Abstract
Background
Current nodal staging (N-staging) of am-pullary carcinoma in the TNM staging system distinguishes between node-negative (N0) and node-positive (N1) disease but does not consider the metastatic lymph node (LN) number.
Methods
Overall, 313 patients who underwent pancreatoduodenectomy for ampullary adenocarcinoma were categorized as N0, N1 (1–2 metastatic LNs), or N2 (≥3 metastatic LNs), as proposed by Kang et al. Clinico-pathological features and overall survival (OS) of the three groups were compared.
Results
The median number of LNs examined was 11, and LN metastasis was present in 142 cases (45 %). When LN-positive cases were re-classified according to the proposed staging system, 82 were N1 (26 %) and 60 were N2 (19 %). There was a significant correlation between proposed N-stage and lymphovascular invasion, perineural invasion, increased tumor size (each p < 0.001), and surgical margin positivity (p = 0.001). The median OS in LN-negative cases was significantly longer than that in LN-positive cases (107.5 vs. 32 months; p < 0.001). Patients with N1 and N2 disease had median survivals of 40 and 24.5 months, respectively (p < 0.0001). In addition, 1-, 3-, and 5-year survivals were 88, 76, 62 %, respectively, for N0; 90, 55, 31.5 %, respectively, for N1; and 68, 34, 30 %, respectively for N2 (p < 0.001). Even with multivariate modeling, the association between higher proposed N stage and shorter survival persisted (hazard ratio 1.6 for N1 and 1.9 for N2; p = 0.018).
Conclusions
Classification of nodal status in ampullary carcinomas based on the number of metastatic LNs has a significant prognostic value. A revised N-staging classification system should be incorporated into the TNM staging of ampullary cancers.
The clinicopathologic features of ampullary carcinoma remain poorly characterized for a number of reasons. Ampullary tumors are relatively rare, and the ampulla is anatomically complex.1–3 Tumors from the pancreas, duodenum, and common bile duct are often misdiagnosed as ampullary. There has not been a uniform definition of what qualifies as ‘ampullary cancer’, and often non-invasive epithelial lesions have been analyzed, together with invasive carcinomas. Many studies use the expression ‘periampullary cancers’, an imprecise term that could refer to any tumor amenable to resection by pancreatoduodenectomy,4–13 including tumors of the ampulla and its immediate vicinity,14 tumors strictly of the ampulla,15 and tumors of the non-ampullary portion of the duodenum.16 Therefore, the results have been highly variable and it is not surprising that some authors continue to question whether ampullary cancer is a distinct entity.17
Recently, the College of American Pathologists proposed a more specific classification of ampullary cancer.18 This new classification was further refined in subsequent studies.19,20 Studies following these classifications clearly identify ampullary cancers as a distinct category with vastly different characteristics and outcomes than cancers of neighboring sites.19,21,22
In this highly inconsistent literature, few prognostic factors of ampullary cancers have been identified.15,23–51 Among these, lymph node (LN) status has been identified as an important predictor of survival in multiple studies.15,26–43 In those studies that found no association between LN involvement and prognosis,25 the negative result is likely attributable to the variable definition of ampullary cancer.
Current N staging of ampullary carcinoma in the American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) TNM classification recognizes only node-negative (N0) and node-positive (N1) disease. However some studies have also shown that among LN-positive cases additional information about prognosis can be gleaned from the number of metastatic LNs.52–58 The prognostic value of the number of positive LNs has been shown for several other cancers and is considered in the TNM staging guidelines for esophageal, gastric, colon, rectal, and breast carcinomas.59 Recently, Kang et al. proposed a new nodal classification for ampullary carcinoma based on analysis of the Surveillance, Epidemiology, and End Results (SEER) database as node-negative (N0), 1–2 LN-positive (N1), and ≥3 LN-positive (N2); this stratification was found to be a significant factor in the survival analysis. They also verified these findings in their institutional database but limited information was provided regarding the criteria of inclusion.60
The aim of this study was to determine the frequency and clinical significance of LN involvement in ampullary adenocarcinomas through analysis of a well-characterized and pathologically verified cohort.
MATERIALS AND METHODS
This study was conducted in accordance with the requirements of the Institutional Review Boards of all institutions involved.
Study Population
A total of 313 cases of invasive ampullary carcinoma (61 % from Emory University, USA; 26 % from the University of California San Francisco, USA; 6 % from Showa University, Japan; 3.5 % from the University of Pittsburgh, USA, and 3.5 % from Marmara University, Turkey) with adequate LN sampling, which met the recently established criteria, were included.18,19 Ampullectomy cases were omitted because of the absence of LN sampling. Pancreatic, common bile duct, and non-ampullary duodenal carcinomas were excluded utilizing the purist’s approach, as previously described.61 Only the cases of ampullary origin with convincing invasive ade-nocarcinoma component verified with pathologic re-review by the authors were included. Patients with unusual carcinoma types such as undifferentiated carcinoma with osteoclast-like giant cells or neuroendocrine neoplasms, and patients who underwent neoadjuvant therapy, were excluded. Demographic, clinical, and survival data were obtained from medical records.
Criteria for Ampullary Origin and the Site-Specific Classification
Five authors (SB, TT, NO, GEK, and VA) re-evaluated the cases to confirm the ampullary origin based on the recently established criteria.18,19 Briefly, a tumor was designated as a primary ampullary carcinoma only if the following criteria were met.
Its epicenter was located in the lumen or walls of the distal ends (intra-ampullary component) of the CBD and/or pancreatic duct, or at the ‘papilla of Vater’ (junction of duodenal and ampullary mucosa, as defined by the College of American Pathologists), or the duodenal surface of the papilla (the duodenal-facing surface of the ampullary protuberance). For the latter, the case was designated as ‘primary ampullary carcinoma’ rather than duodenal, only if the ampullary orifice was located clearly within this lesion.
The epicenter of the tumor or >75 % of the bulk of the lesion was in the ampulla.
Using these strict definitions, there was consensus on ampullary origin of all 313 cases included in the study. In fact, 68 cases that were originally classified as ampullary carcinoma were reclassified as carcinomas secondarily involving the ampulla (33 from the pancreas, 16 from the duodenum, and 19 from the CBD) and were excluded from the study.
