Abstract
The struggle against tuberculosis (TB) is still far from over. TB, caused by Mycobacterium tuberculosis, is one of the deadliest infections worldwide. Co-infection with human immunodeficiency virus (HIV) and the emergence of multidrug-resistant tuberculosis (MDR-TB) and extensively drug-resistant tuberculosis (XDR-TB) strains have further increased the burden for this disease. Herein, we report the discovery of 2-(4-chlorobenzyl)-3-methyl-1-oxo-1H,5H-pyrido[1,2-a]benzimidazole-4-carbonitrile as an effective antitubercular agent and the structural modifications of this molecule that have led to analogues with improved potency and lower toxicity. A number of these derivatives were also active at sub-micromolar concentrations against resistant TB strains and devoid of apparent toxicity to Vero cells, thereby underscoring their value as novel scaffolds for the development of new anti-TB drugs.
Keywords: benzimidazole derivatives, drug design, drug discovery, drug resistance, tuberculosis
Introduction
The resurgence of tuberculosis (TB) is one of the most serious public health concerns worldwide, and the causative pathogen, Mycobacterium tuberculosis (Mtb), is frequently observed in immuno-compromised individuals suffering from human immunodeficiency virus (HIV).[1] The World Health Organization (WHO) has reported that Mtb was responsible for 9.2 million new cases of infection and 1.78 million deaths in 2008, and that one-third of the world’s population is estimated to be infected by latent TB.[2] The treatment of TB must be continued for 6–12 months in order to purge both the active bacteria, as well as the bacteria present in a quiescent state.[3] However, interconnected factors, including the high cost of drugs, the slow expansion of directly observed treatment, short course (DOTS), poor patient compliance with the treatment regimen and physician errors, often result in relapses and the emergence of drug resistance.[4, 5] These resistant strains, in turn, often demand a prolongation of the therapeutic regimen, with the use of additional drugs that leads to an increase in treatment costs.
The number of new cases of drug-resistant TB has increased in the recent years, with 440 000 cases of multidrug-resistant TB (MDR-TB) that have led to an estimated 150 000 deaths in 2008.[6] By definition, MDR-TB strains lose their susceptibility to two of the first-line drugs used in therapy, namely isoniazid (INH) and rifampin (RIF), while extensively drug-resistant tuberculosis (XDR-TB) strains are in addition resistant to at least one fluoroquinolone and one of the injectable anti-TB drugs, such as amikacin, kanamycin (KAN), or capreomycin.[7] Even more problematic is the recent isolation of strains resistant to all of the standard first- and second-line drugs used in TB therapy,[8] giving rise to the likely threat of a virtually incurable infection.
Despite its global impact on world health, TB is considered a neglected disease and no new anti-TB therapeutics have been introduced into the market over the last half century. Although a number of novel agents are fueling the TB pipeline,[9, 10] RIF, used to treat TB since 1966,[11] still represents the last novel class of anti-TB drugs. The foregoing facts strongly highlight the need for the identification and development of agents capable of allowing for a shorter treatment regimen while interacting with a unique biological target, thereby avoiding cross resistance with existing drugs. Many research efforts to identify new antibiotics have made it apparent that a whole-cell phenotypic high-throughput screening (HTS) approach is more likely to yield lead candidates for further advancement than the isolated target approach.[12] Indeed, compounds TMC207[13] and PA824,[14] two of the most promising anti-TB agents in clinical trials, are the result of the improvement of hit compounds identified after a random anti-TB screening of in-house chemical libraries at Johnson & Johnson and Ciba-Geigy, respectively.
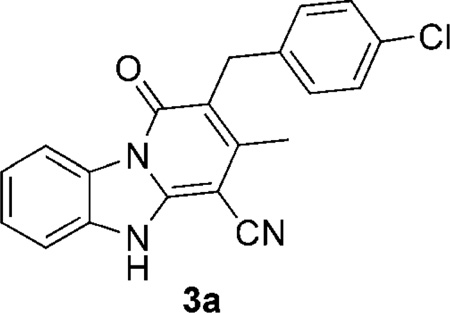
In our own efforts to discover novel anti-TB chemotypes,[15, 16] phenotypic screening of a library of ~6000 compounds was carried out affording a number of new hits with anti-TB activity deemed of interest for further development. Among these structures, compound 3a, containing a pyrido[1,2-a]benzimidazole core, was chosen for further investigation based on its activity, relatively low Vero cell toxicity, and the ease of analogue synthesis. These structure–activity relationship (SAR) elucidation efforts have led to the identification of molecules having both an improved anti-TB potency and selectivity index. Some of the molecules were also investigated for their chemical stability in human liver microsomes, thus providing some insights into the metabolism of these compounds. Of greatest note is our finding that compound 3h was able to maintain good activity when tested against a panel of MDR-TB and XDR-TB strains, thus making these pyrido[1,2-a]benzimidazoles attractive leads for further development in the quest for improved anti-TB drugs.
Results and Discussion
Chemistry
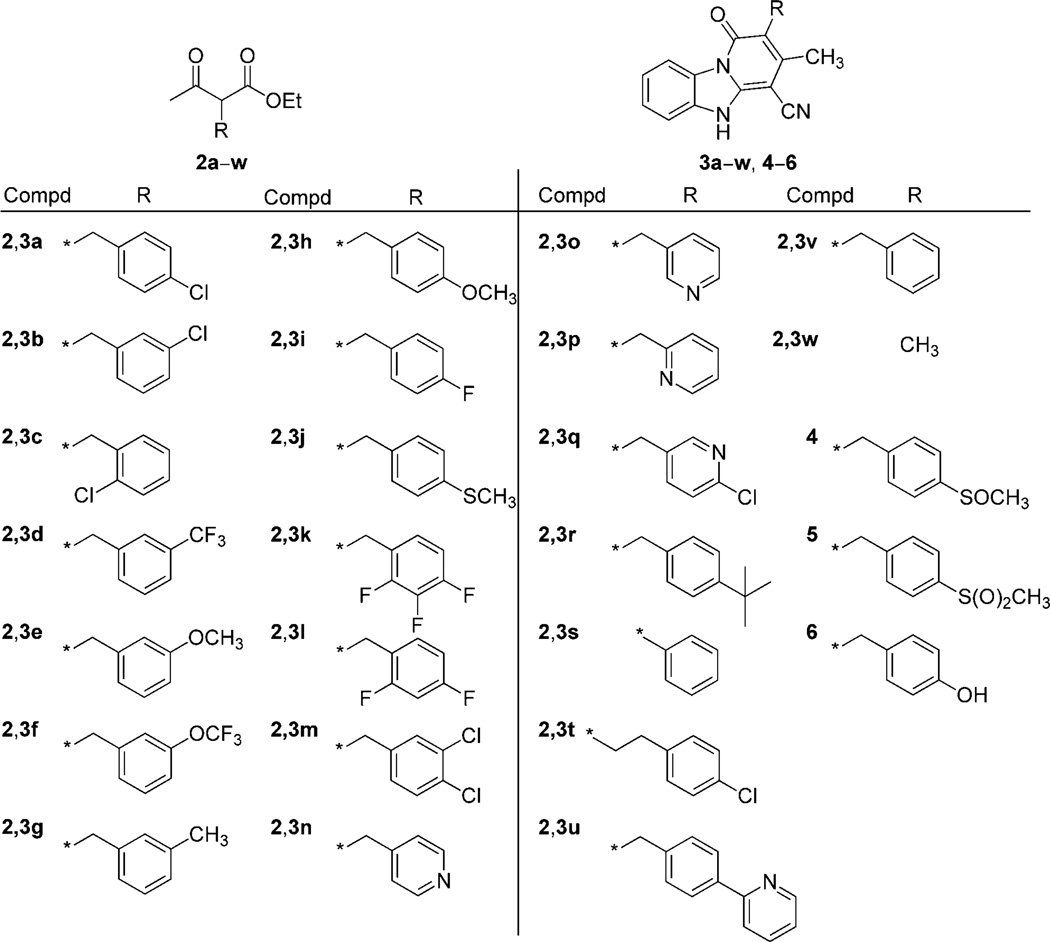
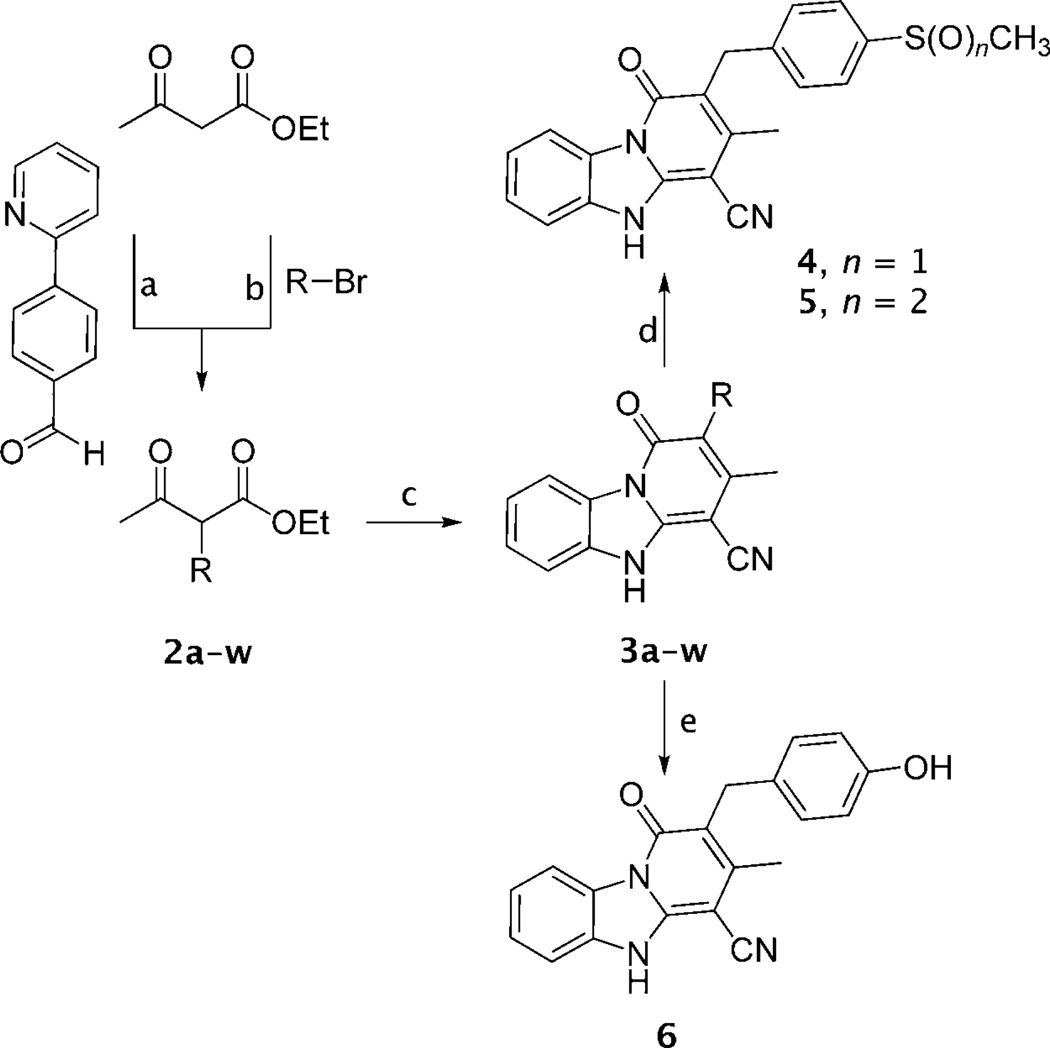
The target compounds (3a–w, 4–6, Figure 1) were synthesized by employing a straightforward and efficient protocol described previously (Scheme 1).[17] Benzimidazole-2-acetonitrile was reacted with an equimolar amount of a suitable α-substituted ethyl acetoacetate (2a–w) in the presence of ammonium acetate either under neat conditions or in anhydrous toluene at 150°C. When not commercially available, α-substituted ethyl acetoacetate derivatives 2 were synthesized by reacting ethyl acetoacetate with a substituted bromomethyl- or bromoethylbenzene, using potassium tert-butoxide as a base in anhydrous tetrahydrofuran (THF) at 70°C.[18] Compound 2u was synthesized by reacting 4-(2-pyridinyl)benzaldehyde with ethyl acetoacetate in the presence of the Me3SiCl/NaI/CH3CN reagent from room temperature to 60°C.[19] Oxidation of 3j with tellurium dioxide/hydrogen peroxide resulted in the sulfoxide 4 in good yields, while the sulfone 5 was obtained using meta-chloroperoxybenzoic acid (mCPBA) as an oxidizing agent in acetone. Demethylation of 3h with boron tribromide in anhydrous dichloromethane at 0°C to room temperature produced the hydroxy derivative 6 in good yield. Product 3b was further examined through 2D-NMR spectroscopy in order to assess which one of the possible tautomeric forms (figure S1 in the Supporting Information) predominates in solution.
