Abstract
Objective
To determine if quantitative ultrashort TE (UTE) T1ρ magnetic resonance (MR) measurements are sensitive to proteoglycan (PG) degradation in human menisci by trypsin digestion.
Methods
Conventional and quantitative UTE-T1ρ MR sequences were performed on four meniscal samples using a 3T scanner. MR imaging was performed before and after 4, 8, and 12 hours of trypsin solution immersion, inducing PG loss. One sample was utilized as a control. Digest solutions were analyzed for glycosaminoglycan (GAG) content. UTE-T1ρ studies were analyzed for quantitative changes.
Results
Images showed progressive tissue swelling, fiber disorganization and increase in signal intensity after GAG depletion. UTE-T1ρ values tended to increase with time after trypsin treatment (p=0.06). Cumulative GAG loss into the bath showed a trend of increased values for trypsin-treated samples (p=0.1).
Conclusion
UTE-T1ρ measurements can non-invasively detect and quantify severity of meniscal degeneration, which has been correlated with progression of osteoarthritis.
Keywords: MRI, T1ρ, meniscus, osteoarthritis, cartilage
Introduction
The progression of osteoarthritis (OA) is now viewed as a whole-joint process that can result from abnormalities in articular cartilage, subchondral bone, synovium, capsule, and meniscus 1. Located between the femoral condyles and tibial plateaus, the menisci play important roles in load transmission, shock absorption, and maintenance of joint stability 2. Mechanical impairment of the meniscus alters the weight-bearing capacities of the knee joint, leading to damage to the adjacent articular cartilage and subchondral bone, eventually contributing to the progression of OA 3–7.
Both articular cartilage (hyaline cartilage) and meniscus (fibrocartilage) consist of a macromolecular framework of collagen fibers, proteoglycan (PG) and water. The onset of OA is primarily associated with biochemical alterations, and in particular, the loss of PG from the cartilage extracellular matrix has been hypothesized to be the initiating event 8, 9. Changes in water content and collagen structure subsequently occur and precede the onset of OA symptoms.
Knowledge of early biochemical changes may allow for targeted therapy before structural and irreversible damage occur. Magnetic resonance (MR) imaging has been established as a sensitive method for detecting structural and functional changes in the early stages of OA, as well as a noninvasive tool for assessing the progression of disease and therapeutic monitoring. Quantitative techniques that have been used include T1ρ and delayed gadolinium enhanced MRI of cartilage (dGEMRIC) for the evaluation of PG content and T2/T2* for the evaluation of water content and collagen integrity.
T1ρ, also known as spin-lattice relaxation in the rotating frame, is the time constant that defines the magnetic relaxation of spins under the influence of a radiofrequency field 10, 11. T1ρ is related to the energy changes between proton spins and the environment and can be used to evaluate the slow-motion interactions between motion-restricted water molecules and their local macromolecular environment 12. The extracellular matrix in the cartilage provides a motion-restricted environment to water molecules. Disruption of this matrix and loss of PG content can lead to an increase in water molecule motion and a corresponding increase in T1ρ values 13, 14.
The menisci of the knee are composed of fibrocartilage with a highly structured matrix, and therefore exhibits a very short mean T2/T2* relaxation time. With ultrashort echo time (UTE) MR imaging sequences, signal can be detected from structures with predominantly short T2/T2* 15. These sequences are now becoming available on clinical MR scanners, along with techniques that provide higher contrast and spatial resolution 15.
The objectives of this study were to determine if quantitative UTE-T1ρ MR properties are sensitive to PG degradation, incurred in human menisci by trypsin digestion, and to discuss the implications in detecting early OA.
Materials and Methods
Specimen Preparation
The Institutional Review Board exempted this anonymized cadaveric study. Three unembalmed, fresh-frozen cadaveric human knees (2 male, 1 female; 74 ± 14.6 years, mean ± standard deviation) were obtained. Radiography was performed on all knees to exclude abnormalities from previous trauma, surgery, or severe osteoarthritis. The knees were stored in an ultralow freezer at −40°C (Forma Bio-Freezer; Forma Scientific, Marietta, Ohio) and subsequently sectioned with a band saw into 3 mm thick sagittal sections. In total, four meniscal pieces were used for this study (two anterior horn and two posterior horn pieces). Before initial MR imaging, the specimens were allowed to thaw at room temperature for 15 minutes and weighed wet. In total, specimens underwent two freeze-thaw cycles. For MR imaging the specimens were secured inside a syringe filled with perfluorooctyl bromide (PFOB; Synquest Labs, Alachua, FL) to prevent dehydration and reduce susceptibility effects 16.