Pathology Evaluation: Histopathologic Parameters and Lymph Node (LN) Assessment
All 313 cases were subjected to pathology re-evaluation by the authors. Pathologic parameters such as tumor size, invasive carcinoma size, typing, and perineural/vascular invasion were reassessed. The cases were classified as intestinal, pancreatobiliary or ‘other’ based on their resemblance to colonic or pancreatic carcinomas, as described previously.1–3,62 This classification was supported by immunohistochemical expression of cell-lineage markers, which have also been used for subclassification of pancreatic and biliary intraductal papillary neoplasms in a subset of the cases (n = 59). A representative formalin-fixed paraffin-embedded tissue section of these cases was immunolabeled using the standard avidin–biotin-peroxidase method with antibodies against the intestinal differentiation markers CK20 (DakoCytomation, Carpinteria, CA, USA), CDX2 (Biogenex, San Ramon, CA, USA), and MUC2 (Leica Microsystems, Bannockburn, IL, USA) and the pancreatobiliary differentiation markers CK7 (DakoCytomation), MUC1 (Leica Microsystems), and MUC5AC (Leica Microsystems).
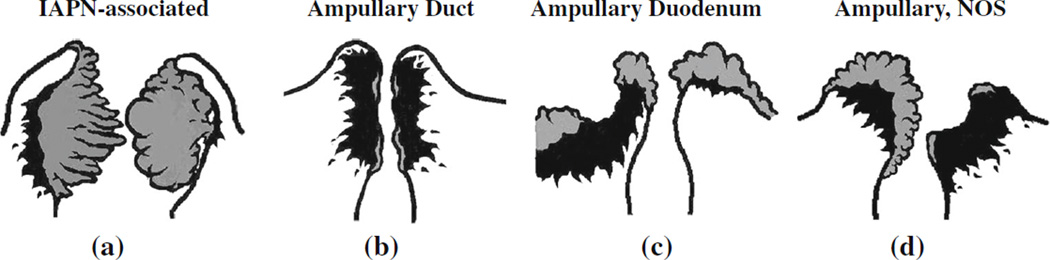
T-stage of the tumors was also re-analyzed. Four site-specific groups comprising the ampullary carcinomas were defined, as recently described (Fig. 1).19
FIG. 1.
Site-specific classification of ampullary carcinoma. a IAPN-associated carcinomas are characterized by a prominent pre-invasive neoplasm (gray areas) that grows predominantly as an exophytic mass within the distal ends of the common bile duct and main pancreatic duct. b Ampullary duct carcinomas have minimal pre-invasive component, and invasive component (black areas) forms a plaque-like stricture at the distal ends of the ducts. c Ampullary duodenum carcinomas form ulcerovegetative tumors that grow predominantly on the duodenal surface of the ampulla. d Carcinomas that do not fit in any of these categories are classified as NOS. IAPN intra-ampullary papillary-tubular neoplasm, NOS not otherwise specified
Since cases were identified retrospectively at multiple different institutions, the LN sampling method utilized at the time of original assessment was not standardized. The orange-peeling method63,64 was used for cases from Emory University.
The revised N-stage protocol proposed by Kang et al.60 was applied to assign patients into node-negative (N0), 1–2 LN-positive (N1), and ≥3 LN-positive (N2) cohorts. The data were analyzed for all patients regardless of the number of LNs examined. Additionally, subset analysis was performed for cases that had ≥12 LNs examined as 12 is the number of LNs advocated by the AJCC as the minimum needed for accurate staging of periampullary cancers treated with pancreaticoduodenectomy.59
Statistical Analysis
Kaplan–Meier survival curves were constructed and compared across all three LN involvement categories using the log-rank test. Additional analyses compared patients with 1–2 metastatic LNs with those with ≥3 affected LNs to further assess the relation between the extent of LN involvement and survival. This analysis was repeated after excluding patients with <12 LNs to control for the extent of LN assessment. The cutoff of 12 LNs was used because it was considered to be an indicator of quality performance.59
Hazard ratios (HRs) and the corresponding 95 % confidence intervals (CIs) reflecting the association between N stage and survival were calculated using Cox models where patients with no metastatic LNs represented the reference group. The adjusted model included age, sex, lymphovascular invasion, T stage, perineural invasion, surgical margin, and site-specific classification. Proportional hazard assumptions were tested by inspecting the log–log curves.
RESULTS
Demographic and Clinicopathologic Data
Overall, 313 patients with invasive ampullary adenocarcinoma were included in this study. The clinicopathologic characteristics of the study cohort are summarized in Table 1. Mean age was 64 years (range 27–89), and 59 % were male (n = 183). The mean overall size of the tumors was 2.7 cm, with mean invasion size of 1.9 cm. Perineural invasion was seen in 35 % (n = 110) and lymphovascular invasion was present in 65 % (n = 202) of cases.
TABLE 1.
Clinicopathologic features of the study cases (n = 313)
| All cases (n = 313) | N0 (n = 171, 55 %) | N1 (n = 82, 26 %) | N2 (n = 60, 19 %) | |
|---|---|---|---|---|
| Age (years) | ||||
| Mean | 64 ± 12 | 65 ± 12 | 65 ± 11 | 62 ± 12 |
| Median | 65 | 66 | 67 | 63 |
| Range | 27–89 | 27–86 | 38–89 | 35–86 |
| Gender [n (%)] | ||||
| Female | 129 (41.5) | 66 (39) | 37 (45) | 26 (43) |
| Male | 183 (58.5) | 104 (61) | 45 (55) | 34 (57) |
| Overall tumor size (mm) | ||||
| Mean | 27 ± 16 | 24 ± 15 | 28 ± 15 | 33 ± 17 |
