Abstract
Objective(s)
This pilot study was conducted to assess the feasibility and acceptability of four adaptive treatment strategies (ATSs) for adolescent depression to plan for a subsequent full-scale clinical trial. The ATSs aim to address two questions that arise when personalizing treatment: (1) for adolescents treated with Interpersonal Psychotherapy for depressed adolescents (IPT-A) (Mufson et al, 2004), at what time point should therapists make the determination that the adolescent is not likely to respond if the initial treatment plan is continued (week 4 or week 8), and (2) for adolescents who are judged to need their treatment augmented, should the therapist increase the number of IPT-A sessions or add pharmacotherapy (fluoxetine).
Method
A 16 week pilot sequential multiple assignment randomized trial (SMART) was conducted with 32 adolescents (mean age = 14.9) who had a diagnosis of Major Depressive Disorder, Dysthymic Disorder, or Depressive Disorder NOS. Adolescents were primarily female (75%) and Caucasian (84.4%). Data regarding the feasibility and acceptability of the study and treatment procedures and treatment response rates was collected.
Results
Week 4 was the more feasible and acceptable decision point for assessing need for a change to treatment. Adolescents, parents, and therapists reported a range of attitudes about medication and more intensive therapy as treatment options.
Conclusions
The ATSs including the week 4 decision point showed promise in terms of their feasibility and acceptability. Results from the pilot study have yielded additional research questions for the full-scale SMART and will improve our ability to successfully conduct the trial.
Keywords: adolescent, depressive disorder, treatment
Adolescent depression is a prevalent disorder that places youth at risk for suicidality, other psychiatric diagnoses, and significant psychosocial impairment both during adolescence and into adulthood (Lewinsohn et al., 1994; Weissman et al., 1999). There are now a number of evidence-based treatments; however, even when depressed adolescents receive these treatments, 30–50% of adolescents do not respond (e.g. (TADS Team, 2004). In practice, this means that clinicians must sequence or combine different treatment approaches until remission is reached, with virtually no empirically-derived guidelines to direct clinicians in this process. As a consequence, clinicians often sequence or augment treatments in a trial and error fashion which could result in extended time to remission, unnecessary experience of adverse side effects, or increased cost or other burdens for adolescents and their families.
Adaptive treatment strategies (ATSs) provide empirical guidelines for sequential clinical decision making by providing decision rules that recommend when, how, and for whom treatments should be applied (Collins, Murphy, & Bierman, 2004; Lavori, Dawson, & Rush, 2000). These decision rules lead to personalized treatment sequences. ATSs have the potential to have a significant public health impact as they can simultaneously improve treatment outcomes and conserve resources by delivering treatments when and for whom they will do the most good. ATSs can be developed and refined within the context of a sequential multiple assignment randomized trial (SMART) (Lavori & Dawson, 2000, 2003; Murphy, 2005). In SMART, subjects can be randomized multiple times and these randomizations occur sequentially through time at selected critical decision points. The results of the SMART trial are then used to define the decision rules that make up the ATS.
Our goal is to use SMART to develop an ATS for adolescent depression that follows a stepped-care model of health care delivery. Stepped-care interventions recommend selecting an initial treatment that is the least restrictive among the available treatments that have empirical support (Sobell & Sobell, 2000). “Least restrictive” refers to all restrictions on the patient’s life and resources, including cost, physical effects of the treatment, and interference in the patient’s lifestyle. More restrictive (but potentially more efficacious) treatments are reserved for patients who do not respond to the initial treatment. Combined treatment (psychotherapy plus medication) is the most intensive evidence-based treatment approach and is associated with the greatest financial burden to families (Domino et al., 2008). As such, a monotherapy was selected as the stage 1 treatment. Specifically, Interpersonal Psychotherapy for depressed adolescents (IPT-A) (Mufson, Dorta, Moreau, & Weissman, 2004), a “well-established” evidence-based treatment for adolescent depression (David-Ferdon & Kaslow, 2008), was selected as the treatment to be provided. The initial treatment plan was twelve IPT-A sessions delivered over 16 weeks (Mufson, Dorta, Wickramaratne, et al., 2004).
In developing an ATS for adolescent depression, we are interested in addressing two specific questions that arise when beginning treatment with IPT-A:
At what time point does one make the determination that the adolescent is not likely to respond if the initial treatment plan is continued? Identifying specific critical treatment decision points is a necessary first step for developing adaptive strategies in order to determine when a treatment change is indicated (Murphy, Oslin, Rush, & Zhu, 2007). Decision points that are specifically operationalized and empirically-based, rather than based on therapist judgment, are more easily replicated and disseminated (Steidtmann et al., 2013). To identify the best time point(s) for assessing whether a change in treatment might be needed, we went back to the data from a previous clinical trial of IPT-A (Mufson, Dorta, Wickramaratne, et al., 2004) and used receiver operating characteristic (ROC) analysis to identify the time point and degree of reduction in depressive symptoms that best predicted treatment response at the end of the trial (week 16). We found that adolescents could be classified as likely to respond or not likely to respond at week 4 or week 8 of treatment (Gunlicks-Stoessel & Mufson, 2011). At week 4, a cutoff of a 20% reduction in depressive symptoms (Hamilton Rating Scale for Depression, HRSD) (Hamilton, 1967) from baseline was found to represent the best combined sensitivity and specificity for predicting response status at the end of the 16 week treatment. At week 8, a 40% reduction in HRSD represented the best combined sensitivity and specificity. We were interested in examining whether it is it better to use an early decision point (week 4) or a late decision point (week 8) for identifying potential insufficient responders to IPT-A.
For adolescents who are insufficient responders, should the therapist increase the number of IPT-A sessions or augment IPT-A with pharmacotherapy? Several studies have found that the combination of psychotherapy and medication results in the greatest response rates (Brent et al., 2008; Clarke et al., 2005; Goodyer et al., 2007; Melvin et al., 2006; TADS Team, 2004). Other studies have found that increasing the number of psychotherapy sessions also increases response rates for patients receiving only psychotherapy (Clarke, Rohde, Lewinsohn, Hops, & Seeley, 1999; TADS Team, 2007). As a consequence, we examine increasing the number of IPT-A sessions from 12 to 16 and augmenting IPT-A with an SSRI, specifically fluoxetine, as options for insufficient responders.
The four adaptive treatment strategies (ATS) considered in this pilot study are:
ATS 1: First treat with weekly IPT-A with an initial treatment plan of 12 sessions. If at week 4, the adolescent has not shown at least a 20% reduction in depression symptoms (HRSD) (i.e. the adolescent is an “insufficient responder”), augment treatment by scheduling biweekly IPT-A sessions for four weeks and then return to weekly sessions for a total of 16 versus standard 12 sessions. Otherwise, if the adolescent has shown at least a 20% reduction in depression symptoms (HRSD) at week 4 (i.e. the adolescent is a “sufficient responder”), maintain initial treatment plan of 12 IPT-A sessions.
ATS 2: First treat with weekly IPT-A with an initial treatment plan of 12 sessions. If at week 4, the adolescent has not shown at least a 20% reduction in depression symptoms (HRSD), augment treatment by adding fluoxetine and continuing IPT-A. Otherwise, if the adolescent has shown at least a 20% reduction in depression symptoms (HRSD) at week 4, maintain initial treatment plan of 12 IPT-A sessions.
ATS 3: First treat with weekly IPT-A with an initial treatment plan of 12 sessions. If at week 8, the adolescent has not shown at least a 40% reduction in depression symptoms (HRSD), augment treatment by scheduling biweekly IPT-A sessions for four weeks for a total of 16 versus standard 12 sessions. Otherwise, if the adolescent has shown at least a 40% reduction in depression symptoms (HRSD) at week 8, maintain initial treatment plan of 12 IPT-A sessions.
