Abstract
Background
Perinatal use of combination antiretroviral therapy dramatically reduces vertical (mother-to-child) transmission of HIV, but has led to a growing population of children with perinatal HIV-exposure but uninfected (HEU). HIV can cause neurological injury among children born with infection, but the neuroanatomical and developmental effects in HEU children are poorly understood.
Methods
We used structural magnetic resonance imaging (MRI) with diffusion tensor imaging (DTI) to compare brain anatomy between 30 HEU and 33 age-matched HIV-unexposed and uninfected (HUU) children from Thailand. Maps of brain volume and microstructural anatomy were compared across groups; associations were tested between neuroimaging measures and concurrent neuropsychological test performance.
Results
Mean (SD) age of children was 10.3 (2.8) years and 58% were male. All were enrolled in school and lived with family members. Intelligence quotient (IQ) did not differ between groups. Caretaker education levels did not differ, but income was higher for HUU (p<0.001). We did not detect group differences in brain volume or DTI metrics, after controlling for sociodemographic factors. The mean (95% confidence interval) fractional anisotropy (FA) in the corpus callosum was 0.375 (0.368–0.381) in HEU compared to 0.370 (0.364–0.375) in HUU. Higher FA and lower mean diffusivity were each associated with higher IQ scores in analyses with both groups combined.
Conclusions
No differences in neuroanatomical or brain integrity measures were detectable in HEU children compared to age- and sex-matched controls (HUU children). Expected associations between brain integrity measures and IQ scores were identified suggesting sufficient power to detect subtle associations that were present.
Keywords: HIV, brain injuries, maternal exposure, children, diffusion tensor imaging
About 1.5 million HIV-infected women annually are in need of antiretroviral medication for the prevention of mother-to-child transmission (PMTCT) of HIV1. PMTCT programs have achieved enormous success in decreasing HIV infection among children across the world. In 2012 alone, about 900,000 women in low- or middle-income countries received necessary ART1. Overall, mother-to-child transmission decreased from 35% without treatment to <1% in the US and 3–4% in developing countries2–4. As a consequence, there is a growing international population of children who are HIV-exposed but uninfected (HEU). For these children, the developmental and neuroanatomical outcomes are poorly understood.
In adults, HIV enters the central nervous system (CNS) soon after infection5. In both children and adults, once in the CNS, HIV instigates an inflammatory cascade with neuronal dysfunction, and among children, leads to neurodevelopmental deficits6, 7. There are consistent neurologic, developmental, cognitive and language deficits in HIV-infected children, and these impairments increase with disease severity8–10. Studies examining neurodevelopment among HEU compared to HIV-unexposed and uninfected (HUU, “control”) children have inconsistent findings that can typically be explained by variations in study design or incomplete consideration of maternal, caregiver, or other environmental factors critical to development9, 11. One study noted a greater frequency of mental, motor and language expression developmental delays in HEU (and other children in affected households) than HUU12. Another failed to identify neurodevelopment differences in the first two years of life13, while yet others find subtle differences14, 15 for which the clinical meaning is unclear. A further evaluation into the neuroanatomical mechanisms potentially affected is therefore needed.
Structural deficits, including cortical and corpus callosum thinning, are frequent in HIV-infected adults as is widespread tissue loss affecting gray and white matter (WM) volumes16, 17. Published studies in children suggest a greater frequency of physical brain damage for vertically infected children than that typically reported among HIV-infected adults18. In the absence of treatment, cortical atrophy with ventriculomegaly (dilation of lateral ventricles), basal ganglia calcification and WM lesions have been observed19, 20.
Diffusion tensor imaging (DTI) offers additional insight into WM microstructure and organization by tracking the diffusion of water molecules through WM tracts of the brain21. Two of the most common measures derived from the tensor representation of DTI scans include fractional anisotropy (FA) and mean diffusivity (MD). Anisotropy and diffusivity parameters from DTI are altered by differences in myelination, WM organization and inflammation22. FA is a normalized measure (ranging from 0 to 1) describing the anisotropic (or non-uniform) degree of water diffusion; higher FA may reflect greater myelination, for example. MD is the average magnitude of diffusive motion in all directions - a higher MD reflects less constrained motion, possibly brought on by damage to the WM. Axial diffusivity (AD) and radial diffusivity (RD) are evaluated to provide greater detail on by measuring water diffusivity parallel or perpendicular to the principal direction of the local dominant fiber orientation, respectively. Abnormal diffusion along the axon (AD) may represent axonal injury, but increased RD may represent loss of myelination23, 24. To this extent, these DTI measures are employed in clinical populations as surrogate markers of pathology and change over time. They provide invaluable insights into the architecture of normal WM development in healthy populations25, 26. In one of the few studies of HIV-infected children, DTI differences were seen in the corpus callosum with lower FA and higher MD among 12 HIV-infected children not on cART compared to controls27. Similar deficits are reported in adults28–30.
To date, no studies have examined the gross and microstructural brain differences in HEU compared to HUU children.
Here we sought to examine whether abnormalities are detectable through maps of local volumetric differences and WM microstructure in HEU children compared to age- and sex-matched HUU children HUU. Anatomical differences, if found, could be a foundation for future neurodevelopmental deficits whereas an absence of measurable differences could provide some guidance to practicing clinicians. To ensure our power to detect associations between DTI measures and cognition, we further sought to demonstrate correlations between WM microstructure and neuropsychological performance among these children. We hypothesized that higher neuronal integrity should reflect more mature neuronal development and be positively associated with better neuropsychological testing performance31, 32.