MR Imaging Protocol
MR imaging was performed with a 3T clinical MR scanner (Signa HDx; GE Healthcare, Milwaukee, WI) with the maximum peak gradient amplitude of 40 mT/m and slew rate of 150 mT/m/sec. Hardware modification included an addition of a custom transmit-receive switch to the receiver preamplifiers for rapid switching after the end of a radiofrequency excitation pulse 17. This modification allowed detection of signal as early as 8 μs after the end of the radiofrequency pulse, which is much shorter than the TE values typically achievable with conventional imaging.
The syringe containing the specimen was placed inside a 2 cm diameter transmit-receive solenoid coil (ProbeTek, San Diego, CA) 18, near the iso-center of the scanner, with the meniscal slice positioned anatomically as it would be expected if it were located inside of a patient. All sequences (conventional and quantitative) were performed in the sagittal plane, with a single slice acquisition, 1 mm slice thickness and a field of view of 4 cm. To ensure comparable regions between scans, the imaging slice was carefully positioned in the center of the meniscal sample. Detailed MR parameters are listed in Table 1. In brief, the conventional morphologic sequences included a fast spin-echo (FSE) fat-suppressed proton-density (PD) weighted sequence (2300 ms TR, 18.7 ms TE) and a FSE T1-weighted sequence (500 ms TR, 18.1 ms TE). The quantitative sequences included the UTE-T1ρ sequence, which is based on a conventional 2D-UTE excitation and acquisition (500 ms TR, 8 μs TE) combined with a 500 Hz spin-lock preparatory pulse, as shown in Figure 1, with scan time of approximately 4–5 minutes per spin lock. To improve the accuracy of UTE-T1ρ measurements, additional total saturation recovery (TSR) UTE measurements were acquired since the use of relatively short TRs can lead to incomplete recovery of the longitudinal magnetization and T1 compensation is required for more accurate estimation of T1ρ 19.
Table 1.
MR Imaging Parameters
| FSE-PD FS | FSE-T1 | UTE-T1ρ | T1-TSR | |
|---|---|---|---|---|
| TR (ms) | 2300 | 550 | 500 | 17, 25, 50, 10, 200, 400, 800, 1600 |
| TE (ms) | 18.7 | 18.1 | 0.012 | 0.012 |
| Matrix | 512 × 512 | 512 × 512 | 512 × 355 | 512 × 255–455 |
| NEX | 1 | 2 | 2 | |
| ETL | 2 | -- | -- | |
| FA | 90 | 60 | 50 | |
| BW | 31 | 25 | 25 | |
| TSL (ms) | -- | -- | 0.02, 2, 7, 14 | -- |
| Thick (mm) | 1 | 1 | 1 | 1 |
| Approximate Time (mins) | 10 | 8 | 18 | 32 |
Abbreviations: PD: Proton-density-weighted; FS: fat-suppressed; T1: T1-weighted; UTE: Ultrashort TE; FSE: fast spin-echo; TSR: Total saturation recovery; TR: Repetition Time; TE: Time of Excitation; NEX: number of excitations; ETL: echo train length; FA: flip angle; BW: bandwidth; TSL: Time of Spin Lock; Thick: slice thickness.
Figure 1.
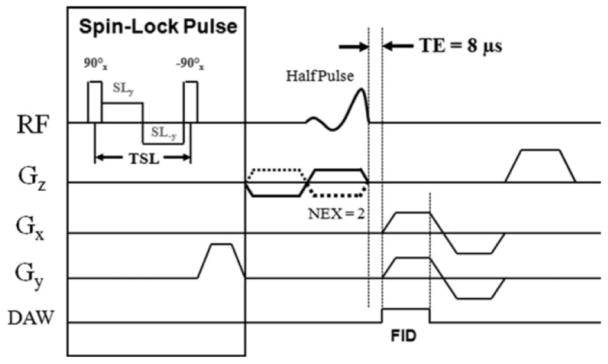
Spin-lock prepared UTE sequence. This sequence combines a regular 2D UTE sequence with a spin-lock preparation pulse, consisting of a hard 90° pulse followed by a composite spin-lock pulse and another −90° hard pulse. The phase of the second half of the composite spin-lock pulse is shifted 180° from the first half to reduce artifacts caused by amplitude of RF field inhomogeneity.