ATS 4: First treat with weekly IPT-A with an initial treatment plan of 12 sessions. If at week 8, the adolescent has not shown at least a 40% reduction in depression symptoms (HRSD), augment treatment by adding fluoxetine and continuing IPT-A. Otherwise, if the adolescent has shown at least a 40% reduction in depression symptoms (HRSD) at week 8, maintain initial treatment plan of 12 IPT-A sessions.
The goal of the current study was to conduct a small-scale pilot SMART to prepare for a larger full-scale trial. Given that this was a pilot study, testing the efficacy of the ATSs was not a study goal. Statistical experts warn against over-interpreting pilot data for estimating power and effect sizes for larger clinical trials (Kraemer, Mintz, Noda, Tinklenberg, & Yesavage, 2006). Instead, they recommend conducting pilot studies with the aim of refining the study design, evaluating the feasibility of both the study procedures and the treatments provided in the trial, and assessing the acceptability of the treatments to the patients (Almirall, Compton, Gunlicks-Stoessel, Duan, & Murphy, 2012; Lancaster, Dodd, & Williamson, 2004). Feasibility refers to the study staffs’ ability to successfully (1) execute the SMART study procedures (e.g. recruitment, randomization), and (2) implement the treatments that comprise the ATSs (Almirall et al., 2012). Acceptability refers to adolescents’ and parents’ willingness to engage in a treatment and their satisfaction with the treatment, as well as the acceptability of the treatments to therapists providing treatment (Almirall et al., 2012). In the current pilot SMART, we addressed the following questions:
Before the start of treatment, will adolescents and parents report that a change in the treatment plan would be acceptable, if the adolescent does not demonstrate sufficient improvement in symptoms?
Will adolescents and parents agree to be randomized to an early decision point (week 4) or a late decision point (week 8) for a potential change in the treatment plan?
Among adolescents who show a sufficient response to treatment at week 4 or week 8, will adolescents and parents agree to continue with the initial treatment plan (12 sessions of IPT-A)?
Among adolescents who show an insufficient response to treatment at week 4 or week 8, will adolescents and parents agree to a second randomization and resultant change in treatment plan (add fluoxetine or increase number of IPT-A sessions from 12 to 16)?
Will adolescents be adherent to treatment and complete the treatment protocol?
Will adolescents and parents report satisfaction with the ATSs?
Will therapists be adherent to the implementation of the ATSs?
Will therapists report satisfaction with the ATSs?
Method
Participants
Adolescents ages 12–17 were recruited from the Minneapolis urban area, by various methods including flyers, radio advertisements, newspaper advertisements, school referrals, and clinic referrals. Staff received phone calls from these participants and returned the calls to provide a description of the study and conduct telephone screens. During the study summary, staff provided families with the following information: “The aim of the study is to develop guidelines for personalizing treatment for adolescent depression. Adolescents who are eligible will be treated with a psychotherapy called Interpersonal Psychotherapy. Interpersonal Psychotherapy is an evidence-based treatment that has been recommended by the American Psychological Association and the American Psychiatric Association. It aims to treat depressive symptoms by helping adolescents improve their relationships and communication skills. Adolescents will attend 12–16 therapy sessions. If they do not show sufficient improvement with the therapy, they may be prescribed Fluoxetine (Prozac). Prozac has been approved by the FDA for treatment of adolescent depression. The initial treatment plan is for adolescents to attend 12 therapy sessions over the course of 16 weeks. If your adolescent improves enough, then your adolescent will continue the initial treatment plan of 12 sessions. If your adolescent is not improving enough, then your adolescent will be randomized to one of two changes in treatment: (1) attend more therapy sessions: your adolescent would come in for therapy twice a week instead of once a week, for a total of 16 sessions instead of 12 sessions, or (2) add antidepressant medication to the therapy. This change will happen either at week 4 or week 8 of therapy, also by chance.” Participants who were interested in the study completed a phone screen to determine eligibility for the baseline evaluation. During the phone screens, parents provided adolescents’ developmental, social, and treatment history, and current psychiatric symptoms. Adolescents who did not meet exclusion criteria on the phone screen were invited to participate in a consent meeting and baseline evaluation to determine eligibility. This study was approved by the site’s institutional review board. Parents gave written informed consent, and adolescents gave written informed assent (and consent after they turned age 18).
Inclusion criteria were as follows: a) age of 12 to 17 years; b) DSM-IV-TR diagnosis of Major Depressive Disorder, Dysthymia, or Depressive Disorder NOS; c) significant symptoms of depression (CDRS-R raw score > 35); d) impairment in general functioning (CGAS < 65); and e) English-speaking adolescent and parent. Exclusion criteria were as follows: a) DSM-IV-TR diagnosis of Schizophrenia, Bipolar Disorder, Psychosis, Substance Abuse, Obsessive Compulsive Disorder, Conduct Disorder, Eating Disorder, or Pervasive Developmental Disorder; b) active suicidal ideation with a plan and/or intent; c) already receiving treatment for depression; d) taking medication for a psychiatric diagnosis other than ADHD (adolescents taking a stable dose of stimulants (> 3 months) were included); e) non-responder to an adequate trial of IPT-A or fluoxetine in the past; f) female adolescents who were pregnant, breastfeeding, or having unprotected sexual intercourse; g) mental retardation.
Study Overview
Eligible adolescents were randomized to an early decision point (week 4) or late decision point (week 8) for identifying potential non-responders to IPT-A. Adolescents who were classified as insufficient responders (< 20% reduction in HRSD at week 4 or < 40% reduction in HRSD at week 8) were randomized a second time to the addition of fluoxetine or an additional 4 IPT-A sessions (increase from 12 to 16 sessions). Assessments were administered by independent evaluators blind to treatment condition at baseline, week 4, week 8, week 12, week 16, and week 32. Clinical measures that were administered as part of the study, but are not reported in this paper which focuses on feasibility and acceptability were: Schedule for Affective Disorders and Schizophrenia for School-aged Children – Present and Lifetime Version (K-SADS-PL) (Kaufman, Birmaher, Brent, & Rao, 1997), Children’s Depression Rating Scale-Revised (CDRS-R) (Poznanski & Mokros, 1996), Global Assessment Scale for Children (C-GAS) (Shaffer et al., 1983), Clinical Global Improvement (CGI) (Guy, 1976), Beck Depression Inventory – II (BDI-II) (Beck, Steer, & Brown, 1996). Adolescents were paid $30 each for the baseline, week 16, and week 32 evaluations. They were paid $20 each for the week 4, 8, and 12 evaluations, which were shorter evaluations. Parents received $10 for each of the 6 evaluations.
Interventions
Early decision point for identifying insufficient responders
Adolescents who were randomized to the early decision point began treatment with IPT-A (Mufson, Dorta, Moreau, et al., 2004). Therapists administered the HRSD at the beginning of the week 1, week 4, and week 8 sessions. At week 4, the therapist calculated the percent reduction in HRSD. Adolescents who demonstrated > 20% reduction in HRSD continued in the IPT-A treatment (12 sessions delivered within 16 weeks). Adolescents who demonstrated < 20% reduction in HRSD were informed that they were randomized to receive an additional four IPT-A sessions or the addition of fluoxetine. Adolescents randomized to receive an additional four IPT-A sessions attended therapy twice a week from week 5 through week 8 (total of 16 sessions). Adolescents randomized to the addition of fluoxetine began pharmacotherapy at week 5 and continued IPT-A (total of 12 sessions).
Late decision point for identifying insufficient responders
Adolescents who were randomized to the late decision point began treatment with IPT-A (Mufson, Dorta, Moreau, et al., 2004). Therapists administered the HRSD at the beginning of the week 1, week 4, and week 8 sessions. At week 8, the therapist calculated the percent reduction in HRSD. Adolescents who demonstrated > 40% reduction in HRSD continued in the IPT-A treatment (12 sessions within 16 weeks). Adolescents who demonstrated < 40% reduction in HRSD were informed that they were randomized to an additional four IPT-A sessions or the addition of fluoxetine. Adolescents randomized to receive an additional four IPT-A sessions attended therapy twice a week from week 9 through week 12 (total of 16 sessions). Adolescents randomized to the addition of fluoxetine began pharmacotherapy at week 9 and continued IPT-A (total of 12 sessions).