| Median | 22 | 20 | 25 | 25 |
| Range | 3–95 | 3–95 | 8–80 | 12–80 |
| Invasive tumor size (mm) | ||||
| Mean | 19 ± 12 | 16 ± 11 | 21 ± 12 | 26 ± 13 |
| Median | 17 | 15 | 20 | 22.5 |
| Range | 2–95 | 2–95 | 3–60 | 7–70 |
| T stage (AJCC 2010 staging) [n (%)] | ||||
| T1 | 38 (12) | 32 (19) | 4 (5) | 2 (3) |
| T2 | 128 (41) | 81 (47) | 34 (42) | 13 (22) |
| T3 | 107 (34) | 48 (28) | 34 (42) | 25 (42) |
| T4 | 39 (13) | 10 (6) | 9 (11) | 20 (33) |
| Perineural invasion [n (%)] | ||||
| Absent | 203 (65) | 128 (75) | 50 (61) | 25 (42) |
| Present | 110 (35) | 43 (25) | 32 (39) | 35 (58) |
| Lymphovascular invasion [n (%)] | ||||
| Absent | 111 (35.5) | 86 (50) | 21 (26) | 4 (7) |
| Present | 202 (64.5) | 85 (50) | 61 (74) | 56 (93) |
| Surgical margin [n (%)] | ||||
| Absent | 298 (96) | 167 (98) | 79 (99) | 52 (87) |
| Present | 12 (4) | 3 (2) | 1 (1) | 8 (13) |
| Harvested lymph node number [n (%)] | ||||
| <12 | 163 (52) | 105 (61) | 45 (55) | 13 (22) |
| ≥12 | 150 (48) | 66 (39) | 37 (45) | 47 (78) |
| Site-specific classification [n (%)] | ||||
| Ampullary, NOS | 159 (51) | 87 (51) | 41 (50) | 31 (52) |
| Ampullary duct | 60 (19) | 25 (15) | 18 (22) | 17 (28) |
| IAPN-associated | 68 (22) | 50 (29) | 13 (16) | 5 (8) |
| Ampullary duodenum | 26 (8) | 9 (5) | 10 (12) | 7 (12) |
| Histologic type [n (%)] | ||||
| Pancreatobiliary | 172 (55) | 95 (56) | 45 (55) | 32 (53) |
| Intestinal | 68 (22) | 46 (27) | 13 (16) | 9 (15) |
| Mixed or other | 73 (23) | 30 (16) | 24 (29) | 19 (32) |
| Outcome [n (%)] | ||||
| Alive | 155 (50) | 97 (58) | 33 (40) | 25 (43) |
| Dead | 153 (50) | 71 (42) | 49 (60) | 33 (57) |
| Median survival (months) | 56 | 107.5 | 40 | 24.5 |
| 1-year survival (%) | 85 | 88 | 90 | 68 |
| 3-year survival (%) | 63 | 76 | 55 | 34 |
| 5-year survival (%) | 49 | 62 | 31.5 | 30 |
AJCC American Joint Committee on Cancer, IAPN intra-ampullary papillary-tubular neoplasm, NOS not otherwise specified
With respect to histologic classification, 55 % of tumors (n = 172) were pancreatobiliary type; 22 % (n = 68) were intestinal type; and 23 % (n = 73) were mixed or other types. Most of the intestinal-type carcinomas revealed CDX2 (90 %) and MUC2 (85 %) expression; however, the specificity of these markers for this phenotype was fairly low (61 and 78 %, respectively). In contrast, all pancreatobiliary-type carcinomas (100 %) were, at least focally, positive for MUC1 and MUC5AC. The cytokeratin profile was entirely non-discriminatory, in that CK7 and CK20 were co-expressed in 53 % of all cases available for immunohistochemical staining. More importantly, CK7, which is regarded as a good marker of pancreatobiliary differentiation, was expressed in a high proportion (63 %) of intestinal cases, and CK20, which is generally considered a good marker of the intestinal phenotype, was expressed in a substantial number (39 %) of pancreatobiliary cases.
Based on the site-specific classification scheme, 159 cases (51 %) were ampullary, not otherwise specified; 68 (22 %) were intra-ampullary papillary-tubular neoplasm (IAPN)-associated; 60 (19 %) were ampullary duct; and 26 (8 %) were ampullary duodenal.
Median follow-up was 56 months, while overall 1-, 3-, and 5-year survival was 85, 63, and 49 %, respectively.
LN Analysis
A total of 142 cases (45 %) had LN metastasis. The median number of LNs examined was 11 (range 1–61), and the number of metastatic LNs among LN-positive cases ranged from 1 to 19 (median 2). Based on the proposed N-staging protocol, 82 cases (26 %) were N1, and 60 (19 %) were N2. In the analysis restricted to cases with more than 12 LNs examined, 66 cases (44 %) were N0, 37 (25 %) were N1, and 47 (31 %) were N2. The percentage of N2 cases at the Emory University, where the orange-peeling method of LN harvesting was performed,63 was 26 %.
There was a statistically significant association between proposed N stage and frequency of aggressive tumor characteristics, including lymphovascular invasion (p < 0.001), perineural invasion (p < 0.001), invasive tumor size (p < 0.001), and surgical margin positivity (p = 0.001).
With respect to histologic classification, 55 % of pan-creatobiliary-type carcinomas were N0, 26 % were N1, and 19 % were N2; 68 % of intestinal-type carcinomas were N0, 19 % were N1, 13 % were N2, and, of the mixed or other type carcinomas, 41 % were N0, 33 % were N1, and 26 % were N2. When only pancreatobiliary and intestinal carcinomas were taken into account, the distribution of N stages was not different between pancreatobiliary and intestinal carcinomas (p = 0.212).
Among the site-specific categories, 74 % of the IAPN-associated carcinoma category were N0, 19 % were N1, and only 7 % were N2. In contrast, the ampullary-ductal group had the highest proportion of N2 cases (28 %).
