Abstract
Current trends of TB rates indicate the Millennium Development Goal of TB elimination by 2050 will not be achieved. The majority of incident cases are occurring in population dense and HIV-endemic regions of Africa and Asia. The persistence of TB in the setting of poor existing health infrastructure has led to a rise in drug-resistant cases, exacerbated by the strong association with HIV co-infection. Spreading drug-resistance threatens to undo decades of progress in controlling the disease. Several significant gaps can be identified in various aspects of national and international-directed TB control efforts. Various governing bodies and international organizations need to address the immediate challenges. This paper highlights some of the major policies lawmakers and funding institutions should consider. Existing economic and social obstacles must be overcome if TB elimination is to be a reachable goal.
Keywords: Mycobacterium tuberculosis, epidemiology, drug-resistance, policy, DOTS
INTRODUCTION
The foundation of current global tuberculosis strategy began in the 1990s when the rising trends of tuberculosis (TB) led to the creation of the directly observed treatment, short course (DOTS) strategy [1,2]. The multidimensional DOTS framework was implemented in 184 countries which led to over 32 million patients being treated and over 25 million being cured (figure 1) [3,4]. Despite these advances, continued challenges to effectively address the epidemic arose [7,8]. In January 2006, the Stop TB Partnership launched its Global Plan to Stop TB 2006–2015 to tackle these issues [9]. The six part strategy detailed a comprehensive approach to halve the 1990 TB death and prevalence rates by 2015 (table 1 and figure 2). The strategy was also in-line with the World Health Organization’s (WHO) prior goals of a 70% detection rate and an 85% cure rate by 2005 [10]. The ultimate aim of the strategy was to achieve the Millennium Developmental Goal of eliminating TB as a public health concern by 2050 (less than one case per million population) [11].
Figure 1. DOTS five-part framework which provided a cost-effective public health strategy on both individual and societal levels.
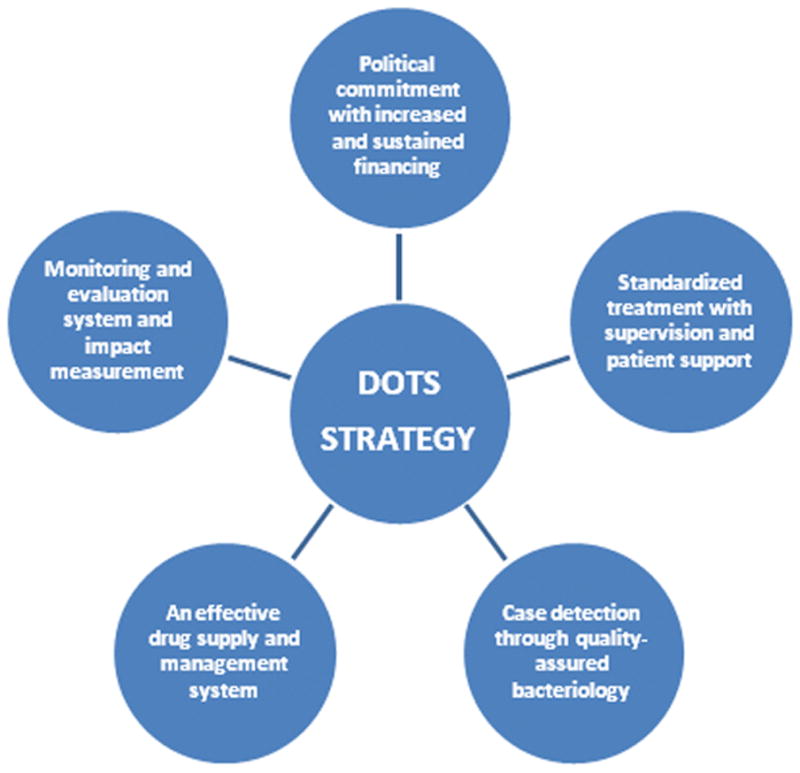
Building on the successes of DOT programs, the WHO and partner agencies developed a strategy for treatment of MDR-TB, termed “DOTS-Plus”, in 1999. The DOTS-Plus strategy adapts the core components of DOTS to the needs of patients with drug-resistant TB [5, 6].
Table 1.
Six components that underlie the Stop TB Partnerships’s Global Plan to Stop TB 2006–2015 [9].
SIX COMPONENTS OF THE STOP TB STRATEGY
|
Figure 2.
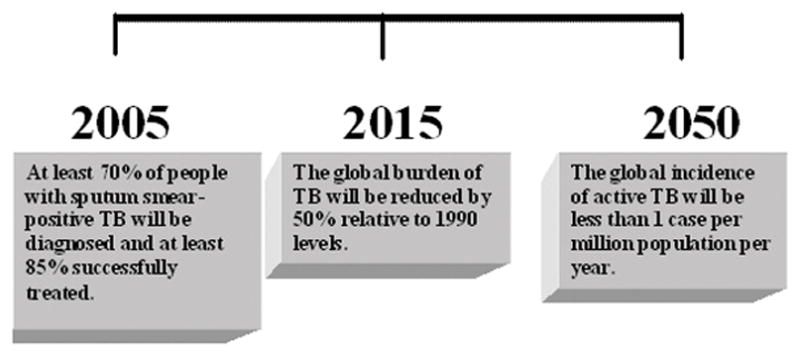
Three key time points in achieving TB elimination by 2050 if all criteria set forth by the Stop TB Partnership are effectively obtained [27,28].
The Global Plan’s total cost was $56 billion, of which $47 billion was slated for implementation of currently available interventions and $9 billion for research and development [9,12]. However, even when launched in 2006 – a time when the global economy was relatively strong - the overall estimated funding gap for the Global Plan was over $30 billion [13]. In past recessions, several countries had reduced spending on overseas developmental programs [14–16]. Thus, there is great concern in 2009 that the burden of TB, which lies largely in developing nations, may be further enhanced in the context of decreased international funding [5,17]. High TB-burden countries will not have the resources to compensate for this monetary loss in funding – especially in the context of an additional 100 million people sinking into poverty because of the ongoing financial crisis [18]. Moreover, poverty brings with it increased household crowding and poor nutrition – known risk factors that enhance TB incidence and transmission [19,20].