IPT-A
IPT-A (Mufson, Dorta, Moreau, et al., 2004) is a 12 session evidence-based psychotherapeutic intervention that aims to decrease depressive symptoms by helping adolescents improve their relationships and interpersonal interactions by addressing one or more of four interpersonal problem areas: grief, role disputes, role transitions, and interpersonal deficits. The initial phase of treatment focuses on exploring the adolescent’s significant relationships and identifying the problem area that will be the focus of treatment. During the middle phase of treatment, the therapist identifies and teaches specific communication and problem-solving skills that can improve the interpersonal difficulties that are most closely related to the onset or maintenance of depression. The adolescent role-plays these skills in session and eventually implements them in their current relationships. During the termination phase, the therapist and adolescent review improvements in depressive symptoms and interpersonal functioning, identify successful strategies used to improve relationships, and foster generalization of skills to future situations.
Pharmacotherapy
Adolescents who received pharmacotherapy were prescribed fluoxetine. The dosage schedule was 10 mg per day for the first week and 20 mg per day for the following 5 weeks. If no treatment response was observed by the sixth week following randomization, the dosage could be increased to 40 mg per day. Pharmacotherapy sessions were scheduled weekly for the first 4 weeks and every other week thereafter. Sessions included assessment of vital signs, adverse effects, safety, and symptomatic response. Adolescents were given enough pills to last until their next scheduled appointment and were asked to bring back the bottle of pills to each appointment. A research coordinator counted the number of pills remaining to assess treatment adherence.
Assessments
Feasibility Measures
Recruitment
The following information was collected: number of participants who called to express interest in the study, number who were eligible/ineligible at the phone screen, number who declined participation at the phone screen and their reasons for declining, number who were eligible/ineligible at the baseline evaluation, and number who withdrew consent after baseline evaluation and their reasons for withdrawing.
Identification of sufficient and insufficient responders
The Hamilton Rating Scale for Depression (HRSD) (Hamilton, 1967; Williams, 1988) is a clinician-administered semi-structured interview measure that assesses the severity of depression symptoms. It was administered by the therapists during therapy sessions at weeks 1, 4, and 8 to assess degree of response to treatment and determine whether a change in treatment plan would be initiated. Compliance with administration of the HRSD at the appropriate session was collected. Audiotapes of the therapists’ administration of the HRSD for each of the therapists’ first three subjects were used to provide inter-rater reliability ratings. The clinical supervisor also provided ratings of these HRSDs, and Pearson's r was used to calculate pairwise correlations among raters. The mean level of agreement for each therapist-supervisor pair was calculated. Correlations between the therapists’ week 4 and week 8 HRSD ratings and the Independent Evaluators’ week 4 and week 8 CDRS-R ratings were used to assess the extent to which there was concordance between therapists’ and Independent Evaluators’ assessments of depressive symptoms. Therapists’ attitudes regarding the use of the HRSD to inform treatment decisions were collected. The percentage of adolescents identified as sufficient and insufficient responders and their confidence intervals were also calculated to assess the extent to which actual response rates matched the expected response rates.
Attrition and adherence
The number of adolescents who dropped out of each treatment condition, average IPT-A session attendance, and average medication session attendance were calculated. For adolescents randomized to add medication, length of time in between when families were informed of the treatment change and when they had their first medication session was calculated. To measure adherence to medication, adolescents were given enough pills to last until their next scheduled appointment and were asked to bring back the bottle of pills to each appointment. The research coordinator counted the number of pills remaining to assess treatment adherence. Average medication dosage at the end of the study was also collected.
Communication of the stage 2 treatment
Therapists’ compliance with communicating whether the adolescent would continue the initial treatment plan, attend an increased number of IPT-A sessions, or being pharmacotherapy was assessed from audiotapes of the therapy sessions. Information on difficulties that arose in communicating the stage 2 treatment was also collected.
Treatment acceptability measures
The Adaptive Treatment Attitudes Questionnaire (ATA) (Authors, unpublished) assesses adolescents’, parents’, and therapists’ attitudes regarding changing the adolescents’ treatment plan and the possible type of change to the treatment plan for insufficient responders. At baseline, adolescents and parents indicated on a 5 point Likert scale how they would feel about a possible change (-2 = very negative, 0 = neutral, 2 = very positive) and whether they would likely agree to the change (-2 = definitely no, 0 = not sure, 2 = definitely yes). At week 5 and week 9, right after the family had been informed whether a change in treatment plan would be initiated and, if so, what kind of change would be made, adolescents and parents also indicated on a 5 point Likert scale how they felt about whether a change was made and how they felt about the type of change that was made (increase IPT-A or add medication). Families also provided these ratings at week 16. At week 5 and week 9, therapists indicated on a 5 point Likert scale how they felt about whether a change was made and how they felt about the type of change that was made (increase IPT-A or add medication).
The Client Satisfaction Questionnaire (CSQ-8) (Larsen, Attkisson, Hargreaves, & Nguyen, 1979) is a well-established self-report instrument used to assess patients’ satisfaction with psychiatric treatment. It was used to assess adolescents’ and parents’ treatment satisfaction. A score of 8–20 indicates low treatment satisfaction, 21–26 indicates medium satisfaction, and 27–32 indicates high satisfaction.
Clinical Outcomes
The Clinical Global Impressions (CGI) (Guy, 1976) is a commonly used measure in clinical trials that tracks change in patients’ clinical status with treatment. It consists of two questions: “how ill do you feel the patient is?” (Severity score) and “how improved is the patient?” (Improvement score) that are rated on a 7-point Likert scale. In keeping with previous clinical trials, treatment response was defined as an Improvement score of 1 or 2 (“much improved” or “very much improved”) (Brent et al., 2008; TADS Team, 2004).