Survival Analysis
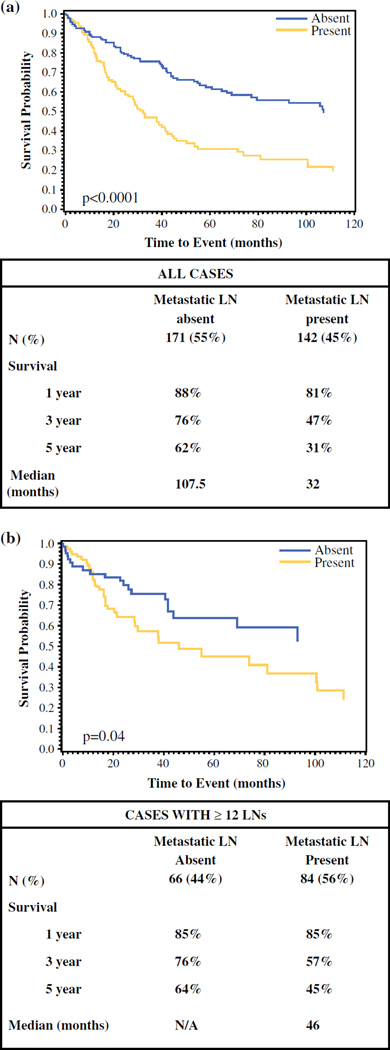
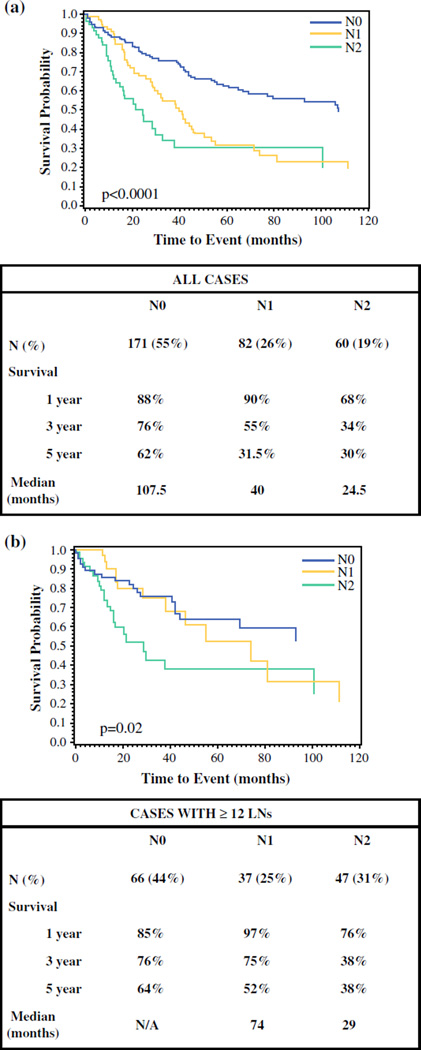
In all patients, the 1-, 3-, and 5-year survival rates were 85, 63, and 49 %, respectively. Median survival of LN-negative cases was significantly longer than that of LN-positive cases (107.5 vs. 32 months; p < 0.0001), with 1-, 3-, and 5-year survival rates of 88, 76, and 62 % versus 81, 47, and 31 %, respectively (p < 0.001; Fig. 2a). In the subanalysis of patients who had 12 or more LNs examined, the difference persisted. The 1-, 3-, and 5-year survival rates were 85, 76 %, and 64 %, respectively, for LN-negative cases, and 85, 57, and 45 %, respectively, for LN-positive cases (p = 0.04; Fig. 2b). The median survival of patients with N1 disease was 40 months compared with 24.5 months for patients with N2 disease (p < 0.0001; Fig. 3a). The 1-, 3-, and 5-year survival rates were 90, 55, and, 31.5 %, respectively, for the N1 group, and 68, 34, and 30 %, respectively, for the N2 group (p < 0.001; Fig. 3a). The corresponding analyses among patients with 12 or more LNs sampled produced similar results, with median survival estimates of 74 months for N1, and 29 months for N2. The 1-, 3-, and 5-year survival rates among N1- and N2-stage groups with sufficient (≥12 LNs) sampling were 97, 75, and 52 %, respectively, and 76, 38, and 38 %, respectively (p = 0.02; Fig. 3b).
FIG. 2.
Comparison of survival between LN-positive and LN-negative cases. a All cases, and b cases with 12 or more LNs sampled only. LN lymph node
FIG. 3.
Comparison of survival between proposed N0 (no positive LNs), N1 (1–2 positive LNs), and N2 (≥3 positive LNs) cases. a All cases, and b cases with 12 or more LNs sampled only. LNs lymph node, NA not applicable
In the multivariable Cox regression model adjusted for age, sex, tumor invasion size, tumor stage, perineural invasion, surgical margin, and site-specific classification, the association between higher proposed N stage and shorter survival persisted. Using the N0 group as the reference, the adjusted HRs (95 % CIs) for N1 and N2 were 1.6 (1.1–2.4) and 1.9 (1.1–3.2), respectively (p value for trend = 0.018) [Table 2]. In these models, age [HR, 1.3 (1.1–1.5, p = 0.004) for a 10 year increase], lymphovascular invasion [HR, 1.7 (1.1–2.5, p = 0.01)], perineural invasion [HR, 1.5 (1.1–2.3, p = 0.04)] and surgical margin positivity [HR, 3.3 (1.6–6.9, p = 0.001)] were also statistically significantly associated with survival.
TABLE 2.
Comparison of hazard ratios by proposed N stage in all cases
| N stage | HRa (95 % CI) | HRa (95 % CI) | p valuec | HRb (95 % CI) | HRb (95 % CI) | p valuec |
|---|---|---|---|---|---|---|
| N0 | 1.0 | 0.5 (0.3–0.7) | <0.0001 | 1.0 | 0.6 (0.4–0.9) | 0.018 |
| N1 | 2.1 (1.4–3.0) | 1.0 | 1.6 (1.1–2.4) | 1.0 | ||
| N2 | 3.0 (1.9–4.6) | 1.4 (0.9–2.2) | 1.9 (1.1–3.2) | 1.2 (0.7–2.0) |
CI confidence interval, HR hazard ratio
HR based on Cox proportional hazard model only, including N stage
HR based on Cox proportional hazard model adjusting for age, sex, invasion size, T stage, surgical margin, site specific lassification
p value based on Chi square test (Wald test)
DISCUSSION
This large, multi-institutional study of ampullary carcinoma examined the prognostic implications of the number of positive LNs using recently proposed nodal substages with the primary outcome of survival after pancreatoduodenectomy for ampullary carcinoma. The proposed N-staging classification was found to stratify patients by survival outcomes in a manner that was clinically and statistically significant. The associations between proposed N stages and survival persisted in a multivariate model that included age, sex, tumor invasion size, tumor stage, perineural invasion, surgical margin, and site-specific classification.
Historically, ampullary carcinoma has been ill-defined. Recent studies have proposed guidelines to identify am-pullary carcinoma, which were used in the present study.18,19 Variability in classifying ampullary tumors generated studies with conflicting results, which led some authors to question the validity that ampullary carcinoma is a distinct entity.17 In this study, we found that the frequency of LN metastasis in ampullary carcinoma was 45 %, which is much lower than the documented LN metastasis rate for pancreatic ductal adenocarcinoma (PDAC) [78 %].22 To account for biases given the retrospective nature of this study, without the ability to control for the number of LNs sampled, cases with ≥12 LNs examined were separately analyzed. The rate of LN metastasis was 56 %, which was still lower than that of PDAC. Furthermore, ampullary carcinoma was found to have a better clinical outcome than is typical of PDACs.19,22 These differences support the clinical significance of the defining criteria employed and reinforce the identity of ampullary carcinoma as a separate clinical entity.
Multiple studies have shown the association between LN metastasis and shorter survival in ampullary carcinoma (Table 3).52–58 In many studies, the absence or presence of LN metastasis was found to be associated with overall survival38,39,41,42 or disease-free survival15,37,40 in univariate analysis. Only some of these associations persisted in multivariate analysis.26–36 As an extension of this, LN ratio was also found to be significant in some studies,44–48 whereas other studies found a different cut-off value of positive LNs to be significant.52–58
TABLE 3.
