Abstract
Mechanisms of plant root tolerance to high temperatures through antioxidant defense are not well understood. The objective of this study was to investigate whether superior root thermotolerance of heat-tolerant Agrostis scabra relative to its congeneric heat-sensitive Agrostis stolonifera was associated with differential accumulation of reactive oxygen species and antioxidant scavenging systems. A. scabra ‘NTAS’ and A. stolonifera ‘Penncross’ plants were exposed to heat stress (35/30°C, day/night) in growth chambers for 24 d. Superoxide (O2 -) content increased in both A. stolonifera and A. scabra roots under heat stress but to a far lesser extent in A. scabra than in A. stolonifera. Hydrogen peroxide (H2O2) content increased significantly in A. stolonifera roots but not in A. scabra roots responding to heat stress. The content of antioxidant compounds (ascorbate and glutathione) did not differ between A. stolonifera and A. scabra under heat stress. Enzymatic activity of superoxide dismutase was less suppressed in A. scabra than that in A. stolonifera under heat stress, while peroxidase and catalase were more induced in A. scabra than in A. stolonifera. Similarly, their encoded transcript levels were either less suppressed, or more induced in A. scabra roots than those in A. stolonifera during heat stress. Roots of A. scabra exhibited greater alternative respiration rate and lower cytochrome respiration rate under heat stress, which was associated with suppression of O2 - and H2O2 production as shown by respiration inhibitors. Superior root thermotolerance of A. scabra was related to decreases in H2O2 and O2 - accumulation facilitated by active enzymatic antioxidant defense systems and the maintenance of alternative respiration, alleviating cellular damages by heat-induced oxidative stress.
Introduction
Heat stress is a major abiotic factor which limits plant growth and productivity, particularly in cool-season (C3) species. Plants undergo various physiological and cellular changes as heat stress progresses including oxidative damage caused by production of reactive oxygen species (ROS), namely hydrogen peroxide (H2O2) and superoxide (O2 -) [1–4]. ROS are typically produced when electrons seeking a lower energy state are transferred to molecular oxygen during inhibition of carbon-fixation in leaves or elevated cytochrome respiration in leaves and roots, among other metabolic processes [5]. Although many of them are important signaling molecules in the regulation of plant growth, overproduction and accumulation of ROS in various plant organs decreases cellular membrane stability leading to oxidative damages of nucleic acid, lipids, and proteins [6–9].Therefore, methods which reduce oxidative damages by limiting production or accumulation of ROS are critical for improving plant tolerance or adaptation to heat stress.
Plant adaptation to oxidative stress may be aided in part by non-enzymatic and enzymatic ROS scavenging systems [6]. Non-enzymatic compounds include glutathione (GSH) and ascorbate (ASA) which possess intrinsic antioxidant properties and serve as electron donors to reduce ROS accumulation[10].There are two distinct antioxidant enzymatic pathways in plants, the first of which utilizes superoxide dismutase (SOD), catalase (CAT), and/or peroxidase (POD) located subcellularly in mitochondria, chloroplasts, and peroxisomes [11–13]. SOD converts O2 - to less-damaging H2O2 which is subsequently split into non-damaging water and oxygen by POD or CAT. The second antioxidant enzymatic pathway utilizes ascorbate peroxidase (APX), glutathione reductase (GR), monodehydroascorbate reductase (MR), and dehydroascorbate reductase (DR)to reduce H2O2 to water and oxygen by controlling the balance of GSH and ASA in plant leaves [11, 12]. Non-enzymatic and enzymatic pathways serve important roles for antioxidant metabolism enhancing plant tolerance to various stresses, though the specific compounds or enzymes contributing to stress defense vary across plant species, varieties, age, organs, and in response to stress type, severity, and duration [9, 14–18]. Most of previous work reported leaf antioxidant mechanisms in relation to stress defense, but limited information is available on how roots may survive high temperature through antioxidant defense.
It is well documented that roots are more sensitive than shoots under heat though the specific mechanisms underlying root susceptibility to heat stress or thermotolerance to tolerate high temperatures are not well understood [4, 19]. In addition, ROS scavenging capacity may differ between roots and shoots due the variations in their subcellular locations in different tissues. ROS is produced and subsequently scavenged in chloroplasts, mitochondria, and peroxisome in leaves, whereas it is mainly generated in mitochondria for root tissues [20–22]. It remains unknown whether the antioxidant scavenging components differ between the two organ types and how individual components affect root thermotolerance. Specifically in plant roots of various species, mitochondrial H2O2 and O2 -increase as root respiration increases during prolonged heat stress [23–25]. There are two distinct pathways by which plant mitochondria carry out respiratory electron transport, the cytochrome pathway which accelerates ROS production and the alternative pathway which slows production rates and reduces net accumulation of ROS [8, 26]. The cytochrome pathway is characterized by electron transfer governing ATP synthesis at the expense of ROS production due to the ubisemiquinone radical which transfers an electron to oxygen prompting superoxide formation [27]. The alternative pathway is characterized by enhanced activity of alternative oxidase (AOX) which effectively diverts high-energy electrons produced by over-reduction of cytochrome oxidase and avoids significant ROS production [28, 29]. Nitric oxide (NO) has been shown to induce alternative respiration by inhibiting cytochrome oxidase activity and is also a complementary partner to ROS for determining cell fate or for signaling responses during stress onset [30–33]. Utilizing NO to prompt a shift towards alternative respiratory pathways and significantly enhance ROS scavenging in various plant organs has not been fully understood and deserves further attention.
Thermal bentgrass (Agrostis scabra) thrives alongside geothermal vents with soil temperature exceeding 40°C in Yellowstone National Park, USA and roots can actively grow under extremely high soil temperatures [34] while many of its congeneric species, such as A. stolonifera, a commonly used turf and forage grass species, are very sensitive to heat stress and majority of roots lost viability as temperature increases above 25°C [19]. The superior root thermotolerance of thermal A. scabra has been attributed to various mechanisms facilitating respiratory acclimation, changes in carbon allocation, cellular membrane stability, and activation of stress defense proteins such as chaperones [19, 35–39].However, many questions remain unanswered regarding the interrelationship of ROS, enzymatic and non-enzymatic antioxidants, and respiratory pathways collectively governing root thermotolerance in cool-season grasses. Comparative analysis between thermal A. scabra and heat-sensitive A. stolonifera will contribute to a better understanding of heat tolerance mechanisms and may aid in future breeding selections of heat-tolerant germplasm in cool-season grasses. Therefore, the objective of this study were to determine whether root thermotolerance was due to activation of non-enzymatic antioxidant production or antioxidant enzymes at both enzymatic activity and gene expression levels and investigate the relationship of root respiration and ROS production in relation to root thermotolerance in thermal A. scabra compared to heat-sensitive A. stolonifera during prolonged heat stress.
Materials and Methods
Plant materials and growth conditions
Tillers (30 per individual plant) of A. stolonifera ‘Penncross’ or A. scabra ‘NTAS’ were harvested from stock plants and transferred to plastic containers(57 x 44 x 30 cm, 12 drainage holes) filled with fritted clay medium (Profile Products, Deerfield, IL). Plants were established for 35 d in a greenhouse set to 23/20°C (day/night), 60% relative humidity (RH), 14 h photoperiod, and 500 μmol m-2 s-1 photosynthetically active radiation (PAR) from natural sunlight and supplemental lighting. Plants were irrigated daily, fertilized twice per week with half-strength Hoagland’s nutrient solution [40], and trimmed to 2 cm once per week during establishment. Plants were not trimmed during the final week of establishment to allow for sufficient regrowth prior to stress imposition, after which time all plants were transferred to controlled-environment growth chambers (Environmental Growth Chamber, Chagrin Falls, Ohio, USA).
Treatments and experimental design
Plants were maintained in controlled-climate growth chambers set to 22/18°C (day/night), 600 μmol m-2 s-1 PAR, 60% RH, and 14 h photoperiod for one week prior to stress imposition, after which time air temperature was raised to 35/30°C to impose heat stress for 24 d. During stress treatment, plants were irrigated daily, fertilized twice per week with half-strength Hoagland’s nutrient solution and not trimmed. The experiment was arranged in a split-plot design with temperature treatment (control or heat) as the main plot and grass species (A. scabra or A. stolonifera) as subplots. Each treatment was replicated in four containers and each container included four individual plants per species. Since each chamber was only able to accommodate two containers, a relocation strategy was introduced between four containers in their respective chambers every 3 d to avoid possible confounding effects of unique chamber environmental variations from occurring.
