REPORT OF A CASE
A 74-year-old patient with metastatic melanoma, with a BRAF kinase V600E mutation, presented to us 2 months into vemurafenib therapy, after developing multiple rapidly enlarging hyperkeratotic papules on his face, trunk, and legs (Figure 1). He denied a history of squamous cell carcinoma (SCC) or keratoacanthoma (KA). On examination, 3- to 5-mm hyperkeratotic papules were distributed on his cheek, shoulder, chest, back, and leg. Some demonstrated central crusting or raised erythematous borders. Clinically, these lesions were suggestive of KAs. Biopsy specimens obtained from these lesions were consistent with well-differentiated invasive SCC-KA type. He also had overall moderate photodamage of his chest and back. Three months into vemurafenib therapy, multiple additional hyperkeratotic lesions continued to appear.
Figure 1.
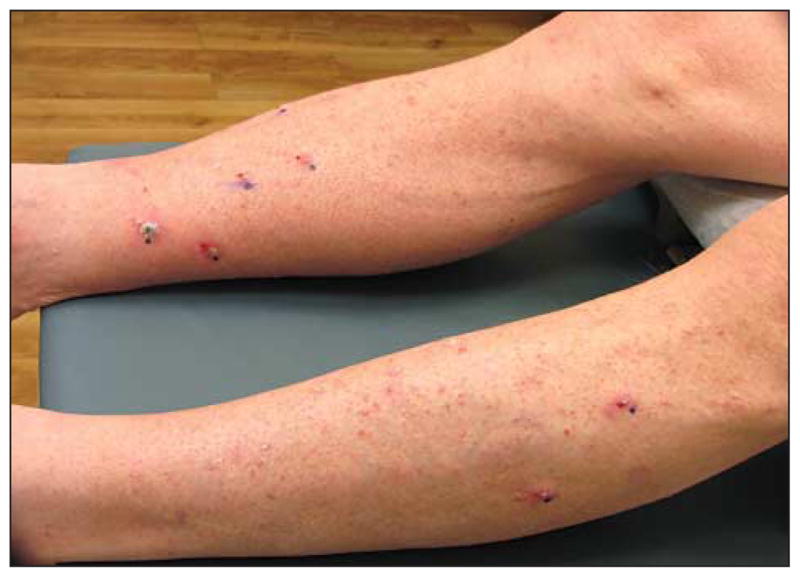
Clinical image before treatment of biopsy-proven squamous cell carcinoma–keratoacanthoma type. Clinical image at initial dermatological evaluation demonstrates multiple keratotic papules measuring from 3 to 5 mm in diameter on the lower extremities, clinically consistent with keratoacanthomas.
THERAPEUTIC CHALLENGE
Vemurafenib provides a much-needed treatment option for patients with metastatic melanoma with a BRAF V600E mutation. In patients with the mutation, vemurafenib is remarkably active compared with dacarbazine.1 However, it has adverse effects, including SCC and KA eruption. Other dermatological adverse effects include alopecia, eruptive nevi, and photosensitivity.1 The current package insert for vemurafenib quotes a 24% rate of SCC development.2 These SCC were mainly KA-type, with a small number of invasive SCCs.1
Keratoacanthoma is characterized by a rapidly growing nodule with central crust. Initially considered benign, many now consider it an SCC variant (“SCC-KA type”) and manage it as such due to similar histologic features and rare reports of metastasis.3 Median time to first KA development is 8 weeks.1 In vermurafenib-treated patients, most lesions are treated by surgical excision, without a significant impact in vemurafenib dosing and schedule vemurafenib dose modification was not required.1
Current recommendations from the vemurafenib package insert2 are that lesions be completely excised, which poses a challenge when multiple lesions are present and new lesions continue to develop. Thus far, the only treatments described for vemurafenib-induced KAs are excision and photodynamic therapy (PDT). Light therapy poses the challenge of penetrating the KAs deeply enough for an effective intervention. In addition, vemurafenib has demonstrated photosensitizing properties, although it is unclear if this includes sensitivity to blue or red light as used in PDT. Excision of multiple lesions may be impractical, and incompletely excised KAs may frequently recur. Our patient’s KAs required consideration of alternative therapies that would both treat active lesions and reduce the number of new KAs. Optimally, a prophylactic approach would be implemented to minimize the need for destructive methods.
