Abstract
Purpose
To compare ultrasound echo intensity (EI) to high-resolution T1-weighted MRI and to establish calibration equations to estimate percent intramuscular fat from EI.
Methods
Thirty-one participants underwent both ultrasound and MRI testing of 4 muscles: rectus femoris (RF), biceps femoris (BF), tibialis anterior (TA), and medial gastrocnemius (MG).
Results
Strong correlations were found between MRI percent fat and muscle EI after correcting for subcutaneous fat thickness (r = 0.91 in RF, r = 0.80 in BF, r = 0.80 in TA, r = 0.76 in MG). Three types of calibration equations were established.
Conclusion
Muscle ultrasound is a practical and reproducible method that can be used as an imaging technique for examination of percent intramuscular fat. Future ultrasound studies are needed to establish equations for other muscle groups to enhance its use in both research and clinical settings.
Keywords: Muscle, ultrasound, MRI, muscle EI, intramuscular fat, muscle composition
Introduction
Overweight and obesity are associated with many secondary health conditions1-3. Recent studies have suggested that adipocytes deposited in different areas of the body have different physiological activities, and the health risks of obesity are related closely to the location of fat depots in addition to the total amount of adipose tissue4,5. For example, visceral abdominal fat has been found to be related to dyslipidemia, glucose intolerance, and higher risk of cardiovascular disease6. Increased fat depots within skeletal muscle have also been found to be associated with functional decline and metabolic disorders7. These studies indicate the importance of examining the distribution of adipose tissue and deposition of ectopic fat.
Muscle ultrasound is emerging as an imaging technique for measurement of muscle quality8. Lean muscle tissue has low echogenicity, while intramuscular fat and connective tissue have high echogenicity9. This technique quantifies total muscle echo intensity (EI) using gray scale analysis with the assumption that the higher the mean pixel intensity of a muscle region of interest, the lower the muscle quality (i.e. more intramuscular fat)8,10,11. Muscle ultrasound is a low cost and easily accessible technology that can be applied in individuals who cannot undergo other imaging technologies such as MRI, computed tomography (CT), or dual-energy X-ray absorptiometry (DXA). Several studies have shown that increased EI is correlated negatively with muscle strength and cardiovascular health in people across different age groups10,11. Some studies have also established analytical methods to improve the consistency and compatibility of the ultrasound technique across different ultrasound devices12.
Ultrasound has been recognized as a valuable tool to evaluate marbling in cattle and swine with the establishment of percent intramuscular fat prediction equations13-15. Similar studies have not been conducted in humans due to the difficulty of obtaining biopsy samples. In addition, no study has compared muscle EI to MRI, an imaging technique that provides a comprehensive picture of the structure and composition of skeletal muscle. The other limitation of current ultrasound techniques is the use of arbitrary EI units as an outcome measure, which makes it difficult to compare ultrasound to other body and muscle composition techniques. Although utilizing the prediction equations established from animal subjects can be convenient, whether they are appropriate for human subjects is not known. The purpose of this study was to compare EI from the ultrasound technique with percent intramuscular fat measurements derived from high-resolution T1-weighted MRI images. The study also aimed to establish muscle-specific calibration equations to be used in humans to quantify muscle EI into percent intramuscular fat.
Materials and Methods
Participants
Thirty-one participants (14 men, 17 women) between the ages of 20 and 61 years were recruited in this study. Participants with diverse body mass index (BMI) and physical activity level were recruited to provide a wide range of body adiposity. The study was approved by the Institutional Review Board. We certify that all applicable instructional and governmental regulations concerning the ethical use of human volunteers were followed in this study. Written informed consent was obtained from all participants prior to any data collection and after a detailed description of the study was provided. Data were collected from March 2014 to April 2014.
Study Design
All participants completed 2 or 3 test sessions. The first and second sessions involved an ultrasound study and an MRI scan. The third session was an optional session to test reproducibility of the ultrasound technique. Tests on individual participants were completed within a 1-week period.
Ultrasound Experimental Protocol
An ultrasound test was performed on 4 lower extremity muscles: rectus femoris (RF), biceps femoris (BF), tibialis anterior (TA), and medial gastrocnemius (MG) using a LOGIQ e ultrasound-imaging device (GE Healthcare UK Ltd., Chalfont, Buckinghamshire, England). The dominant leg was tested. Participants were examined while resting supine on an examining table. Ultrasound Brightness mode (B-mode) with musculoskeletal scanning preset and a multi-frequency linear transducer (8-12 MHz) with 12.7 × 47.1mm footprint were used. The beam width of the transducer was approximately 2.0mm at its narrowest point. Gain and transducer frequency were adjusted to 58-dB and 8 MHz, respectively. Scanning depth was set to 4 cm with an apparent spatial resolution of 80 μm/pixel. The scanning depth was only increased when testing participants with greater subcutaneous fat to allow for capturing enough muscle area. Time gain compensation was adjusted to neutral position. Focus number and area were increased to maximum and kept consistent across all participants to adjust for differences in muscle size among participants. Other ultrasound settings were unchanged from the preset.
Before starting the ultrasound study, the upper and lower leg length of each participant was measured from the superior lateral aspect of the patella to the anterior superior iliac spine and from the inferior lateral aspect of the patella to the calcaneus, respectively. Marks were made on the anterior and posterior parts of the 1/3 and 1/4 of upper and lower leg length, measured from the patella. The purpose of the marks was to ensure that the scanning locations between ultrasound and MRI as well as between participants were consistent. A generous amount of ultrasound gel was applied to avoid excessive pressure on the skin. Each scan involved a 16-second ultrasound clip on 1 of the marks, and each muscle was scanned twice (both 1/3 and 1/4 marks). A total of 8 scans were obtained from each participant. Each ultrasound clip was reviewed, and 1 frame with the best focus was chosen and saved into a JPEG image for analysis. Muscle EI was determined by gray-scale analysis using ImageJ16. A muscle of interest was circled manually while avoiding surrounding fascia and bone. The mean voxel intensity of the selected muscle region was obtained from each measurement, and an average of 3 measurements was calculated. Subcutaneous fat thickness, muscle thickness, and area of the muscle of interest were also recorded. Images were analyzed by 2 investigators to test for the inter-rater reliability.
