Abstract
Objective
The objective of this study was to assess craving and mood related to opioid and cocaine use among asymptomatic hepatitis C virus (HCV)+ and HCV− methadone patients who have not started antiviral treatment.
Methods
In this 28-week prospective ecological momentary assessment (EMA) study, 114 methadone-maintained, heroin- and cocaine-abusing individuals reported from the field in real time on their mood, craving, exposure to drug-use triggers, and drug use via handheld computers.
Results
Sixty-one percent were HCV+; none were overtly symptomatic or receiving HCV treatment. HCV status was not associated with age, sex, race, or past-30-day or lifetime heroin or cocaine use. In event-contingent EMA entries, HCV+ individuals more often attributed use to having been bored, worried, or sad; feeling uncomfortable; or others being critical of them compared with HCV− participants. In randomly prompted EMA entries, HCV+ participants reported significantly more exposure to drug-use triggers, including handling ≥$10, seeing cocaine or heroin, seeing someone being offered/use cocaine or heroin, being tempted to use cocaine, and wanting to see what would happen if they used just a little cocaine or heroin.
Conclusions
HCV+ individuals experienced more negative moods and more often cited these negative moods as causes for drug use. HCV+ individuals reported greater exposure to environmental drug-use triggers, but they did not more frequently cite these as causes for drug use. The EMA data reported here suggest that HCV+ intravenous drug users may experience more labile mood and more reactivity to mood than HCV− intravenous drug users. The reason for the difference is not clear, but HCV status may be relevant to tailoring of treatment.
Keywords: hepatitis C, ecological momentary assessment, craving, triggers, mood
Infection with hepatitis C virus (HCV) is a complex public health problem with a high prevalence of chronic infection, an increasing prevalence of HCV-associated disease, low rates of testing and treatment, and the prospect of increasing incidence associated with intravenous drug use (IVDU; Smith, Jorgensen, Zibbell, & Beckett, 2012). IVDU is a major risk factor for HCV, and HCV prevalence is exceedingly high among intravenous drug users (Schreuder et al., 2010). A study conducted in Baltimore, MD, found that the seroprevalence of HCV among intravenous drug users was 77%, with most individuals becoming positive within the first year of initiating IVDU (Garfein, Vlahov, Galai, Doherty, & Nelson, 1996). IVDU does not directly influence HCV progression despite the potential for multiple repeat exposures to various HCV genotypes, but HCV progression may be accelerated by several factors associated with IVDU, including excess alcohol intake, reduced access and adherence to treatment, and poor nutrition (Cooper & Mills, 2006).
Given the adverse public health consequences of HCV infection and its link to HIV, improved approaches to reducing the spread of HCV, particularly among intravenous drug users, are needed. Although there is strong evidence that medication-assisted addiction treatment reduces drug misuse, HIV-risk behaviors, and HIV infection rates (Metzger, Woody, & O’Brien, 2010; Millson et al., 2007), the corresponding evidence for HCV remains controversial (Crofts, Nigro, Oman, Stevenson, & Sherman, 1997; Selvey, Denton, & Plant, 1997; Thiede, Hagan, & Murrill, 2000), suggesting that approaches beyond medication-assisted treatment alone are needed to curb HCV-risk behaviors and infection rates. Given that risk behaviors persist during addiction treatment (Chaudhry et al., 2011) and are more likely among patients who do not know their HCV status (Vidal-Trécan, Coste, Varescon-Pousson, Christoforov, & Boissonnas, 2000), one approach might be to test people for hepatitis C upon addiction-treatment admission and inform them of the results. Unawareness of HCV status is common. In one study, 72% of HCV+ and 46% of HCV− intravenous drug users were unaware of their HCV status (Hagan et al., 2006). However, even after diagnosis with HCV, high-risk behaviors continue (Hagan et al., 2006; Ompad, Fuller, Vlahov, Thomas, & Strathdee, 2002). The initial diagnosis of HCV had a strong, negative emotional effect in almost half of a patient group that was not primarily composed of intravenous drug users (Fabris et al., 2006). For intravenous drug users, an HCV diagnosis is sometimes thought to be less momentous than for nonintravenous drug users, but that may not always be true (Treloar & Rhodes, 2009). As for possible emotional effects of the disease process itself, there seems to be no published research; phenomenological studies of hepatitis C focus on patients who have begun antiviral treatment, which can produce psychiatric side effects of its own (Sgorbini, O’Brien, & Jackson, 2009). What is unknown is whether ongoing risk behavior is increased by the negative emotional effect of HCV diagnosis itself or by other factors in the lived experience of HCV+ individuals not on antiviral treatment.
The analyses presented here are part of a project to better understand the factors influencing drug use and lapse and improve approaches to reducing high-risk behaviors. We have followed up on prior research in our laboratory showing that HCV+ patients in methadone maintenance report higher drug-related HIV-risk behaviors and are more likely to test positive for opioids compared with HCV− patients (Willner-Reid, Belendiuk, Epstein, Schmittner, & Preston, 2008). Our treatment-research clinic uses ecological momentary assessment (EMA), a technique for collection of in-the-field, first-person data using handheld computers. EMA largely eliminates recall bias by collecting self-report in near real time and produces time-stamped data that are amenable to statistical aggregation (Shiffman, Stone, & Hufford, 2008). We have previously reported success with EMA in a study of over 100 heroin- and cocaine-using methadone-maintained patients and shown that cocaine-use events are preceded by increasing exposure to drug-use triggers and are associated with craving, stress, and tobacco use (Epstein, Marrone, Heishman, Schmittner, & Preston, 2010; Epstein & Preston, 2010; Epstein et al., 2009; Preston et al., 2009). In the analyses reported here, we determine whether HCV+ and HCV− individuals are differentially exposed, in their daily lives, to triggers of craving and drug use, and we explore the relationship of HCV status with demographics and momentary moods in patients whose HCV is not yet overtly symptomatic in an effort to inform treatment interventions for reduction of HCV-risk behavior and infection rates.
Method
Participants and Setting
Participants were heroin- and cocaine-using individuals in an outpatient methadone treatment setting in Baltimore, MD, enrolled in a prospective cohort study from 2006 to 2007. Participants received daily directly observed methadone, weekly individual counseling, and abstinence reinforcement (weeks 7–19 only). All individuals were recruited from the community via newspaper and personal referral. Participants were eligible for inclusion in the study if they met the following criteria: (a) age between 18 and 65; (b) evidence of physical dependence on opioids (self-report and physical exam); and (c) evidence of cocaine and opiate use (self-report and urine screen). Exclusion criteria included (a) schizophrenia or any other Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM–IV) psychotic disorder, history of bipolar disorder, or current major depressive disorder; (b) current dependence on alcohol or sedative-hypnotic (e.g., benzodiazepine [by DSM–IV criteria]); (c) cognitive impairment severe enough to preclude informed consent or valid self-report; (d) medical illness that in the view of the investigators would compromise participation in research; or (e) urologic conditions that would inhibit urine collection.
The National Institute on Drug Abuse Intramural Research Program Institutional Review Board approved this study, and written informed consent was obtained before participation. The study is in the National Institutes of Health Clinical Trial registry (NCT00292136).
