Abstract
We investigated time trends in extended-spectrum cephalosporin-resistant Escherichia coli and Klebsiella pneumoniae isolates from different patient settings in The Netherlands from 2008–2012. E. coli and K. pneumoniae isolates from blood and urine samples of patients > = 18 years were selected from the Dutch Infectious Disease Surveillance System-Antimicrobial Resistance (ISIS-AR) database. We used multivariable Poisson regression to study the rate per year of blood stream infections by susceptible and resistant isolates, and generalized estimating equation (GEE) log-binomial regression for trends in the proportion of extended-spectrum cephalosporin-resistant isolates. Susceptibility data of 197,513 E. coli and 38,244 K. pneumoniae isolates were included. The proportion of extended-spectrum cephalosporin-resistant E. coli and K. pneumoniae isolates from urine and blood samples increased in all patient settings, except for K. pneumoniae isolates from patients admitted to intensive care units. For K. pneumoniae, there was a different time trend between various patient groups (p<0.01), with a significantly higher increase in extended-spectrum cephalosporin-resistant isolates from patients attending a general practitioner than in isolates from hospitalized patients. For E. coli, the increasing time trends did not differ among different patient groups. This nationwide study shows a general increase in extended-spectrum cephalosporin-resistant E. coli and K. pneumoniae isolates. However, differences in trends between E. coli en K. pneumoniae underline the importance of E. coli as a community-pathogen and its subsequent influence on hospital resistance level, while for K. pneumoniae the level of resistance within the hospital seems less influenced by the resistance trends in the community.
Introduction
Treatment of infections by Escherichia coli and Klebsiella pneumoniae has become more challenging because of increasing resistance to extended-spectrum cephalosporin resulting from the production of Extended-Spectrum β-lactamases (ESBLs) [1]. Resistance has important implications for clinicians and patients due to a higher chance of inadequate treatment, increased length of hospital stay and additional healthcare costs [2,3,4]. Traditionally, long-term hospitalization, admission on an intensive care unit (ICU) and the presence of invasive medical devices were considered risk factors for colonization or infection with ESBL-producing organisms [1]. Since CTX-M ESBL types have replaced TEM and SHV mutants as the predominant ESBL type in Europe [5], the epidemiology of infections by ESBL-producing bacteria has changed from predominantly healthcare-associated risk factors to community-associated risk factors [6], and from K. pneumoniae to E. coli as the main hosting pathogen [1,5,6]. ESBL-producing E. coli isolates are increasingly detected in nursing homes and community-based health-care facilities and have been associated with travel [7]. This facilitates a potential influx of ESBL-producing microorganisms from the community into the hospital [6,8].
This worldwide spread of ESBL-producers into the community [9,10] possibly requires new approaches for the surveillance and prevention of ESBL-producing E. coli and K. pneumoniae in hospital settings.
We therefore assessed trends in extended-spectrum cephalosporin-resistant K. pneumoniae, representing nosocomial resistance and E. coli isolates, representing community-onset resistance, from hospitalized patients as well as patients attending a general practitioner (GP).
Materials and Methods
Data
We used data from the Dutch Infectious Disease Surveillance Information System–Antibiotic Resistance (ISIS-AR) over a five-year period (2008 to 2012). The ISIS-AR database is described in detail elsewhere [11]. Briefly, the 30 currently participating laboratories cover approximately 65% of the Dutch laboratories with a wide geographic range and provide on a monthly basis data on bacterial identification and antimicrobial susceptibility of all routinely cultured isolates. Over 50% of the Dutch population is covered by ISIS-AR and its antimicrobial susceptibility data are considered representative for the Netherlands. The data include Minimum Inhibitory Concentrations (MICs) of automated susceptibility testing systems or E-tests and patient data (i.e., age, gender, hospital department, specimen site). Data quality is assured by structural quality control and monthly feedback of unusual findings. Additionally laboratories are regularly audited by independent external experts of the Dutch institute for the promotion of quality in laboratory research and for the accreditation of laboratories in the health care sector (CCKL [http://www.cckl.nl/]).
Isolates
E. coli and K. pneumoniae isolates from blood and urine samples of adult patients (i.e., age > = 18 years) attending a general practitioner (GP) or an outpatient department (OPD) or admitted at an inpatient department (IPD) or intensive care unit (ICU) were included. For some hospitals included in the ISIS-AR database no distinction between the emergency department and OPD/IPD can be made, resulting in isolates from emergency departments to be included in both OPD and IPD settings. Only isolates from continuously reporting laboratories over the period 2008 to 2012 were selected, resulting in the inclusion of data from 22 participating laboratories, still representing over 40% of the Dutch population). MIC results of automated susceptibility testing systems for cefotaxime/ceftriaxone or ceftazidime were re-interpret using the European Committee for Antimicrobial Susceptibility Testing (EUCAST) 2012 breakpoints (version 2.0 valid until Dec 31, 2012) for classifying organisms as susceptible or resistant, including non-susceptible isolates [12]. Isolates were defined as extended-spectrum cephalosporin resistant if they were non-susceptible to at least one of the two types of agents recommended for ESBL screening in The Netherlands, namely cefotaxime/ceftriaxone and ceftazidime. To avoid overrepresentation caused by multiple isolates from the same patient, isolates obtained in the 30-day period following the first positive blood isolate were excluded and we assumed that each blood isolate included represented a bacteremia episode. For urine isolates, the first isolate per patient per year was included, with at least a 90 days period between the included isolate and an isolate selected from the previous year [13].