Figure 1.
Structures of intermediates 2a–w and target compounds 3a–w, 4–6. * indicates the point of attachment.
Scheme 1.
Reagents and conditions: a) (CH3)3SiCl/NaI/CH3CN, CH3CN, RT→60°C, 24 h, 57%; b) tBuOK, tBuOH, THF, 70°C, 1–2.5 h, 26–92 %; c) Benzimidazole-2-acetonitrile, CH3COONH4, neat or toluene, 150 °C, 0.5–1.5 h, 51–91 %; d) for 4: TeO2/H2O2, MeOH/H2O, RT, 72 h, 66%; for 5: m-CPBA, acetone, RT, 3 h; e) BBr3, CH2Cl2, 0°C!RT, 2 h, 79%. For complete structures see Figure 1.
In vitro biological studies
All of the target compounds synthesized were first tested against the Mtb H37Rv strain to obtain the minimum inhibitory concentration (MIC) as detected using the microplate Alamar Blue assay (MABA), that is, the lowest concentration leading to at least a 90% reduction of bacterial growth (MICMABA). In addition, for compounds with low MICMABA values, the MIC was measured using a broth dilution (BD) method, that is the lowest concentration of drug preventing visible bacterial growth in a 5 mL Tween-free Middlebrook broth dilution culture (MICBD). The apparent cytotoxicity was obtained using Vero cells. Several derivatives were found to be highly active in inhibiting the growth of actively replicating Mtb with MICMABA values of less than 1 µg mL−1 (Table 1). For these compounds, the MICBD values were the same or slightly higher than those of the corresponding MICMABA, thereby indicating a cidal effect for this class of derivatives.
Table 1.
Anti-TB activity of compounds 3a–w, 4–6 against M. tuberculosis strain H37Rv.
| Compd | MICMABA[a] [µg mL−1] |
MICBD[b] [µg mL−1] |
IC50 Vero cells[c] [µg mL−1] |
Compd | MICMABA [µg mL−1] |
MICBD [µg mL−1] |
IC50 Vero cells [µg mL−1] |
|---|---|---|---|---|---|---|---|
| 3a | 0.5 | 1.0 | 8.0 | 3n | >128 | nt | nt |
| 3b | >128 | >128 | nt[d] | 3o | 4.0 | nt | nt |
| 3c | >128 | >128 | nt | 3p | 4.0 | nt | nt |
| 3d | >128 | nt | nt | 3q | 0.5 | nt | nt |
| 3e | >128 | nt | nt | 3r | 0.5 | 1.0 | >64 |
| 3 f | 64 | nt | nt | 3s | 8.0 | nt | nt |
| 3g | 8.0 | nt | nt | 3t | >128 | nt | nt |
| 3h | 0.25 | 0.25 | >64 | 3u | 4.0 | nt | nt |
| 3i | 2.0 | 2.0 | 16 | 3v | 8.0 | nt | nt |
| 3j | 0.5 | nt | nt | 3w | >128 | nt | nt |
| 3k | 0.5 | nt | nt | 4 | 2.0 | nt | nt |
| 3l | 0.25 | 1.0 | 8.0 | 5 | 1.0 | nt | nt |
| 3m | 0.25 | 0.5 | 4.0 | 6 | >128 | nt | nt |
| INH[e] | 0.04 | nt | nt |
The lowest concentration of drug leading to at least a 90% reduction of bacterial growth signal by microplate Alamar Blue assay (MABA); values reported are the average of three individual measurements;
The lowest concentration of drug preventing visible bacterial growth in a 5 mL Tween-free Middlebrook broth dilution (BD) culture; values reported are the average of three individual measurements;
Cytotoxicity against Vero cells;
Not tested;
Isoniazid.
From the HTS studies, compound 3a had an MICMABA value of 0.12 µg mL−1 versus a toxicity toward Vero cells of > 100 µm. However, upon repeat testing, 3a exhibited a higher MICMABA value of 0.5 µg mL−1 and showed a sharp decrease in the selectivity index (SI=16) with an IC50 value of only 8 µg mL−1 against the Vero cells, which is not consistent with the data obtained from the initial HTS study. Improvements were thus needed, and the first round of modifications aimed at improving the activity and toxicity profile were made at the site of the benzyl ring, as the pyrido[1,2-a]benzimidazole nucleus along with the keto moiety at C1 and the nitrile group at C4 were believed to be important determinants of activity.
We first evaluated the effect of removal of the benzyl appendage. As it is apparent from the activity of compound 3w (MICMABA > 128 µg mL−1) that the benzyl ring plays an important role in conferring the desired anti-TB activity. We next investigated the role of the chlorine atom and its position on the benzyl ring. Moving the chlorine from the para to the meta and ortho positions (3b, 3c, MICMABA=128 µg mL−1) also resulted in a loss of the anti-TB activity, while the removal of the halogen atom led to a smaller drop in potency, with 3v having an MICMABA value of 8.0 µg mL−1. In the majority of cases, introduction of various substituents on the benzyl ring at positions other than the para position resulted in an increase in the MICMABA value. In particular, meta substitution with electron-withdrawing groups, such as CF3 (3d, MICMABA=128 µg mL−1, XCF3=3.18)[20] and OCF3 (3 f, MICMABA=64 µg mL−1, XOCF3= 3.53),[20] was less tolerated than small electron-donor groups, such as CH3 (3g, MICMABA=8 µg mL−1, XCH3=2.47).[20] On the other hand, modifications at the para position of the benzyl group played a key role in enhancing the anti-TB potency with a variety of substituents being tolerated. Among the analogues synthesized, the para methoxy-substituted compound 3h exhibited one of the best activities in the series with an MICMABA value of 0.25 µg mL−1, only fivefold higher than that of INH, the most effective drug against actively replicating Mtb.
More importantly, 3h also had a favorable selectivity index with an IC50 value against Vero cells of greater than 128 µg mL−1 and improved physicochemical parameters (table S2 in the Supporting Information) compared to 3a such as the ClogP (4.0 vs 4.8), topological polar surface area (TPSA; 70.3 Å2 vs 61.1 Å2), and the predicted aqueous solubility (AlogpS; −3.72 [65 mgL−1] vs −4.08 [29 mgL−1]). Analogue 3j, bearing a para methylthioether group, retained good potency (MICMABA=0.5 µg mL−1), almost comparable with that of the corresponding methoxy derivative. Oxidation of the sulfur to the sulfoxide 4 (MICMABA= 2.0 µg mL−1) and the sulfone 5 (MICMABA=1.0 µg mL−1) caused a small reduction in anti-TB activity. Interestingly, the introduction of a polar p-hydroxy group caused a loss of activity, the MICMABA value of 6 being greater than 128 µg mL−1.