MATERIALS AND METHODS
Participant selection
Enrollment in this substudy was designed to provide two control groups (HUU and HEU) for the Pediatric Randomized Early vs. Deferred Initiation in Cambodia and Thailand (PREDICT Cohort study33, an ongoing investigation of HIV-infected children. Children from the HEU and HUU arms were recruited from: (1) siblings of the HIV-infected children enrolled in the PREDICT study; (2) children born to HIV-infected mothers at the PREDICT study sites; and (3) well-child clinics in hospitals associated with the respective study sites. Thirty-one HEU and thirty-three HUU children were enrolled and matched by age and sex to the HIV-infected children in the PREDICT study. All children satisfied the following inclusion criteria: (1) age <18 years; (2) able to tolerate MRI; and (3) written informed consent signed by a caregiver and assent by participant, if age > 8 years. Children were excluded from enrollment for: (1) previous or current brain infection; (2) known neurological or psychiatric disorder; (3) any congenital abnormality (one child inadvertently enrolled with a congenital limb deficit was not included in these analyses); or (4) head injury with a loss of consciousness. Images were collected at Chulalongkorn University Hospital in Bangkok and Chiang Mai University Hospital in Chiang Mai, Thailand. The Institutional Review Boards (IRBs) of each study site, the Thai Ministry of Public Health, the University of California San Francisco, and the University of Southern California, in Los Angeles each approved the study.
Clinical assessment
Caregivers provided medical history and a pediatrician completed a physical examination, collecting information on neurodevelopmental milestones. ART exposure of the HEU children, however, was not recalled by the majority of caregivers and therefore unavailable. Fourteen of 30 HEU children were born before the year 2000, when PMTCT was implemented in Thailand, and thus, were less likely to be exposed to ART. School attendance was unavailable for two children (1 HEU and 1 HUU), caregivers’ education level was unavailable for three children (2 HEU and 1 HUU), and caregiver income was unavailable for two children (1 HEU and 1 HUU). All children completed age-appropriate neuropsychological assessments as previously described14, 15. The battery included: the Wechsler Preschool and Primary Scale of Intelligence-III (WPPSI-III, ages 2 to 6 years) and the Wechsler Intelligence Scale for Children (WISC-III, ages 6 to 17 years).34–36. To ensure the same cognitive testing, four children under age 6 at enrollment were not included in the analyses comparing brain measures to neuropsychological test performance.
Brain image acquisition
Whole brain structural T1-weighted MRI and diffusion-weighted imaging (DWI) were performed on GE 1.5 tesla scanners at both study sites. The structural imaging used the following protocol: axial plane, 3D SPGR images with a minimum TE at full echo, TR = 11.2 ms, slice thickness =1.0mm; 256×256 imaging matrix. DWI was collected in duplicate using a single-shot echoplanar imaging protocol; 25 diffusion-sensitized directions were collected with a b-value of 1000 s/mm2.
Image preprocessing
Extra-cerebral tissue was removed from anatomical scans using either the brain extraction tool (BET;37) from the Functional MRI of the Brain (FMRIB) Software Library (FSL; www.fmrib.ox.ac.uk/fsl) for DWI or FreeSurfer38 for T1-weighted images. T1-weighted scans were corrected for intensity inhomogeneities using the MNI nu_correct tool (www.bic.mni.mcgill.ca/software/). Each image was aligned to a common space (the Colin2739 template with 1mm isotropic voxels) using FSL’s flirt40. DWI were corrected for head motion and eddy current distortions using the FSL eddy_correct tool. To correct for echo-planar imaging induced susceptibility artifacts, b0 images were linearly aligned and then elastically registered to their respective down-sampled T1-weighted structural scans as previously described41, 42. Gradient vectors were corrected to account for the linear registration. All images were visually inspected for quality; both DWI sets were concatenated to boost the signal-to-noise when estimating diffusion parameters. A diffusion tensor was modeled at each voxel to calculate scalar anisotropy and diffusivity maps using FSL43.
Brain-wide image analyses
Detailed procedures can be found elsewhere28. Briefly, using 36 maps from a mix of HUU and HEU children, a study-specific minimal deformation template (MDT) was created for T1-weighted images and FA images, as previously described44. Each child’s pre-processed T1-weighted scan was then linearly and elastically registered to the T1-weighted MDT and a 3D Jacobian expansion factor map was created, the tensor based morphometry map (TBM) used to characterize local volumetric differences. A similar process was conducted for FA-based registration. To further ensure alignment of WM across subjects, we applied a second round of registrations, where the regions of low FA (FA<0.2) were removed from the subjects’ maps and the MDT before registration. The resulting FA-based deformation fields were applied to all DTI maps including FA, MD, AD and RD (as described above) to ensure all maps are in the same space.
Statistical Analysis
Voxelwise regression models were used to test for effects of HIV exposure as well as neuropsychological score associations with structural variations in TBM maps and DTI measures (FA, MD, RD, and AD). We adjusted for imaging site and both the linear and non-linear effects of age and sex. We controlled for education level of the primary caretaker (greater than elementary school level or not) and caregiver income (below, at, or above average). As there are three groupings for income level, our regression models used a random effects model grouped by income status. We accounted for total brain volume in morphometry analyses.
