Abstract
High-fat diets (HFD) are commonly used in rodents to induce obesity, increase serum fatty acids, and induce lipotoxicity in various organs. In-vitro studies commonly utilize individual free fatty acids (FFA) to study lipid exposure in an effort to model what is occurring in-vivo, however, these approaches are not physiological as tissues are exposed to multiple fatty acids in-vivo. Here we characterize circulating lipids in obese-prone rats fed a HFD in both fasted and fed states with the goal of developing physiologically relevant fatty acid mixtures for subsequent in-vitro studies. Rats were fed a HFD (60% kcal fat) or a control diet (10% kcal fat) for 3 weeks; liver tissue, and both portal and systemic blood was collected. Fatty acid profiles and absolute concentrations of triglycerides (TAG) and FFA in the serum and TAG, diacylglycerol (DAG), and phospholipids (PL) in the liver were measured. Surprisingly, both systemic and portal serum TAG were ~40% lower in HFD-fed compared to controls. Overall, compared to the control diet, HFD feeding consistently induced an increase in the proportion of circulating polyunsaturated fatty acids (PUFA) with a concomitant decline in monounsaturated fatty acids (MUFA), and saturated fatty acids (SFA) in both serum TAG and FFA. The elevations of PUFA were mostly attributed to increases in n-6 PUFA, linoleic acid and arachidonic acid. In conclusion, fatty acid mixtures enriched with linoleic and arachidonic acid in addition to SFA and MUFA should be utilized for in-vitro studies attempting to model lipid exposures that occur during in-vivo HFD condition.
Keywords: fatty acids, HFD, development of obesity, PUFA
Introduction
Increased ectopic lipid storage is a hallmark of high-fat diet (HFD)-induced obesity and metabolic disease [1–3]. FFA exposure has frequently been used to increase lipid storage and insulin resistance in various cell culture models of tissues known to participate in the development of obesity associated metabolic diseases (e.g. muscle, liver, adipose, endothelial, pancreas). The liver plays a central role in handling dietary lipids; it utilizes lipids as an energy source, stores them as TAG, or repackages them into lipoproteins for export to the periphery. Excessive lipid accumulation in the liver, or hepatic steatosis, can impair factors that suppress VLDL secretion and eventually lead to chronic dyslipidemia [4, 5]. Liver receives exposure to fatty acids from the blood stream of both systemic circulation and the portal vein. However, the differences of fatty acid profiles between systemic circulation and portal blood in relation to a HFD or obesity are still unclear. Obesity has been casually linked with elevated circulating lipids including both FFA [6] and TAG [7–9] which correlates strongly with insulin resistance [10]. These elevated lipids travel through the circulation and, after being hydrolyzed by lipoprotein lipase, can putatively result in tissue lipotoxicity that is associated with impaired insulin action and inflammation [11]. Due to the important regulatory roles of liver, primary hepatocytes, and immortalized hepatocyte cell lines have been widely used to study hepatic lipid metabolism and insulin resistance following FFA exposures [12, 13].
Many different fatty acid species or fatty acid mixtures have been used to study lipotoxicity in-vitro. Palmitic acid (16:0) and oleic acid (18:1, n-9) are the two most commonly used FFA [14, 15], as they are the dominant saturated fatty acid (SFA) and mono-unsaturated fatty acids (MUFA) in circulation and in most tissues, respectively [16]. The SFA palmitic acid has been most commonly used to induce increased lipid accumulation and insulin resistance [17–19] despite potential problems with cell toxicity if concentrations are too high. Oleic acid is sometimes added with palmitic acid to increase physiological relevancy [20]. However, tissues are exposed to more than one or two fatty acids in-vivo [16], therefore the most commonly used approaches to induce lipid accumulation in-vitro ignore the importance of fatty acid combinations in cellular lipid metabolism. Different fatty acid species cause various toxicity and/or inflammatory reactions to cells that may eventually alter cell functions [21], thus, it would be most logical to recapitulate in-vivo events that occur during HFD feeding conditions during the development of obesity. Here, we quantified the in-vivo circulating systemic and portal fatty acid profiles of obesity-prone rats that were fed a commonly used lard-based HFD (60% kcal fat) for 3 weeks to capture the initial stage of obesity. We made these measurements in rats who were fasted overnight, or who were allowed ad libitum access to food up until sample analysis to determine if the fasting or fed state impacted results. We also made measurements to compare the fatty acid profile in the portal blood versus systemic circulation to elucidate the fatty acids that liver is exposed to when fed a HFD. The results demonstrate the unique fatty acid profiles develop in the circulation following a short-term HFD feeding and strongly suggest that in-vitro work should utilize PUFA in addition to SFA and MUFA, if the goal is to model in-vivo conditions. The proportion of certain fatty acid species dramatically differs between portal serum and systemic serum, suggesting that both fatty acid pools should be considered when treating hepatocytes, whereas systemic fatty acid profile may be used in cell culture studies involving other cell types such as adipocytes, myocytes, endothelial cells…etc.