In an increasingly globalized environment, where local and international travel is available to a variety of populations, the problems in high TB-burden countries may be easily translated to a multitude of world settings [21,22]. This scenario, along with increasing amounts of drug-resistance, has led to a greater resolve among various governments and international organizations to act against the ongoing epidemic [23–25]. The challenges that must be addressed by the numerous governmental and nongovernmental bodies must be framed in the most recent available epidemiologic indicators.
EPIDEMIOLOGY
A 2009 report on global tuberculosis control published by the WHO describes the most recent figures available on TB epidemiology. The comprehensive data derives from 196 countries and territories that reported information in 2008. The countries account for 99.6% of the world’s estimated TB cases and 99.7% of the world’s population [26]. The report provides a comprehensive analysis of the current state of the epidemic at the global, national and local levels. The results are derived from a standard data collection form reported to the WHO in 2008 from individual nations. The impact of current TB control methods continue to be measured by incidence, prevalence and mortality indicators. It is these three which frame the ultimate aims of TB Control as determined by the Millennium Developmental Goals and Stop TB Partnership [26–28].
An estimated 9.27 million incident cases of TB were documented in 2007, thirty thousand more than in 2006. Over 80% of those cases were in Africa and Asia (figure 3). Although the absolute number of incident cases is increasing, the number of cases per capita is falling. The global rate of decline is slightly less than 1% per year which precludes any possibility of achieving TB elimination by 2050 [26]. In the USA, the average annual percentage decline in the TB rate decreased from 7.3% per year during 1993–2000 to 3.8% during 2000–2008. The slowing of the decline in TB was largely due to persistence of TB in foreign born populations and racial/ethnic minorities. The TB rate was nearly ten times higher in foreign-born compared to USA-born persons – most prominently among the Asian populations. TB prevalence among Hispanic and blacks were nearly eight times higher than among non-Hispanic whites [30].
Figure 3. Estimated number of new TB cases in 2007.
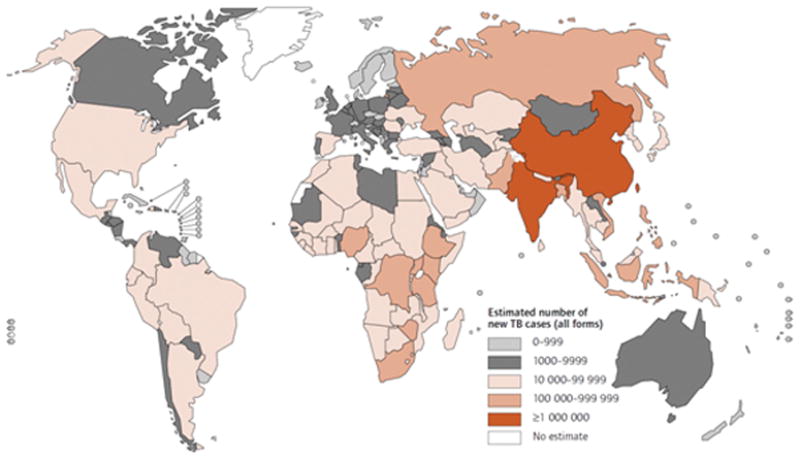
The five countries that rank first to fifth in terms of total numbers of cases in 2007 are India (2.0 million), China (1.3 million), Indonesia (0.53 million), Nigeria (0.46 million) and South Africa (0.46 million). The TB in such high burdened countries usually affects members in their economic prime. Besides the loss of productivity, the cost of treating TB in such areas may involve mean household spending of as much as 8---20 percent of annual household income [29].
Reprinted with permission from World Health Organization. Global Tuberculosis Control 2009. Geneva: World Health Organization 2009.
The worldwide prevalence of TB in 2007 was 206 per 100,000 population (13.7 million cases of TB in 2007). This decreased from the 13.9 million cases reported in 2006. However, the African and European regions have prevalence rates above and almost equivalent to the 1990 levels, respectively. Neither region will be able to achieve the 2015 target goal of halving prevalence rates from 1990 [26].
Of the 13.7 million prevalent cases, an estimated 687 000 (5%) were HIV-positive. Approximately 35% (456,000) of the over 1.7 million total TB-related deaths were among incident TB cases co-infected with HIV. The estimated mortality directly attributed to TB among the HIV-infected population is approximately double in 2008 compared to 2007 data [4]. The increased prevalence and mortality within this subset of patients is likely not due to an increase in absolute cases but due to a greater worldwide focus on HIV testing – primarily in Africa. Of the 64 countries reporting co-infection, HIV-positive individuals are about 20 times more likely than HIV negative individuals to develop TB in HIV-endemic regions. In sub-Saharan Africa, a region which is encountering an HIV-pandemic, most countries have reported over 30% of patients with active tuberculosis are co-infected with HIV. In areas of Lesotho, almost 90% of patients with tuberculosis have HIV infection [26,31].
Approximately 500,000 cases of multi-drug resistant TB (MDR-TB) were noted in 2007 of which 85% of cases occurred in 27 countries (table 2). MDR-TB is classified as resistance to isoniazid and rifampin among the first-line TB drugs [34]. Unfortunately, less than 5% of the estimated MDR-TB cases are appropriately receiving treatment according to international guidelines [35]. To meet the targets set in the Global Plan, the diagnosis and treatment of MDR-TB needs to be scaled up in the three countries that account for 57% of cases (China, India and Russian Federation). Until that time, the documented cases of extensively drug resistant TB (XDR-TB) will continue to rise from its current prevalence in a record 55 countries. XDR-TB is classified as meeting the criteria of MDR-TB along with resistance to any fluoroquinolones and at least one of three injectable drugs (amikacin, capreomycin or kanamycin) [36]. The fourth report of the Global Project on Anti-Tuberculosis Drug Resistance Surveillance released in February 2008 was remarkable for showing that in high burden countries like South Africa, Tomsk Oblast (Russian Federation) and Estonia that 5.7%, 6.6% and 23.7% of all MDR-TB cases were reclassified as XDR-TB [37]. The continued rise in drug-resistance has presented a growing barrier to achieving any sustainable path to TB elimination [26].
