruction that may cause DGE is evident through an upper gastrointestinal series or by endoscope. The diagnostic methods listed below can be used to diagnose DGE.
Gastric-emptying scintigraphy
This is the gold standard for measuring gastric motility; diagnoses are made by measuring the quantity of remaining radioisotope in stomach at the specific time intervals after instructing patients take low-fat solid food (scrambled egg meal) labeled with its radioisotope (Tc-99m). In general, the diagnosis is made at 4 hours after food is first taken - when 10% of it remains in stomach. However, the results may differ dependent upon the type of food, the posture of a patient, and the time of measurement; therefore, testing should occur according to a standardized protocol and keeping other factors in mind that might affect the results, such as smoking behavior, medicine administration, and an increase in blood sugar.10,11
Wireless capsule motility (Smart Pill Gastrointestinal Monitoring System; Smart Pill Corporation, Buffalo, NY)
This is a diagnostic method developed for the purpose of diagnosing patients with DGE and chronic constipation, and was approved by the US Food and Drug Administration (FDA) in 2006. Patients are instructed to swallow a capsule that has a sensor embedded, and is approximately the size of a pill measuring 1.3 cm by 2.6 cm. In turn a sensor was embedded in the capsule, which senses a change in pH, pressure, and temperature, within their stomach. Data recorded by the capsule, which is discharged 1-2 days later, are analyzed by computer and used for diagnosis.12 The diagnostic accuracy of this method is similar to that of gastric-emptying scintigraphy (GES).13
Antroduodenal manometry
Antroduodenal mobility is measured by recording the change in temperature within the stomach before and after the meal. A transducer containing a pressure sensor is placed at a specific site in the stomach by using radiographic fluoroscopy or an endoscope, and is recorded if the antral, pyloric, and duodenal pressures are balanced. If DGE is present, the antral, pyloric, and duodenal pressure waves lead to disturbance in their relationship, and there is a lower antral mobility index.14 This method in particular, helps diagnose systemic sclerosis and diabetic gastric malfunction.10
Breath test
The breath test is a non-invasive examination and an indirect examination equivalent to gastric-emptying scintigraphy in terms of reliability. This method measures the amount of CO2 that is released from the lungs in the form of 13C-CO2 metabolized in the small intestine after the intake of a commercialized solid food (ex. muffin) that is combined with 13C-labeled octanoic acid.15 The strength of breath testing is that unlike gastric-emptying scintigraphy, there is no risk of being exposed to radiation. However, the disadvantage of this method is not applicable to patients with liver cirrhosis, who are not able to metabolize octanoate into CO2.16
Other imaging studies
Both 2D- and 3D- ultrasonography are simple and non-invasive methods to assess structural and functional gastric motility. During fasting or after a meal, further expansion of the gastric antrum can be observed, and this is the time to diagnose.17 The recent development of magnetic resonance (MR) image technology provides higher image resolution, allowing measure gastric emptying, gastroduodenal motility, and gastric secretion all together. However, the cost of these imaging techniques is very high, and it requires a specialist in MR image interpretation and is mostly used in laboratories rather than clinical environments due to the absence of a standardized test method to be commonly adopted by all medical institutions.18
PROPOSED CAUSES OF DELAYED GASTRIC EMPTYING AFTER SURGERY
DGE is a complex process caused by a disorder and incoordination in any one of various factors in charge of gastric motility, such as smooth muscle cells (myogenic), enteric neurons (hormonal), and the autonomic nervous system (neural). DGE often occurs after operative maneuvers that cause the loss of organs responsible for gastric motility and emptying or kinetic muscular or neuromuscular ischemia. A variety of clinical conditions - for instance diabetes, virus infection, medication, and scleroderma - can be factors that induce DGE.19
The etiologic mechanism of DGE after PPPD remains poorly understood, but can be summarized as follows. First, DGE occurs because the receptor for motilin (a hormone that stimulates the migratory motor complex [MMC]) plays a major role in gastric emptying movement during fasting and is removed by surgery. Second, it emerges because excessive contraction of the pyloric sphincter or antrum asthenia that occurs after the vagus nerve is removed or damaged during the dissection. Third, it is because ischemic damage is done as gastric antral or pyloric bloodstreams are blocked. Furthermore, both gastroduodenal dysrhythmia following infection after anastomotic leakage and torsion or angulation of the reconstructed intestinal loops after radical surgery are suggested as being the causes of DGE.