Materials and Methods
Animals
The protocol was approved by the Institutional Animal Care and Use Committee at the University of Missouri–Columbia and the Subcommittee for Animal Safety for the Research Service at the Harry S Truman Memorial VA Hospital. Thirty-four 5-wk-old obesity-prone Sprague-Dawley male rats were purchased from Taconic (Hudson, NY, USA) and housed in a room controlled for temperature (75°F), humidity (50%), and light cycle (12 hour light/dark). Obesity-prone rats were chosen so that there would be a consistent development of obesity upon feeding a HFD. Rats had access to ad libitum standard rodent chow (Purina Formulab 5008, St. Louis, MO, USA) and water. At the age of 7 weeks, rats were switched to a low-fat control diet with 10% kcal of total energy from fat ([50% of the fat from lard, 50% from soybean oil]; 20% kcal protein, 70% kcal carbohydrate, D12450B, Research Diet, Inc., New Brunswick, NJ, USA) for three weeks. At 10 weeks of age, half of the animals were switched to a HFD with 60% kcal fat ([92% of the fat from lard, 8% from soybean oil], 20% kcal protein, 20% kcal carbohydrate, D12492, Research Diets Inc., New Brunswick, NJ, USA) for three weeks while the other half were maintained on the control diet. This specific HFD was chosen because it is one of the most commonly reported diets in the literature to induce obesity and ectopic lipid storage. Diet composition and fatty acid profiles obtained from the manufacturer are shown in Supplemental Tables S1 and S2. We chose to perform a 3-week HFD feeding to access the changes of fatty acid profiles while animals were developing obesity, in which the changes of fatty acid profiles most likely reflect more from the dietary intervention instead of causing by diseases. Fourteen rats (n=7/group) were euthanized at 9am after a 12-h food withdraw (fasted state) whereas the other twenty rats (n=10/group) were fed ad libitum until euthanasia at 7am in the morning (fed state). More rats were used in the fed state group because more variation was expected due to ad libitum feeding prior to sacrifice. Blood was collected at both times to determine if fatty acid profiles differed during fed and fasted conditions. Both systemic circulation blood (cardiac puncture under anesthesia) and portal blood samples (portal vein catheterization) were collected and centrifuged (7000g, 10 min, 4°C) to separate serum after allowing clotting at room temperature for 20 min. Serum samples were aliquoted and stored in −80°C until analysis.
Lipid Extraction and Separation
Lipids were extracted using the method described by Folch et al. [22] with slight modifications as described previously [23]. Serum and liver samples from each animal were mixed or homogenized in ice-cold Trizma/EDTA buffer (50 mmol·L−1 Tris and 1 mmol·L−1 EDTA; pH 7.4). Twenty μg of 17:0 NEFA (Sigma, St. Louis, MO, USA) in chloroform and 25 μg of 17:0 TG (Nu-chek, Elysian, MN, USA) were added into each serum sample as internal standards. Similarly, 100 μg of 17:0 TG, 25μg of 17:0 DG (Nu-chek, Elysian, MN, USA), and 100μg of 17:0 phospholipids (PL; Avanti, Alabaster, AL, USA) were added into each liver sample as internal standards. Samples were mixed with 3mL of 0.5% of acetic acid in methanol, and with 6mL of chloroform. The mixtures were then centrifuged (190g, 10 min), and the organic phase was transferred into another glass conical tube through 1PS filter (Whatman, Piscataway, NJ, USA) and evaporated under a nitrogen stream before being reconstituted with 100μL of 2:1 chloroform: methanol. Extracted lipids were run on a silica gel 60Å analytical thin-layer chromatography (TLC) plate (Sigma, St. Louis, MO, USA) in a solvent system containing hexane/diethyl ether/acetic acid (70:30:1, by vol.). Lipid class standards were also spotted for detecting target bands. 2, 7-dichlorofluorescein was used to visualize individual lipid classes on the plate under a UV lamp. TAG and FFA bands in the serum samples and the TAG, DAG and PL bands in the liver samples were scraped and collected.
