Abstract
Purpose.
To identify stem cells in the chamber angle of the monkey eye by detection of 5-bromo-2′-deoxyuridine (BrdU) long-term retention.
Methods.
Four cynomolgus monkeys were treated with BrdU via subcutaneous pumps for 4 weeks. The eyes of two animals were processed immediately thereafter (group 1) while in the other animals, BrdU treatment was discontinued for 4 weeks to allow identification of cells with long-term BrdU retention (group 2). The number of BrdU-positive nuclei was quantified, and the cells were characterized by immunohistochemistry and transmission electron microscopy (TEM).
Results.
The number of BrdU-positive cells was higher at Schwalbe's line covering the peripheral end of Descemet's membrane than in Schlemm's canal (SC) endothelium, trabecular meshwork (TM), and scleral spur (SS). Labeling with BrdU in SC, TM, and SS was less intense and the number of labeled cells was smaller in group 2 than in group 1. In contrast, in cells of Schwalbe's line the intensity of BrdU staining and the number of BrdU-positive cells was similar when group 1 and 2 monkeys were compared with each other, indicating long-term BrdU retention. Cells that were BrdU-positive in Schwalbe's line region stained for the stem cell marker OCT4. Details of a stem cell niche in Schwalbe's line region were identified by TEM.
Conclusions.
We provide evidence for a niche in the Schwalbe's line region harboring cells with long-term BrdU retention and OCT4 immunoreactivity. The cells likely constitute a population of adult stem cells with the capability to compensate for the loss of TM and/or corneal endothelial cells.
Keywords: Schwalbe's line, stem cells, BrdU retention, monkey eye
Schwalbe's line region in the monkey eye harbors cells with long-term BrdU retention and OCT4 immunoreactivity. The cells likely constitute a population of adult stem cells with the capability to compensate for the loss of TM and/or corneal endothelial cells.
Introduction
The cells of the conventional or trabecular outflow pathways, trabecular meshwork (TM) cells and Schlemm's canal (SC) endothelial cells are under constant mechanical stress or strain. A major contributing factor to mechanical load in the trabecular outflow pathways is the immediate neighborhood of the ciliary muscle and its anterior tendons that connect with the posterior parts of the trabecular meshwork.1,2 Experimental studies using cholinergic drugs identified a considerable stretch and distension of the trabecular meshwork outflow pathways during ciliary muscle contraction,3–5 a scenario that likely occurs constantly during a lifetime while the ciliary muscle contracts in accommodation. Another mechanical factor acting in the aqueous humor outflow pathways is the passage of aqueous humor. In response to aqueous humor flow, cells of the SC inner wall partially detach from their underlying extracellular matrix to form characteristic outpouchings into the lumen of SC that have been termed giant vacuoles.1 It seems reasonable to assume that mechanical stress or strain continuously leads to the loss of individual TM or SC cells that need to be replaced in order to guarantee continuous function of the trabecular outflow pathways. At least for the TM, the replacement appears not to be sufficient over the lifetime as a continuous loss of TM cells at a constant rate of 0.56% per year6,7 or at a loss rate of 6000 TM cells per year8 has been shown. The loss of TM cells is higher in the TM of patients with primary open-angle glaucoma (POAG), a factor that has been hypothesized to cause or contribute to the increase in resistance of the trabecular outflow pathways, and to the idea of POAG as a consequence of aging.6
Throughout the body, cells that have been lost because of wear and tear can be replaced by mitosis of neighboring differentiated cells. Indeed, results from autoradiographic studies using 3H-thymidine labeling indicated a slow ongoing rate of DNA synthesis and presumably trabecular cell replication in the TM of the normal eye. In vivo studies showed a 0.1% to 0.4% baseline incorporation rate in the TM of cynomolgus monkeys,9 and a higher (0.82% to 2.17%) in the TM of cats.10 In a study of organ-cultured human eyes, incorporation rates in TM cells of 0.34% to 0.44% were found in control eyes and of 0.59% in laser-treated fellow eyes.11,12
Alternatively, lost cells may be replaced by proliferation and subsequent migration of adult stem cells that typically reside in an adjacent niche. The stem cell niche is thought to constitute an instructive or permissive environment by expressing certain growth factors and/or extracellular matrix molecules.13,14 Adult stem cells are usually defined as proliferative cells that maintain their own numbers (self-maintenance) while dividing a large number of times during which they can produce daughter cells that are capable of differentiating down various lineages (pluripotency).15 Stem cells can also alter their self-maintenance probability to ensure an expansion of stem cell numbers if required following injury (clonogenic capacity). Adult stem cells that are responsible for the maintenance of tissue integrity and cell renewal throughout adulthood have been described for various organs and tissues such as the intestinal epithelium, the epidermis, the corneal epithelium and the hematopoietic system.16–21 In all these tissues, there is a relatively high basic cellular turnover. More recently, adult stem cells were also identified in brain22,23 and retina24 where cell division is extremely rare and the situation is more comparable to that in the TM.
There is evidence that adult stem cells reside somewhere in the trabecular meshwork outflow pathways. Cells that expressed stem cell markers were detected in cell cultures that were initiated following isolation from fresh human TM by fluorescence-activated cell sorting.25 Moreover, primary cultures initiated from human trabecular meshwork contain relatively undifferentiated or progenitor cells which are capable of forming spherical clusters or free-floating spheres that may contain undifferentiated multipotent progenitor cells.26 However, it is unclear if adult TM stem cells exist in vivo or where they have their niche in the anterior eye. In general, adult stem cells are difficult to identify in vivo, because they usually express only a limited amount of cell-specific markers. A well-established noninvasive method to identify stem cells monitors the incorporation of the nucleotide analogue 5-bromo-2′-deoxyuridine (BrdU) into the DNA of cycling cells. Under steady-state conditions in vivo, most stem cell populations are believed to divide infrequently and to have a long cell cycle time. Murine hematopoietic stem cells, for example, have been shown to slowly divide over a period of 1.5 to 3.0 months.27 Quite similarly, epithelial stem cells in the skin rarely divide within their niche but change properties abruptly when stimulated to exit.28 Hence, stem cells in the S-phase that have incorporated BrdU will retain it over many weeks, in contrast to more rapidly dividing cells, in which BrdU becomes diluted over time.
Here we provide evidence that cells with long-term BrdU retention reside in close association with the trabecular outflow pathways in the eyes of cynomolgus monkeys. The cells are localized at the peripheral end of Descemet's membrane in region of the Schwalbe's line and stain for the stem cell marker OCT4. Our results provide essential support for the concept that Schwalbe's line constitutes the niche for adult TM stem cells in the primate eye.