Root physiological analysis
Following 24 d stress treatment, roots were washed free of fritted clay and a subset from each plant was collected to quantify root electrolyte leakage (EL) and malondialdehyde (MDA) content. EL was measured according to the procedure described by Blum and Ebercon [41] and used to indicate the cellular membrane stability or membrane status following treatment. A subset of roots was rinsed with deionized water to remove exogenous solutes and placed in a test tube containing 30 mL deionized water. Tubes were agitated in a conical flask shaker for 12 h and the initial conductance (Ci) of incubation solution measured using a conductivity meter (YSI Model 32, Yellow Springs, OH). Tubes containing root tissue were then autoclaved at 121°C for 20 min and again agitated for 12 h. The maximal conductance (Cmax) of incubation solution was then measured and EL (%) was calculated as ((Ci/Cmax) × 100).
MDA is the final product of lipid peroxidation in plant tissue and was measured according to the procedure described by Zhang and Kirkham [17] with slight modifications. A subset of roots (0.5 g) was homogenized in 6 mL 0.1% trichloroacetic acid (TCA) and the homogenate was centrifuged at 10,000 g for 10 min. 1mL supernatant was added to 4 mL 10% TCA containing 0.5% thiobarbituric acid (TBA). The mixture was incubated at 95°C for 30 min, quickly cooled on ice, and centrifuged at 10,000 g for 10 min at 4°C. The absorbance of supernatant was measured at 532 and 600 nm using a spectrophotometer (Spectronic Instruments, Rochester, NY). The concentration of MDA was calculated using MDA’s extinction coefficient of 155 mM-1 cm-1[42]. All content levels were expressed as the mean (the average content in g fresh weight (FW)) ± SE (the standard error) of four biological replicates.
Histochemical staining for presence of hydrogen peroxide and superoxide
Histochemical staining for the presence of hydrogen peroxide and superoxide was performed following 24 d stress treatment, using procedures described in Thordal-Christensen et al. [43] and Dunand et al. [44], with slight modifications respectively. To evaluate the presence of hydrogen peroxide (H2O2), a subset of roots was stained with 1% (w/v) 3-diaminobenzinidine (DAB; pH 3.8) for 2 h and subsequently rinsed with deionized water. To evaluate the presence of superoxide (O2 -), a subset of roots was stained with2 mM nitroblue tetrozolium (NBT) in 20 mM phosphate-buffered saline (PBS; pH 6.8) for 30 min and subsequently rinsed with deionized water. DAB or NBT-stained roots were observed visually using an Olympus FSX100 Bio-imaging navigator (Central Valley, PA) and pictures were captured using bright-field single-shot mode at 4.2x magnification.
Quantification of reactive oxygen species and antioxidant compounds
Superoxide (O2 -) production rate was measured according to the procedure described by Bian and Jiang [15] with slight modifications. Root tissue (0.5 g)was ground to a powder in liquid nitrogen, homogenized in 1 mL 50 mM Tris-HCl (pH 7.5), and centrifuged at 5,000 g for 10 min at 4°C. 200 μL supernatant was added to 800 μL 0.5 mM 3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrozolium-5-carboxanilide inner salt (XTT). XTT reduction was recorded once per minute for 3 min at 470 nm using a spectrophotometer (Spectronic Instruments, Rochester, NY) and the background absorbance was corrected with 50 units of superoxide dismutase (SOD). The O2 - production rate was calculated using 2.16 ×104 M-1 cm-1 extinction coefficient and expressed as μmol O2 - min-1 g-1 FW [45].
Hydrogen peroxide (H2O2) content was measured according to the procedure described by Zhou et al. [46] with slight modifications. Root tissue (0.5 g) was homogenized in 5 mL 5% (w/v) TCA and the homogenate was centrifuged at 10,000 g for 20 min at 4°C. The supernatant was adjusted to pH 8.4 with 17 M ammonia solution, briefly centrifuged to remove large particles, and divided into 1 mL aliquots. 8 μg catalase was added to one aliquot to serve as the blank and not added to other aliquots. 1 mL of colorimetric reagent solution containing 10 mg 4-aminoantipyrine, 10 mg phenol, and 5 mg peroxidase in 100 mM acetic acid buffer (pH 5.6) was added to each aliquot and the color reaction was incubated for 10 min at 30°C. Following incubation, the absorbance was measured at 505 nm using a spectrophotometer (Spectronic Instruments, Rochester, NY) and H2O2 content determined based on standard curve generated with known H2O2concentrations.All content levels were expressed as the mean (the average content in g FW) ± SE of four biological replicates.
Quantification of non-enzymatic antioxidant content
Glutathione (GSH) and ascorbate (ASA) contents were measured according to the procedure described by Guri [47] with slight modifications. Root tissue (0.5 g) was homogenized in 5 mL 5% (w/v) TCA on ice and centrifuged at 16,000 g for 20 min at 4°C. The homogenate was titrated to a pH range of 6–8 with 1.5 mL 0.1 M NaOH. 2 mL titrated homogenate was added to0.5 mL 0.2 M sodium phosphate buffer (pH 7.0), 0.4 mL deionized water, and 0.1 mL 0.5% (w/v) dithiobis-2-nitrobenzoic acid (DTNB) and absorbance measured at 412 nm using a spectrophotometer (Spectronic Instruments, Rochester, NY). Titrated homogenate containing sodium phosphate buffer, deionized water, but lacking DTNB served as the blank. GSH and ASA content were determined based on standard curves generated with known concentrations of each compound.
Reduced and total ASA content were measured according to the procedure described by Ma et al. [48] with slight modifications. Root tissue (0.5 g) was homogenized in 8 ml 5% (w/v) TCA on ice, centrifuged at 10,000 g for 10 min at 4°C, and resulting supernatant used immediately for analysis. For total ASA quantification, the supernatant was incubated in 200 mM sodium phosphate buffer (pH 7.4) and 1.5 mM dithiothreitol (DTT) for 50 min to reduce all oxidized ascorbate (DHA) to ASA. Following incubation, 200 μL 0.5% (w/v) N-ethylmaleimide (NEM) was added to remove excess DTT. The resulting solution (0.8 ml)was then added to a reaction mixture containing 1 mL 10% (w/v) TCA, 800 μL 42% (w/v) o-phosphoric acid, 800 μL 65 mM 2,2’-dipyridyl in 70% (v/v) ethanol, and 400 μL 3% (w/v) iron (III) chloride. The reaction was incubated at 42°C for 1 h, and absorbance measured at 525 nm using a spectrophotometer (Spectronic Instruments, Rochester, NY).Reduced ASA was measured using the procedure described above with DTT and NEM substituted with 400 μL deionized water. Reduced and total ASA content were determined based on standard curves generated with known ASA concentrations. All content levels were expressed as the mean (the average content in g FW) ± SE of four biological replicates.
Quantification of enzymatic activity
Enzyme activity of CAT, POD, SOD, APX, DR, MR, and GR was measured according to the procedures described by Zhang and Kirkham [17]. For each CAT, POD, and SOD assay, 0.5 g root tissue was homogenized in 6 ml 50 mM sodium phosphate buffer (pH 7.0) containing 0.2 mM ethylenediaminetetraacetic acid (EDTA) and 1% (w/v) polyvinylpyrrolidone (PVP) on ice and the homogenates were centrifuged at 15,000 g for 20 min at 4°C. The absorbance were measured at 240, 470, and 560 nm for CAT, POD, and SOD, respectively, using a spectrophotometer (Spectronic Instruments, Rochester, NY).For each APX, DR, MR, and GR assay, 0.5 g root tissue was homogenized in 6 ml 25 mM sodium phosphate buffer (pH 7.8) containing 0.2 mM EDTA and 1% (w/v) PVP and the homogenates were centrifuged at 15,000 g for 20 min at 4°C. Absorbance were measured at 290, 265, 340, and 340 nm for APX, DR, MR, and GR, respectively, using a spectrophotometer (Spectronic Instruments, Rochester, NY). All enzymatic activity levels were expressed as the mean (the average content in mg of protein) x SE of four biological replicates.
Gene expression analysis of enzymatic antioxidants
Gene expression analysis was performed by quantitative reverse transcriptase polymerase chain reaction (qRT-PCR).Total RNA was isolated from root tissue using TRIzol reagent (Life Technologies, Grand Island, NY) and treated with DNase (TURBO DNA-free kit; Life Technologies, Grand Island, NY) to remove contaminating genomic DNA. 2 μg total RNA was reverse-transcribed using a high-capacity cDNA reverse transcription kit (Life Technologies, Grand Island, NY) and the synthesized cDNA was amplified in a StepOnePlus Real-Time PCR system (Life Technologies, Grand Island, NY) using the following parameters: pre-heat cycle of 95°C for 3 min, 40 cycles of 95°C denaturation for 30 sec, and 60°C annealing/extension for 60 sec. Power SYBR Green PCR Master Mix (Life Technologies, Grand Island, NY) was the intercalating dye used to detect gene expression level. Gene name, accession number, forward and reverse primer sequences are provided in Table 1. A melting curve analysis was performed for each primer pair to confirm its specificity. Actin2 was used as the reference gene, since its expression was constant throughout treatments. A ΔΔCt method was used to calculate the relative expression level between genes of interest and reference gene, respectively. All transcript levels were expressed as the mean (the average relative expression level) ± SE of four biological replicates.