It was believed that DGE occurs as a result of reduced stimulation of a motilin-like hormonal receptor,20,21,22 the gastric antrum was removed, and antroduodenal movement was lost after the removal of duodenum following the standard pancreaticoduodenectomy. More recent, PPPD has become generalized and the results of studies on the adoption of this method have reported a higher incidence of DGE than occurs following standard pancreaticoduodenectomy. It has been estimated that DGE occurred because the remaining gastric antrum and pylorus were damaged during the surgery.23 However, the recent meta-analysis of the clinical impact of pancreaticoduodenectomy and PPPD have concluded that there was no statistically significant difference in the incidence of DGE between these two surgical techniques.24
The surgical methods of pancreaticojejunostomy, which is a pancreaticojejunal anastomosis and pancreaticogastrostomy, are likely to influence the phenomenon of DGE, and the past two randomized controlled trials (RCTs) have shown no difference in the incidence of DGE.25,26 Recently, two RCT reports have been published that PPPD with pancreaticogastrostomy lowers the incidence of DGE. These studies have suggested that the postsurgical perianatomic space following pancreaticogastrostomy is relatively smaller compared with pancreaticojejunostomy, so the risk of developing infectious complications, which causes DGE such as fluid collection by leakage, is reduced.27,28
There was the some RCTs concluded that antecolic duodenojejunostomy prevented DGE about ten times greater than retrocolic duodenojejunostomy,29 and it supposed to be the anastomosis was kept in more vertical posture, generating less temporal torsion, angulation or compression.30 And another potential reason suggested was because the antecolic anastomosis was located relatively farther away from intraperitoneal inflammation such as leakage or abscess following pancreaticojejunostomy than retrocolic anastomosis.31 Few prospective studies have been carried out so far, so the results remain to be seen.
The relationship between DGE and the incidence of complications following PPPD was reported, and it was because the secondary intraperitoneal inflammation facilitated fibrotic process in antrum and duodenum and triggered dysrhythmia by the gastroduodenal emptying movement;5,32 30-65% of patients with complications from this method were related to DGE, although the percentage varies from researcher to researcher.33 Up to this point, there has been no well-established level 1 study on this subject. Studies have been reported that DGE was affected by a conduction defect that was caused by damage to the vagus nerves around the pylorus ring or the lesser curvature after the dissection of gastroduodenal lymphatic tissues during radical surgery,34 and that the incidence of DGE was lowered if preventive pyloromyotomy was performed after PPPD on the assumption that the branches of vagus nerves damaged during the surgery would set off excessive contraction in pyloric ring and this would be the cause of DGE.35 Based on this theoretical background, Kawai and colleagues36 reported a RCT by comparing the 2 groups that had the pylorus ring removed after PPPD and the control group confirmed that there was a significant difference in the incidence of DGE in the pyloric ring resection group (4.5% vs. 17.2%, p=0.0244).
It has been suggested that ischemic damage to the blood vessels of antropyloric region which occurred during the surgery might induce DGE. In other words, the antral-pyloric motility disorder occurred by pyloric sphincter or antral ischemia after the ligation of right gastric artery or gastroduodenal artery, which was performed during the surgery might cause DGE,37 but currently there is no solid information that has supported this argument.