MRI Experimental Protocol
T1-weighted MRI images (TR = 800 ms; TE = min full) were obtained using a 3.0 Tesla whole body MR system (GE Healthcare, Waukesha, WI) at the Biomedical Health and Sciences Institute. Images were obtained with 1024 × 1024 matrix on both lower leg and upper leg of each participant. A field of view (FOV) of 18 × 18 cm (in-plane resolution of 176 × 176 μm /voxel) was set for the upper leg scans and 16 × 16 cm (in-plane resolution of 156 × 156 μm /voxel) for the lower leg scans unless changes were necessary (i.e. participants with larger size of thigh or calf). A volume knee coil was placed on the lower leg with the centerline of the coil aligned to the ultrasound marks described above. A lower leg scan involved a total of 4 imaging slices with 3.0 mm slice thickness and 10.0 mm spacing. The knee coil was then repositioned with the center line aligned with another ultrasound mark on the upper leg. An upper leg scan was done with the same settings as the lower leg scan except that the FOV and slice spacing were changed to 18 × 18 cm and 15.0 mm, respectively. The entire MRI testing procedure including positioning and the upper and lower leg scans was approximately 30 minutes per participant.
MRI images were analyzed using ImageJ16 and with a similar protocol published in a previous study17. The muscle of interest was circled, and a histogram of voxel intensity of the muscle of interest was obtained. Fat, muscle, and connective tissue were determined by visual judgment, which was based on the voxel intensity of tissues, anatomical appearance, and anatomical locations. The determined voxel intensity (DVI) of fat (DVIfat) was calculated by averaging the intensities of 3 selected areas of fat. The same procedure was followed for acquiring the DVI of muscle (DVImuscle) and connective tissue. The DVIs were then used to differentiate each tissue within the muscle of interest. To calculate the percent intramuscular fat of a muscle, a weighted percent fat (percent fatweighted) associated with each raw voxel intensity (VIraw) was first calculated using
| (Equation 1) |
Weighted fat voxel counts (FVCweighted) and weighted muscle voxel counts (MVCweighted) were then determined using equations 2 and 3:
| (Equation 2) |
| (Equation 3) |
Sums of FVCweighted and MVCweighted of a muscle region of interest (FVCweighted.ROI and MVCweighted.ROI) were then used to calculate percent intramuscular fat of the muscle using the equation 4:
| (Equation 4) |
Correcting for Subcutaneous Fat Thickness
During the initial data analysis, an independent influence of subcutaneous fat thickness on muscle EI was observed. To further examine this potential influence, the subcutaneous fat thickness of 5 participants was reduced by applying 4 different levels of external pressure on the skin. Care was taken to ensure minimal change in muscle shape. The associations of EI and different level of subcutaneous fat thickness were compared (Figure 1). The averages of the 5 slopes and y-intercepts were calculated. A correction factor was generated from the following equation, where cf = correction factor and x = subcutaneous fat thickness. The cf represents the addition of EI with every 1.0cm subcutaneous fat thickness:
| (Equation 5) |
The cf was then applied to correct for the potential influence of subcutaneous fat on EI using equation 6, where y1 = raw EI, x = subcutaneous fat thickness, cf = 40.5278, and y2 = corrected EI,
| (Equation 6) |
Figure 1.
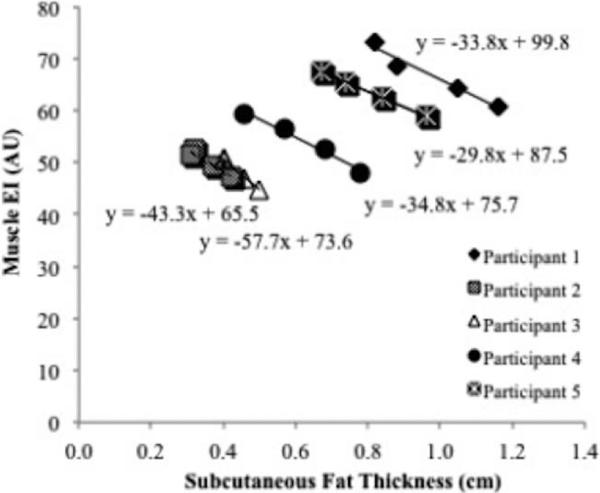
Correlations between subcutaneous fat thickness and muscle echo intensity (EI). The regression equations were averaged and used to establish a correction factor for subcutaneous fat thickness.
Generation of Calibration Equations
Correlations between muscle EI and MRI percent fat were obtained, and the linear regression equations were used to generate calibration equations. Different calibration equations were established by combining all the muscle groups (group equation) and by examining separately based on muscle groups (muscle-specific equation) and gender (gender-specific equation). The ultrasound percent fat calculated with each equation was compared.
Statistical Analysis
Data are presented as means ± SD. Correlation between muscle EI- and MRI- measured percent fat of each muscle was analyzed using Pearson correlation. To examine the influence of subcutaneous fat thickness on muscle EI and to evaluate the use of the correction factor, the correlations between MRI percent fat and muscle EI were compared using 3 approaches: 1) simple linear correlation between MRI percent fat and raw EI, 2) simple linear correlation between MRI percent fat and corrected EI, and 3) multiple regression analysis between MRI percent fat (dependent variable), raw EI, and subcutaneous fat thickness (independent variables). Ultrasound test-retest and inter-rater reliabilities were analyzed using coefficient of variation (CV) and 2-way random intraclass correlation coefficients (ICC) with absolute agreement. CV of the EI of the 2 ultrasound scanning locations and 3 MRI slices were also computed. Statistical analyses were performed using SPSS 19.0 (IBM®, Armonk, NY). Significance was accepted when P < 0.05.
