Abstract
Purpose.
To assess the suitability of human donor corneas maintained in long-term organ culture for the isolation and expansion of viable and functional corneal stromal stem cells (CSSCs). These cells display properties similar to mesenchymal stem cells and demonstrate the ability to reproduce an organized matrix in vitro. Therefore, CSSCs have great potential for the development of cell-based therapies for corneal blindness or stromal tissue bioengineering.
Methods.
Human donor corneas that had been stored either in organ-culture medium (OC) up to 4 weeks (n = 3) or in Optisol medium (OS) up to 6 days (n = 3) were used for isolation of CSSCs and maintained in culture until passage 4. Cell phenotype of isolated CSSCs was assessed with light microscopy and immunocytochemistry (PAX6, CD73, and CD90). PAX6 protein expression was further confirmed with immunoblot analysis.
Results.
A comparison of CSSCs isolated from corneas stored under OC and OS conditions revealed no obvious differences in their morphology. Immunocytochemistry revealed CSSCs from both OC and OS corneas maintained positive staining for PAX6 and mesenchymal stem cell markers CD73 and CD90. Immunoblotting confirmed protein expression of PAX6 in cells from both tissue types.
Conclusions.
Human CSSCs exhibit survival capacity by retaining their phenotype following isolation from long storage, OC corneas. This advantageous property enables the retrieval of CSSCs from OC corneas that are more abundantly available for research than OS-stored corneas. Organ-culture corneas are also often discarded for retrieval of other cell types, such as corneal epithelial and endothelial cells, which require high tissue quality for their preservation.
Keywords: keratocytes, corneal stromal stem cells, corneal organ culture, bioengineering
This study demonstrates that long-term organ culture storage of corneas does not affect the viability and functionality of human corneal stromal stem cells.
Introduction
The cornea is the outermost transparent surface of the eye and acts as our window to the world, refracting light onto the retina enabling healthy vision.1 The stroma is the main component of the cornea and consists of a network of lamellae made up of tightly packed, aligned collagen fibrils.2 This highly ordered organization of collagen fibrils is thought to be essential to corneal transparency.3 Situated between these lamellae are the resident cells of the corneal stroma, the keratocytes.
Keratocytes are mesenchymal-derived cells that remain quiescent for the most part of adult life. Upon activation following injury or infection, keratocytes can adopt a fibroblast or myofibroblast phenotype, producing a disorganized extracellular matrix causing vision disrupting corneal scarring. During normal wound healing these cells apoptose or dedifferentiate back into keratocytes, which remodel the temporary disorganized matrix into a healthy corneal stroma.4 In severe cases of corneal opacity such as limbal stem cell deficiency (LSCD), the myofibroblasts remain activated causing a permanently altered stromal matrix.5,6
Currently, the most common approach to restore vision in scarred corneas is to surgically replace the corneal stroma with allogeneic donor corneal tissue (penetrating keratoplasty). However, alternative approaches are becoming increasingly necessary as donor shortage is a major problem in most countries. The supply of donor corneas in particular is expected to decrease further due to current advances in corrective eye surgery, which renders potential donor corneas unsuitable for transplantation. Techniques that use tissue engineering and regenerative medicine technologies are being developed as alternative approaches to using donor tissue.7,8 There are numerous studies focusing on the development of a tissue engineered human cornea using a variety of substrates populated with cells of the cornea.9–11 However, some stromal cells, particularly keratocytes are difficult to culture in vitro as they quickly differentiate into fibroblasts in response to expansion.12 More recently, a small population of human corneal stromal stem cells (CSSCs) has been identified in the corneal limbal stroma, which display properties similar to mesenchymal stem cells. These were the first human cells to be identified with keratocyte progenitor potential.13 Unlike keratocytes, these cells replicate in vitro and maintain a corneal phenotype over a very high number of population doublings. Previous studies by Du and colleagues14 have demonstrated that these cells have the ability to restore collagen fibril organization and transparency in lumican-deficient mice (a mouse scar model), without immune rejection. These properties make CSSCs an ideal candidate for a direct cell-based therapy of corneal scarring or in the development of a bioengineered corneal stromal equivalent.
