Abstract
In many synapses of the CNS, mobile zinc is packaged into glutamatergic vesicles and co-released with glutamate during neurotransmission. Following synaptic release, the mobilized zinc modulates ligand- and voltage-gated channels and receptors, functioning as an inhibitory neuromodulator. However, the origin and role of tonic, as opposed to phasically released, zinc are less well understood. We investigated tonic zinc in the dorsal cochlear nucleus (DCN), a zinc-rich, auditory brainstem nucleus. Our results show that application of a high-affinity, extracellular zinc chelator (ZX1) enhances spontaneous firing in DCN principal neurons (fusiform cells), consistent with inhibition of this neuronal property by tonic zinc. The enhancing effect was prevented by prior application of strychnine, a glycine receptor antagonist, suggesting that ZX1 interferes with zinc-mediated modulation of spontaneous glycinergic inhibition. In particular, ZX1 decreased the amplitude and the frequency of glycinergic miniature inhibitory postsynaptic currents in fusiform cells, from which we conclude that tonic zinc enhances glycinergic inhibitory neurotransmission. The observed zinc-mediated inhibition in spontaneous firing is present in mice lacking the vesicular zinc transporter (ZnT3), indicating that non-vesicular zinc inhibits spontaneous firing. Noise-induced increase in the spontaneous firing of fusiform cells is crucial for the induction of tinnitus. In this context, tonic zinc provides a powerful break of spontaneous firing that may protect against pathological run-up of spontaneous activity in the DCN.
Keywords: Zinc, Vesicular zinc transporter, Glycinergic neurotransmission, Spontaneous firing, Auditory brainstem, Dorsal cochlear nucleus
Introduction
Since the discovery that zinc is loaded into glutamatergic vesicles and is exocytosed with glutamate during synaptic transmission, numerous studies have investigated the role of mobilized zinc in synapses. These studies are consistent with a model whereby zinc serves as an inhibitory neuromodulatory neurotransmitter, inhibiting NMDA receptors (NMDARs), reducing release probability in excitatory synapses, and potentiating glycinergic and GABAergic inhibitory neurotransmission (Xie and Smart, 1991; Hirzel et al., 2006; Nozaki et al., 2011; Pan et al., 2011; Perez-Rosello et al., 2013; Vergnano et al., 2014). Unlike many other neurotransmitter systems, where the actions of tonic levels of neurotransmitters are well studied, the role of tonic zinc remains less understood. Moreover, prior electrophysiological studies are limited and present conflicting results. For example, one study reports that, in mossy fiber synapses, ZnT3-dependent tonic zinc levels modulate NMDARs (Vogt et al., 2000), but more recent work at the same synapses, finds that ambient zinc levels are too low for NMDAR modulation (Vergnano et al., 2014). The role of ambient zinc therefore remains enigmatic.
A major obstacle in determining the role of zinc (synaptic and tonic) has been the lack of a chelator with appropriate kinetic and thermodynamic properties for probing the temporal and spatial changes of mobile zinc at synapses (Radford and Lippard, 2013), as illustrated by the contrasting findings about the role of tonic zinc in previous work. Quite different zinc chelators were applied in these studies: a kinetically slow one (Vogt et al., 2000) and a faster, but low-affinity chelator (Vergnano et al., 2014). To assess the role and origin of tonic zinc we utilized the fast, high-affinity zinc chelator ZX1 (Pan et al., 2011; Radford and Lippard, 2013) as well as transgenic mice lacking ZnT3. We studied the role of tonic zinc on DCN fusiform cells. Fusiform cells generate spontaneous action potentials (Rhode et al., 1983; Hancock and Voigt, 2002; Leao et al., 2012) and, because they are embedded in a zinc-rich nucleus (Frederickson et al., 1988; Rubio and Juiz, 1998; Oertel and Young, 2004), they provide an ideal assay for testing the effects of tonic zinc on neuronal excitability.
Methods
Slice preparation
Experiments were conducted according to the methods approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. Coronal brainstem slices were prepared from ICR mice and ZnT3 knockout (ZnT3 KO) mice (P17–P25). ICR mice were purchased from Harlan and ZnT3 KO mice were purchased from The Jackson Laboratory. The preparation of coronal slices containing DCN has been described in detail previously (Tzounopoulos et al., 2004).