Table 1. Primer sequences of ROS scavenging genes used in qRT-PCR.
Proposed gene names, GenBank accession numbers, best BLAST hit names, E-values, sequence identity scores are also listed.
| Gene | Accession number | Best BLAST hit | E-value | Identity | Primer sequence | |
|---|---|---|---|---|---|---|
| CuZn-SOD | DV867103 | JQ269675.1 (Triticum aestivum) | 3e-161 | 87% | Forward | CACTGGACCTCACTTCAAC |
| Reverse | GTAGCAACACCATCCACTC | |||||
| POD2 | DV867327 | XM_010230345.1 (Brachypodium distachyon) | 6e-153 | 89% | Forward | CTTCGACAACGCCTACTAC |
| Reverse | TTTGCCCATGTTCACCA | |||||
| CAT1 | DY543619 | AJ634002.1 (Schedonorus arundinaceus) | 0 | 93% | Forward | TTGCCAATAAGAGGGAGAATG |
| Reverse | CGAAGCCGAGCATGTAAG | |||||
| APX2 | GR281667 | KP852178.1 (Beckmannia syzigachne) | 0 | 94% | Forward | AGGACATTGTTGCCCTTTC |
| Reverse | GCTCCGTGAAGTAAGAGTTG | |||||
| GR | AB277097 | AB277097 (Hordeum vulgare) | 0 | 100% | Forward | GATGGAGGCTACTTGCTTTG |
| Reverse | GCTAAGACCCACGACAGATA | |||||
| MR | DV865077 | KC884831.1 (Triticum aestivum) | 5e-160 | 90% | Forward | CCATGAAGCTCTACAACGAG |
| Reverse | GTAGAAGTAGGGCAGGTAGT | |||||
| DR | DV853556 | HM125046.1 (Puccinellia tenuiflora) | 0 | 90% | Forward | GAAAGGTGCCTGTGTTTAATG |
| Reverse | GTGATGGAGTTGGGTACTTC | |||||
| ACT2 | DY543529 | XM_003578821.2 (Brachypodium distachyon) | 0 | 93% | Forward | CCTTTTCCAGCCATCTTTCA |
| Reverse | GAGGTCCTTCCTGATATCCA | |||||
Quantification of root respiration rate
Root respiration rate was measured according to the procedure described by Rachmilevitch et al. [35] with slight modifications. A subset of roots was detached from shoots, washed free of fritted clay, and immediately transferred into 500 ml Buchner flasks containing 400 ml half-strength Hoagland’s nutrient solution. The nutrient solution contained either 200 μM sodium nitroprusside (SNP) to inhibit the cytochrome respiratory pathway or 10 mM salicylhydroxamic acid (SHAM) to inhibit the alternative respiratory pathway. Solutions containing SNP or SHAM were maintained as an open-flow system by aerating with circulating pumps (Apollo Enterprises Inc., Oxnard, CA) for 30 min, after which time a closed-flow system was created by connecting the terminal air tube to the circulating pump inlet. Vacuum grease and Teflon tape were used to maintain an air-tight seal around the rubber stoppers. CO2 evolution rate was measured every 30 min for 2 h by extracting 1 ml air samples from the flasks using air-tight syringes and re-sealable septa affixed to flask side arms. Air samples were then injected into a Shimazu GC-8AIT gas chromatograph (Shimazu, Kyoto, Japan) equipped with a thermal conductivity detector and a stainless steel column (length: 6 ft; I.D.: 0.085”; O.D.: 1/8”) packed with Porapack Q (80/100 mesh).The temperatures for injector, column, and detector were set at 30°C, 150°C, and 150°C, respectively. Helium was used as a carrier gas at a flow rate of 30 mLminute-1. Remaining root tissue was dried in an oven at 80°C for 72 h and subsequently weighed on a mass balance. Root respiration rates were expressed as O2 uptake rate (mmol h-1 g-1root dry weight, DW) converted proportionally from CO2 evolution rate [34].
Statistical analysis
Temperature treatment effects and species variations were tested with the analysis of variance using the general linear model in a statistical program (SAS9.0, Cary, NC). Differences in mean values between treatments and between species were determined using the student’s t-test. A P-value of ≤0.05 was considered as statistically significant.
Results
Physiological responses for differential root thermotolerance of A. scabra and A. stolonifera
Heat stress treatment significantly increased root EL for both grass species compared to respective non-stress controls (Fig 1A). EL increased by 95 and 76% for A. stolonifera and A. scabra, respectively, due to heat stress. EL did not differ between A. stolonifera and A. scabra under non-stress conditions whereas it remained significantly lower (11% decrease) in A. scabra compared to A. stolonifera following heat stress treatment. Heat stress treatment significantly increased root MDA content for A. stolonifera but not A. scabra compared to respective non-stress controls (Fig 1B). Root MDA content increased by 26 and 11% for A. stolonifera and A. scabra, respectively, due to heat stress. MDA content did not differ between A. stolonifera and A. scabra under non-stress conditions whereas it remained significantly lower (12% decrease) in A. scabra compared to A. stolonifera following heat stress treatment.
Fig 1. Root electrolyte leakage (A) and MDA content (B) of A. stolonifera and A. scabra following control and heat stress treatment.
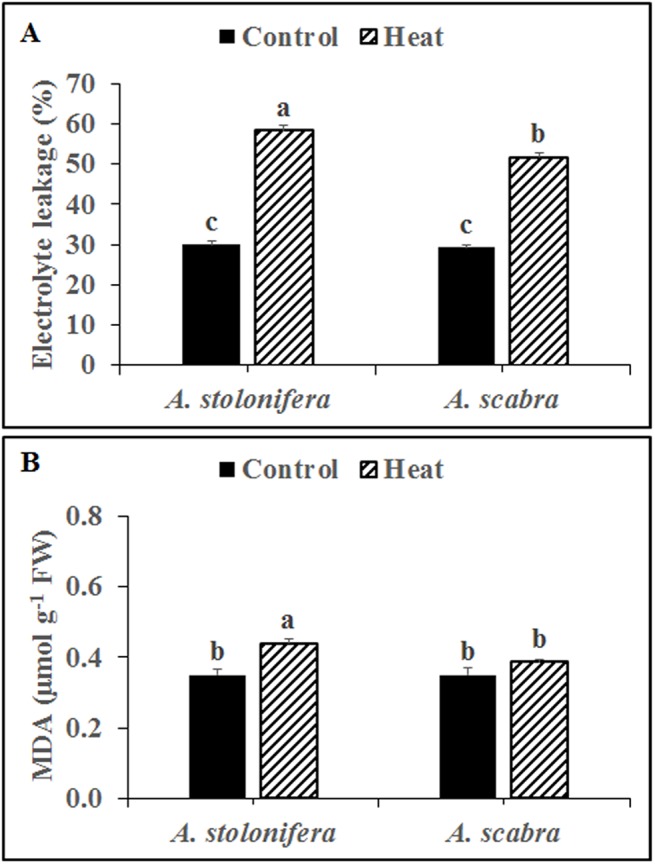
Data shown are the mean ± SE of four biological replicates. Different letters atop bars indicate significant differences exist at the P ≤ 0.05 level.
ROS production for differential root thermotolerance of A. scabra and A. stolonifera
Heat stress treatment significantly increased root superoxide (O2 -) content for both grass species compared to respective non-stress controls (Fig 2A). Superoxide (O2 -) content increased by 94 and 15% for A. stolonifera and A. scabra, respectively, due to heat stress. O2 - content was significantly higher (27% increase) in A. scabra than A. stolonifera under non-stress conditions whereas it remained significantly lower (25% decrease) in A. scabra compared to A. stolonifera following heat stress treatment. Heat stress treatment significantly increased root hydrogen peroxide H2O2 content in A. stolonifera but not in A. scabra compared to respective non-stress controls (Fig 2B). H2O2 content increased by 117 and 5% for A. stolonifera and A. scabra, respectively, due to heat stress.H2O2 content did not differ between the species under non-stress conditions whereas it remained significantly lower (41% decrease) in A. scabra compared to A. stolonifera following heat stress treatment. Histochemical staining for O2 - and H2O2 (Fig 2C and 2D, respectively) visually depicted decreased staining intensity for both ROS in A. scabra roots relative to A. stolonifera roots following heat stress.