Statistical analyses
First, we calculated the time trend in the rate of bloodstream infections by E. coli and K. pneumoniae per year for extended-spectrum cephalosporin susceptible (ESC-S) and resistant isolates combined and for resistant isolates separately. The rate was defined as the absolute number of isolates, assuming a stable at-risk population. The yearly rate ratio (yRaR) was calculated using multivariable Poisson regression, forcing a log-linear trend over time, with a robust standard error and adjusted for patient characteristics (i.e., age, gender, patient setting and the geographic location of the laboratory within the Netherlands, see Table 1 for definitions). Secondly, we analyzed the time trend in the yearly proportion of extended-spectrum cephalosporin resistant E. coli and K. pneumoniae isolates (expressed as yearly risk ratios [yRiR) by a multivariable log-binomial regression analysis, that included patient characteristics. These analyses were performed separately for blood samples, urine samples of female patients and urine samples of male patients. Urine isolates from male and female patients were analyzed separately, because the Dutch College of General Practitioners guidelines on urinary tract infections recommend routine culture of urine for all males with urinary tract symptoms, since urinary tract infections in males are considered complicated infections. For females, testing is only recommend in case of non-response to first-line antimicrobial treatment or in case of a complicated infection [14].
Table 1. Background characteristics for Escherichia coli and Klebsiella pneumoniae isolates, ISIS-AR The Netherlands, 2008–2012.
| E. coli | K. pneumoniae | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | |||||||
| blood isolates | male urine isolates | female urine isolates | blood isolates | male urine isolates | female urine isolates | |||||||
| Variable | n = 14461 | n = 42270 | n = 140782 | n = 2492 | n = 9995 | n = 25553 | ||||||
| ESC-R a | 863 | (6.0) | 2481 | (5.2) | 5331 | (3.6) | 204 | (8.2) | 631 | (6.3) | 1000 | (3.8) |
| Age in years | ||||||||||||
| 19–64 | 4049 | (28.0) | 19379 | (41.0) | 69121 | (47.3) | 774 | (31.1) | 3169 | (31.7) | 8902 | (33.5) |
| ≥65 | 10412 | (72.0) | 27891 | (59.0) | 76992 | (52.7) | 1718 | (68.9) | 6826 | (68.3) | 17651 | (66.5) |
| Gender b | ||||||||||||
| Female | 7282 | (50.4) | - | - | - | - | 1087 | (43.6) | - | - | - | - |
| Male | 7179 | (49.6) | - | - | - | - | 1405 | (56.4) | - | - | - | - |
| Patient setting c | ||||||||||||
| GP | - | - | 24244 | (51.3) | 89704 | (61.4) | - | - | 3702 | (37.0) | 14494 | (54.6) |
| OPD | 4630 | (32.0) | 13045 | (27.6) | 29640 | (20.3) | 690 | (27.7) | 3728 | (37.3) | 6737 | (25.4) |
| IPD | 8638 | (59.7) | 9558 | (20.2) | 25885 | (17.7) | 1515 | (60.8) | 2419 | (24.2) | 5042 | (19.0) |
| ICU | 1186 | (8.2) | 423 | (0.9) | 884 | (0.6) | 287 | (11.5) | 146 | (1.5) | 280 | (1.1) |
| Geographic Region | ||||||||||||
| Northwest | 3727 | (25.8) | 15384 | (32.5) | 47351 | (32.4) | 618 | (24.8) | 2604 | (26.1) | 6440 | (24.3) |
| Northeast | 1953 | (13.5) | 2200 | (4.7) | 5346 | (3.7) | 259 | (10.4) | 1420 | (14.2) | 4295 | (16.2) |
| Middle | 2523 | (17.4) | 9991 | (21.2) | 31233 | (21.4) | 388 | (15.6) | 1559 | (15.6) | 4119 | (15.5) |
| Southwest | 1814 | (12.5) | 7860 | (16.7) | 26901 | (18.4) | 347 | (13.9) | 1165 | (11.7) | 3316 | (12.5) |
| South | 4423 | (30.6) | 11766 | (24.9) | 35018 | (24.0) | 873 | (35.0) | 3233 | (32.3) | 8358 | (31.5) |
aESC-R: Extended-spectrum cephalosporin-resistant
bUrine samples are stratified for gender.
cThe patient settings are: General Practitioner (GP), Outpatient Department (OPD), Inpatient Department (IPD) and Intensive Care Unit (ICU). The dataset did not contain blood isolates from general practitioners.