Previously, it has been shown that CSSCs can be harvested from fresh donor tissue that is less than 6-days old and stored in Optisol (OS), a medium typically used for donor corneal tissue in the United States.13 This type of hypothermic storage condition maintains the donor cornea at 4°C in a medium supplemented with antibiotics and dehydrating agents such as chondroitin sulfate and dextran which prevent the corneas from swelling. Optisol-stored corneas have been shown to have minimal damage to the epithelium when stored up to 6 days with extensive epithelial loss observed beyond 10 days of storage.15 In Europe however, the corneoscleral donor rims remaining after penetrating keratoplasty are commonly stored in organ culture (OC) medium at temperatures of 31°C to 37°C for up to 4 weeks.16 Tissue preserved in this way, although useful for transplantation, is often disregarded for epithelial and endothelial cell isolation for in vitro cultivation. This is because generally these cell types do not survive longer storage culture conditions. Currently, OS-stored corneal rims are typically used to harvest CSSCs and human limbal epithelial stem cells (HLESCs) for engineering cultured corneal epithelium.17
Given the prevalence of OC storage in the European Union, the purpose of this study was to assess the suitability of donor corneas maintained in long-term OC for the isolation and expansion of viable and functional CSSCs. These tissues are abundantly available, but are potentially overlooked due to extended storage times compared with OS-stored corneas. Cell phenotype was assessed by comparing morphology of cells in culture, expression of PAX6 protein, (a homeobox transcription factor specific for eye development), and expression of the mesenchymal stem cell markers CD73 and CD90 (specific surface antigen markers used to define mesenchymal stem cells).18 Differentiation of isolated CSSCs into keratocytes was also investigated to confirm their progenitor phenotype by assessing cells in culture for keratocyte marker expression.
Methods
Ethical Conduct of Research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. For investigations involving human subjects, informed consent has been obtained from the participants involved.
Human CSSCs Culture and Characterization
Isolation of Human CSSCs.
Human CSSCs were isolated from six donor corneas using a method similar to that described by Du and colleagues.13 The ages of corneal donors ranged from 50–75 years old from both males and females (Table). At the time of isolation, three donor corneas had been stored in OC medium up to 4 weeks at ambient temperature and the remaining three donor corneas had been stored in OS, at 4°C, for up to 6 days. Briefly, the superficial corneal limbal region was dissected into small fragments and digested in Dulbecco's modified Eagle's medium (DMEM; Sigma-Aldrich, Dorset, UK) supplemented with 50 ug/mL gentamicin (Gibco, Life Technologies, Paisley, UK), Penicillin-Streptomycin solution (1×; Gibco, Life Technologies, Paisley, UK), containing collagenase type L (0.5 mg/mL; Sigma-Aldrich) and incubated at 37°C overnight. Primary stromal cells were plated into flasks coated with fibronectin-collagen (FNC; Athena Enzyme System, Baltimore, MD, USA) and cultured in CSSC medium (modified from the method described by Jiang and colleagues19) consisting of a mixture of DMEM low glucose (Gibco, Life Technologies) and MCDB-201 (Sigma-Aldrich) medium, supplemented with 2% fetal bovine serum (Invitrogen, Life Technologies, Paisley, UK), 10 ng/mL epidermal growth factor (Sigma-Aldrich), 10 ng/mL platelet-derived growth factor (PDGF-BB; R&D Systems, Abingdon, Oxford, UK), Insulin-Transferrin-Selenium (ITS) solution (1×; Gibco, Life Technologies), 0.1 mM ascorbic acid-2-phosphate (Sigma-Aldrich), 10−8 M dexamethasone (Sigma-Aldrich), penicillin-streptomycin solution (1×; Corning Cellgro), 50 ug/mL gentamicin (Gibco, Life Technologies), and 100 ng/mL cholera toxin (Sigma-Aldrich). Cells were trypsinised and subcultured when colonies of small polygonal cells were visible. Cultures were not allowed to reach confluence.