Enzymatic Digestion and Biochemical Analysis
After MR imaging, three meniscal specimens were weighed wet and immersed at 37°C for 4 hours in a 5 mL 0.5 mg/mL solution of trypsin (T1426; Sigma-Aldrich, St. Louis, MO) in normal saline, in order to induce proteoglycan loss. Trypsin depletes proteoglycan across different species 20. A non-physiologically high concentration was used in order to achieve rapid degradation in a short amount of time. The digestion was stopped by extricating the specimens from the trypsin solution and rinsing with normal saline solution. Each meniscal sample was then re-imaged using the same MR protocol. In total each of the three specimens underwent longitudinal analysis with imaging at four time points: pre-digestion and after 4, 8 and 12 hours of sequential trypsin digestion (Figure 2A). As a control, one specimen was immersed in normal saline and subjected to the same procedure described above. Small volumes of digest solution at 4, 8, and 12 hours of digestion were analyzed for contents of sulfated glycosaminoglycan (GAG) using a dimethylmethylene blue (DMMB) assay 21, adapted to a microplate reader 22. Specifically, GAG quantification is based on the shift in absorption observed when DMMB dye associates with repeating negative charges on the GAGs, resulting in stacking of the dye molecules and a metachromatic shift 21, 22.
Figure 2.
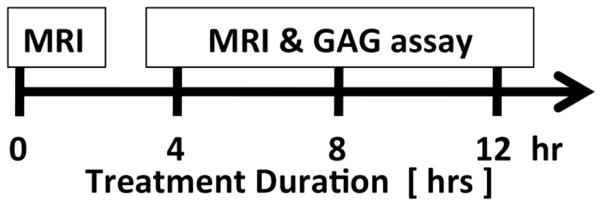
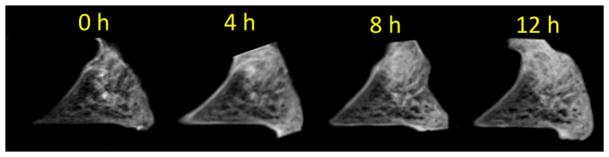
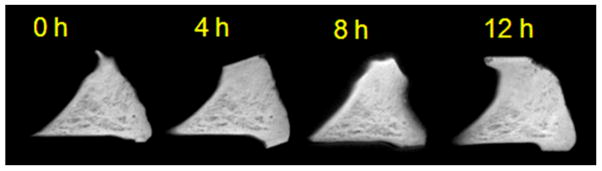
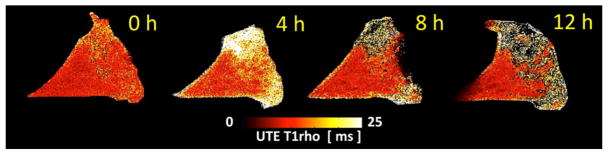
(A) Study design. (B) Meniscal morphologic and signal changes on PD-weighted imaging, after progressive enzymatic GAG digestion. The image at t=0h (prior to enzymatic digestion) depicts the detailed collagen fiber network as well as the normal triangular shape of the meniscal horn. As the trypsin digestion time progresses (from t=4h to t=12h), progressive shape and contour changes are observed (secondary to swelling), as well as fiber disorganization and increase in signal intensity (related to GAG depletion). (C) UTE-T1ρ images profile similar morphologic changes with increasing digestion times, but clearly have increased signal as compared with the PD-weighted images. (D) UTE-T1ρ color maps show altered quantitative values corresponding to the morphologic changes.
MR Image Analysis
The MR images were reviewed in consensus by two musculoskeletal radiologists, both with 10 years of experience. The conventional sequences (PD fat-suppressed and T1-weighted sequences) were used to characterize changes in the morphology and signal of the menisci, before and after enzymatic digestion. In addition, contrast and edge delineation between the hypointense circumferential hoop fibers and hyperintense radial tie fibers was evaluated. They also served as a baseline for comparison with the quantitative UTE imaging techniques.
UTE-T1ρ images were analyzed with MATLAB (R2009a with Image Processing Toolbox) in a similar manner to that described previously 19. In brief, the MR source images obtained at multiple TSLs were analyzed by voxel-wise mono-exponential fitting of signal intensities to create T1ρ maps. Regions of interest (ROI) were placed over the entire meniscal specimen, avoiding areas of artifact or partial-volume effect, and were analyzed by mono-exponential fitting of the data with T1 compensation 19. The measurements were calculated for all specimens, before and after 4, 8 and 12 hours of enzymatic digestion.
Statistical Analysis
To determine the effects of trypsin-digestion duration on UTE-T1ρ MR properties and GAG release, repeated measures ANOVA (α=0.05) was performed. In addition, to account for sample variations in initial (pre-digestion) quantitative MR properties, data were normalized to time-zero values and re-analyzed. Statistical analysis was performed using Systat (v10, Systat Software Inc., San Jose, CA).