Results
Feasibility of Study and Treatment Procedures
Recruitment: patient disposition
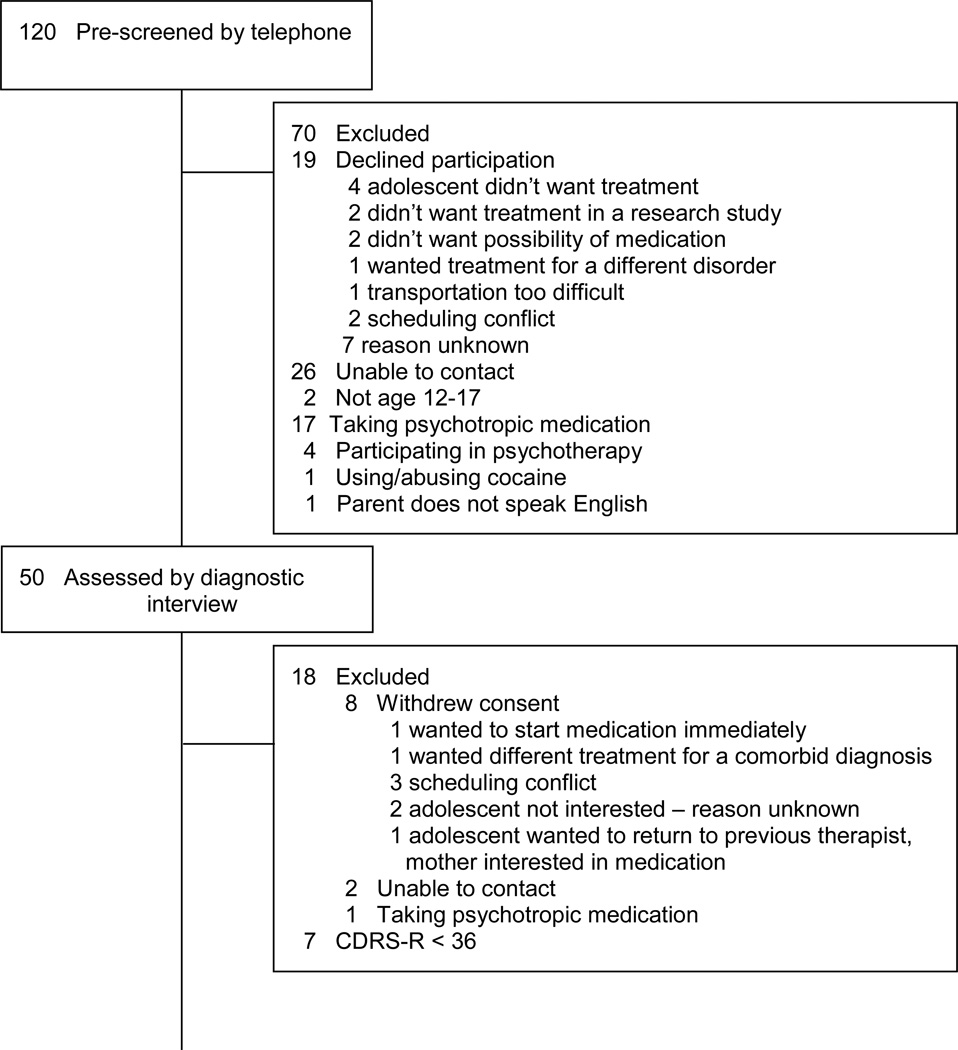
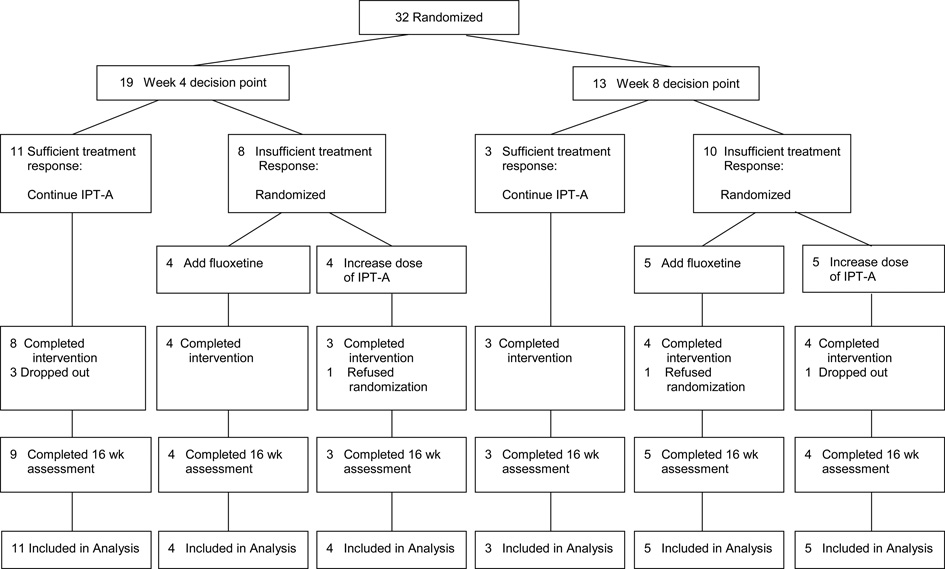
Of the 120 adolescents pre-screened by telephone, 50 consented to a diagnostic evaluation to determine study eligibility (see Figure 2). Of the families who chose not to participate after listening to information about the study, two gave reasons for declining that were specific to the ATSs provided in the study: the families did not want the adolescent to have the possibility of taking psychotropic medication. Eight families withdrew consent after completing the evaluation to determine eligibility. One of these families gave a reason for withdrawing consent that was specific to the ATSs provided in the study: the family wanted the adolescent to start psychotropic medication right away. Thirty-two adolescents began treatment and were randomized to a week 4 or a week 8 decision point for determining if a change in treatment would be made. Twenty-six adolescents (81.3%) completed treatment and twenty-eight (87.5%) completed the week 16 assessment.
Recruitment: demographic and clinical characteristics
Participants were an average of 14.9 years of age (SD = 1.7) and 75% female. Three (9.4%) adolescents identified as American Indian/Alaskan Native, 27 (84.4%) as Caucasian, and two (6.3%) as biracial (African American/Caucasian). Modal family income was $90,000-$179,000. Depression diagnoses were as follows: 30 (93.8%) with Major Depressive Disorder (MDD), one (3.1%) with MDD and dysthymic disorder, and one (3.1%) with Depressive Disorder NOS. Depression severity at baseline ranged from mild (CDRS-R raw score = 38) to severe (CDRS-R raw score = 71). Mean (SD) severity was 55.28 (10.46), which translates to a normed t score of 72, indicating a moderate severity of depression. Most adolescents were in their first depressive episode (79.31%), with a mean episode duration of 18.5 (14.13) months. Comorbid diagnoses were as follows: six (18.8%) with Generalized Anxiety Disorder, seven with Social Phobia (21.9%), two (6.3%)with Specific Phobia, two (6.3%) with Oppositional Defiant Disorder, and three (9.4%) with Attention-Deficit Hyperactivity Disorder.
Identification of Sufficient and Insufficient Responders
Compliance with administering the HRSD at the appropriate session was 100%. Inter-rater reliability (Pearson’s r) was .82. Therapists’ HRSD ratings at week 4 and week 8 were significantly correlated with Independent Evaluators’ CDRS-R ratings (week 4: r = .78, p = .00; week 8: r = .63, p = .00). Therapists expressed some dissatisfaction with how long the HRSD took to administer and felt it sometimes hindered therapeutic progress.
Based on data from Mufson et al (2004), it was estimated that 63% (95% CI: 46.7%-80.0%) of adolescents would demonstrate a sufficient response to treatment at week 4. In our current pilot study, amongst adolescents randomized to week 4 decision point, 57.9% (95% CI: 33.3%-78.9%) of the sample demonstrated a sufficient response to treatment and continued with the initial treatment plan. It was estimated that at week 8, 49% (95% CI: 26.7%-63.3%) would demonstrate a sufficient response to treatment. In our current study, amongst adolescents randomized to a week 8 decision point, 23.1% (95% CI: 0.0%-46.7%) of the sample demonstrated a sufficient response to treatment and continued with the initial treatment plan.
Attrition and adherence
All families in the study agreed to the week 4 versus week 8 randomization (i.e. they did not drop out because they wanted the decision regarding whether to change the treatment plan to be made at a different time point). Amongst the adolescents who showed a sufficient response to therapy so that a change in treatment plan was not triggered (n = 14), all fourteen families agreed to continue treatment without making a change. Three of these adolescents later dropped out of treatment (4 and 6 weeks after the decision to not change treatment was made). One adolescent felt she was not getting better and wanted to discontinue therapy and take medication instead. One adolescent reported that she was feeling better and did not wish to continue therapy. The other adolescent had scheduling difficulties and was lost. Amongst the adolescents who demonstrated an insufficient response to treatment and were randomized to add medication (n = 9), one family refused the treatment assignment. Amongst the adolescents who demonstrated an insufficient response to treatment and were randomized to increase the number of IPT-A sessions (n = 9), one family refused the treatment assignment. One adolescent showed an insufficient response to treatment, but dropped out before we could tell the family the type of treatment change to which the adolescent had been randomized.
Adolescents who demonstrated a sufficient response to treatment and did not receive a change in treatment (n = 14) could attend a possible 12 IPT-A sessions. Mean (SD) number of completed IPT-A sessions for these adolescents was 11.00 (1.84). Adolescents who demonstrated an insufficient response to treatment and were randomized to add medication (n = 9) could attend a possible 12 IPT-A sessions. Mean (SD) session attendance for the adolescents who agreed to the change was 11.78 (1.56). Adolescents who demonstrated an insufficient response to treatment and were randomized to increase the number of IPT-A sessions (n = 9) could attend a possible 16 IPT-A sessions. All adolescents who consented to the change increased their session attendance, and their mean (SD) session attendance was 15.29 (.95).