Electrophysiological recordings and analysis
Loose cell-attached voltage-clamp and whole cell voltage-clamp recordings were obtained from visually identified fusiform cells at a temperature of 31–33 °C. Fusiform cells were identified on the basis of morphological and electrophysiological criteria (Tzounopoulos et al., 2004). In preparing the external solution we removed contaminating zinc from our solutions with Chelex 100 resin (Biorad). After applying Chelex to the ACSF, high purity calcium and magnesium salts were added (99.995% purity, Sigma). All plastic and glassware were washed with 5% high purity nitric acid (Sigma). The external solution contained the following (in mM): 130 NaCl, 3 KCl, 2.4 CaCl2, 1.3 MgSO4, 21 NaHCO3, 3.5 HEPES, and 10 glucose, saturated with 95% O2/5% CO2. For loose cell-attached voltage-clamp recordings assessing spontaneous firing, pipettes (1.5–2.5 MΩ) were filled with modified external solution containing (in mM): 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 2 CaCl2·2H2O, 1 MgCl2, 25 NaHCO3, and 25 glucose. Seal resistance was maintained between 10 and 30 MΩ with command potential at 0 mV. To measure the time course of ZX1 (100 μM, purchased from Strem) and strychnine (500 nM) on spontaneous firing frequency, instantaneous frequency was measured, averaged every minute, and then normalized to baseline. For miniature IPSCs (mIPSCs) and spontaneous IPSCs (sIPSCs) experiments, fusiform cells were clamped at the reversal of excitatory responses (~0 mV) with pipettes containing a cesium-based internal solution (containing in mM: Cs (CH3O3S) 126, KCl 4, HEPES 10, Na2ATP 4, Tris-GTP 0.3, Tris-phosphocreatine 10, CsEGTA 1, QX-314 1, sodium ascorbate 3, pH = 7.25, 295 mOsm). Miniature IPSCs were recorded in the presence of tetrodotoxin (TTX, sodium channel blocker, 0.5 μM), SR95531 (GABAA receptor antagonist, 20 μM), AP-5 (NMDA receptor antagonist, 50 μM), and DNQX (AMPA receptor antagonist, 50 μM). Spontaneous IPSCs were recorded in the presence of SR95531 (20 μM). Ten-second blocks of mIPSCs and sIPSCs were acquired at a sample rate of 50 kHz and low pass filtered at 10 kHz. Negative-voltage pulses (−5 mV, 50 ms) were delivered every 10 s to monitor input and access resistance. Voltage-clamp experiments were not included if the series and/or input resistance changed by more than 20% during the experiment. mIPSCs and sIPSCs were detected and analyzed using Mini-analysis software (Synaptosoft) with amplitude and area thresholds set at 3 times the noise level. All events were verified by visual inspection. Amplitude values were obtained by subtracting the average baseline from the amplitude at the local maximum during the event. Electrophysiological data were acquired and analyzed using pClamp, IGOR PRO (Wavemetrics), and GraphPad Prism (GraphPad Software). Statistical comparisons were made using analysis of variance, and paired and unpaired two-tailed Student’s t tests. Statistical significance was based on p values <0.05. All means are reported ±SEM.
Results
Tonic zinc decreases spontaneous firing in fusiform cells by enhancing glycinergic neurotransmission
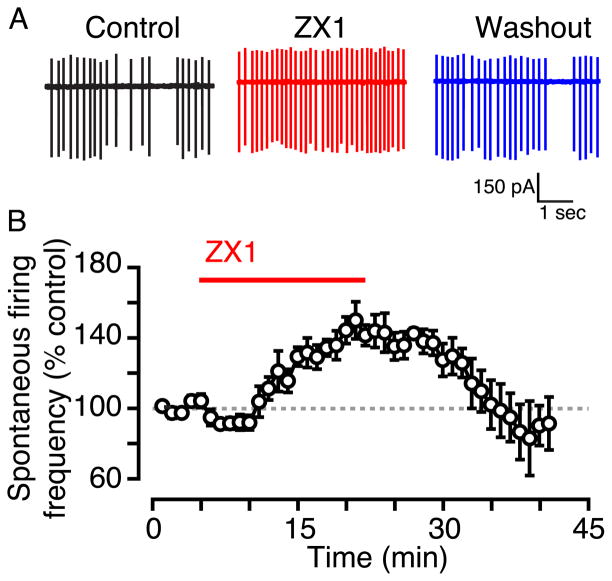
To test whether tonic zinc modulates spontaneous firing in fusiform cells, we examined the effect of ZX1, a high-affinity extracellular zinc chelator (Pan et al., 2011; Radford and Lippard, 2013), on the rate of spontaneous action potentials. In cell-attached recordings, 100 μM ZX1 increased the spontaneous firing rate in fusiform cells; this effect was reversed upon removal of ZX1 from the bath (Figs. 1A, B; ZX1: 148 ± 14% of control, n = 7, p = 0.01 when compared to control; wash out: 95 ± 10%, p = 0.5 when compared to control). These results indicate that tonic zinc inhibits spontaneous firing rates in fusiform cells.
Fig. 1.
Tonic zinc decreases fusiform cell spontaneous firing rate. A) Representative cell-attached recordings showing fusiform cell spontaneous firing in control (black), in the presence of ZX1 (red), and after ZX1 washout (blue). B) Time course of the effect of ZX1. ZX1 significantly increased fusiform cell spontaneous firing rate; the firing rate returned to baseline after ZX1 washout.