To determine whether time trends between the different patient settings (i.e., GP, OPD, IPD and ICU) differed significantly, an interaction term between patient setting and time in years was tested. To correct for repeated measures of patients contributing more than once to the dataset, generalized estimating equation (GEE) was used. This GEE analyses was used and for all patient categories and settings, except for the analysis of urine isolates from female patients admitted at the ICU departments, due to the low number of repeated measures. A limit of six repeated measures was set to avoid sparse data problems, which implied the exclusion of less than 1% of all isolates. Proportions point-estimates with 95% confidence intervals and time trends were plotted using time in years as a categorical variable. A p-value <0.05% was considered as statistically significant. Data analysis was performed using SPSS software version 19 (IBM) and Stata/SE software version 12 (StataCorp).
Ethics statement
The data of the bacterial isolates and their susceptibility results used in this study belong to the microbiological laboratories participating in ISIS-AR and was obtained as part of routine clinical care in the past. Written or verbal consent of patients was therefore not obtained. Furthermore, all patient identifiers had been previously removed and data were analysed anonymously. According to the Dutch Medical Research Involving Human Subjects Act (WMO) this study was considered exempt from review by an Institutional Review Board.
Results
Characteristics
Table 1 summarizes the background characteristics of isolates included in this study. The number of included E. coli isolates (n = 197,513) was substantially higher than the number of included K. pneumoniae isolates (n = 38,040). Most of the isolates originated from urine samples of female patients. The overall proportion of extended-spectrum cephalosporin resistant isolates was lower in E. coli isolates (6.0, 5.2 and 3.6%, for blood, urine from males and urine from females respectively) compared to K. pneumoniae isolates (8.2, 6.3 and 3.8%, respectively). Resistance was highest among blood isolates and lowest among urine isolates of female patients.
Time trends in the rate of bloodstream infections
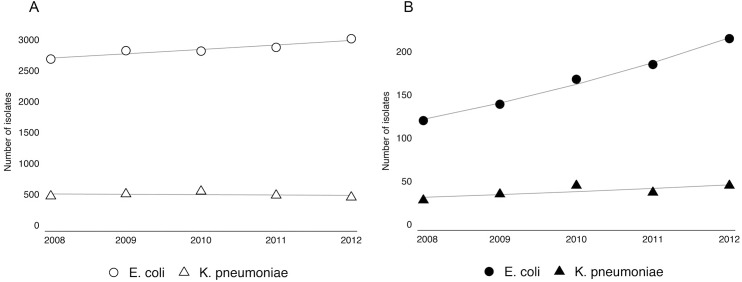
Fig 1 shows a significant increase in the total number of E. coli bloodstream infections per year from 2008–2012; from 2687 in 2008 to 3016 in 2012 (yRaR 1.03 [95% confidence interval (95% CI) 1.03–1.03]), while there was no time trend in the total number of bloodstream infections by K. pneumoniae, from 472 in 2008 to 454 in 2012 (yRaR 0.99 [95% CI 0.95–1.03]). When limiting analysis to extended-spectrum cephalosporin resistant isolates, there was an increase in the total number of bloodstream infections by both E. coli (yRaR 1.15 [95% CI 1.09–1.22]) and K. pneumoniae (yRaR 1.09 [95% CI 1.09–1.10]). The degree of increase in resistant E. coli isolates was higher than the increase in susceptible isolates (Fig 1).
Fig 1. The rate of bloodstream infections by Escherichia coli and Klebsiella pneumoniae (A) and the rate of bloodstream infections by extended-spectrum cephalosporin-resistant Escherichia coli and Klebsiella pneumoniae (B) from 2008–2012, ISIS-AR, the Netherlands, adjusted for patient characteristics, including age, gender, patient setting and geographic region.
The plots show the point-estimates and log-linear trends generated by a Poisson regression with robust standard error.
Trends in the proportion of extended-spectrum cephalosporin resistance
There was a significant increase in the proportion of extended-spectrum cephalosporin resistant E. coli and K. pneumoniae isolates (Table 2 and Fig 2). The yearly increase in the proportion of resistant E. coli in blood isolates (yRiR 1.15 [95% CI 1.09–1.20]) and K. pneumoniae in blood isolates (yRiR 1.11 [95% CI 1.00–1.23]) was similar. The increase in resistance among E. coli and K. pneumoniae was also similar for urine isolates from male patients (Table 2). However, when analyzing urine isolates from female patients there was a stronger increase in resistant K. pneumoniae isolates than in resistant E. coli isolates.
Table 2. Adjusted risk ratio’s (yRiR) per year for the time trends in proportion of Escherichia coli and Klebsiella pneumoniae isolates resistant to third generation cephalosporins, ISIS-AR, The Netherlands, 2008–2012.
| yRiR a | [95% CI] | |
|---|---|---|
| Blood | ||
| E. coli | 1.15 | [1.09–1.20] |
| K. pneumoniae | 1.11 | [1.00–1.23] |
| Urine from males | ||
| E. coli | 1.13 | [1.10–1.16] |
| K. pneumoniae | 1.12 | [1.07–1.18] |
| Urine from females | ||
| E. coli | 1.07 | [1.05–1.09] |
| K. pneumoniae | 1.16 | [1.11–1.21] |
aAdjusted yearly risk ratio’s (yRiR) and 95% confidence intervals (95% CI) are generated with a log-binomial regression, adjusted for patient characteristics, including age, gender, patient setting and geographic region, and corrected for repeated measures using generalized estimating equation (GEE).