Different number (cut-off value) of positive lymph nodes deemed to be significant in the literature
| Author, year | No. of | Cut-off | Comments | Risk assessment |
|---|---|---|---|---|
| cases | values | determined by | ||
| Shroff et al. 52 | 92 | 0, 1–3, or > 3 | Disease-free and overall survival | |
| Sakata et al.53 | 71 | 0, 1–3, or ≥4 | The number of positive LNs better predicts the outcome than the LN ratio |
|
| Lee et al.54 | 52 | ≥3 | In univariate analysis, the number of positive LNs, and LN ratio and LN location are significantly correlated with survival |
Recurrence and survival |
| In multivariate analysis, the factor of ≥3 metastatic LNs is the only independent prognostic factor |
||||
| Choi et al.55 | 78 | 0–1 or ≥ 2 | LN number and presence of metastatic LNs do not affect overall survival |
Recurrence and overall survival |
| ≥2 metastatic LNs significantly affects disease-free survival | ||||
| Sommerville et al.56 | 39 | 0, 1–3, or > 3 | >3 vs. 0 metastatic LN hazard ratio is significant | Overall survival |
| Sierzega et al.57 | 111 | ≥4 | In univariate analysis, the presence of metastatic LNs, and their number and ratio are significantly correlated with survival |
Overall survival |
| Sakata et al.58 | 62 | 0, 1–3, or ≥4 | The number, not the location, of metastatic LNs independently affects long-term survival |
Overall survival |
LNs lymph nodes
The current AJCC staging system for ampullary carcinoma stratifies patients into N-stage groups reflecting only the absence or presence of LN metastasis without consideration of the number of positive LNs.59 For many other types of carcinomas, the number of positive LNs had been found to be of prognostic significance and is included in their respective N-stage guidelines.59 For ampullary cancer, previous studies have found that the number of positive LNs is associated with survival; however, the value differed in studies with >2, >3, and >4 being advocated.52–58 In their analysis of the SEER database, Kang et al. recently determined that stratifying positive LNs as N1 (1 or 2 LNs) versus N2 (3 or more LNs) had significant prognostic value.60 Their findings were separately validated in an institutional cohort, although the detailed criteria of patient selection for the latter were not provided. In this study, the value of this nodal substaging was analyzed, and we validated this proposal in a well-characterized, pathologically-verified cohort, both in univariate and multivariate survival analysis.
In addition to the established relationship to survival, the proposed N substage protocol suggested an association with features observed in aggressive disease, including lymphovascular invasion, perineural invasion, surgical margin positivity, and invasive tumor size. This observation is similar to the results found by Kang et al.60
The prognostic differences in three N-stage groups persist in subset analysis of cases with ≥12 LNs examined. The rate of LN-positive cases was 56 % in the ≥12 LN group compared with 45 % in all patients, suggesting that lack of LN sampling could lead to understaging. Furthermore, in this subset, the survival curve of N2 category separates more dramatically from the N0 and N1 groups. Intriguingly, also in this subset, the N0 and N1 groups begin to have overlapping survival curves. The fact that N0 and N1 (1–2 metastatic LNs) cases have similar survival in this better-sampled subset with ≥12 LNs may be attributable to various factors, one of which is direct invasion of LN being regarded as ‘metastasis’ by AJCC TNM classification. Some authors believe that direct invasion does not imply the same thing as true LN metastasis as the cells do not yet have the ability to grow and survive within lymphatic channels, and extravasate and form self-sustaining colonies in LNs.65 Of note, due to anatomic complexity, it may be difficult to determine whether an LN is involved directly or represents true metastasis in the periampullary region. Another possibility for the similar survivals of N0 and N1 cases in this better-documented group might be that only cases with the ability to metastasize to multiple LNs represent the true biologic aggressiveness of the disease. This may also explain why N2 cases are more common than N1 cases. Regardless, the implication is that an improved LN sampling of pancreatoduodenectomy specimens enhances the prognostic value of separating LN-positive cases into N1 and N2 substages. Of note, LN sampling in the pathology gross room can be augmented by the orange-peeling method yielding improved LN harvest and, consequently, a better assessment of LN status.63,64 This can then have an impact on prognostication, as illustrated in the study by Partelli et al.66 In fact, in our study, in cases from the institution where the orange-peeling method is routinely applied, the frequency of N2 cases was higher (26 vs. 13.5 % in the remainder); thus, an orange-peeling approach, or other approach that generates more accurate LN harvest, should be adopted.
We acknowledge the limitation of this study design. This was a retrospective, multi-institutional study, therefore the gross dissection method of inspecting and sampling for LNs and data for the adjuvant therapy was not available. Additionally, retrospective analysis of data is limited to determining associations between factors and outcomes, and causality cannot be confirmed. Despite these drawbacks, the present study is important because it compares the current N-stage guidelines for ampullary carcinoma with proposed guidelines to create substages within the LN-positive population. The results from the current study validate the prognostic significance of the substaging proposed by Kang et al., which should be integrated into future N-stage guidelines for ampullary carcinoma.60
CONCLUSIONS
In summary, defined with the revised criteria, ampullary carcinoma is a distinct entity. LN status is strongly associated with survival. Furthermore, substaging N-stage, as proposed by Kang et al., with 1–2 LN-positive (N1) and ≥3 LN-positive (N2) cases offers improved stratification of survival in ampullary carcinoma. Considering that there are very few established predictors of outcome in ampullary carcinoma, incorporating substaging of LN-positive cases is strongly recommended as a part of the TNM stage of these tumors.
ACKNOWLEDGMENT
The authors would like to thank Allyne Manzo and Lorraine Biedrzycki for assistance with the figures.
Footnotes
DISCLOSURES None of the authors have no affiliation with, or financial involvement in, any organization with a direct financial interest in the subject matter or materials discussed in the manuscript.
This study was presented in part at the annual meeting of the United States and Canadian Academy of Pathology, San Diego, CA, USA, 1–7 March 2014.