Fatty Acid Analyses
Collected lipid fractions were reconstituted in 500μL toluene. For TAG, DAG and PL fractions — 1mL of 0.5M sodium methoxide in anhydrous methanol was mixed with each sample for 1 min, and then mixed with 750μL distilled deionized water and 2 mL iso-octane. The samples were vortexed thoroughly and centrifuged under 190g for 10 min. After centrifugation, the upper layer was transferred into another glass tube through a sodium sulfate-filled glass pipette before blowing down under a nitrogen stream. For the FFA fractions—1.2 mL of methanol and 100μL of acetyl chloride were mixed with the scraped FFA fractions, the mixture was heated in a 100°C water bath for 60 min. Two mL hexane and 750μL distilled deionized water were mixed thoroughly with the FFA mixture. The upper phase was transferred into another conical glass tube through a sodium sulfate-filled glass pipette before blowing down under a nitrogen stream. Fatty acid methyl esters were reconstituted with 100μL heptane and transferred into auto-injector vials for analysis by gas chromatography (Agilent Technologies, Wilmington, DL, USA).
Statistical Analysis
One-way ANOVA was performed using SPSS software (IBM SPSS Statistics, V.19.0, IBM Corporation, Armonk, NY, USA) to determine differences in body weight, body weight gain, fat pad weight, food intake, and each fatty acid in the liver between groups. The differences between SFA, MUFA and PUFA in each diet group were also determined by one-way ANOVA. Two-way ANOVA was performed to compare serum fatty acids in control diet versus HFD and portal versus systemic blood samples within the fasted state or within the fed state. Post hoc analysis was performed by LSD comparisons where significant differences were found by ANOVA. Data are shown as mean ± SEM. Statistical significance was set at p<0.05.
Results
Body weight, fat pad weight and food intake
As expected, both final mean body weight and weight gain over the 3-wk period were higher in the HFD group compared to the control group (Table 1). HFD fed rats had significantly heavier fat pad mass (sum of epididymal and retroperitoneal fat pads) compared to the control group. Food intake by weight in the HFD group was markedly lower (p<0.05) than the control diet group, but caloric intake was higher (p<0.05) due to the greater energy density of the HFD.
Table 1.
Animal Characteristics.
| Control diet | HFD | |
|---|---|---|
| Final body weight (g) | 440.1 ± 8.9 | 479.0 ± 8.7* |
| Body weight change (g) | 126.8 ± 10.6 | 141.9 ± 14.1* |
| Fat pad weight (g) | 18.4 ± 0.7 | 25.6 ± 1.0* |
| Avg. Daily Food intake (g/day) | 25.4 ± 0.6 | 21.4 ± 0.7* |
| Avg. Daily Energy intake (kcal/day) | 97.7 ± 2.1 | 111.9 ± 3.7* |
Data are shown as mean ± SEM, n=17. Fat pad weight was the sum of epididymal and retroperitoneal fat pads.
indicated statistically significant difference (p<0.05) in the HFD group compare to the control diet group.
Total serum TAG and FFA concentrations
Total serum concentrations of TAG and FFA are shown in Fig. 1. Total serum TAG level was significantly lower (p<0.05) in the HFD fed rats compared to the control under both fasted and fed states (Fig. 1a). Total circulating TAG level was significantly higher (p<0.05) in the portal serum compared to systemic serum in the fed state, but no difference was found in the fasted state. In the fasted state, no significant differences were found either between diets or between the serum sources of total FFA (Fig. 1b). However, the HFD group had markedly higher total FFA concentration compared to the control diet in the fed state. Furthermore, total FFA level in the portal serum was significantly higher than systemic serum in the HFD fed rats.
Figure 1. Total serum (a) TAG and (b) FFA concentrations.
Data are shown as mean ± SEM, n=5–10 per group. * denoted diet effect (control vs. HFD); + denoted serum source effect (systemic vs. portal) within the diet group; $ denoted interaction, p<0.05.
Fatty acid profile in systemic serum TAG
Complete serum fatty acid profile of serum TAG is shown in Supplemental Table S3. Total SFA, MUFA and PUFA were calculated to evaluate global changes in fatty acid composition. Control diet fed animals sacrificed under the fasted state had 54% of MUFA, 23% of PUFA and 23% of SFA of total fatty acids in their systemic serum TAG samples, whereas systemic serum of HFD fed rats had 32% of MUFA, 48% PUFA and 21% SFA in total fatty acids of serum TAG (Fig. 2a). Thus, HFD drastically shifted the dominant fatty acids from MUFA to PUFA in the systemic TAG after three weeks of HFD feeding. The PUFA in HFD serum was comprised of 72% linoleic acid, and 15% arachidonic acid. In the fasted state, the majority of the TAG in serum comes from liver suggesting that livers of HFD fed rats were releasing more n-6 PUFA than MUFA or SFA in TAG.