Table 2. First and second-line drug regimens used in the treatment of TB.
First line regimens are used for 6–9 months to treat active drug-susceptible tuberculosis. A combination of first and second line drugs are used for 18–24 months to treat drug-resistant tuberculosis [25,32,33].
| 1st Line Regimen | 2nd Line Regimen |
|---|---|
|
| |
| Isoniazid | Aminoglycosides (Amikacin, Kanamycin) |
| Rifampin/Rifampicin/Rifapentine | Polypeptides (Capreomycin) |
| Ethambutol | Fluoroquinolones (Moxifloxacin, Levofloxacin, Gatifoxacin) |
| Pyrazinamide | Thioamides (Ethionamide, Prothionamide) |
| Streptomycin | Cycloserine P-aminosalicylic acid |
CHALLENGES TO GLOBAL TB ELIMINATION
The current epidemiologic data mandates further discussion of the means to enhance current TB control and treatment efforts. Several solutions may be found within the framework delineated in the Stop TB Partnership’s Goals (table 1). A detailed discussion of the various policies that ought to be considered to enhance the goal of eliminating TB by 2050 is beyond the scope of this paper. This paper highlights some of the major policies worthy of consideration by lawmakers and funding institutions.
TB PREVENTION ISSUES
Better Diagnostics to Increase Case Detection
While a treatment success rate of 85% has been achieved in most nations, including the WHO regions of Africa, Americas and Europe, case detection rate of new smear-positive cases under DOTS in 2008 was 63% - a minimal increase compared to the 62% noted in 2006 [26]. Furthermore, instead of culture-methods, most TB program in high burden countries rely on traditional methodologies like tuberculin skin testing, sputum smear microscopy and clinical symptoms [35,38]. The sensitivity of such tests is very poor, especially in those with HIV or extrapulmonary infection [39–41]. A key aspect of improving detection and further enhancing cure rates is the implementation of accurate point-of-care TB diagnostics [40].
Reduce Systems-wide Risks
Effective national TB control plans are often found to be united with national healthcare strategies [26]. Such programs realize that numerous risk factors enhance the transmission and advancement of the TB disease process. Such risk factors include malnutrition, tobacco smoke, air pollution and lack of respiratory infection control programs [42,43]. The relative risk of developing active TB in the setting of malnutrition, tobacco smoke and indoor pollution is estimated at 4.0 (range=2.0–6.0), 2.6 (range=1.6–4.3) and 1.5 (range=1.2–3.2), respectively [3,44–46]. Recent modelling has shown that if China reduced smoking and solid fuel use and maintained 80% DOTS coverage, they would realize a projected reduction of annual TB incidence by 14–52% by 2033 [47]. Analysis by the WHO suggests that greater than 20% of the global TB burden may be attributable to tobacco smoke [48].
Other risk factors that enhance TB are most prominent in those who are socioeconomically disadvantaged [3,49]. These populations often live in crowded settings and work in poorly ventilated areas. They are generally less educated regarding healthy behavior and have livelihoods that are vulnerable to economic instability [50,51]. Any effective TB elimination effort, sponsored through various non-governmental and governmental public health authorities, must address all such socioeconomic determinants of disease (i.e. free and easily accessible HIV/TB treatment).
Implement Sound Infection Control Policies
Infection control has been a growing concern due to increasingly appreciated community and nosocomial spread of tuberculosis. A key means by which drug-resistant TB (MDR-TB and XDR-TB) epidemics have continued to progress has been through primary infection [52,53]. Infection control practices in both community and health care facilities are required to disrupt the cycle of global TB transmission. Mathematical modelling of an XDR-TB outbreak in a rural community hospital in South Africa has shown that a combination of mask use and improved ventilation may avert over 30% of XDR-TB cases. Identifying health care workers at risk of TB (i.e. HIV-positive) is also an essential aspect of any elimination effort and therefore demands better a better administrative response. Health care workers serve as the base of any TB elimination effort and protecting their welfare is critical [54,55]
TB MANAGEMENT ISSUES
Bring Private Practitioners in the DOTS Care Team
Efforts must be made at the governmental level to encourage public-private partnerships to enhance DOTS [42]. Private health care providers are major participants in the care of peoples in the developing world [56,57]. In Pakistan, for example, 79% of first-line health care is provided by these practitioners [58]. Unfortunately, private health care providers are often poorly trained or monitored for adhering to national TB programs or international TB guidelines [59]. If any effective diagnostic and therapeutic modality to enhance DOTS coverage in the world is to occur, then private health care providers must be inline with any current and newly adopted standard of care [35].
Increase Early Detection of TB among those HIV-infected
HIV co-infection increases the likelihood of progression to active TB following primary infection and reactivation of latent TB infection [60–62]. The immunocompromised state increases the bacillary burden of disease and enhances the evolution of drug-resistant TB. [63,64]. Reliable diagnosis of TB in HIV populations is difficult using conventional diagnostics (i.e. tuberculin skin testing and sputum conversion) [65–67]. Novel diagnostic technologies may be utilized but the applicability in resource-limited settings, where there is a high burden of HIV disease, must be immediately addressed.
Increase Early Detection of HIV among those with TB Disease
Detecting HIV infection in those diagnosed with TB is often neglected but is critical to success. TB accounts for the majority of the mortality seen in those diagnosed with HIV. Only thirty seven percent (500,000) TB patients in the African region knew of their HIV status in 2007 [26]. Identifying HIV infection provides an opportunity to initiate ARV as well as cotrimoxazole prophylaxis. Among those co-infected with HIV and TB, co-trimoxazole prophylaxis and anti-retroviral therapy was instituted in only 63% and 34% of cases, respectively (figure 4) [26].
Figure 4. Antiretroviral therapy coverage in sub-Saharan Africa in 2007.