Fig 2. Quantification of O2- (A) and H2O2 (B) content of A. stolonifera and A. scabra following control or heat-stress treatment and histochemical staining of A. stolonifera and A. scabra roots with NBT (C) or DAB (D).
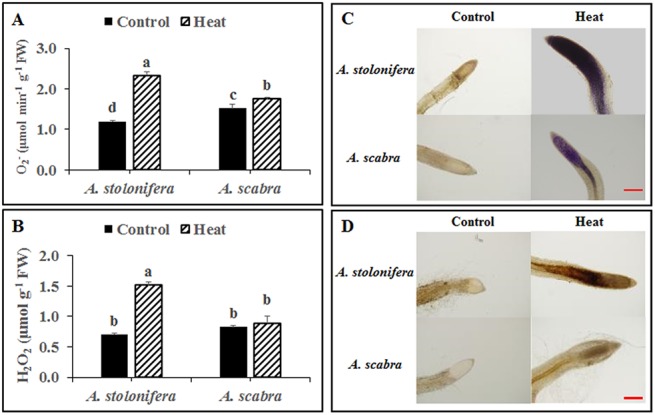
Data shown are the mean ± SE of four biological replicates. Different letters atop bars indicate significant differences exist at the P ≤ 0.05 level. Bar represents 100 μm.
Non-enzymatic antioxidant content and antioxidant enzyme activities for differential root thermotolerance of A. scabra and A. stolonifera
Heat stress treatment significantly increased reduced ASA content for both A. stolonifera (28%) and A. scabra (23%), compared to respective non-stress controls (Fig 3A). However, there was no difference in reduced ASA content between A. scabra and A. stolonifera under heat or non-stress conditions. There was no significant change in total ASA or GSH content for either species due to heat stress compared to respective non-stress controls (Fig 3B and 3C, respectively). Additionally, there was no difference in total ASA or GSH content between A. scabra and A. stolonifera under heat or non-stress conditions.
Fig 3. Reduced ascorbate (A), total ascorbate (B) and glutathione (C) content in A. stolonifera and A. scabra roots under control and heat stress condition.
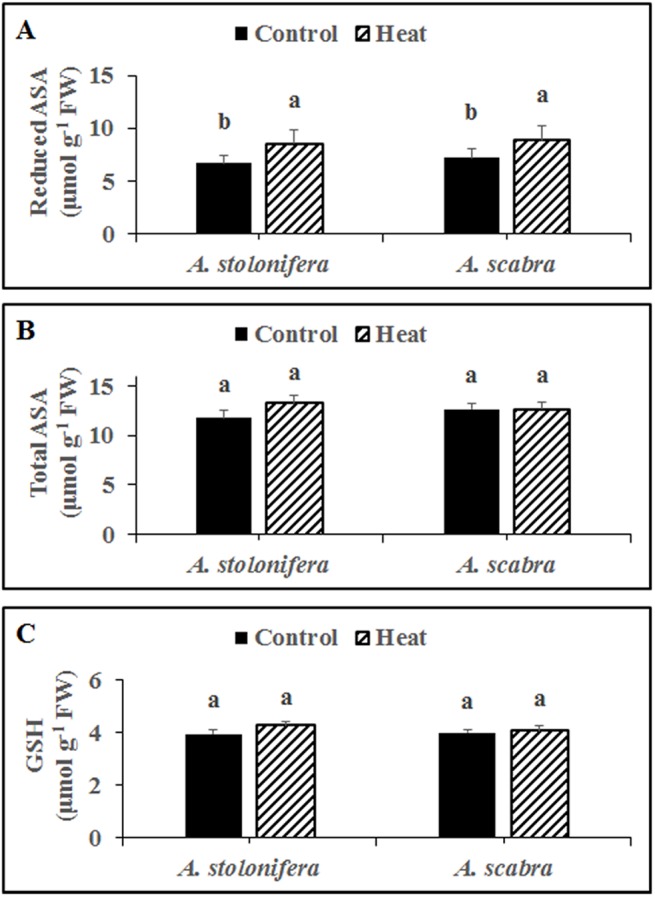
Data shown are the mean ± SE of four biological replicates. Different letters atop bars indicate significant differences exist at the P ≤ 0.05 level.
Heat stress treatment significantly decreased SOD activity for both grass species compared to respective non-stress controls (Fig 4A). SOD activity decreased by 81 and 41% for A. stolonifera and A. scabra, respectively, due to heat stress. SOD activity was significantly higher (31% increase) in A. scabra compared to A. stolonifera under non-stress conditions and remained significantly higher (2.98 fold) in A. scabra compared to A. stolonifera following heat stress treatment. Heat stress treatment significantly decreased POD activity for both grass species compared to respective non-stress controls (Fig 4B). POD activity decreased by 57 and 43% for A. stolonifera and A. scabra, respectively, due to heat stress. There was no difference in POD activity between A. scabra and A. stolonifera under non-stress conditions whereas POD activity was significantly higher (27% increase) in A. scabra compared to A. stolonifera following heat stress treatment. Heat stress treatment significantly increased CAT activity for both grass species compared to respective non-stress controls (Fig 4C). CAT activity increased 2.5 and 2.4-fold for A. stolonifera and A. scabra, respectively, due to heat stress. CAT activity was significantly higher (80% increase) in A. scabra compared to A. stolonifera under non-stress conditions and remained significantly higher (70% increase) in A. scabra compared to A. stolonifera following heat stress treatment.
Fig 4. Enzymatic activity of SOD (A), POD (B) and CAT (C) in roots of control or heat-stressed A. stolonifera and A. scabra.
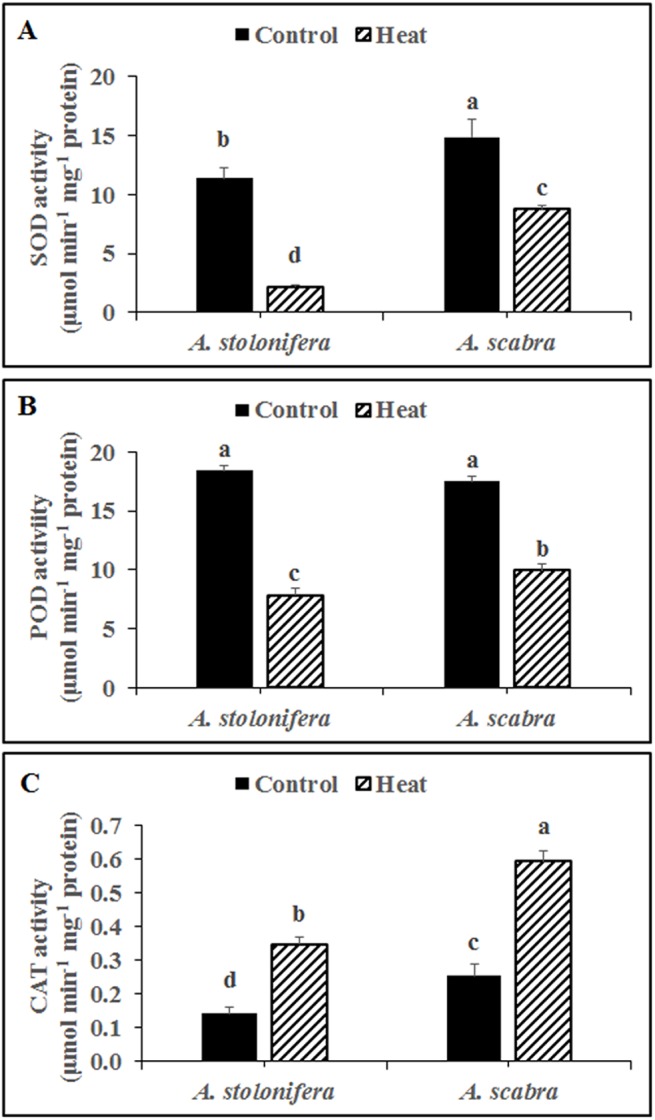
Data shown are the mean ± SE of four biological replicates. Different letters atop bars indicate significant differences exist at the P ≤ 0.05 level.