REFERENCES
- 1.Klimstra DS, Albores-Saavedra J, Hruban RH, Zamboni G. Tumours of the ampullary region. In: FT F, Bosman RH, Carneiro ND, Hruban RH, Theise ND, editors. WHO classification of tumours of the digestive system. Lyon: International Agency for Research on Cancer (IARC); 2010. pp. 81–94. [Google Scholar]
- 2.Adsay NV, Basturk O. Tumors of major and minor ampulla. In: Odze R, Goldblum J, editors. Surgical pathology of the GI tract, liver, biliary tract, and pancreas. Philadelphia: Elsevier; 2014. pp. 1120–1139. [Google Scholar]
- 3.Thompson LDR, Basturk O, Adsay NV. Pancreas. In: Mills SE, editor. Sternberg’s diagnostic surgical pathology. Philadelphia: Lippincott Williams & Wilkins; 2015. pp. 1577–1662. [Google Scholar]
- 4.Shi HY, Wang SN, Lee KT. Temporal trends and volume-outcome associations in periampullary cancer patients: a propensity score-adjusted nationwide population-based study. Am J Surg. 2014;207:512–519. doi: 10.1016/j.amjsurg.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 5.Bronsert P, Kohler I, Werner M, et al. Intestinal-type of differentiation predicts favourable overall survival: confirmatory clinicopathological analysis of 198 periampullary adenocarcino-mas of pancreatic, biliary, ampullary and duodenal origin. BMC Cancer. 2013;13:428. doi: 10.1186/1471-2407-13-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumari N, Prabha K, Singh RK, Baitha DK, Krishnani N. Intestinal and pancreatobiliary differentiation in periampullary carcinoma: the role of immunohistochemistry. Hum Pathol. 2013;44:2213–2219. doi: 10.1016/j.humpath.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Lee SR, Kim HO, Park YL, Shin JH. Lymph node ratio predicts local recurrence for periampullary tumours. ANZ J Surg. 2014;84:353–358. doi: 10.1111/ans.12129. [DOI] [PubMed] [Google Scholar]
- 8.Bornstein-Quevedo L, Gamboa-Dominguez A. Carcinoid tumors of the duodenum and ampulla of vater: a clinicomorphologic, immunohistochemical, and cell kinetic comparison. Hum Pathol. 2001;32:1252–1256. doi: 10.1053/hupa.2001.28955. [DOI] [PubMed] [Google Scholar]
- 9.Gutierrez JC, Franceschi D, Koniaris LG. How many lymph nodes properly stage a periampullary malignancy? J Gastrointest Surg. 2008;12:77–85. doi: 10.1007/s11605-007-0251-7. [DOI] [PubMed] [Google Scholar]
- 10.Kim K, Chie EK, Jang JY, et al. Prognostic significance of tumour location after adjuvant chemoradiotherapy for periampullary ade-nocarcinoma. Clin Transl Oncol. 2012;14:391–395. doi: 10.1007/s12094-012-0814-2. [DOI] [PubMed] [Google Scholar]
- 11.Maithel SK, Khalili K, Dixon E, et al. Impact of regional lymph node evaluation in staging patients with periampullary tumors. Ann Surg Oncol. 2007;14:202–210. doi: 10.1245/s10434-006-9041-9. [DOI] [PubMed] [Google Scholar]
- 12.Westgaard A, Pomianowska E, Clausen OP, Gladhaug IP. Intestinal-type and pancreatobiliary-type adenocarcinomas: how does ampullary carcinoma differ from other periampullary malignancies? Ann Surg Oncol. 2013;20:430–439. doi: 10.1245/s10434-012-2603-0. [DOI] [PubMed] [Google Scholar]
- 13.Riall TS, Cameron JL, Lillemoe KD, et al. Resected peri-ampullary adenocarcinoma: 5-year survivors and their 6- to 10-year follow-up. Surgery. 2006;140:764–772. doi: 10.1016/j.surg.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Ahn DH, Bekaii-Saab T. Ampullary cancer: an overview. Am Soc Clin Oncol Educ Book. 2014:112–115. doi: 10.14694/EdBook_AM.2014.34.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams JA, Cubilla A, Maclean BJ, Fortner JG. Twenty-two year experience with periampullary carcinoma at Memorial Sloan-Kettering Cancer Center. Am J Surg. 1979;138:662–665. doi: 10.1016/0002-9610(79)90341-6. [DOI] [PubMed] [Google Scholar]
- 16.Cubilla AL, Fitzgerald PJ. Surgical pathology aspects of cancer of the ampulla-head-of-pancreas region. Monogr Pathol. 1980;21:67–81. [PubMed] [Google Scholar]
- 17.Perysinakis I, Margaris I, Kouraklis G. Ampullary cancer: a separate clinical entity? Histopathology. 2014;64:759–768. doi: 10.1111/his.12324. [DOI] [PubMed] [Google Scholar]
- 18.Washington K, Berlin J, Branton P for the College of American Pathologists (CAP) [Accessed 24 Jan 2014];Protocol for the examination of specimens from patients with carcinoma of the ampulla of Vater. 2012 Available at: http://www.cap.org/apps/docs/committees/cancer/cancer_protocols/2012/Ampulla_12protocol_3101.pdf.