Statistical testing of DTI was limited to regions where FA was > 0.2 to exclude gray matter and CSF45, 46. Due to a higher level of motion artifacts, the cerebellum and brain stem were not evaluated. We used the false discovery rate (FDR) method to control the false positive rate of each map at q=0.0547. Prior studies identified significant associations between HIV serostatus and corpus callosum (CC) FA27. To provide effect-sizes to interpret our negative findings, we hypothesized similar relationships in the present sample. We masked the midsagittal CC on the MDT to calculate the average FA for each subject and completed a focused comparison of this value across groups using linear regression. We calculated the standardized mean difference effect-size, or Cohen’s d, by calculating the difference in means divided by the pooled standard error.
Neuropsychological test scores were evaluated in a hierarchical manner with both groups combined in a regression model. Full Scale IQ (FSIQ) scores were examined first, then Performance IQ (PIQ) and Verbal IQ (VIQ) followed by subtests within each domain; each score was tested separately.
RESULTS
Imaging cohort characteristics
By design, the two groups were similar on most demographic characteristics (Table 1). Age ranged from 5 to 15 years with a median of 10.5; about half (58%) were male. All attended school and had parents or relatives as caretakers. Children in the HEU group were more likely to live in an environment with lower caregiver income. No differences were observed in neuropsychological test scores between HEU and HUU children.
Table 1.
Socio-demographic factors for all HUU and HEU children. Mean and standard deviation of data is presented; percentage is presented where appropriate.
| Variables | HIV-unexposed uninfected (HUU -33) | HIV-exposed uninfected (HEU -30) | p-value |
|---|---|---|---|
| 33 | 30 | ||
| Age mean (standard deviation) in years | 10.1 +/− 2.6 | 10.5+/− 3.0 | 0.50 |
| Gender (% male) | 66% | 47% | 0.11 |
| Imaging Site n | 0.99 | ||
| Chiang Mai University Hospital: Chiang Mai | 17 | 15 | |
| Chulalongkorn University Hospital: Bangkok | 16 | 15 | |
| School attendance (% attended)* | 97% | 97% | 1.0 |
| Caretaker income* | <0.001 | ||
| Less than average | 5 | 15 | |
| Average | 20 | 9 | |
| Greater than average | 7 | 5 | |
| Caretaker education* | 0.13 | ||
| None or elementary | 12 | 16 | |
| Higher | 20 | 12 | |
| WISC-III Full Scale IQ mean (SD) | 84.3 +/− 12.6 | 82.9 +/− 15.4 | 0.71 |
| WISC-III Verbal IQ (VIQ) | 81.5 +/− 12.0 | 79.4 +/− 14.1 | 0.58 |