MANAGEMENT OF DELAYED GASTRIC EMPTYING
Dietary manipulation
In the event of delayed gastric emptying, dietary modification is needed for the purposes of treatment. According to the American Gastroenterological Association,38 patients with vomiting symptoms must undergo a differential diagnosis from regurgitation, rumination, and bulimia. If the symptoms have already occurred, efforts should be made to relieve the patients' anxiety, fear, and depression. In the context of food, patients need to eat small amount of food, on a frequent basis; liquid nutrition (soups) is better than solid nutrition because the ability of gastric emptying tends to be preserved for liquid food; the food that is low in fat and fiber is better because these increase the emptying time; an isotonic food is better than a hypertonic food; and the food that is moderate in temperature (not cold or hot) is recommended.19
Prokinetic medications
Prokinetics plays a role in accelerating gastric contractility, enhancing gastric dysrhythmia, and improving the coordination of antral and duodenal movement.38 The representative promotility agents include metoclopramide, domperidone, and erythromycin. First, in the case of erythromycin, emptying movement is most active during phase III out of a total 4 phases that involve gastric emptying movement in the fasting state. The gastric MMC is initiated in this phase, and the combined motilin receptor with erythromycin, which is a motilin agonist, will further stimulate the gastric emptying movement.39 The dosage must be kept low (1-3 mg/kg/hr), and attention must be given because the long-term use of erythromycin may cause symptoms of abdominal cramps, nausea, diarrhea, and prolongation of the QT interval.40 Several RCTs, which have used erythromycin to prevent the postoperative DGE, include level 1 study that reported a significant reduction of the incidence of DGE,8,39 but the effects of erythromycin felt in the clinical environment are not that positive.
Metoclopramide is the only drug approved by FDA for the treatment of gastroparesis. As dopamine D2 receptor antagonist, metoclopramide blocks dopamine D2 receptor and stimulates 5-HT4 receptor to increase the secretion of acetylcholine.41 In addition, it increases lower esophageal sphincter tone, accelerates antral and fundal contractility, and encourages antroduodenal peristalsis. Since it passes through the blood-brain barrier (BBB), it may cause extrapyramidal movement disorders, such as Parkinsonism, tardive dyskinesia, and akathisia, which is a disadvantage of this agent.42 Domperidone is approved in Canada, Mexico, and European countries except in the US, which permits the use of domperidone on an investigational basis, only. Domperidone's action mechanism is similar to that of metoclopramide, but it does not pass through the BBB, producing few central nervous system side effects. The effect and efficacy of domperidone are known to be similar to those of metoclopramide.43 Prokinetics and antiemetics should be administered along with domperidone; the symptoms of nausea and vomiting need to be alleviated. Metoclopramide has an antiemetic effect. Besides phenothiazine derivatives, serotonin 5-HT3 receptor antagonists, histamine H1 receptor antagonists, and benzodiazepines can be used.44 In addition to these medications, endoscopic intrapyloric Botox injection, which is an invasive intervention, is found to be an effective,45 but not a recommended method at present.
Papers recently published by large medical institutions tend to report a relatively lower incidence of DGE. It seems to be that the surgical techniques have been standardized and developed in such a manner as to reduce complications.33,46,47 Since definitions about delayed gastric emptying are not unified so that different standards are applied by researchers, the direct or indirect comparison of the results impedes the evaluation of DGE. Indeed, the incidence of DGE, when standard surgical technique defined recently by the International Study Group for Pancreatic Surgery (ISGPS) has been applied and was reported to be twice as high as when different standards were applied in other studies.
CONCLUSIONS
There seems to be no preferred method to prevent delayed gastric emptying after pancreaticoduodenectomy. However, some RCTs have reported that preventive administration of erythromycin and an antecolic duodenojejunostomy over transverse colon may contribute to preventing DGE. It is most important to develop and to universalize a standardized surgical technique in a way to reduce factors that are considered to cause DGE after pancreaticoduodenectomy. Moreover, if it is suspected that a delay in gastric emptying occurred after pancreaticoduodenectomy and a differential diagnosis from diseases with similar symptoms via an accurate diagnostic approach should be implemented. Based on these, RCT needs to be carried out through the cooperation of many medical institutions to align the terms and definitions of the delayed gastric emptying after surgery and to develop a unified standard.