Results
Morphologic Analysis
Using the high-resolution, thin slice (1 mm) PD fat-suppressed and T1-weighted sequences, the detailed meniscal ultrastructure was evident. The meniscal collagen fiber network was visible and obvious change was demonstrated on all specimens after progressive enzymatic GAG digestion. Due to GAG depletion and resultant tissue swelling, there were progressive contour abnormalities, fiber disorganization and generalized increase in signal intensity (Figure 2B). The morphologic changes after digestion were also evident with the UTE-T1ρ sequence (Figure 2C), representing an additional advantage to this technique.
Quantitative and Biochemical Analysis
The meniscal specimens that were to be subjected to trypsin degradation demonstrated baseline quantitative UTE-T1ρ of 8.58 ± 1.36 ms (mean ± standard deviation). After 4, 8, and 12 hours of trypsin treatment, mean UTE-T1ρ values increased to 10.96 ± 3.1 ms, 11.64 ± 3.38 ms, and 12.2 ± 3.8 ms, respectively. Although values tended to increase with time, they did not reach significance (p=0.06, Figures 2D and 3A). Normalized UTE-T1ρ values for 4, 8, and 12 hours were 1.27 ± 0.25 ms, 1.35 ± 0.28 ms, and 1.41 ± 0.33 ms, respectively. Normalized values increased significantly with time (p=0.04, Figure 3B). In contrast, the saline-treated specimen did not show any notable changes in values with UTE-T1ρ values at baseline and after 4, 8, and 12 hours of treatment of 18.79, 18.93, 19.0, and 19.35 ms, respectively. Cumulative GAG loss into the bath was consistent with the MR changes, with GAG after 4, 8, and 12 hours of trypsin digestion measuring 0.98 ± 1.3 mg/g, 1.47 ± 1.73 mg/g, and 1.61 ± 1.76 mg/g, respectively. GAG loss showed a trend of increase with time only for the trypsin-treated samples (p=0.1, Figure 4).
Figure 3.
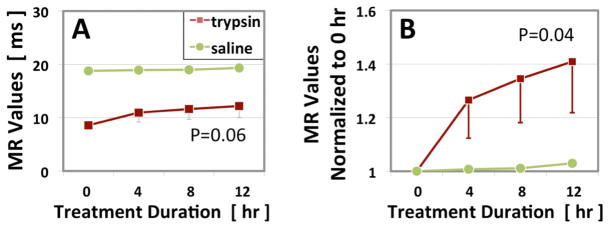
Changes in (A) raw and (B) normalized UTE-T1ρ MR values of the three trypsin- and single saline-treated specimens at each time point (mean ± standard error of the mean).
Figure 4.
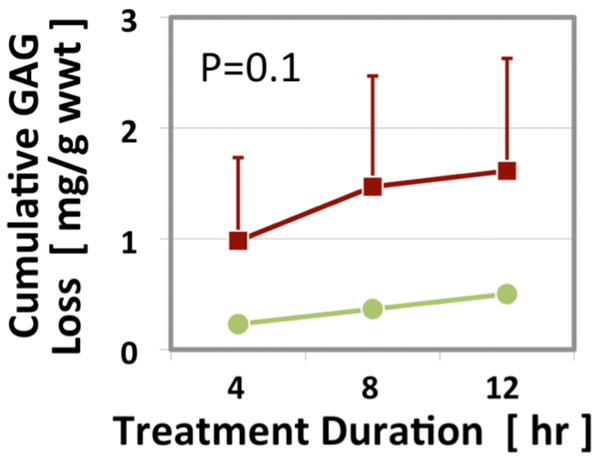
Cumulative GAG loss in the three trypsin- and single saline-treated specimens (mean ± standard error of the mean).
Discussion
The results of our study showed PG loss in meniscal tissue exposed to longitudinal trypsin digestion, with a corresponding increase in UTE-T1ρ values. We also observed progressive morphologic changes in the menisci after trypsin digestion with the UTE-T1ρ sequence, representing an additional advantage to this technique.
To our knowledge, ours is the first “in vitro” study to investigate the T1ρ relaxation changes in human menisci after enzymatic PG digestion, utilizing UTE pulse sequences. Novel UTE techniques are tailored for short T2/T2* tissues, and when compared to existing sequences tailored for long T2/T2* tissues, have been shown to be accurate for morphologic evaluation and provide sensitive quantitative measures of changes in biochemical properties 23.
Both articular cartilage and meniscus consist of a macromolecular framework of collagen fibers, PG and water. While both articular hyaline cartilage and meniscus contain similar fractions of water and collagen (with cartilage primarily type II and meniscus type I), the PG concentration in hyaline cartilage is much higher than in the meniscus (approximately 12% versus as little as 1–2%) 15. However, the various factors that contribute to T1ρ changes in the meniscus are not fully understood. Therefore, our understanding of the biochemical mechanism of T1ρ is primarily based upon the body of work in hyaline cartilage 11.