Amongst adolescents randomized to add medication, the mean length of time between when they were informed of the change in treatment and their first medication session was 6.11 days (SD = 2.37). Adolescents who demonstrated an insufficient response to treatment at week 4 and were randomized to add medication (n = 4) could attend a possible 8 medication sessions. Mean (SD) session attendance for these adolescents was 7.67 (1.53). Adolescents who demonstrated an insufficient response to treatment at week 8 and were randomized to add medication (n = 5) could attend a possible 6 medication sessions. Mean (SD) session attendance for these adolescents was 3.8 (1.64). Based on pill counts, overall mean adherence for adolescent randomized to week 4 was 98.09% (SD = 2.00%), and week 8 was 96.07% (SD = 6.8%). The mean post-treatment dose for adolescents randomized to week 4 was 35.0 mg (SD = 10.0), and week 8 was 22.5 mg (SD = 5.0).
Communication of the Stage 2 Treatment
For all subjects, therapists were adherent in communicating whether the adolescent would continue the initial treatment plan, attend an increased number of IPT-A sessions, or begin pharmacotherapy. Several types of concerns about the stage 2 treatments were raised by some adolescents and parents, including concern that the adolescent had not shown sufficient improvement, surprise that a change in treatment would be made because the family perceived the adolescent to have improved, and amongst adolescents randomized to increase IPT-A, concern about the feasibility of attending therapy twice a week.
Treatment Acceptability
Baseline adaptive treatment attitudes
While there was some variability, most adolescents reported on the Adaptive Treatment Attitudes Questionnaire (ATA) that they would feel positive about increasing the number of IPT-A sessions or adding medication (see Table 1). Most adolescents indicated that they would agree to both changes. Parents reported that they would feel better about increasing the IPT-A than they would about adding medication. Most indicated that they would agree to either change, but more parents indicated that they would agree to increase IPT-A than to add medication.
Table 1.
Adolescents’ and Parents’ Adaptive Treatment Attitudes
| Pre-treatment | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Feelings about change | Agree to change | |||||||||||
| Adolescent | Parent | Adolescent | Parent | |||||||||
| Pos | Neu | Neg | Pos | Neu | Neg | Yes | Not Sure |
No | Yes | Not Sure |
No | |
| Increase IPT-A | 53.9 | 23.1 | 23.1 | 70.3 | 22.2 | 7.4 | 84.7 | 11.5 | 3.8 | 96.3 | 3.7 | 0 |
| Add Meds | 46.1 | 30.8 | 23.1 | 29.6 | 33.3 | 37.0 | 73.1 | 7.7 | 19.2 | 62.9 | 33.3 | 3.7 |
| Post-decision point Feelings about change |
Post-treatment Feelings about change |
|||||||||||
| Adolescent | Parent | Adolescent | Parent | |||||||||
| Pos | Neu | Neg | Pos | Neu | Neg | Pos | Neu | Neg | Pos | Neu | Neg | |
| Week 4 | 66.7 | 22.2 | 11.1 | 70.0 | 10.0 | 20.0 | 58.8 | 41.2 | 0 | 64.3 | 7.1 | 28.6 |
| Week 8 | 33.3 | 50.0 | 16.7 | 25.0 | 50.0 | 25.0 | 62.5 | 25.0 | 12.5 | 66.7 | 22.2 | 11.1 |
| Increase IPT-A | 50.0 | 25.0 | 25.0 | 100.0 | 0 | 0 | 80.0 | 20.0 | 0 | 100.0 | 0 | 0 |
| Add Meds | 20.0 | 60.0 | 20.0 | 20.0 | 20.0 | 60.0 | 71.4 | 14.2 | 14.2 | 50.0 | 25.0 | 25.0 |
Note. Pos = positive, Neu = neutral, Neg = negative.
Adolescents’ and parents’ post-decision point and post-treatment adaptive treatment attitudes
Adolescents’ and parents’ attitudes right after they were informed of whether or not there would be a change to the treatment plan and their attitudes post-treatment are reported in Table 1. Regarding attitudes about the week 4 versus the week 8 decision point, immediately after families were informed whether the adolescent had been randomized to a week 4 or a week 8 decision point, both adolescents and parents reported more positive feelings if the adolescent had been randomized to week 4 as compared to week 8. Adolescents and parents in the week 8 condition mostly reported feeling neutral. However, by the end of treatment, the time point did not seem to matter: both adolescents and parents reported mostly positive feelings about both week 4 and week 8 decision points.
Amongst the insufficient responders, half of the adolescents who were randomized to increased IPT-A felt positively about it right after they were informed of the change, with the other half split between negative and neutral. By the end of treatment, 80% of adolescents who were randomized to increased IPT-A felt positively. All parents of adolescents randomized to increase IPT-A felt positively about the change both right after they were informed of the change and post-treatment. Amongst the adolescents who were randomized to add medication, most reported feeling neutral about the change right after they were informed of the change; however, by post-treatment, most adolescents felt positively about the change. Most parents reported feeling negative about adding medication right after they were informed of the change. By post-treatment, half felt positive, and the other half were split between negative and neutral.
Therapists’ post-decision point adaptive treatment attitudes
Most therapists felt positive about both the week 4 and the week 8 decision point (week 4: 76.9% positive, 23.1% negative; week 8: 66.7% positive, 33.3% negative). Regarding the type of change in treatment for insufficient responders, most therapists felt positive about adding medication (75.0% positive, 25.0% negative). They were split between feeling positive and negative about increasing the number of IPT-A sessions (50.0% positive, 50.0% negative).
Overall Treatment Satisfaction
Overall, adolescents’ and parents’ treatment satisfaction (CSQ-8) was high (adolescents: M = 26.83, SD = 3.89; parents: mean = 26.72, SD = 4.26). Identifying insufficient responders at week 4 appeared to lead to slightly higher treatment satisfaction for adolescents than waiting until week 8 (Week 4 adolescents: M = 27.53, SD = 3.38; parents: mean = 26.93, SD = 4.27. Week 8 adolescents: M = 25.67, SD = 4.58; parents: mean = 26.40, SD = 4.45). Amongst the insufficient responders, both adolescents and parents reported slightly higher treatment satisfaction with increase IPT-A as compared to add medication. (Adolescent increase IPT-A: mean = 26.00, SD = 5.02; adolescent add medication: mean = 25.14, SD = 4.45; parent increase IPT-A: mean = 26.50, SD = 3.21; parent add medication: mean = 24.13, SD = 4.32).
Clinical Outcomes
Treatment response rates are reported in Table 3. Response rates for the week 4 decision point were higher than the week 8 decision point, though there was overlap in their 95% confidence intervals.
Table 3.
Treatment Response Rates Based on CGI Improvement
| Treatment Group | Response Rate | 95% CI |
|---|---|---|
| Week 4 Decision Point (n = 19) | 68.4% | 45.5% – 88.2% |
| Sufficient responders (n = 11) | 72.7% | |
| Insufficient, add meds (n = 4) | 50% | |
| Insufficient, increase IPT-A (n = 4) | 75% | |
| Week 8 Decision Point (n = 13) | 46.2% | 20.0% – 75.0% |
| Sufficient responders (n = 3) | 33.3% | |
| Insufficient, add meds (n = 5) | 40.0% | |
| Insufficient, increase IPT-A (n = 5) | 60.0% |
Note. Response defined as CGI-I of 1 or 2.