- 19.Adsay V, Ohike N, Tajiri T, et al. Ampullary region carcinomas: definition and site specific classification with delineation of four clinicopathologically and prognostically distinct subsets in an analysis of 249 cases. Am J Surg Pathol. 2012;36:1592–1608. doi: 10.1097/PAS.0b013e31826399d8. [DOI] [PubMed] [Google Scholar]
- 20.Ohike N, Kim GE, Tajiri T, et al. Intra-ampullary papillary-tubular neoplasm (IAPN): characterization of tumoral intraepithelial neoplasia occurring within the ampulla: a clinicopathologic analysis of 82 cases. Am J Surg Pathol. 2010;34:1731–1748. doi: 10.1097/PAS.0b013e3181f8ff05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tajiri T, Basturk O, Krasinskas A, et al. Prognostic differences between ampullary carcinomas and pancreatic ductal adenocarcinomas: the importance of size of invasive component [abstract] Mod Pathol. 2009;22:323A. [Google Scholar]
- 22.Saka B, Tajiri T, Ohike N, et al. Clinicopathologic comparison of ampullary versus pancreatic carcinoma: preinvasive component, size of invasion, stage, resectability and histologic phenotype are the factors for the significantly favorable outcome of ampullary carcinoma [abstract] Mod Pathol. 2013;26:429A. [Google Scholar]
- 23.Mori K, Ikei S, Yamane T, et al. Pathological factors influencing survival of carcinoma of the ampulla of Vater. Eur J Surg Oncol. 1990;16:183–188. [PubMed] [Google Scholar]
- 24.Shyr YM, Su CH, Wu LH, Li AF, Chiu JH, Lui WY. DNA ploidy as a major prognostic factor in resectable ampulla of Vater cancers. J Surg Oncol. 1993;53:220–225. doi: 10.1002/jso.2930530406. [DOI] [PubMed] [Google Scholar]
- 25.Woo SM, Ryu JK, Lee SH, et al. Recurrence and prognostic factors of ampullary carcinoma after radical resection: comparison with distal extrahepatic cholangiocarcinoma. Ann Surg Oncol. 2007;14:3195–3201. doi: 10.1245/s10434-007-9537-y. [DOI] [PubMed] [Google Scholar]
- 26.Zhou J, Zhang Q, Li P, Shan Y, Zhao D, Cai J. Prognostic factors of carcinoma of the ampulla of Vater after surgery. Tumour Biol. 2014;35(2):1143–1148. doi: 10.1007/s13277-013-1153-9. [DOI] [PubMed] [Google Scholar]
- 27.Kim WS, Choi DW, Choi SH, Heo JS, You DD, Lee HG. Clinical significance of pathologic subtype in curatively resected ampulla of vater cancer. J Surg Oncol. 2012;105:266–272. doi: 10.1002/jso.22090. [DOI] [PubMed] [Google Scholar]
- 28.Inoue Y, Hayashi M, Hirokawa F, Egashira Y, Tanigawa N. Clinicopathological and operative factors for prognosis of carcinoma of the ampulla of vater. Hepatogastroenterology. 2012;59:1573–1576. doi: 10.5754/hge10742. [DOI] [PubMed] [Google Scholar]
- 29.Kohler I, Jacob D, Budzies J, et al. Phenotypic and genotypic characterization of carcinomas of the papilla of Vater has prognostic and putative therapeutic implications. Am J Clin Pathol. 2011;135:202–211. doi: 10.1309/AJCPCTCUQSYI89YT. [DOI] [PubMed] [Google Scholar]
- 30.Ohike N, Coban I, Kim GE, et al. Tumor budding as a strong prognostic indicator in invasive ampullary adenocarcinomas. Am J Surg Pathol. 2010;34:1417–1424. doi: 10.1097/PAS.0b013e3181f0b05a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsu HP, Shan YS, Jin YT, Lai MD, Lin PW. Loss of E-cadherin and beta-catenin is correlated with poor prognosis of ampullary neoplasms. J Surg Oncol. 2010;101:356–362. doi: 10.1002/jso.21493. [DOI] [PubMed] [Google Scholar]
- 32.de Paiva Haddad LB, Patzina RA, Penteado S, et al. Lymph node involvement and not the histophatologic subtype is correlated with outcome after resection of adenocarcinoma of the ampulla of vater. J Gastrointest Surg. 2010;14:719–728. doi: 10.1007/s11605-010-1156-4. [DOI] [PubMed] [Google Scholar]
- 33.Lowe MC, Coban I, Adsay NV, et al. Important prognostic factors in adenocarcinoma of the ampulla of Vater. Am Surg. 2009;75:754–760. discussion 761. [PubMed] [Google Scholar]
- 34.Yao HS, Wang Q, Wang WJ, Hu ZQ. Intraoperative allogeneic red blood cell transfusion in ampullary cancer outcome after curative pancreatoduodenectomy: a clinical study and meta-analysis. World J Surg. 2008;32:2038–2046. doi: 10.1007/s00268-008-9675-9. [DOI] [PubMed] [Google Scholar]
- 35.Shimizu Y, Kimura F, Shimizu H, Yoshidome H, Ohtsuka M, Miyazaki M. The morbidity, mortality, and prognostic factors for ampullary carcinoma and distal cholangiocarcinoma. Hepatogastroenterology. 2008;55:699–703. [PubMed] [Google Scholar]
- 36.Qiao QL, Zhao YG, Ye ML, et al. Carcinoma of the ampulla of Vater: factors influencing long-term survival of 127 patients with resection. World J Surg. 2007;31:137–143. doi: 10.1007/s00268-006-0213-3. discussion 144–136. [DOI] [PubMed] [Google Scholar]
- 37.Chen J, Cai S, Dong J. Predictors of recurrence after pancreaticoduodenectomy for carcinoma of the ampulla of Vater [in Chinese] Nan Fang Yi Ke Da Xue Xue Bao. 2012;32:1242–1244. [PubMed] [Google Scholar]
- 38.Showalter TN, Zhan T, Anne PR, et al. The influence of prognostic factors and adjuvant chemoradiation on survival after pancreaticoduodenectomy for ampullary carcinoma. J Gastroin-test Surg. 2011;15:1411–1416. doi: 10.1007/s11605-011-1518-6. [DOI] [PubMed] [Google Scholar]
- 39.Casaretto E, Andrada DG, Granero LE. Results of cephalic pancreaticoduodenectomy for ampullary carcinoma. Analysis of 18 consecutive cases [in Spanish] Acta Gastroenterol Latinoam. 2010;40:22–31. [PubMed] [Google Scholar]
- 40.Sudo T, Murakami Y, Uemura K, et al. Prognostic impact of perineural invasion following pancreatoduodenectomy with lymphadenectomy for ampullary carcinoma. Dig Dis Sci. 2008;53:2281–2286. doi: 10.1007/s10620-007-0117-6. [DOI] [PubMed] [Google Scholar]
- 41.Barauskas G, Gulbinas A, Pranys D, Dambrauskas Z, Pundzius J. Tumor-related factors and patient’s age influence survival after resection for ampullary adenocarcinoma. J Hepatobiliary Pan-creat Surg. 2008;15:423–428. doi: 10.1007/s00534-007-1313-7. [DOI] [PubMed] [Google Scholar]
- 42.Sessa F, Furlan D, Zampatti C, Carnevali I, Franzi F, Capella C. Prognostic factors for ampullary adenocarcinomas: tumor stage, tumor histology, tumor location, immunohistochemistry and mi-crosatellite instability. Virchows Arch. 2007;451:649–657. doi: 10.1007/s00428-007-0444-1. [DOI] [PubMed] [Google Scholar]