In hyaline cartilage, T1ρ relaxation is inversely related to PG content 24–28. Duvvuri et al 13 demonstrated an increase in T1ρ relaxation times following articular hyaline cartilage PG degradation with trypsin. They postulated that the increase in the relaxation time following PG degradation is directly related to the amount of free water present in the tissue and that the T1ρ relaxation measurements are selectively sensitive to changes in PG concentration.
Bolbos et al speculated that, in the meniscus, besides the influence of PG content, there might be additional contributions of collagen (including content and fiber orientation) as well as hydration to the T1ρ characteristics 29. Son et al found that T1ρ was mostly associated with differences in meniscal water content, rather than PG or collagen content 30. It is notable that their meniscal samples were obtained from late-stage osteoarthritis patients undergoing total knee replacement, and that any specific sensitivity of T1ρ to a biochemical constituent may have been overwhelmed by a change in water content 30.
Some consider T1ρ to be a hybrid between T1 and T2/T2* 11, and in fact, the boundaries for T1ρ are always between T1 and T2 if a refocusing pulse (spin-echo) is used in the acquisition and between T1 and T2* if gradient recalled echo (GRE) sequences are used. When comparing T1ρ values obtained with GRE sequences to T1ρ obtained with UTE sequences, it is expected that UTE-T1ρ values would be lower due to the additional contribution to the mean value from short T2* components, which cannot be detected by conventional T1ρ sequences with relatively long TEs. Our results are consistent with this (initial UTE-T1ρ values of ~10–20 ms) when comparing with those recently reported by Wang et al using a GRE sequence (mean T1ρ values of 25–33 ms) 31. Du et al reported a mean meniscal UTE-T1ρ value of 7.98 ms using healthy volunteers 19, and our values are likely higher due to the use of elderly cadaveric specimens and likely degenerative changes.
The association between knee OA and meniscal damage and tears is well known 32–38. Meniscal damage can alter joint biomechanics and correlates with progression of cartilage degradation and OA. Ding et al 39 showed that untreated meniscal tears were an independent risk factor for knee OA and associated with cartilage defects/volume loss in asymptomatic patients, similar to other risk factors such as age, sex, BMI, and family history. This study suggested that a meniscal tear is an early event in the disease process.
Quantitative MR sequences, such as T1ρ, that could indicate biochemical meniscal damage prior to substantial structural defects would be of great clinical utility. Wang et al found that T1ρ measurements of the menisci were useful in distinguishing between patients with acute anterior cruciate ligament (ACL) injuries and healthy controls, suggesting that T1ρ may detect degeneration 31. Stehling et al found that meniscal T1ρ values significantly increased in runners after a marathon and remained at a high level after 3 months, indicating persistent changes in matrix composition 40. Zarins et al found a trend for T1ρ values to be higher in menisci with intra-substance signal abnormality (indicating early degeneration) compared with menisci of normal intensity 41. The authors also found that quantitative meniscal T1ρ measurements demonstrated good clinical correlation with WOMAC (Western Ontario and McMasters Universities Arthritis Index) scores 41.
Quantitative T1ρ measurements in the menisci may be used to differentiate healthy subjects from individuals with early OA. Rauscher and coworkers prospectively evaluated the differences in T1ρ values in the meniscus in patients with varying degrees of OA and healthy control subjects, and showed that meniscal T1ρ values were increased in the patients with OA when compared with values in the healthy subjects, and also tended to correlate with the degree of OA 42. In addition, significant correlations between matrix measurements in the meniscus and clinical scores were found. Wang et al found higher T1ρ values in specific meniscus and femorotibial cartilage subregions in patients with OA, suggesting that T1ρ might detect meniscal abnormalities in patients with OA, and that regional damage of both hyaline cartilage and meniscus may be associated with OA 43. Bolbos et al retrospectively evaluated the T1ρ relaxation time in the lateral meniscus and its relationship with T1ρ of the adjacent cartilage in knees with acute ACL injuries and in healthy controls, and found a strong injury-related relationship between meniscus and cartilage biochemical changes 29.
The human knee meniscus has a short T2/T2* relaxation time, due to restricted water mobility. UTE pulse sequences allow earlier acquisition after excitation compared with conventional sequences, facilitating detection of short T2* relaxation components in tissue before they have decayed to a low level. Using the novel UTE-T1ρ technique, high signal can be acquired from short T2* tissues such as the menisci, and there is now the opportunity for improved detection of structural and biochemical alterations. The image quality with the UTE-T1ρ sequences is competitive with clinical sequences.