| WISC-III Performance IQ (PIQ) | 90.4 +/− 15.8 | 89.7 +/− 17.2 | 0.74 |
| Verbal comprehension index (VCI) | 78.6 +/− 12.2 | 79.1 +/−13.7 | 0.89 |
| Processing speed index (PSI) | 104.6 +/− 16.2 | 100.0 +/− 20.9 | 0.37 |
| Freedom from distractibility (FDI) | 99.9 +/− 10.0 | 95.5 +/− 13.6 | 0.18 |
| Perceptual organization index (POI) | 88.5 +/− 15.0 | 88.8 +/− 16.5 | 0.94 |
Denotes missing data (see Methods section for details). Statistical analyses performed on non-missing data.
Brain structure of HEU compared to HUU children
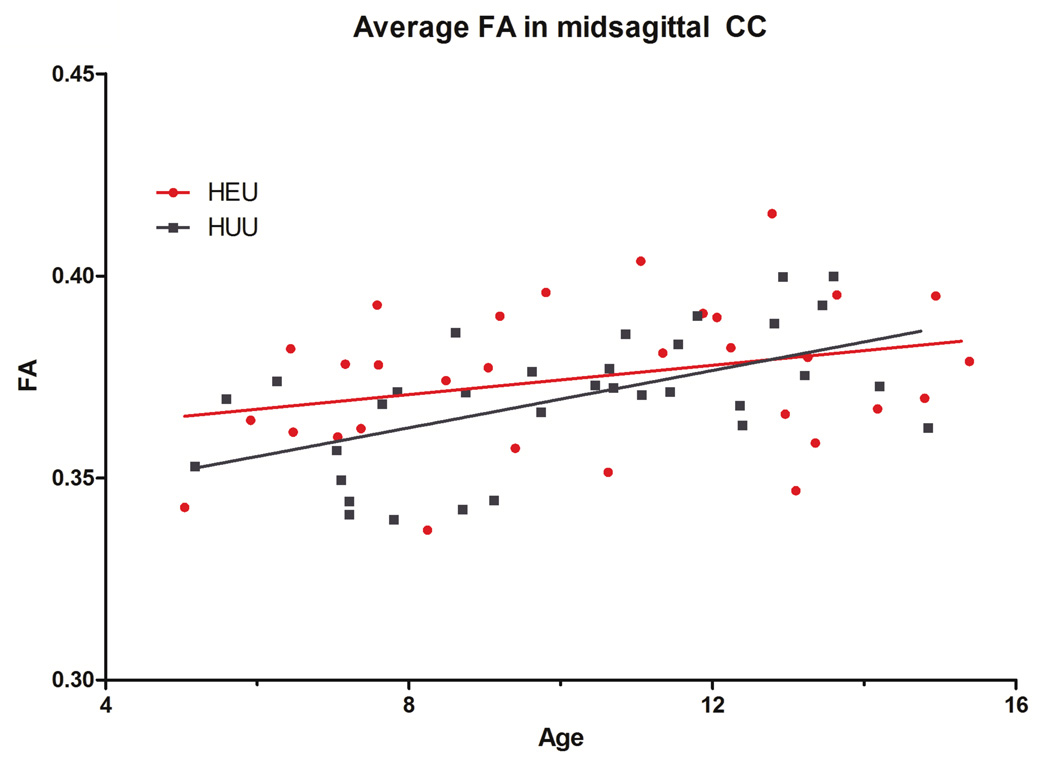
We did not identify structural brain differences between HEU and HUU children. No volumetric differences met our criteria for significance across the entire brain. Similarly, we did not identify differences in WM integrity measures observed with DTI, including FA, MD, RD and AD. We first examined the full group of children (33 HUU vs. 30 HEU), then, those ages 6 years and up (31 HUU vs. 28 HEU), and finally those with all socio-demographic data available (caretaker education and income, 30 HUU and 24 HEU). In all situations, brain structure was not significantly different between groups. We did not identify differences in the average FA in the CC in HEU as compared to HUU (Figure 1). The mean (95% confidence interval) FA in the CC was 0.375 (0.368–0.381) and 0.370 (0.364–0.375) for HEU and HUU children, respectively (p=0.23); the corresponding effect size for the between group mean difference in CC FA was d=0.33 (standard deviations).
Figure 1.
No significant voxelwise differences were found in FA between HEU and HUU children across the full white matter or averaged in the midsagittal corpus callosum (p = 0.23). Both groups show similar increases in the mean FA of the corpus callosum with age, as expected.
Association between DTI and neuropsychological test performance
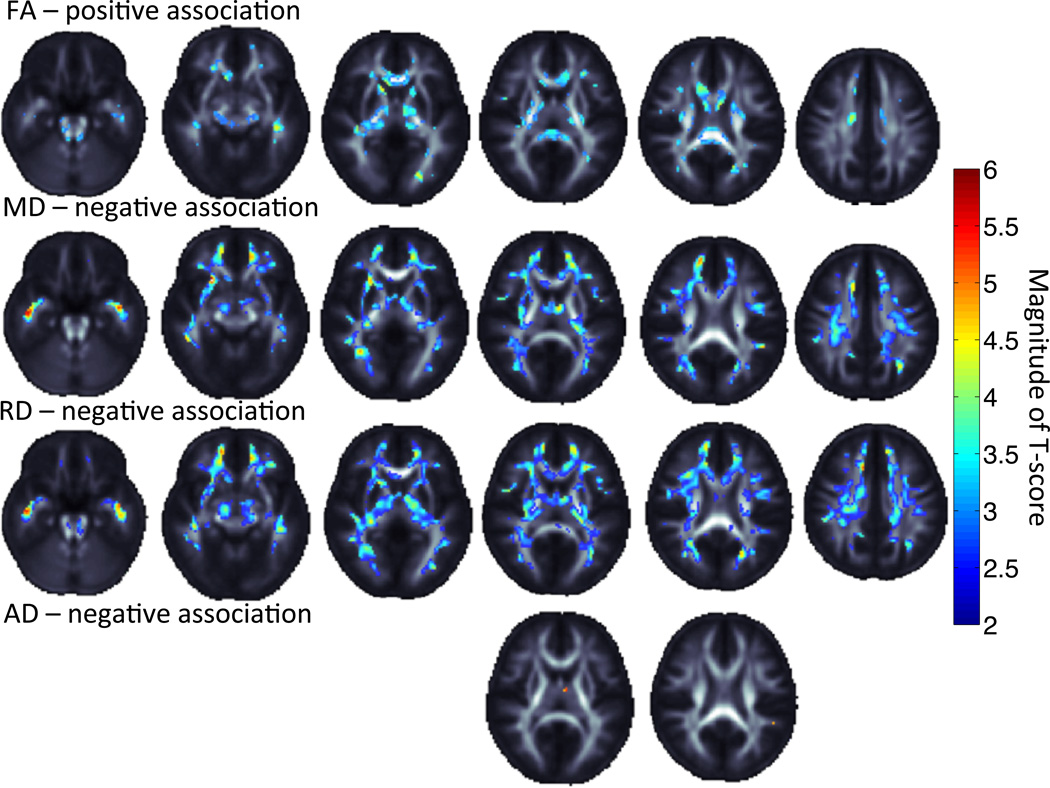
No associations were found between neuropsychological test performance and brain volume by TBM. However, DTI measures were significantly associated with FSIQ and PIQ but not VIQ (Table 2). Positive associations were found for FA with PIQ, while negative associations were found between both MD and RD with Full and Performance IQ (Figure 2; SI Figure 1). No associations were significant with VIQ. All FA maps showed direct associations with IQ while all diffusivity maps showed an indirect association. While RD showed in general strong associations, minimal associations were seen with AD (Figure 2; SI Figure 1).