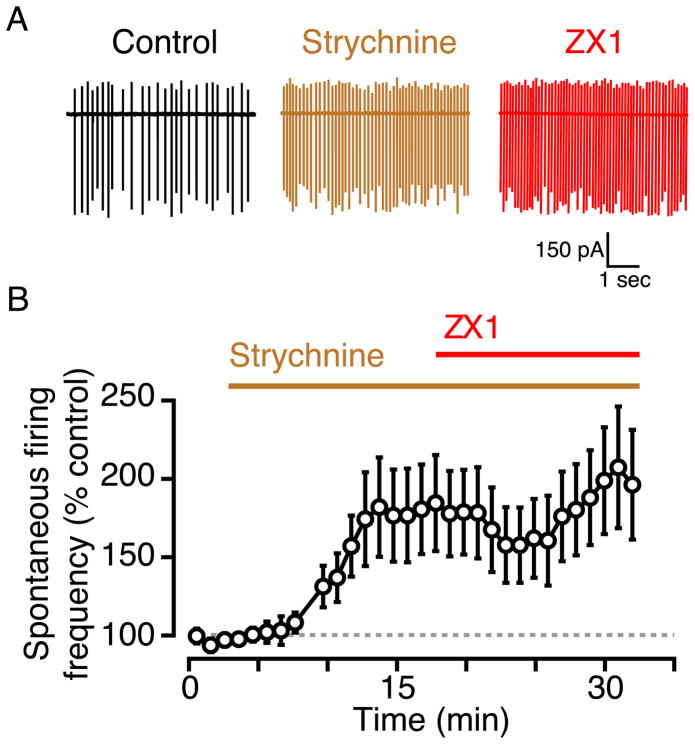
Spontaneous firing in fusiform cells is generated by intrinsic neuronal properties (Leao et al., 2012); however, synaptic activity, mainly inhibitory, modulates spontaneous firing in fusiform cells (Roberts and Trussell, 2010). Fusiform cells receive robust glycinergic (and GABAergic inhibition) from cartwheel, stellate, and tuberculoventral cells (Oertel and Young, 2004; Mancilla and Manis, 2009; Roberts and Trussell, 2010; Kuo et al., 2012; Apostolides and Trussell, 2013, 2014a), and zinc potentiates glycinergic neurotransmission in other brain areas (Laube et al., 2000; Suwa et al., 2001). We therefore hypothesized that tonic zinc exerts its inhibitory effect on fusiform cell firing by potentiating spontaneous glycinergic inhibition. Consistent with this hypothesis, we found that 500 nM strychnine, a glycine receptor (GlyR) antagonist, increased spontaneous firing and blocked the effect of zinc chelation (Figs. 2A, B; strychnine: 179 ± 28% of control, n = 5, p = 0.03 when compared to control; ZX1: 112 ± 8% of strychnine, n = 5, p = 0.24 when compared to strychnine). The absence of an effect of ZX1 on spontaneous firing after strychnine application is not the result of a “ceiling effect”, because the magnitude of ZX1 enhancement was not correlated with the initial spontaneous firing frequency (Suppl Fig. 1A, r2 = 0.004, p = 0.88). Moreover, the effect of ZX1 in the presence of strychnine was smaller than in control conditions even when the initial firing frequency prior to ZX1 application was similar between control and strychnine (Suppl. Fig. 1B). Together, these results suggest that tonic zinc inhibits spontaneous firing rates in fusiform cells, at least in part by potentiating spontaneous glycinergic neurotransmission.
Fig. 2.
Pharmacological blockade of glycinergic inhibition increases fusiform cell spontaneous firing rate and occludes the effect of tonic zinc. A) Representative cell-attached recordings showing fusiform cell spontaneous firing in control (black), in the presence of strychnine (brown), and in the presence of strychnine and ZX1 (red). B) Time course of the effect of strychnine and ZX1. Strychnine significantly increased fusiform cell spontaneous firing rate; ZX1 did not increase spontaneous firing rate when applied after strychnine.
Tonic zinc potentiates GlyRs and increases Pr in glycinergic terminals
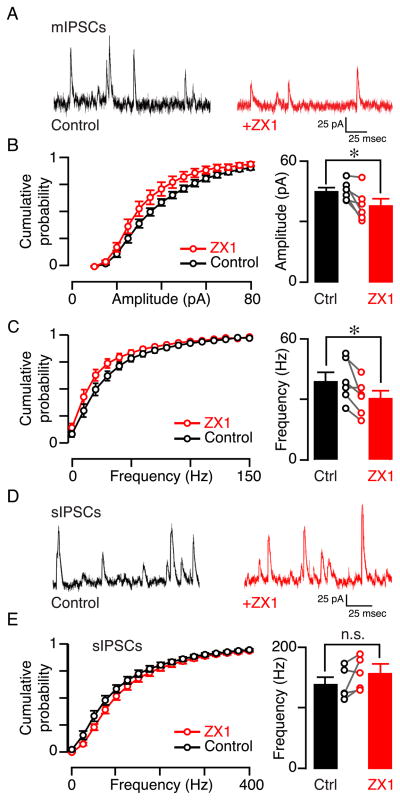
Tonic zinc may increase glycinergic inhibition onto fusiform cells presynaptically, either by increasing glycine release and/or by increasing the spontaneous firing rate of glycinergic interneurons that contact fusiform cells, or postsynaptically, by potentiating glycine receptors (GlyRs). To determine whether tonic zinc causes a potentiation in postsynaptic GlyRs we measured the effect of ZX1 on the amplitude of miniature, action-potential-independent, IPSCs (mIPSCs). We performed these experiments in the presence of 500 nM tetrodotoxin, a sodium channel blocker, ZX1 decreased the mean mIPSC amplitude, suggesting that tonic zinc potentiates postsynaptic GlyRs (Figs. 3A, B; control: 44.5 ± 1.7 pA; ZX1: 37.6 ± 2.9 pA, n = 6, p = 0.024). To assess whether tonic zinc influences the probability of release in glycinergic terminals we evaluated the frequency of mIPSCs. ZX1 reduced mIPSC frequency, suggesting a tonic zinc-mediated increase in glycine release probability (Figs. 3A, C; control: 39. 