Heat stress treatment significantly decreased APX activity for both grass species compared to respective non-stress controls (Fig 5A). APX activity decreased by 55 and 58% for A. stolonifera and A. scabra, respectively, due to heat stress. APX activity was significantly higher (47% increase) in A. scabra compared to A. stolonifera under non-stress conditions and remained significantly higher (41% increase) in A. scabra compared to A. stolonifera following heat stress treatment. Heat stress treatment significantly decreased GR activity for both grass species compared to respective non-stress controls (Fig 5B). GR activity decreased by 50 and 23% for A. stolonifera and A. scabra, respectively, due to heat stress. GR activity was significantly higher (29% increase) in A. scabra compared to A. stolonifera under non-stress conditions and remained significantly higher (97% increase) in A. scabra compared to A. stolonifera following heat stress treatment. Heat stress treatment significantly increased MR activity for A. scabra but not for A. stolonifera compared to respective non-stress controls (Fig 5C). MR activity increased by 32% for A. scabra due to heat stress. MR activity was significantly higher (41% increase) in A. scabra compared to A. stolonifera under non-stress conditions and remained significantly higher (65% increase) in A. scabra compared to A. stolonifera following heat stress treatment. Heat stress treatment significantly increased DR activity for A. scabra but not for A. stolonifera compared to respective non-stress controls (Fig 5D). DR activity increased by 43% for A. scabra due to heat stress. DR activity was significantly higher (37% increase) in A. scabra compared to A. stolonifera under non-stress conditions and remained significantly higher (116% increase) in A. scabra compared to A. stolonifera following heat stress treatment.
Fig 5. Enzymatic activity of APX (A), GR (B), MR (C) and DR (D) in roots of A. stolonifera and A. scabra under control or heat stress condition.
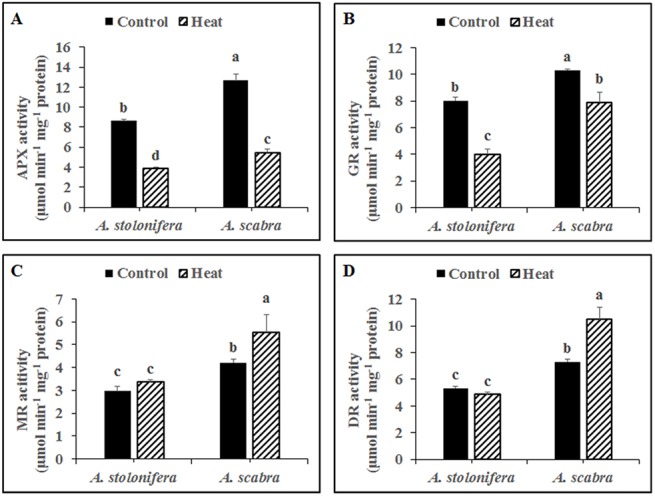
Data shown are the mean ± SE of four biological replicates. Different letters atop bars indicate significant differences exist at the P ≤ 0.05 level.
Enzyme gene expression for differential root thermotolerance of A. scabra and A. stolonifera
Antioxidant enzyme gene transcript levels quantified by qRT-PCR exhibited significant differences between A. scabra and A. stolonifera in response to heat stress treatment. Heat stress treatment significantly down-regulated SOD expression for both grass species compared to respective non-stress controls (Fig 6A). SOD expression decreased by 93 and 51% for A. stolonifera and A. scabra, respectively, due to heat stress. SOD expression was significantly higher (11% increase) in A. scabra compared to A. stolonifera under non-stress conditions and remained significantly higher (6.93 fold) in A. scabra compared to A. stolonifera following heat stress treatment. Heat stress treatment significantly up-regulated POD expression for both grass species compared to respective non-stress controls (Fig 6B). POD expression increased 2.1 and 4.1-fold for A. stolonifera and A. scabra, respectively, due to heat stress. POD expression was significantly higher (83% increase) in A. scabra compared to A. stolonifera under non-stress conditions and remained significantly higher (2.55 fold) in A. scabra compared to A. stolonifera following heat stress treatment. Heat stress treatment significantly up-regulated CAT expression for A. scabra but not for A. stolonifera compared to respective non-stress controls (Fig 6C). CAT expression increased by 45% for A. scabra in response to heat stress. CAT expression was significantly lower (38% decrease) in A. scabra compared to A. stolonifera under non-stress conditions and remained significantly lower (16% decrease) in A. scabra compared to A. stolonifera following heat stress treatment.
Fig 6. Transcript level of SOD (A), POD (B) and CAT (C) in roots of control or heat-stressed A. stolonifera and A. scabra.
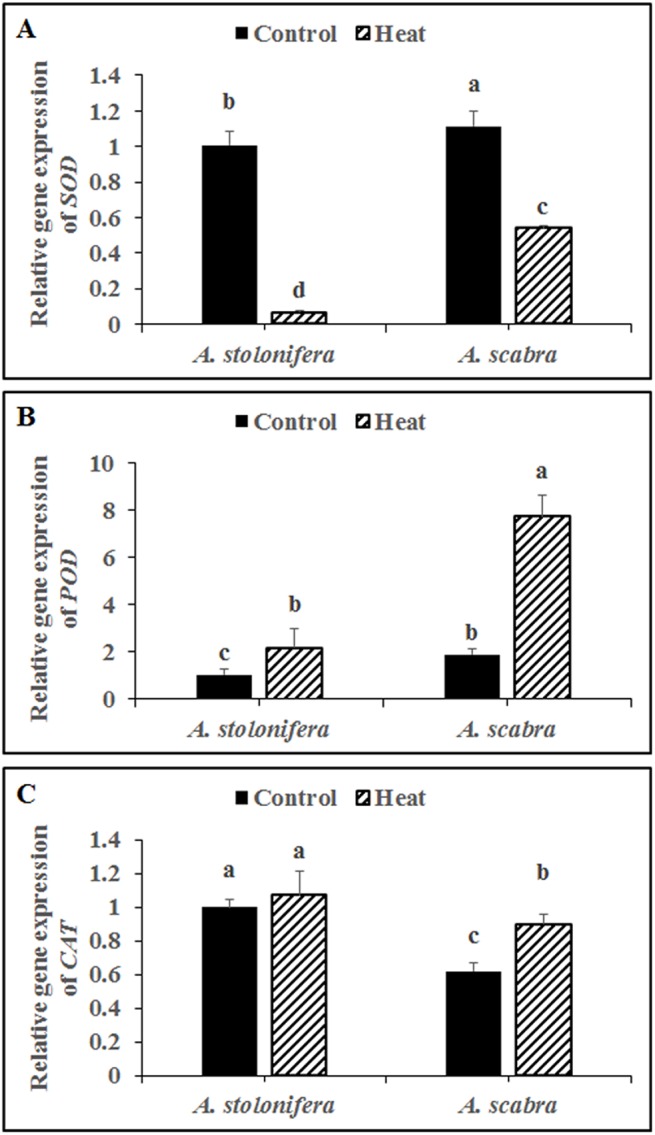
Data shown are the mean ± SE of four biological replicates. Different letters atop bars indicate significant differences exist at the P ≤ 0.05 level.
Heat stress treatment significantly down-regulated APX expression for both grass species compared to respective non-stress controls (Fig 7A). APX expression decreased by 92 and 92% for A. stolonifera and A. scabra, respectively, due to heat stress. APX expression was significantly lower (24% decrease) in A. scabra compared to A. stolonifera under non-stress conditions whereas no significant differences existed between A. scabra and A. stolonifera following heat stress treatment. There were no significant differences in GR or MR expression levels for either grass species compared to respective non-stress controls (Fig 7B and 7C, respectively). GR expression was significantly lower (62% decrease) in A. scabra compared to A. stolonifera under non-stress conditions and remained significantly lower (72% decrease) in A. scabra compared to A. stolonifera following heat stress treatment. There was no difference in MR expression between A. scabra and A. stolonifera under heat or non-stress conditions. Heat stress treatment significantly up-regulated DR expression for both grass species compared to respective non-stress controls (Fig 7D). DR expression increased 2.75 and 1.94-fold for A. stolonifera and A. scabra, respectively, due to heat stress. DR expression was significantly lower (12% decrease) in A. scabra compared to A. stolonifera under heat stress conditions whereas no significant differences existed between A. scabra and A. stolonifera under non-stress conditions.
Fig 7. Transcript levels of APX (A), GR (B), MR (C) and DR (D) in roots of A. stolonifera and A. scabra under control or heat stress condition.
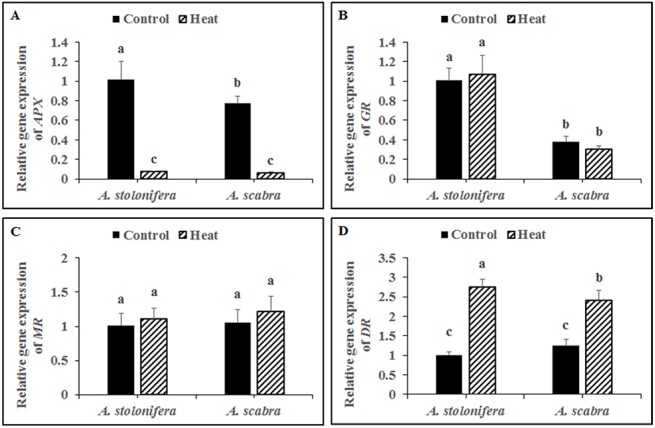
Data shown are the mean ± SE of four biological replicates. Different letters atop bars indicate significant differences exist at the P ≤ 0.05 level.