Data Collection
Demographic data and drug-use history were collected at baseline using the Addiction Severity Index (ASI; McLellan, Luborsky, O’Brien, & Woody, 1980) and psychiatric history was collected using the Diagnostic Interview Schedule for the DSM–IV (Robins, Helzer, Croughan, & Ratcliff, 1981). Hepatitis C serostatus was determined by HCV antibody blood test on admission as part of general screening for medical illness; test results were not otherwise used as part of inclusion/exclusion criteria. Drug use was monitored via drug screens on urine specimens collected 3 times per week under observation.
EMA data were collected during weeks 3–28 on handheld personal digital assistants (PDAs; Palm Zire or Palm Zire 21, Palm, Inc., Sunnyvale, CA) that ran our Transactional Electronic Diary software (Vahabzadeh, Epstein, Mezghanni, Lin, & Preston, 2004; Vahabzadeh, Lin, Mezghanni, Epstein, & Preston, 2009). EMA methods are described briefly in the following paragraph; a more detailed description has been previously published (Epstein et al., 2009).
Participants were asked to make two kinds of EMA entries. The first type of entry was an event-contingent entry, which was initiated by the participant when he or she either craved or used heroin, cocaine, or both. The second type of entry was a random-prompt entry, which was prompted by the PDA and occurred 2–5 times per day during preset hours of wakefulness determined for each participant.
Drug-use triggers were assessed in both types of entries. In each event-contingent entry for heroin or cocaine craving or drug use, participants responded to a series of statements that began with, “I think it happened because …” Each random prompt consisted of the same statements that began “Within the past hour …” The drug-use triggers and the wording of the items were based on a published taxonomy by Marlatt and Gordon (Heather, Stallard, & Tebbutt, 1991; Marlatt & Gordon, 1985) and our clinical experience (Epstein et al., 2009). The following items were answered yes or no: I felt bored; I felt angry or frustrated; I felt worried, anxious, or tense; I felt sad; I felt others were being critical of me; I handled $10 or more in cash; I was in a good mood and felt like celebrating; I felt ill, in pain, or uncomfortable. The possible responses were heroin, cocaine, both, or neither on the following items: I saw …; I saw someone using …; Someone offered me …; I wanted to see what would happen if I used just a little …; out of the blue I felt tempted to use …. The questions were always presented in the same order, and the participants could endorse multiple questions.
In the random prompts only, mood was assessed with adjectives (happy, relaxed, tired, irritated, stressed, and bored) derived from the Circumplex Model of Affect (Russell, 1980) in six items worded “Right now, do you feel …?” and rated on a 4-point scale using Shiffman’s “NO, no, yes, YES” response anchors (Epstein & Preston, 2010; Shiffman et al., 2002). Craving for heroin, cocaine, and tobacco were posed as “Right now do you crave …?” and rated on the same scale (Shiffman et al., 2002).
Data Analyses
Analyses were done with Stata 10.0 (Stata, 2007) and SAS 9.1. Demographics and drug-use characteristics were compared across HCV+ and HCV− groups with Pearson χ2 tests, Fisher’s exact tests, and independent-sample t tests.
EMA craving and use data for event-contingent and randomly prompted entries were analyzed with generalized linear mixed models (SAS Proc Glimmix) for dichotomous outcomes and general linear mixed models (SAS Proc Mixed) for continuous outcomes. Participants were treated as a random factor. The models were adjusted for education, which was one of the few significant demographic differences between groups in bivariate analyses. Bivariate predictors with p ≤ .20 were included in the multivariate analyses. We conducted diagnostic tests on all multivariate models and found no evidence of collinearity (variance inflation factors all <10).
Urine drug-use data were analyzed with generalized linear mixed models (SAS Proc Glimmix) and are reported as model-adjusted percent-negative. Proc Glimmix handles incomplete repeated-measures data without requiring imputation of missing data points. Participants were treated as a random factor. Each model included two predictors: HCV (positive or negative) and day, along with their interaction. We included up to 58 urine data points for each participant; after the 58th urine (week 19 on a thrice-weekly collection schedule) many of the participants had transferred to other treatment programs or were starting their methadone dose tapers.
Effect sizes are given in terms of positive correlation coefficients, which we derived from the F tests and denominator degrees of freedom when the numerator degrees of freedom equaled 1 (Rosnow, Rosenthal, & Rubin, 2000).
To adjust for multiple tests of significance (45 in all for the group comparisons on EMA responses relevant to moods and triggers, of which 34 are shown here), we entered all obtained p values into the SAS procedure Multtest to obtain false-discovery rate (FDR) p values, which are the ones we report here. FDR correction reduced the number of p values <.05 from 32 to 31. All tests were two-tailed.
Results
Demographics and Drug-Use History
In our sample of 114 heroin and cocaine users, 69 (61%) were HCV+ at baseline. In bivariate analysis, most baseline demographic measures did not differ by HCV status (see Table 1). There were significant differences in drug-use histories, with HCV+ participants spending more on drugs, more likely to use by the intravenous route, and more likely to be current cigarette smokers compared with HCV− individuals. Two HCV+ individuals were on antiviral medications for HIV. No participants were on ribavirin, interferon, or protease inhibitors used to treat chronic hepatitis C. The average maximum methadone dose was not different in HCV− participants (96.2 ± 1.4 mg/day) compared with HCV+ participants (98.3 ± 0.7 mg/day), p = .157.
Table 1.
Baseline Demographics and Drug-Use Characteristics by HCV Status (N = 114)
| Category | HCV+ | HCV− | p |
|---|---|---|---|
| HCV status (n, %) | 69 (61%) | 45 (39%) | |
| Age, years (M ± SD) | 41.5 ± 8.3 | 39.8 ± 7.7 | .28 |
| Male (n, %) | 45 (65%) | 27 (60%) | .57 |
| Non-Caucasian (n, %) | 40 (58%) | 32 (71%) | .16 |
| Education (M ± SD) | 11.6 ± 1.5 | 12.1 ± 1.4 | .10 |
| Net income, 30 days (M ± SD) | $694 ± 1280 | $560 ± 813 | .53 |
| Heroin use, years (M ± SD) | 13.7 ± 8.8 | 13.7 ± 8.2 | .63 |
| Cocaine use, years (M ± SD) | 12.6 ± 9.0 | 10.0 ± 7.8 | .15 |
| Other opiate use, 30 days (M ± SD) | 0.1 ± 0.4 | 0.8 ± 2.1 | .03 |
| Money spent on drugs, 30 days (M ± SD) | $2,074.30 ± 1,332.00 | $1,612.40 ± 1,001.90 | .04 |
| Overdose (M ± SD) | 0.8 ± 2.1 | 0.1 ± 0.5 | .013 |
| Route of administration of heroin (n, %) | <.001 | ||
| Intranasal | 9 (13%) | 36 (80%) | |
| Intravenous | 60 (87%) | 9 (20%) | |
| Route of administration of cocaine (n, %) | <.001 | ||
| Intranasal | 1 (1%) | 8 (19%) | |
| Intravenous | 43 (62%) | 5 (11%) | |
| Smoking | 24 (35%) | 31 (55%) | |
| Drinking to intoxication, 30 days (M ± SD) | 0.1 ± 0.8 | 0.5 ± 1.5 | .12 |
| Current tobacco smoking (n, %) | 64 (93%) | 34 (76%) | <.02 |
Note. HIV prevalence was very low in HCV+ and HCV− groups.