The sharp difference in potency between 6 and the other pyrido[1,2-a]benzimidazole analogues may be rationalized by the existence of a hydrophobic pocket in the target binding site, located within the vicinity of the para position of the benzyl moiety. This notion is to some extent further supported by the activity of a small series of derivatives for which the benzyl moiety has been substituted with a methylpyridinyl group. When the nitrogen of the pyridine ring is in the para position in relation to the linker (3n, MICMABA > 128 µg mL−1), activity is lost, whereas the anti-TB activities are somehow restored when the nitrogen atom of the pyridine ring is moved around the ring as in derivatives 3o, 3p (MICMABA=4.0 µg mL−1), and 3q (MICMABA=0.5 µg mL−1). Replacement of the chlorine atom with a smaller fluorine atom (3i, MICMABA=2.0 µg mL−1) did not significantly affect the potency. However, compounds containing two or three halogen atoms, namely the fluorine containing analogues 3k (MICMABA=0.5 µg mL−1) and 3l (MICMABA=0.25 µg mL−1), and the dichloro derivative 3m (MICMABA=0.25 µg mL−1), were found to be more potent than the corresponding monosubstituted derivatives. Interestingly, the biological data of compounds 3a, 3b and 3m support the notion that the activity is dependent upon the presence of a lipophilic substituent at the para position of the benzyl ring.
Next, bulkier substituents were introduced at the same attachment point so as to obtain further insights regarding the hypothesized lipophilic pocket and its size. The tert-butyl derivative 3r retained excellent activity, with an MICMABA value of 0.5 µg mL−1, whereas compound 3u, bearing a 2-pyridyl ring as the substituent, was found to be less active with an MICMABA value of 4.0 µg mL−1. These findings give further support to the hypothesis of the presence of a hydrophobic pocket in the target binding site in proximity to the para position of the benzyl ring. Likely, this pocket is important for the interaction with the present molecules and is able to accommodate a range of substituents mainly of a nonpolar character. Finally, we investigated the effect of the methylene bridge on the anti-TB activity. Removal of the methylene linker (3s, MICMABA= 8.0 µg mL−1) did not have any apparent effect on the potency (3v vs 3s), whereas the elongation of the linker with an additional methylene group caused the complete loss of the anti- TB activity (3t, MICMABA > 128 µg mL−1).
For selected compounds, MICBD values were determined, which further confirmed the promising activity of these molecules with SAR consistent with that of the MICMABA values. Some basic considerations regarding the structure–toxicity relationships for this class of derivatives can be surmised from the small set of compounds synthesized. Compounds such as 3a, 3i, 3l and 3m, bearing halogen substituents on the benzyl ring, although exhibiting high anti-TB potency, were found to be toxic to Vero cells. Compounds such as 3r and the lead 3h, bearing a tert-butyl and a methoxy group, respectively, did not show any apparent toxicity in the same cell line (Table 1). Thus, it might be hypothesized that the electron-withdrawing nature of the substituent on the benzyl ring is responsible for the cytotoxicity, while electron-donating groups afford compounds with potent anti-TB activity but lacking toxicity to Vero cells.
Although phenotypic screening is a powerful tool in the discovery of new antibacterial leads, in the majority of cases it fails to provide any insights into the mechanism of action of the hits. Some of the pyrido[1,2-a]benzimidazoles that we have indentified here have previously been reported to inhibit the biosynthesis of β-1,6-glucan, a key component of the mycetes cell wall.[21] This fact, along with the bactericidal nature of these compounds, leads to the hypothesis that a mycobacterial orthologue of the fungal β-1,6-glucan synthases might be the target for these molecules. Although the presence of potential β-glucan synthase orthologue(s) in Mtb cannot be excluded, it is known that the mycobacterial genome does not appear to contain genes encoding the enzymes traditionally associated with β-glucan synthesis,[22] and, in addition, it is debatable whether or not glucans are crucial components of the mycobacterial cell wall, thus leaving open the question, what is the actual biosynthetic pathway inhibited by these compounds?
The most promising compound of the series, i.e., 3h, was also tested for its ability to inhibit the growth of selected MDR-TB and XDR-TB strains, namely KZN494, V2475[23] (MDR-TB), TF-274,[23] and R-506[23] (XDR-TB). It was extremely encouraging to find that 3h maintained the same activity, as found for the susceptible strain H37rv, in three of the mutated Mtb strains (KZN494, TF-274, and R-506), whereas it failed to show appreciable activity against the MDR strain V2475 (Table 2). Although this may be considered a weakness for the further advancement of 3h and related analogues, this information can be used to better understand the molecular target(s) of these molecules, which is(are) as yet undetermined. However, the data at present would appear to suggest that the activity of this novel class of antimycobacterial agents might depend on a novel mechanism of action, different from those of the most commonly used drugs.
Table 2.
Comparative anti-TB activity of compounds 3h and selected antitubercular drugs against susceptible, MDR and XDR strains of M. tuberculosis.
| Compd | V4207 (DS)[a] |
TF274 (XDR)[b] |
R506 (XDR)[b] |
KZN494 (MDR)[c] |
V2475 (MDR)[c] |
|---|---|---|---|---|---|
| MICMABA[d] [µg mL−1] | |||||
| 3h | 0.5–2 | 0.5 | 0.5 | 0.5 | 16 |
| INH[e] | 0.04 | 4–8 | 8 | 16 | 16 |
| RIF[f] | 0.063 | >128 | >128 | 128 | 2–4 |
| LEV[g] | 0.125 | 1 | 2 | nt[l] | nt |
| OFL[h] | 0.5 | 4–8 | 4 | 0.5 | 0.5 |
| KAN[i] | 2–4 | >128 | >128 | 2 | 2 |
A drug-susceptible strain of Mtb;
An extensively drug-resistant strain of Mtb;
A multidrug resistant strain of Mtb;
The lowest concentration of drug leading to at least a 90% reduction of bacterial growth signal by microplate Alamar Blue assay (MABA); values reported are the average of three individual measurements;
Isoniazid;
Rifampin;
Levofloxacin;
Ofloxacin;
Kanamycin;
Not tested.
Selected compounds, namely 3a, 3d, 3e, 3v and 3w, were also tested for their metabolic stability. After 30 min incubation in the presence of mouse liver microsomes, the half life and the ratio of predicted clearance to plasma flow were derived and calculated based on the well-stirred model (Table 3; see also table S4 in the Supporting Information). Although the set of derivatives tested is modest, basic conclusions about the metabolism of these compounds can be made. The presence of electron-withdrawing groups in the para position leads to a shorter half life, as does the unsubstituted benzyl group. Substituents in the meta position are somehow able to enhance the stability of these compounds, especially in the case of a strong electron-withdrawing group. The methyl derivative 3w was found to be the most stable member of the set of compounds tested, leading to the conclusion that the benzyl moiety is crucial for the anti-TB activity, but also constitutes the metabolic “softspot” for this series of compounds.
Table 3.
Metabolic stability of selected compounds in mouse liver microsomes.
| Compd | Half life [min] | Qp,h [%][a] |
|---|---|---|
| 3a | 6.50 | 95.4 |
| 3d | 14.96 | 90.0 |
| 3e | 9.43 | 93.5 |
| 3v | 7.82 | 94.5 |
| 3w | 51.21 | 72.5 |
Percentage ratio of predicted clearance over plasma flow.
Although the synthesis of these pyrido[1,2-a]benzimidazoles has been described earlier, the question as to which one of the possible tautomers is present in solution has not been addressed. Therefore, compound 3b was investigated in order to assign the exchangeable proton at δH 13.47 ppm and the neighboring protons thereby determining the structure of the molecule (figure S2 in the Supporting Information). We first used a combination of correlation spectroscopy (COSY) and heteronuclear multiple bond correlation (HMBC) to assign each proton’s resonance signal to the corresponding carbon. Once each proton of the structure was characterized (table S3 in the Supporting Information), selective 1D-nuclear Overhauser effect spectroscopy (NOESY) was used to determine each proton’s proximity to δH 13.47 ppm. Briefly, the resonance signal at δH 13.47 ppm was irradiated with a selective shaped pulse, so as to detect only those protons that are proximal to it. Only one peak, at δH 7.52 ppm, was revealed in the experiment, thus establishing that the exchangeable proton was attached at the nitrogen of the benzimidazole ring (figure S2 in the Supporting Information), and that the pyridone form is the major tautomer in solution. However, as is well known, the tautomeric equilibrium can be shifted upon binding of the compound to its molecular target.
Conclusions
Based on data from a phenotypic screening assay of about 6 000 compounds, which identified compound 3a as a good lead candidate, we constructed a small set of analogous pyrido[1,2-a]benzimidazoles. A number of the synthesized compounds were found to exhibit excellent bactericidal activities, with several of them having MICMABA values lower than 1 µg mL−1 and MICBD values similar or slightly higher than the corresponding MICMABA. Modifications of the benzyl moiety led to a range of activities and allowed us to draft a preliminary SAR. Removal of the benzyl group caused a loss in activity, whereas substitution at its para position led to improved anti-TB activity, with a variety of substituents being tolerated. Halogens, a methoxy group, an alkyl appendage, and a heteroaromatic ring were found to be suitable substituents, whereas the more hydrophilic hydroxy group caused a loss in activity. The biological results may be consistent with the existence of a hydrophobic region in the target binding site within the proximity of the benzyl ring. However, the introduction of halogens resulted in compounds that are toxic to Vero cells, whereas electron- donating substituents coupled strong activities with an apparent lack of cytotoxicity. Removal of the linker methylene unit did not affect the potency, while elongation of the linker with an additional methylene group (3t) led to an inactive compound. More importantly, against the two XDR-TB and one MDR-TB strains tested in this study, 3h showed equal in vitro activities as found against the susceptible control strain, thereby suggesting potential for use in MDR-TB and XDR-TB chemotherapy. The molecular target, mechanism of action, and the in vivo efficacy of such compounds are currently under investigation. Overall, the high bactericidal potency against drug-susceptible and drug-resistant Mtb strains establishes these pyrido[1,2-a]benzimidazole derivatives as a novel promising anti-TB chemotype.