Table 2.
Summary of associations between DTI measures and IQ measures and subscores, and the percent of the brain that was significantly associated to IQ. 0.0 denotes no significant association when correcting for voxelwise comparisons at a FDR q-value of 0.05. In parentheses, we note the critical p-value which controls the false discovery rate at 0.05. Please note that the close this value is to 0.05, the greater the statistical effect of the variable being tested. All IQ measures were positively associated with FA and negatively associated with diffusion measures MD, RD and AD.
| FA (%) (pFDR) |
MD(%) (pFDR) |
RD(%) (pFDR) |
AD(%) (pFDR) |
|
|---|---|---|---|---|
| Full Scale IQ | 0.0 (N/A) |
0.7 (3.3×10−4) |
9.8 (4.9×10−3) |
0.0 (N/A) |
| Performance IQ | 12.2 (6.2×10−3) |
31.5 (1.6×10−2) |
43.6 (2.2×10−2) |
0.05 (2.1×10−5) |
| Verbal IQ | 0.0 (N/A) |
0.0 (N/A) |
0.0 (N/A) |
0.0 (N/A) |
| Processing Speed IQ | 0.0 (N/A) |
0.0 (N/A) |
0.0 (N/A) |
0.0 (N/A) |
| Perceptual Organization Index | 0.0 (N/A) |
29.6 (1.4×10−2) |
36.8 (1.8×10−2) |
0.05 (2.7×10−5) |
| Freedom From Distractibility | 14.6 (7.1×10−3) |
0.0 (N/A) |
0.5 (2.0×10−4) |
0.0 (N/A) |
Figure 2.
Performance IQ is associated positively with FA and negatively with MD, RD and AD. Warmer colors represent a greater effect of association. Images are shown in radiological convention with left hemisphere displayed on the right and right on the left.
As an exploratory investigation of specific intelligence measures, we assessed the effects of Perceptual Organization Index, Processing Speed Index and Freedom From Distractibility Index on brain microstructure (Table 2). We did not assess the Verbal Comprehension index or TBM measures as they were not significant in primary models. The largest effects were seen with performance and perceptual organization scores, and not with verbal intelligence or processing speed. The strongest effects for these were found in negative associations with RD and MD in the internal capsule, cingulum and optic/temporal regions including the uncinate and thalamic radiations. Positive FA associations were found in the corpus callosum, in particular the splenium.
DISCUSSION
Here we compared brain structure and WM microstructure between groups of HEU and carefully age- and sex- matched HUU children. Brain volume and WM microstructural integrity were similar among children of similar ages, independent of HIV exposure. This study complements prior reports of no detectable differences in neurodevelopment between HEU and HUU children13. Our previous findings in the full sample (not only imaged children) demonstrated small, although statistically significant group differences in neuropsychological test performance14, 15; our current study mapping the neuropsychiatric scores across the brain enables us to observe the neuroanatomical pathways where these group differences may reside. However, the clinical significance of our previous findings remains unclear.
Despite the absence of neuroanatomical differences by group, subsequent analysis found statistical significance in analyses of microstructural variation associated with age and neuropsychiatric test scores of intellectual ability, as would be expected among developing children48. These findings add confidence that our methodologies had sufficient power to detect associations between brain measures and cognition. DTI studies of similar age groups and with similar or smaller sample sizes have successfully identified microstructural abnormalities in children with neurogenetic disorders49 or behavior problems50. The differences observed in these studies highlight the power of DTI to identify neuroanatomical changes associated with childhood conditions and adds confidence that we had the power to identify differences in the current study.
The strength of evidence for a negative finding - in this case that there is no strong brain difference between groups - is restricted by the available sample size. The CC measure demonstrates tight overlap of confidence intervals between groups, and a small effect size, so groups differences in mean FA levels, are small if present at all. If differences exist, they may be smaller than necessary to be clinically meaningful. Consequently, these findings are reassuring for clinicians caring for HEU youth; but continued monitoring for long-term neurological effects remains essential, particularly as these children enter later adolescence.
Non-verbal IQ measures are associated with DTI measures of WM microstructure in cognitively normal children and in young adults48, 51. In all of these studies, a higher intelligence score was generally associated with higher FA and lower MD, reflecting “more intact” WM fibers. Our study similarly found markers of greater microstructural integrity associated with higher neuropsychological performance in tracts previously identified in DTI studies of IQ48, 51 including the internal capsule and optic/thalamic radiations. We also explored specific subtests that may be reflected in the structure of the developing brain. We found greater IQ associations with radial than axial diffusivity, suggesting a greater influence of myelination than axonal development on IQ. Our findings suggest that DTI measures could be reliably used to monitor neurodevelopment in HEU children.