There are some limitations to our study. The first limitation is the small sample size, which may have caused selection bias. Furthermore, with more samples the trends seen in our results may have reached significance. However, ours was a longitudinal pilot study and utilized four cross-sectional measurements per sample (pre-digestion and after 4, 8, and 12 hours of digestion). Second, the 2D UTE-T1ρ acquisition is subject to eddy currents, field inhomogeneity and gradient nonlinearity, although placement of a specimen or body part near the magnet iso-center can improve image quality. Third, the 2D UTE-T1ρ sequence performs single-slice imaging and quantification, although this can potentially be overcome with 3D imaging techniques. Fourth, we included the entire meniscal horn in the ROI measurements, without considering zonal variations. More studies are needed to evaluate the T1ρ values in the different anatomical subregions of the menisci. Fifth, histology was not performed in our trypsinated menisci. However, trypsin is known to induce PG degradation 20 and this was macroscopically evident in our samples. Finally, the factors that contribute to T1ρ changes in the meniscus are not fully understood and need further investigation.
Meniscus damage is associated with adjacent cartilage degeneration and has been implicated in OA progression. UTE-T1ρ relaxation correlates with changes in PG content in the meniscus. Early detection of meniscal damage, represented by elevations in meniscal UTE-T1ρ relaxation measurements, may identify subjects at increased risk for OA.
Acknowledgments
Funding
The authors acknowledge funding from the National Institutes of Health grant #R01AR064321 and from the VA Clinical Science Research and Development Service (Career Development Award IK2CX000749).
Footnotes
Conflict of Interest
The authors declare that there is no conflict of interest
Contributor Information
Juliana C. Campos, Email: camposju@hotmail.com.
Won C. Bae, Email: wbae@ucsd.edu.
Richard Znamirowski, Email: znamirowski@ucsd.edu.
Sheronda Statum, Email: sherondastatum@msn.com.
Jiang Du, Email: jiangdu@ucsd.edu.
Christine B. Chung, Email: cbchung@ucsd.edu.
References
- 1.Brandt KD, Radin EL, Dieppe PA, et al. Yet more evidence that osteoarthritis is not a cartilage disease. Ann Rheum Dis. 2006;65:1261–4. doi: 10.1136/ard.2006.058347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fukubayashi T, Kurosawa H. The contact area and pressure distribution pattern of the knee. A study of normal and osteoarthrotic knee joints. Acta Orthop Scand. 1980;51:871–9. doi: 10.3109/17453678008990887. [DOI] [PubMed] [Google Scholar]
- 3.Berthiaume MJ, Raynauld JP, Martel-Pelletier J, et al. Meniscal tear and extrusion are strongly associated with progression of symptomatic knee osteoarthritis as assessed by quantitative magnetic resonance imaging. Ann Rheum Dis. 2005;64:556–63. doi: 10.1136/ard.2004.023796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hellio Le Graverand MP, Vignon E, Otterness IG, et al. Early changes in lapine menisci during osteoarthritis development: Part I: cellular and matrix alterations. Osteoarthritis Cartilage. 2001;9:56–64. doi: 10.1053/joca.2000.0350. [DOI] [PubMed] [Google Scholar]
- 5.Hellio Le Graverand MP, Vignon E, Otterness IG, et al. Early changes in lapine menisci during osteoarthritis development: Part II: molecular alterations. Osteoarthritis Cartilage. 2001;9:65–72. doi: 10.1053/joca.2000.0351. [DOI] [PubMed] [Google Scholar]
- 6.Lo GH, Hunter DJ, Nevitt M, et al. Strong association of MRI meniscal derangement and bone marrow lesions in knee osteoarthritis: data from the osteoarthritis initiative. Osteoarthritis Cartilage. 2009;17:743–7. doi: 10.1016/j.joca.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma L, Eckstein F, Song J, et al. Relationship of meniscal damage, meniscal extrusion, malalignment, and joint laxity to subsequent cartilage loss in osteoarthritic knees. Arthritis Rheum. 2008;58:1716–26. doi: 10.1002/art.23462. [DOI] [PubMed] [Google Scholar]
- 8.Bashir A, Gray ML, Burstein D. Gd-DTPA2- as a measure of cartilage degradation. Magn Reson Med. 1996;36:665–73. doi: 10.1002/mrm.1910360504. [DOI] [PubMed] [Google Scholar]