Psychiatric History
There was no indication of a group difference in psychiatric history. For illustration, we present some data on depression from the Diagnostic Interview Schedule for DSM-IV (DIS-IV) interviews. At admission, the mean number of depressive symptoms was 0.96 (SEM 0.28, range 0–9) in the HCV+ participants and 0.69 (SEM 0.24, range 0–8) in the HCV− participants, F(1, 112) = 0.45, p = .50, effect size r = .06. “Current” depression, defined as within the past 12 months, was 3% in HCV+ and 0% in HCV− participants; remitted depression was 6% and 7%, respectively, in HCV+ and HCV− participants. Ninety-one percent of HCV+ and 93% of HCV− participants endorsed no history of depression (p = .73). No participants were receiving medication for depression on admission or during the course of the study.
EMA: Event-Contingent Entries
EMA data were collected for 10,781 person-days. There was no difference in the total number of event-contingent entries reported by HCV+ individuals when compared with HCV− individuals (see Table 2). Furthermore, there was no significant difference by HCV status when looking at the six categories of events: heroin craving, heroin use, cocaine craving, cocaine use, both craving, and both use. Although the numbers of events did not differ between the two groups, participants’ reports of the reasons (triggers) for events did differ. Compared with HCV− participants, HCV+ participants attributed their use more often to mood states such as being bored, uncomfortable, worried, sad, feeling others were critical of them, or being in a good mood and wanting to celebrate (see Table 3). There was no difference by HCV status among other putative triggers for use, such as handling cash, seeing cocaine or heroin, seeing someone using cocaine or heroin, someone offering cocaine or heroin, feeling tempted out of blue, or wanting to test self-control by using just a little (data not shown).
Table 2.
EMA Entries by HCV Status (N = 114)
| Category | HCV+ | HCV− | p |
|---|---|---|---|
| Event-contingent entries (per participant) | |||
| Total number entries (M ± SD) | 23.0 ± 20.2 | 24.7 ± 28.2 | .72 |
| Heroin craving events (M ± SD) | 1.6 ± 3.0 | 4.3 ± 9.8 | .08 |
| Heroin use events (M ± SD) | 0.7 ± 2.0 | 0.5 ± 1.2 | .56 |
| Cocaine craving (M ± SD) | 5.7 ± 8.7 | 6.6 ± 9.6 | .62 |
| Cocaine use events (M ± SD) | 6.6 ± 10.1 | 6.5 ± 10.2 | .98 |
| Both craving events (M ± SD) | 6.0 ± 9.6 | 5.3 ± 11.4 | .75 |
| Both use events (M ± SD) | 2.5 ± 4.7 | 1.6 ± 3.0 | .19 |
| Random-prompt entries | |||
| Compliance (M ± SD; range)a | 73% (16.6%; 8%–98%) | 76% (13.4%; 38%–96%) | .43 |
| Total number of entries | 222.6 ± 113.3 | 258.3 ± 122.2 | .11 |
| Drinking at random prompt (95% CI) | 2% (1.6%, 2.1%) | 1% (1.0%, 1.7%) | .02 |
| Smoking at random prompt (95% CI) | 34% (33%, 35%) | 24% (23%, 26%) | <.0001 |
Group differences reported here include adjustment for differences in education (but are also statistically significant without that adjustment).
Table 3.
Reason for Drug Use in Event-Contingent Entries and Exposure to Mood-Related Cues in Random-Prompt Entries by HCV Status (Adjusted for Education)
| Mood Triggers | Event-Contingent Entries—It Happened Because …
|
Random-Prompt Entries—Within the Past Hour …
|
||||||
|---|---|---|---|---|---|---|---|---|
| HCV+ (%, 95% CI) | HCV− (%, 95% CI) | F Value (df) | p | HCV+ (%, 95% CI) | HCV− (%, 95% CI) | F Value (df) | p | |
| Bored | 52% (46%, 57%) | 32% (26%, 39%) | F (1, 83) = 16.62 | .0002 | 14% (13%, 15%) | 9% (8%, 9.4%) | F (1, 111) = 67.5 | .002 |
| Felt others critical | 23% (19%, 28%) | 12% (8%, 17%) | F(1, 83) = 10.38 | .004 | 11% (10%, 12%) | 4% (3.8%, 4.6%) | F (1, 111) = 145,6 | .0002 |
| Frustrated | 38% (33%, 44%) | 34% (27%, 41%) | F (1, 83) = 0.96 | .37 | 16% (15%, 17%) | 10% (9%, 11%) | F (1, 111) = 81.1 | .0002 |
| Sad | 32% (27%, 38%) | 21% (15%, 27%) | F (1, 83) = 6.62 | .018 | 13% (12%, 14%) | 7% (6%, 8%) | F (1, 111) = 78.1 | .0002 |
| Worried | 42% (36%, 48%) | 28% (21%, 35%) | F (1, 83) = 8.48 | .008 | 17% (16%, 18%) | 9% (8%, 10%) | F (1, 111) = 112.0 | .0002 |
| Uncomfortable | 24% (19%, 29%) | 12% (8%, 18%) | F (1, 83) = 7.58 | .012 | 11% (10%, 12%) | 10% (9%, 11%) | F (1, 111) = 5.0 | .041 |
| Good mood and felt like celebrating | 21% (17%, 26%) | 11% (7%, 16%) | F (1, 83) = 8.71 | .007 | 6% (5%, 7%) | 3% (2.7%, 4%) | F (1, 111) = 49.8 | .002 |
EMA: Random-Prompt Entries
We used a Glimmix model with a logit link to test the difference in percentages of random prompts responded to by HCV+ individuals (73%) versus HCV− individuals (76%); there was no group difference in this measure of compliance, F(1, 112) = 0.73, p = .39, effect size r = .08. EMA-reported use of alcohol and tobacco was more frequent in HCV+ compared with HCV− participants. Drinking alcohol at the time of a random prompt was reported significantly less frequently by HCV+ participants than by HCV− participants, F(1, 111) = 5.37, p = .02, effect size r = .21. Smoking at random prompts was endorsed on significantly more occasions by HCV+ participants than by HCV− participants, F(1, 111) = 101.1, p < .001, effect size r = .69.