Table.
Donor Information of OS and OC Corneas
|
Donor |
1 |
2 |
3 |
4 |
5 |
6 |
| Storage medium | OS | OS | OS | OC | OC | OC |
| Age | 58 | 74 | 50 | 62 | 51 | 75 |
| Sex | F | M | M | M | F | M |
The age of donors ranged from 50–75 years and tissue was from both male and female donors.
Assessing Morphology of Human CSSCS.
Cultures of human CSSCs were assessed using light microscopy. Photographs of CSSCs from OC- and OS-stored corneas were taken at every passage to compare the morphology of cells.
Immunocytochemistry of Human CSSCs.
Cells were plated onto permanox slides at a density of 40,000 cells per slide, cultured for a further 3 to 4 days in CSSC media, then fixed with 4% paraformaldehyde (at passage 2–4) for 15 minutes. Cells were subsequently washed with PBS (Invitrogen, Life Technologies) and blocked for 1 hour with 5% goat serum in PBS with 0.25% Triton X-100 (Sigma-Aldrich) at room temperature. Following a further wash with PBS, samples were incubated overnight at 4°C with the primary antibody diluted in 2% goat serum with PBS (PAX6; Covance, Princeton, NJ, USA) at a concentration of 1:70, CD90 and CD73 at a concentration of 10 μg/mL). Samples were washed three times with PBS prior to incubation with the secondary goat anti-rabbit 594 Alexa Fluor antibody (1:500 dilution; Invitrogen) and FITC-labelled phalloidin (1:1000 concentration; Sigma-Aldrich), which binds to the actin cytoskeleton. These were incubated for 1 hour at room temperature in the dark. To visualize nuclei, samples were mounted underneath coverslips in Vectashield mounting medium containing 4′6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Inc., Burlingame, CA, USA). Samples were viewed and analyzed on a confocal Zeiss LSM 710 microscope (Zeiss, Cambridge, UK).
Immunoblotting of CSSCS.
Protein was extracted from CSSCs samples from both cultures using a lysis buffer and a protease inhibitor. Samples were then separated on a 4% to 12% Bis-Tris gel (NuPAGE gel; Invitrogen, Paisley, UK). Appropriate molecular weight markers were placed into the gel together with a positive control for PAX6. Gels were prepared for Western blotting followed by electrotransferral to polyvinylidine difluoride (PVDF) membranes. PAX6 was identified with incubation of PAX6 antibody (Covance) at 1:70 concentrations. Visualization of immunoreactivity was achieved using an immunodetection kit (ECL Western Blotting immunodetection reagents; Amersham Biosciences, London, UK).
Keratocyte Culture and Characterization
Differentiation of CSSCS Into Keratocytes.
Corneal stromal stem cells at passage 2 through 4 were cultured for 3 weeks in keratocyte differentiation medium (KDM) consisting of Advanced DMEM (Sigma-Aldrich), 10 ng/mL fibroblast growth factor (Sigma-Aldrich), 0.1 mM L-ascorbic acid-2-phosphate (Sigma-Aldrich), 50 ug/mL gentamicin (Invitrogen), Penicillin-Streptomycin solution (1×; Corning Cellgro), GlutaMAX (1×; Invitrogen, Life Technologies). Media was replaced every 2 to 3 days.
Assessing Morphology of Keratocytes.
To observe changes in the morphology of CSSCs as they differentiate into keratocytes, cultures were assessed with light microscopy. Photographs of cultures were taken over 3 weeks.
Immunocytochemistry of Keratocytes.