References
- 1.Traverso LW, Longmire WP., Jr Preservation of the pylorus in pancreaticoduodenectomy. Surg Gynecol Obstet. 1978;146:959–962. [PubMed] [Google Scholar]
- 2.Whipple AO, Parsons WB, Mullins CR. Treatment of carcinoma of the ampulla of vater. Ann Surg. 1935;102:763–779. doi: 10.1097/00000658-193510000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McPhee JT, Hill JS, Whalen GF, et al. Perioperative mortality for pancreatectomy: a national perspective. Ann Surg. 2007;246:246–253. doi: 10.1097/01.sla.0000259993.17350.3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akamatsu N, Sugawara Y, Komagome M, et al. Risk factors for postoperative pancreatic fistula after pancreaticoduodenectomy: the significance of the ratio of the main pancreatic duct to the pancreas body as a predictor of leakage. J Hepatobiliary Pancreat Sci. 2010;17:322–328. doi: 10.1007/s00534-009-0248-6. [DOI] [PubMed] [Google Scholar]
- 5.van Berge Henegouwen MI, van Gulik TM, Dewit LT, et al. Delayed gastric emptying after standard pancreaticoduodenectomy versus pylorus-preserving pancreaticoduodenectomy: an analysis of 200 consecutive patients. J Am Coll Surg. 1997;185:373–379. doi: 10.1016/s1072-7515(97)00078-1. [DOI] [PubMed] [Google Scholar]
- 6.Yeo CJ, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226:248–257. doi: 10.1097/00000658-199709000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akizuki E, Kimura Y, Nobuoka T, et al. Reconsideration of postoperative oral intake tolerance after pancreaticoduodenectomy: prospective consecutive analysis of delayed gastric emptying according to the ISGPS definition and the amount of dietary intake. Ann Surg. 2009;249:986–994. doi: 10.1097/SLA.0b013e3181a63c4c. [DOI] [PubMed] [Google Scholar]
- 8.Yeo CJ, Barry MK, Sauter PK, et al. Erythromycin accelerates gastric emptying after pancreaticoduodenectomy. A prospective, randomized, placebo-controlled trial. Ann Surg. 1993;218:229–237. doi: 10.1097/00000658-199309000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wente MN, Bassi C, Dervenis C, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS) Surgery. 2007;142:761–768. doi: 10.1016/j.surg.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Camilleri M, Hasler WL, Parkman HP, Quigley EM, Soffer E. Measurement of gastrointestinal motility in the GI laboratory. Gastroenterology. 1998;115:747–762. doi: 10.1016/s0016-5085(98)70155-6. [DOI] [PubMed] [Google Scholar]
- 11.Miller G, Palmer KR, Smith B, Ferrington C, Merrick MV. Smoking delays gastric emptying of solids. Gut. 1989;30:50–53. doi: 10.1136/gut.30.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuo B, McCallum RW, Koch KL, et al. Comparison of gastric emptying of a nondigestible capsule to a radio-labelled meal in healthy and gastroparetic subjects. Aliment Pharmacol Ther. 2008;27:186–196. doi: 10.1111/j.1365-2036.2007.03564.x. [DOI] [PubMed] [Google Scholar]
- 13.Cassilly D, Kantor S, Knight LC, et al. Gastric emptying of a non-digestible solid: assessment with simultaneous SmartPill pH and pressure capsule, antroduodenal manometry, gastric emptying scintigraphy. Neurogastroenterol Motil. 2008;20:311–319. doi: 10.1111/j.1365-2982.2007.01061.x. [DOI] [PubMed] [Google Scholar]
- 14.Fraser RJ, Horowitz M, Maddox AF, Dent J. Postprandial antropyloroduodenal motility and gastric emptying in gastroparesis--effects of cisapride. Gut. 1994;35:172–178. doi: 10.1136/gut.35.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedenberg FK, Parkman HP. Delayed gastric emptying: whom to test, how to test, and what to do. Curr Treat Options Gastroenterol. 2006;9:295–304. doi: 10.1007/s11938-006-0011-x. [DOI] [PubMed] [Google Scholar]