This study focused on children aged 5–15 years in Thailand with caregiver consent to participate and therefore our results may not be generalizable to all HEU children globally where nutritional and environmental issues may differ. Also, we did not have information on the maternal history, perinatal experience, or antiretroviral exposure in the perinatal period. Although our conclusions remain the same, these measures may have been helpful in studying possible brain correlates of perinatal ART exposure, HIV parameters during pregnancy, pregnancy outcomes, prenatal and postnatal care, maternal mental health history and drug and alcohol use, among others. Fourteen of 30 HEU children born before the year 2000, when PMTCT was implemented in Thailand, thus, were less likely to be exposed to ART. Among the remaining children, exposure may have been varied: 11 were born between 2000 and 2003 when only zidovudine was part of the program, and 5 were born after 2004 when administration of zidovudine with single dose nevirapine became standard. Complementing our work, a recent study where ART exposure was known did not identify adverse neurodevelopmental outcomes among children exposed to ART in utero52. Other studies evaluating developmental differences in HEU children have shown minimal effects of ART. Mental and motor functioning was not lower in a large cohort of ART exposed children compared to unexposed children in the United States54. While substance abuse (and not ART) affected cognitive outcome in one study55, no evidence was found for association of prenatal drug use with neurological deficits in another56. In contrast, neuroimaging studies of the effects of perinatal exposure to heavy alcohol and drug use on the brain have identified abnormalities in the structure of the developing brain57–59.
We imaged 63 children living in resource-limited regions of Thailand with MRI and DTI to examine potential differences in the neuroanatomical organization and microstructural integrity associated with prenatal exposure to HIV. Our study found no significant differences between brain volumes or microstructure associated with HIV exposure supportive that if differences exist, they are likely of limited magnitude and unlikely to manifest as clinically meaningful developmental differences. We also confirm a positive association between microstructural integrity and neuropsychological testing performance among children in Thailand. Our study investigated children only into early adolescence; continued longitudinal monitoring of HEU children will be necessary to determine if these findings hold through later stages of neurodevelopment.
Supplementary Material
Acknowledgments
Funding Source: This work was supported by grant R01 MH089722; NJ, TMN and PT were additionally supported by grants R01 HD050735, R01 AG040060, R01 MH097268, EB008432, EB008281, and EB007813 (to PT).
Footnotes
Financial Disclosure Statement: Authors have no financial disclosures.
Conflict of Interest: Authors have no conflicts of interest.
REFERENCES
- 1.WHO. Global Update on HIV Treatment 2013: Results, Impact and Opportunities. Published 2013.
- 2.UNAIDS. UNAIDS World AIDS Day Report 2012. Geneva, Switzerland; 2012. [Google Scholar]
- 3.MRC SAMRC. SA PMTCT Evaluation shows that virtual elimination of paediatric HIV is possible with intensified effort. 2011 [Google Scholar]
- 4.CDC. Mother-to-Child (Perinatal) HIV Transmission and Prevention. 2007 [Google Scholar]
- 5.Valcour V, Chalermchai T, Sailasuta N, Marovich M, Lerdlum S, Suttichom D, et al. Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis. 2012;206(2):275–282. doi: 10.1093/infdis/jis326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5(1):69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 7.Epstein LG, Gelbard HA. HIV-1-induced neuronal injury in the developing brain. J Leukoc Biol. 1999;65(4):453–457. doi: 10.1002/jlb.65.4.453. [DOI] [PubMed] [Google Scholar]
- 8.Chase C, Ware J, Hittelman J, Blasini I, Smith R, Llorente A, et al. Early cognitive and motor development among infants born to women infected with human immunodeficiency virus. Women and Infants Transmission Study Group. Pediatrics. 2000;106(2):E25. doi: 10.1542/peds.106.2.e25. [DOI] [PubMed] [Google Scholar]
- 9.Le Doare K, Bland R, Newell ML. Neurodevelopment in children born to HIV-infected mothers by infection and treatment status. Pediatrics. 2012;130(5):e1326–e1344. doi: 10.1542/peds.2012-0405. [DOI] [PubMed] [Google Scholar]
- 10.Smith R, Malee K, Leighty R, Brouwers P, Mellins C, Hittelman J, et al. Effects of perinatal HIV infection and associated risk factors on cognitive development among young children. Pediatrics. 2006;117(3):851–862. doi: 10.1542/peds.2005-0804. [DOI] [PubMed] [Google Scholar]
- 11.Walker SP, Wachs TD, Gardner JM, Lozoff B, Wasserman GA, Pollitt E, et al. Child development: risk factors for adverse outcomes in developing countries. Lancet. 2007;369(9556):145–157. doi: 10.1016/S0140-6736(07)60076-2. [DOI] [PubMed] [Google Scholar]
- 12.Van Rie A, Mupuala A, Dow A. Impact of the HIV/AIDS epidemic on the neurodevelopment of preschool-aged children in Kinshasa, Democratic Republic of the Congo. Pediatrics. 2008;122(1):e123–e128. doi: 10.1542/peds.2007-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomez C, Archila ME, Rugeles C, Carrizosa J, Rugeles MT, Cornejo JW. A prospective study of neurodevelopment of uninfected children born to human immunodeficiency virus type 1 positive mothers. Rev Neurol. 2009;48(6):287–291. [PubMed] [Google Scholar]