3± 4.3 Hz; ZX1: 30.9 ± 3.5 Hz, n = 6, p = 0.03). Finally, to evaluate whether tonic zinc affects the overall firing of spontaneously active presynaptic glycinergic interneurons, which are also contacting each other (Roberts et al., 2008; Apostolides and Trussell, 2014b), we evaluated the effect of ZX1 on the frequency of spontaneous glycinergic IPSCs (sIPSCs), no TTX was present in these experiments. ZX1 did not affect sIPSC frequency suggesting that the overall spontaneous firing rate of presynaptic inhibitory neurons was not affected by tonic zinc (Figs. 3D, E; control: 140.8 ± 10.6 Hz; ZX1: 158.9 ± 10.7 Hz, n = 5, p = 0.153). However, because tonic zinc increased mIPSC frequency, the lack of effect on sIPSC frequency may reflect a zinc-mediated reduction in the overall firing of presynaptic interneurons that balances the increase in mIPSC frequency or a differential regulation of action potential-driven and non-action potential driven neurotransmitter release (Ramirez and Kavalali, 2011). Together these results suggest that tonic zinc causes a potentiation of postsynaptic GlyRs and an increase in release probability of glycine, at least during non-action potential-driven release. These effects act synergistically to enhance spontaneous glycinergic inhibition to fusiform cells.
Fig. 3.
Tonic zinc increases mIPSC amplitude and frequency. A) Left — example trace showing control mIPSCs (black); right — example trace showing mIPSCs after ZX1 application (red). B) Left — cumulative probability plot of the amplitudes of mIPSCs in control and in the presence of ZX1; right — group data show that ZX1 significantly reduced the average amplitude of mIPSCs. C) Left — cumulative probability plot of the frequencies of mIPSCs in control and in the presence of ZX1; right — group data show that ZX1 significantly reduced the mean frequency of mIPSCs. D) Left — example trace showing control sIPSCs (black); right — example trace showing sIPSCs after ZX1 application (red). E) Left — cumulative probability plot of the frequency of sIPSCs in control and in the presence of ZX1; right —group data show that ZX1 did not significantly increase the mean frequency of sIPSCs.
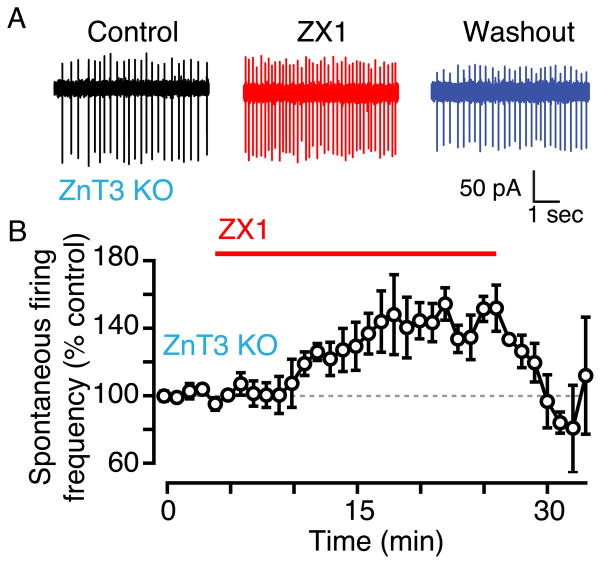
Tonic zinc-mediated inhibition in spontaneous firing is not ZnT3-dependent
To determine the source of tonic zinc mediating the modulation on spontaneous firing, we examined the effect of ZX1 on fusiform cells from ZnT3 knockout mice, which lack synaptic (vesicular) zinc. Application of ZX1 caused a significant increase in spontaneous firing frequency that was reversed upon ZX1 removal (Figs. 4A, B; ZX1: 128 ± 8%, n = 5, p = 0.000001). Moreover, this increase is not quantitatively different from the increase observed in wild type mice, suggesting that ZnT3-independent zinc is capable of inhibiting spontaneous firing in fusiform cells (p = 0.31 when compared to Fig. 1).
Fig. 4.
Tonic, ZnT3-independent zinc decreases fusiform cell spontaneous firing rate in ZnT3 knockout (KO) mice. A) Representative cell-attached recordings showing fusiform cell spontaneous firing in control (black), in the presence of ZX1 (red), and after ZX1 washout (blue). B) Time course of the effect of ZX1. ZX1 significantly increased fusiform cell spontaneous firing rate in ZnT3 KO mice; the firing rate returned to baseline after ZX1 washout.