Results
Study Participants
All participants completed the study without any adverse events. The physical characteristics of the participants are summarized in Table 1.
Table 1.
Physical characteristics of participants
| Men (n = 14) | Women (n = 17) | |
|---|---|---|
| Age, yrs | 27.9 ± 14.9 (20-64) | 21.9 ± 2.5 (20-29) |
| Height, cm | 181.3 ± 7.8 (170-198.1) | 165.3 ± 5.5 (157.5-175) |
| Weight, kg | 77.6 ± 11.1 (62.6-95.8) | 63.7 ± 9.3 (46.8-90.3) |
| BMI, kg/m2 | 23.4 ± 3.5 (19.4-29.4) | 23.6 ± 2.6 (18.9-33.1) |
Values are expressed as mean ± SD (range). BMI = body mass index
Ultrasound and MRI Results
Representative muscle images obtained from both MR and ultrasound are shown in Figure 2. Both MRI and ultrasound images were acquired from the same location of each muscle group so that comparisons between muscle EI and MRI-measured percent intramuscular fat could be made. Examined muscle area and muscle EI of each muscle group are shown in Table 2. It should be noted that the entire areas of BF, TA, and MG could not be examined fully due to the size limitation of the ultrasound window.
Figure 2.
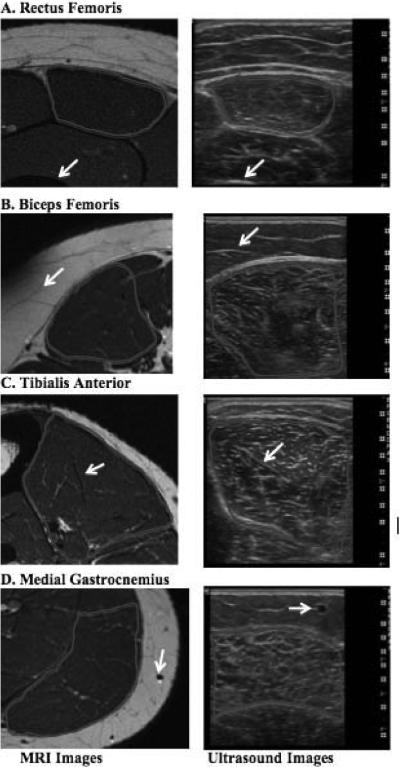
Representative T1-weighted MRI (left column) and ultrasound B-mode images (right column) demonstrate the muscle site comparability between the 2 imaging techniques. White arrows indicate the same anatomical features in the two images.
Table 2.
Outcomes of ultrasound and MRI testing
| Rectus Femoris (n= 28) | Biceps Femoris (n= 27) | Tibialis Anterior (n= 27) | Medial Gastrocnemius (n=26) | |
|---|---|---|---|---|
| Ultrasound | ||||
| Examined Muscle Area, cm2 | 4.8 ± 1.4 | 13.2 ± 2.1 | 10.1 ± 1.5 | 8.8 ± 1.6 |
| Echo intensity, AU | 55.1 ± 7.4 | 42.6 ± 7.3 | 56.1 ± 8.0 | 51.5 ± 8.5 |
| (37.0-66.3) | (23.1-56.9) | (32.8-68.8) | (32.2-65.9) | |
| MRI | ||||
| Examined Muscle Area, cm2 | 4.3 ± 1.5 | 16.0 ± 3.6 | 15.9 ± 2.9 | 16.9 ± 4.8 |
| Examined Muscle Volume, cm3 | 1.3 ± 0.5 | 4.8 ± 1.1 | 4.8 ± 0.9 | 5.1 ± 1.4 |
| Intramuscular fat, percent | 13.1 ± 2.5 | 15.6 ± 4.6 | 14.7 ± 4.3 | 14.6 ± 4.8 |
| (7.8-16.3) | (7.5-21.7) | (8.5-25.3) | (7.9-30.1) |
Values are expressed as mean ± SD (range). AU = arbitrary unit. MRI = magnetic resonance imaging.
Uncorrected Echo Intensity vs. MRI-measured percent Intramuscular Fat
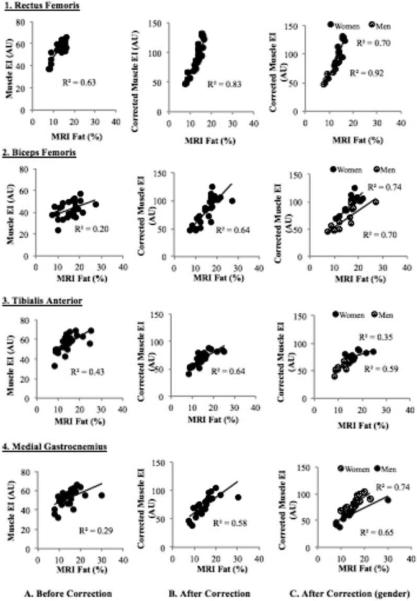
Comparisons between the muscle EI without correction for subcutaneous fat thickness and percent intramuscular fat measured with MRI are shown in Figures 3A1-A4. Moderate to strong correlations were found between muscle EI and MRI percent fat when examining RF (r = 0.79) and TA (r = 0.66). Weak to moderate correlations were found in BF (r = 0.45) and MG (r = 0.54).
Figure 3.
Correlations between MRI-measured percent intramuscular fat and muscle echo intensity (EI) in the 4 muscles (A1-A4). Correlations between MRI-measured percent intramuscular fat and muscle echo intensity (EI) after correcting for subcutaneous fat thickness in the 4 muscles (B1-B4). Correlations between MRI-measured percent intramuscular fat and muscle echo intensity (EI) after correcting for subcutaneous fat thickness in the 4 muscles, separated by gender (C1-C4).