Experimental Section
Biology
The minimum inhibitory concentration (MIC) values were determined using M. tuberculosis H37Rv and the Kwa-Zulu Natal strains. Initial MIC screening was done with the microplate Alamar Blue assay (MABA) according to published procedures.[23b] MICBD determinations were made by using broth dilution methods. Reported MIC values are an average of three individual measurements. For a description of the biological assays, see the Supporting Information.
Chemistry
1H NMR and 13C NMR spectra were recorded on a Bruker spectrometer at 400 MHz and 100 MHz, respectively, with tetramethylsilane (TMS) as an internal standard. 19F NMR spectra were recorded on a Bruker spectrometer at 376 MHz with trifluoroacetic acid (TFA) as an external standard. Standard abbreviations indicating multiplicity were used as follows: s=singlet, d= doublet, dd=doublet of doublets, t=triplet, q=quadruplet, m= multiplet and br=broad. High-resolution mass spectrometry (HRMS) was performed on Q-TOF-2TM (Micromass). TLC was performed with Merck 60 F254 silica gel plates. Column chromatography was performed using CombiFlash Rf system with RediSep columns or alternatively using Merck silica gel (40–60 mesh). Preparative HPLC was carried out on a Shimadzu SCL-10A VP instrument with an ACE 5-AQ (21.2 mm×150 mm) column. Analytical HPLC was carried out on Agilent 1100 HPLC system with a Synergi 4 µm Hydro-RP 80 A column, on a variable wavelength detector G1314A. Method 1: Flow rate=1.4 mL min−1; gradient elution over 20 min, from 30% CH3CN/H2O to 100% CH3CN with 0.05% TFA. For 2D-NMR experiments a spectrometer Bruker at 900 MHz was used: 3b was dissolved in 0.75 mL of [D6]DMSO (99.96% deuterium) and transferred into a 5 mm NMR tube. A Bruker AV900 spectrometer equipped with a 5 mm ATM BBO probe was used to collect 1H, DEPTQ, COSY, HSQC, HMBC and sel 1D-NOESY data used in resonance assignment. Spectral information was referenced to the corresponding residual solvent peak.
The purity of the target compounds was determined to be > 98% by analytical HPLC (table S1 in the Supporting Information).
General procedure for the synthesis of 2d, f, g, i, j, k, l, m, p, t
To a suspension of tBuOK (1.2 equiv) in anhydrous THF (2 mL mmol−1), ethyl acetoacetate (1.1 equiv) and tBuOH (0.1 equiv) were added at 0°C under argon. The resulting clear solution was stirred for 30 min, and then the appropriate benzyl bromide (1 equiv) was added dropwise at 0°C. The reaction mixture was stirred at 70°C until consumption of the starting material as determined by TLC, and then the reaction mixture was quenched with H2O and saturated aq. NaHCO3. The aqueous layer was extracted with Et2O (3×10 mL) and the combined organic extracts were washed with brine, dried over MgSO4, and concentrated in vacuo. The residue was purified by flash chromatography to obtain the desired product. Compounds 2a, b, c, e, h, o, q, r, s, v, w were commercially available, while analytical data for compounds 2d,[24] f,[24] i,[25] m,[24] n,[26] matched the data previously published.
Ethyl 2-[3-(trifluoromethyl)benzyl]-3-oxobutanoate (2 d):[24]
Purified by column chromatography (EtOAc-hexane 1:4). Yield 67% (colorless oil); 1H NMR (400 MHz, CDCl3): δ=1.21 (t, J=7.2 Hz, 3 H), 2.23 (s, 3H), 3.15–3.25 (m, 2 H), 3.78 (t, J=8.0 Hz, 1H), 4.47 (q, J= 7.2 Hz, 2H), 7.30–7.55 ppm (m, 4H).
Ethyl 2-[3-(trifluoromethoxy)benzyl]-3-oxobutanoate (2 f):[24]
Purified by column chromatography (EtOAc-hexane 1:4). Yield 87% (colorless oil); 1H NMR (400 MHz, CDCl3): δ=1.24 (t, J=7.2 Hz, 3 H), 2.20 (s, 3H), 2.32 (s, 3 H), 3.12–3.20 (m, 2 H), 3.78 (t, J=7.6 Hz, 1H), 4.16 (q, J=7.2 Hz, 2H), 6.80–7.00 (m, 3H), 7.15–7.25 ppm (m, 1 H).
Ethyl 2-(3-methylbenzyl)-3-oxobutanoate (2 g)
Purified by column chromatography (EtOAc-hexane 1:4). Yield 69% (colorless oil); 1H NMR (400 MHz, CDCl3): δ=1.22 (t, J=7.2 Hz, 3 H), 2.23 (s, 3 H), 3.15–3.25 (m, 2 H), 3.78 (t, J=8.0 Hz, 1 H), 4.47 (q, J=7.2 Hz, 2 H), 7.30–7.55 ppm (m, 4H).
Ethyl 2-(4-fluorobenzyl)-3-oxobutanoate (2 i):[25]
Purified by column chromatography (EtOAc-hexane 1:4). Yield 65% (colorless oil); 1H NMR (400 MHz, CDCl3): δ=1.27 (t, J=7.2 Hz, 3 H), 2.26 (s, 3 H), 2.55–2.65 (m, 2 H), 3.46 (t, J=7.2 Hz, 1 H), 4.28 (q, J=7.2 Hz, 2 H), 7.12 (d, J=8.4 Hz, 2 H), 7.22 ppm (d, J=8.4 Hz, 2 H).
Ethyl 2-[4-(methylthio)benzyl]-3-oxobutanoate (2 j)
Purified by column chromatography (EtOAc-hexane 1:4). Yield 62% (colorless oil); 1H NMR (400 MHz, CDCl3): δ=1.23 (t, J=7.2 Hz, 3 H), 2.20 (s, 3 H), 3.09–3.20 (m, 2 H), 3.75 (t, J=7.6 Hz, 1 H), 4.47 (q, J=7.2 Hz, 2 H), 7.11 (d, J=8.4 Hz, 2 H), 7.22 ppm (d, J=8.4 Hz, 2H).
Ethyl 2-(2,3,4-trifluorobenzyl)-3-oxobutanoate (2 k)
Purified by column chromatography (EtOAc-hexane 1:4). Yield 81% (white solid); 1H NMR (400 MHz, CDCl3): δ=1.23 (t, J=7.2 Hz, 3 H), 2.25 (s, 3 H), 3.10–3.22 (m, 2 H), 3.80 (t, J=8.0 Hz, 1 H), 4.20 (q, J=7.2 Hz, 2 H), 6.85–6.95 ppm (m, 2H).
Ethyl 2-(2,4-difluorobenzyl)-3-oxobutanoate (2 l)
Purified by column chromatography (EtOAc-hexane 1:4). Yield 61% (colorless oil); 1H NMR (400 MHz, CDCl3): δ=1.22 (t, J=7.2 Hz, 3 H), 2.23 (s, 3 H), 3.05–3.15 (m, 2 H), 3.81 (t, J=8.0 Hz, 1 H), 4.15 (q, J=7.2 Hz, 2 H), 6.70–6.80 (m, 2H), 7.05–7.20 ppm (m, 1 H).
Ethyl 2-(3,4-dichlorobenzyl)-3-oxobutanoate (2m):[24]
Purified by column chromatography (EtOAc-hexane 1:4). Yield 70% (colorless oil); 1H NMR (400 MHz, CDCl3): δ=1.22 (t, J=7.2 Hz, 3 H), 2.23 (s, 3 H), 3.05–3.20 (m, 2 H), 3.73 (t, J=7.6 Hz, 1 H), 4.16 (q, J=7.2 Hz, 2 H), 7.03 (dd, J1=2.0 HzJ2=8.0 Hz, 1 H), 7.25–7.30 (m, 1 H), 7.30–7.35 ppm (m, 1 H).
Ethyl 2-(pyridin-2-yl-methyl)-3-oxobutanoate (2 p)
Purified by column chromatography (EtOAc-hexane 1:4). Yield 92% (colorless oil); 1H NMR (400 MHz, CDCl3): δ=1.21 (t, J=7.2 Hz, 3 H), 2.32 (s, 3 H), 3.25–3.45 (m, 2 H), 4.16 (q, J=7.2 Hz, 2H), 4.29 (t, J=7.6 Hz, 1 H), 7.10 (m, 1 H), 7.18 (d, J=7.6 Hz, 1H), 7.57 (m, 1H), 8.46 ppm (m, 1 H).
Ethyl 2-[(4-chlorophenyl)ethyl]-3-oxobutanoate (2 t)
Purified by column chromatography (EtOAc-hexane 1:4). Yield 26% (colorless oil); 1H NMR (400 MHz, CDCl3): δ=1.28 (t, J=7.2 Hz, 3H), 2.10–2.20 (m, 2H) 2.21 (s, 3H), 2.55–2.65 (m, 2H) 3.38 (t, J=7.2 Hz, 1 H), 4.20 (q, J=7.2 Hz, 2H), 7.10 (d, J=8.4 Hz, 2H), 7.24 ppm (d, J=8.4 Hz, 2 H).