Discussion
Our results identify tonic zinc as an endogenous modulator of spontaneous firing in principal DCN neurons. Previous studies established that ~50% of fusiform cells are spontaneously active, firing action potentials in the absence of evoked synaptic stimulation. Spontaneous firing is mediated by intrinsic properties (Leao et al., 2012). In particular, fusiform cells with resting membrane potential above or approximately at −65 mV, which is set by the variable expression of inwardly rectifying potassium currents, activate “persistent” sodium channels, which in turn initiate spontaneous firing. Fusiform cells with slightly more hyperpolarized resting membrane potential remain quiet. Although intrinsic conductances mediate spontaneous firing, spontaneous activity from inhibitory interneurons, such as cartwheel and stellate cells, is a key modulator of fusiform cell spontaneous firing rates (Roberts et al., 2008; Roberts and Trussell, 2010; Apostolides and Trussell, 2014a, b). Our results show that, by potentiating glycinergic mIPSCs and increasing the probability of release of glycine, tonic zinc inhibits spontaneous firing.
These findings complement and extend previous studies showing that zinc acts as an allosteric modulator of GlyRs and as an enhancer of glycine release. Previous reports detail a bimodal effect of zinc, where at concentrations between 10 nM and 1 μM it potentiates glycinergic currents, including mIPSCs (Laube et al., 2000; Suwa et al., 2001), whereas at higher concentrations zinc decreases glycinergic currents (Bloomenthal et al., 1994; Laube et al., 1995). The potentiating effect of zinc, which is consistent with our findings, is due to a zinc-mediated increase in the affinity of glycine, resulting from decreased glycine dissociation, whereas higher zinc concentrations cause a voltage-independent block through reduction in the efficacy in channel opening (Laube et al., 2000; Lynch, 2004). Moreover, low zinc levels enhance glycinergic neurotransmission via an increase in the probability of release (Suwa et al., 2001; Laube, 2002), which is also consistent with our findings. Although these studies have revealed that the increase in the probability of release is mediated by presynaptic P2X in rat spinal neurons (Laube, 2002), further studies are needed to determine the mechanism of zinc-mediated modulation of the probability of release in DCN glycinergic synapses.
Tonic zinc in synapses
We identified a separate pool of tonic zinc that is not ZnT3-dependent and that modulates fusiform cell spontaneous firing. We, therefore, propose that zinc may modulate neuronal excitability even in neuronal areas that are not in the vicinity of synaptic vesicles loaded with zinc.
Our results show that tonic zinc levels potentiate GlyRs, which in turn suggests that tonic zinc levels must be at least 10 nM. Ten nanomolar tonic zinc levels are capable of modulating NMDARs containing NR2A subunits (Hansen et al., 2014) and GlyRs containing the a1 subunit (Miller et al., 2005). Moreover, our results indicate that the tonic zinc level must be lower than 10 μM, because above this concentration zinc inhibits GlyRs. Our estimated tonic zinc levels are consistent with those reported in the brain in vivo (Frederickson et al., 2006; Su et al., 2014).
Our results show that the source of tonic zinc is not ZnT3-dependent in DCN parallel fiber synapses. However, the origin of DCN tonic zinc remains unknown. The SLC30 (ZnT) and SLC39 (ZIP) families of zinc transport proteins control intracellular and extracellular zinc levels (Huang and Tepaamorndech, 2013; Jeong and Eide, 2013). Although the neuronal localization of ZIP transporters is less well understood, from the 10 known members of the ZnT transporter family that export the ion away from the cytosol into organelles or into the extracellular space, ZnT1 clusters at synaptic sites (Mellone et al., 2014; Sindreu et al., 2014). We therefore propose that it may participate in establishing tonic ZnT3-independent zinc levels within the synaptic cleft. ZnT1 is not restricted to synapses that contain ZnT3, consistent with our suggestion that tonic zinc may modulate NMDARs and GlyRs at synaptic areas that do not express the vesicular zinc transporter. Alternatively, the origin of tonic zinc may be non-neuronal. In either case, more studies are needed for determining the source of tonic, ZnT3-independent zinc.
Tonic zinc, spontaneous firing and tinnitus
Previous studies have linked the lack of potentiating effect of zinc in GlyRs to hyperekplexia in a mouse animal model (Hirzel et al., 2006). Moreover, recent studies have linked mutations that abolish zinc-mediated allosteric GlyR potentiation with human hyperekplexia (Zhou et al., 2013). We propose that zinc mediated allosteric enhancement of GlyRs may be involved in tinnitus.
Previous studies have linked the DCN with the induction of tinnitus; ablation of the DCN during the noise-exposure, but not after the establishment of tinnitus, prevents the development of tinnitus (Brozoski and Bauer, 2005; Brozoski et al., 2012). Importantly, increases in spontaneous fusiform cell firing rates are associated with behavioral evidence of tinnitus (Li et al., 2013). Noise-induced decreases in GABAergic/glycinergic inhibition, increases in glutamatergic excitation, and decreases in KCNQ2/3 potassium channel activity contribute to tinnitus-related DCN hyperexcitability (Wang et al., 2009; Middleton et al., 2011; Zeng et al., 2012; Li et al., 2013). Given that zinc levels are lowered by sensory activity (Brown and Dyck, 2005; Nakashima and Dyck, 2010), we propose that, if similar noise-induced reduction in zinc levels occurs in the DCN, which is not known, it would lead to decreased glycinergic inhibition and increased spontaneous firing rates. Such behavior would point to zinc as a potential therapeutic for tinnitus patients.