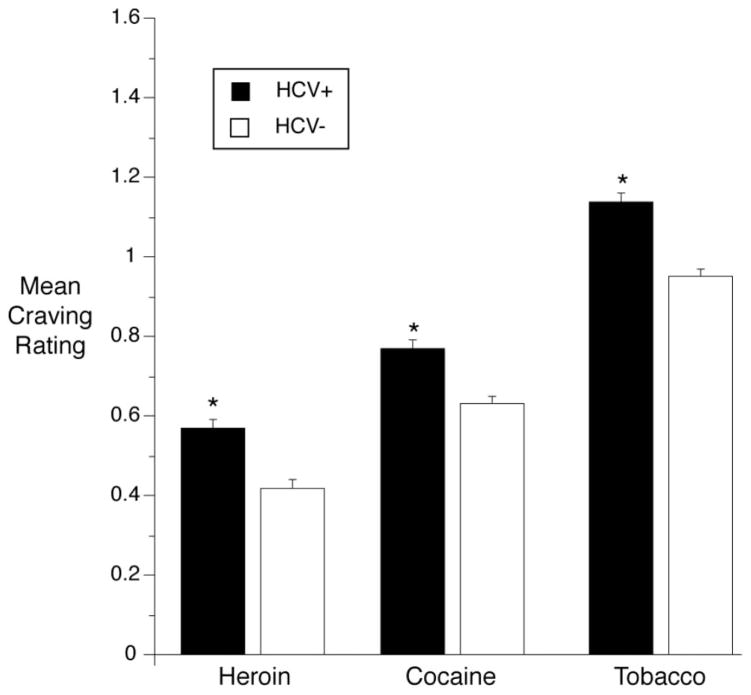
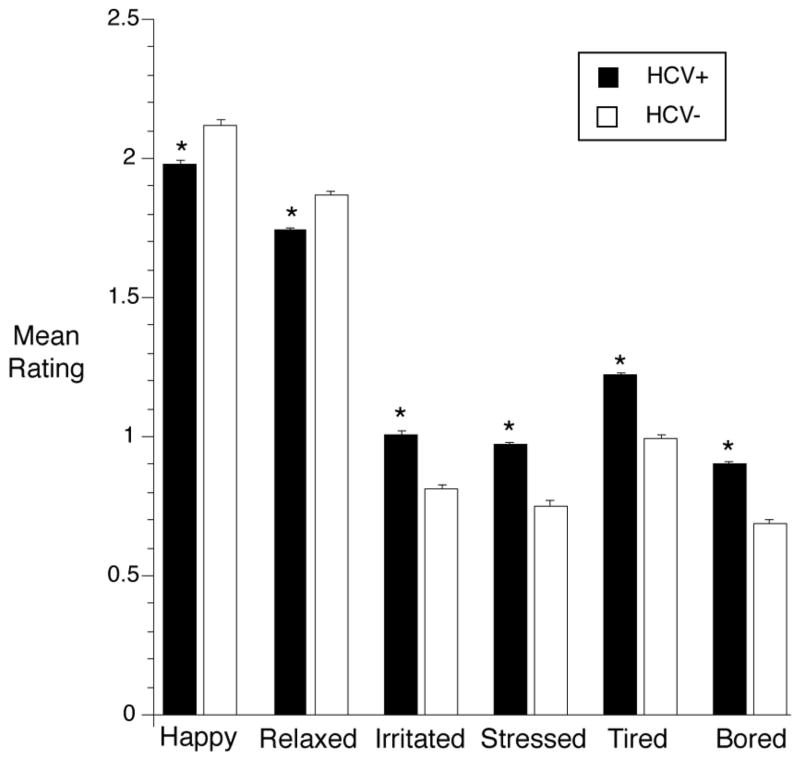
Compared with HCV− participants, HCV+ participants reported higher mean heroin craving, F(1, 112) = 42.7, p = .0002, effect size r = .53; higher mean cocaine craving, F(1, 112) = 27.2, p = .0002, effect size r = .44; and higher mean tobacco craving, F(1, 112) = 44.5, p = .0002, effect size r = .53 (see Figure 1). Mood at the time of random prompt also significantly differed by HCV status, with HCV+ individuals giving lower ratings of feeling happy (F(1, 111) = 39.7, p = .0002, effect size r = .26) and relaxed (F(1, 111) = 45.5, p = .0002, effect size r = .29) as well as higher ratings of feeling irritated (F(1, 111) = 90.0, p = .0002, effect size r = .67), stressed (F(1, 111) = 99.3, p = .0002, effect size r = .69), tired (F(1, 111) = 139.8, p = .0002, effect size r = .75), and bored (F(1, 111) = 117.2, p = .0002, effect size r = .72; see Figure 2).
Figure 1.
Cravings for heroin, cocaine, and tobacco, reported in randomly prompted EMA entries, had higher intensities in HCV+ participants than in HCV− participants. Model-adjusted means and SEM are from linear mixed models (SAS Proc Mixed). Error bars indicate SEM. Response anchors were 0 = NO!, 1 = no??, 2 = yes??, 3 = YES!! * p < .05.
Figure 2.
Mood ratings in randomly prompted EMA reports were more negative in HCV+ participants than in HCV− participants. Details are the same as those for Figure 1.
Past-hour exposure to putative drug-use triggers was higher in HCV+ participants than in HCV− participants. This was true for all of the mood-related triggers we assessed, including not only the negative-mood triggers (feeling bored, criticized by others, frustrated, sad, worried, or uncomfortable) but (also) the positive-mood trigger (being in a good mood and wanting to celebrate; see Table 3).
In the same set of past-hour drug-use-trigger items, HCV+ participants reported higher rates of what could broadly be termed “drug-related triggers”: handling $10 or more, seeing cocaine/ heroin, being offered cocaine/heroin, seeing someone use cocaine/ heroin, being tempted out of the blue to use cocaine, and wanting to see what would happen upon using just a little cocaine (or, at trend level, heroin; see Table 4).
Table 4.
Exposure to Drug Cues by HCV Status in Random-Prompt Entries
| Past-Hour Drug Trigger | HCV+ (%, 95% CI) | HCV− (%, 95% CI) | F Value (df) | p |
|---|---|---|---|---|
| Handled ≥$10 | 41% (39%, 42%) | 29% (28%, 31%) | F (1, 111) = 108.7 | .0002 |
| Saw cocaine | 6% (6%, 7%) | 4% (3.2%, 4.1%) | F (1, 111) = 54.51 | .0002 |
| Saw heroin | 3% (3%, 4%) | 2% (2%, 3%) | F (1, 111) = 8.99 | .006 |
| Offered cocaine | 5% (5%, 6%) | 3% (2.8%, 3.6%) | F (1, 111) = 43.83 | .0002 |
| Offered heroin | 3% (2%, 3%) | 2% (1.7%, 2.4%) | F (1, 111) = 6.21 | .021 |
| Saw cocaine use | 2% (1%, 2%) | 0.4% (0.3%, 0.5%) | F (1, 111) = 60.78 | .0002 |
| Saw heroin use | 1% (0.9%, 1.3%) | 0.3% (0.2%, 0.4%) | F (1, 111) = 38.17 | .002 |
| Tempted cocaine | 8% (7.8%, 9.1%) | 7% (6%, 8%) | F (1, 111) = 7.42 | .012 |
| Tempted heroin | 4% (3.8%, 4.8%) | 4% (3.2%, 4.2%) | F (1, 111) = 2.82 | .12 |
| Wanted see happen cocaine | 6% (5.8%, 6.9%) | 4% (3.3%, 4.4%) | F (1, 111) = 38.14 | .0002 |
| Wanted to see happen heroin | 3% (2.8%, 3.6%) | 2.5% (2%, 3%) | F (1, 111) = 4.02 | .065 |
Urine Drug Screen Results
Urine drug screen data from the first 19 weeks of the study showed greater heroin abstinence in HCV− participants: the model-adjusted percentage of opiate-negative urines was 70% in the HCV− group (95% confidence interval [CI] 66%, 75%) and 62% in the HCV+ group (95% CI 58%, 66%), F(1, 112) = 6.89, p = .0099, effect size r = .24. Heroin abstinence increased over time in the whole cohort as reflected in a significant main effect of time, F(57, 5,225) = 3.18, p < .0001. However, there was no significant interaction of HCV status × time, F(57, 5,225) = 0.78, p = .88.