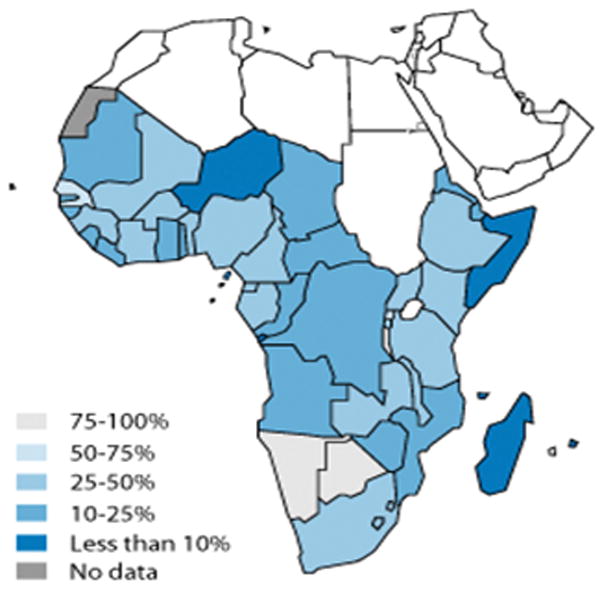
Major progress in HIV testing has been undertaken in the African region compared to previous surveillance data. However, thirty seven percent (500,000) TB patients in the African region knew of their HIV status in 2007. Of the 250,000 HIV-positive TB patients in this region, only 100,000 were started on HIV anti-retroviral therapy (ART). The progress in HIV testing, which still requires improvement, is outpacing treatment with ART. If this continues, the TB epidemic will be sustained in these settings [23,26,33,62].
Reprinted with permission from [68]. World Health Organization. Antiretroviral therapy coverage in sub-Saharan Africa. http://www.who.int/hiv/data/art_coverage/en/index.html.
Ramp Up Isoniazid Prophylactic Therapy
Isoniazid prophylactic therapy has also been vastly underutilized in the HIV population; its focused implementation may serve as a control strategy by reducing a major reservoir of disease [69,70]. Isoniazid has been shown to reduce TB incidence but is only taken by less than 1% of HIV patients infected with latent TB [71]. It is a cost-effective strategy that could be utilized in resource-limited settings where latent TB is likely to be INH-susceptible. INH prophylaxis further enhances the probability of reducing TB when taking antiretroviral therapy [72,73]. If included with comprehensive contact investigations in low-income and middle-income countries, the latent TB reservoir that underlies the continued epidemic could be effectively addressed [74].
Support Strong National TB Programs to Prevent Emergence of Drug-resistant TB
When any piece of a national TB control strategy is weak, the concern for amplification of drug-resistance arises [75]. Insufficient public health measures have led to an estimated 289,000 new MDR-TB cases (3.1% of all new TB patients) in 2007 [26,76]. Cases of MDR-TB were unevenly distributed with 27 countries representing 85% of all cases. The rise in MDR-TB inferred a rise in XDR-TB via the continued implementation of poor case detection and inappropriate/incomplete treatment regimens (figure 5)[53,63]. In 2008, the number of prevalent cases of XDR-TB rose to a record 55 countries and territories [26]. Fewer than 3% of those diagnosed with drug-resistant TB receive treatment according to international guidelines [26]. The cost of treatment has been a major barrier as it is 100-times more expensive to treat MDR-TB as opposed to drug-susceptible TB due to the number of medications and clinical management of its prolonged and potentially toxic treatment course [77,78].
FIGURE 5. MDR-TB treatment outcomes in nine countries in a 2004 cohort.
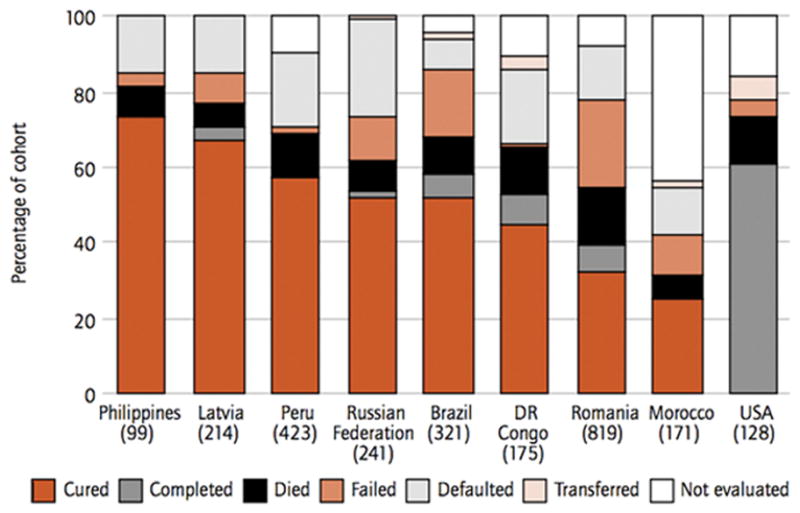
<>brThe number of patients in the cohort is shown under each bar. A notable feature in all categories is the prominent percentage of death and defaulted/failed treatment. Patients with MDR and XDR-TB have a notably greater amount of morbidity and mortality [37,38,39]. The countries that contain the highest amount of MDR-TB cases include India (131,000), China (112,000), Russian Federation (43,000), South Africa (16,000) and Bangladesh (15,000). Reprinted with permission from World Health Organization. Global Tuberculosis Control 2009. Geneva: World Health Organization 2009.
A recent meeting in April 2009 in Beijing, China was convened among 27 countries with a high-burden of TB drug-resistant disease [79]. A unifying theme during the conference was the necessity of strengthening the overall health systems of all affected countries. Nations and international organizations must improve health services to simultaneously address TB surveillance, treatment and prevention mechanisms [80]. For example, individual nations like China have developed an internet-based disease information system that requires hospitals to report details of all patients presenting to TB dispensaries and therefore contributing to its 70% case detection target [75]. International bodies like the Stop TB Department have also contributed to strengthening of health systems through such programs as the Global Laboratory Initiative. This program works at country and regional levels to create a multifaceted approach to laboratory capacity strengthening to diagnose drug-resistant TB in even resource-limited settings [81].