Although the association of zinc and tinnitus has been previously hypothesized, dietary zinc supplements are not an effective treatment (Coelho et al., 2013). However, these studies are related to the advanced stages of tinnitus. As tinnitus becomes chronic, the locus of expression shifts from the DCN to higher order structures such as the cortex and the limbic system (Rauschecker et al., 2010; Leaver et al., 2011). Therefore, even if dietary zinc supplements were to increase the levels of synaptic zinc in the DCN in humans, the perception of tinnitus would not be affected for chronic tinnitus sufferers because the DCN, a key site for the induction of tinnitus, is not involved in its maintenance. Future experiments are required to examine the potential role of zinc in mediating tinnitus-related hyperexcitability in animal models of tinnitus and to learn whether changes in dietary zinc may play a role in the induction of tinnitus in humans.
Conclusions
Application of ZX1, an extracellular zinc chelator, reveals that tonic zinc inhibits spontaneous firing in fusiform cells. A GlyR antagonist occluded this inhibition, suggesting that it is mediated by GlyRs. Consistent with this conclusion, tonic zinc enhances glycinergic neuro-transmission via pre- and postsynaptic mechanisms. The source of tonic zinc that enhances fusiform cell spontaneous firing rates is not ZnT3-dependent, thus raising the possibility that zinc may influence neuronal excitability even in neuronal areas that are not in the vicinity of ZnT3.
Supplementary Material
Acknowledgments
This work was supported by funding from the NIH: TT: RO1-DC007905; TPR: F32-DC011664; CTA: F32-DC013734-01A1; and SJL: RO1-GM065519.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nbd.2015.03.012.
References
- Apostolides PF, Trussell LO. Regulation of interneuron excitability by gap junction coupling with principal cells. Nat Neurosci. 2013;16:1764–1772. doi: 10.1038/nn.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolides PF, Trussell LO. Superficial stellate cells of the dorsal cochlear nucleus. Front Neural Circ. 2014a;8:63. doi: 10.3389/fncir.2014.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolides PF, Trussell LO. Chemical synaptic transmission onto superficial stellate cells of the mouse dorsal cochlear nucleus. J Neurophysiol. 2014b;111:1812–1822. doi: 10.1152/jn.00821.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomenthal AB, Goldwater E, Pritchett DB, Harrison NL. Biphasic modulation of the strychnine-sensitive glycine receptor by Zn2+ Mol Pharmacol. 1994;46:1156–1159. [PubMed] [Google Scholar]
- Brown CE, Dyck RH. Modulation of synaptic zinc in barrel cortex by whisker stimulation. Neuroscience. 2005;134:355–359. doi: 10.1016/j.neuroscience.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Bauer CA. The effect of dorsal cochlear nucleus ablation on tinnitus in rats. Hear Res. 2005;206:227–236. doi: 10.1016/j.heares.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Wisner KW, Sybert LT, Bauer CA. Bilateral dorsal cochlear nucleus lesions prevent acoustic-trauma induced tinnitus in an animal model. J Assoc Res Otolaryngol. 2012;13:55–66. doi: 10.1007/s10162-011-0290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho C, Witt SA, Ji H, Hansen MR, Gantz BRT. Zinc to treat tinnitus in the elderly: a randomized placebo controlled crossover trial. Otol Neurotol. 2013;34:1146–1154. doi: 10.1097/MAO.0b013e31827e609e. [DOI] [PubMed] [Google Scholar]
- Frederickson CJ, Howell GA, Haigh MD, Danscher G. Zinc-containing fiber systems in the cochlear nuclei of the rat and mouse. Hear Res. 1988;36:203–211. doi: 10.1016/0378-5955(88)90062-7. [DOI] [PubMed] [Google Scholar]
- Frederickson CJ, Giblin LJ, III, Balaji RV, Masalha R, Zeng Y, Lopez EV, Koh JY, Chorin U, Besser L, Hershfinkel M, Li Y, Thompson RB, Krezel A. Synaptic release of zinc from brain slices: factors governing release, imaging, and accurate calculation of concentration. J Neurosci Methods. 2006;154:19–29. doi: 10.1016/j.jneumeth.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Hancock KE, Voigt HF. Intracellularly labeled fusiform cells in dorsal cochlear nucleus of the gerbil. II Comparison of physiology and anatomy. J Neurophysiol. 2002;87:2520–2530. doi: 10.1152/jn.2002.87.5.2520. [DOI] [PubMed] [Google Scholar]