Materials and Methods
Animals and Treatment
Four cynomolgus monkeys (Macaca fascicularis) and two rhesus monkeys (Macaca mulatta) that were housed at the Wisconsin National Primate Research Center were used for the present study. Rhesus monkeys were used for transmission electron microscopy (see below). We administered BrdU (Sigma-Aldrich Corp., St. Louis, MO, USA) to the four cynomolgus monkeys via subcutaneous minipumps (ALZET model 2ML4, ideally 1 pump/0.75 kg body weight; DURECT Corp., Cupertino, CA, USA) in order to yield a rate of 8 mg/kg/d (2.65 μL/h/pump) for 4 weeks. Pumps were removed from animals that had BrdU treatment discontinued. Two animals were killed immediately thereafter. In the other two animals, BrdU treatment was discontinued for another 4 weeks before they were killed (Fig. 1A). Animals were fixed under deep anesthesia (intramuscular ketamine 25 mg/kg followed by intravenous [IV] pentobarbital Na 15 mg/kg) by perfusion fixation via the heart. Whole body exsanguination (resulting in death) and fixation was accomplished by cannulating the left ventricle of the heart, starting the infusion, then cutting the right atrium to allow blood and fluid to escape. An entire liter of 0.1 M PBS was perfused through the system resulting in all the blood being removed. Then the fixative solution (1 L 4% paraformaldehyde/0.1 M PBS) was allowed to perfuse through. After perfusion, the 12:00 limbus was marked with a suture, the eyes were enucleated, immersed in 4% PFA, and sent to Germany for further analysis. A window was cut in the cornea after enucleation to allow the fixative to penetrate. All experiments were conducted in compliance with the ARVO Statement on the Use of Animals in Ophthalmic and Vision Research and institutional guidelines.
Figure 1.
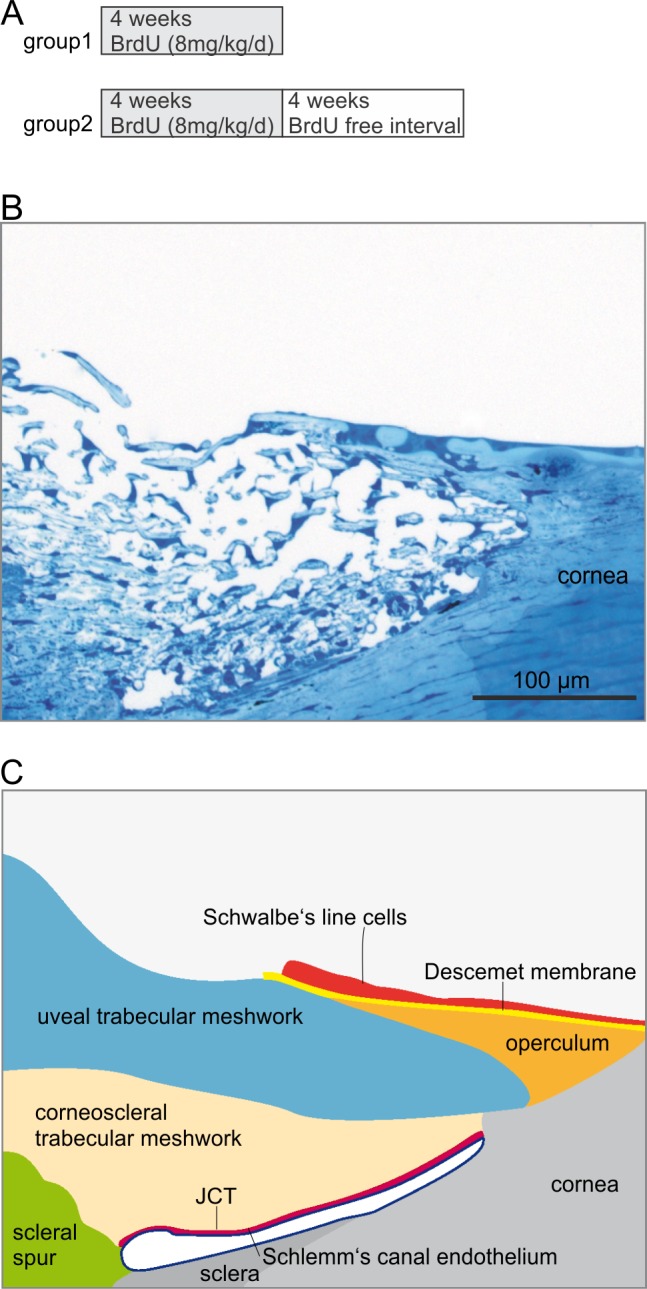
Experimental setup and data analysis. (A) Schematic of the BrdU treatment protocol. (B, C) 1-μm semi-thin section through the chamber angle of a monkey eye (B) and schematic drawing (C) of the different regions that were analyzed for presence of BrdU-positive cells.
Immunohistochemistry
Eyes were dissected into quadrants (superior, inferior, temporal, and nasal) and embedded in paraffin. Paraffin sections at a thickness of 6 μm were dewaxed, washed for 5 minutes each in H2O and phosphate buffer (PhP), blocked with 5% dry milk in 0.1 M PhP for 45 minutes, and incubated with primary antibodies (BrdU 1:50; Invitrogen, Life Technologies, Darmstadt, Germany; CD31 1:100; Dako Deutschland GmbH, Hamburg, Germany; OCT4 1:50; Santa Cruz Biotechnology, Dallas, TX, USA) in 0.5% dry milk at 4°C overnight. After three washes in PhP (5 minutes each), secondary antibodies (anti-mouse biotinylated 1:500 [Vector Laboratories, Inc., Burlingame, CA, USA] followed by Streptavidin AlexaFluor 488 1:1000 [Invitrogen] for BrdU, anti-rabbit AlexaFluor 546 [Invitrogen] 1:1000 for CD31 and OCT4) were applied for 1 hour at room temperature. If required, double staining with antibodies against CD31 or OCT4 was performed following counterstaining of cell nuclei with DAPI (Vectashield; Vector Laboratories, Inc.) diluted 1:10 in fluorescent mounting medium (Serva Electrophoresis GmbH, Heidelberg, Germany). The sections were analyzed using a fluorescence light microscope (AxioVision; Carl Zeiss Meditec MicroImaging GmbH, Jena, Germany) and the appropriate software (AxioVision 4.8).
Quantification of BrdU-Positive Cells
Cells that were BrdU-positive were counted with respect to their localization in the four quadrants of the eye and the different subareas of the trabecular outflow pathways (Fig. 1). For subareas, we selected the different regions of the TM; uveal and corneoscleral TM, juxtacanalicular tissue (JCT), scleral spur, and the endothelial layer of SC (Figs. 1B, 1C). In addition, we counted cells in the most anterior, nonfiltering part of the TM localized close to Schwalbe's line and the transition zone to the cornea. This region is known in the cynomolgus and rhesus monkey as “operculum.” We distinguished operculum cells (i.e., cells that are underneath the peripheral end of Descemet's membrane), from Schwalbe's line cells (i.e., cells that reside on the inner surface of Descemet's membrane). The number of BrdU-positive cells was normalized to the number of DAPI-positive cells in the respective quadrant, in the respective subarea of the trabecular meshwork outflow pathways or in the Schwalbe's line region. A minimum of 15 different sections was analyzed per quadrant. To avoid double counting of BrdU-positive cells, sections were separated by at least 12 μm. Possible differences between the four quadrants were tested with a linear mixed regression model accounting for the nonindependence of sections from the same region and eye, and of sections from differing regions within the same eye (see “Statistics” section). For the data presentation in the diagrams, we calculated the mean value of these 15 or more different sections per quadrant for all eyes of the treatment group (as defined in Fig. 1A). Possible differences between the different subareas of the chamber angle outflow pathway were again analyzed using a linear mixed regression model (see “Statistics” section). For the data presentation in the diagrams, the mean value for each subarea (as defined in Fig. 1C) across all eyes per group (as defined in Fig. 1A) is shown.