Ethyl 2-[4-(pyridin-2-yl)-benzyl]-3-oxobutanoate (2 u)
To a stirred mixture of (CH3)3SiCl (3.44 mL, 27.3 mmol), NaI (4.1 g, 27.3 mmol), ethyl acetoacetate (0.689 mL, 5.45 mmol) and CH3CN (1 mL mmol−1 of (CH3)3SiCl), 4-(pyridin-2-yl)benzaldehyde (1 g, 5.45 mmol) was added at 0°C. The mixture was stirred for 6 h at RT, and then overnight at 60 °C. After cooling, H2O was added and the organic layer was extracted with EtOAc, washed with aq. Na2S2O3, brine, dried over MgSO4, filtered, and concentrated in vacuo. The resulted yellow powder was used for the next reaction step without further purification (0.88 g, yield 57%); 1H NMR (400 MHz, CDCl3): δ=1.27 (t, J=7.2 Hz, 3H), 2.27 (s, 3 H), 3.25–3.45 (m, 2H), 4.16 (q, J=7.2 Hz, 2 H), 4.36 (t, J=7.6 Hz, 1H), 7.30–7.40 (m, 1 H), 7.72 (d, J=8.0 Hz, 2 H), 8.00 (d, J=8.4 Hz, 2 H), 8.18 (d, J=8.4 Hz, 2H), 8.75 ppm (d, J=7.6 Hz, 1 H).
General procedure for the synthesis of 3a–w
Benzimidazole-2-acetonitrile (1 equiv), ammonium acetate (2.2 equiv) and the appropriate substituted ethyl acetoacetate 2a–w (1.2 equiv) were reacted either in neat or in anhydrous toluene at 150°C for 0.5–1.5 h. After cooling, the solid was washed with a mixture of EtOH/Et2O (1:1, 3×10 mL), collected by filtration and purified either through HPLC or recrystallized from EtOH/DMF. In the cases of the reactions in toluene, after cooling the solvent was evaporated, and the resulting solid washed with a mixture of EtOH/Et2O (1:1, 3×10 mL), collected by filtration and purified through HPLC. Analytical data for compounds 3s[22] and 3v[17] matched the data published previously.
2-(4-Chlorobenzyl)-3-methyl-1-oxo-1H,5H-pyrido[1,2-a]benzimidazole-4-carbonitrile (3 a)
Neat; purified by preparative HPLC. Yield 73% (white powder); 1H NMR (400 MHz, [D6]DMSO): δ=2.32 (s, 3 H), 3.91 (s, 2 H), 7.22 (d, J=8.4 Hz, 2H); 7.26 (d, J=8.4 Hz, 2 H), 7.33 (m, 1H), 7.45–7.55 (m, 2H), 8.56 (d, J=8.0 Hz, 1 H), 13.47 ppm (s, 1H); 13C NMR (400 MHz, [D6]DMSO): δ=18.3, 30.6, 69.8, 111.3, 114.0, 116.3, 116.8, 122.2, 126.6, 127.8, 128.2, 129.9, 130.4, 131.7, 139.5, 145.5, 147.4, 158.7 ppm; HRMS (ESI) calcd for C20H14ClN3O: 348.0898 [M+H]+, found: 348.0896.
2-(3-Chlorobenzyl)-3-methyl-1-oxo-1H,5H-pyrido[1,2-a]benzimidazole-4-carbonitrile (3 b)
Neat; purified by preparative HPLC. Yield 62% (white powder); 1H NMR (400 MHz, [D6]DMSO): δ=2.33 (s, 3 H), 3.95 (s, 2 H), 7.17–7.26 (m, 4H); 7.34 (m, 1H), 7.51 (m, 2H), 8.58 (d, J=8.0 Hz, 1 H), 13.47 ppm (s, 1H); 13C NMR (400 MHz, [D6]DMSO): δ=18.3, 30.8, 69.8, 111.2, 113.5, 116.2, 116.8, 122.1, 125.8, 126.5, 126.7, 127.7, 127.7, 130.0, 131.6, 132.9, 143.1, 145.5, 147.3, 158.7 ppm; HRMS (ESI) calcd for C20H14ClN3O: 348.0898 [M+ H]+, found: 348.0900.
2-(2-Chlorobenzyl)-3-methyl-1-oxo-1H,5H-pyrido[1,2-a]benzimidazole-4-carbonitrile (3 c)
Neat; recrystallized from EtOH/DMF. Yield 81% (white powder); 1H NMR (400 MHz, [D6]DMSO): δ=2.26 (s, 3 H), 3.98 (s, 2H), 6.91 (d, J=7.2 Hz, 1H), 7.14 (t, J=7.2 Hz, 1 H), 7.21 (t, J=7.2 Hz, 1H), 7.35 (m, 1H), 7.45 (d, J=8.0 Hz, 1H), 7.46– 7.55 (m, 2H), 8.55 (d, J=8.0 Hz, 1H), 13.57 ppm (s, 1H); 13C NMR (400 MHz, [D6]DMSO) δ=18.3, 29.0, 70.0, 111.3, 112.0, 116.3, 116.9, 122.3, 126.7, 127.3, 127.7, 127.8, 128.5, 129.1, 131.8, 133.2, 137.1, 145.8, 148.1, 158.7 ppm; HRMS (ESI) calcd for C20H14ClN3O: 348.0898 [M+H]+, found: 348.0898.
3-Methyl-1-oxo-2-[3-(trifluoromethyl)benzyl]-1H,5H-pyrido[1,2-a]-benzimidazole-4-carbonitrile (3 d)
Neat; recrystallized from EtOH/DMF. Yield 83% (white powder); 1H NMR (400 MHz, [D6]DMSO): δ=2.35 (s, 3H), 4.05 (s, 2 H), 7.30–7.40 (m, 1H); 7.40–7.55 (m, 5 H), 7.60 (s, 1 H), 8.58 (d, J=8.4 Hz, 1H), 13.51 ppm (s, 1H); 13C NMR (400 MHz, [D6]DMSO): δ=18.4, 31.1, 69.9, 99.6, 111.4, 113.5, 116.3, 122.3, 123.7 (q, J=270 Hz), 124.6 (q, J=3.2 Hz), 126.7, 127.9, 128.9, 129.2, 129.4 (q, J=40 Hz), 131.8, 132.1, 142.1, 145.7, 147.5, 158.8 ppm; HRMS (ESI) calcd for C21H14F3N3O: 380.1016 [M+H]+, found: 380.1035.
3-Methyl-2-(3-methoxybenzyl)-1-oxo-1H,5H-pyrido[1,2-a]benzimidazole-4-carbonitrile (3 e)
Neat; purified by preparative HPLC. Yield 83% (white powder); 1H NMR (400 MHz, [D6]DMSO): δ=2.32 (s, 3 H), 3.68 (s, 3H), 3.92 (s, 2H), 6.70–6.85 (m, 3H); 7.10–7.15 (m, 1 H), 7.33 (m, 1 H), 7.45–7.55 (m, 2 H), 8.58 (d, J=8.4 Hz, 1 H), 13.60 ppm (s, 1H); 13C NMR (400 MHz, [D6]DMSO): δ=18.3, 31.2, 54.9, 69.8, 110.9, 111.3, 114.0, 114.3, 116.2, 116.9, 120.2, 122.4, 126.5, 127.9, 129.3, 131.8, 142.1, 145.6, 147.2, 158.8, 159.4 ppm; HRMS (ESI) calcd for C21H17N3O2 : 344.1394 [M+H]+, found 344.1395.
3-Methyl-1-oxo-2-[3-(trifluoromethoxy)benzyl]-1H,5H-pyrido[1,2-a]-benzimidazole-4-carbonitrile (3 f)
Neat; purified by preparative HPLC. Yield 63% (grey powder); 1H NMR (400 MHz, [D6]DMSO): δ= 2.34 (s, 3H), 4.01 (s, 2H), 7.15–7.40 (m, 5H); 7.45–7.55 (m, 2H), 8.58 (d, J=8.4 Hz, 1H), 13.66 ppm (s, 1H); 13C NMR (400 MHz, [D6]DMSO): δ=18.4, 30.9, 69.9, 111.3, 113.5, 116.3, 116.9, 118.2, 120.5, 121.4, 122.3, 126.7, 127.0, 127.9, 130.1, 131.8, 143.5, 145.7, 147.5, 148.5, 158.8 ppm; HRMS (ESI) calcd for C21H14F3N3O2 : 398.1088 [M+H]+, found 398.1099.
3-Methyl-2-(3-methylbenzyl)-1-oxo-1H,5H-pyrido[1,2-a]benzimidazole-4-carbonitrile (3 g)
Neat; recrystallized from EtOH/DMF. Yield 91% (white powder); 1H NMR (400 MHz, [D6]DMSO): δ=2.22 (s, 3 H), 2.34 (s, 3 H), 3.92 (s, 2H), 6.90–7.10 (m, 3 H), 7.11 (m, 1H); 7.36 (m, 1H), 7.45–7.55 (m, 2H), 8.60 (d, J=8.0 Hz, 1 H), 13.49 ppm (s, 1H); 13C NMR (400 MHz, [D6]DMSO): δ=18.4, 21.1, 31.1, 69.8, 111.3, 114.5, 116.3, 117.0, 122.2, 125.0, 126.5, 126.6, 127.9, 128.2, 128.6, 131.8, 137.3, 140.4, 147.2, 158.8 ppm; HRMS (ESI) calcd for C21H17N3O: 328.1444 [M+H]+, found 328.1450.
3-Methyl-2-(4-methoxybenzyl)-1-oxo-1H,5H-pyrido[1,2-a]benzimidazole-4-carbonitrile (3 h)
Neat; purified by preparative HPLC. Yield 74% (white powder); 1H NMR (400 MHz, [D6]DMSO): δ=2.33 (s, 3H), 3.67 (s, 3 H), 3.87 (s, 2H), 6.79 (d, J=8.0 Hz, 2H); 7.03 (d, J= 8.0 Hz, 2 H), 7.33 (m, 1 H), 7.45–7.55 (m, 2 H), 8.58 (d, J=8.0 Hz, 1 H), 13.61 ppm (s, 1H); 13C NMR (400 MHz, [D6]DMSO): δ=18.3, 30.3, 55.0, 69.6, 111.2, 113.7, 114.9, 116.3, 116.9, 122.2, 126.6, 127.9, 129.0, 131.8, 132.3, 145.5, 146.9, 157.4, 158.8 ppm; HRMS (ESI) calcd for C21H17N3O2 : 344.1394 [M+H]+, found 344.1395.