- Hansen KB, Ogden KK, Yuan H, Traynelis SF. Distinct functional and pharmacological properties of Triheteromeric GluN1/GluN2A/GluN2B NMDA receptors. Neuron. 2014;81:1084–1096. doi: 10.1016/j.neuron.2014.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirzel K, Muller U, Latal AT, Hulsmann S, Grudzinska J, Seeliger MW, Betz H, Laube B. Hyperekplexia phenotype of glycine receptor alpha1 subunit mutant mice identifies Zn2+ as an essential endogenous modulator of glycinergic neuro-transmission. Neuron. 2006;52:679–690. doi: 10.1016/j.neuron.2006.09.035. [DOI] [PubMed] [Google Scholar]
- Huang L, Tepaamorndech S. The SLC30 family of zinc transporters — a review of current understanding of their biological and pathophysiological roles. Mol Aspects Med. 2013;34:548–560. doi: 10.1016/j.mam.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Jeong J, Eide DJ. The SLC39 family of zinc transporters. Mol Aspects Med. 2013;34:612–619. doi: 10.1016/j.mam.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo SP, Lu HW, Trussell LO. Intrinsic and synaptic properties of vertical cells of the mouse dorsal cochlear nucleus. J Neurophysiol. 2012;108:1186–1198. doi: 10.1152/jn.00778.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube B. Potentiation of inhibitory glycinergic neurotransmission by Zn2+: a synergistic interplay between presynaptic P2X2 and postsynaptic glycine receptors. Eur J Neurosci. 2002;16:1025–1036. doi: 10.1046/j.1460-9568.2002.02170.x. [DOI] [PubMed] [Google Scholar]
- Laube B, Kuhse J, Rundstrom N, Kirsch J, Schmieden V, Betz H. Modulation by zinc ions of native rat and recombinant human inhibitory glycine receptors. J Physiol. 1995;483 (Pt 3):613–619. doi: 10.1113/jphysiol.1995.sp020610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube B, Kuhse J, Betz H. Kinetic and mutational analysis of Zn2+ modulation of recombinant human inhibitory glycine receptors. J Physiol. 2000;522 (Pt 2):215–230. doi: 10.1111/j.1469-7793.2000.t01-1-00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leao RM, Li S, Doiron B, Tzounopoulos T. Diverse levels of an inwardly rectifying potassium conductance generate heterogeneous neuronal behavior in a population of dorsal cochlear nucleus pyramidal neurons. J Neurophysiol. 2012;107:3008–3019. doi: 10.1152/jn.00660.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver AM, Renier L, Chevillet MA, Morgan S, Kim HJ, Rauschecker JP. Dysregulation of limbic and auditory networks in tinnitus. Neuron. 2011;69:33–43. doi: 10.1016/j.neuron.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Choi V, Tzounopoulos T. Pathogenic plasticity of Kv7.2/3 channel activity is essential for the induction of tinnitus. Proc Natl Acad Sci U S A. 2013;110:9980–9985. doi: 10.1073/pnas.1302770110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JW. Molecular structure and function of the glycine receptor chloride channel. Physiol Rev. 2004;84:1051–1095. doi: 10.1152/physrev.00042.2003. [DOI] [PubMed] [Google Scholar]
- Mancilla JG, Manis PB. Two distinct types of inhibition mediated by cartwheel cells in the dorsal cochlear nucleus. J Neurophysiol. 2009;102:1287–1295. doi: 10.1152/jn.91272.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellone M, Pelucchi S, Alberti L, Genazzani A, Di Luca M, Gardoni F. Zinc transporter-1 (ZNT-1): a novel NMDA receptor-binding protein at postsynaptic density. J Neurochem. 2014;132 (2):159–168. doi: 10.1111/jnc.12968. [DOI] [PubMed] [Google Scholar]
- Middleton JW, Kiritani T, Pedersen C, Turner JG, Shepherd GM, Tzounopoulos T. Mice with behavioral evidence of tinnitus exhibit dorsal cochlear nucleus hyperactivity because of decreased GABAergic inhibition. Proc Natl Acad Sci U S A. 2011;108:7601–7606. doi: 10.1073/pnas.1100223108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller PS, Da Silva HM, Smart TG. Molecular basis for zinc potentiation at strychnine-sensitive glycine receptors. J Biol Chem. 2005;280:37877–37884. doi: 10.1074/jbc.M508303200. [DOI] [PubMed] [Google Scholar]
- Nakashima AS, Dyck RH. Dynamic, experience-dependent modulation of synaptic zinc within the excitatory synapses of the mouse barrel cortex. Neuroscience. 2010;170:1015–1019. doi: 10.1016/j.neuroscience.2010.08.020. [DOI] [PubMed] [Google Scholar]