- 9.Taylor C, Carballido-Gamio J, Majumdar S, et al. Comparison of quantitative imaging of cartilage for osteoarthritis: T2, T1rho, dGEMRIC and contrast-enhanced computed tomography. Magn Reson Imaging. 2009;27:779–84. doi: 10.1016/j.mri.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Redfield AG. Nuclear spin thermodynamics in the rotating frame. Science. 1969;164:1015–23. doi: 10.1126/science.164.3883.1015. [DOI] [PubMed] [Google Scholar]
- 11.Wang L, Regatte RR. T MRI of human musculoskeletal system. J Magn Reson Imaging. 2014 doi: 10.1002/jmri.24677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makela HI, Grohn OH, Kettunen MI, et al. Proton exchange as a relaxation mechanism for T1 in the rotating frame in native and immobilized protein solutions. Biochem Biophys Res Commun. 2001;289:813–8. doi: 10.1006/bbrc.2001.6058. [DOI] [PubMed] [Google Scholar]
- 13.Duvvuri U, Reddy R, Patel SD, et al. T1rho-relaxation in articular cartilage: effects of enzymatic degradation. Magn Reson Med. 1997;38:863–7. doi: 10.1002/mrm.1910380602. [DOI] [PubMed] [Google Scholar]
- 14.Regatte RR, Akella SV, Borthakur A, et al. Proteoglycan depletion-induced changes in transverse relaxation maps of cartilage: comparison of T2 and T1rho. Acad Radiol. 2002;9:1388–94. doi: 10.1016/s1076-6332(03)80666-9. [DOI] [PubMed] [Google Scholar]
- 15.Chang EY, Du J, Chung CB. UTE imaging in the musculoskeletal system. J Magn Reson Imaging. 2014 doi: 10.1002/jmri.24713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang EY, Du J, Bae WC, et al. Effects of Achilles tendon immersion in saline and perfluorochemicals on T2 and T2*. J Magn Reson Imaging. 2014;40:496–500. doi: 10.1002/jmri.24360. [DOI] [PubMed] [Google Scholar]
- 17.Du J, Hamilton G, Takahashi A, et al. Ultrashort echo time spectroscopic imaging (UTESI) of cortical bone. Magn Reson Med. 2007;58:1001–9. doi: 10.1002/mrm.21397. [DOI] [PubMed] [Google Scholar]
- 18.Carl M, Chiang JTA. Investigations of the Origin of Phase Differences Seen with Ultrashort TE Imaging of Short T2 Meniscal Tissue. Magnetic Resonance in Medicine. 2012;67:991–1003. doi: 10.1002/mrm.23075. [DOI] [PubMed] [Google Scholar]
- 19.Du J, Carl M, Diaz E, et al. Ultrashort TE T1rho (UTE T1rho) imaging of the Achilles tendon and meniscus. Magn Reson Med. 2010;64:834–42. doi: 10.1002/mrm.22474. [DOI] [PubMed] [Google Scholar]
- 20.Raya JG, Melkus G, Adam-Neumair S, et al. Change of diffusion tensor imaging parameters in articular cartilage with progressive proteoglycan extraction. Invest Radiol. 2011;46:401–9. doi: 10.1097/RLI.0b013e3182145aa8. [DOI] [PubMed] [Google Scholar]
- 21.Farndale RW, Sayers CA, Barrett AJ. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9:247–8. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- 22.Hollander AP, Atkins RM, Eastwood DM, et al. Human Cartilage Is Degraded by Rheumatoid-Arthritis Synovial-Fluid but Not by Recombinant Cytokines Invitro. Clinical and Experimental Immunology. 1991;83:52–57. doi: 10.1111/j.1365-2249.1991.tb05587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bae WC, Dwek JR, Znamirowski R, et al. Ultrashort echo time MR imaging of osteochondral junction of the knee at 3 T: identification of anatomic structures contributing to signal intensity. Radiology. 2010;254:837–45. doi: 10.1148/radiol.09081743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akella SV, Regatte RR, Gougoutas AJ, et al. Proteoglycan-induced changes in T1rho-relaxation of articular cartilage at 4T. Magn Reson Med. 2001;46:419–23. doi: 10.1002/mrm.1208. [DOI] [PubMed] [Google Scholar]
- 25.Duvvuri U, Charagundla SR, Kudchodkar SB, et al. Human knee: in vivo T1(rho)-weighted MR imaging at 1. 5 T--preliminary experience. Radiology. 2001;220:822–6. doi: 10.1148/radiol.2203001662. [DOI] [PubMed] [Google Scholar]
- 26.Mosher TJ, Zhang Z, Reddy R, et al. Knee articular cartilage damage in osteoarthritis: analysis of MR image biomarker reproducibility in ACRIN-PA 4001 multicenter trial. Radiology. 2011;258:832–42. doi: 10.1148/radiol.10101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Regatte RR, Akella SV, Wheaton AJ, et al. 3D-T1rho-relaxation mapping of articular cartilage: in vivo assessment of early degenerative changes in symptomatic osteoarthritic subjects. Acad Radiol. 2004;11:741–9. doi: 10.1016/j.acra.2004.03.051. [DOI] [PubMed] [Google Scholar]