Fig 2. The proportion (in %) of extended-spectrum cephalosporin-resistant Escherichia coli and Klebsiella pneumoniae isolates, for blood isolates (A), male urine isolates (B) and female urine isolates (C) from 2008–2012, ISIS-AR, the Netherlands.
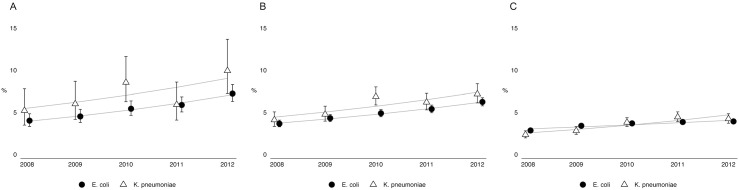
The point-estimates and log-linear trends per year were estimated with a generalized estimation equation (GEE) for log-binominal regression including time in years, and patient characteristics, including age, gender, patient setting and geographic region. The error bars represent the 95% confident intervals of each of the point-estimates.
Trends in the proportion of extended-spectrum cephalosporin resistant isolates per patient setting
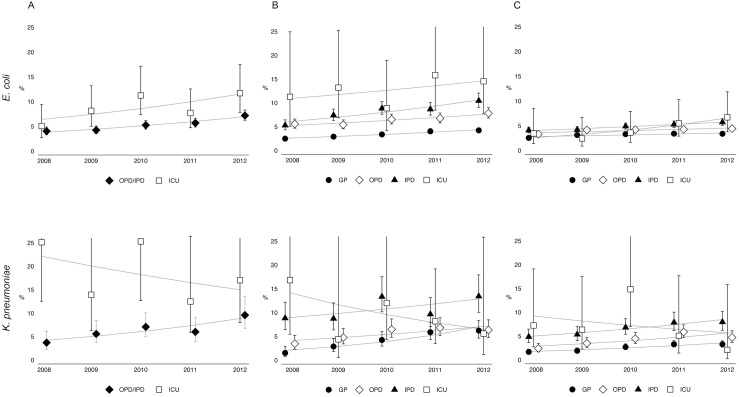
Fig 3 and Table 3 show the point-estimates and log-linear trends in the proportion of extended-spectrum cephalosporin resistant isolates per patient setting, namely GP, OPD, IPD and ICU. There was a general tendency towards increasing resistance for both E. coli and K. pneumoniae isolates from all patient settings, except for K. pneumoniae isolates of patients admitted at an ICU. Furthermore, within the different sample types, there were differences in time trends between both organisms when analyzing per setting. For example, the increase in the proportion of extended-spectrum cephalosporin resistant E. coli isolates from male urine samples was present in all patient settings and did not differ significantly between the different settings (p = 0.77). However, for K. pneumoniae, there was a significant difference in time trend in the proportion of resistance between isolates from hospital sites and isolates from the GP (p<0.01 [Table 3]); the increase in resistance was significantly higher among isolates from the GP. For isolates from females, a similar pattern was seen, although not statistically significant. For blood isolates, there was a difference in the resistance time trend between K. pneumoniae isolates from outpatient and hospital departments and isolates from the ICUs (p = 0.07 [Table 3]), while there were no differences in time trend between the different hospital settings for E. coli isolates (p = 0.84).
Fig 3. The proportion (in %) extended-spectrum cephalosporin-resistant Escherichia coli and Klebsiella pneumoniae isolates, for blood isolates (A), male urine isolates (B) and female urine isolates (C), per patient setting (general practitioner [GP], outpatient department [OPD], inpatient department [IPD] and intensive care units [ICU]), from 2008–2012, ISIS-AR, the Netherlands.
The point-estimates are generated with a generalized estimation equation (GEE) for log-binominal regression, including time in years, and patient characteristics, including age, gender, and geographic region. The error bars represent the 95% confident intervals of each of the point-estimates. To produce plots with comparable y-axis, the upper error bar is cut-off for some of the point-estimates.