However, significant monetary and personnel resources are required for such programs. A gap of $800 million exists between the available funding in 2009 and necessary requirements to fulfill the Global Plan initiatives for 22 of the high TB burden countries – the regions with the highest amount of drug-resistance. The majority of funding deficits are notably for MDR-TB diagnosis and treatment in the South-East Asia and Western Pacific regions. Two specific areas that are notably underfunded for drug-resistant TB programs include the economic engines and increasingly populous countries of India and China [26].
TB FUNDING ISSUES
Close the Funding Gap for DOTS
The existing funding gap among the 94 countries with 93% of global cases in 2009 is $1.6 billion [26]. According to the World Bank, the economic burden of deaths associated with TB (including HIV co-infection) in Sub-Saharan Africa is $519 billion (95% CI, $475–$563) when there is no DOTS coverage. The report noted that if DOTS was sustained at 2005 coverage levels in key high-burden countries, then there would be an estimated economic gain of around $1.6 trillion (over the period 2006–2015) – ranging from $0.74 billion (95% CI, $0.64–$0.84) in Zimbabwe to $748 billion (95% CI, $638–$857) in China [5].
Fully Fund TB Control Programs
Of the 94 countries that reported 93% of TB cases, a total of $4.2 billion is required for full implementation of country plans in 2009. 87% of funding derives from national governments (including loans), 9% from the Global Plan and 4% from donors other than the Global Plan. The current economic crisis has directly impacted financial spending on various TB and other health-related programs whose impact is most directly felt on impoverished populations [82,83]. If high-burden countries’ governments cannot support these efforts, then the burden of funding will inevitably fall upon non-governmental agencies, international bodies and resource-rich countries [84–86].
Greater Funding to Integrate TB-HIV Services
Of the total of $2.9 billion required for full implementation of the country plans in the 22 most high-burden countries in 2009, solely 3% is dedicated to joint TB/HIV activities [26]. This budgetary percentage is likely insufficient when considering a high TB burden country like South Africa where prevalence of HIV at antenatal clinics varies from 15% to 39% [87,88]. Cost-effective strategies may be immediately implemented utilizing currently available technologies in resource-limited settings. For example, a centralized TB culture program serving HIV-positive patients in urban Brazil suggests that solid TB media culture alone has substantial impact and reasonable cost-effectiveness when deployed in this setting. It was estimated that solid TB culture could avert an estimated 37 disability-adjusted life years (DALYs) per 1,000 TB suspects and prevent 49% of all TB deaths occurring after initial presentation, at a cost of $962 per DALY averted [89].
CONCLUSION
Several critical gaps can be identified in worldwide TB control efforts. These gaps are evident in the global rate of decline in TB cases being less than what would be required to achieve the Millennium Development goal of TB elimination by 2050. Moreover, this has led to the rise in drug-resistant cases most prominently in population-dense countries like China and India. The deadly duo of TB and HIV co-infection is fuelling the TB epidemic in many countries. Although the greater burden of disease has largely fallen on resource-limited nations, our increasingly globalized environment has shown that the responsibility for action must be sought in all international arenas. Many solutions are immediately available but the funding gaps to realize these initiatives are a major obstacle.
Strategies that should be considered include policies that enhance DOTS by improving diagnostics to increase case detection, including private practitioners and closing the funding gap in DOTS-based programs. Risk factors for continued TB transmission could be addressed by reducing socioeconomic health disparities and improving financial support to national TB control programs. Better management of HIV and TB co-infection may occur by enhancing diagnostic applications of both disease processes and utilizing isoniazid prophylactic therapy. These strategies could serve as part of the foundation to address the emergence of drug-resistant TB and ultimately the elimination of the disease. Underlying any effort in TB elimination though will be increased funding and political will from both the international community and national health sectors.
Acknowledgments
Financial Support
The support of NIAID awards 30036, 079590, 37856, and 36973 is gratefully acknowledged.
Footnotes
Potential Conflicts of Interests
We declare that we have no conflicts of interest
References
- 1.Raviglione MC, Pio A. Evolution of WHO policies for tuberculosis control, 1948–2001. Lancet. 2002;359:775–780. doi: 10.1016/s0140-6736(02)07880-7. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Forty-fourth World Health Assembly. Geneva: World Health Organization; 1991. [Google Scholar]
- 3.Lönroth K, Raviglione M. Global epidemiology of tuberculosis: Prospects for control. Semin Respir Crit Care Med. 2008;29:481–491. doi: 10.1055/s-0028-1085700. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Global Tuberculosis Control. Geneva: World Health Organization; 2008. [Google Scholar]
- 5.Laxminarayan R, Klein E, Dye C, Floyd K, Darley S, Adeyi O. Policy research working paper 4295. Washington: World Bank; 2007. Economic benefit of tuberculosis control. [Google Scholar]
- 6.World Health Organization. Guidelines for establishing DOTS-Plus pilot projects for the management of multidrug-resistant tuberculosis (MDR-TB) Geneva: World Health Organization; 2000. [Google Scholar]
- 7.Raviglione MC, Uplekar M. WHO’s new Stop TB Strategy of WHO. Lancet. 2006;367:952–955. doi: 10.1016/S0140-6736(06)68392-X. [DOI] [PubMed] [Google Scholar]