SNP and SHAM effects on root respiration and ROS production for differential root thermotolerance of A. scabra and A. stolonifera
Incubating A. scabra or A. stolonifera roots in solutions containing SNP or SHAM revealed significant differences in cytochrome (SHAM-resistant, SNP-inhibited) and alternative (SNP-resistant, SHAM-inhibited) respiration rates responding to heat stress. Heat stress treatment significantly increased total root respiration for both grass species compared to respective non-stress controls (Fig 8). The addition of SNP to incubation solutions reduced respiration rates by 15 and 41% in A. scabra and A. stolonifera, respectively, compared to heat-stressed roots with water only. Alternatively, the addition of SHAM to incubation solutions reduced respiration rates by 74 and 60% in A. scabra and A. stolonifera, respectively, compared to heat-stressed roots with water only. Furthermore, A. scabra root respiration rates were significantly lower than non-stressed roots incubated in water only. The results suggest that the alternative respiratory pathway was more involved in the respiratory activities for root tissues of A. scabra than that of A. stolonifera under heat stress. Histochemical staining for O2 - and H2O2 in SNP and SHAM-incubated roots demonstrated variable accumulation of ROS resulting from changes in cytochrome or alternative respiration rates. A. stolonifera roots with increased cytochrome respiratory rates and less-active alternative respiration visually depicted an increased staining intensity by NBT for O2 - (Fig 9A to 9L) and DAB for H2O2 (Fig 10A to 10L) as compared to A. scabra roots.
Fig 8. Root respiration rate for A. stolonifera and A. scabra under heat stress condition as affected by SNP or SHAM.
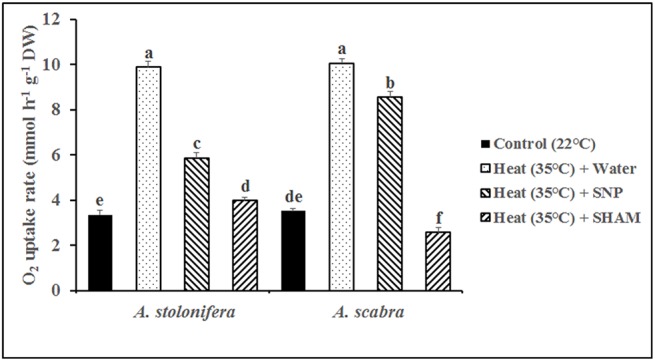
Data shown are the mean ± SE of four biological replicates. Different letters atop bars indicate significant differences exist at the P ≤ 0.05 level.
Fig 9. Histochemical staining of A. stolonifera (A to F) and A. scabra (G to L) root tips under control and heat stress conditions using NBT.
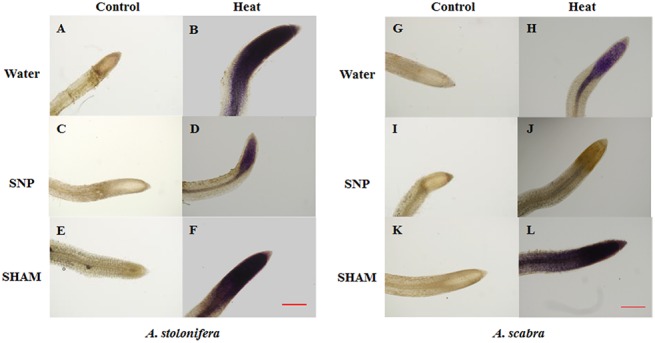
Bar represents 100 μm.
Fig 10. Histochemical staining of A. stolonifera (A to F) and A. scabra (G to L) root tips under control and heat stress conditions using DAB.
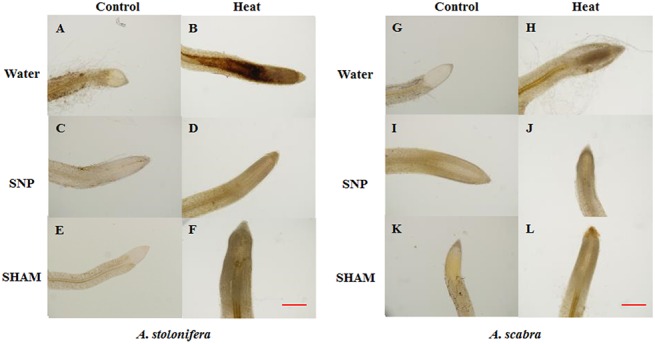
Bar represents 100 μm.
Discussion
It has been widely recognized that there exists large genetic variability in how roots of different plant species tolerate prolonged period of heat stress, though the contributing underlying mechanisms are not well understood [4]. The current study performed comparative analysis for roots of heat-tolerant A. scabra and heat-sensitive A. stolonifera to better describe specific mechanisms contributing to root thermotolerance in cool-season grasses. The commonly-used indicators of cellular membrane stability and integrity (EL and MDA, respectively) showed that A. scabra roots sustained less membrane damage compared to A. stolonifera following heat stress treatment. Changes in antioxidant activities and respiratory patterns may prevent or mitigate ROS accumulation to protect cellular membranes and enhance plant root thermotolerance, as discussed below.
Heat-induced ROS formation is a major factor contributing to cellular damages throughout various tissues and organs of many plant species [49]. ROS production results from excessive reductions occurring during respiratory electron transport inducing oxidative damage to nucleic acids, lipids, and proteins [7, 9, 50]. Heat-tolerant A. scabra accumulated significantly less H2O2 and O2 - in roots whereas ROS content was significantly higher in A. stolonifera roots following heat stress treatment of the current study. The ability of A. scabra to avoid or minimize H2O2 and O2 -accumulation in roots may be dependent upon enhanced antioxidant capabilities and/or utilization of efficient respiratory pathways limiting ROS during periods of heat stress.
The plant antioxidant defense system is composed of non-enzymatic (ASA and GSH) and enzymatic (SOD, APX, CAT, POD, GR, MR, and DR) components which effectively scavenge and disable ROS throughout the plant [3]. Root ASA and GSH content did not differ between A. scabra and A. stolonifera responding to heat stress suggesting that non-enzymatic antioxidants were either ineffective or not utilized to rid the roots of ROS during heat stress treatment of the current study. Root SOD, POD, CAT, APX, GR, MR, and DR enzyme activities as well as SOD and POD transcript abundances were significantly higher in A. scabra compared to A. stolonifera following heat stress. However, APX, GR, MR, and DR activities showed opposite trends compared to the respective transcript levels for each enzyme which may result from significant post translation modification [51]. Another important reason for this discrepancy is the lack of gene family information in these two species. The current knowledge of ROS scavenging-related genes are from previous reports of EST database, which contains incomplete information of Agrostis stolonifera genome [52]. In fact, there is no report on the classification and dissection of ROS scavenging enzyme transcripts in Agrostis spp., which calls for high-throughput sequencing and annotation efforts in future. Nevertheless, the results suggest that enhanced antioxidant activity, particularly for SOD, POD, and CAT, serve important roles for ROS scavenging and cellular maintenance in heat-tolerant A. scabra roots whereas non-enzymatic components do not.
In addition to the carbon metabolism and energy production needed to support a myriad of plant growth functions, respiratory metabolism may also serve as a major ROS source if left unchecked by various regulatory pathways [9, 53]. Abiotic stressors such as heat, drought, and salinity may increase plant respiration rates and contribute largely towards increased ROS production and accumulation [54–56]. The alterative respiratory pathway involves alternative oxidase (AOX) accepting electrons from ubiquinone and reducing oxygen to water preventing over-reduction of accumulated ubiquinone when cytochrome respiration is otherwise inhibited or restricted by stress [57–59]. Root cytochrome and alternative respiration rates increased for both grass species responding to heat stress in the current study. Incubation in SNP or SHAM to specifically inhibit cytochrome or alternative respiration, respectively, revealed that A. scabra roots maintained lower cytochrome and higher alternative respiration rates while A. stolonifera had higher cytochrome and lower alternative respiration rates following heat stress treatment. Minimizing the ROS-producing cytochrome respiratory pathway may contribute to the differential root thermotolerance observed between A. scabra and A. stolonifera [60]. Histochemical staining of roots treated with SNP or SHAM further confirmed that ROS were reduced when cytochrome respiration was suppressed in Agrostis spp. responding to heat stress treatment.