SOLUTION
Organ transplant recipients are known to have high rates of SCC and actinic keratosis. Although presentation differs from vemurafenib-treated patients, both show diffuse keratinocytic neoplasm eruptions. We believed it was feasible to attempt similar therapeutic approaches in our patient. Acitretin is a systemic retinoid that inhibits the development of epithelial carcinomas, including SCC, in renal transplant recipients.4 On the basis of this rationale, we offered the patient acitretin in an attempt to decrease rate of new lesion development. We also recommended a local approach with intralesional fluorouracil for established KAs. By injecting intralesionally, deeper penetration is achieved compared with topical therapy. This therapy is especially advantageous for large, multiple, or recurrent KAs.3
After initial biopsy findings confirmed the diagnosis of SCC-KA type, 16 SCC-KA type lesions were documented. Thirteen of the most prominent of these lesions were treated with intralesional fluorouracil, using a total of 2.5 mL at a concentration of 50 mg/mL (average of 0.2 mL/lesion). The patient was started on systemic acitretin therapy at this time, at a dose of 25 mg daily. His dose was decreased to 25 mg every other day after 2 weeks owing to the development of skin exfoliation, a known adverse effect of acitretin therapy. His liver function remained within normal limits during treatment, and his lipid levels were mildly elevated.
The lesions treated with intralesional fluorouracil nearly all resolved, and those that remained demonstrated a significant reduction in size. During his 5 weeks on acitretin therapy, the patient developed only 3 new lesions (Figure 2). His rate of previous lesion development before presenting to our clinic was not definitively known, but the patient reported a significant rate of decrease of incident lesions. At the time of acitretin therapy discontinuation, he showed an overall decrease from 16 lesions to 4, representing a 75% clinical improvement. It would be useful to assess if the rate of lesion development increased again after stopping acitretin use; however, owing to progression of his melanoma, vemurafenib therapy was discontinued just 2 weeks after the acitretin therapy was stopped. The patient developed 2 new lesions after acitretin therapy was discontinued, but following discontinuation of vemurafenib therapy, he had complete resolution of all lesions with no future KA development. This fact, combined with the short duration of treatment, make it difficult to reach a definitive conclusion about the efficacy of acitretin in the prevention and treatment of vemurafenib-induced SCCs. Intralesional fluorouracil, however, demonstrated a more objective alternative to excision or PDT for these lesions. Our initial findings show that both intralesional fluorouracil and acitretin should be further evaluated in vemurafenib-treated melanoma patients.
Figure 2.
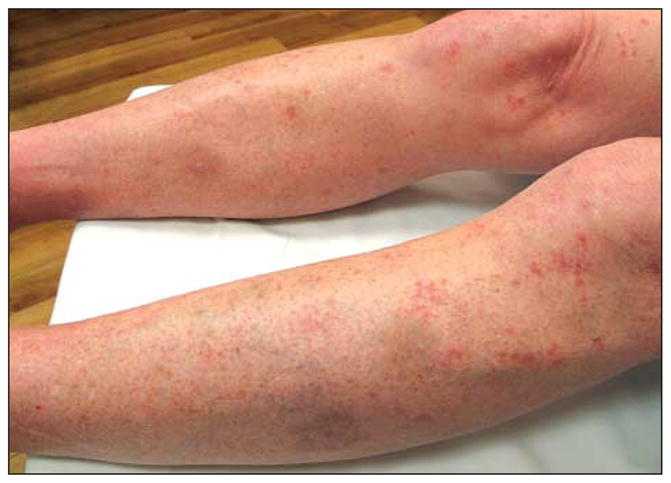
Clinical image after treatment of biopsy-proven squamous cell carcinoma–keratoacanthoma type. The follow-up image taken 2 weeks later demonstrates a significant resolution of the keratoacanthomas following treatment with intralesional fluorouracil and acitretin.