Cocaine abstinence did not differ across groups: The model-adjusted percentage of cocaine-negative urines was 26% in the HCV− group (95% CI 21%, 32%) and 29% in the HCV+ group (95% CI 25%, 33%), F(1, 112) = 0.59, p = .44, effect size r = .07 There was no main effect of time, F(57, 5225) = 0.90, p = .69, nor was there an interaction of HCV status × Time, F(57, 5225) = 0.83, p = .81.
Discussion
Our principal findings were that HCV+ individuals reported more past-hour and current negative mood states and attributed more specific drug-use episodes to mood. Furthermore, although HCV+ individuals reported exposure to putative environmental drug-use triggers more often than HCV− individuals, they were less likely to identify these putative triggers as the cause of episodes of drug use. Finally, HCV+ individuals had higher absolute rates of opiate, tobacco, and alcohol use and higher craving for heroin, cocaine, and tobacco.
Our sample had an HCV seroprevalence rate of 61%, which is consistent with other studies, in which rates have ranged from 60% to 80% (Nelson et al., 2011). Route of drug administration was strongly associated with HCV status, a finding that is also consistent with other studies (Willner-Reid et al., 2008). We found no association between HCV status and years of prior drug use, although other studies have shown longer lifetime heroin, cocaine, and polydrug use associated with positive HCV status (Crofts et al., 1993; Quaglio et al., 2003; Willner-Reid et al., 2008). A possible explanation is that more than 75% of our sample had used heroin for more than 4.8 years, which, given the ease of HCV transmission, may have created something of a ceiling effect. The fact that this “ceiling” was only 61% may reflect the availability of needle exchange in Baltimore.
In event-contingent EMA entries, we found no significant difference by HCV status in the numbers of reported drug-use episodes or craving episodes. However, we did find differences by HCV status in participants’ causal attributions for drug-use episodes. HCV+ individuals attributed their use more often to mood states such as being bored, uncomfortable, worried, sad, or feeling others were critical of them. There was no such difference for putative environmental triggers such as handling cash, seeing cocaine or heroin, seeing someone using cocaine or heroin, or being offered cocaine or heroin. There was also no such difference for internal triggers such as feeling tempted out of blue or wanting to see what would happen upon using just a small amount. The fact that any differences were found is especially interesting given that all of our participants were receiving the same treatment (methadone, weekly psychosocial counseling, and contingency management).
In randomly prompted EMA entries, our HCV+ participants reported on a 4-point scale a greater degree of craving for heroin, cocaine, and tobacco and they were more likely to report use of alcohol or tobacco at the moment of the prompt. HCV+ individuals’ primary route of heroin administration was intravenous, and HCV− individuals’ primary route of heroin administration was intranasal. It is possible that the differences in the effects of heroin by those routes (e.g., more rapid onset and offset of high by the intravenous route) were responsible for some of the differences in craving between the two groups. However, differences in route of administration do not seem to account for the difference in cocaine craving. Cocaine’s route of administration was predominantly intravenous in HCV+ individuals and smoked in HCV− individuals. If anything, smoking is associated with a more rapid onset of high. These findings suggest that HCV+ individuals may particularly benefit from treatment interventions tailored to emphasize craving management and address the role of alcohol and tobacco in craving and use of heroin and cocaine. The increased craving for and use of tobacco among HCV+ individuals also provides an opportunity for focused smoking-cessation efforts.
The randomly prompted EMA entries also showed that our HCV+ participants more often reported their mood “right now” as negative (irritated, stressed, tired, or bored) and less often reported it as positive (relaxed or happy). Many, although not all, studies have shown that depression is common in individuals with untreated HCV and may result in poorer health-related quality of life (Batki, Canfield, Smyth, & Ploutz-Snyder, 2009; Dwight et al., 2000; Erim et al., 2010; Golden, O’Dwyer, & Conroy, 2005; Nelligan et al., 2008). Our data showed no differences between HCV+ and HCV− individuals in depressive symptoms, current (past 12 months) depression, remitted depression, history of depression, or use of current or past antidepressant medications. The discrepancy might be explained in part by recent evidence of mild but significant neurocognitive impairment in HCV infection that cannot be attributed to substance abuse, coexistent depression, or hepatic encephalopathy. Studies using in vivo magnetic resonance spectroscopy have suggested a biological mechanism for these cognitive findings, the case for which is bolstered by the detection of HCV genetic sequences in postmortem brain tissue (Forton, Taylor-Robinson, & Thomas, 2003).
It is also possible that our HCV+ participants were undergoing a more general, bidirectional lability of mood. In reporting past-hour experiences of putative triggers, our HCV+ participants more often reported that they had experienced a negative mood at some point in the past hour (bored, frustrated, sad, uncomfortable, worried, or felt criticized by others), but also more often reported that they had been “in a good mood and felt like celebrating” at some point in the past hour. Although bipolar disorder is over-represented in patients with hepatitis C, (Klinkenberg et al., 2003; Rosenberg et al., 2001), the exclusion criteria for our study make it unlikely that our mood findings can be explained by mood disorders. We also cannot attribute our mood findings to the side effects of antiviral medications because only two of our HCV+ participants were taking such medications and neither was taking medications effective in hepatitis C treatment. One possibility is that mood lability occurred in reaction to knowledge of the HCV diagnosis (Fabris et al., 2006; Treloar & Rhodes, 2009); we cannot determine that because we did not systematically assess our participants’ awareness of their HCV status before screening for the study presented here. When we administered semistructured interviews to our participants to assess their ongoing symptoms of substance-use disorders (data not reported in this paper), we sometimes had to remind them that they had been diagnosed with HCV, suggesting that their awareness of their HCV status fluctuates. Perhaps the disease process itself had subtle, direct effects on participants’ moods, or perhaps there were preexisting mood differences between our HCV+ and HCV− participants beyond what we detected. Regardless of cause, the differences in past-hour experiences of emotional triggers were present and could be of clinical interest.
Given the nature of our data, we cannot draw causal conclusions, but we can speculate on reasons for the differences we observed in reported triggers for drug use between HCV+ and HCV− individuals. One possibility is that HCV infection itself is causing the differences. There is neuropsychological, brain imaging, and virological evidence that HCV infection has a direct effect on cognitive function that can occur in the early stages of chronic infection (Thomas, Lok, Locarnini, & Zuckerman, 2013). Another possibility is that group differences in cognitive function predated the HCV infection, accounting for the risky behaviors that led to infection and for the differences observed in current behavior. Either way, a relevant area of cognitive function is impulsivity. For example, Huckans et al. (2011) found that regardless of substance-abuse history, adults with hepatitis C were significantly more likely to exhibit steep delay discounting. Delay discounting has been shown to correlate with loss of control and impulsivity across various disease processes including addiction (Bickel, Jarmolowicz, Mueller, Koffarnus, & Gatchalian, 2012; Bickel & Marsch, 2001). If individuals with hepatitis C are more impulsive than those without it, then they may then more often put themselves in risky situations, including those fraught with environmental triggers as demonstrated in this study. Although this addresses our finding of differential exposure to environmental triggers on the basis of HCV status, it does not address why there was not differential attribution of drug use due to these environmental triggers by HCV status. Regardless of the origin of the difference in trigger exposure and triggers resulting in use by HCV status, the existence of these differences has implications for treatment of addiction and HCV.