Corrected Echo Intensity vs. MRI-measured percent Intramuscular Fat
Figure 3B1-B4 shows the correlations between MRI percent intramuscular fat and muscle EI after corrected for subcutaneous fat thickness. Stronger correlations were found in all muscle groups when the correction factor was applied (r = 0.91 in RF, r = 0.80 in BF, r = 0.80 in TA, r = 0.76 in MG). When examining the correlations separately by gender (Figure 3C1-C4), similar and stronger correlations were found in all muscles in men (r = 0.96 in RF, r = 0.86 in BF, r = 0.77 in TA, r = 0.86 in MG) when compared to women (r = 0.84 in RF, r = 0.84 in BF, r = 0.59 in TA, r = 0.81 in MG). A moderate to strong correlation (r = 0.61) was found between corrected muscle EI and MRI percent intramuscular fat after combining all the examined muscles (Figure 4).
Figure 4.
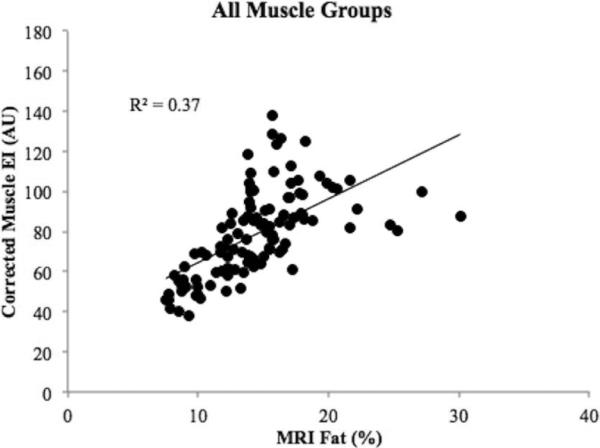
A correlation graph between MRI-measured percent intramuscular fat and corrected muscle echo intensity (EI) of all four muscles.
Examining the Influence of Subcutaneous Fat Thickness on Muscle Echo Intensity
Table 3 summarizes the relationship between MRI percent fat and muscle EI. The correlations between MRI percent fat and muscle EI improved after accounting for subcutaneous fat thickness using both the multiple regression analysis and application of the correction factor. The multiple regression analysis and the correction factor provided similar correlations between MRI percent fat and muscle EI (multiple regression analysis (r) vs. correction factor (r): 0.90 vs. 0.92 in RF; 0.80 vs. 0.71 in BF; 0.80 vs. 0.80 in TA; 0.76 vs. 0.73 in MG).
Table 3.
The Influence of Subcutaneous Fat Thickness on Muscle EI
| Simple linear correlation | Multiple Regression | ||
|---|---|---|---|
| Raw EI | Corrected EI | Independent variables: Raw EI & fat thickness Dependent variable: MRI percent fat | |
| MRI percent Fat vs. Muscle EI | |||
| Rectus Femoris | 0.79 | 0.90 | 0.92 |
| Biceps Femoris | 0.45 | 0.80 | 0.71 |
| Tibialis Anterior | 0.66 | 0.80 | 0.80 |
| Medial Gastrocnemius | 0.54 | 0.76 | 0.73 |
Values are correlation coefficients (r). EI = echo intensity; MRI = magnetic resonance imaging; Raw EI = echo intensity before correcting for subcutaneous fat thickness; Corrected EI = echo intensity after the correction factor was applied.
Calibration Equations
Three types of calibration equations were developed, and all the equations are presented in Table 4. The 95 % CI of the slope for muscle specific equations was 0.077 to 0.112 for RF, 0.107 to 0.202 for BF, 0.195 to 0.366 for TA, and 0.147 to 0.2221 for MG. For gender specific equations, the 95% CI of the slope was 0.038 to 0.085 in women and 0.111 to 0.177 in men for RF, 0.095 to 0.208 in women and 0.048 to 0.256 in men for BF, 0.046 to 0.454 in women and 0.055 to 0.340 in men for TA, and 0.134 to 0.344 in women and 0.120 to 0.261in men for MG.
Table 4.
Calibration Equations
| Rectus Femoris | Biceps Femoris | Tibialis Anterior | Medial Gastrocnemius | |
|---|---|---|---|---|
| Muscle specific | y = [0.093 * (40 * z) + x]+4.698 | y = [0.143 * (40 * z) + x]+3.459 | y = [0.256 * (40 * z) + x]-2.991 | y = [0.184 * (40 * z) + x]+0.673 |
| Gender specific (women) | y = [0.062 * (40 * z) + x]+7.901 | y = [0.177 * (40 * z) + x]+1.823 | y = [0.250 * (40 * z) + x]-2.366 | y = [0.239 * (40 * z) + x]+4.221 |
| Gender specific (men) | y = [0.144 * (40 * z) + x]+1.126 | y = [0.152 * (40 * z) + x]+2.368 | y = [0.198 * (40 * z) + x]+0.094 | y = [0.198 * (40 * z) + x]+0.384 |
| Group (all muscles) | y = [0.114 * (40 * z) + x] + 5.231 | |||
x = raw echo intensity; y = percent intramuscular fat; z = subcutaneous fat thickness.