Figure 2. Fatty acid composition of systemic serum in TAG fraction under (a) fasted and (b) fed state in rats fed control or HFD.
Data are shown as mean ± SEM, n=5–10 per group. a,b,cMeans with different subscripts differ by p<0.05 within control diet. x,y,zMeans with different subscripts differ by p<0.05 within HFD.
In the fed state, systemic serum of control rats had 51% of MUFA, 19% of PUFA and 30% of SFA of the total fatty acids in TAG, whereas the HFD group had 42% MUFA, 36% PUFA and 22% SFA in the TAG fraction of their systemic serum (Fig. 2b). It appeared that a similar shift from MUFA in the control group to more PUFA in the HFD group also occurred in the fed state; however, the shifting was not as dramatic as seen in the fasted state. This is likely due to the serum TAG fraction measured in the ad libitum fed state reflecting the fatty acid composition of the diet, which is characterized by a MUFA-dominant profile.
Fatty acid profile of portal serum TAG
Similar to what we observed in the systemic serum TAG, control diet fed animals displayed a fatty acid profile with 53% of MUFA, 18% PUFA and 29% SFA in the portal serum under fasted state (Fig. 3a). HFD also shifted the predominant fatty acids to PUFA in the portal serum TAG during the fasted state. In this condition, the fatty acid profile of fasted portal serum TAG was composed of 32% MUFA, 39% PUFA and 29% SFA in the total fatty acids. In the fed state, the majority of fatty acids in the portal serum TAG of the control diet fed animals were MUFA (42% of total fatty acid), while PUFA and SFA accounted for 22% and 36%, respectively. HFD fed animals had 42% MUFA of total fatty acid, 34% of PUFA and 24% of SFA in their portal serum TAG (Fig. 3b).
Figure 3. Fatty acid composition of portal serum in TAG fraction under (a) fasted and (b) fed state in rats fed a control or HFD.
Data are shown as mean ± SEM, n=5–10 per group. a,b,cMeans with different subscripts differ by p<0.05 within control diet. x,y,zMeans with different subscripts differ by p<0.05 within HFD.
Overall, the profile in the portal serum was identical with systemic serum and the HFD group had a similar degree of shifting from MUFA to PUFA in both portal serum and systemic serum TAG. The proportion of SFA under the fasted state did not change between control diet and HFD in both serum sources. In the fed state, however, HFD fed rats had a lower proportion of SFA and higher proportion of PUFA compared to control diet.
Fatty acid profile of systemic serum FFA
Complete serum fatty acid profile in percent fatty acid of FFA fraction is shown in Supplemental Table S4. In the fasted state, the dominant fatty acids were SFA (45% of total fatty acids), followed by MUFA (32%), and then PUFA (23%) in the systemic serum FFA of control diet fed animals (Fig. 4a). The majority of the fatty acids in the SFA were stearic acid (50%) and palmitic acid (40%). In the systemic serum FFA of HFD fed animals, SFA was still the dominant fatty acids that represented 47% of the total fatty acids in the fasted state. However, there were more PUFA (31% of total fatty acids) in the systemic serum FFA of HFD group compared to the control group. MUFA only accounted for 22% of the total FFA in the systemic serum of the animals fed with HFD under fasted state (Fig. 4a). This finding suggested that a similar shift from MUFA to PUFA with HFD feeding still existed in the FFA fraction of systemic serum samples.
Figure 4. Fatty acid composition of systemic serum in FFA fraction under both (a) fasted and (b) fed state in rats fed a control or HFD.<.
br>Data are shown as mean ± SEM, n=5–10 per group. a,b,cMeans with different subscripts differ by p<0.05 within control diet. x,y,zMeans with different subscripts differ by p<0.05 within HFD.
In the fed state, 61% of the total fatty acids were SFA in the FFA fraction of systemic serum; 21% were MUFA; 18% were PUFA in the control diet fed animals (Fig. 4b). Whereas in the HFD fed rats, 45% of the total fatty acids in the FFA fraction of systemic serum were SFA, 25% were MUFA, and 30% were PUFA (Fig. 4b). The proportion of PUFA in systemic FFA increased significantly with HFD feeding in both fasted and fed states.