Respiration inhibitors, such as SNP and SHAM, have been utilized to study the relationship between cytochrome and alternative respiratory pathways in other plant species [61, 62]and have also been used to study the role of alternative respiration in NO signaling [63, 64]. The cytochrome respiration activity in isolated soybean mitochondria was inhibited by the addition of NO solution, while alternative respiration activity was not [30]. Arabidopsis thaliana cell cultures incubated with NO donor had increased AOX1a expression and alternative respiration rates [65]. Whether or not AOX expression and alternative respiration are stimulated in cool-season grasses treated with NO deserves further attention in future research. Nevertheless, the results suggest that the collective effects of alternative respiration limitingH2O2 and O2 - production rates plus enzymatic antioxidants significantly detoxifying ROS contribute to superior root thermotolerance in A. scabra.
In summary, A. scabra roots exhibited superior heat tolerance compared to A. stolonifera, as demonstrated through physiological and biochemical analysis. The superior root thermotolerance may be due to a highly-efficient enzymatic antioxidant defense system detoxifying ROS from plant roots and active alternative respiration suppressing ROS production. Identifying and characterization of the specific mechanisms of antioxidant scavenging systems is of great benefits for future heat-screening efforts for developing heat-tolerant temperate grass species through genetic modification or molecular breeding.
Abbreviations
- AOX
alternative oxidase
- APX
ascorbate peroxidase
- ASA
ascorbate
- CAT
catalase
- DAB
3-diaminobenzinidine
- DR
dehydroascorbate reductase
- DTNB
dithiobis-2-nitrobenzoic acid
- DTT
dithiothreitol
- EDTA
ethylenediaminetetraacetic acid
- EL
electrolyte leakage
- GR
glutathione reductase
- GSH
glutathione
- H2O2
hydrogen peroxide
- MDA
malondialdehyde
- MR
monodehydroascorbate reductase
- NBT
nitroblue tetrozolium
- NEM
N-ethylmaleimide
- NO
nitrogen oxide
- O2-
superoxide
- PAR
photosynthetically active radiation
- POD
peroxidase
- PVP
polyvinylpyrrolidone
- qRT-PCR
quantitative reverse transcriptase polymerase chain reaction
- RH
relative humidity
- ROS
reactive oxygen species
- SHAM
salicylhydroxamic acid
- SNP
sodium nitroprusside
- SOD
superoxide dismutase
- TBA
thiobarbituric acid
- TCA
trichloroacetic acid
- XTT
3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrozolium-5-carboxanilide inner salt
Data Availability
All relevant data are within the paper.
Funding Statement
The authors have no support or funding to report.
References
- 1. Foyer CH, Descourvieres P, Kunert KJ. Protection against oxygen radicals: an important defense mechanism studied in transgenic plants. Plant Cell Environ. 1994; 17(5):507–23. [Google Scholar]
- 2. Vacca RA, de Pinto MC, Valenti D, Passarella S, Marra E, De Gara L. Production of reactive oxygen species, alteration of cytosolic ascorbate peroxidase, and impairment of mitochondrial metabolism are early events in heat shock-induced programmed cell death in tobacco Bright-Yellow 2 cells. Plant Physiol. 2004; 134(3):1100–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Suzuki N, Mittler R. Reactive oxygen species and temperature stresses: A delicate balance between signaling and destruction. Physiol Plant. 2006(126):45–51. [Google Scholar]
- 4. Wahid A, Gelani S, Ashraf M, Foolad M. Heat tolerance in plants: An overview. Environ Exp Bot. 2007; 61(3):199–223. [Google Scholar]
- 5. Asada K, Takahashi M. Production and scavenging of active oxygen in chloroplasts In: Kyle DJ, Osmond CB, Arntzen C, editors. Photoinhibition. Amsterdam: Elsevier; 1987. pp. 227–87. [Google Scholar]
- 6. Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002; 7(9):405–10. [DOI] [PubMed] [Google Scholar]
- 7. Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004; 55:373–99. [DOI] [PubMed] [Google Scholar]
- 8. Moller IM, Jensen PE, Hansson A. Oxidative modifications to cellular components in plants. Annu Rev Plant Biol. 2007; 58:459–81. [DOI] [PubMed] [Google Scholar]
- 9. Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. PPB / Societe francaise de physiologie vegetale. 2010; 48(12):909–30. [DOI] [PubMed] [Google Scholar]
- 10. Noctor G, Foyer CH. Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol. 1998; 49:249–79. [DOI] [PubMed] [Google Scholar]
- 11. Bowler C, Montagu MV, Inze D. Superoxide dismutase and stress tolerance. Annu Rev Plant Physiol Plant Mol Biol. 1992; 43(1):83–116. [Google Scholar]
- 12. Asada K. Ascorbate peroxidase–a hydrogen peroxide-scavenging enzyme in plants. Physiol Plant. 1992; 85(2):235–41. [Google Scholar]
- 13. Willekens H, Chamnongpol S, Davey M, Schraudner M, Langebartels C, Van Montagu M, et al. Catalase is a sink for H2O2 and is indispensable for stress defense in C3 plants. EMBO J. 1997; 16(16):4806–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chaitanya KV, Sundar D, Masilamani S, Reddy AR. Variation in heat stress-induced antioxidant enzyme activities among three mulberry cultivars. Plant Growth Regul. 2001; 36(2):175–80. [Google Scholar]
- 15. Bian S, Jiang Y. Reactive oxygen species, antioxidant enzyme activities and gene expression patterns in leaves and roots of Kentucky bluegrass in response to drought stress and recovery. Sci Hort. 2009; 120(2):264–70. [Google Scholar]
- 16. He Y, Huang B. Differential responses to heat stress in activities and isozymes of four antioxidant enzymes for two cultivars of Kentucky bluegrass contrasting in heat tolerance. J Amer Soc Hort Sci. 2010; 135(2):116–24. [Google Scholar]
- 17. Zhang J, Kirkham MB. Antioxidant responses to drought in sunflower and sorghum seedlings. New Phytol. 1996(132):361–73. [DOI] [PubMed] [Google Scholar]
- 18. Zhang X, Schmidt R. Hormone-containing products' impact on antioxidant status of tall fescue and creeping bentgrass subjected to drought. Crop Sci. 2000; 40(5):1344–9. [Google Scholar]
- 19. Huang B, Rachmilevitch S, Xu J. Root carbon and protein metabolism associated with heat tolerance. J Exp Bot. 2012; 63(9):3455–65. 10.1093/jxb/ers003 [DOI] [PubMed] [Google Scholar]
- 20. Yamamoto Y, Kobayashi Y, Devi SR, Rikiishi S, Matsumoto H. Aluminum toxicity is associated with mitochondrial dysfunction and the production of reactive oxygen species in plant cells. Plant Physiol. 2002; 128(1):63–72. [PMC free article] [PubMed] [Google Scholar]
- 21. Rhoads DM, Umbach AL, Subbaiah CC, Siedow JN. Mitochondrial reactive oxygen species. Contribution to oxidative stress and interorganellar signaling. Plant Physiol. 2006; 141(2):357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Navrot N, Rouhier N, Gelhaye E, Jacquot J-P. Reactive oxygen species generation and antioxidant systems in plant mitochondria. Physiol Plant. 2007; 129(1):185–95. [Google Scholar]
- 23. Atkin OK, Edwards EJ, Loveys BR. Response of root respiration to changes in temperature and its relevance to global warming. New Phytol. 2000(147):141–54. [Google Scholar]
- 24. Konigshofer H, Tromballa HW, Loppert HG. Early events in signaling high-temperature stress in tobacco BY2 cells involve alterations in membrane fluidity and enhanced hydrogen peroxide production. Plant Cell Environ. 2008; 31(12):1771–80. 10.1111/j.1365-3040.2008.01880.x [DOI] [PubMed] [Google Scholar]
- 25. Savicka M, Skute N. Effects of high temperature on malondialdehyde content, superoxide production and growth changes in wheat seedlings (Triticum aestivum L.). Ekologija. 2010; 56(1):26–33. [Google Scholar]
- 26. Maxwell DP, Wang Y, Macintosh L. The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc Natl Acad Sci. 1999; 96(14):8271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Halliwell B, Gutteridge J. The chemistry of free radicals and related ‘reactive species’. Free Radic Biol Med. 1999; 3. [Google Scholar]
- 28. Purvis AC, Shewfelt RL. Does the alternative pathway ameliorate chilling injury in sensitive plant tissues? Physiol Plant. 1993; 88(4):712–8. [DOI] [PubMed] [Google Scholar]
- 29. Wagner AM, Krab K. The alternative respiration pathway in plants: Role and regulation. Physiol Plant. 1995; 95(2):318–25. [Google Scholar]