- 8.Maher D, Dyee C, Floyd K, et al. Planning to improve global health: the next decade of tuberculosis control. Bull World Health Organ. 2007;85:341–347. doi: 10.2471/06.037820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raviglione MC. The new Stop TB Strategy and the Global Plan to Stop TB, 2006–2015. Bull World Health Organ. 2007;85:327. doi: 10.2471/06.038513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stop TB Partnership. The Global Plan to Stop TB 2006–2015. Geneva: World Health Organization; 2006. [Google Scholar]
- 11.Komatsu R, Low-Beer D, Schwartländer B. Global Fund-supported programmes contribution to international targets and the Millenium Development Goals: an initial analysis. Bull World Health Organ. 2007;10:805–811. doi: 10.2471/BLT.06.038315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young DB, Perkins MD, Duncan K, Barry CE. Confronting the scientific obstacles to global control of tuberculosis. J Clin Invest. 2008;4:1255–1265. doi: 10.1172/JCI34614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dye C, Hosseini M, Watt C. Did we reach the 2005 targets for tuberculosis control? Bull World Health Organ. 2007;85:364–369. doi: 10.2471/06.037580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.International Monetary Fund. The implications of the global financial crisis for developing countries. Washington, DC: IMF; 2009. [Google Scholar]
- 15.Calì M, Massa I, te Velde DW. ODI Background Paper. London: Overseas Development Institute; 2009. The global financial crisis: Financial flows to developing countries set to fall by one quarter. [Google Scholar]
- 16.Overseas Development Institute. A development charter for the G-20. London: Overseas Development Institute; 2009. [Google Scholar]
- 17.Morris K. Global tuberculosis control amid the world economic crisis. Lancet Infect Dis. 2009;3:144–145. doi: 10.1016/s1473-3099(09)70030-1. [DOI] [PubMed] [Google Scholar]
- 18.World Bank. New & Broadcast. World Bank; 2008. [accessed on April 14, 2009]. Food price crisis imperils 100 million in poor countries, Zoellick says. http://go.worldbank.org/5W9U9WTJB0. [Google Scholar]
- 19.Frieden TR. Lessons from tuberculosis control for public health. Int J Tuberc Lung Dis. 2009;4:421–428. [PubMed] [Google Scholar]
- 20.Lönnroth K, Holtz TH, Cobelens F, et al. Inclusion of information on risk factors, socio-economic status and health seeking in a tuberculosis prevalence survey. Int J Tuberc Lung Dis. 2009;2:171–176. [PubMed] [Google Scholar]
- 21.Bobashev G, Morris RJ, Goedecke DM. Sampling for global epidemic models and the topology of an international airport network. PLoS ONE. 2008;9:e3154. doi: 10.1371/journal.pone.0003154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. Updated guidelines on tuberculosis and air travel. Weekly epidemiological record. 2008;83:209–216. [Google Scholar]
- 23.Kaplan JE, Benson C, Holmes KH, et al. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Disease Society of America. MMWR Recomm Rep. 2009;58:1–207. [PubMed] [Google Scholar]
- 24.Stuckler D, King LP, Basu S. International Monetary Fund programs and tuberculosis outcomes in post-communist countries. PLoS Med. 2008;7:e143. doi: 10.1371/journal.pmed.0050143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention (CDC) Plan to combat extensively drug-resistant tuberculosis: recommendations of the Federal Tuberculosis Task Force. MMWR Recomm Rep. 2009;58:1–43. [PubMed] [Google Scholar]
- 26.World Health Organization. Global Tuberculosis Control 2009. Geneva: World Health Organization; 2009. [Google Scholar]
- 27.Dye C. Targets for global tuberculosis control. International Journal of Tuberculosis and Lung Disease. 2006;10:460–462. [PubMed] [Google Scholar]
- 28.The Global Plan to Stop TB. 2006–2015: actions for life towards a world free of tuberculosis. Geneva: World Health Organization; 2006. [Google Scholar]
- 29.Russell S. The economic burden of illness for households in developing countries: a review of studies focusing on malaria, tuberculosis, and human immunodeficiency virus/acquired immunodeficiency syndrome. Am J Trop Med Hyg. 2004;71:147–155. [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention (CDC). . Trend in tuberculosis --- United States, 2008. MMWR. 2009;58:249–253. [Google Scholar]
- 31.World Health Organization. Global tuberculosis: surveillance, planning, financing. Geneva: World Health Organization; 2006. [Google Scholar]
- 32.Taylor Z, Nolan CM, Blumberg HM, et al. Controlling tuberculosis in the United States. Recommendations from the American Thoracic Society, CDC, and the Infectious Diseases Society of America. MMWR Recomm Rep. 2005;54:1–81. [PubMed] [Google Scholar]
- 33.Mukherjee JS, Rich ML, Socci AR, et al. Programmes and principles in treatment of multidrug-resistant tuberculosis. Lancet. 2004;363:474–481. doi: 10.1016/S0140-6736(04)15496-2. [DOI] [PubMed] [Google Scholar]
- 34.Frieden TR, Fujiwara PI, Washko RM, Hamburg MA. Tuberculosis in New York City. N Eng J Med. 1995;333:521–526. doi: 10.1056/NEJM199507273330406. [DOI] [PubMed] [Google Scholar]
- 35.Hopewell PC, Pai M, Maher D, Uplekar M, Raviglione MC. International Standards for Tuberculosis Care. Lancet Infect Dis. 2006;6:710–725. doi: 10.1016/S1473-3099(06)70628-4. [DOI] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Preventation (CDC). . Emergence of Mycobacterium Tuberculosis with extensive resistance to second-line drugs-worldwide, 2000–2004. MMWR Morb Mortal Wkly Rep. 2006;55:301–305. [PubMed] [Google Scholar]
- 37.WHO, IUATLD. Fourth global report. Geneva: World Health Organization; 2008. Anti-tuberculosis drug resistance in the world. [Google Scholar]
- 38.Siddiqi K, Lambert ML, Walley J. Clinical diagnosis of smear-negative pulmonary tuberculosis in low-income countries: the current evidence. Lancet Infect Dis. 2003;3:2888–296. doi: 10.1016/s1473-3099(03)00609-1. [DOI] [PubMed] [Google Scholar]
- 39.Mtei L, Matee M, Herfort O, et al. High rates of clinical and subclinical tuberculosis among HIV-infected ambulatory subjects in Tanzania. Clin Infect Dis. 2005;40:1500–1507. doi: 10.1086/429825. [DOI] [PubMed] [Google Scholar]
- 40.Getahun H, Harrington M, O’Brien R, Nunn P. Diagnosis of smear-negative pulmonary tuberculosis in people with HIV infection or AIDS in resource-constrained settings: informing urgent policy changes. Lancet. 2007;369:2042–2049. doi: 10.1016/S0140-6736(07)60284-0. [DOI] [PubMed] [Google Scholar]