Keratocyte markers keratocan, lumican, and ALDH1A1 were used to confirm keratocyte phenotype. Cells were seeded onto permanox slides at a seeding density of 40,000 cells per slide, cultured, and subsequently fixed with 4% paraformaldehyde for 15 minutes. Cells were permeabilized with 0.5% Triton in PBS for 15 minutes at room temperature. Samples were treated with 0.4 U/mL chondroitinase ABC (Sigma-Aldrich) for 1 hour at 37°C followed by treatment with 0.4 U/mL endo-β-galactosidase (Sigma-Aldrich) for 1 hour at 37°C. Cells were washed with PBS and incubated with blocking agent (2% BSA in PBS) for 30 minutes at room temperature. Primary antibodies anti-Keratocan, anti-Lumican (1:20 concentration; Sigma-Aldrich), and anti-ALDH1A1 (1:50 concentration; Abcam, Cambridge, UK) were prepared in blocking agent and slides incubated overnight at 4°C. These were rinsed three times with PBS prior to incubating with secondary goat anti-rabbit 594 Alexa Fluor antibody (1:500 dilution; Invitrogen Ltd.), and FITC-labelled phalloidin (1:1000 concentration; Sigma-Aldrich) for 1 hour at room temperature in the dark. Slides were mounted underneath coverslips in Vectashield mounting medium containing DAPI (Vector Laboratories Inc.). Samples were viewed and analyzed on a confocal Zeiss LSM 710 microscope.
Results
Characterization of CSSCs
Morphology of CSSCS.
There was no observable difference in the morphology of CSSCs isolated and cultured from organ culture and OS-stored corneas. The Table contains all donor information including age, sex, time of storage and type of storage for each donor used. Cells from both cultures appeared small and polygonal and grew in sparsely arranged colonies (Fig. 1). Other stromal cells including more dendritic-shaped cells indicative of the keratocyte phenotype and longer spindle-shaped fibroblasts were visible in both primary cultures but disappeared at later passages.
Figure 1.

Light microscope images of human CSSCs (passage 2) isolated from (A) OS- or (B) OC-stored corneas. Corneal stromal stem cells (C) appear small and square among some keratocyte (K) and fibroblast (F) cells. Scale bar: 200 μm.
Immunocytochemistry of Human CSSCS.
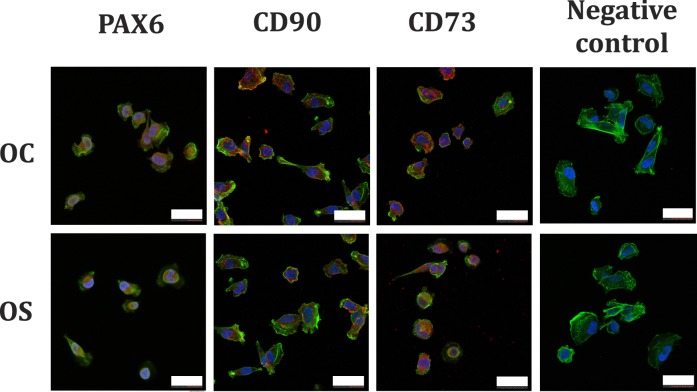
Immunocytochemistry revealed CSSCs (up to passage 4) expressed positive nuclear staining for PAX6 (positive PAX6 staining in CSSCs was expected since PAX6 is a homeobox transcription factor expressed in embryonic ocular precursor cells and epithelial cells). Figure 2 contains confocal micrographs illustrating positive PAX6 expression in cultured CSSCs from both OC- and OS-stored corneas. Expression of CD73 and CD90 (mesenchymal stem cell markers) were observed in CSSCs isolated from corneas in both storage conditions (Fig. 2). Expression of PAX6, CD73, and CD90 was observed in cells with a small polygonal morphology, indicative of the mesenchymal stem cell phenotype.
Figure 2.
Confocal micrographs of human CSSCs isolated from OS- or OC-stored corneas. Cells display positive nuclear expression of PAX6 (gene expressed in early eye development), and CD73 and CD90 (mesenchymal stem cell markers). Scale bars: 40 μm.
Immunoblotting of Human CSSCS.