Transmission Electron Microscopy
For transmission electron microscopy (TEM), untreated eyes from two rhesus monkeys (Macaca mulatta) aged 19 to 20 years were studied. The contralateral eye had been surgically treated by viscocanalostomy.29 The anterior chambers of each monkey were exchanged with cationic 5 nm and noncationized 10 nm gold solution at an intraocular pressure of ~15 mm Hg, and then perfused at 25 mm Hg with Ito's solution30 from an elevated reservoir. Under deep general anesthesia with IV pentobarbital sodium, 15 mg/kg, these animals were then perfused through the heart with phosphate buffered saline, 0.1 mol/L (pH 7.4) followed by Ito's solution. The eyes were enucleated, windows were cut in the cornea and sclera, and the eyes placed in the same fixative and sent to Germany for electron microscopy. Upon arrival, the eyes were placed in cacodylate buffer (pH 7.4) for 24 hours to wash out fixative. Each eye was bisected and the anterior halves were cut into quadrants by meridional sectioning. Each quadrant was further dissected into wedge-shaped specimens of 1- to 1.2-mm width that contained trabecular meshwork, ciliary muscle, iris and adjacent cornea and sclera. All wedges were dehydrated in ascending concentrations of alcohol and embedded in epoxy resin according to standard protocols. A least three specimens from each quadrant were analyzed. All semi-thin sections were stained with Richardson's stain31 and examined by light microscopy. Subsequently, meridional and equatorial ultrathin sections were cut from each specimen that had been investigated by light microscopy and stained with lead citrate and uranyl acetate for TEM.
Statistics
All results are expressed as mean ± SEM. We used R 3.0.332 and lme4 1.1.733 to perform a linear mixed effects analysis of the relationship between the BrdU-positive cell count normalized to the total number of DAPI-stained cells and quadrant location (super, inferior, nasal, temporal). Quadrant location was specified as a fixed effect. As random effects, intercepts for eyes and random slopes for quadrants per eye were entered. Residual plots and quantile-quantile plots were visually inspected to confirm homoscedasticity and normality of residuals across groups. Statistical (P) values for the main effect of quadrant were obtained by likelihood ratio testing of the full model against the model without the fixed effect of quadrant. Analyses were separately conducted for groups 1 and 2.
A similar linear mixed effects analysis was performed to analyze the relationship between the BrdU-positive cell count normalized to the total number of DAPI-stained cells and trabecular meshwork regions or the chamber angle outflow pathways (trabecular meshwork and Schwalbe's line), respectively. Analyses were again conducted separately for group 1 and 2 animals. Chamber angle outflow pathway localization was specified as a fixed effect, and random intercepts for localizations nested in eyes were specified. It was not possible to expand the random effects structure to include random slopes due to model convergence failure. Statistical values for the main effect of chamber angle outflow pathway were obtained by likelihood ratio testing of the full model against the model without the fixed effect of chamber angle outflow pathway localizations. The Kenward-Roger approximation was used to calculate approximate degrees of freedom34,35 and P values for all pairwise comparisons were obtained from the t-distribution with approximated degrees of freedom. Bonferroni's post hoc adjustment to P values was used to control the family-wise error rate. Values of P ≤ 0.05 were considered to be statistically significant.
Results
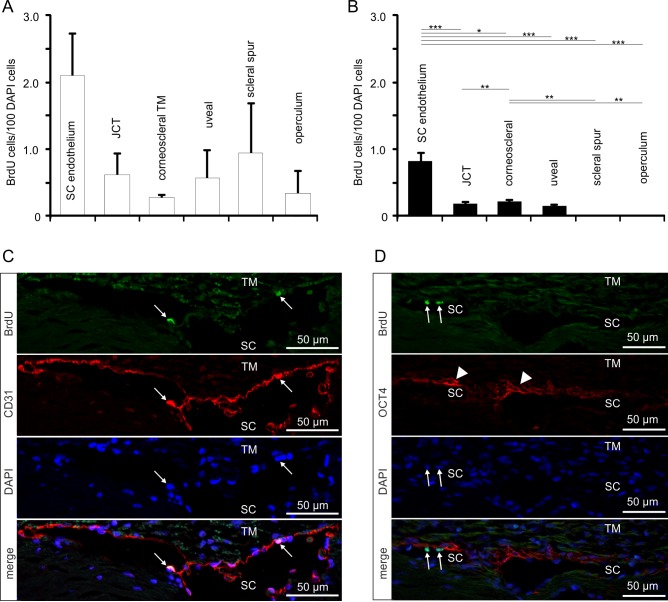
We used four cynomolgus monkeys (Macaca fascicularis) to identify adult stem cells in the trabecular meshwork outflow pathways. To this end, BrdU was administered via subcutaneous minipumps for 4 weeks. Two animals were killed immediately thereafter (group 1, chronic BrdU). In the two other animals, BrdU treatment was discontinued for another 4 weeks before they were killed (group 2, chronic BrdU and long-term retention; Fig. 1A). When sections through the chamber angle were labeled for BrdU, positively stained nuclei were regularly observed in the different regions of the trabecular outflow pathways (Fig. 2A). Quantitative analysis showed no significant preference in the number of BrdU-positive cells for the different quadrants of the eyes (Fig. 2B). This was true for both groups of monkeys. We next distinguished BrdU-positive cells with regard to their specific location in the TM outflow pathways and observed positively labeled cells in all the different regions that were investigated, (e.g., SC endothelium, JCT, corneoscleral and uveal TM, scleral spur, and operculum; Fig. 3A). In the two monkeys of group 2 (chronic BrdU and long-term retention), the number of BrdU-positive cells in the different regions was smaller than in group 1 (chronic BrdU). The highest number of BrdU-positive cells in the eyes of group 2 was observed in SC endothelium, in which the number of BrdU-positive cells was significantly higher than in JCT, corneoscleral TM, and corneoscleral and uveal TM. Very few BrdU-positive cells were observed in the scleral spur and operculum of group 1 monkeys, while no positive cells were observed in group 2 monkeys (Fig. 3B).
Figure 2.