Comparisons Between Different Equations
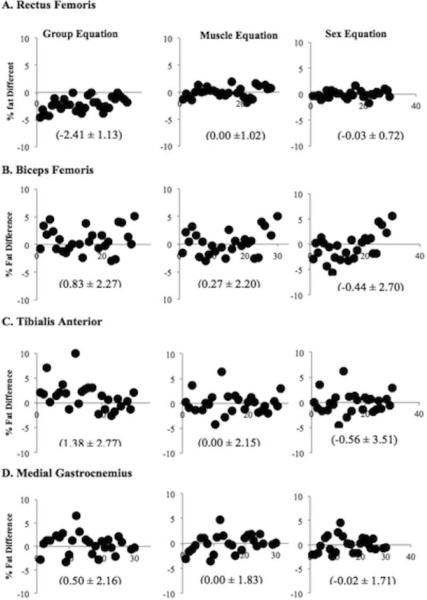
Relationships between calibrated ultrasound percent fat and MRI percent intramuscular fat were examined. Figure 5A-D shows the difference between MRI percent intramuscular fat and ultrasound percent fat calculated using each equation. When converting muscle EI into percent fat using the group equation, the mean and standard deviation of percent fat difference were larger across all muscle groups (-2.41 ± 1.13 in RF; 0.83 ± 2.27 in BF; 1.38 ± 2.77 in TA; 0.50 ± 2.16 in MG) when compared to that using muscle specific (0.00 ± 1.02 in RF; 0.27 ± 2.20 in BF; 0.00 ± 2.15 in TA; 0.00 ± 1.83 in MG) and gender specific equations (-0.03 ± 0.72 in RF; -0.44 ± 2.70 in BF; -0.56 ± 3.51 in TA; -0.02 ± 1.71 in MG).
Figure 5.
Differences between MRI-measured percent fat and ultrasound percent fat calculated using the three types of calibration equations.
Within Muscle Variability
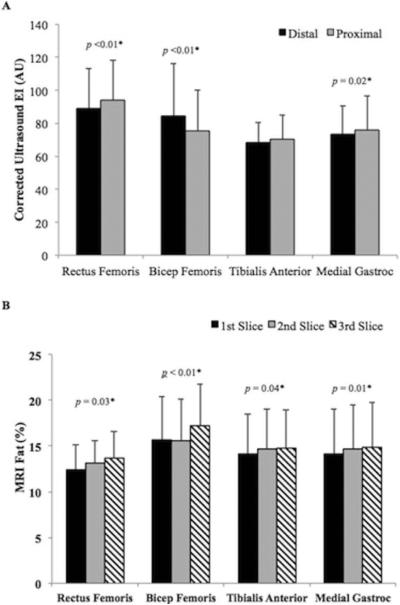
Muscle EIs between the 2 scanning locations were compared. The mean CV between the 2 locations was 5.6% in RF, 6.3% in BF, 5.0% in TA, and 4.8% in MG. After correcting for subcutaneous fat thickness, the mean coefficient of variation was 5.7% in RF, 8.7% in BF, 4.9% in TA, and 5.2% in MG (Figure 6A). Percent intramuscular fat differences between the 3 MRI slices were also compared (Figure 6B). The mean CV between the 3 MRI slices was 11.0 % in RF, 7.6 % in BF, 5.6 % in TA, and 5.1% in MG.
Figure 6.
Two representative graphs showing variability of muscle EIs between two different scanning sites within a muscle (A) as well as variability of percent intramuscular fat between the three MRI slices (B).
Ultrasound Reproducibility and Inter-Analyzer Reliability
The reproducibility of the ultrasound technique was examined by repeating the same testing procedure on 10 participants on 2 different days within a week. The ultrasound technique demonstrated high reproducibility between the 2 testing days across all muscle groups. Results are reported as mean CV & ICC (95% CI): RF = 3.3 ± 3.0 & 0.91 (0.64-0.98); BF = 13.1 ± 8.7 & 0.72 (-0.28-0.93); TA = 2.6 ± 1.6 & 0.92 (0.57-0.97); MG = 5.6 ± 4.9 & 0.71 (0.12-0.95). The inter-analyzer reliability of the ultrasound technique was tested on the ultrasound images of 23 participants. A high inter-analyzer reliability was observed in all muscle groups. Results are reported as mean CV & ICC (95% CI): RF = 4.3 ± 2.6 & 0.93 (0.66-0.98); BF = 4.5 ± 2.7 & 0.96 (-0.88-0.99); TA = 3.5 ± 2.2 & 0.98 (0.89-0.99); MG = 3.7 ± 3.1 & 0.95 (0.28-0.99).
Discussion
We have generated calibration equations to quantify muscle EI into percent intramuscular fat on 4 muscles in the lower extremity. In this study, moderate to strong correlations were found between MRI-measured percent intramuscular fat and muscle EI. This is consistent with previous literature that compared muscle EI to percent intramuscular fat measured with muscle biopsy samples18,19. Reimers et al. examined muscle echogenicity and biopsy samples of 86 uninjured muscles and concluded that the increased muscle EI was mainly caused by elevated intramuscular lipid content19. Previous studies have also reported the associations of higher muscle EI with reduced muscle strength, neuromuscular diseases, and lower cardiovascular performance10,11,20-22. While muscle EI has provided valuable clinical information, the arbitrary EI units make comparisons between the ultrasound technique and other body and muscle composition techniques difficult and thus limit its use as an alternative technique to examine muscle composition. The calibration equations established in this study can help address this limitation. It is important to emphasize that the applicability of the calibration equations will be reduced if a different ultrasound device is used. This is due to different manufacturer settings which make muscle EI variable among ultrasound devices. Care should be taken when applying the equations generated from these data.
We also observed an independent influence of subcutaneous fat thickness on muscle EI, since all the settings were kept consistent, and we were able to develop a correction factor to correct for the potential influence. This phenomenon has been reported previously12,23. As Wattjes et al. pointed out in their review article, reflection or absorption of ultrasound sound waves made visualization of deeper tissues difficult, which limits its application to examining superficial muscle groups23. In our study, a correction factor was established after examining the associations of EIs and subcutaneous fat thickness altered by applying different levels of pressure on the skin. After the correction factor was applied to raw EIs, the correlations between muscle EI and MRI percent intramuscular fat improved. Similar correlations were found when comparing the relationship between MRI percent fat, raw muscle EI, and subcutaneous fat thickness using multiple regression analysis, which further supports the use of the correction factor. It should be noted that we developed 1 correction factor and applied it to all muscle groups. While this is one way to correct for the influence of subcutaneous fat thickness on muscle EI, it remains possible that different muscle groups require specific correction factors. This is due to the variability in muscle composition (i.e. amount of connective tissues) and depth of individual muscle groups. Future studies are needed to further examine the applicability of the correction factor.