- 16.Perri F, Bellini M, Portincasa P, et al. (13)C-octanoic acid breath test (OBT) with a new test meal (EXPIROGer): Toward standardization for testing gastric emptying of solids. Dig Liver Dis. 2010;42:549–553. doi: 10.1016/j.dld.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Undeland KA, Hausken T, Svebak S, Aanderud S, Berstad A. Wide gastric antrum and low vagal tone in patients with diabetes mellitus type 1 compared to patients with functional dyspepsia and healthy individuals. Dig Dis Sci. 1996;41:9–16. doi: 10.1007/BF02208577. [DOI] [PubMed] [Google Scholar]
- 18.Feinle C, Kunz P, Boesiger P, Fried M, Schwizer W. Scintigraphic validation of a magnetic resonance imaging method to study gastric emptying of a solid meal in humans. Gut. 1999;44:106–111. doi: 10.1136/gut.44.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang DM, Friedenberg FK. Gastroparesis: approach, diagnostic evaluation, and management. Dis Mon. 2011;57:74–101. doi: 10.1016/j.disamonth.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Fox JE, Daniel EE, Jury J, Robotham H. The mechanism of motilin excitation of the canine small intestine. Life Sci. 1984;34:1001–1006. doi: 10.1016/0024-3205(84)90305-9. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka M, Sarr MG. Total duodenectomy: effect on canine gastrointestinal motility. J Surg Res. 1987;42:483–493. doi: 10.1016/0022-4804(87)90022-9. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka M, Sarr MG. Role of the duodenum in the control of canine gastrointestinal motility. Gastroenterology. 1988;94:622–629. doi: 10.1016/0016-5085(88)90232-6. [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi K, Kishinaka M, Nagai E, et al. Pancreatoduodenectomy for pancreatic head carcinoma with or without pylorus preservation. Hepatogastroenterology. 2001;48:1479–1485. [PubMed] [Google Scholar]
- 24.Kawai M, Yamaue H. Pancreaticoduodenectomy versus pylorus-preserving pancreaticoduodenectomy: the clinical impact of a new surgical procedure; pylorus-resecting pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci. 2011 doi: 10.1007/s00534-011-0427-0. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Yeo CJ, Cameron JL, Maher MM, et al. A prospective randomized trial of pancreaticogastrostomy versus pancreaticojejunostomy after pancreaticoduodenectomy. Ann Surg. 1995;222:580–588. doi: 10.1097/00000658-199510000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duffas JP, Suc B, Msika S, et al. French Associations for Research in Surgery. A controlled randomized multicenter trial of pancreatogastrostomy or pancreatojejunostomy after pancreatoduodenectomy. Am J Surg. 2005;189:720–729. doi: 10.1016/j.amjsurg.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 27.Bassi C, Falconi M, Molinari E, et al. Reconstruction by pancreaticojejunostomy versus pancreaticogastrostomy following pancreatectomy: results of a comparative study. Ann Surg. 2005;242:767–771. doi: 10.1097/01.sla.0000189124.47589.6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernández-Cruz L, Cosa R, Blanco L, López-Boado MA, Astudillo E. Pancreatogastrostomy with gastric partition after pylorus-preserving pancreatoduodenectomy versus conventional pancreatojejunostomy: a prospective randomized study. Ann Surg. 2008;248:930–938. doi: 10.1097/SLA.0b013e31818fefc7. [DOI] [PubMed] [Google Scholar]
- 29.Tani M, Terasawa H, Kawai M, et al. Improvement of delayed gastric emptying in pylorus-preserving pancreaticoduodenectomy: results of a prospective, randomized, controlled trial. Ann Surg. 2006;243:316–320. doi: 10.1097/01.sla.0000201479.84934.ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horstmann O, Markus PM, Ghadimi MB, Becker H. Pylorus preservation has no impact on delayed gastric emptying after pancreatic head resection. Pancreas. 2004;28:69–74. doi: 10.1097/00006676-200401000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Sugiyama M, Abe N, Ueki H, Masaki T, Mori T, Atomi Y. A new reconstruction method for preventing delayed gastric emptying after pylorus-preserving pancreatoduodenectomy. Am J Surg. 2004;187:743–746. doi: 10.1016/j.amjsurg.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 32.Riediger H, Makowiec F, Schareck WD, Hopt UT, Adam U. Delayed gastric emptying after pylorus-preserving pancreatoduodenectomy is strongly related to other postoperative complications. J Gastrointest Surg. 2003;7:758–765. doi: 10.1016/s1091-255x(03)00109-4. [DOI] [PubMed] [Google Scholar]