Discussion
In this study, we examined the feasibility and acceptability of four ATSs for adolescent depression with the goal of refining the ATSs and study procedures to prepare for a larger full-scale SMART that will be used to compare their efficacy. The results of the study support the continued clinical importance of examining the efficacy of adaptive treatments for adolescent depression. Rates of insufficient responders at week 4 or week 8 of IPT-A in this trial were comparable to or higher than estimates based on a previous clinical trial of IPT-A (Mufson, Dorta, Wickramaratne, et al., 2004), indicating a need to identify efficacious second-stage treatments to improve clinical outcomes. Parents, adolescents, and therapists also had clear feelings about medication and more intensive therapy as treatment options. This suggests that knowing the relative efficacy of adding medication versus intensifying the therapy would be clinically meaningful for families and treatment providers.
Our pilot study also provided us with the opportunity to discover prior to conducting a large-scale clinical trial that one of our decision points for identifying insufficient responders was preferable to the other. The feasibility, acceptability, and clinical outcomes for the week 4 decision point were better than the week 8 decision point. The week 4 guideline identified the expected number of sufficient responders, whereas the week 8 guideline identified significantly fewer sufficient responders than expected. Adolescents randomized to add medication at week 8 had less time before the end of the study to schedule follow-up appointments with the psychiatrist. As a result, they had fewer medication appointments and fewer opportunities to increase the dose of medication than adolescents randomized at week 4, and they ended the study on a lower dose of medication. Adolescents and parents reported more positive feelings about the week 4 decision point than the week 8 decision point right after they were informed of their randomized time point. Post-treatment, adolescents randomized to week 4 reported higher overall treatment satisfaction than adolescents randomized to week 8. Treatment response rates were also higher for the week 4 decision point than the week 8 decision point.
Based on these results, we decided to move forward in the subsequent full-scale SMART with assessing the relative efficacy of the two ATSs that included the week 4 decision point and drop the two ATSs that included the week 8 decision point. Because we were able to use the pilot study to identify the more feasible and acceptable decision point for determining whether a change in treatment might be needed, this enabled us to plan to use the subsequent full-scale SMART to address a different important question that arises when treating depressed adolescents in a stepped-care model: amongst the two evidence-based monotherapies for adolescent depression, psychotherapy (IPT-A) and medication (fluoxetine), for which adolescents is it better to start treatment with IPT-A and for which adolescents is it better to start with fluoxetine? In the subsequent full-scale SMART, adolescents will be randomized to begin treatment with IPT-A or fluoxetine, and we will explore the moderating role of two major mechanisms of adolescent depression, interpersonal and positive affective processes (Eshel & Roiser, 2010; Hammen, 1999), in treatment that begins with IPT-A or fluoxetine. At week 4, insufficient responders to IPT-A or fluoxetine will be randomized a second time to increase the dose of the initial treatment or add the other treatment (e.g. add fluoxetine to IPT-A, or add IPT-A to fluoxetine). In this way, we will increase the precision of the adaptive treatment strategy by providing guidelines for both initial treatment selection and selection of the augmentation approach for insufficient responders.
In addition to this substantial change to the study design, some additional needed modifications to the study procedures and to the ATSs were identified (see Table 2). Therapists and adolescents were dissatisfied with the HRSD as the measure used to identify sufficient and insufficient responses, as it took, on average, 15–20 minutes to complete, and reduced the time available to focus on the therapeutic work. In the subsequent full-scale SMART, therapists will extend the length of the sessions in which the HRSD is administered from 45 to 60 minutes. Given that most insurance panels will increase payments for 60 minute sessions, this should be feasible to implement in clinical practice.
Table 2.
Issues Identified in the SMART Pilot and Proposed Changes to the Subsequent Full-Scale SMART.
| Topic | Issues Discovered in the SMART Pilot | Changes in Preparation for the Full-Scale SMART |
|---|---|---|
| Recruitment | Our sample consisted primarily of Caucasian upper middle-class adolescents. While this sample is fairly representative of depressed adolescents who seek treatment, our aims is to develop treatment guidelines that will benefit the broader population of depressed adolescents. | Greater efforts will be made to recruit from pediatric clinics, mental health clinics, and schools that serve low income and/or minority populations. In addition, conversations with lower income families, as well as community providers who serve lower income communities revealed that transportation to the medical center is often a barrier to treatment. In the full-scale SMART, participants will receive vouchers to pay for parking, and public transportation costs will be reimbursed, if needed. The availability of these benefits will be added to all recruitment materials. |
| Identification of Sufficient and Insufficient Responders | The HRSD took, on average, 15–20 minutes to complete. Both therapists and adolescents expressed frustration at having to spend so much of a therapy session focused on assessment rather than therapy, and felt it hindered therapeutic progress. | In the subsequent full-scale SMART, therapists will extend the length of the sessions in which the HRSD is administered from 45 to 60 minutes. Given that most insurance panels will increase payments for 60 minute sessions, this should be feasible to implement in clinical practice. |
| Communication of the Stage 2 Treatment | Some families expressed concern about the timing of and change (or no change) to the treatment plan. These concerns are described below. | Revisions were made to the treatment manual to direct therapists on how to talk about the change to the treatment plan (or lack of change) and how to address families’ questions and concerns. These revisions are described below. |
| 1. Some families whose adolescents were classified as week 4 sufficient responders expressed surprise that a change in treatment would not be made, as they did not perceive the adolescent to have demonstrated a significant improvement in symptoms. | 1. Therapists now describe the specific symptoms that have improved rather than just saying that enough improvement was seen. In addition, the therapists explain that this early in therapy (week 4), we wouldn’t expect to see a very large change in depressive symptoms and that we are not looking for a full response to treatment at that point, but rather a “signal” that the depressive symptoms are starting to reduce a bit. | |
| 2. Some families of adolescents who had not demonstrated a sufficient mid-treatment response with the initial treatment plan expressed worry that not enough improvement had been seen yet. | 2. Therapists now work to address these concerns by blaming the depression rather than the adolescent (“there are different types of depression that respond to different treatments in different ways”), indicating that steps are being taken to address the insufficient response (“we’re getting a signal that we need to make an adjustment to your treatment plan to better treat your depression. We’re going to make a change now rather than waiting until the end of the study so that we can catch this early.”), and attempting to instill hope (“If you continue to attend your appointments and actively engage in your treatment, you are very likely to feel better.”). | |
| 3. Some families expressed surprise that a change in treatment would be made, as they perceived the adolescent to have been improving. | 3. Therapists now work to acknowledge the improvement (“You have been working hard in therapy and you can feel proud of the progress you’ve made. We can see that this work is starting to improve your mood.”), and explain that the goal is recovery rather than just improvement (“Our goal is for you to feel completely better at the end of treatment and not just mostly better. We’re getting a signal that we need to intensify your treatment (by either increasing the number of therapy sessions or adding medication) to help increase your chances of feeling completely better.”). | |
| 4. Some adolescents who were randomized to increased number of IPT-A sessions expressed ambivalence or disappointment because they felt coming to therapy twice a week interfered with other activities they’d rather be doing. | 4. Therapists now work to better engage adolescents in therapy and increase motivation by reviewing the ways in which the depression is interfering in the adolescent’s life and the potential benefits of feeling better. The therapist describes the research indicating that increasing the number of therapy sessions can improve depression outcomes. Therapists also help adolescents to problem-solve potential scheduling conflicts. | |
| Identification of Additional Tailoring Variables | 1. Therapists noted anecdotally that some adolescents reported little or no lift in their mood after engaging in a positive interpersonal interaction or social activity (even when the adolescent reported that the interaction or activity had gone well). For these adolescents, this lack of mood reactivity was apparent early on in treatment and did not seem to get better over the course of therapy. Therapists expressed concern when these adolescents weren’t randomized to receive medication. | 1. In the full-scale SMART, we will measure mood reactivity as a potential additional tailoring variable. |
| 2. Therapists noted anecdotally that some adolescents were not willing to work on some of their difficult relationships, despite the therapist’s best efforts. It just seemed like the adolescents weren’t ready yet. This made the increased IPT-A condition difficult, as the therapist had more therapy sessions, but had trouble engaging the adolescent in the therapy process. | 2. In the full-scale SMART, we will include a measure of readiness to change as a potential additional tailoring variable. | |
| 3. Therapists noted that some adolescents were actively working in therapy, completing their interpersonal experiments in between sessions (IPT-A’s equivalent of homework), and having success with their interpersonal experiments. When these adolescents were randomized to add medication, the therapists felt the adolescents could have done well with increased IPT-A and might not have needed medication (and could have avoided potential side effects). | 3. In the full-scale SMART, we will include a measure of homework completion and homework success as a potential additional tailoring variable. | |
| 4. Therapists noted that some adolescents were not completing their interpersonal experiments in between sessions and this did not seem to be due to lack of engagement in therapy, but rather the interpersonal experiments made them too anxious and so they avoided doing them. For adolescents who didn’t receive medication, therapists wondered if the adolescents had received medication, if it might have reduced their anxiety and enabled them to attempt their interpersonal experiments. | 4. In the full-scale SMART, we will include a measure of anxiety as a potential additional tailoring variable. | |
Experiences from the pilot have also led us to revise the treatment manual which guides therapists on how to communicate the stage 2 treatments to families. While most families were happy with their stage 2 treatment, some families expressed concern about the change (or no change) to the treatment plan. Revisions were made to the treatment manual to direct therapists on how to talk about the change to the treatment plan (or lack of change) and how to address families’ questions and concerns (see Table 3).