PAX6 production was demonstrated by immunoblotting of proteins collected from CSSCs isolated from both OC- and OS-stored corneas (Fig. 3). A band showing the positive control of PAX6 can be seen at the predicted weight of 47 kDa. Bands from CSSCs were slightly lower than 47 kDa as observed in other studies by D'elia and colleagues20 that showed a similar pattern of PAX6 expression in immunoblots and could be due to post-translational modifications.
Figure 3.
Immunoblot of PAX6 protein in CSSCs isolated from OC and OS donor corneas. The first lane shows a MagicMark standard (M). The last 3 lanes show expression from cells known to be positive for PAX6 protein; human limbal fibroblasts (HLF), limbal epithelial cells (LEC), and a positive (+VE) control of PAX6, consisting of rat brain lysate, respectively.
Differentiation Potential of Human CSSCS Into Keratocytes.
To confirm differentiation potential, CSSCs were cultured under serum-free conditions to induce their differentiation into keratocytes. Figure 4 includes light microscopy images illustrating the morphology of CSSCs cultured in KDM for 1 and 3 weeks. The small, polygonal morphology characteristic of CSSCs changed to a more dendritic morphology typical of keratocytes. Immunocytochemistry analysis (Fig. 5) revealed CSSCs gain keratocyte characteristics through exposure to KDM. Cells cultured for 3 weeks in KDM exhibited positive expression of keratocyte markers ALDH1A1, keratocan, and lumican. Similar to recent studies by Stagos and colleagues,21 ALDH1A1 was expressed in the nucleus of keratocytes.
Figure 4.
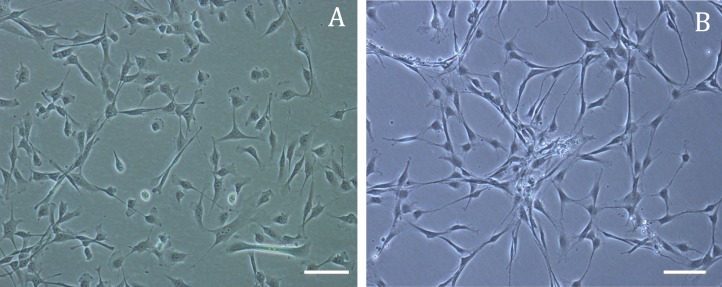
Light microscopy images illustrating change in morphology of CSSCs to keratocytes. (A) Corneal stromal stem cells cultured in CSSC media (passage 2), (B) CSSCs cultured in KDM for 3 weeks. Scale bars: 100 μm.
Figure 5.

Confocal micrographs of CSSCs that have differentiated into keratocytes. Corneal stromal stem cells were cultured in KDM for 3 weeks prior to seeding and fixing on permanox slides. Positive marker expression for ALDH1A1, Keratocan, and Lumican are shown in red. (FITC Phalloidin staining cytoplasm in green, DAPI staining nuclei in blue). Scale bars: 20 μm.
Discussion
In this study, we have demonstrated that CSSCs can be isolated reliably from stored OC donor corneas. This capacity to survive such long-term storage conditions has not been previously reported for CSSCs. Since human CSSCs have the ability to reorganize a disorganized matrix typical of corneal scarring,14 they hold great potential for bioengineering applications but have only been isolated from fresh OS-stored corneas. This finding is of importance as cells can now be isolated from OC-stored donor corneas, which are more readily available than OS-stored corneas. Organ cultured–stored corneas are often disregarded for ex vivo expansion of other cell types such as corneal epithelial and endothelial cells, which require fresher tissue quality for their preservation. There is also the potential to culture a sufficient number of cells from one OC-stored donor cornea to treat multiple patients with corneal scarring.