2-(4-Fluorobenzyl)-3-methyl-1-oxo-1H,5H-pyrido[1,2-a]benzimidazole-4-carbonitrile (3 i)
Neat; recrystallized from EtOH/DMF. Yield 91% (white powder); 1H NMR (400 MHz, [D6]DMSO): δ=2.34 (s, 3 H), 3.93 (s, 2 H), 7.04 (m, 2H); 7.20–7.30 (m, 2H), 7.35 (m, 1H), 7.45–7.55 (m, 2H), 8.58 (d, J=8.0 Hz, 1H), 13.50 ppm (s, 1H); 19F NMR (376 MHz, [D6]DMSO): δ=−116.2; HRMS (ESI) calcd for C20H14FN3O: 332.1194 [M+H]+, found 332.1203.
3-Methyl-1-oxo-2-[4-(thiomethyl)benzyl]-1H,5H-pyrido[1,2-a]benzimidazole-4-carbonitrile (3 j)
Neat; purified by preparative HPLC. Yield 81% (white powder); 1H NMR (400 MHz, [D6]DMSO): δ=2.32 (s, 3H), 2.39 (s, 3 H), 3.89 (s, 2H), 7.12 (d, J=8.4 Hz, 2H); 7.15 (d, J= 8.4 Hz, 2 H), 7.35 (m, 1 H), 7.45–7.53 (m, 2 H), 8.57 (d, J=8.0 Hz, 1 H), 13.67 ppm (s, 1H); 13C NMR (400 MHz, [D6]DMSO): δ=15.1, 18.4, 30.6, 69.7, 111.3, 114.4, 116.3, 116.9, 122.3, 126.4, 126.7, 127.9, 128.7, 131.8, 134.9, 137.4, 145.5, 147.1, 158.8 ppm; HRMS (ESI) calcd for C21H17N3OS: 360.1165 [M+H]+, found 360.1153.
3-Methyl-1-oxo-2-(2,3,4-trifluorobenzyl)-1H,5H-pyrido[1,2-a]benzimidazole-4-carbonitrile (3 k)
Neat; purified by preparative HPLC. Yield 50% (white powder); 1H NMR (400 MHz, [D6]DMSO): δ=2.33 (s, 3 H), 3.93 (s, 2 H), 6.90 (m, 1H), 7.11 (m, 1H); 7.33 (m, 1 H), 7.45–7.55 (m, 2H), 8.53 (d, J=8.0 Hz, 1 H), 13.54 ppm (s, 1H); 19F NMR (376 MHz, [D6]DMSO): δ=−161.0 (app t, J=24 Hz), −137.5 (dd, J1=4.0 Hz, J2=16.0 Hz), −137.2 ppm (dd, J1=4.0 Hz, J2=16.0 Hz); HRMS (ESI) calcd for C20H12F3N3O: 366.0884 [M+H]+, found 366.0874.
2-(2,4-Difluorobenzyl)-3-methyl-1-oxo-1H,5H-pyrido[1,2-a]benzimidazole-4-carbonitrile (3 l)
Neat; recrystallized from EtOH/DMF. Yield 75% (white powder); 1H NMR (400 MHz, [D6]DMSO): δ=2.33 (s, 3H), 3.90 (s, 2H), 7.10–7.20 (m, 2 H), 7.33 (m, 1 H), 7.45–7.55 (m, 2 H), 8.55 (d, J=8.4 Hz, 1 H), 13.54 ppm (s, 1H); 19F NMR (376 MHz, [D6]DMSO): δ=−112.4 (d, J=4.0 Hz), −112.1 ppm (d, J=8.0 Hz); HRMS (ESI) calcd for C20H13F2N3O: 350.1088 [M+H]+, found 350.1092.
2-(3,4-Dichlorobenzyl)-3-methyl-1-oxo-1H,5H-pyrido[1,2-a]benzimidazole-4-carbonitrile (3m)
Neat; purified by preparative HPLC. Yield 51% (white powder); 1H NMR (400 MHz, [D6]DMSO): δ=2.36 (s, 3H), 3.98 (s, 2H), 7.20–7.25 (m, 1 H), 7.36 (m, 1 H), 7.45–7.55 (m, 4 H), 8.59 (d, J=8.0 Hz, 1H), 13.61 ppm (s, 1H); 13C NMR (400 MHz, [D6]DMSO): δ=18.4, 30.4, 69.9, 99.6, 111.3, 113.3, 116.3, 116.9, 122.3, 126.7, 127.8, 128.5, 129.9, 130.4, 130.8, 131.7, 141.9, 145.7, 147.6, 158.8 ppm; HRMS (ESI) calcd for C20H13Cl2N3O: 380.0363 [M−H]+, found 380.0375.
3-Methyl-1-oxo-2-[(pyridin-4-yl)methyl]-1H,5H-pyrido[1,2-a]benzimidazole-4-carbonitrile (3 n)
Neat; recrystallized from EtOH/DMF. Yield 78% (white powder); 1H NMR (400 MHz, [D6]DMSO): δ=2.33 (s, 3 H), 3.98 (s, 2H), 7.22 (m, 2H), 7.34 (m, 1 H), 7.45–7.55 (m, 2 H), 8.40, (m, 2H), 8.56 (d, J=7.6 Hz, 1 H), 13.50 ppm (s, 1H); 13C NMR (400 MHz, [D6]DMSO): δ=18.4, 30.7, 70.0, 99.6, 111.4, 112.5, 116.3, 122.2, 123.6, 126.6, 127.9, 132.0, 145.8, 147.7, 149.4, 149.8, 158.8 ppm; HRMS (ESI) calcd for C19H14N4O: 315.1240 [M+H]+, found 315.1245.
3-Methyl-1-oxo-2-[(pyridin-3-yl)methyl]-1H,5H-pyrido[1,2-a]benzimidazole-4-carbonitrile (3 o)
Toluene; purified by preparative HPLC. Yield 85% (white powder); 1H NMR (400 MHz, [D6]DMSO): δ=2.39 (s, 3 H), 3.99 (s, 2 H), 7.27 (m, 1H), 7.36 (m, 1H), 7.45–7.55 (m, 2 H), 7.61 (d, J=8.0 Hz, 1H), 8.37, (s, 1 H), 8.50 (s, 1H), 8.60 (d, J=8.0 Hz, 1 H), 13.52 ppm (s, 1H); 13C NMR (400 MHz, [D6]DMSO): δ=18.8, 29.1, 70.3, 111.8, 113.6, 116.6, 117.4, 122.5, 123.9, 127.0, 128.3, 132.6, 135.9, 136.5, 146.2, 147.5, 147.7, 149.8, 159.2 ppm; HRMS (ESI) calcd for C19H14N4O: 315.1240 [M+H]+, found 315.1254.
3-Methyl-1-oxo-2-[(pyridin-2-yl)methyl]-1H,5H-pyrido[1,2-a]benzimidazole-4-carbonitrile (3 p)
Toluene; purified by preparative HPLC. Yield 71% (white powder); 1H NMR (400 MHz, [D6]DMSO): δ=2.41 (s, 3 H), 4.10 (s, 2 H), 7.18 (m, 1 H), 7.24 (d, J=8.0 Hz, 1 H), 7.35 (m, 1 H), 7.45–7.55 (m, 2 H), 7.65 (t, J=7.6 Hz, 1 H), 8.44, (m, 1 H), 8.58 (d, J=8.0 Hz, 1H), 13.52 ppm (s, 1H); 13C NMR (400 MHz, [D6]DMSO): δ=19.0, 34.6, 70.2, 111.8, 113.5, 116.6, 117.4, 121.7, 122.6, 122.8, 127.0, 128.3, 132.4, 137.0, 146.1, 148.1, 149.2, 159.1, 160.0 ppm; HRMS (ESI) calcd for C19H14N4O: 315.1240 [M+H]+, found 315.1255.
2-[(6-Chloropyridin-3-yl)methyl]-3-methyl-1-oxo-1H,5H-pyrido-[1,2-a]benzimidazole-4-carbonitrile (3 q)
Toluene; purified by preparative HPLC. Yield 88% (white powder); 1H NMR (400 MHz, [D6]DMSO): δ=2.37 (s, 3H), 3.94 (s, 2 H), 7.35–7.37 (m, 2H), 7.45–7.55 (m, 2 H), 7.67 (d, J=8.0 Hz, 1H), 8.31 (s, 1 H), 8.56 (d, J=8.0 Hz, 1 H), 13.51 ppm (s, 1H); 13C NMR (400 MHz, [D6]DMSO): δ=18.8, 28.3, 70.3, 111.7, 113.4, 116.6, 117.2, 122.7, 124.3, 127.0, 128.2, 132.1, 136.1, 139.3, 146.0, 147.8, 148.2, 149.8, 159.0 ppm; HRMS (ESI) calcd for C19H13ClN4O: 349.0851 [M+H]+, found 349.0867.
3-Methyl-1-oxo-2-[(4-tert-butyl)benzyl]-1H,5H-pyrido[1,2-a]benzimidazole-4-carbonitrile (3 r)
Neat; purified by preparative HPLC. Yield 79% (white powder); 1H NMR (400 MHz, [D6]DMSO): δ=1.22 (s, 9H), 2.36 (s, 3 H), 3.91 (s, 2H), 7.13 (d, J=8.0 Hz, 2H); 7.24 (d, J= 8.0 Hz, 2 H), 7.34 (m, 1 H), 7.44–7.55 (m, 2 H), 8.59 (d, J=8.0 Hz, 1 H), 13.48 ppm (s, 1H); 13C NMR (400 MHz, [D6]DMSO): δ=18.4, 30.7, 31.2, 34.1, 69.6, 99.6, 111.3, 114.7, 116.3, 117.0, 122.2, 125.0, 126.6, 127.7, 127.9, 131.8, 137.4, 145.5, 146.9, 148.0, 158.8 ppm; HRMS (ESI) calcd for C24H23N3O: 370.1914 [M+H]+, found 370.1915.