- 28.Tsushima H, Okazaki K, Takayama Y, et al. Evaluation of cartilage degradation in arthritis using T1rho magnetic resonance imaging mapping. Rheumatol Int. 2012;32:2867–75. doi: 10.1007/s00296-011-2140-3. [DOI] [PubMed] [Google Scholar]
- 29.Bolbos RI, Link TM, Ma CB, et al. T1rho relaxation time of the meniscus and its relationship with T1rho of adjacent cartilage in knees with acute ACL injuries at 3 T. Osteoarthritis Cartilage. 2009;17:12–8. doi: 10.1016/j.joca.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Son M, Goodman SB, Chen W, et al. Regional variation in T1rho and T2 times in osteoarthritic human menisci: correlation with mechanical properties and matrix composition. Osteoarthritis Cartilage. 2013;21:796–805. doi: 10.1016/j.joca.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L, Chang G, Bencardino J, et al. T1rho MRI at 3T of menisci in patients with acute anterior cruciate ligament (ACL) injury. J Magn Reson Imaging. 2014 doi: 10.1002/jmri.24594. [DOI] [PubMed] [Google Scholar]
- 32.Bhattacharyya T, Gale D, Dewire P, et al. The clinical importance of meniscal tears demonstrated by magnetic resonance imaging in osteoarthritis of the knee. J Bone Joint Surg Am. 2003;85-A:4–9. doi: 10.2106/00004623-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Christoforakis J, Pradhan R, Sanchez-Ballester J, et al. Is there an association between articular cartilage changes and degenerative meniscus tears? Arthroscopy. 2005;21:1366–9. doi: 10.1016/j.arthro.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 34.Crema MD, Guermazi A, Li L, et al. The association of prevalent medial meniscal pathology with cartilage loss in the medial tibiofemoral compartment over a 2-year period. Osteoarthritis Cartilage. 2010;18:336–43. doi: 10.1016/j.joca.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 35.Englund M, Guermazi A, Roemer FW, et al. Meniscal tear in knees without surgery and the development of radiographic osteoarthritis among middle-aged and elderly persons: The Multicenter Osteoarthritis Study. Arthritis Rheum. 2009;60:831–9. doi: 10.1002/art.24383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Felson DT. An update on the pathogenesis and epidemiology of osteoarthritis. Radiol Clin North Am. 2004;42:1–9. v. doi: 10.1016/S0033-8389(03)00161-1. [DOI] [PubMed] [Google Scholar]
- 37.Messner K, Gao J. The menisci of the knee joint. Anatomical and functional characteristics, and a rationale for clinical treatment. J Anat. 1998;193(Pt 2):161–78. doi: 10.1046/j.1469-7580.1998.19320161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raynauld JP, Martel-Pelletier J, Berthiaume MJ, et al. Quantitative magnetic resonance imaging evaluation of knee osteoarthritis progression over two years and correlation with clinical symptoms and radiologic changes. Arthritis Rheum. 2004;50:476–87. doi: 10.1002/art.20000. [DOI] [PubMed] [Google Scholar]
- 39.Ding C, Martel-Pelletier J, Pelletier JP, et al. Meniscal tear as an osteoarthritis risk factor in a largely non-osteoarthritic cohort: a cross-sectional study. J Rheumatol. 2007;34:776–84. [PubMed] [Google Scholar]
- 40.Stehling C, Luke A, Stahl R, et al. Meniscal T1rho and T2 measured with 3. 0T MRI increases directly after running a marathon. Skeletal Radiol. 2011;40:725–35. doi: 10.1007/s00256-010-1058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zarins ZA, Bolbos RI, Pialat JB, et al. Cartilage and meniscus assessment using T1rho and T2 measurements in healthy subjects and patients with osteoarthritis. Osteoarthritis Cartilage. 2010;18:1408–16. doi: 10.1016/j.joca.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rauscher I, Stahl R, Cheng J, et al. Meniscal measurements of T1rho and T2 at MR imaging in healthy subjects and patients with osteoarthritis. Radiology. 2008;249:591–600. doi: 10.1148/radiol.2492071870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang L, Chang G, Xu J, et al. T1rho MRI of menisci and cartilage in patients with osteoarthritis at 3T. Eur J Radiol. 2012;81:2329–36. doi: 10.1016/j.ejrad.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]