Fatty acid profile in portal serum FFA
The pattern of fatty acids composition in the FFA fraction of portal serum, as shown in Fig. 5, was similar to the systemic serum results. In the portal serum FFA of the control diet fed rats, the predominant fatty acids were still SFA, represented 53% of the total fatty acids in the fasted state; 32% of the total fatty acids were MUFA; 15% were PUFA under fasted state. In the HFD group, 50% of the total fatty acids in the portal serum FFA were SFA, 26% were MUFA, and 24% were PUFA under fasted state (Fig. 5a). Overall, the percentage of MUFA decreased in the HFD group and shifted to PUFA compared to control diet group. Fed state fatty acid profiles nearly mimicked those found with fasting. In the fed state, 59% of the total fatty acids in the FFA fraction of portal serum were SFA, 24% were MUFA, and the rest 17% were PUFA in the control diet group (Fig. 5b). In the HFD fed rats, 48% of the total fatty acids were SFA, 27% were MUFA, and 25% were PUFA in the FFA fraction of their portal serum under fed state.
Figure 5. Fatty acid composition of portal serum in FFA fraction under (a) fasted and (b) fed state in rats fed a control or HFD.<.
br>Data are shown as mean ± SEM, n=5–10 per group. a,b,cMeans with different subscripts differ by p<0.05 within control diet. x,y,zMeans with different subscripts differ by p<0.05 within HFD.
Palmitic acid (16:0) in the serum
Percent palmitic acid in the TAG and FFA fraction are shown in Table 2. HFD fed animals had significantly lower (p<0.05) palmitic acid in the TAG fraction under both fasted and fed states. Additionally, the HFD fed portal serum TAG palmitic acid was lower vs. systemic TAG palmitic acid (p<0.05) under fasted state. However, HFD fed portal serum TAG palmitic acid was not lower under fed state when compare to systemic TAG palmitic acid. A significant interaction between diet interventions and serum sources was observed in the fed state (p<0.05). The increased palmitic acid in the control diet fed animals might indicate an increase in de novo lipogenesis which is logical given their high carbohydrate intake. Similar with TAG fraction, HFD group had significantly lower (p<0.05) palmitic acid in the FFA fraction in both fasted and fed state. The portal serum had higher (p<0.05) palmitic acid under fasted state; no difference was found between serum sources in the fed state.
Table 2.
Percent fatty acid in TAG and FFA fractions of systemic and portal serum under fasted and fed state.
| Fasted State | Fed State | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Control | HFD | Control | HFD | ||||||
|
| |||||||||
| Fatty Acid | Lipid Fraction | Systemic | Portal | Systemic | Portal | Systemic | Portal | Systemic | Portal |
| 16:0 | TAG (%) | 17.4 ± 1.6 | 27.0 ± 0.9† | 12.5 ± 1.5* | 21.6 ± 1.4†,* | 26.3 ± 1.0 | 31.6 ± 1.9† | 15.5 ± 0.9* | 15.6 ± 1.1* |
| FFA (%) | 18.7 ± 3.9 | 34.0 ± 1.8† | 15.1 ± 1.5* | 25.8 ± 2.8†,* | 25.8 ± 2.6 | 19.8 ± 2.2 | 14.4 ± 1.4* | 16.1 ± 1.2* | |
|
| |||||||||
| 18:0 | TAG (%) | 2.6 ± 0.2 | 2.0 ± 0.2 | 8.1 ± 0.9* | 7.3 ± 2.5* | 3.3 ± 0.2 | 4.4 ± 0.5 | 7.9 ± 0.3* | 7.9 ± 0.3* |
| FFA (%) | 22.4 ± 2.5 | 17.2 ± 2.6† | 27.6 ± 1.9 | 22.0 ± 2.3† | 33.9 ± 2.9 | 36.4 ± 3.5 | 29.5 ± 1.4 | 30.8 ± 2.5 | |
|
| |||||||||
| 18:1, n-9 | TAG (%) | 39.7 ± 1.4 | 34.1 ± 1.0 | 28.4 ± 1.0* | 28.5 ± 1.9* | 35.0 ± 0.6 | 28.0 ± 3.2 | 38.4 ± 0.3* | 39.2 ± 0.4* |
| FFA (%) | 24.4 ± 1.8 | 19.8 ± 1.8 | 20.3 ± 2.1 | 22.4 ± 2.4 | 16.7 ± 1.4 | 17.6 ± 1.5 | 23.4 ± 1.4* | 26.2 ± 1.6* | |
|
| |||||||||
| 18:2, n-6 | TAG (%) | 15.9 ± 1.2 | 14.3 ± 1.4 | 29.6 ± 0.8* | 28.3 ± 0.9* | 15.7 ± 0.9 | 19.4 ± 1.6† | 26.5 ± 0.7* | 27.9 ± 0.2* |