- Nozaki C, Vergnano AM, Filliol D, Ouagazzal AM, Le Goff A, Carvalho S, Reiss D, Gaveriaux-Ruff C, Neyton J, Paoletti P, Kieffer BL. Zinc alleviates pain through high-affinity binding to the NMDA receptor NR2A subunit. Nat Neurosci. 2011;14:1017–1022. doi: 10.1038/nn.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel D, Young ED. What’s a cerebellar circuit doing in the auditory system? Trends Neurosci. 2004;27:104–110. doi: 10.1016/j.tins.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Pan E, Zhang XA, Huang Z, Krezel A, Zhao M, Tinberg CE, Lippard SJ, McNamara JO. Vesicular zinc promotes presynaptic and inhibits postsynaptic long-term potentiation of mossy fiber-CA3 synapse. Neuron. 2011;71:1116–1126. doi: 10.1016/j.neuron.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Rosello T, Anderson CT, Schopfer FJ, Zhao Y, Gilad D, Salvatore SR, Freeman BA, Hershfinkel M, Aizenman E, Tzounopoulos T. Synaptic Zn2+ inhibits neurotransmitter release by promoting endocannabinoid synthesis. J Neurosci. 2013;33:9259–9272. doi: 10.1523/JNEUROSCI.0237-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radford RJ, Lippard SJ. Chelators for investigating zinc metalloneurochemistry. Curr Opin Chem Biol. 2013;17:129–136. doi: 10.1016/j.cbpa.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez DM, Kavalali ET. Differential regulation of spontaneous and evoked neurotransmitter release at central synapses. Curr Opin Neurobiol. 2011;21:275–282. doi: 10.1016/j.conb.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker JP, Leaver AM, Muhlau M. Tuning out the noise: limbic–auditory interactions in tinnitus. Neuron. 2010;66:819–826. doi: 10.1016/j.neuron.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode WS, Oertel D, Smith PH. Physiological response properties of cells labeled intracellularly with horseradish peroxidase in cat ventral cochlear nucleus. J Comp Neurol. 1983;213:448–463. doi: 10.1002/cne.902130408. [DOI] [PubMed] [Google Scholar]
- Roberts MT, Trussell LO. Molecular layer inhibitory interneurons provide feedforward and lateral inhibition in the dorsal cochlear nucleus. J Neurophysiol. 2010;104 (5):2462–2473. doi: 10.1152/jn.00312.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MT, Bender KJ, Trussell LO. Fidelity of complex spike-mediated synaptic transmission between inhibitory interneurons. J Neurosci. 2008;28:9440–9450. doi: 10.1523/JNEUROSCI.2226-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio ME, Juiz JM. Chemical anatomy of excitatory endings in the dorsal cochlear nucleus of the rat: differential synaptic distribution of aspartate aminotransferase, glutamate, and vesicular zinc. J Comp Neurol. 1998;399:341–358. doi: 10.1002/(sici)1096-9861(19980928)399:3<341::aid-cne4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Sindreu C, Bayes A, Altafaj X, Perez-Clausell J. Zinc transporter-1 concentrates at the postsynaptic density of hippocampal synapses. Mol Brain. 2014;7:16. doi: 10.1186/1756-6606-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su CK, Hsia SC, Sun YC. Three-dimensional printed sample load/inject valves enabling online monitoring of extracellular calcium and zinc ions in living rat brains. Anal Chim Acta. 2014;838:58–63. doi: 10.1016/j.aca.2014.06.037. [DOI] [PubMed] [Google Scholar]
- Suwa H, Saint-Amant L, Triller A, Drapeau P, Legendre P. High-affinity zinc potentiation of inhibitory postsynaptic glycinergic currents in the zebrafish hindbrain. J Neurophysiol. 2001;85:912–925. doi: 10.1152/jn.2001.85.2.912. [DOI] [PubMed] [Google Scholar]
- Tzounopoulos T, Kim Y, Oertel D, Trussell LO. Cell-specific, spike timing-dependent plasticities in the dorsal cochlear nucleus. Nat Neurosci. 2004;7:719–725. doi: 10.1038/nn1272. [DOI] [PubMed] [Google Scholar]
- Vergnano AM, Rebola N, Savtchenko LP, Pinheiro PS, Casado M, Kieffer BL, Rusakov DA, Mulle C, Paoletti P. Zinc dynamics and action at excitatory synapses. Neuron. 2014;82:1101–1114. doi: 10.1016/j.neuron.2014.04.034. [DOI] [PubMed] [Google Scholar]
- Vogt K, Mellor J, Tong G, Nicoll R. The actions of synaptically released zinc at hippocampal mossy fiber synapses. Neuron. 2000;26:187–196. doi: 10.1016/s0896-6273(00)81149-6. [DOI] [PubMed] [Google Scholar]
- Wang H, Brozoski TJ, Turner JG, Ling L, Parrish JL, Hughes LF, Caspary DM. Plasticity at glycinergic synapses in dorsal cochlear nucleus of rats with behavioral evidence of tinnitus. Neuroscience. 2009;164:747–759. doi: 10.1016/j.neuroscience.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie XM, Smart TG. A physiological role for endogenous zinc in rat hippocampal synaptic neurotransmission. Nature. 1991;349:521–524. doi: 10.1038/349521a0. [DOI] [PubMed] [Google Scholar]
- Zeng C, Yang Z, Shreve L, Bledsoe S, Shore S. Somatosensory projections to cochlear nucleus are upregulated after unilateral deafness. J Neurosci. 2012;32:15791–15801. doi: 10.1523/JNEUROSCI.2598-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N, Wang CH, Zhang S, Wu DC. The GLRA1 missense mutation W170S associates lack of Zn2+ potentiation with human hyperekplexia. J Neurosci. 2013;33:17675–17681. doi: 10.1523/JNEUROSCI.3240-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.