Our results revealed no obvious differences between CSSCs isolated from OS- and OC-stored corneas when comparing cell morphology, expression of PAX6, CD73, or CD90. Studies by Du and colleagues13 in which human CSSCs were isolated from fresh OS-stored donor corneas, demonstrated that CSSCs exhibited a morphology and positive PAX6 expression comparable with that observed in our study. Since PAX6 is a transcription factor essential for ocular development and present in most embryonic ocular tissues but not adult keratocytes,22 positive expression in isolated cells confirms the CSSC phenotype from both OC- and OS-stored corneas. Positive expression of mesenchymal stem cell markers CD73 and CD90, further confirms the CSSC phenotype. In addition, CSSCs isolated from OC- and OS-stored corneas were successfully shown to differentiate into keratocytes by culturing in serum-free media. These differentiated cells exhibited positive expression of keratocyte markers keratocan, lumican, and ALDH1A1, similar to that observed by Park and colleagues,23 confirming the keratocyte phenotype and demonstrating their progenitor potential.
We hypothesize that this survival capacity demonstrated by CSSCs isolated from OC-stored corneas may be attributed to their location, residing inside the matrix where they are better protected from postmortem changes than the epithelial and endothelial corneal surface layers. Keratocytes are considered to remain quiescent throughout adult life24 and, thus, have very low metabolic activity. Therefore, we could postulate that CSSCs, as the progenitor cells of keratocytes, may have similar metabolic levels and require less energy for survival. This property may give CSSCs the advantage over other cell types, enabling them to survive extended storage conditions.
Importantly, the OC corneas in our study were maintained at ambient temperature rather than 31°C to 37°C for the majority of the storage duration. Donor corneas are typically placed into OC medium following retrieval and stored at 31°C under GMP conditions, prior to being used for transplantation. Following penetrating keratoplasty, the remaining corneoscleral rim is placed back into OC medium in the operating theatre but stored at ambient temperature to avoid compromising the sterile GMP conditions. From here on, the donor tissue is maintained at ambient temperature until used for research purposes. The survival of CSSCs under these storage conditions can be compared with the survival of human limbal epithelial stem cells (HLESCs) in studies by Raeder and colleagues,25 which showed that OC storage of cultured HLESCs at ambient temperature was superior to OC storage at 31°C and Optisol-GS (Bausch & Lomb, Rochester, NY, USA) storage at 5°C.
Conclusions
This novel finding that CSSCs can be isolated from OC corneas and survive long-term storage enables greater use of donor corneal limbal rims that would otherwise be discarded. It also highlights a greater advantage CSSCs have over other cell types including corneal epithelial and endothelial cells, which require higher tissue quality for their preservation for ex vivo expansion.
Acknowledgments
Supported by funding received from The Special Trustees of Moorfields Eye Hospital in addition to partial funding from the National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital, NHS Foundation Trust, and UCL Institute of Ophthalmology.
Disclosure: A.K. Kureshi, None; J.L. Funderburgh, None; J.T. Daniels, None
References
- 1. Notara M, Alatza A, Gilfillan J, et al. In sickness and in health: corneal epithelial stem cell biology, pathology and therapy. Exp Eye Res. 2010; 90: 188–195. [DOI] [PubMed] [Google Scholar]
- 2. Freegard TJ. The physical basis of transparency of the normal cornea. Eye (Lond). 1997; 11( pt. 4); 465–471. [DOI] [PubMed] [Google Scholar]