- 14.Puthanakit T, Ananworanich J, Vonthanak S, Kosalaraksa P, Hansudewechakul R, van der Lugt J, et al. Cognitive function and neurodevelopmental outcomes in HIV-infected Children older than 1 year of age randomized to early versus deferred antiretroviral therapy: the PREDICT neurodevelopmental study. Pediatr Infect Dis J. 2013;32(5):501–508. doi: 10.1097/INF.0b013e31827fb19d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kerr SJ, Puthanakit T, Vibol U, Aurpibul L, Vonthanak S, Kosalaraksa P, et al. Neurodevelopmental outcomes in HIV-exposed-uninfected children versus those not exposed to HIV. AIDS Care. 2014 doi: 10.1080/09540121.2014.920949. under revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson PM, Dutton RA, Hayashi KM, Toga AW, Lopez OL, Aizenstein HJ, et al. Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4+ T lymphocyte decline. Proc Natl Acad Sci U S A. 2005;102(43):15647–15652. doi: 10.1073/pnas.0502548102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiang MC, Dutton RA, Hayashi KM, Lopez OL, Aizenstein HJ, Toga AW, et al. 3D pattern of brain atrophy in HIV/AIDS visualized using tensor-based morphometry. Neuroimage. 2007;34(1):44–60. doi: 10.1016/j.neuroimage.2006.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tardieu M, Le Chenadec J, Persoz A, Meyer L, Blanche S, Mayaux MJ. HIV-1-related encephalopathy in infants compared with children and adults. French Pediatric HIV Infection Study and the SEROCO Group. Neurology. 2000;54(5):1089–1095. doi: 10.1212/wnl.54.5.1089. [DOI] [PubMed] [Google Scholar]
- 19.Safriel YI, Haller JO, Lefton DR, Obedian R. Imaging of the brain in the HIV-positive child. Pediatr Radiol. 2000;30(11):725–732. doi: 10.1007/s002470000338. [DOI] [PubMed] [Google Scholar]
- 20.George R, Andronikou S, du Plessis J, du Plessis AM, Van Toorn R, Maydell A. Central nervous system manifestations of HIV infection in children. Pediatr Radiol. 2009;39(6):575–585. doi: 10.1007/s00247-009-1170-4. [DOI] [PubMed] [Google Scholar]
- 21.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111(3):209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- 22.Thomason M, Thompson PM. Diffusion Imaging, White Matter and Psychopathology. Annual Review of Clinical Psychology. 2010 doi: 10.1146/annurev-clinpsy-032210-104507. [DOI] [PubMed] [Google Scholar]
- 23.Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, et al. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging. 2001;13(4):534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- 24.Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17(3):1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 25.Hermoye L, Saint-Martin C, Cosnard G, Lee SK, Kim J, Nassogne MC, et al. Pediatric diffusion tensor imaging: normal database and observation of the white matter maturation in early childhood. Neuroimage. 2006;29(2):493–504. doi: 10.1016/j.neuroimage.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 26.Dennis EL, Thompson PM. Typical and atypical brain development: a review of neuroimaging studies. Dialogues in clinical neuroscience. 2013;15(3):359–384. doi: 10.31887/DCNS.2013.15.3/edennis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoare J, Fouche JP, Spottiswoode B, Donald K, Philipps N, Bezuidenhout H, et al. A diffusion tensor imaging and neurocognitive study of HIV-positive children who are HAART-naive "slow progressors". J Neurovirol. 2012;18(3):205–212. doi: 10.1007/s13365-012-0099-9. [DOI] [PubMed] [Google Scholar]
- 28.Nir TM, Jahanshad N, Busovaca E, Wendelken L, Nicolas K, Thompson PM, et al. Mapping white matter integrity in elderly people with HIV. Human Brain Mapping. 2013 doi: 10.1002/hbm.22228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gongvatana A, Cohen RA, Correia S, Devlin KN, Miles J, Kang H, et al. Clinical contributors to cerebral white matter integrity in HIV-infected individuals. J Neurovirol. 2011;17(5):477–486. doi: 10.1007/s13365-011-0055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfefferbaum A, Rosenbloom MJ, Rohlfing T, Kemper CA, Deresinski S, Sullivan EV. Frontostriatal fiber bundle compromise in HIV infection without dementia. AIDS. 2009;23(15):1977–1985. doi: 10.1097/QAD.0b013e32832e77fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaddock-Heyman L, Erickson KI, Voss MW, Powers JP, Knecht AM, Pontifex MB, et al. White matter microstructure is associated with cognitive control in children. Biol Psychol. 2013;94(1):109–115. doi: 10.1016/j.biopsycho.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tamnes CK, Ostby Y, Walhovd KB, Westlye LT, Due-Tonnessen P, Fjell AM. Intellectual abilities and white matter microstructure in development: a diffusion tensor imaging study. Hum Brain Mapp. 2010;31(10):1609–1625. doi: 10.1002/hbm.20962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puthanakit T, Saphonn V, Ananworanich J, Kosalaraksa P, Hansudewechakul R, Vibol U, et al. Early versus deferred antiretroviral therapy for children older than 1 year infected with HIV (PREDICT): a multicentre, randomised, open-label trial. The Lancet infectious diseases. 2012;12(12):933–941. doi: 10.1016/S1473-3099(12)70242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wechsler D. Wechsler Preschool and Primary Scale of Intelligence-Revised (WPPSI-R) San Antonio, TX: TP Corporation; 1989. [Google Scholar]