Table 3. Adjusted risk ratio’s (yRiR) per year for the time trends in proportion of Escherichia coli and Klebsiella pneumoniae isolates resistant to third generation cephalosporins per patient setting, ISIS-AR, The Netherlands, 2008–2012.
| E. coli | K. pneumoniae | |||
|---|---|---|---|---|
| yRiR a | [95% CI] | yRiR | a [95% CI] | |
| Blood | ||||
| OPD/IPD b | 1.15 | [1.10–1.21] | 1.19 | [1.06–1.35] |
| ICU | 1.14 | [1.00–1.31] | 0.92 | [0.77–1.10] |
| Urine from males | ||||
| GP | 1.14 | [1.08–1.19] | 1.34 | [1.18–1.51] |
| OPD | 1.09 | [1.04–1.14] | 1.14 | [1.03–1.23] |
| IPD | 1.14 | [1.09–1.19] | 1.08 | [1.00–1.18] |
| ICU | 1.06 | [0.88–1.29] | 0.85 | [0.61–1.17] |
| Urine from females | ||||
| GP | 1.06 | [1.03–1.09] | 1.19 | [1.10–1.28] |
| OPD | 1.06 | [1.02–1.10] | 1.17 | [1.08–1.28] |
| IPD | 1.09 | [1.05–1.13] | 1.13 | [1.05–1.22] |
| ICU | 1.25 | [0.99–1.57] | 0.91 | [0.86–1.21] |
aAdjusted risk ratio’s (yRiR) and 95% confidence intervals (95% CI) are generated with a log-binomial regression, adjusted patient characteristics, including age, gender and geographic region, and corrected for repeated measures using generalized estimating equation (GEE).
The patient settings are: General Practitioner (GP), Outpatient Department (OPD), Inpatient Department (IPD) and Intensive Care Unit (ICU)
b Because the absence of blood samples from GPs and the low number of isolates from OPD and IPD this both hospital locations were combined.
Discussion
Recent literature suggests a shift in the source of ESBL-producing bacteria from mainly hospital-acquired towards the emergence of these microorganisms in the community [1,6,15]. This study focused on differences in time trends between extended-spectrum cephalosporin resistant E. coli and K. pneumoniae isolates from different patient settings. First, time trends in the rate of bloodstream infections were studied, showing no increase in the total number of K. pneumoniae infections, while the number of infections with E. coli increased. The rate of bloodstream infections by extended-spectrum cephalosporin resistant E. coli and K. pneumoniae increased for both species. The proportion of extended-spectrum cephalosporin-resistant E. coli isolates from urine and blood samples increased in all patient settings in contrast to K. pneumoniae isolates showing different time trends between various patient settings. For K. pneumoniae isolates there was a significantly higher increase in resistant isolates from patients attending a general practitioner than in isolates from hospitalized patients. Especially in the ICU departments, there was a clear difference in time trend between both species, with an increase in the proportion of resistant E. coli isolates and a non-significant decrease in resistance in K. pneumoniae isolates.
The lower rise in the proportion of extended-spectrum cephalosporin resistant K. pneumoniae isolates in hospital setting indicates that perhaps, a plateau has been reached. However, due to the small timespan and the low numbers of K. pneumoniae isolates in this study, it might also reflect a temporal decline in the percentage of resistant isolates as shown in a recent study by Kronenberg et al., [16]. They showed that the increasing resistance time trends for both E. coli and K. pneumoniae are similar in Switzerland from 2004–2011. However, the percentages of resistant K. pneumoniae isolates showed more variations then the yearly percentages of resistant E. coli, due to multiple outbreaks in several institutions [16]. On the other hand, a recent study from the United States, studying K. pneumoniae isolates from 1999 to 2010, showed a plateauing trend of extended-spectrum cephalosporin resistant K. pneumoniae isolates [13]. Additionally, data from resistance surveillance of bloodstream infections in the United Kingdom (UK), 1990–2010, describes a dip in ESBL prevalence among E. coli isolates around 2006 with a stable epidemiology thereafter [17]. The author suggests that this effect might reflect the massive prescribing changes in UK hospitals, with a move away from cephalosporins and quinolones towards beta-lactamase inhibitor combinations. These prescribing changes, like reducing the number of Methicillin-Resistant Staphylococcus aureus (MRSA), became a major subject in national reduction targets [17] and the simultaneous increased attention on contact precautions for patients infected with or carrying MRSA, might also have benefitted the prevention of spread of resistant E. coli and K. pneumoniae isolates.
The Dutch Working Party on Antibiotic Policy (SWAB) and the Dutch Working Party on Infection Prevention (WIP) fight the same battle for rational and restrained antibiotic policy and the control of highly resistant microorganisms (HRMO) in Dutch hospitals as the UK [18,19]. Data from the European Antimicrobial Surveillance Network (EARS-net) shows a steady, almost linear, rise in the percentages of extended-spectrum cephalosporin resistance in E. coli isolates between neighboring European countries, including the Netherlands and the UK [20]. However, a more variable pattern is visible for K. pneumoniae isolates and the lowest percentages of resistance were found in the UK and the Netherlands in 2012, which might reflect the common efforts in infection control.
Differences in time trends between E. coli and K. pneumoniae isolates likely reflect the different characteristics of these two bacteria. K. pneumoniae is a bacterium that has adapted especially to the hospital environment and survives longer than other Enterobacteriaceae on hands and environmental surfaces, making it easier to cause cross-infections within hospitals [21, 22]. E. coli is ubiquitous in the community and therefore less affected by infection prevention policies. Subsequently, the rise in extended-spectrum cephalosporin resistance within hospital settings is probably explained by the rise of resistant E. coli isolates within the community. In elderly patients from long-term care facilities in the United States, sequence type (ST) 131, a clone of extended-spectrum cephalosporin resistant E. coli that has been implicated as an important source of the emergence of extended-spectrum cephalosporin resistance in E. coli, has become dominant [23, 24]. Additionally, it has been suggested that the ST131 clone has become more common in the community due to the antibiotic pressure [7, 24, 25].