- 3. Maurice DM. The structure and transparency of the cornea. J Physiol. 1957; 136: 263–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wilson SE, Chaurasia SS, Medeiros FW. Apoptosis in the initiation, modulation and termination of the corneal wound healing response. Exp Eye Res. 2007; 85: 305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Espana EM, Ti SE, Grueterich M, Touhami A, Tseng SC. Corneal stromal changes following reconstruction by ex vivo expanded limbal epithelial cells in rabbits with total limbal stem cell deficiency. Br J Ophthalmol. 2003; 87: 1509–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saito T, Nishida K, Sugiyama H, et al. Abnormal keratocytes and stromal inflammation in chronic phase of severe ocular surface diseases with stem cell deficiency. Br J Ophthalmol. 2008; 92: 404–410. [DOI] [PubMed] [Google Scholar]
- 7. Germain L, Carrier P, Auger FA, Salesse C, Guerin SL. Can we produce a human corneal equivalent by tissue engineering? Prog Retin Eye Res. 2000; 19: 497–527. [DOI] [PubMed] [Google Scholar]
- 8. Langer R. Vacanti. JP. Tissue engineering. Science. 1993; 260: 920–926. [DOI] [PubMed] [Google Scholar]
- 9. Krishnan S, Sekar S, Katheem MF, Krishnakumar S, Sastry TP. Fish scale collagen–a novel material for corneal tissue engineering. Artif Organs. 2012; 36: 829–835. [DOI] [PubMed] [Google Scholar]
- 10. Harkin DG, George KA, Madden PW, Schwab IR, Hutmacher DW, Chirila TV. Silk fibroin in ocular tissue reconstruction. Biomaterials. 2011; 32: 2445–2458. [DOI] [PubMed] [Google Scholar]
- 11. Deshpande P, McKean R, Blackwood KA, Senior RA, Ogunbanjo A, Ryan AJ, et al. Using poly(lactide-co-glycolide) electrospun scaffolds to deliver cultured epithelial cells to the cornea. Regen Med. 2010; 5: 395–401. [DOI] [PubMed] [Google Scholar]
- 12. Jester JV, Barry-Lane PA, Cavanagh HD, Petroll WM. Induction of alpha-smooth muscle actin expression and myofibroblast transformation in cultured corneal keratocytes. Cornea. 1996; 15: 505–516. [PubMed] [Google Scholar]
- 13. Du Y, Funderburgh ML, Mann MM, SundarRaj N, Funderburgh JL. Multipotent stem cells in human corneal stroma. Stem Cells. 2005; 23: 1266–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Du Y, Carlson EC, Funderburgh ML, et al. Stem cell therapy restores transparency to defective murine corneas. Stem Cells. 2009; 27: 1635–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Means TL, Geroski DH, L'Hernault N, et al. The corneal epithelium after Optisol-GS storage. Cornea. 1996; 15: 599–605. [PubMed] [Google Scholar]
- 16. Pels L. Organ culture: the method of choice for preservation of human donor corneas. Br J Ophthalmol. 1997; 81: 523–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Levis HJ, Brown RA, Daniels JT. Plastic compressed collagen as a biomimetic substrate for human limbal epithelial cell culture. Biomaterials. 2010; 31: 7726–7737. [DOI] [PubMed] [Google Scholar]
- 18. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006; 8: 315–317. [DOI] [PubMed] [Google Scholar]
- 19. Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002; 418: 41–49. [DOI] [PubMed] [Google Scholar]
- 20. D'elia AV, Puppin C, Pellizzari L, et al. Molecular analysis of a human PAX6 homeobox mutant. Eur J Hum Genet. 2006; 14: 744–751. [DOI] [PubMed] [Google Scholar]
- 21. Stagos D, Chen Y, Cantore M, Jester JV, Vasiliou V. Corneal aldehyde dehydrogenases: multiple functions and novel nuclear localization. Brain Res Bull. 2010; 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Funderburgh ML, Du Y, Mann MM, SundarRaj N, Funderburgh JL. PAX6 expression identifies progenitor cells for corneal keratocytes. FASEB J. 2005; 19: 1371–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Park SH, Kim KW, Chun YS, Kim JC. Human mesenchymal stem cells differentiate into keratocyte-like cells in keratocyte-conditioned medium. Exp Eye Res. 2012; 101: 16–26. [DOI] [PubMed] [Google Scholar]
- 24. Jester JV, Barry PA, Lind GJ, Petroll WM, Garana R, Cavanagh HD. Corneal keratocytes: in situ and in vitro organization of cytoskeletal contractile proteins. Invest Ophthalmol Vis Sci. 1994; 35: 730–743. [PubMed] [Google Scholar]
- 25. Raeder S, Utheim TP, Utheim OA, et al. Effects of organ culture and Optisol-GS storage on structural integrity, phenotypes, and apoptosis in cultured corneal epithelium. Invest Ophthalmol Vis Sci. 2007; 48: 5484–5493. [DOI] [PubMed] [Google Scholar]