- 43.Dahl S, Bendixen M, Fristrup CW, Mortensen MB. Treatment outcomes for patients with papilla of Vater cancer [in Danish] Ugeskr Laeger. 2010;172:1361–1365. [PubMed] [Google Scholar]
- 44.Pomianowska E, Westgaard A, Mathisen O, Clausen OP, Gladhaug IP. Prognostic relevance of number and ratio of metastatic lymph nodes in resected pancreatic, ampullary, and distal bile duct carcinomas. Ann Surg Oncol. 2013;20:233–241. doi: 10.1245/s10434-012-2592-z. [DOI] [PubMed] [Google Scholar]
- 45.Roland CL, Katz MH, Gonzalez GM, et al. A high positive lymph node ratio is associated with distant recurrence after surgical resection of ampullary carcinoma. J Gastrointest Surg. 2012;16:2056–2063. doi: 10.1007/s11605-012-2015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hurtuk MG, Hughes C, Shoup M, Aranha GV. Does lymph node ratio impact survival in resected periampullary malignancies? Am J Surg. 2009;197:348–352. doi: 10.1016/j.amjsurg.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 47.Zhou J, Zhang Q, Li P, Shan Y, Zhao D, Cai J. Prognostic relevance of number and ratio of metastatic lymph nodes in resected carcinoma of the ampulla of Vater. Chin J Cancer Res. 2013;25:735–742. doi: 10.3978/j.issn.1000-9604.2013.12.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Falconi M, Crippa S, Dominguez I, et al. Prognostic relevance of lymph node ratio and number of resected nodes after curative resection of ampulla of Vater carcinoma. Ann Surg Oncol. 2008;15:3178–3186. doi: 10.1245/s10434-008-0099-4. [DOI] [PubMed] [Google Scholar]
- 49.Bogoevski D, Chayeb H, Cataldegirmen G, et al. Nodal mi-croinvolvement in patients with carcinoma of the papilla of vater receiving no adjuvant chemotherapy. J Gastrointest Surg. 2008;12:1830–1837. doi: 10.1007/s11605-008-0683-8. discussion 1837–1838. [DOI] [PubMed] [Google Scholar]
- 50.van der Gaag NA, ten Kate FJ, Lagarde SM, Busch OR, van Gulik TM, Gouma DJ. Prognostic significance of extracapsular lymph node involvement in patients with adenocarcinoma of the ampulla of Vater. Br J Surg. 2008;95:735–743. doi: 10.1002/bjs.6076. [DOI] [PubMed] [Google Scholar]
- 51.Hornick JR, Johnston FM, Simon PO, et al. A single-institution review of 157 patients presenting with benign and malignant tumors of the ampulla of Vater: management and outcomes. Surgery. 2011;150:169–176. doi: 10.1016/j.surg.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shroff S, Overman MJ, Rashid A, et al. The expression of PTEN is associated with improved prognosis in patients with ampullary adenocarcinoma after pancreaticoduodenectomy. Arch Pathol Lab Med. 2013;137:1619–1626. doi: 10.5858/arpa.2012-0418-OA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sakata J, Shirai Y, Wakai T, Ajioka Y, Akazawa K, Hatakeyama K. Assessment of the nodal status in ampullary carcinoma: the number of positive lymph nodes versus the lymph node ratio. World J Surg. 2011;35:2118–2124. doi: 10.1007/s00268-011-1175-7. [DOI] [PubMed] [Google Scholar]
- 54.Lee JH, Lee KG, Ha TK, et al. Pattern analysis of lymph node metastasis and the prognostic importance of number of metastatic nodes in ampullary adenocarcinoma. Am Surg. 2011;77:322–329. [PubMed] [Google Scholar]
- 55.Choi SB, Kim WB, Song TJ, Suh SO, Kim YC, Choi SY. Surgical outcomes and prognostic factors for ampulla of Vater cancer. Scand J Surg. 2011;100:92–98. doi: 10.1177/145749691110000205. [DOI] [PubMed] [Google Scholar]
- 56.Sommerville CA, Limongelli P, Pai M, et al. Survival analysis after pancreatic resection for ampullary and pancreatic head carcinoma: an analysis of clinicopathological factors. J Surg Oncol. 2009;100:651–656. doi: 10.1002/jso.21390. [DOI] [PubMed] [Google Scholar]
- 57.Sierzega M, Nowak K, Kulig J, Matyja A, Nowak W, Popiela T. Lymph node involvement in ampullary cancer: the importance of the number, ratio, and location of metastatic nodes. J Surg Oncol. 2009;100:19–24. doi: 10.1002/jso.21283. [DOI] [PubMed] [Google Scholar]
- 58.Sakata J, Shirai Y, Wakai T, et al. Number of positive lymph nodes independently affects long-term survival after resection in patients with ampullary carcinoma. Eur J Surg Oncol. 2007;33:346–351. doi: 10.1016/j.ejso.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 59.Edge SB. AJCC cancer staging manual. 7th ed. New York: Springer; 2010. American Joint Committee on Cancer. [DOI] [PubMed] [Google Scholar]
- 60.Kang HJ, Eo SH, Kim SC, et al. Increased number of metastatic lymph nodes in adenocarcinoma of the ampulla of Vater as a prognostic factor: a proposal of new nodal classification. Surgery. 2014;155(1):74–84. doi: 10.1016/j.surg.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 61.Saka B, Bagci P, Krasinskas A, et al. Duodenal carcinomas of non-ampullary origin are significantly more aggressive than ampullary carcinomas. Mod Pathol. 2013;26:176A–177A. [Google Scholar]
- 62.Balci S, Kim GE, Ohike N, et al. Applicability and prognostic relevance of ampullary carcinoma histologic typing as pancre-atobiliary versus intestinal [abstract] Mod Pathol. 2010;23:350A. [Google Scholar]
- 63.Adsay NV, Basturk O, Altinel D, et al. The number of lymph nodes identified in a simple pancreatoduodenectomy specimen: comparison of conventional vs orange-peeling approach in pathologic assessment. Mod Pathol. 2009;22:107–112. doi: 10.1038/modpathol.2008.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Adsay NV, Basturk O, Saka B, et al. Whipple made simple for surgical pathologists: orientation, dissection, and sampling of pancreaticoduodenectomy specimens for a more practical and accurate evaluation of pancreatic, distal common bile duct, and ampullary tumors. Am J Surg Pathol. 2014;38(4):480–493. doi: 10.1097/PAS.0000000000000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pai RK, Beck AH, Mitchem J, et al. Pattern of lymph node involvement and prognosis in pancreatic adenocarcinoma: direct lymph node invasion has similar survival to node-negative disease. Am J Surg Pathol. 2011;35:228–234. doi: 10.1097/PAS.0b013e318206c37a. [DOI] [PubMed] [Google Scholar]
- 66.Partelli S, Crippa S, Capelli P, et al. Adequacy of lymph node retrieval for ampullary cancer and its association with improved staging and survival. World J Surg. 2013;37:1397–1404. doi: 10.1007/s00268-013-1995-8. [DOI] [PubMed] [Google Scholar]