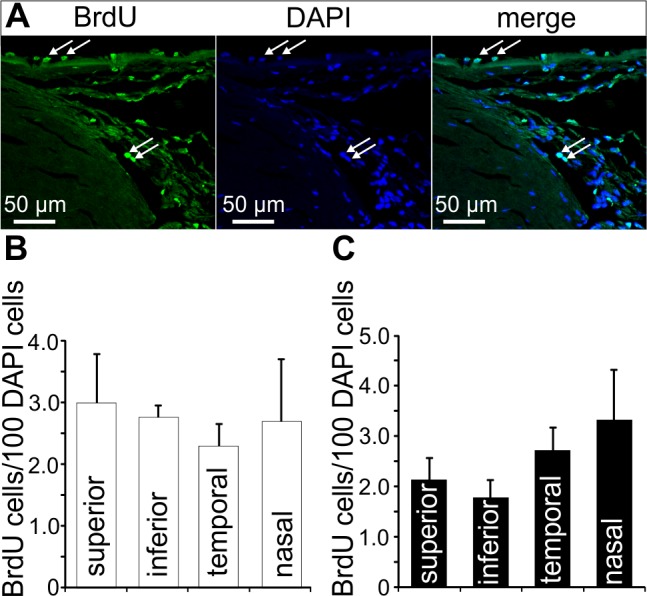
BrdU-positive cells in the chamber angle. (A) Immunohistochemical staining for BrdU (green) in cells of the TM outflow pathway. Nuclei are stained with DAPI (blue). Arrows indicate BrdU-positive cells in Schlemm's canal endothelium and in the region of Schwalbe's line. (B, C) Quantification and statistical analysis of BrdU-positive cells in the different quadrants of group 1 ([B], chronic BrdU) and group 2 ([B], chronic BrdU and long-term retention) eyes. Means ± SEM are shown.
Figure 3.
BrdU-positive cells in the trabecular meshwork outflow pathways. (A, B) Relative number of BrdU-positive cells in the different regions of the TM outflow pathways in group 1 (A) and group 2 (B) eyes. Means ± SEM are shown. *P < 0.05. **P < 0.01. ***P < 0.001. (C) Immunohistochemical staining of Schlemm's canal endothelium in a group 2 eye for BrdU (green) and CD31 (red). Cell nuclei are stained with DAPI (blue). The arrows point toward a BrdU-/CD31-positive cell in Schlemm's canal endothelium. (D) Immunohistochemical staining of Schlemm's canal endothelium in a group 2 eye for BrdU (green) and OCT4 (red). Nuclei are stained with DAPI (blue). The arrows point toward a BrdU-positive cell in Schlemm's canal endothelium, arrowheads mark nonnuclear labeling in the JCT.
Next we performed double immunohistochemistry to identify the nature of BrdU-stained cells. All BrdU-labeled cells in the SC endothelial layer stained for CD31, a marker for differentiated vascular endothelium (Fig. 3C). In contrast, SC BrdU-positive cells did not react with antibodies against octamer-binding transcription factor 4 (OCT4),36 a homeodomain transcription factor that is critically involved in the self-renewal of stem cells (Fig. 3D). Some highly reproducible, non-nuclear and presumably extracellular OCT4 labeling was observed in the JCT, which we regarded as nonstem cell relevant since OCT4 is a transcription factor that localizes to the nucleus to serve its function (Fig. 3D). Noteworthy, similar to nuclei of SC cells, BrdU-positive nuclei in the different regions of the TM outflow pathways were not immunoreactive for OCT4.
We next turned our attention to Schwalbe's line cells that cover the peripheral end of Descemet's membrane and which do not constitute an anatomic part of the TM outflow pathways. The relative number of BrdU-positive cells in this area was significantly higher than among the cells of all the different regions of the TM outflow pathways in both group 1 and 2 monkeys (Figs. 4A, 4B). We observed no difference in the relative number of BrdU-labeled Schwalbe's line cells between groups 1 and 2 (Figs. 4A, 4B), a finding that strongly indicated long-term BrdU retention. Double immunohistochemistry showed that all BrdU-positive Schwalbe's line cells were immunoreactive for the stem cell marker OCT4 (Fig. 4C). Some nuclei in the operculum area also stained for OCT4 (Fig. 4C).
Figure 4.
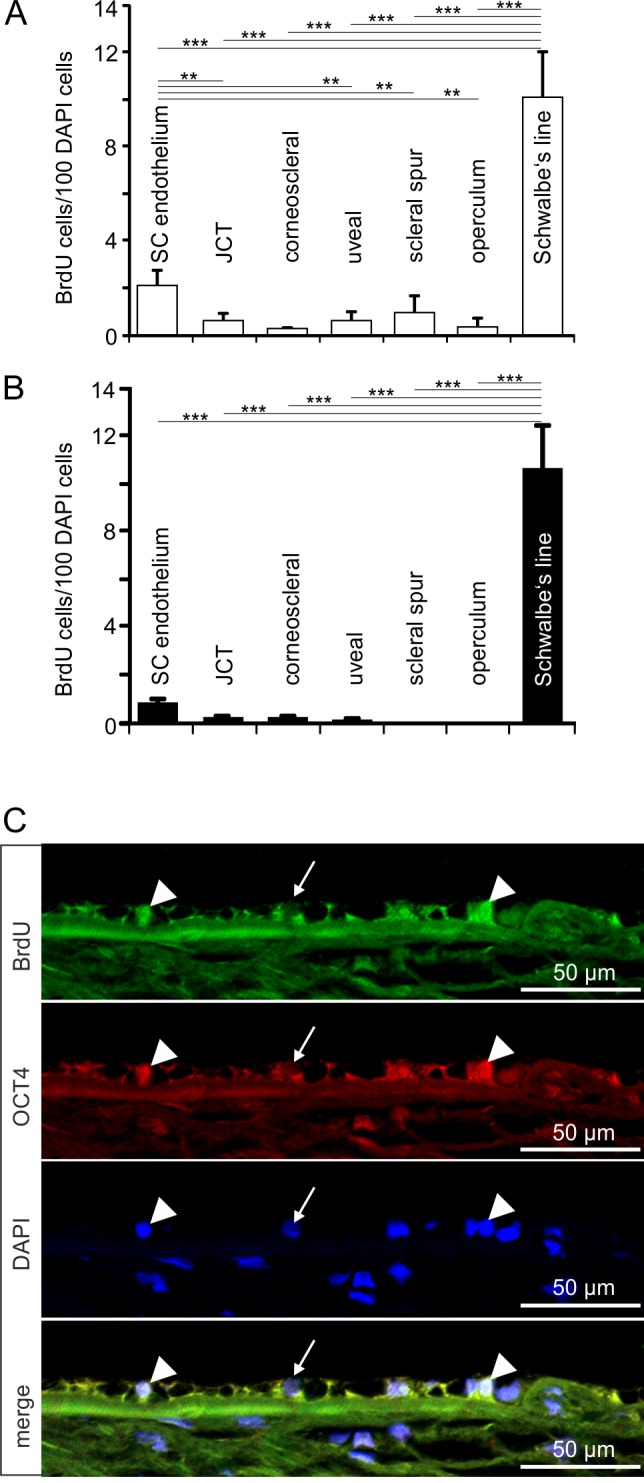
BrdU-positive cells in Schwalbe's line region. (A, B) Relative number of BrdU-positive cells in Schwalbe's line region in comparison with that in the different regions of the TM outflow pathways in group 1 (A) and group 2 (B) eyes. Means ± SEM are shown, **P < 0.01. ***P < 0.001. Due to structural damage at the Schwalbe's line, one eye could not be included in this analysis. (C) Immunohistochemical staining of Schwalbe's line cells in a group 2 eye for BrdU (green) and OCT4 (red). Nuclei are stained with DAPI (blue). Arrowheads indicate BrdU/OCT4-positive cells in Schwalbe's line region, while the arrow points toward a BrdU/OCT4-negative nucleus that is stained with DAPI.