3-Methyl-1-oxo-2-phenyl-1H,5H-pyrido[1,2-a]benzimidazole-4-carbonitrile (3 s):[25]
Neat; purified by preparative HPLC. Yield 81% (white powder); 1H NMR (400 MHz, [D6]DMSO): δ=2.18 (s, 3 H), 7.17 (t, J=7.6 Hz, 1H); 7.25–7.35 (m, 3 H), 7.35–7.45 (m, 3H), 7.50 (d, J=8.0 Hz, 1H), 8.49 (d, J=8.0 Hz, 1H), 13.48 ppm (s, 1H); 13C NMR (400 MHz, [D6]DMSO): δ=19.7, 70.9, 113.5, 115.7, 120.0, 125.2, 126.4, 127.8, 129.4, 131.5, 137.1, 146.5, 158.8, 172.1 ppm; HRMS (ESI) calcd for C19H13N3O: 300.1131 [M+H]+, found 300.1132.
2-[(4-Chlorophenyl)ethyl]-3-methyl-1-oxo-1H,5H-pyrido[1,2-a]-benzimidazole-4-carbonitrile (3 t)
Toluene; purified by preparative HPLC. Yield 78% (white powder); 1H NMR (400 MHz, [D6]DMSO): δ=2.22 (s, 3 H), 2.70 (m, 2H), 2.78 (m, 2H), 7.24 (d, J=8.4 Hz, 2H), 7.25–7.40 (m, 5H), 7.45–7.55 (m, 2 H), 8.59 (d, J=8.0 Hz, 1H), 13.57 ppm (s, 1H); 13C NMR (400 MHz, [D6]DMSO): δ=17.8, 28.4, 33.5, 69.3, 111.2, 114.6, 116.2, 117.0, 122.1, 126.5, 127.7, 128.2, 130.3, 130.5, 131.7, 140.8, 145.3, 146.2, 158.3 ppm; HRMS (ESI) calcd for C21H16ClN3O: 362.1055 [M+H]+, found 362.1039.
3-Methyl-1-oxo-2-[4-(pyridin-2-yl)benzyl]-1H,5H-pyrido[1,2-a]-benzimidazole-4-carbonitrile (3 u)
Toluene; purified by preparative HPLC. Yield 52% (yellow powder); 1H NMR (400 MHz, [D6]DMSO): δ=2.34 (s, 3H), 4.01 (s, 2 H), 7.20–7.40 (m, 4H), 7.45– 7.55 (m, 2 H), 7.80–8.00 (m, 4H), 8.10 (m, 1 H), 8.50–8.60 (m, 3H), 13.50 ppm (s, 1H); 13C NMR (400 MHz, [D6]DMSO): δ=18.1, 31.0, 69.8, 111.3, 114.1, 116.3, 116.9, 121.0, 122.3, 122.9, 126.7, 126.9, 127.9, 128.6, 131.8, 135.0, 138.9, 142.3, 145.6, 147.4, 148.2, 155.2, 158.9 ppm; HRMS (ESI) calcd for C25H18N4O: 391.1540 [M+H]+, found 391.1539.
2-Benzyl-3-methyl-1-oxo-1H,5H-pyrido[1,2-a]benzimidazole-4-carbonitrile (3 v):[18]
Neat; recrystallized from EtOH/DMF. Yield 90% (white powder); 1H NMR (400 MHz, [D6]DMSO): δ=2.35 (s, 3 H), 3.97 (s, 2 H), 7.10–7.30 (m, 5H), 7.35 (m, 1 H), 7.45–7.55 (m, 2H), 8.60 (d, J=8.0 Hz, 1 H), 13.49 ppm (s, 1H); 13C NMR (400 MHz, [D6]DMSO): δ=18.3, 31.1, 69.6, 111.2, 114.4, 116.2, 116.9, 122.2, 125.8, 126.5, 127.8, 128.0, 128.3, 131.7, 140.5, 145.5, 147.0, 158.7 ppm; HRMS (ESI) calcd for C20H15N3O: 314.1288 [M+H]+, found 314.1301.
2,3-Dimethyl-1-oxo-1H,5H-pyrido[1,2-a]benzimidazole-4-carbonitrile (3w)
Neat; recrystallized from EtOH/DMF. Yield 73% (white powder); 1H NMR (400 MHz [D6]DMSO): δ=2.01 (s, 3 H), 2.30 (s, 3 H), 7.32 (bd s, 1H), 7.45–7.55 (m, 2H), 8.55 (d, J=6.8 Hz, 1 H), 13.33 ppm (s, 1H); 13C NMR (400 MHz, [D6]DMSO): δ=11.7, 18.5, 69.3, 111.0, 111.2, 116.2, 117.2, 122.1, 126.5, 127.8, 131.8, 145.3, 145.9, 158.6 ppm; HRMS (ESI) calcd for C14H11N3O: 238.0975 [M+ H]+, found 238.0973.
3-Methyl-2-[4-(methylsulfinyl)benzyl]-1-oxo-1H,5H-pyrido[1,2-a]-benzimidazole-4-carbonitrile (4)
To a flask containing 3j (61 mg, 0.17 mmol) suspended in MeOH (2 mL) were added TeO2 (27 mg, 0.17 mmol), 30% H2O2 (0.1 mL), and a drop of concd HCl. The mixture was stirred at RT for 72 h. Water (10 mL) was then added, and the resulting precipitate was collected by filtration. The precipitate was washed with H2O (3×5 mL) and purified by preparative HPLC to afford 4 as a white solid (42 mg, 66%); 1H NMR (400 MHz, [D6]DMSO): δ=2.37 (s, 3H), 2.68 (s, 3 H), 4.08 (s, 2H), 7.35 (m, 1 H), 7.42 (d, J=8.0 Hz, 2H); 7.45–7.53 (m, 2H), 8.59 (d, J=8.0 Hz, 1H), 13.54 ppm (s, 1H); 13C NMR (400 MHz, [D6]DMSO): δ=18.8, 31.5, 43.6, 70.2, 111.7, 114.2, 116.7, 117.3, 122.7, 124.1, 127.1, 127.5, 128.2, 129.2, 132.2, 144.0, 146.0, 147.8, 159.2 ppm; HRMS (ESI) calcd for C21H17N3O2S: 374.0969 [M−H]+, found 374.0983.
3-Methyl-2-[4-(methylsulfonyl)benzyl]-1-oxo-1H,5H-pyrido[1,2-a]-benzimidazole-4-carbonitrile (5)
To a solution of 3j (50 mg, 0.14 mmol) in acetone (3 mL), a solution of m-CPBA (80 %, 36 mg, 0.21 mmol) in acetone (1 mL) was added at 0 °C, and the reaction mixture was stirred at RT for 3 h. Water (7 mL) was then added, the solvent concentrated, and the resulting precipitate was collected by filtration. The precipitate was washed with H2O (3×5 mL), Et2O (3×5 mL) and purified by preparative HPLC to afford 5 as a white solid; 1H NMR (400 MHz, [D6]DMSO): δ=2.41 (s, 3H), 3.15 (s, 3 H), 4.08 (s, 2 H), 7.36 (m, 1 H), 7.42 (d, J=8.0 Hz, 1H), 7.45–7.55 (m, 4 H), 7.79 (d, J=8.4 Hz, 1H), 8.59 (d, J=8.0 Hz, 1 H), 13.54 ppm (s, 1H); 13C NMR (400 MHz, [D6]DMSO): δ=18.8, 31.9, 43.6, 70.2, 112.9, 114.9, 116.7, 117.3, 123.5, 124.0, 127.1, 127.5, 128.2, 130.2, 132.2, 144.0, 146.1, 147.8, 159.2 ppm; HRMS (ESI) calcd for C21H17N3O3S: 390.0918 [M−H]+, found 390.0933.
2-(4-Hydroxybenzyl)-3-methyl-1-oxo-1H,5H-pyrido[1,2-a]benzimidazole-4-carbonitrile (6)
To a suspension of 3h (100 mg, 0.29 mmol) in anhydrous CH2Cl2 (8 mL), BBr3 (2.32 mL, 2.33 mmol) was added dropwise at 0 °C and the reaction mixture was stirred for 2 h at RT. Upon completion, the reaction mixture was quenched with H2O (5 mL), and the organic solvent was evaporated. The solid resulted was collected and purified by preparative HPLC to give 6 as white solid (75 mg, 79%); 1H NMR (400 MHz, [D6]DMSO): δ=2.34 (s, 3 H), 3.84 (s, 2H), 6.62 (d, J=8.0 Hz, 2H); 7.00 (d, J= 8.0 Hz, 2 H), 7.35 (m, 1 H), 7.45–7.55 (m, 2 H), 8.60 (d, J=8.0 Hz, 1 H), 9.10 (s, 1 H), 13.46 ppm (s, 1H); 13C NMR (400 MHz, [D6]DMSO): δ= 18.3, 30.2, 69.6, 111.3, 115.1, 116.3, 117.0, 122.3, 126.7, 127.8, 128.9, 130.5, 131.7, 145.5, 146.9, 155.3, 158.8 ppm; HRMS (ESI) calcd for C20H15N3O2 : 330.1237 [M+H]+, found 330.1225.