Taken together, our event-contingent and random-prompt data suggest that our HCV+ participants did not crave drugs more often than other participants, but they did crave more intensely. This appears consistent with our finding that HCV+ participants cited emotional triggers for drug use more frequently than environmental triggers, despite greater exposure emotional and environmental triggers, when compared with HCV− individuals.
Urine drug screens provided some objective corroboration of EMA self-reports of drug use. Consistent with the EMA data, HCV+ participants had more heroin-positive urines than HCV–participants and no difference in urine-verified cocaine use by HCV status. The difference occurred despite both groups being maintained on methadone at equivalent daily doses. This finding lends credence to the idea that HCV+ individuals may be differentially sensitive to drug-use triggers.
The main limitation of our study is that we cannot draw causal conclusions. However, our correlational findings are stronger than many other correlational findings because our methods minimize recall bias. Another limitation of our study is that we did not systematically assess symptoms of depression throughout the study, although we do provide evidence of similar low rates of depressive symptoms, current depression, history of depression, and remitted depression at baseline in HCV+ and HCV− individuals. Another limitation is that we determined HCV status only at baseline using an antibody test. Given that HCV antibodies are detectable approximately 8 weeks after exposure (Strader, Wright, Thomas, & Seeff, 2004), we may have missed individuals with acute infection, although this number is likely small. Also, the HCV antibody test determines only exposure to the virus, not infection, which develops in 55–85% of those exposed (Hoofnagle, 2002). Differentiating between exposure and infection might have further implications in regards to drug craving and use triggers. It is also possible that because of limitations of the HCV antibody test and the prevalence of immunosuppression in a drug-using population, we may have underdiagnosed HCV in our population, which, if the case, would make our findings more robust than those presented here. It is also possible that some of the relationships found in this paper were due to the invariant order of the presentation of the EMA questions, but it is unlikely that this would vary by HCV status. A final limitation is that our results might not generalize to other populations. Strengths of the study include its prospective design, large sample size, use of EMA, and corroboration of drug-use self-reports with urine screens.
Evidence has shown that individualized addiction treatment is more effective than a one-size-fits-all approach; it increases treatment retention and rates of abstinence initiation and decreases the likelihood of relapse (Alexander, Nahra, Lemak, Pollack, & Campbell, 2008; American Society of Addiction Medicine, 2001; Institute of Medicine, 1990, 2001; National Institute on Drug Abuse, 1999). Addiction-treatment outcomes are better when the treatment specifically addresses the needs of particular populations, such as women (Gjestad, Franck, Lindberg, & Haver, 2011; Niv & Hser, 2007), the elderly (Poppele & Anders, 2009), adolescents (Weinberg, Rahdert, Colliver, & Glantz, 1998), or individuals with comorbidities (Drake, Wallach, & McGovern, 2005; Minkoff, 2000). Given our findings, it might be beneficial to consider HCV+ individuals a special population and to tailor treatment accordingly. In addition, because a diagnosis of hepatitis C may increase receipt of addiction-treatment services (Orwat et al., 2011), and because addiction treatment creates an opportunity to expand access to hepatitis C treatment (Dore & Thomas, 2005; Swan et al., 2010), identification of HCV+ individuals may increase engagement with both types of treatment.
In conclusion, using EMA we found that when compared with HCV− individuals, HCV+ individuals reported experiencing more negative moods and more often cited mood as cause for drug use; although HCV+ individuals also reported greater exposure to negative environmental drug triggers, they did not more often cite these as triggers for drug use. Although further confirmatory research is needed, our findings support the need for tailored prevention and treatment interventions that account for HCV status. Future studies might account for HCV false-positive and false-negative rates, include an assessment of HCV status knowledge, examine more closely the interrelationship between depression and HCV status, and assess the role of HCV on neurocognition.
Acknowledgments
This research was supported by the Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health.
Footnotes
These data were presented at the College on Problems of Drug Dependence meeting in June 2011 and the Association of Medical Education and Research in Substance Abuse meeting in November 2011.
Contributor Information
Karran A. Phillips, National Institute on Drug Abuse, Intramural Research Program, National Institutes of Health, Baltimore, Maryland
David H. Epstein, National Institute on Drug Abuse, Intramural Research Program, National Institutes of Health, Baltimore, Maryland
Massoud Vahabzadeh, National Institute on Drug Abuse, Intramural Research Program, National Institutes of Health, Baltimore, Maryland.
Mustapha Mezghanni, Johns Hopkins Bayview Medical Center, Baltimore, Maryland.
Jia-Ling Lin, National Institute on Drug Abuse, Intramural Research Program, National Institutes of Health, Baltimore, Maryland.
Kenzie L. Preston, National Institute on Drug Abuse, Intramural Research Program, National Institutes of Health, Baltimore, Maryland
References
- Alexander JA, Nahra TA, Lemak CH, Pollack H, Campbell CI. Tailored treatment in the outpatient substance abuse treatment sector: 1995–2005. Journal of Substance Abuse Treatment. 2008;34:282–292. doi: 10.1016/j.jsat.2007.04.009. [DOI] [PubMed] [Google Scholar]
- American Society of Addiction Medicine. Patient placement criteria for the treatment of substance related disorders. 2. Chevy Chase, MD: Author; 2001. Rev. ed. [Google Scholar]
- Batki SL, Canfield KM, Smyth E, Ploutz-Snyder R. Health-related quality of life in methadone maintenance patients with untreated hepatitis C virus infection. Drug and Alcohol Dependence. 2009;101:176–182. doi: 10.1016/j.drugalcdep.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Jarmolowicz DP, Mueller ET, Koffarnus MN, Gatchalian KM. Excessive discounting of delayed reinforcers as a trans-disease process contributing to addiction and other disease-related vulnerabilities: Emerging evidence. Pharmacology & Therapeutics. 2012;134:287–297. doi: 10.1016/j.pharmthera.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Marsch LA. Toward a behavioral economic understanding of drug dependence: Delay discounting processes. Addiction. 2001;96:73–86. doi: 10.1046/j.1360-0443.2001.961736.x. [DOI] [PubMed] [Google Scholar]
- Chaudhry AA, Botsko M, Weiss L, Egan JE, Mitty J, Estrada B, Fiellin DA. Participant characteristics and HIV risk behaviors among individuals entering integrated buprenorphine/naloxone and HIV care. Journal of Acquired Immune Deficiency Syndrome. 2011;56(Suppl 1):S14–S21. doi: 10.1097/QAI.0b013e318209d3b9. [DOI] [PubMed] [Google Scholar]