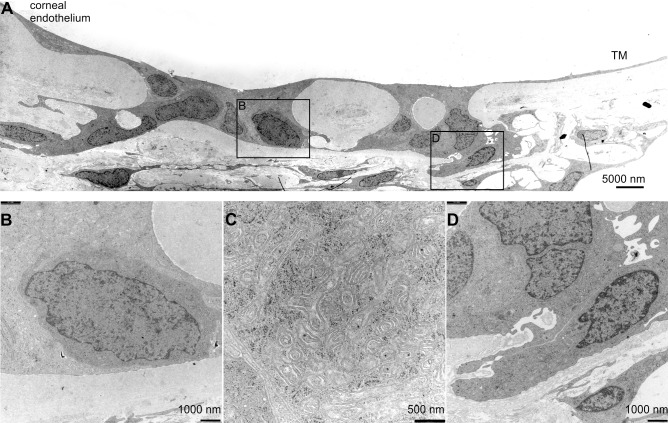
Finally, we investigated by light and electron microscopy the area of Schwalbe's line region in which we had previously observed cells with long-term BrdU retention and OCT4 immunoreactivity. Since the fixation protocol that had been used for BrdU detection did not allow preservation of ultrastructural details, we used untreated eyes from two rhesus monkeys that had been fixed for TEM studies. In the area close to the peripheral end of Descemet's membrane, where most of the BrdU/OCT4-positive cells reside (Fig. 5A), we regularly observed cuboidal epithelial cells that differed in shape from the flat adjacent corneal endothelial cells (Fig. 5B). The cells frequently formed small clusters that were embedded in furrows of Descemet's membrane, a finding that was most obvious when equatorial (frontal) sections were studied (Fig. 5C). In places, the cells completely filled gaps in Descemet's membrane and were in direct contact on their basal side with TM cells from the nonfiltering operculum region of the TM (Figs. 6A, 6B). Most of the cells in the Schwalbe's line region were characterized by the presence of numerous mitochondria of the tubular type (Fig. 6C). In addition, we frequently observed smaller cells with considerably less cytoplasm than the mitochondria-rich cell type. The smaller cells were typically engulfed by a mitochondria-rich cell (Fig. 6D).
Figure 5.
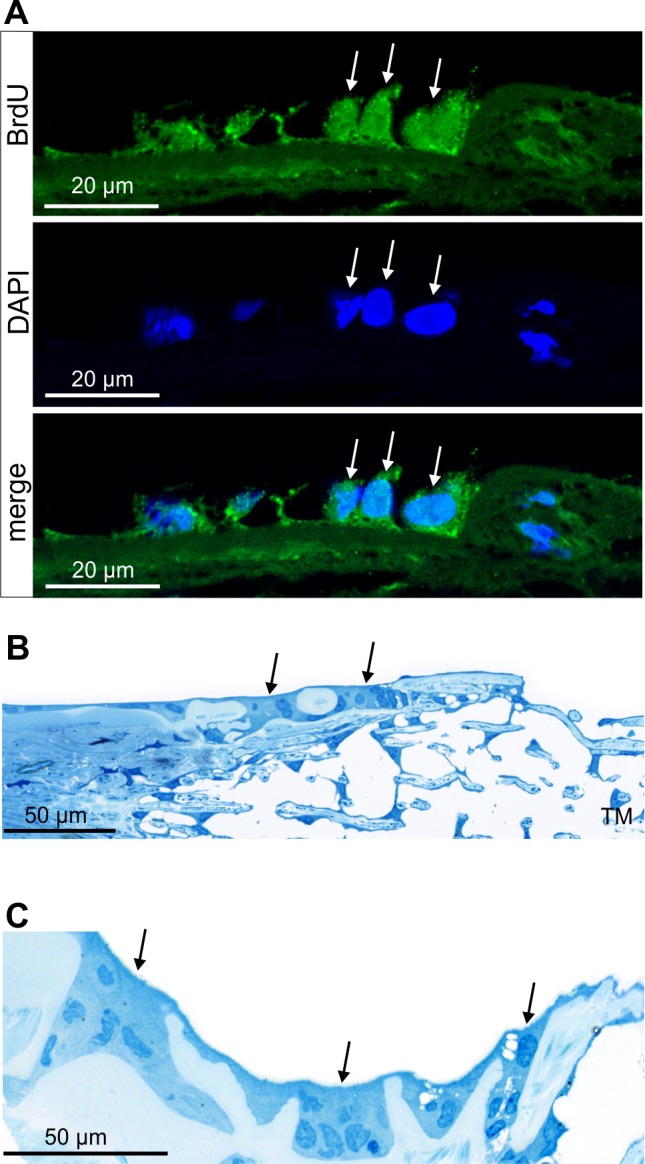
Structural characteristics of Schwalbe's line region. (A) Immunohistochemistry for BrdU (green) in Schwalbe's line cells (arrows). Nuclei are stained with DAPI (blue). (B, C) Semi-thin meridional (B) and equatorial (C) sections through the same area as in (A) in the eye of a different monkey. Cuboidal epithelial cells in Schwalbe's line form small clusters which are embedded in furrows of Descemet's membrane.
Figure 6.
Ultrastructural characteristics of Schwalbe's line cells and the putative stem cell niche. (A) Transmission electron micrographs of Schwalbe's line cells in the same monkey as in Figures 5B and 5C. Boxed areas in (A) are shown at higher magnification in (B) and (D). Schwalbe's line cells fill gaps in Descemet's membrane and are in direct contact on their basal side with TM cells from the nonfiltrating operculum region of the TM (A, D). Most of the cells in Schwalbe's line region are characterized by the presence of numerous mitochondria of the tubular type (C). In addition, smaller cells are observed with considerably less cytoplasm than the mitochondria-rich cell type (B). The smaller cells are typically engulfed by a mitochondria-rich cell.
Discussion
We conclude that adult stem cells reside at the peripheral edge of Descemet's membrane in the primate eye, a region that is commonly referred to as Schwalbe's line. This conclusion rests upon (1) the discovery of cells with long-term retention of BrdU; (2) the fact that cells with BrdU retention are immunoreactive for OCT4, a marker for stem cells; and (3) the identification of ultrastructural characteristics for a stem cell niche in the Schwalbe's line region.