Some additional needed alterations to the study design and procedures were also identified. The sample that we recruited consisted primarily of upper middle class white females. The preponderance of females is an issue with treatment of depression generally. Adolescent girls have more positive help-seeking attitudes and lower perceived barriers to initiating treatment than males (Cohen, 1999; Kuhl, Jarkon-Horlick, & Morrissey, 1997). Racial and ethnic minority youth are also less likely to receive mental health treatment (Garland et al., 2005). While our sample is representative of who is likely to receive an ATS in a clinic setting, we would like to develop treatment guidelines that also benefit under-served, under-studied populations. As such, greater efforts will be made in the subsequent full-scale SMART to recruit a more diverse sample of adolescents by recruiting from sites that that serve low income and/or minority populations (see Table 3). We will also provide compensation for parking and/or public transportation to make it easier for lower income families to come to treatment.
Therapists’ attitudes about the ATSs were extremely valuable. We were surprised to discover that 50% of therapists felt negative about the stage 2 treatment strategy of increasing the dose of IPT-A. Therapists’ concerns about some adolescents’ stage 2 treatments and their reasons for concern led to interesting and important discussions about additional characteristics of the adolescent that might be used to decide whether to augment treatment with medication or additional therapy sessions, including mood reactivity to positive interpersonal events, successful completion of therapy homework, readiness to work on their problems, and comorbid anxiety. Homework completion and readiness to change have been identified as predictors of treatment outcome with CBT (Gaynor, Lawrence, & Nelson-Gray, 2006; Lewis et al., 2009), but have not yet been examined as predictors of outcome with IPT-A. Comorbid anxiety has been found to predict poorer treatment outcome with IPT-A (Young et al., 2012; Young, Mufson, & Davies, 2006), but its impact on IPT-A augmentation strategies of adding medication or increasing the dose of IPT-A has not been examined. Mood reactivity to positive interpersonal events that were prescribed in therapy has not been previously examined as a predictor of treatment outcome; however, pre-treatment levels of anhedonia has been shown to be a predictor (McMakin et al., 2012). Measures of these characteristics will be included in the subsequent full-scale SMART as potential additional tailoring variables (see Table 3).
In sum, much was learned from this pilot study. Evaluating the efficacy of adaptive treatment strategies continues to be clinically important, and the ATSs that include the week 4 decision point show promise in terms of their feasibility to implement and acceptability to families. Families’ and therapists’ experiences with the ATSs in the pilot study have led to meaningful improvements to the ATSs themselves. Results from the pilot study have yielded additional research questions to examine in the full-scale SMART and will improve our ability to successfully propose and conduct the trial.
Figure 1.
CONSORT Diagram
Acknowledgements
Research reported in this publication was supported by the following Award Numbers from the National Institutes of Health: K23MH090216 from the National Institute of Mental Health (Gunlicks-Stoessel), P50DA010075 from the National Institute on Drug Abuse (Murphy, Almirall), R03MH097954 from the National Institute of Mental Health (Almirall), and UL1TR000114 from the National Center for Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the National Center for Research Resources. Study data were collected and managed using REDCap electronic data capture tools hosted at the University of Minnesota.
Contributor Information
Meredith Gunlicks-Stoessel, Email: mgunlick@umn.edu, Department of Psychiatry, University of Minnesota, Minneapolis, MN.
Laura Mufson, Email: mufsonL@nyspi.columbia.edu, Department of Psychiatry, Columbia University College of Physicians & Surgeons and New York State Psychiatric Institute, 10510 Riverside Dr Unit 74, New York, NY 10032.
Ana Westervelt, Email: bortn005@umn.edu, Department of Psychiatry, University of Minnesota, Minneapolis, MN.
Daniel Almirall, Email: Dalmiral@isr.umich.edu, Institute for Social Research, University of Michigan, Ann Arbor, MI.
Susan Murphy, Email: samurphy@umich.edu, Department of Statistics, Department of Psychiatry, and Institute for Social Research, University of Michigan, Ann Arbor, MI.