Better correlations were observed when we compared MRI percent fat to corrected muscle EI of each muscle group than when we compared MRI percent fat to corrected muscle EI of all muscle groups. This finding is consistent with previous studies that suggested variability in EI across different muscle groups8,24. Pillen & van Alfen suggested the differences in fibrous tissue distribution and muscle fiber orientation of each muscle group, which resulted in a muscle's unique range of EI8. In addition, a better relationship was found between MRI percent intramuscular fat and corrected muscle EI of each muscle group within men compared to women. Arts et al. reported that the relationship between age and EI was gender- and muscle-dependent24, and our results agree with their findings.
In this study, we report 3 types of calibration equations: group equation, muscle-specific equation, and gender-specific equation. A better relationship was observed when we compared MRI percent fat and ultrasound percent fat calculated using the muscle-specific equation to that calculated using the group equation. This again supports the notion that the normal range of EI is muscle specific. Muscle-specific and gender-specific equations were compared, and similar correlations were found between MRI percent intramuscular fat and ultrasound percent fat calculated using the 2 equations, suggesting both equations can be used in the future. Furthermore, a better relationship between MRI percent fat and ultrasound percent fat was observed in RF. A possible explanation for this is the limitation of the ultrasound window. As previously mentioned, the ultrasound window captured the entire area of RF but not the other muscles, which could result in potential measurement error. Future studies can address this issue by investigating the relationship between MRI percent fat and muscle EI using ultrasound probes with wider FOV, e.g. probes that are used to scan bigger muscle groups in adult full sized cattle, or by combining ultrasound scans of different sites of a muscle to obtain a more representative image of a muscle.
We investigated the percent intramuscular fat measured with MRI in 4 different muscle groups: RF, BF, TA, and MG. We found a range of approximately 13.0 to 16.0% mean intramuscular fat from all muscle groups. These results are comparable to percent intramuscular fat reported by other studies using different imaging techniques. Kovanlikaya et al. examined the relationships between insulin levels and fat accumulation in the soleus muscle, liver, and pancreas using the three-point Dixon MRI technique in 15 young, healthy Mexican-American women and reported an average intramuscular fat of approximately 15% in the lean group and 23% in the obese group, classified based on BMI25. Wren et al.26 investigated percent intramuscular fat of several muscle groups in 9 boys with Duchene muscular dystrophy and found that the percent intramuscular fat was highly correlated with each muscle group (r = 0.83 to 0.98). Overall, the levels of intramuscular fat we found are consistent with results reported in previous studies.
We also demonstrated the high reproducibility and inter-rater reliability of the ultrasound technique. We found a strong ICC between days 1 and 2 in all muscle groups. This finding is comparable to the study conducted by Reimers et al. in which they found a test-retest correlation coefficient of 0.94 for EI in calf muscle27. A higher CV was found in BF (CV = 13.1 percent) between the 2 different days. A possible explanation is the difficulty to locate the same ultrasound scanning area of BF. Ultrasound has a limited FOV, which makes it difficult to capture the entire muscle area of larger muscle groups transversely. In addition, we found significant variability of muscle EIs between 2 locations of each muscle group as well as percent intramuscular fat between 3 different MRI slices (Figure 6A & B), which indicate the importance of ensuring the consistency of scanning locations. As Scholten et al. pointed out in their study, measuring the exact muscle site was necessary to obtain comparable and reliable results across individuals28. In this study, although care was taken to ensure the same measurement sites were scanned across different days and participants, the potential discrepancy can contribute to the larger CV observed in BF. Future studies will need to ensure scanning site consistency and consider the variability between different sites within a muscle to accurately interpret muscle composition.
We also found a larger variability in participants with higher percent intramuscular fat (above 15percent) measured by MRI. We have some possible explanations for this observation. As Pillen et al. reported, attenuation of the ultrasound beam occurs when the sound wave encounters different tissues such as muscle, connective tissue, and adipose tissue8. Our hypothesis is that when the amount of intramuscular fat reaches approximately 15%, it may begin to affect the reflection and absorption of sound non-systematically based on the differing distribution patterns of intramuscular fat and connective tissue. This produces an underestimation of the actual EI of a muscle. In addition, we examined the association between muscle EI and percent intramuscular fat without separating the potential influence of connective tissue. Previous studies have suggested a strong correlation between muscle EI and interstitial fibrous tissue measured with biopsy samples in animal models29,30. While a smaller amount of connective tissue was assumed in the relatively young and healthy participants in this study, any potential effect of connective tissue on EI is unknown. Future studies are needed to establish methods such as texture analysis to identify different tissues within a muscle using ultrasound. The second explanation is the potential limitation of the current MRI percent intramuscular fat analysis. We relied on manually-determined PIs to differentiate pure muscle, pure fat, and connective tissue and calculated percent intramuscular fat based on DPIs of the 3 tissues17. When a muscle contains a high amount of adipose tissue, identification of pure muscle is difficult. As a result, underestimation of intramuscular fat can occur. Further investigations that use better analytical methods such as the Dixon MRI technique are needed to validate the current manually-determined PI method25.
Our study has some limitations. First, as mentioned previously, the potential influence of connective tissue on muscle EI was not addressed in this study. Future studies with a combination of imaging techniques and, potentially, muscle biopsies are needed to further examine the role of connective tissue on muscle EI. The second limitation of the study is the number of muscles examined. In this study we only examined 4 muscle groups. While this study shows the possibility of estimating percent intramuscular fat from muscle EI, future studies are required to establish calibration equations for other muscle groups. Another limitation is that the calibration equations established in this study can only be applied to muscle EI obtained with the specific ultrasound device and settings we used. A potential solution for this is to establish correction factors or analytical techniques to convert muscle EI among ultrasound devices, as reported by previous studies12,31. Another possible solution is to develop a phantom with a range of percent fat to calibrate different ultrasound devices. Nevertheless, future studies are needed to improve the compatibility between ultrasound devices to enhance the practicality of the ultrasound technique.