- 41.Millen SJ, Uys PW, Hargrove J, van Helden PD, Williams BG. The effect of diagnostic delays on the drop-out rate and the total delay to diagnosis of tuberculosis. PLoS ONE. 2008;3:e1933. doi: 10.1371/journal.pone.0001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malmborg R, Mann G, Thomson R, Squire SB. Can public-private collaboration promote tuberculosis case detection among the poor and vulnerable? Bull Who Health Org. 2006;84:752–758. [PMC free article] [PubMed] [Google Scholar]
- 43.Lin H, Ezzati M, Murray M. Tobacco smoke, indoor air pollution and tuberculosis: a systematic review and metanalysis. PLoS Med. 2007;4:e142. doi: 10.1371/journal.pmed.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cegielski JP, McMurray DN. The relationship between malnutrition and tuberculosis: evidence from studies in humans and experimental animals. Int J Tuberc Lung Dis. 2004;8:286–298. [PubMed] [Google Scholar]
- 45.Slama K, Chiang CY, Enarson D, et al. Tobacco and tuberculosis: a qualitative systematic review and meta-analysis. Int J Tuberc Lung Dis. 2007;11:1049–1061. [PubMed] [Google Scholar]
- 46.Rehfuess E. Fuel for life: Household Energy and Health. Geneva: World Health Organization; 2006. [Google Scholar]
- 47.Hsien-Ho L, Murray M, Cohen T, Colijn C, Ezzati Effects of smoking and solid-fuel use on COPD, lung cancer and tuberculosis in China: a time-based multiple risk factor, modeling study. Lancet. 2008;372:1473–1483. doi: 10.1016/S0140-6736(08)61345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brands A, Ottmani S, Lönroth K, et al. Reply to Addressing smoking cessation in tuberculosis control. Bull World Health Organ. 2007;85:647–648. doi: 10.2471/BLT.07.044982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.World Health Organization. Reaching the Poor: Challenges for TB Programmes in the Western Pacific Region. Manila: World Health Organization; 2004. [Google Scholar]
- 50.Muniyandi M, Ramachandran R, Gopi PG, et al. The prevalence of tuberculosis in different economic strata: a community survey from South India. Int J Tuberc Lung Dis. 2007;11:1042–1045. [PubMed] [Google Scholar]
- 51.World Health Organization. Commission on Social Determinants of Health. Geneva: World Health Organization; 2006. [Google Scholar]
- 52.Bock N, Jensen PA, Miller B, Nardell E. Tuberculosis infection control in resource-limited settings in the era of expanding HIV care and treatment. J Infect Dis. 2007;196:s108–113. doi: 10.1086/518661. [DOI] [PubMed] [Google Scholar]
- 53.Jassal M, Bishai WR. Extensively drug resistant tuberculosis. Lancet Infect Dis. 2009;9:19–30. doi: 10.1016/S1473-3099(08)70260-3. [DOI] [PubMed] [Google Scholar]
- 54.Jensen PA, Lambert LA, Iademarco MF, Ridzon F. Guideline for preventing the transmission of Mycobacterium tuberculosis in health-care settings. MMWR Recomm Rep. 2005;55 (RR-17):1–141. [PubMed] [Google Scholar]
- 55.Basu S, Andrews JR, Poolman EM, et al. Prevention of nosocomial transmission of extensively drug-resistant tuberculosis in rural South African district hospitals: an epidemiological modeling study. Lancet. 2007;370:1500–1507. doi: 10.1016/S0140-6736(07)61636-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Uplekar MW, Shepard DS. Treatment of tuberculosis by private general practitioners in India. Tubercle. 1991;72:284–290. doi: 10.1016/0041-3879(91)90055-w. [DOI] [PubMed] [Google Scholar]
- 57.Impact of advocacy on the tuberculosis management practices of private practitioners in Chennai City, India. Int J Tuberc Lung Dis. 2009;13:112–118. [PubMed] [Google Scholar]
- 58.Luby S, Zaidi N, Rehman S, Northrup R. Improving private practitioners sick-child case management in two urban communities in Pakistan. Trop Med Int Health. 2002;7:210–219. doi: 10.1046/j.1365-3156.2002.00859.x. [DOI] [PubMed] [Google Scholar]
- 59.Hongoro C, Kumaranayake L. Do they work? Regulating for-profit providers in Zimbabwe. Health Policy Plan. 2000;15:368–377. doi: 10.1093/heapol/15.4.368. [DOI] [PubMed] [Google Scholar]
- 60.Goldfeld A, Ellner JJ. Pathogenesis of management of HIV/TB co-infection in Asia. Tuberculosis (Edib) 2007;87 (Suppl):S26–S30. doi: 10.1016/j.tube.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 61.Nunn P, Williams B, Floyd K, Dye C, Elzinga G, Raviglione M. Tuberculosis control in the era of HIV. Nat Rev immunol. 2005;5:819–826. doi: 10.1038/nri1704. [DOI] [PubMed] [Google Scholar]
- 62.Reid A, Scano F, Getahun H. Towards universal access to HIV prevention, treatment, care, and support: the role of tuberculosis/HIV collaboration. Lancet Infect Dis. 2006;6:483–95. doi: 10.1016/S1473-3099(06)70549-7. [DOI] [PubMed] [Google Scholar]
- 63.Gillespie SH. Evolution of drug resistance in Mycobacterium tuberculosis: clinical and molecular perspective. Antimicrob Agents Chemother. 2002;46:267–274. doi: 10.1128/AAC.46.2.267-274.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dorman SE, Chaisson RE. From magic bullets back to the Magic Mountain: the rise of extensively drug-resistant tuberculosis. Nat Med. 2007;13:295–298. doi: 10.1038/nm0307-295. [DOI] [PubMed] [Google Scholar]
- 65.Mtei L, Matee M, Herfort O, et al. High rates of clinical and subclinical tuberculosis among HIV-infected ambulatory subjects in Tanzania. Clin Infect Dis. 2005;40:1500–1507. doi: 10.1086/429825. [DOI] [PubMed] [Google Scholar]
- 66.Getahun H, Harrington M, O’Brien R, Nunn P. Diagnosis of smear-negative pulmonary tuberculosis in people with HIV infection or AIDS in resource-constrained settings: informing urgent policy changes. Lancet. 2007;369:2042–2049. doi: 10.1016/S0140-6736(07)60284-0. [DOI] [PubMed] [Google Scholar]
- 67.Mwandumba HC, Bertel Squire S, White SA, et al. Association between sputum smear status and local immune responses at the site of disease in HIV-infected patients with pulmonary tuberculosis. Tuberculosis. 2008;88:58–63. doi: 10.1016/j.tube.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 68.World Health Organization. [accessed on May 14, 2009];Antiretroviral therapy coverage in sub-Saharan Africa. http://www.who.int/hiv/data/art_coverage/en/index.html.