The cells in the Schwalbe's line region that we identified are very likely identical to those described by Guiseppina Raviola in the rhesus monkey more than 30 years ago.37 Raviola termed the cells “Schwalbe's line cells” and described them as being arranged in a discontinuous cord of variable thickness oriented circumferentially at the corneal periphery. Similar to our study, the cells were reported as rich in mitochondria and to form clusters at the tapering end of Descemet's membrane. Based on her additional observation of secretory granules and osmiophilic lamellated bodies in Schwalbe's line cells, Raviola hypothesized that the cells produce a phospholipid material which is released in the aqueous humor and thus facilitates its movement through the tissues of the sclerocorneal angle. This hypothesis is not supported by the data of our study, since in the two monkey eyes that were investigated by TEM, we did not observe secretory granules and/or osmiophilic lamellated bodies as a characteristic structural element of Schwalbe's line cells.
We did, however, observe cells with a high nuclear-cytoplasmic ratio, heterochromatin-rich nuclei and a sparse cytoplasm that were in close contact with Descemet's membrane and engulfed by the larger, mitochondria-rich cells. Comparable ultrastructural characteristics have been observed in other types of stem cells in or outside the eye.38,39 It has been hypothesized that adult stem cells are maintained in a state of “stemness” by the presence of controlled intrinsic and extrinsic factors in their local microenvironment, the so-called stem cell niche.14 Factors that are required for such a niche are extracellular matrix components and cell-cell contacts. Based on this concept, we hypothesize that Descemet's membrane at its periphery and the mitochondria-rich cell type are critical components of the stem cell niche in Schwalbe's line region.
In the monkey eye, Descemet's membrane forms discontinuities at its peripheral end.29 Schwalbe's line cells are often seen to be in direct contact with the TM cells of its nonfiltering anterior part through those discontinuities. It seems reasonable to propose that this is also the route which is used after stem cell division by resulting progenitor cells to migrate to the TM in order to replace cells. In support of this hypothesis are observations in monkey eyes in which TM damage leading to cell loss was induced by long-term treatment with echothiophate40 or timolol.41 In these eyes, large clusters of elongated cells strands were seen in the nonfiltering operculum part of the TM, which were not present at similar amounts in controls. Overall, this observation is in agreement with the concept that stem cell numbers expand following injury (clonogenic capacity).
Several reports indicate that cells comparable to those characterized in the present study in the monkey eye are similarly localized in the Schwalbe's line region of the human eye. Acott and colleagues11 studied 3H-thymidine incorporation into trabecular cell DNA in a human corneoscleral explant organ culture system that was treated by laser trabeculoplasty. The authors observed a 4-fold increase in cell division and nearly 60% of this cell division was localized to the anterior, nonfiltering region of the trabecular meshwork where it inserts into the cornea beneath Schwalbe's line. In other studies using human corneas with attached scleral rims obtained from eye banks, BrdU-labeling and the expression of stem cell markers like OCT4 were observed in an area just at and adjacent to the trabecular meshwork, especially when the tissue had been wounded earlier.42,43 Clearly, in fresh human tissues studies on a characteristic stem cell property such as long-term BrdU retention are difficult if impossible to perform. Moreover, in organ culture of human tissue the expression of stem cell molecules or the incorporation of BrdU might be under the influence of the growth factors which are added as supplement to the culture medium. Still, taking into account the overall anatomical and structural similarities between the monkey and human eye, it appears to be very likely that cells with stem cell properties reside in region of Schwalbe's line in the human eye.
The two cellular populations that are in the immediate neighborhood of Schwalbe's line cells, TM cells and the corneal endothelium, both take their origin from neural crest cells that migrate to this area of the eye during development.44,45 It is tempting to speculate that Schwalbe's line cells provide a population of pluripotent stem cells that is capable of differentiating down a TM and a corneal endothelial lineage to replace both cell types (Fig. 7). In contrast to TM cells, SC cells appear to be replaced by differentiated vascular endothelial cells that are capable of mitosis. The specific environment of SC inner wall cells that do not reside on a complete basal lamina,2 and are subject to continuous stretch and strain induced by aqueous humor flow may require SC cell regeneration when SC cells detach and become lost.
Figure 7.
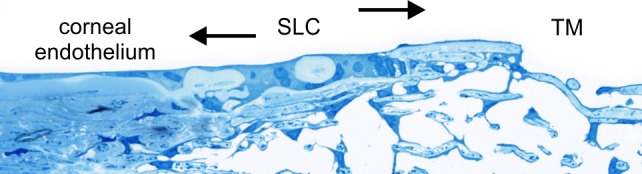
Semi-thin section of Schwalbe's line cells illustrating the concept that the cells provide a population of pluripotent stem cells that are capable of differentiating down a TM and a corneal endothelial lineage to replace both cell types.
In untreated POAG, the number of TM cells is significantly smaller than in age-matched normal eyes.6 Another characteristic finding in POAG is the reduction of SC cross-sectional area, SC perimeter, and SC inner wall length.46 It is plausible that a cell-based therapy that leads to a repopulation of the TM outflow pathways with differentiated TM and/or SC cells may help to prevent or reverse the structural and functional changes in patients that suffer from POAG.47 The results of the present study provide evidence for this concept and the need for further study.
We realize that the central hypothesis of our study would be supported considerably if, in addition to OCT4, the expression of other molecular markers for stem cells would be shown in Schwalbe's line cells. While the transcription factor OCT4 alone is sufficient to reprogram human neural stem cells to pluripotency indicating its key role as regulator of stemness,48,49 its expression has been observed in adult differentiated mononuclear cells, a finding that questioned somewhat its relevance as reliable marker for adult stem cells.50 In general, the expression of markers in adult stem cells depends on their specific nature and context. The cells of the trabecular meshwork, and of the corneal stroma and endothelium including Schwalbe's line, all derive from the neural crest,44,51–53 a cell population that gives origin to an extremely broad variety of very different tissues including but not limited to peripheral glia and neurons, melanocytes, and cranial mesenchyme.54–56 Accordingly, adult neural crest-derived stem cells, which have been isolated from various tissues such as cornea,57,58 iris,59 skin,60 palate,61 nasal mucosa62 or periodontal ligament,63 express a multitude of markers that are characteristic for their broad progeny. Still, to our knowledge, a clear-cut marker that identifies undifferentiated adult neural crest-derived stem cells in situ has not been identified so far.64 We are confident that the data of our study, which characterizes in detail for the first time the specific in situ localization of a niche for neural crest-derived stem cells in the adult eye, will greatly facilitate the isolation of the cells allowing their detailed molecular characterization in future studies.
Acknowledgments
The authors thank Elke Stauber, Angelika Pach, Margit Schimmel, Silvia Babl, and Galen Heyne for technical assistance.
Supported by grants from The Glaucoma Foundation, New York, the Deutsche Forschungsgemeinschaft (FOR 1075, TP5), and the Ocular Physiology Research & Education Foundation.