References
- Almirall D, Compton SN, Gunlicks-Stoessel M, Duan N, Murphy S. Preparing for a sequential multiple assignment randomized trial for developing an adaptive treatment strategy: Designing a SMART pilot study. Statistics in Medicine. 2012;31:1887–1902. doi: 10.1002/sim.4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory. 2nd edition. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- Brent D, Emslie G, Clarke G, Wagner KD, Asarnow JR, Keller M, Zelazny J. Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: The TORDIA randomized controlled clinical trial. Journal of the American Medical Association. 2008;299:901–913. doi: 10.1001/jama.299.8.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke G, Debar L, Lynch F, Powell J, Gale J, O’Connor E. A randomized effectiveness trial of brief cognitive-behavioral therapy for depressed adolescents receiving antidepressant medication. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44:888–898. [PubMed] [Google Scholar]
- Clarke G, Rohde P, Lewinsohn P, Hops H, Seeley J. Cognitive-behavioral treatment of adolescent depression: Efficacy of acute group treatment and booster sessions. Journal of the American Academy of Child & Adolescent Psychiatry. 1999;38:272–279. doi: 10.1097/00004583-199903000-00014. [DOI] [PubMed] [Google Scholar]
- Cohen B. Measuring the willingness to seek help. Journal of Social Service Research. 1999;26:67–82. [Google Scholar]
- Collins LM, Murphy SA, Bierman KA. A conceptual framework for adaptive preventive interventions. Prevention Science. 2004;5:185–196. doi: 10.1023/b:prev.0000037641.26017.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David-Ferdon C, Kaslow NJ. Evidence-based psychosocial treatments for child and adolescent depression. Journal of Clinical Child and Adolescent Psychology. 2008;37:62–104. doi: 10.1080/15374410701817865. [DOI] [PubMed] [Google Scholar]
- Domino ME, Burns BJ, Silva SG, Kratochvil CJ, Vitiello B, Reinecke MA, March JS. Cost-effectiveness of treatments for adolescent depression: Results from TADS. American Journal of Psychiatry. 2008;47:1369–1373. doi: 10.1176/appi.ajp.2008.07101610. [DOI] [PubMed] [Google Scholar]
- Eshel N, Roiser JP. Reward and punishment processing in depression. Biological Psychiatry. 2010;38:118–124. doi: 10.1016/j.biopsych.2010.01.027. [DOI] [PubMed] [Google Scholar]
- Garland AF, Lau AS, Yeh M, McCabe KM, Hough RL, Landsverk JA. Racial and ethnic differences in utilization of mental health services among high-risk youths. American Journal of Psychiatry. 2005;162:1336–1343. doi: 10.1176/appi.ajp.162.7.1336. [DOI] [PubMed] [Google Scholar]
- Gaynor ST, Lawrence P, Nelson-Gray R. Measuring homework compliance in cognitive-behavioral therapy for adolescent depression: Review, preliminary findings, and Implications for theory and practice. Behavior Modification. 2006;30:647–672. doi: 10.1177/0145445504272979. [DOI] [PubMed] [Google Scholar]
- Goodyer IM, Dubicka B, Wilkinson P, Kelvin R, Roberts C, Byford S, et al. Selective serotonin reuptake inhibitors (SSRIs) and routine specialist care with and without cognitive behaviour therapy in adolescents with major depression: Randomised controlled trial. British Medical Journal. 2007 doi: 10.1136/bmj.39224.494340.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunlicks-Stoessel ML, Mufson L. Early patterns of symptom change signal remission with interpersonal psychotherapy for depressed adolescents. Depression & Anxiety. 2011;28:525–531. doi: 10.1002/da.20849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy W. ECDEU assessment manual for psychopharmacology. 2 ed. Washington, DC: US Government Printing Office; 1976. [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. British Journal of Social and Clinical Psychology. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Hammen C. The emergence of an interpersonal approach to depression. In: Joiner T, Coyne JC, editors. The interactional nature of depression: Advances in interpersonal approaches. Washington, DC: American Psychological Association; 1999. pp. 21–35. [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Mintz J, Noda A, Tinklenberg J, Yesavage JA. Caution regarding the use of pilot studies to guide power calculations for study proposals. Archives of General Psychiatry. 2006;63:484–489. doi: 10.1001/archpsyc.63.5.484. [DOI] [PubMed] [Google Scholar]
- Kuhl J, Jarkon-Horlick L, Morrissey RF. Measuring barriers to help-seeking behavior in adolescents. Journal of Youth and Adolescence. 1997;26:637–650. [Google Scholar]
- Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. Journal of Evaluation in Clinical Practice. 2004;10:307–312. doi: 10.1111/j..2002.384.doc.x. [DOI] [PubMed] [Google Scholar]
- Larsen DL, Attkisson CC, Hargreaves WA, Nguyen TD. Assessment of client/patient satisfaction: Development of a general scale. Evaluation and Program Planning. 1979;2:197–207. doi: 10.1016/0149-7189(79)90094-6. [DOI] [PubMed] [Google Scholar]
- Lavori PW, Dawson R. A design for testing clinical strategies: Biased individually tailored within-subject randomization. Journal of the Royal Statistics Society. 2000;163:29–38. [Google Scholar]
- Lavori PW, Dawson R. Dynamic treatment regimes: Practical design considerations. Clinical Trials. 2003;1:9–20. doi: 10.1191/1740774s04cn002oa. [DOI] [PubMed] [Google Scholar]
- Lavori PW, Dawson R, Rush AJ. Flexible treatment strategies in chronic disease: Clinical and research implications. Biological Psychiatry. 2000;48:605–614. doi: 10.1016/s0006-3223(00)00946-x. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Roberts RE, Seeley JR, Rohde P, Gotlib IH, Hops H. Adolescent psychopathology II: Psychosocial risk factors for depression. Journal of Abnormal Psychology. 1994;103:302–315. doi: 10.1037//0021-843x.103.2.302. [DOI] [PubMed] [Google Scholar]
- Lewis CC, Simons AD, Silva SG, Rohde P, Small DM, Murakami JL, March JS. The role of readiness to change in response to treatment of adolescent depression. Journal of Consulting and Clinical Psychology. 2009;77:422–428. doi: 10.1037/a0014154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMakin DL, Olino TM, Porta G, Dietz LJ, Emslie G, Clarke G, Brent DA. Anhedonia predicts poorer recovery among youth with selective serotonin reupdate inhibitor treatment-resistant depression. Journal of the American Academy of Child & Adolescent Psychiatry. 2012;51:404–411. doi: 10.1016/j.jaac.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melvin GA, Tonge BJ, King NJ, Heyne D, Gordon MS, Klimkeit E. A comparison of cognitive-behavioral therapy, sertraline, and their combination for adolescent depression. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:1151–1161. doi: 10.1097/01.chi.0000233157.21925.71. [DOI] [PubMed] [Google Scholar]
- Mufson L, Dorta KP, Moreau D, Weissman MM. Interpersonal psychotherapy for depressed adolescents. 2 ed. New York: Guilford Press; 2004. [Google Scholar]
- Mufson L, Dorta KP, Wickramaratne P, Nomura Y, Olfson M, Weissman MM. A randomized effectiveness trial of interpersonal psychotherapy for depressed adolescents. Archives of General Psychiatry. 2004;61:577–584. doi: 10.1001/archpsyc.61.6.577. [DOI] [PubMed] [Google Scholar]
- Murphy SA. An experimental design for the development of adaptive treatment stratgies. Statistics in Medicine. 2005;24:1455–1481. doi: 10.1002/sim.2022. [DOI] [PubMed] [Google Scholar]
- Murphy SA, Oslin DW, Rush AJ, Zhu J. Methodological challenges in constructing effective treatment sequences for chronic psychiatric disorders. Neuropsychopharmacology. 2007;32:257–262. doi: 10.1038/sj.npp.1301241. [DOI] [PubMed] [Google Scholar]
- Poznanski EO, Mokros HB. The Children's Depression Rating Scale - Revised (CDRS-R) Los Angeles: Western Psychological Services; 1996. [Google Scholar]
- Shaffer D, Gould MS, Brasic J, Ambrosini P, Bird HR, Aluwahlia S. A children's Global Assessment Scale (CGAS) Archives of General Psychiatry. 1983;40:1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- Sobell M, Sobell L. Stepped care as a heuristic approach to the treatment of alcohol problems. Journal of Consulting and Clinical Psychology. 2000;68:573–579. [PubMed] [Google Scholar]
- Steidtmann D, Manber R, Blasey C, Markowitz JC, Klein DN, Rothbaum BO, Arnow BA. Detecting critical decision points in psychotherapy and psychotherapy & medication for chronic depression. Journal of Consulting and Clinical Psychology. 2013;81:783–792. doi: 10.1037/a0033250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TADS Team. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression. Journal of the American Medical Association. 2004;292:807–820. doi: 10.1001/jama.292.7.807. [DOI] [PubMed] [Google Scholar]
- TADS Team. The Treatment for Adolescents with Depression Study (TADS): Long-term effectiveness and safety outcomes. Archives of General Psychiatry. 2007;64:1132–1144. doi: 10.1001/archpsyc.64.10.1132. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Wolk S, Goldstein RB, Moreau D, Adams P, Greenwald S, Wickramaratne P. Depressed adolescents grown up. Journal of the American Medical Association. 1999;281:1707–1713. doi: 10.1001/jama.281.18.1707. [DOI] [PubMed] [Google Scholar]
- Williams JB. A structured interview guide for the Hamilton Depression Rating Scales. Archives of General Psychiatry. 1988;45:742–747. doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]
- Young JF, Makover HB, Cohen JR, Mufson L, Gallop RJ, Benas JS. Interpersonal psychotherapy-adolescent skills training: Anxiety outcomes and impact of comorbidity. Journal of Clinical Child and Adolescent Psychology. 2012;41:640–653. doi: 10.1080/15374416.2012.704843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JF, Mufson L, Davies M. Impact of comorbid anxiety in an effectiveness study of interpersonal psychotherapy for depressed adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:904–912. doi: 10.1097/01.chi.0000222791.23927.5f. [DOI] [PubMed] [Google Scholar]