This study has some limitations, including the limited timespan of the study and the low number of resistant isolates, in particular for K. pneumoniae, due to low prevalence of resistance in the Netherlands. Results found might therefore not be representative for countries with higher levels of resistance. Furthermore, sampling bias might have resulted in the overrepresentation of resistant isolates. However, this bias is likely to have been constant over time, resulting in no effect on the yearly trend. Third, the data has been corrected for repeated measures. Since there is no standardized patient identification across laboratories within our dataset, unidentified double sampling of individual persons may have occurred. However, we assume that the effects of double sampling are limited considering the organization of healthcare in The Netherlands, where patients generally have one GP. Fourth, for comparing differences in time trends between community-onset and hospital-associated infections and resistant bacteria it would be preferable to have better coverage of patients within long-term care facilities, given their potential role in spread of resistance [23]. Fifth, to confirm that the influx of ESBL-producing bacteria from the community into hospital settings occurs, genotypic information is needed, whereas ISIS-AR only includes phenotypic data. On the other hand, results in our study are robust due to the large numbers of isolates present in the dataset and the wide geographic distribution of the participating laboratories ensuring a good representation of the Dutch population. By the inclusion of laboratories that have continuously delivered data it was possible to compare the year-to-year proportion and to give reliable time trends.
Conclusion
This nationwide study shows a general increase in extended-spectrum cephalosporin-resistant E. coli and K. pneumoniae isolates. However, differences in trends between E. coli en K. pneumoniae underline the importance of E. coli as a community-pathogen and its subsequent influence on hospital resistance level, while for K. pneumoniae the level of resistance within the hospital seems less influenced by the resistance trends in the community.
This increase in the proportion of resistant E. coli isolates within the community and hospital settings implies that preventing the spread of extended-spectrum cephalosporin-resistance should not only focus on hospital settings, but also on preventing the spread of resistant species within the community. These efforts could prevent the influx of resistant species into the hospital setting and help to reduce the chance of inadequate treatment, increased length of hospital stay and additional healthcare costs due to infection with extended-spectrum cephalosporin-resistance bacteria.
Acknowledgments
Members of the ISIS-AR study group
Alkmaar, Department of Microbiology, Medical Center Alkmaar (F. Vlaspolder, J.W.T. Cohen Stuart)
Apeldoorn, Department of Medical Microbiology and Infection Prevention, Gelre Hospitals (B.C. van Hees)
Bergen op Zoom, Department of Medical Microbiology, Lievensberg Hospital (R.G.F. Wintermans)
Bilthoven, Centre for Infectious Disease Control, National Institute for Public Health and the Environment (W. Altorf-van der Kuil, J. Alblas, A.K. van der Bij, D. Frentz, T. Leenstra, J.C. Monen, J. Muilwijk, D.W. Notermans, S.C. de Greeff).
Breda, Laboratory for Microbiology and Infection Control, Amphia Hospital (P.H.J. van Keulen, J.A.J.W. Kluytmans)
Delft, Department of Medical Microbiology, Diagnostic Centre SSDZ (E.E. Mattsson)
Deventer, Department of Medical Microbiology, Deventer Hospital (F.W. Sebens)
Dordrecht, Department of Medical Microbiology, Albert Schweitzer Hospital (H.M.E. Frénay, B. Maraha)
Enschede, Laboratory for Microbiology and Public Health (F.G.C. Heilmann, T. Halaby)
Goes, Department of Medical Microbiology and Immunology, Admiraal De Ruyter Hospital, (D. Versteeg)
Groningen, Laboratory for Infectious Diseases (R. Hendrix, J.F.P. Schellekens)
Haarlem, Regional Laboratory of Public Health (B.M.W. Diederen).