In conclusion, we established calibration equations to quantify muscle EI into percent intramuscular fat after assessing 4 different lower extremity muscles with high-resolution T1-weighted MRI and ultrasound. A correction factor for subcutaneous fat thickness was developed to correct for its potential influence on muscle EI. Future studies are required to test the validity and reliability of the calibration equations. In addition, variability of muscle EIs and percent intramuscular fat between different ultrasound scanning sites and MRI slices was found, suggesting the need to examine multiple sites of a muscle to obtain a comprehensive composition of a muscle. Muscle ultrasound is a low cost, easily accessible, and highly reproducible method that can be an option for evaluating skeletal muscle health. More studies are required to enhance the utility of the ultrasound technique in both research and clinical settings.
Acknowledgements
This study was funded in part by National Institutes of Health Grant (HD-039676). The authors would like to thank Kim Mason at the Bio-imaging Research Center for her assistance in MRI data collection, the graduate research assistants in the Exercise Muscle Physiology Laboratory for their assistant and support, and the participants for their enthusiastic commitment to this study.
Abbreviations
- EI
Echo Intensity
- RF
Rectus Femoris
- BF
Biceps Femoris
- TA
Tibialis Anterior
- MG
Medial Gastrocnemius
- CT
Computed Tomography
- DXA
Dual-energy X-ray Absorptiometry (DXA)
- FOV
Field of View
- DVI
Determined Voxel Intensity
- DVIfat
Determined Voxel Intensity of Pure Fat
- DVImuscle
Determined Voxel Intensity of Pure Muscle
- percent fatweighedt
Weighted percent fat
- VIraw
Raw Voxel Intensity
- FVCweighted
Weighted Fat Voxel Counts
- MVCweighted
Weighted Muscle Voxel Counts
- FVCweighted.ROI
Sum of FVCweighted in the Muscle Region of Interest
- MVCweighted.ROI
Sum of MVCweighted in the Muscle Region of Interest
- cf
Correction Factor
- CV
Coefficient of Variation
- ICC
Intraclass Correlation Coefficients
- SD
Standard Deviation
Footnotes
Financial Disclosure
No author has a financial or proprietary interest in any material or method mentioned
Conflict of interest statement
The authors report no conflicts of interest
References
- 1.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease From the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006 Feb 14;113(6):898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. 2006. [DOI] [PubMed] [Google Scholar]
- 2.Goya Wannamethee S, Gerald Shaper A, Whincup PH, Walker M. Overweight and obesity and the burden of disease and disability in elderly men. International Journal Of Obesity And Related Metabolic Disorders: Journal Of The International Association For The Study Of Obesity. 2004;28(11):1374–1382. doi: 10.1038/sj.ijo.0802775. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM. Obesity, metabolic syndrome, and cardiovascular disease. Journal of Clinical Endocrinology & Metabolism. 2004 Jun 1;89(6):2595–2600. doi: 10.1210/jc.2004-0372. 2004. [DOI] [PubMed] [Google Scholar]
- 4.Hermsdorff HH, Monteiro JB. [Visceral, subcutaneous or intramuscular fat: where is the problem?]. Arquivos brasileiros de endocrinologia e metabologia. 2004;48(6):803–811. doi: 10.1590/s0004-27302004000600005. [DOI] [PubMed] [Google Scholar]
- 5.Goodpaster BH, Krishnaswami S, Harris TB, Katsiaras A, Kritchevsky SB, Simonsick EM, et al. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Archives of Internal Medicine. 2005;165(7):777–783. doi: 10.1001/archinte.165.7.777. [DOI] [PubMed] [Google Scholar]
- 6.Pillen S, van Keimpema M, Nievelstein RA, Verrips A, van Kruijsbergen- Raijmann W, Zwarts MJ. Skeletal muscle ultrasonography: Visual versus quantitative evaluation. Ultrasound in medicine & biology. 2006;32(9):1315–1321. doi: 10.1016/j.ultrasmedbio.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 7.Shaw CS, Clark J, Wagenmakers AJ. The effect of exercise and nutrition on intramuscular fat metabolism and insulin sensitivity. Annual Review of Nutrition. 2010;30(1):13–34. doi: 10.1146/annurev.nutr.012809.104817. [DOI] [PubMed] [Google Scholar]
- 8.Pillen S, van Alfen N. Skeletal muscle ultrasound. Neurological Research. 2011;33(10):1016–1024. doi: 10.1179/1743132811Y.0000000010. [DOI] [PubMed] [Google Scholar]
- 9.Mayans D, Cartwright MS, Walker FO. Neuromuscular ultrasonography: quantifying muscle and nerve measurements. Physical Medicine and Rehabilitation Clinics of North America. 2012;23(1):133–148. doi: 10.1016/j.pmr.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukumoto Y, Ikezoe T, Yamada Y, Tsukagoshi R, Nakamura M, Kimura M, et al. Skeletal muscle quality assessed from echo intensity is associated with muscle strength of middle-aged and elderly persons. Eur J Appl Physiol. 2012;112(4):1519–1525. doi: 10.1007/s00421-011-2099-5. [DOI] [PubMed] [Google Scholar]