- Cooper CL, Mills EJ. Therapeutic challenges in hepatitis C-infected injection drug using patients. Harm Reduction Journal. 2006;3:31. doi: 10.1186/1477-7517-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofts N, Hopper JL, Bowden DS, Breschkin AM, Milner R, Locarnini SA. Hepatitis-C virus-infection among a cohort of Victorian injecting drug users. Medical Journal of Australia. 1993;159:237–241. doi: 10.5694/j.1326-5377.1993.tb137822.x. [DOI] [PubMed] [Google Scholar]
- Crofts N, Nigro L, Oman K, Stevenson E, Sherman J. Methadone maintenance and hepatitis C virus infection among injecting drug users. Addiction. 1997;92:999–1005. doi: 10.1111/j.1360-0443.1997.tb02979.x. [DOI] [PubMed] [Google Scholar]
- Dore GJ, Thomas DL. Management and treatment of injection drug users with hepatitis C virus (HCV) infection and HCV/human immunodeficiency virus coinfection. Seminars in Liver Disease. 2005;25:18–32. doi: 10.1055/s-2005-864779. [DOI] [PubMed] [Google Scholar]
- Drake RE, Wallach MA, McGovern MP. Future directions in preventing relapse to substance abuse among clients with severe mental illnesses. Psychiatric Services. 2005;56:1297–1302. doi: 10.1176/appi.ps.56.10.1297. [DOI] [PubMed] [Google Scholar]
- Dwight MM, Kowdley KV, Russo JE, Ciechanowski PS, Larson AM, Katon WJ. Depression, fatigue, and functional disability in patients with chronic hepatitis C. Journal of Psychosomatic Research. 2000;49:311–317. doi: 10.1016/S0022-3999(00)00155-0. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Marrone GF, Heishman SJ, Schmittner J, Preston KL. Tobacco, cocaine, and heroin: Craving and use during daily life. Addictive Behaviors. 2010;35:318–324. doi: 10.1016/j.addbeh.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Preston KL. Daily life hour by hour, with and without cocaine: An ecological momentary assessment study. Psychopharmacology. 2010;211:223–232. doi: 10.1007/s00213-010-1884-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Willner-Reid J, Vahabzadeh M, Mezghanni M, Lin JL, Preston KL. Real-time electronic diary reports of cue exposure and mood in the hours before cocaine and heroin craving and use. Archives of General Psychiatry. 2009;66:88–94. doi: 10.1001/archgenpsychiatry.2008.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erim Y, Tagay S, Beckmann M, Bein S, Cicinnati V, Beckebaum S, Schlaak JF. Depression and protective factors of mental health in people with hepatitis C: A questionnaire survey. International Journal of Nursing Studies. 2010;47:342–349. doi: 10.1016/j.ijnurstu.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Fabris P, Tositti G, Giordani MT, Baldo V, Grasso A, Pignattari E, Floreani A. Assessing patients’ understanding of hepatitis C virus infection and its impact on their lifestyle. Alimentary Pharmacology & Therapeutics. 2006;23:1161–1170. doi: 10.1111/j.1365-2036.2006.02882.x. [DOI] [PubMed] [Google Scholar]
- Forton DM, Taylor-Robinson SD, Thomas HC. Cerebral dysfunction in chronic hepatitis C infection. Journal of Viral Hepatitis. 2003;10:81–86. doi: 10.1046/j.1365-2893.2003.00416.x. [DOI] [PubMed] [Google Scholar]
- Garfein RS, Vlahov D, Galai N, Doherty MC, Nelson KE. Viral infections in short-term injection drug users: The prevalence of the hepatitis C, hepatitis B, human immunodeficiency, and human T-lymphotropic viruses. American Journal of Public Health. 1996;86:655–661. doi: 10.2105/AJPH.86.5.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjestad R, Franck J, Lindberg S, Haver B. Early treatment for women with alcohol addiction (EWA) reduces mortality: A randomized controlled trial with long-term register follow-up. Alcohol and Alcoholism. 2011;46:170–176. doi: 10.1093/alcalc/agq097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden J, O’Dwyer AM, Conroy RM. Depression and anxiety in patients with hepatitis C: Prevalence, detection rates and risk factors. General Hospital Psychiatry. 2005;27:431–438. doi: 10.1016/j.genhosppsych.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Hagan H, Campbell J, Thiede H, Strathdee S, Ouellet L, Kapadia F, Garfein RS. Self-reported hepatitis C virus antibody status and risk behavior in young injectors. Public Health Reports. 2006;121:710–719. doi: 10.1177/003335490612100611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heather N, Stallard A, Tebbutt J. Importance of substance cues in relapse among heroin users: Comparison of two methods of investigation. Addictive Behaviors. 1991;16:41–49. doi: 10.1016/0306-4603(91)90038-J. [DOI] [PubMed] [Google Scholar]
- Hoofnagle JH. Course and outcome of hepatitis C. Hepatology. 2002;36(Suppl 1):S21–S29. doi: 10.1002/hep.1840360704. [DOI] [PubMed] [Google Scholar]
- Huckans M, Seelye A, Woodhouse J, Parcel T, Mull L, Schwartz D, Hoffman W. Discounting of delayed rewards and executive dysfunction in individuals infected with hepatitis C. Journal of Clinical and Experimental Neuropsychology. 2011;33:176–186. doi: 10.1080/13803395.2010.499355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine. Broadening the base of treatment for alcohol problems. Washington, DC: National Academy Press; 1990. [PubMed] [Google Scholar]
- Institute of Medicine. Crossing the quality chasm: A new health system for the 21st century. Washington, DC: National Academy Press; 2001. [PubMed] [Google Scholar]
- Klinkenberg WD, Caslyn RJ, Morse GA, Yonker RD, McCudden S, Ketema F, Constantine NT. Prevalence of human immunodeficiency virus, hepatitis B, and hepatitis C among homeless persons with co-occurring severe mental illness and substance use disorders. Comprehensive Psychiatry. 2003;44:293–302. doi: 10.1016/S0010-440X(03)00094-4. [DOI] [PubMed] [Google Scholar]
- Marlatt G, Gordon J. Relapse prevention: Maintenance strategies in the treatment of addictive behaviors. New York, NY: Guilford Press; 1985. [Google Scholar]
- McLellan AT, Luborsky L, O’Brien CP, Woody GE. An improved diagnostic instrument for substance abuse patients: The Addiction Severity Index. Journal of Nervous and Mental Diseases. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- Metzger DS, Woody GE, O’Brien CP. Drug treatment as HIV prevention: A research update. Journal of Acquired Immune Deficiency Syndrome. 2010;55(Suppl 1):S32–S36. doi: 10.1097/QAI.0b013e3181f9c10b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millson P, Challacombe L, Villeneuve PJ, Strike CJ, Fischer B, Myers T, Hopkins S. Reduction in injection-related HIV risk after 6 months in a low threshold methadone treatment program. AIDS Education and Prevention. 2007;19:124–136. doi: 10.1521/aeap.2007.19.2.124. [DOI] [PubMed] [Google Scholar]
- Minkoff K. An integrated model for the management of co-occurring psychiatric and substance disorders in managed-care systems. Disease Management & Health Outcomes. 2000;8:251–257. doi: 10.2165/00115677-200008050-00001. [DOI] [Google Scholar]
- National Institute on Drug Abuse. Principles of addiction treatment: A research-based guide. Bethesda, MD: National Institutes of Health; 1999. [Google Scholar]