Heerlen, Department of Medical Microbiology, Atrium MC Parkstad, (E.I.G.B. de Brauwer, F.S. Stals)
Hilversum, Department of Medical Microbiology, CBSL (L.J. Bakker, J.W. Dorigo-Zetsma)
Leeuwarden, Izore, Centre for Infectious Diseases Friesland (J.H. van Zeijl)
Leiden, Department of Medical Microbiology and Immunology, University Medical Center Leiden (A.T. Bernards)
Nieuwegein, Department of Medical Microbiology, St Antonius Hospital, (B.M. de Jongh, B.J.M. Vlaminckx)
Nijmegen, Department of Medical Microbiology, Canisius Wilhelmina Hospital (A. Horrevorts)
Nijmegen, Department of Medical Microbiology, Radboud University Nijmegen Medical Center, (S. Kuipers)
Roosendaal, Department of Medical Microbiology, Franciscus Hospital (R.G.F. Wintermans)
Schiedam, Department of Medical Microbiology, Vlietland Hospital (B. Moffie)
's-Gravenhage, Department of Medical Microbiology, Haga Teaching Hospital (R.W. Brimicombe)
's-Gravenhage, Department of Medical Microbiology, MCH Westeinde Hospital (C.L. Jansen)
's-Hertogenbosch, Department of Medical Microbiology and Infection Control, Jeroen Bosch Hospital (N.H.M. Renders)
Terneuzen, Department of MedicalMicrobiology, ZorgSaamHospital Zeeuws-Vlaanderen (B.G.A. Hendrickx)
Tilburg, Department of Medical Microbiology, St. Elisabeth Hospital (A.G.M. Buiting)
Utrecht, Laboratory of Medical Microbiology and Immunology, Diakonessenhuis (J.A. Kaan, S.F.T. Thijsen)
Utrecht, Department of Medical Microbiology, Saltro, Primary Health Care Laboratory (M.P.D. Deege)
Utrecht, Department of MedicalMicrobiology, University Medical Center Utrecht (M.B. Ekkelenkamp)
Veldhoven, Department of Medical Microbiology, PAMM (H.T. Tjhie)
Velp, Laboratory for Medical Microbiology and Immunology, Rijnstate Hospital (A.A. van Zwet)
Woerden, Department of Medical Microbiology, ZuweHofpoort Hospital (G.P. Voorn)
Zwolle, Laboratory of Medical Microbiology and Infectious Diseases, Isala Clinics (G.J.H.M. Ruijs, M.J.H.M. Wolfhagen)
Data Availability
Data are available in aggregated format at the public available interactive website of ISIS-AR (https://www.isis-web.nl). A request for data in unaggregated format can be done by the submission of a data request form to the ISIS-AR registration committee. This data request form is available at https://www.isis-web.nl/interactieve_rapporten/ and can be sent by e-mail to tjalling.leenstra@rivm.nl. The raw data cannot be made publicly available within the manuscript or in Supporting Information files due to written agreement between the RIVM and the laboratories participating in the ISIS-AR database, in which it is stated that the data in the isis-ar database is owned by the individual laboratories and that it is not allowed to provide them to other parties unless approved by the registration committee on individual basis.
Funding Statement
ISIS-AR is supported by the Dutch Ministry of Health. This study was conducted as part of the authors’ routine work, and the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Paterson DL, Bonomo RA. (2005) Extended-spectrum beta-lactamases: a clinical update. Clin Microbiol Rev 18: 657–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee SY, Kotapati S, Kuti JL, Nightingale CH, Nicolau DP. (2006) Impact of extended-spectrum b-lactamase–producing Escherichia coli and Klebsiella species on clinical outcomes and hospital costs: a matched cohort study. Infect Control Hosp Epidemiol 27: 1226–1232. [DOI] [PubMed] [Google Scholar]
- 3. Kang CI, Kim SH, Park WB, Lee KD, Kim HB, KIM EC, et al. (2004) Bloodstream infections due to extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae: risk factors for mortality and treatment outcome, with special emphasis on antimicrobial therapy. Antimicrob Agents Chemother 38: 4574–4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schwaber MJ, Navon-Venezia S, Kaye KS, Ben-Ami R, Schwartz D, Carmeli Y. (2006) Clinical and economic impact of bacteremia with extended- spectrum-β-lactamase-producing Enterobacteriaceae. Antimicrob Agents Chemother 50: 1257–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Livermore DM, Canton R, Gniadkowski M, Nordmann P, Rossolini GM, Arlet G, et al. (2007) CTX-M: changing the face of ESBLs in Europe. J Antimicrob Chemother 59: 165–174. [DOI] [PubMed] [Google Scholar]
- 6. Rodriguez-Bano J, Navarro MD, Romero L, Muniain MA, De Cueto M, Ríos MJ, et al. (2006) Bacteremia due to extended-spectrum beta-lactamase-producing Escherichia coli in the CTX-M era: a new clinical challenge. Clin Infect Disease 43: 1407–14. [DOI] [PubMed] [Google Scholar]
- 7. Van der Bij AK, Pitout JDD. (2012) The role of international travel in the worldwide spread of multiresistant Enterobacteriaceae. J Antimicrob Chemother 67: 2090–2100. 10.1093/jac/dks214 [DOI] [PubMed] [Google Scholar]
- 8. Boyd DA, Tyler S, Christianson S, McGeer A, Muller MP, Willey BM, et al. (2004) Complete nucleotide sequence of a 92-kilobase plasmid harboring the CTX-M-15 extended-spectrum beta-lactamase involved in an outbreak in long-term-care facilities in Toronto, Canada. Antimicrob Agents Chemother 48: 3758–3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ben-Ami R, Rodríguez-Baño J, Arslan H, Pitout JD, Quentin C, Calbo ES, et al. (2009) A multinational survey of risk factors for infection with extended-spectrum beta-lactamase-producing enterobacteriaceae in nonhospitalized patients. Clin Infect Dis 49: 682–90. 10.1086/604713 [DOI] [PubMed] [Google Scholar]
- 10. Hawser SP, Bouchillon SK, Hoban DJ, Badal RE, Cantón R, Baquero F. (2010) Incidence and antimicrobial susceptibility of Escherichia coli and Klebsiella pneumoniae with extended-spectrum β-lactamases in community- and hospital-associated intra-abdominal infections in Europe: results of the 2008 Study for Monitoring Antimicrobial Resistance Trends (SMART). Antimicrob Agents Chemother 54: 3043–3046. 10.1128/AAC.00265-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Van der Bij AK, van Dijk K, Muilwijk J, Thijssen SFT, Notermans DW, de Greeff S, et al. (2012) Clinical breakpoint changes and their impact on surveillance of antimicrobial resistance in Escherichia coli causing bacteraemia. Clin Microbiol Infect 18: 466–472. [DOI] [PubMed] [Google Scholar]
- 12.European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint tables for interpretation of MICs and zone diameters. Version 2.0, valid from 2012-01-01. Available: http://www.eucast.org/antimicrobial_susceptibility_testing/previous_versions_of_tables/ (20 May 2013, date last accessed).