Supplementary Material
Acknowledgements
The support of US National Institutes of Health (grants AI30036 and AI079590) is gratefully acknowledged. We are grateful to Prof. P. Moodley, W. R. Jacobs, Jr., and M. Larsen for generously sharing clinical isolates of M. tuberculosis from KwaZulu Natal and for providing their drug susceptibility phenotypes. Dr. S. C. Cho and Prof S. G. Franzblau are acknowledged for the pheno typic HTS of the ASDI library. Dr A. Krunic is acknowledged for the 2D-NMR spectrometry assistance.
Footnotes
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/cphc.201000490.
References
- 1.a) Brooks JT, Kaplan JE, Holmes KK, Benson C, Pau A, Masur H. Clin. Infect. Dis. 2009;48:609–611. doi: 10.1086/596756. [DOI] [PubMed] [Google Scholar]; b) Lawn SD, Churchyard G. Curr. Opin. HIV AIDS. 2009;4:325–333. doi: 10.1097/COH.0b013e32832c7d61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization (WHO) [Last accessed: December 20, 2010];Global Tuberculosis Control: A short update to the 2009 report. 2009 http://whqlibdoc.who.int/publications/2009/9789241598866_eng.pdf.
- 3.a) Boshoff HIM, Barry CE., III Nat. Rev. Microbiol. 2005;3:70–80. doi: 10.1038/nrmicro1065. [DOI] [PubMed] [Google Scholar]; b) Wayne LG, Sohaskey CD. Annu. Rev. Microbiol. 2001;55:139–163. doi: 10.1146/annurev.micro.55.1.139. [DOI] [PubMed] [Google Scholar]
- 4.Johnson R, Streicher EM, Louw GE, Warren RM, Van Helden PD, Victor TC. Curr. Issues Mol. Biol. 2006;8:97–112. [PubMed] [Google Scholar]
- 5.Jassal MS, Bishai WR. Clin. Infect. Dis. 2010;50:S156–S164. doi: 10.1086/651486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization (WHO) [Last accessed: December 20, 2010];Multidrug and extensively drug-resistant tuberculosis: 2010 global report on surveillance and response. 2010 http://whqlibdoc.who.int/publications/2010/9789241599191_eng.pdf.
- 7.a) Dorman SE, Chaisson RE. Nat. Med. 2007;3:295–298. doi: 10.1038/nm0307-295. [DOI] [PubMed] [Google Scholar]; b) Extensively Drug-Resistant Tuberculosis: United States, 1993–2006, Morb. Mortal. Wkly Rep. 2007;56:250–253. [PubMed] [Google Scholar]
- 8.a) Migliori GB, De Iaco G, Besozzi G, Centis R, Cirillo DM. Euro Surveill. 2007;12(20):3194. doi: 10.2807/esw.12.20.03194-en. [DOI] [PubMed] [Google Scholar]; b) Velayati AA, Masjedi MR, Farnia P, Tabarsi P, Ghanavi J, Ziazarifi AH, Hoffner SE. Chest. 2009;136:420–425. doi: 10.1378/chest.08-2427. [DOI] [PubMed] [Google Scholar]
- 9.a) Check E. J. Nat. Med. 2007;13:266. doi: 10.1038/nm0307-266. [DOI] [PubMed] [Google Scholar]; b) Spigelman MK. J. Infect. Dis. 2007;196:S28–S34. doi: 10.1086/518663. [DOI] [PubMed] [Google Scholar]; c) Van den Boogaard J, Kibiki GS, Kisanga ER, Boeree MJ, Aarnoutse RE. Antimicrob. Agents Chemother. 2009;53:849–862. doi: 10.1128/AAC.00749-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma Z, Lienhardt C, McIlleron H, Nunn AJ, Wang X. Lancet. 2010;375:2100–2109. doi: 10.1016/S0140-6736(10)60359-9. [DOI] [PubMed] [Google Scholar]
- 11.Maggi N, Pasqualucci CR, Ballotta R, Sensi P. Farmaco Sci. 1966;21:68–75. [PubMed] [Google Scholar]
- 12.Ananthan S, Faaleolea ER, Goldman RC, Hobrath JV, Kwong CD, Laughon BE, Maddry JA, Metha A, Rasmussen L, Reynolds RC, Secrist JA, III, Shindo N, Showe DN, Sosa MI, Suling WJ, White EL. Tuberculosis. 2009;89:334–353. doi: 10.1016/j.tube.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andries K, Verhasselt P, Guillemont J, Goehlmann HWH, Neefs J-M, Winkler H, Van Gestel J, Timmerman P, Zhu M, Lee E, Williams P, de Chaffoy D, Huitric E, Hoffner S, Cambau E, Truffot-Pernot C, Lounis N, Jarlier VA. Science. 2005;307:223–227. doi: 10.1126/science.1106753. [DOI] [PubMed] [Google Scholar]
- 14.Lenaerts AJ, Gruppo V, Marietta KS, Johnson CM, Driscoll DK, Tompkins NM, Rose JD, Reynolds RC, Orme IM. Antimicrob. Agents Chemother. 2005;49:2294–2301. doi: 10.1128/AAC.49.6.2294-2301.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.a) Mao J, Wang Y, Wan B, Kozikowski AP, Franzblau SG. Chem Med-Chem. 2007;2:1624–1630. doi: 10.1002/cmdc.200700112. [DOI] [PubMed] [Google Scholar]; b) Mao J, Wan B, Wang Y, Franzblau SG, Kozikowski AP. Chem Med Chem. 2007;2:811–813. doi: 10.1002/cmdc.200700048. [DOI] [PubMed] [Google Scholar]
- 16.a) Lilienkampf A, Mao J, Wan B, Wang Y, Franzblau SG, Kozikowski AP. J. Med. Chem. 2009;52:2109–2118. doi: 10.1021/jm900003c. [DOI] [PubMed] [Google Scholar]; b) Pieroni M, Lilienkampf A, Wan B, Wang Y, Franzblau SG, Kozikowski AP. J. Med. Chem. 2009;52:6287–6296. doi: 10.1021/jm900513a. [DOI] [PubMed] [Google Scholar]; c) Lilienkampf A, Pieroni M, Wan B, Wang Y, Franzblau SG, Kozikowski AP. J. Med. Chem. 2010;53:678–688. doi: 10.1021/jm901273n. [DOI] [PubMed] [Google Scholar]
- 17.Rida SM, Soliman FSG, Badawey EAM, Kappe T. J. Heterocycl. Chem. 1988;25:1725–1728. [Google Scholar]
- 18.Lee H-S, Park J-S, Kim BM, Gellman SH. J. Org. Chem. 2003;68:1575–1578. doi: 10.1021/jo026738b. [DOI] [PubMed] [Google Scholar]
- 19.Sakai T, Miyata K, Tsuboi S, Utaka M. Bull. Chem. Soc. Jpn. 1989;62:4072–4074. [Google Scholar]
- 20.Xie Q, Sun H, Xie G, Zhou J. J. Chem. Inf. Comput. Sci. 1995;35:106–109. [Google Scholar]
- 21.a) Takemura M, Takahashi H, Kawakami K, Takeshita H, Kimura Y, Watanabe J, Sugimoto Y, Kitamura A, Nakajima R, Kanai K, Fujisawa T. Chem. Abstr. Vol. 139. Tokyo, Japan: Daiichi Pharmaceutical Co., Ltd; 2003. p. 164813. 2003, PCT Int. Appl. WO/2003/064422A1 20030807. [Google Scholar]; b) Kitamura A, Higuchi S, Hata M, Kawakami K, Kumi Y, Namba K, Nakajima R. Antimicrob. Agents Chemother. 2009;53:3963–3971. doi: 10.1128/AAC.00435-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rothfuchs AG, Bafica A, Feng CG, Egen JG, Williams DL, Brown GD, Sher A. J. Immunol. 2007;179:3463–3471. doi: 10.4049/jimmunol.179.6.3463. [DOI] [PubMed] [Google Scholar]
- 23.a) Ioerger TR, Koo S, No EG, Chen X, Larsen MH, Jacobs WR, Jr, Pillay M, Sturm AW, Sacchettini JC. PLoS One. 2009;4:e7778. doi: 10.1371/journal.pone.0007778. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Collins LA, Franzblau SG. Antimicrob. Agents Chemother. 1997;41:1004–1009. doi: 10.1128/aac.41.5.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bremberg U, Lindén A, Lundbäck T, Nilsson J, Wiik M, Bergner M, Brandt P, Hammer K, Ringom R. Chem. Abstr. Vol. 151. Stockholm, Sweden: Biovitrum AB; 2008. p. 313387. 2008, PCT Int. Appl. WO/2008/116898A1. [Google Scholar]
- 25.Hynd G, Harris NV, Bull RJ, Gardan S, Handa BK. Chem. Abstr. Vol. 148. Harlow, UK: Argenta Discovery Ltd; 2007. p. 78884. 2007, PCT Int. Appl. WO/2007/144625A1. [Google Scholar]
- 26.Moloney GP, Robertson AD, Martin GR, MacLennan S, Mathews N, Dodsworth S, Sang PY, Knight C, Glen RA. J. Med. Chem. 1997;40:2347–2362. doi: 10.1021/jm9605849. [DOI] [PubMed] [Google Scholar]
- 27.Tetko IV, Gasteiger J, Todeschini R, Mauri A, Livingstone D, Ertl P, Rohde B, Selzer P. J. Med. Chem. 2000;43:3714–3717. doi: 10.1021/jm000942e. [DOI] [PubMed] [Google Scholar]; Radchenko EV, Zefirov NS, Makarenko AS, Tanchuk VY, Prokopenko VV. J. Comput. Aid. Mol. Des. 2005;19:453–463. doi: 10.1007/s10822-005-8694-y. [DOI] [PubMed] [Google Scholar]
- 28.Ertl P, Rohde B, Selzer P. J. Med. Chem. 2000;43:3714–3717. doi: 10.1021/jm000942e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.