Disclosure: B.M. Braunger, None; B. Ademoglu, None; S.E. Koschade, None; R. Fuchshofer, None; B.T. Gabelt, None; J.A. Kiland, None; E.A. Hennes-Beann, None; K.G. Brunner, None; P.L. Kaufman, None; E.R. Tamm, None
References
- 1. Tamm ER. The trabecular meshwork outflow pathways: structural and functional aspects. Exp Eye Res. 2009; 88: 648–655. [DOI] [PubMed] [Google Scholar]
- 2. Tamm ER. The trabecular meshwork outflow pathways. Functional morphology and surgical aspects. In: Shaarawy TM, Sherwood MB, Hitchings RA, Crowston JG. eds Glaucoma. London: Saunders Elsevier; 2009: 31–44. [Google Scholar]
- 3. Grierson I, Lee WR, Abraham S. Effects of pilocarpine on the morphology of the human outflow apparatus. Br J Ophthalmol. 1978; 62: 302–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grierson I, Lee WR, Abraham S. The effects of topical pilocarpine on the morphology of the outflow apparatus of the baboon (Papio cynocephalus). Invest Ophthalmol Vis Sci. 1979; 18: 346–355. [PubMed] [Google Scholar]
- 5. Rohen JW. Ciliarkörper (Corpus ciliare). In: von Möllendorf W, Bargmann W. eds Handbuch der mikroskopischen Anatomie des Menschen. Vol 3, Part 4. Haut und Sinnesorgane Das Auge und seine Hilfsorgane. Heidelberg, New York: Springer Verlag; 1964: 189–237. [Google Scholar]
- 6. Alvarado J, Murphy C, Juster R. Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology. 1984; 91: 564–579. [DOI] [PubMed] [Google Scholar]
- 7. Alvarado J, Murphy C, Polansky J, Juster R. Age-related changes in trabecular meshwork cellularity. Invest Ophthalmol Vis Sci. 1981; 21: 714–727. [PubMed] [Google Scholar]
- 8. Grierson I, Howes RC. Age-related depletion of the cell population in the human trabecular meshwork. Eye. 1987; 1 (part 2); 204–210. [DOI] [PubMed] [Google Scholar]
- 9. Dueker DK, Norberg M, Johnson DH, Tschumper RC, Feeney-Burns L. Stimulation of cell division by argon and Nd: YAG laser trabeculoplasty in cynomolgus monkeys. Invest Ophthalmol Vis Sci. 1990; 31: 115–124. [PubMed] [Google Scholar]
- 10. Kimpel MW, Johnson DH. Factors influencing in vivo trabecular cell replication as determined by 3H-thymidine labelling; an autoradiographic study in cats. Curr Eye Res. 1992; 11: 297–306. [DOI] [PubMed] [Google Scholar]
- 11. Acott TS, Samples JR, Bradley JM, Bacon DR, Bylsma SS, Van Buskirk EM. Trabecular repopulation by anterior trabecular meshwork cells after laser trabeculoplasty. Am J Ophthalmol. 1989; 107: 1–6. [DOI] [PubMed] [Google Scholar]
- 12. Bylsma SS, Samples JR, Acott TS, Van Buskirk EM. Trabecular cell division after argon laser trabeculoplasty. Arch Ophthalmol. 1988; 106: 544–547. [DOI] [PubMed] [Google Scholar]
- 13. Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature. 2001; 414: 98–104. [DOI] [PubMed] [Google Scholar]
- 14. Watt FM, Hogan BL. Out of Eden: stem cells and their niches. Science. 2000; 287: 1427–1430. [DOI] [PubMed] [Google Scholar]
- 15. Serafini M, Verfaillie CM. Pluripotency in adult stem cells: state of the art. Semin Reprod Med. 2006; 24: 379–388. [DOI] [PubMed] [Google Scholar]
- 16. Marshman E, Booth C, Potten CS. The intestinal epithelial stem cell. BioEssays. 2002; 24: 91–98. [DOI] [PubMed] [Google Scholar]
- 17. Watt FM. Epidermal stem cells: markers, patterning and the control of stem cell fate. Philos Trans R Soc Lond B Biol Sci. 1998; 353: 831–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dua HS, Azuara-Blanco A. Limbal stem cells of the corneal epithelium. Surv Ophthalmol. 2000; 44: 415–425. [DOI] [PubMed] [Google Scholar]
- 19. Daniels JT, Dart JK, Tuft SJ, Khaw PT. Corneal stem cells in review. Wound Repair Regen. 2001; 9: 483–494. [DOI] [PubMed] [Google Scholar]
- 20. Wolosin JM, Xiong X, Schutte M, Stegman Z, Tieng A. Stem cells and differentiation stages in the limbo-corneal epithelium. Prog Retin Eye Res. 2000; 19: 223–255. [DOI] [PubMed] [Google Scholar]
- 21. Orkin SH, Zon LI. Hematopoiesis and stem cells: plasticity versus developmental heterogeneity. Nat Immunol. 2002; 3: 323–328. [DOI] [PubMed] [Google Scholar]
- 22. Temple S. The development of neural stem cells. Nature. 2001; 414: 112–117. [DOI] [PubMed] [Google Scholar]
- 23. Gritti A, Vescovi AL, Galli R. Adult neural stem cells: plasticity and developmental potential. J Physiol Paris. 2002; 96: 81–90. [DOI] [PubMed] [Google Scholar]
- 24. Reh TA, Fischer AJ. Stem cells in the vertebrate retina. Brain Behav Evol. 2001; 58: 296–305. [DOI] [PubMed] [Google Scholar]
- 25. Du Y, Roh DS, Mann MM, Funderburgh ML, Funderburgh JL, Schuman JS. Multipotent stem cells from trabecular meshwork become phagocytic TM cells. Invest Ophthalmol Vis Sci. 2012; 53: 1566–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gonzalez P, Epstein DL, Luna C, Liton PB. Characterization of free-floating spheres from human trabecular meshwork (HTM) cell culture in vitro. Exp Eye Res. 2006; 82: 959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cheshier SH, Morrison SJ, Liao X, Weissman IL. In vivo proliferation and cell cycle kinetics of long-term self- renewing hematopoietic stem cells. Proc Natl Acad Sci USA. 1999; 96: 3120–3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tumbar T, Guasch G, Greco V, et al. Defining the epithelial stem cell niche in skin. Science. 2004; 303: 359–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tamm ER, Carassa RG, Albert DM, et al. Viscocanalostomy in rhesus monkeys. Arch Ophthalmol. 2004; 122: 1826–1838. [DOI] [PubMed] [Google Scholar]
- 30. Ito S, Karnovsky MJ. Formaldehyde-glutaraldehyde fixatives containing trinitro compounds. J Cell Biol. 1968; 39: 168A–169A. [Google Scholar]
- 31. Richardson KC, Jarret L, Finke H. Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Stain Technol. 1960; 35: 313–323. [DOI] [PubMed] [Google Scholar]
- 32. R Core Team. A language and environment for statistical computing. Vienna, Austria: Foundation for Statistical Computing; Available at: http://www.R-project.org/. [Google Scholar]
- 33. Bates D, Maechler M, Bolker B, Walker S. lme4: Linear mixed-effects models using Eigen and S4. R package version 1.1-7. 2014. http://CRAN.R-project.org/package=lme4. [Google Scholar]