- 33.Lytras D, Paraskevas KI, Avgerinos C, et al. Therapeutic strategies for the management of delayed gastric emptying after pancreatic resection. Langenbecks Arch Surg. 2007;392:1–12. doi: 10.1007/s00423-006-0096-7. [DOI] [PubMed] [Google Scholar]
- 34.Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg. 2002;236:355–366. doi: 10.1097/00000658-200209000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim DK, Hindenburg AA, Sharma SK, et al. Is pylorospasm a cause of delayed gastric emptying after pylorus-preserving pancreaticoduodenectomy? Ann Surg Oncol. 2005;12:222–227. doi: 10.1245/ASO.2005.03.078. [DOI] [PubMed] [Google Scholar]
- 36.Kawai M, Tani M, Hirono S, et al. Pylorus ring resection reduces delayed gastric emptying in patients undergoing pancreatoduodenectomy: a prospective, randomized, controlled trial of pylorus-resecting versus pylorus-preserving pancreatoduodenectomy. Ann Surg. 2011;253:495–501. doi: 10.1097/SLA.0b013e31820d98f1. [DOI] [PubMed] [Google Scholar]
- 37.Itani KM, Coleman RE, Meyers WC, Akwari OE. Pylorus-preserving pancreatoduodenectomy. A clinical and physiologic appraisal. Ann Surg. 1986;204:655–664. doi: 10.1097/00000658-198612000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parkman HP, Hasler WL, Fisher RS American Gastroenterological Association. American Gastroenterological Association medical position statement: diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1589–1591. doi: 10.1053/j.gastro.2004.09.054. [DOI] [PubMed] [Google Scholar]
- 39.Ohwada S, Satoh Y, Kawate S, et al. Low-dose erythromycin reduces delayed gastric emptying and improves gastric motility after Billroth I pylorus-preserving pancreaticoduodenectomy. Ann Surg. 2001;234:668–674. doi: 10.1097/00000658-200111000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Donovan D, Feinle-Bisset C, Jones K, Horowitz M. Idiopathic and diabetic gastroparesis. Curr Treat Options Gastroenterol. 2003;6:299–309. doi: 10.1007/s11938-003-0022-9. [DOI] [PubMed] [Google Scholar]
- 41.Rao AS, Camilleri M. Review article: metoclopramide and tardive dyskinesia. Aliment Pharmacol Ther. 2010;31:11–19. doi: 10.1111/j.1365-2036.2009.04189.x. [DOI] [PubMed] [Google Scholar]
- 42.Ganzini L, Casey DE, Hoffman WF, McCall AL. The prevalence of metoclopramide-induced tardive dyskinesia and acute extrapyramidal movement disorders. Arch Intern Med. 1993;153:1469–1475. [PubMed] [Google Scholar]
- 43.Sugumar A, Singh A, Pasricha PJ. A systematic review of the efficacy of domperidone for the treatment of diabetic gastroparesis. Clin Gastroenterol Hepatol. 2008;6:726–733. doi: 10.1016/j.cgh.2008.02.065. [DOI] [PubMed] [Google Scholar]
- 44.Quigley EM, Hasler WL, Parkman HP. AGA technical review on nausea and vomiting. Gastroenterology. 2001;120:263–286. doi: 10.1053/gast.2001.20516. [DOI] [PubMed] [Google Scholar]
- 45.Friedenberg FK, Palit A, Parkman HP, Hanlon A, Nelson DB. Botulinum toxin A for the treatment of delayed gastric emptying. Am J Gastroenterol. 2008;103:416–423. doi: 10.1111/j.1572-0241.2007.01676.x. [DOI] [PubMed] [Google Scholar]
- 46.Gouma DJ, Obertop H. Centralization of surgery for periampullary malignancy. Br J Surg. 1999;86:1361–1362. doi: 10.1046/j.1365-2168.1999.01275.x. [DOI] [PubMed] [Google Scholar]
- 47.Birkmeyer JD, Finlayson SR, Tosteson AN, Sharp SM, Warshaw AL, Fisher ES. Effect of hospital volume on in-hospital mortality with pancreaticoduodenectomy. Surgery. 1999;125:250–256. [PubMed] [Google Scholar]