- 35.Wechsler D. Wechsler Intelligence Scale for Children. Third Edition. San Antonio, TX: TP Corporation; 1991. [Google Scholar]
- 36.Puthanakit. International AIDS Conference. Bangkok, Thailand: 2004. Psychosocial and behavioral functioning of Thai school-aged children with perinatally acquired HIV infection. [Google Scholar]
- 37.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- 39.Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr. 1998;22:324–333. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- 40.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 41.Leow AD, Yanovsky I, Chiang MC, Lee AD, Klunder AD, Lu A, et al. Statistical properties of Jacobian maps and the realization of unbiased large-deformation nonlinear image registration. IEEE Trans Med Imaging. 2007;26(6):822–832. doi: 10.1109/TMI.2007.892646. [DOI] [PubMed] [Google Scholar]
- 42.Jahanshad N, Lee AD, Barysheva M, McMahon KL, de Zubicaray GI, Martin NG, et al. Genetic influences on brain asymmetry: a DTI study of 374 twins and siblings. Neuroimage. 2010;52(2):455–469. doi: 10.1016/j.neuroimage.2010.04.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66(1):259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gutman B, Svarer C, Leow AD, Yanovsky I, Toga AW, Thompson PM. Creating unbiased minimal deformation templates for brain volume registration. OHBM. 2010 [Google Scholar]
- 45.Wakana S, Nagae-Poetscher LM, Jiang H, van Zijl P, Golay X, Mori S. Macroscopic orientation component analysis of brain white matter and thalamus based on diffusion tensor imaging. Magn Reson Med. 2005;53(3):649–657. doi: 10.1002/mrm.20386. [DOI] [PubMed] [Google Scholar]
- 46.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 47.Benjamini Y, Hochberg Y. Controlling the false discovery rate - a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B-Methodological. 1995;57:289–300. [Google Scholar]
- 48.Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Cognitive functions correlate with white matter architecture in a normal pediatric population: a diffusion tensor MRI study. Hum Brain Mapp. 2005;26(2):139–147. doi: 10.1002/hbm.20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Villalon-Reina J, Jahanshad N, Beaton E, Toga AW, Thompson PM, Simon TJ. White matter microstructural abnormalities in girls with chromosome 22q11.2 deletion syndrome, Fragile X or Turner syndrome as evidenced by diffusion tensor imaging. Neuroimage. 2013;81:441–454. doi: 10.1016/j.neuroimage.2013.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y, Horst KK, Kronenberger WG, Hummer TA, Mosier KM, Kalnin AJ, et al. White matter abnormalities associated with disruptive behavior disorder in adolescents with and without attention-deficit/hyperactivity disorder. Psychiatry Res. 2012;202(3):245–251. doi: 10.1016/j.pscychresns.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 51.Chiang MC, Barysheva M, Shattuck DW, Lee AD, Madsen SK, Avedissian C, et al. Genetics of brain fiber architecture and intellectual performance. J Neurosci. 2009;29(7):2212–2224. doi: 10.1523/JNEUROSCI.4184-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sirois PA, Huo Y, Williams PL, Malee K, Garvie PA, Kammerer B, et al. Safety of Perinatal Exposure to Antiretroviral Medications: Developmental Outcomes in Infants. Pediatr Infect Dis J. 2013 doi: 10.1097/INF.0b013e318284129a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watts DH, Huang S, Culnane M, Kaiser KA, Scheuerle A, Mofenson L, et al. Birth defects among a cohort of infants born to HIV-infected women on antiretroviral medication. J Perinat Med. 2011;39(2):163–170. doi: 10.1515/JPM.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Williams PL, Marino M, Malee K, Brogly S, Hughes MD, Mofenson LM, et al. Neurodevelopment and in utero antiretroviral exposure of HIV-exposed uninfected infants. Pediatrics. 2010;125(2):e250–e260. doi: 10.1542/peds.2009-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alimenti A, Forbes JC, Oberlander TF, Money DM, Grunau RE, Papsdorf MP, et al. A prospective controlled study of neurodevelopment in HIV-uninfected children exposed to combination antiretroviral drugs in pregnancy. Pediatrics. 2006;118(4):e1139–e1145. doi: 10.1542/peds.2006-0525. [DOI] [PubMed] [Google Scholar]
- 56.Knight WG, Mellins CA, Levenson RL, Jr, Arpadi SM, Kairam R. Brief report: effects of pediatric HIV infection on mental and psychomotor development. J Pediatr Psychol. 2000;25(8):583–587. doi: 10.1093/jpepsy/25.8.583. [DOI] [PubMed] [Google Scholar]
- 57.Roussotte F, Soderberg L, Warner T, Narr K, Lebel C, Behnke M, et al. Adolescents with prenatal cocaine exposure show subtle alterations in striatal surface morphology and frontal cortical volumes. J Neurodev Disord. 2012;4(1):22. doi: 10.1186/1866-1955-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lebel C, Roussotte F, Sowell ER. Imaging the impact of prenatal alcohol exposure on the structure of the developing human brain. Neuropsychol Rev. 2011;21(2):102–118. doi: 10.1007/s11065-011-9163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Colby JB, Smith L, O'Connor MJ, Bookheimer SY, Van Horn JD, Sowell ER. White matter microstructural alterations in children with prenatal methamphetamine/polydrug exposure. Psychiatry Res. 2012;204(2–3):140–148. doi: 10.1016/j.pscychresns.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.