- 69.Dye C, Watt CJ, Bleed DM, et al. Evolution of tuberculosis control and prospects for reducing tuberculosis incidence, prevalence and deaths globally. JAMA. 2005;293:2767–2775. doi: 10.1001/jama.293.22.2767. [DOI] [PubMed] [Google Scholar]
- 70.Golub JE, Saraceni V, Cavalcante SC, et al. The impact of antiretroviral therapy and isoniazid preventive therapy on tuberculosis incidence in HIV-infected patients in Rio de Janeiro, Brazil. AIDS. 2007;21:1441–1448. doi: 10.1097/QAD.0b013e328216f441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Szakacs TA, Wilson D, Cameron DW, Clark M, Kocheleff P, Muller FJ, McCarthy AE. Adherence with isoniazid for prevention of tuberculosis among HIV-infected adults in South Africa. BMC Infect Dis. 2006;6:97. doi: 10.1186/1471-2334-6-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lim HJ, Okwera A, Mayanja-Kizza H, Ellner JJ, Mugerwa RD, Whalen CC. Effect of tuberculosis preventive therapy on HIV disease progression and survival in HIV-infected adults. HIV Clin Trials. 2006;7:172–183. doi: 10.1310/hct0704-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gary DM, Zar H, Cotton M. Impact of tuberculosis preventative therapy on tuberculosis mortality in HIV-infected children. Cochrane Database Syst Rev. 2009;1:CD006418. doi: 10.1002/14651858.CD006418.pub2. [DOI] [PubMed] [Google Scholar]
- 74.Morrison J, Pai M, Hopewell Tuberculosis and latent tuberculosis infection in close contacts of people with pulmonary tuberculosis in low-income and middle-income countries: a systematic review and meta-analysis. Lancet. 2008;8:359–368. doi: 10.1016/S1473-3099(08)70071-9. [DOI] [PubMed] [Google Scholar]
- 75.Dye C. Doomsday postponed? Preventing and reversing epidemics of drug-resistant tuberculosis. Nat Rev Microbiol. 2009;7:81–87. doi: 10.1038/nrmicro2048. [DOI] [PubMed] [Google Scholar]
- 76.World Health Organization. A ministerial meeting of high M/XDR-TB burden. 2009. countries; 1–3 April 2009; Beijing China. World Health Organization; 2009. [Google Scholar]
- 77.Ranjbhandary SS, Marks SM, Bock NN. Costs of patients hospitalized for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2004;8:1012–1016. [PMC free article] [PubMed] [Google Scholar]
- 78.Tupasi TE, Gupta R, Quelapio MI, et al. Feasibility and cost-effectiveness of treating multidrug-resistant tuberculosis: a cohort study in the Phillipines. PLoS Med. 2006;3:e352. doi: 10.1371/journal.pmed.0030352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cheng M. Ministerial meeting agrees plan for tuberculosis control. Lancet. 2009;373:1328. doi: 10.1016/s0140-6736(09)60757-1. [DOI] [PubMed] [Google Scholar]
- 80.Das Gupta M, Gostin L. Donors’ roles in building of global public goods in health. Lancet. 2009:1395–1397. doi: 10.1016/S0140-6736(09)60245-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.World Health Organization. [Accessed: May 12, 2009];Global laboratory initiative. Available at: http://www.who.int/tb/dots.laboratory/gli/en/index.html.
- 82.Long Q, Li Y, Wang Y, et al. Barriers to accessing TB diagnosis for rural-to-urban migrants with chronic cough in Chongqing, China: a mixed methods study. BMC Health Serv Res. 2008;8:202. doi: 10.1186/1472-6963-8-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee JW, Loevinsohn E, Kumaresan JA. Response to a major disease of poverty: the Global Partnership to Stop TB. Bull World Health Organ. 2002;80:428. [PMC free article] [PubMed] [Google Scholar]
- 84.Marmot MG, Bell R. How will the financial crisis affect health? BMJ. 2009 doi: 10.1136/bmj.b1314. [DOI] [PubMed] [Google Scholar]
- 85.Erhola ML, Brimkulov N, Chubakov T, et al. Development process of the Practical Approach to Lung Health in Kyrgyzstan. Int J Tuberc Lung Dis. 2009;13:540–4. [PubMed] [Google Scholar]
- 86.Vassall A, Chechulin Y, Raykhert I, et al. Reforming tuberculosis control in Ukraine: results of pilot projects and implications for the national scale-up of DOTS. Health Policy Plan. 2009;24:55–62. doi: 10.1093/heapol/czn045. [DOI] [PubMed] [Google Scholar]
- 87.Martinson N, Mohapi L, Bakos D, Gray GE, McIntyre JA, Holmes CB. Costs of providing care for HIV-infected adults in an urban HIV clinic in Soweto, South Africa. J Acquir Immune Defic Syndr. 2009;3:327–330. doi: 10.1097/QAI.0b013e3181958546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.UNAIDS/WHO. [Accessed: May 8, 2008];AIDS epidemic update 2007 regional summaries. 2008 Apr 16; Available at: http://data.unaids.org/pub/Report/2008/jc1526_epibriefs_ssafrica_en.pdf.
- 89.Dowdy DW, Lourenço MC, Cavalcante SC, et al. Impact and cost-effectiveness of culture for diagnosis of tuberculosis in HIV-infected Brazilian adults. PLoS ONE. 2008;12:e4057. doi: 10.1371/journal.pone.0004057. [DOI] [PMC free article] [PubMed] [Google Scholar]