- 13. Laupland KB, Ross T, Pitout JDD, Church DL, Gregson DB. (2007) Investigation of sources of potential bias in laboratory surveillance for anti-microbial resistance. Clin Invest Med 30: 159–166. [DOI] [PubMed] [Google Scholar]
- 14.Dutch College of General Practitioners. Guideline urinary tract infection. Available: https://www.nhg.org/standaarden/volledig/nhg-standaard-urineweginfecties (13 December 2014, date last accessed)
- 15. Braykov NP, Eber MR, Klein EY, Morgan DJ, Laxminarayan R. (2013) Trends in resistance to carbapenems and third-generation cephalosporins among clinical isolates of Klebsiella pneumoniae in the United States, 1999–2010. Infect Control Hospital Epidemiol 34: 259–268. [DOI] [PubMed] [Google Scholar]
- 16. Kronenberg A, Hilty M, Endimiani A, Mühlemann K. (2013) Temporal trends of extended-spectrum cephalosporin-resistant Escherichia coli and Klebsiella pneumoniae isolates in in- and outpatients in Switzerland, 2004 to 2011. Euro Surveill 18: pii 20484. [PubMed] [Google Scholar]
- 17. Livermore DM. (2012) Fourteen years in resistance. Int J Antimicrob Agents 39: 283–294. 10.1016/j.ijantimicag.2011.12.012 [DOI] [PubMed] [Google Scholar]
- 18.The Dutch Working Party on Antibiotic Policy (SWAB). Guidelines. Available: http://www.swab.nl/guidelines (20 May 2013, date last accessed).
- 19.Dutch Working party on Infection Prevention. Documentation. Available: http://www.wip/UK/document.htm (20 May 2013, date last accessed).
- 20.European Centre for Disease Prevention and Control (ECDC). European Antimicrobial Surveillance Network (EARS-Net). Interactive database. Available: http://www.rivm.nl/earss/ (02 April 2014, date last accessed).
- 21. Casewell MW, Desai N. (1983) Survival of multiply-resistant Klebsiella aerogenes and other Gram-negative bacilli on finger-tips. J Hosp Infect 4: 350–360. [DOI] [PubMed] [Google Scholar]
- 22. Kamer A, Schwebke I, Kampf G. (2006) How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis 6:130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Banerjee R, Johnston B, Lohse C, Porter SB, Clabots C, Johnson JR. (2013) Escherichia coli Sequence Type 131 Is a Dominant, Antimicrobial-Resistant Clonal Group Associated with Healthcare and Elderly Hosts. Infect Control Hosp Epidemiol 34: 361–268. 10.1086/669865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peirano G, Pitout JDD. (2010) Molecular epidemiology of Escherichia coli producing CTX-M B-lactamases: the worldwide emergence of clone ST131 O25:H4. Int J of Antimicrob Agents 35: 316–321. [DOI] [PubMed] [Google Scholar]
- 25. Pitout JD, Gregson DB, Campbell L, Laupland KB. (2009) Molecular characteristics of extended-spectrum-beta-lactamase-producing Escherichia coli isolates causing bacteremia in the Calgary Health Region from 2000 to 2007: emergence of clone ST131 as a cause of community-acquired infections. Antimicrob Agents Chemother 53: 2846–2851. 10.1128/AAC.00247-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available in aggregated format at the public available interactive website of ISIS-AR (https://www.isis-web.nl). A request for data in unaggregated format can be done by the submission of a data request form to the ISIS-AR registration committee. This data request form is available at https://www.isis-web.nl/interactieve_rapporten/ and can be sent by e-mail to tjalling.leenstra@rivm.nl. The raw data cannot be made publicly available within the manuscript or in Supporting Information files due to written agreement between the RIVM and the laboratories participating in the ISIS-AR database, in which it is stated that the data in the isis-ar database is owned by the individual laboratories and that it is not allowed to provide them to other parties unless approved by the registration committee on individual basis.