- 30. Millar AH, Day DA. Nitric oxide inhibits the cytochrome oxidase but not the alternative oxidase of plant mitochondria. FEBS lett. 1996; 398(2):155–8. [DOI] [PubMed] [Google Scholar]
- 31. Wang H, Huang J, Bi Y. Induction of alternative respiratory pathway involves nitric oxide, hydrogen peroxide and ethylene under salt stress. Plant Signal Behav. 2010; 5(12):1636–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cvetkovska M, Vanlerberghe GC. Alternative oxidase modulates leaf mitochondrial concentrations of superoxide and nitric oxide. New Phytol. 2012; 195(1):32–9. 10.1111/j.1469-8137.2012.04166.x [DOI] [PubMed] [Google Scholar]
- 33. Gupta KJ, Shah JK, Brotman Y, Jahnke K, Willmitzer L, Kaiser WM, et al. Inhibition of aconitase by nitric oxide leads to induction of the alternative oxidase and to a shift of metabolism towards biosynthesis of amino acids. J Exp Bot. 2012; 63(4):1773–84. 10.1093/jxb/ers053 [DOI] [PubMed] [Google Scholar]
- 34. Stout RG, Al-Niemi TS. Heat-tolerant flowering plants of active geothermal areas in Yellowstone National Park. Ann Bot. 2002; 90(2):259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rachmilevitch S, Lambers H, Huang B. Root respiratory characteristics associated with plant adaptation to high soil temperature for geothermal and turf-type Agrostis species. J Exp Bot. 2006; 57(3):623–31. [DOI] [PubMed] [Google Scholar]
- 36. Rachmilevitch S, Lambers H, Huang B. Short-term and long-term root respiratory acclimation to elevated temperatures associated with root thermotolerance for two Agrostis grass species. J Exp Bot. 2008; 59(14):3803–9. 10.1093/jxb/ern233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xu C, Huang B. Root proteomic responses to heat stress in two Agrostis grass species contrasting in heat tolerance. J Exp Bot. 2008; 59(15):4183–94. 10.1093/jxb/ern258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xu C, Huang B. Differential proteomic response to heat stress in thermal Agrostis scabra and heat-sensitive Agrostis stolonifera . Physiol Plant. 2010; 139(2):192–204. 10.1111/j.1399-3054.2010.01357.x [DOI] [PubMed] [Google Scholar]
- 39. Xu Y, Du HM, Huang BR. Identification of metabolites associated with superior heat tolerance in thermal bentgrass through metabolic profiling. Crop Sci. 2013; 53(4):1626–35. [Google Scholar]
- 40. Hoagland DR, Arnon DI. The water-culture method for growing plants without soil. Circular California Agricultural Experiment Station. 1950; 347(2nd edit). [Google Scholar]
- 41. Blum A, Ebercon A. Cell-membrane stability as a measure of drought and heat tolerance in wheat. Crop Sci. 1981; 21(1):43–7. [Google Scholar]
- 42. Heath RL, Packer L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Archives of biochemistry and biophysics. 1968; 125(1):189–98. [DOI] [PubMed] [Google Scholar]
- 43. Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 1997; 11(6):1187–94. [Google Scholar]
- 44. Dunand C, Crèvecoeur M, Penel C. Distribution of superoxide and hydrogen peroxide in Arabidopsis root and their influence on root development: possible interaction with peroxidases. New Phytol. 2007; 174(2):332–41. [DOI] [PubMed] [Google Scholar]
- 45. Sutherland MW, Learmonth BA. The tetrazolium dyes MTS and XTT provide new quantitative assays for superoxide and superoxide dismutase. Free Radical Res. 1997; 27(3):283–9. [DOI] [PubMed] [Google Scholar]
- 46. Zhou B, Wang J, Guo Z, Tan H, Zhu X. A simple colorimetric method for determination of hydrogen peroxide in plant tissues. Plant Growth Regul. 2006; 49(2–3):113–8. [Google Scholar]
- 47. Guri A. Variation in glutathione and ascorbic acid content among selected cultivars of Phaseolus Vulgaris prior to and after exposure to ozone. Can J Plant Sci. 1983; 63(3):733–7. [Google Scholar]
- 48. Ma Y-H, Ma F-W, Zhang J-K, Li M-J, Wang Y-H, Liang D. Effects of high temperature on activities and gene expression of enzymes involved in ascorbate–glutathione cycle in apple leaves. Plant Sci. 2008; 175(6):761–6. [Google Scholar]
- 49. Anderson JA. Catalase activity, hydrogen peroxide content and thermotolerance of pepper leaves. Sci Hortic. 2002; 95(4):277–84. [Google Scholar]
- 50. Davidson JF, Schiestl RH. Mitochondrial respiratory electron carriers are involved in oxidative stress during heat stress in Saccharomyces cerevisiae . Mol Cell Biol. 2001; 21(24):8483–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yannarelli GG, Fernandez-Alvarez AJ, Santa-Cruz DM, Tomaro ML. Glutathione reductase activity and isoforms in leaves and roots of wheat plants subjected to cadmium stress. Phytochem. 2007; 68(4):505–12. [DOI] [PubMed] [Google Scholar]
- 52. Rotter D, Bharti A, Li H, Luo C, Bonos S, Bughrara S, et al. Analysis of EST sequences suggests recent origin of allotetraploid colonial and creeping bentgrasses. Mol Genet Genomics. 2007; 278(2):197–209. [DOI] [PubMed] [Google Scholar]
- 53. Juszczuk I, Malusà E, Rychter AM. Oxidative stress during phosphate deficiency in roots of bean plants (Phaseolus vulgaris L.). J Plant Physiol. 2001; 158(10):1299–305. [Google Scholar]
- 54. Naya L, Ladrera R, Ramos J, Gonzalez EM, Arrese-Igor C, Minchin FR, et al. The response of carbon metabolism and antioxidant defenses of alfalfa nodules to drought stress and to the subsequent recovery of plants. Plant Physiol. 2007; 144(2):1104–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Huve K, Bichele I, Rasulov B, Niinemets U. When it is too hot for photosynthesis: heat-induced instability of photosynthesis in relation to respiratory burst, cell permeability changes and H2O2 formation. Plant Cell Environ. 2011; 34(1):113–26. 10.1111/j.1365-3040.2010.02229.x [DOI] [PubMed] [Google Scholar]
- 56. Redha A, Al-Mansor N, Suleman P, Al-Hasan R, Afzal M. Modulation of antioxidant defenses in Conocarpus lancifolius under variable abiotic stress. Biochem System Ecol. 2012; 43:80–6. [Google Scholar]
- 57. Millenaar FF, Lambers H. The alternative oxidase: in vivo regulation and function. Plant Biol. 2003; 5(1):2–15. [Google Scholar]
- 58. Fiorani F, Umbach AL, Siedow JN. The alternative oxidase of plant mitochondria is involved in the acclimation of shoot growth at low temperature. A study of Arabidopsis AOX1a transgenic plants. Plant Physiol. 2005; 139(4):1795–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ribas-Carbo M, Taylor NL, Giles L, Busquets S, Finnegan PM, Day DA, et al. Effects of water stress on respiration in soybean leaves. Plant Physiol. 2005; 139(1):466–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rachmilevitch S, Xu Y, Gonzalez-Meler MA, Huang BR, Lambers H. Cytochrome and alternative pathway activity in roots of thermal and non-thermal Agrostis species in response to high soil temperature. Physiol Plant. 2007; 129(1):163–74. [Google Scholar]
- 61. Atkin OK, Zhang QS, Wiskich JT. Effect of temperature on rates of alternative and cytochrome pathway respiration and their relationship with the redox poise of the quinone pool. Plant Physiol. 2002; 128(1):212–22. [PMC free article] [PubMed] [Google Scholar]
- 62. Schwarzländer M, Fricker MD, Sweetlove LJ. Monitoring the in vivo redox state of plant mitochondria: Effect of respiratory inhibitors, abiotic stress and assessment of recovery from oxidative challenge. BBA-Bioenergetics. 2009; 1787(5):468–75. 10.1016/j.bbabio.2009.01.020 [DOI] [PubMed] [Google Scholar]
- 63. Beligni MV, Lamattina L. Nitric oxide protects against cellular damage produced by methylviologen herbicides in potato plants. Nitric Oxide. 1999; 3(3):199–208. [DOI] [PubMed] [Google Scholar]
- 64. Ederli L, Morettini R, Borgogni A, Wasternack C, Miersch O, Reale L, et al. Interaction between nitric oxide and ethylene in the induction of alternative oxidase in ozone-treated tobacco plants. Plant Physiol. 2006; 142(2):595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Huang X, von Rad U, Durner J. Nitric oxide induces transcriptional activation of the nitric oxide-tolerant alternative oxidase in Arabidopsis suspension cells. Planta. 2002; 215(6):914–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.