- Nelligan JA, Loftis JM, Matthews AM, Zucker BL, Linke AM, Hauser P. Depression comorbidity and antidepressant use in veterans with chronic hepatitis C: Results from a retrospective chart review. Journal of Clinical Psychiatry. 2008;69:810–816. doi: 10.4088/JCP.v69n0514. [DOI] [PubMed] [Google Scholar]
- Nelson PK, Mathers BM, Cowie B, Hagan H, Des Jarlais D, Horyniak D, Degenhardt L. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: Results of systematic reviews. Lancet. 2011;378:571–583. doi: 10.1016/S0140-6736(11)61097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niv N, Hser YI. Women-only and mixed-gender drug abuse treatment programs: Service needs, utilization and outcomes. Drug and Alcohol Dependence. 2007;87:194–201. doi: 10.1016/j.drugalcdep.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Ompad DC, Fuller CM, Vlahov D, Thomas D, Strathdee SA. Lack of behavior change after disclosure of hepatitis C virus infection among young injection drug users in Baltimore, MD. Clinical Infectious Diseases. 2002;35:783–788. doi: 10.1086/342063. [DOI] [PubMed] [Google Scholar]
- Orwat J, Saitz R, Tompkins CP, Cheng DM, Dentato MP, Samet JH. Substance abuse treatment utilization among adults living with HIV/AIDS and alcohol or drug problems. Journal of Substance Abuse Treatment. 2011;41:233–242. doi: 10.1016/j.jsat.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppele G, Anders H. Qualified Withdrawal Treatment in Patients Aged Over 60 Years of Age - Developments, Experiences and Prospects in an Age Specific Motivational Offer. Suchttherapie. 2009;10:28–31. doi: 10.1055/s-0028-1128153. [DOI] [Google Scholar]
- Preston KL, Vahabzadeh M, Schmittner J, Lin JL, Gorelick DA, Epstein DH. Cocaine craving and use during daily life. Psychopharmacology. 2009;207:291–301. doi: 10.1007/s00213-009-1655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaglio GL, Lugoboni F, Pajusco B, Sarti M, Talamini G, Mezzelani P, Des Jarlais DC. Hepatitis C virus infection: Prevalence, predictor variables and prevention opportunities among drug users in Italy. Journal of Viral Hepatology. 2003;10:394–400. doi: 10.1046/j.1365-2893.2003.00448.x. [DOI] [PubMed] [Google Scholar]
- Robins LN, Helzer JE, Croughan J, Ratcliff KS. National Institute of Mental Health Diagnostic Interview Schedule. Its history, characteristics, and validity. Archives of General Psychiatry. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- Rosenberg SD, Goodman LA, Osher FC, Swartz MS, Essock SM, Butterfield MI, Salyers MP. Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness. American Journal of Public Health. 2001;91:31–37. doi: 10.2105/ajph.91.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosnow RL, Rosenthal R, Rubin DB. Contrasts and correlations in effect-size estimation. Psychological Science. 2000;11:446–453. doi: 10.1111/1467-9280.00287. [DOI] [PubMed] [Google Scholar]
- Russell JA. A circumplex model of affect. Journal of Personality and Social Psychology. 1980;39:1161–1178. doi: 10.1037/h0077714. [DOI] [PubMed] [Google Scholar]
- Schreuder I, van der Sande MA, de Wit M, Bongaerts M, Boucher CA, Croes EA, van Veen MG. Seroprevalence of HIV, hepatitis B, and hepatitis C among opioid drug users on methadone treatment in the Netherlands. Harm Reduction Journal. 2010;7:25. doi: 10.1186/1477-7517-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvey LA, Denton M, Plant AJ. Incidence and prevalence of hepatitis C among clients of a Brisbane methadone clinic: Factors influencing hepatitis C serostatus. Australian and New Zealand Journal of Public Health. 1997;21:102–104. doi: 10.1111/j.1467-842X.1997.tb01664.x. [DOI] [PubMed] [Google Scholar]
- Sgorbini M, O’Brien L, Jackson D. Living with hepatitis C and treatment: The personal experiences of patients. Journal of Clinical Nursing. 2009;18:2282–2291. doi: 10.1111/j.1365-2702.2009.02806.x. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Gwaltney CJ, Balabanis MH, Liu KS, Paty JA, Kassel JD, Gnys M. Immediate antecedents of cigarette smoking: An analysis from ecological momentary assessment. Journal of Abnormal Psychology. 2002;111:531–545. doi: 10.1037/0021-843X.111.4.531. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annual Review of Clinical Psychology. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- Smith BD, Jorgensen C, Zibbell JE, Beckett GA. Centers for Disease Control and Prevention initiatives to prevent hepatitis C virus infection: A selective update. Clinical Infectious Diseases. 2012;55:S49–S53. doi: 10.1093/cid/cis363. [DOI] [PubMed] [Google Scholar]
- Stata Corporation. StataCorp (Version Release 10.0) College Station, TX: Author; 2007. [Google Scholar]
- Strader DB, Wright T, Thomas D, Seeff L. Diagnosis, management, and treatment of hepatitis. Hepatology. 2004;39:1147–1171. doi: 10.1002/hep.20119. [DOI] [PubMed] [Google Scholar]
- Swan D, Long J, Carr O, Flanagan J, Irish H, Keating S, Cullen W. Barriers to and facilitators of hepatitis C testing, management, and treatment among current and former injecting drug users: A qualitative exploration. AIDS Patient Care and STDs. 2010;24(12):753–762. doi: 10.1089/apc.2010.0142. [DOI] [PubMed] [Google Scholar]
- Thiede H, Hagan H, Murrill CS. Methadone treatment and HIV and hepatitis B and C risk reduction among injectors in the Seattle area. Journal of Urban Health. 2000;77:331–345. doi: 10.1007/BF02386744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas HC, Lok ASF, Locarnini SA, Zuckerman AJ, editors. Viral hepatitis. 4. Wiley; 2013. [DOI] [Google Scholar]
- Treloar C, Rhodes T. The lived experience of hepatitis C and its treatment among injecting drug users: Qualitative synthesis. Qualitative Health Research. 2009;19:1321–1334. doi: 10.1177/1049732309341656. [DOI] [PubMed] [Google Scholar]
- Vahabzadeh M, Epstein DH, Mezghanni M, Lin J-L, Preston KL. An electronic diary software for ecological momentary assessment (EMA) in clinical trials. Proceedings of the 17th IEEE Symposium on Computer-Based Medical Systems (CBMS); 2004. pp. 167–172. [DOI] [Google Scholar]
- Vahabzadeh M, Lin JL, Mezghanni M, Epstein DH, Preston KL. Automation in an addiction treatment research clinic: Computerised contingency management, ecological momentary assessment and a protocol workflow system. Drug and Alcohol Review. 2009;28:3–11. doi: 10.1111/j.1465-3362.2008.00007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Trécan G, Coste J, Varescon-Pousson I, Christoforov B, Boissonnas A. HCV status knowledge and risk behaviours amongst intravenous drug users. European Journal of Epidemiology. 2000;16:439–445. doi: 10.1023/A:1007622831518. [DOI] [PubMed] [Google Scholar]
- Weinberg NZ, Rahdert E, Colliver JD, Glantz MD. Adolescent substance abuse: A review of the past 10 years. Journal of the American Academy of Child & Adolescent Psychiatry. 1998;37:252–261. doi: 10.1097/00004583-199803000-00009. [DOI] [PubMed] [Google Scholar]
- Willner-Reid J, Belendiuk KA, Epstein DH, Schmittner J, Preston KL. Hepatitis C and human immunodeficiency virus risk behaviors in polydrug users on methadone maintenance. Journal of Substance Abuse Treatment. 2008;35:78–86. doi: 10.1016/j.jsat.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]