- 34. Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997; 53: 983–997. [PubMed] [Google Scholar]
- 35. Halekoh U, Højsgaard SA. Kenward-Roger approximation and parametric bootstrap methods for tests in linear mixed models–The R Package pbkrtest. J Sta Soft. 2014; 49: 1–30. [Google Scholar]
- 36. Nichols J, Zevnik B, Anastassiadis K, et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998; 95: 379–391. [DOI] [PubMed] [Google Scholar]
- 37. Raviola G. Schwalbe line's cells: a new cell type in the trabecular meshwork of Macaca mulatta. Invest Ophthalmol Vis Sci. 1982; 22: 45–56. [PubMed] [Google Scholar]
- 38. Schlotzer-Schrehardt U, Kruse FE. Identification and characterization of limbal stem cells. Exp Eye Res. 2005; 81: 247–264. [DOI] [PubMed] [Google Scholar]
- 39. Lee ST, Gong SP, Yum KE, et al. Transformation of somatic cells into stem cell-like cells under a stromal niche. FASEB J. 2013; 27: 2644–2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lütjen-Drecoll E, Kaufman PL. Echothiophate-induced structural alterations in the anterior chamber angle of the cynomolgus monkey. Invest Ophthalmol Vis Sci. 1979; 18: 918–929. [PubMed] [Google Scholar]
- 41. Lütjen-Drecoll E, Kaufman PL. Long-term timolol and epinephrine in monkeys. II. Morphological alterations in trabecular meshwork and ciliary muscle. Trans Ophthalmol Soc U K. 1986; 105: 196–207. [PubMed] [Google Scholar]
- 42. McGowan SL, Edelhauser HF, Pfister RR, Whikehart DR. Stem cell markers in the human posterior limbus and corneal endothelium of unwounded and wounded corneas. Mol Vis. 2007; 13: 1984–2000. [PubMed] [Google Scholar]
- 43. Whikehart DR, Parikh CH, Vaughn AV, Mishler K, Edelhauser HF. Evidence suggesting the existence of stem cells for the human corneal endothelium. Mol Vis. 2005; 11: 816–824. [PubMed] [Google Scholar]
- 44. Cvekl A, Tamm ER. Anterior eye development and ocular mesenchyme: new insights from mouse models and human diseases. Bioessays. 2004; 26: 374–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tamm ER. Genetic changes and their influence on structure and function of the eye in glaucoma. In: Grehn FJ, Stamper R. eds Essentials in Ophthalmology Glaucoma. Berlin: Springer; 2004. [Google Scholar]
- 46. Allingham RR, de Kater AW, Ethier CR. Schlemm's canal and primary open angle glaucoma: correlation between Schlemm's canal dimensions and outflow facility. Exp Eye Res. 1996; 62: 101–109. [DOI] [PubMed] [Google Scholar]
- 47. Kelley MJ, Rose AY, Keller KE, Hessle H, Samples JR, Acott TS. Stem cells in the trabecular meshwork: present and future promises. Exp Eye Res. 2009; 88: 747–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kim JB, Greber B, Arauzo-Bravo MJ, et al. Direct reprogramming of human neural stem cells by OCT4. Nature. 2009; 461: 649–643. [DOI] [PubMed] [Google Scholar]
- 49. Kim JB, Sebastiano V, Wu G, et al. Oct4-induced pluripotency in adult neural stem cells. Cell. 2009; 136: 411–419. [DOI] [PubMed] [Google Scholar]
- 50. Zangrossi S, Marabese M, Broggini M, et al. Oct-4 expression in adult human differentiated cells challenges its role as a pure stem cell marker. Stem Cells. 2007; 25: 1675–1680. [DOI] [PubMed] [Google Scholar]
- 51. Creuzet S, Vincent C, Couly G. Neural crest derivatives in ocular and periocular structures. Int J Dev Biol. 2005; 49: 161–171. [DOI] [PubMed] [Google Scholar]
- 52. Kanakubo S, Nomura T, Yamamura K, Miyazaki J, Tamai M, Osumi N. Abnormal migration and distribution of neural crest cells in Pax6 heterozygous mutant eye, a model for human eye diseases. Genes Cells. 2006; 11: 919–933. [DOI] [PubMed] [Google Scholar]
- 53. Gage PJ, Rhoades W, Prucka SK, Hjalt T. Fate maps of neural crest and mesoderm in the mammalian eye. Invest Ophthalmol Vis Sci. 2005; 46: 4200–4208. [DOI] [PubMed] [Google Scholar]
- 54. Bronner ME, LeDouarin NM. Development and evolution of the neural crest: an overview. Dev Biol. 2012; 366: 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rogers CD, Jayasena CS, Nie S, Bronner ME. Neural crest specification: tissues, signals, and transcription factors. Wiley Interdiscip Rev Dev Biol. 2012; 1: 52–68. [DOI] [PubMed] [Google Scholar]
- 56. Bronner ME. Formation and migration of neural crest cells in the vertebrate embryo. Histochem Cell Biol. 2012; 138: 179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yoshida S, Shimmura S, Nagoshi N, et al. Isolation of multipotent neural crest-derived stem cells from the adult mouse cornea. Stem Cells. 2006; 24: 2714–2722. [DOI] [PubMed] [Google Scholar]
- 58. Brandl C, Florian C, Driemel O, Weber BH, Morsczeck C. Identification of neural crest-derived stem cell-like cells from the corneal limbus of juvenile mice. Exp Eye Res. 2009; 89: 209–217. [DOI] [PubMed] [Google Scholar]
- 59. Kikuchi M, Hayashi R, Kanakubo S, et al. Neural crest-derived multipotent cells in the adult mouse iris stroma. Genes Cells. 2011; 16: 273–281. [DOI] [PubMed] [Google Scholar]
- 60. Hunt DP, Morris PN, Sterling J, et al. A highly enriched niche of precursor cells with neuronal and glial potential within the hair follicle dermal papilla of adult skin. Stem Cells. 2008; 26: 163–172. [DOI] [PubMed] [Google Scholar]
- 61. Widera D, Zander C, Heidbreder M, et al. Adult palatum as a novel source of neural crest-related stem cells. Stem Cells. 2009; 27: 1899–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hauser S, Widera D, Qunneis F, et al. Isolation of novel multipotent neural crest-derived stem cells from adult human inferior turbinate. Stem Cells Dev. 2012; 21: 742–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Techawattanawisal W, Nakahama K, Komaki M, Abe M, Takagi Y, Morita I. Isolation of multipotent stem cells from adult rat periodontal ligament by neurosphere-forming culture system. Biochem Biophys Res Commun. 2007; 357: 917–923. [DOI] [PubMed] [Google Scholar]
- 64. Dupin E, Sommer L. Neural crest progenitors and stem cells: from early development to adulthood. Dev Biol. 2012; 366: 83–95. [DOI] [PubMed] [Google Scholar]