- 11.Cadore EL, Izquierdo M, Conceicao M, Radaelli R, Pinto RS, Baroni BM, et al. Echo intensity is associated with skeletal muscle power and cardiovascular performance in elderly men. Experimental Gerontology. 2012;47(6):473–478. doi: 10.1016/j.exger.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Pillen S, van Dijk JP, Weijers G, Raijmann W, de Korte CL, Zwarts MJ. Quantitative gray-scale analysis in skeletal muscle ultrasound: A comparison study of two ultrasound devices. Muscle & Nerve. 2009;39(6):781–786. doi: 10.1002/mus.21285. [DOI] [PubMed] [Google Scholar]
- 13.Newcom DW, Baas TJ, Lampe JF. Prediction of intramuscular fat percentage in live swine using real-time ultrasound. Journal of Animal Science. 2002 Dec 1;80(12):3046–3052. doi: 10.2527/2002.80123046x. 2002. [DOI] [PubMed] [Google Scholar]
- 14.Hassen A, Wilson DE, Amin VR, Rouse GH, Hays CL. Predicting percentage of intramuscular fat using two types of real-time ultrasound equipment. Journal of Animal Science. 2001 Jan 1;79(1):11–18. doi: 10.2527/2001.79111x. 2001. [DOI] [PubMed] [Google Scholar]
- 15.Parish JA, Rhinehart JD, Vann RC. Ultrasound Scanning Beef Cattle for Body Composition. Mississippi State University Extension Service; Starkville, MS: 2011. [Google Scholar]
- 16.ImageJ [computer program] U.S. National Institutes of Health; Bethesda, Maryland, USA: 1197-2014. [Google Scholar]
- 17.Ryan TE, Brizendine JT, Backus D, McCully KK. Electrically induced resistance training in individuals with motor complete spinal cord injury. Archives of Physical Medicine and Rehabilitation. 2013;94(11):2166–2173. doi: 10.1016/j.apmr.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 18.Reimers CD, Fleckenstein JL, Witt TN, Muller-Felber W, Pongratz DE. Muscular ultrasound in idiopathic inflammatory myopathies of adults. Journal of the neurological sciences. 1993;116(1):82–92. doi: 10.1016/0022-510x(93)90093-e. [DOI] [PubMed] [Google Scholar]
- 19.Reimers K, Reimers CD, Wagner S, Paetzke I, Pongratz DE. Skeletal muscle sonography: a correlative study of echogenicity and morphology. Journal of Ultrasound in Medicine. 1993 Feb 1;12(2):73–77. doi: 10.7863/jum.1993.12.2.73. 1993. [DOI] [PubMed] [Google Scholar]
- 20.Heckmatt J, Dubowitz V, Leeman S. Detection Of pathological change in dystrophic muscle with B-scan ultrasound imaging. The Lancet. 1980;315(8183):1389–1390. doi: 10.1016/s0140-6736(80)92656-2. [DOI] [PubMed] [Google Scholar]
- 21.Pillen S, Arts IM, Zwarts MJ. Muscle ultrasound in neuromuscular disorders. Muscle & Nerve. 2008;37(6):679–693. doi: 10.1002/mus.21015. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe Y, Yamada Y, Fukumoto Y, Ishihara T, Yokoyama K, Yoshida T, et al. Echo intensity obtained from ultrasounography images reflecting muscle strength in elderly men. Clinical Interventions in Aging. 2013;8:993–998. doi: 10.2147/CIA.S47263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wattjes M, Kley R, Fischer D. Neuromuscular imaging in inherited muscle diseases. Eur Radiol. 2010;20(10):2447–2460. doi: 10.1007/s00330-010-1799-2. 2010/10/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arts IM, Pillen S, Schelhaas HJ, Overeem S, Zwarts MJ. Normal values for quantitative muscle ultrasonography in adults. Muscle & Nerve. 2010;41(1):32–41. doi: 10.1002/mus.21458. [DOI] [PubMed] [Google Scholar]
- 25.Kovanlikaya A, Mittelman S, Ward A, Geffner M, Dorey F, Gilsanz V. Obesity and fat quantification in lean tissues using three-point Dixon MR imaging. Pediatr Radiol. 2005;35(6):601–607. doi: 10.1007/s00247-005-1413-y. 2005/06/01. [DOI] [PubMed] [Google Scholar]
- 26.Wren TA, Bluml S, Tseng-Ong L, Gilsanz V. Three-point technique of fat quantification of muscle tissue as a marker of disease progression in Duchenne muscular dystrophy: preliminary study. American Journal of Roentgenology. 2008;190(1):W8–W12. doi: 10.2214/AJR.07.2732. 2008/01/01. [DOI] [PubMed] [Google Scholar]
- 27.Reimers CD, Schlotter B, Eicke BM, Witt TN. Calf enlargement in neuromuscular diseases: a quantitative ultrasound study in 350 patients and review of the literature. Journal of the neurological sciences. 1996;143(1):46–56. doi: 10.1016/s0022-510x(96)00037-8. [DOI] [PubMed] [Google Scholar]
- 28.Scholten RR, Pillen S, Verrips A, Zwarts MJ. Quantitative ultrasonography of skeletal muscles in children: Normal values. Muscle & Nerve. 2003;27(6):693–698. doi: 10.1002/mus.10384. [DOI] [PubMed] [Google Scholar]
- 29.Pillen S, Tak RO, Zwarts MJ, Lammens MM, Verrijp KN, Arts IM, et al. Skeletal muscle ultrasound: correlation between fibrous tissue and echo intensity. Ultrasound in medicine & biology. 2009;35(3):443–446. doi: 10.1016/j.ultrasmedbio.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 30.Hu CF, Chen CP, Tsai WC, Hu LL, Hsu CC, Tseng ST, et al. Quantification of skeletal muscle fibrosis at different healing stages using sonography: a morphologic and histologic study in an animal model. Journal of Ultrasound in Medicine. 2012 Jan 1;31(1):43–48. doi: 10.7863/jum.2012.31.1.43. 2012. [DOI] [PubMed] [Google Scholar]
- 31.Zaidman CM, Holland MR, Anderson CC, Pestronk A. Calibrated quantitative ultrasound imaging of skeletal muscle using backscatter analysis. Muscle & Nerve. 2008;38(1):893–898. doi: 10.1002/mus.21052. [DOI] [PubMed] [Google Scholar]