COMMENT
The observed development of SCC during vemurafenib treatment was recently studied by Su et al5 who found that RAS mutations were present in 60% of patients who developed cutaneous SCC while taking a BRAF inhibitor. The RAS mutations occurred frequently in lesions on photodamaged skin, like that of our patient, suggesting that vemurafenib accelerates the growth of preexisting damage, rather than initiating carcinogenesis directly. This helps explain the large variability of SCC development observed among patients receiving vemurafenib. Functional studies have suggested that these RAS mutations lead to cutaneous SCC through mitogen-activated protein kinase (MAPK) pathway activation. Current trials by Flaherty et al6 are testing the combination of BRAF inhibitors combined with inhibition of the MAPK pathway via a MAPK kinase (MEK) inhibitor, with phase 2 results showing a decrease of cutaneous SCC development from 19% to 7%. MEK inhibition is thus an exciting future treatment option; however, there is continued clinical need to identify effective options to prevent and/or treat the SCCs developed in the setting of BRAF inhibition.
A 75% improvement was seen in the number of KAs by using both intralesional fluorouracil for established KAs and systemic acitretin in an attempt to prevent new lesion development. The patient ultimately experienced complete cessation of KA development following discontinuation of vemurafenib therapy, confirming the role of vemurafenib in his KA development. A decreased rate of new lesion development was observed during treatment with systemic acitretin. However, the short duration of treatment with acitretin, combined with concomitant intralesional fluorouracil therapy and early vemurafenib therapy discontinuation, make the association of acitretin and lesion improvement difficult to confirm in a definitive manner. Intralesional fluorouracil showed more objective efficacy by leading to clinical resolution of established lesions. This suggests the potential value of combination therapy with intralesional fluorouracil and systemic acitretin for patients on vemurafenib therapy with KA eruption. Systemic acitretin is not without adverse effects, and indeed our patient was not able to tolerate it for longer than 5 weeks; however, acitretin showed a potential benefit by reducing the number of new KA-type lesions. This approach will need to be evaluated in additional patients to determine if similar SCC prevention or treatment-limiting adverse effects are observed. Further studies are needed to evaluate and optimize therapeutic regimens for both intralesional fluorouracil and systemic acitretin when used for vemurafenib-induced KAs and SCCs.
Footnotes
Conflict of Interest Disclosure: Dr Cranmer discloses that he is a paid speaker for Genentech.
Author Contributions: All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Cranmer and Curiel-Lewandrowski. Acquisition of data: Cranmer, Morrison, Erickson, and Curiel-Lewandrowski. Analysis and interpretation of data: LaPresto, Erickson, and Curiel-Lewandrowski. Drafting of the manuscript: LaPresto, Cranmer, Morrison, and Curiel-Lewandrowski. Critical revision of the manuscript for important intellectual content: Cranmer and Erickson. Administrative, technical, and material support: Cranmer, Morrison, Erickson, and Curiel-Lewandrowski. Study supervision: Curiel-Lewandrowski.
References
- 1.Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366(8):707–714. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zelboraf [package insert] South San Francisco, CA: Genentech, Inc; 2012. [Google Scholar]
- 3.Good LM, Miller MD, High WA. Intralesional agents in the management of cutaneous malignancy: a review. J Am Acad Dermatol. 2011;64(2):413–422. doi: 10.1016/j.jaad.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Bavinck JN, Tieben LM, Van der Woude FJ, et al. Prevention of skin cancer and reduction of keratotic skin lesions during acitretin therapy in renal transplant recipients: a double-blind, placebo-controlled study. J Clin Oncol. 1995;13(8):1933–1938. doi: 10.1200/JCO.1995.13.8.1933. [DOI] [PubMed] [Google Scholar]
- 5.Su F, Viros A, Milagre C, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med. 2012;366(3):207–215. doi: 10.1056/NEJMoa1105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367(18):1694–1703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]