| FFA (%) | 10.5 ± 0.8 | 9.5 ± 1.0 | 15.8 ± 1.8* | 17.5 ± 2.1* | 8.3 ± 1.4 | 10.8 ± 1.2 | 17.9 ± 1.0* | 19.5 ± 1.2* | |
|
| |||||||||
| 20:4, n-6 | TAG (%) | 3.3 ± 0.6 | 2.0 ± 0.3† | 7.9 ± 0.8* | 5.9 ± 0.9†,* | 0.7 ± 0.1 | 1.0 ± 0.1 | 3.3 ± 0.3* | 3.9 ± 0.4* |
| FFA (%) | 8.1 ± 1.4 | 3.8 ± 0.4† | 8.8 ± 1.0 | 5.0 ± 0.5† | 6.3 ± 0.6 | 5.1 ± 1.2† | 8.4 ± 0.8 | 5.0 ± 0.8† | |
Data are shown as mean ± SEM, n=5–10 per group.
denotes serum source effect (systemic vs. portal serum) within the diet group,
denotes main diet effect (control vs. HFD), p<0.05
Stearic acid (18:0) in the serum
Percent stearic acid in the TAG and FFA fraction are shown in Table 2. The HFD group had significantly higher (p<0.05) stearic acid in the TAG fraction in both fasted and fed state, however, the percent steric acid in the portal vs. systemic serum TAG did not differ. The proportion of steric acid in the portal serum FFA was significantly lower compared to the systemic serum FFA in both diet conditions in the fasted state. Neither diet nor serum source effects were found in FFA fraction under the fed state.
Oleic acid (18:1, n-9) in the serum
Percent oleic acid in the TAG and FFA fraction are shown in Table 2. The HFD group had significantly lower (p<0.05) oleic acid in the TAG fraction in the fasted state, but markedly higher in the fed state. As for percent oleic acid in the FFA, the fed state, HFD group had higher (p<0.05) levels in the FFA fraction compared to the control diet group; neither diet nor serum source effects were found in FFA fraction under the fasted state.
Linoleic acid (18:2, n-6) in the serum
Percent linoleic acid in the TAG and FFA fraction are shown in Table 2. The percentages of linoleic acid in the serum TAG of HFD fed animals were significantly higher (p<0.05) compared to the control diet group in both fasted and fed state. Portal serum TAG had higher (p<0.05) linoleic acid than systemic serum in the fed state. The percent linoleic acid in the FFA fraction was also significant higher (p<0.05) in the HFD group in both fasted and fed states compared to control diet fed animals, but no difference were found between serum sources.
Arachidonic acid (20:4, n-6) in the serum
Significantly higher (p<0.05) percent of arachidonic acid in the serum TAG were found in the HFD fed compared to control diet group in both fasted and fed states (Table 2). Portal serum had significantly lower (p<0.05) percentage of arachidonic acid compared to systemic serum TAG in the fasted state. The percent of arachidonic acid in the portal serum was lower (p<0.05) than systemic serum in the FFA fraction under both fasted and fed states. No diet effect was found in the percent of arachidonic acid in the FFA fraction.
Liver TAG, DAG and PL
In the fasted state, total liver TAG level was higher in the HFD group compared to the control diet fed animals (Fig. 6a). In contrast, total liver TAG level did not differ between diet groups under fed state. However, liver TAG was higher in the fed state compared to the fasted state. Diet interventions did not change the level of total DAG in the liver, but the total liver DAG in the fed state was higher than the fasted state (Fig. 6b). Total PL concentration in the liver did not differ between diet groups or fasted/fed status (Fig. 6c). Fatty acid profile in the liver TAG, DAG and PL fractions are shown in the supplemental materials (Supplemental Table S5, S6, S7). Fasted liver TAG data in Supplemental Table S5 has been previously published from our group [24].
Figure 6. Total liver (a) TAG, (b) DAG and (c) PL concentrations.
Data are shown as mean ± SEM, n=4 per group. * denotes diet effect (control vs. HFD); + denoted feeding status (fasted vs. fed), p<0.05
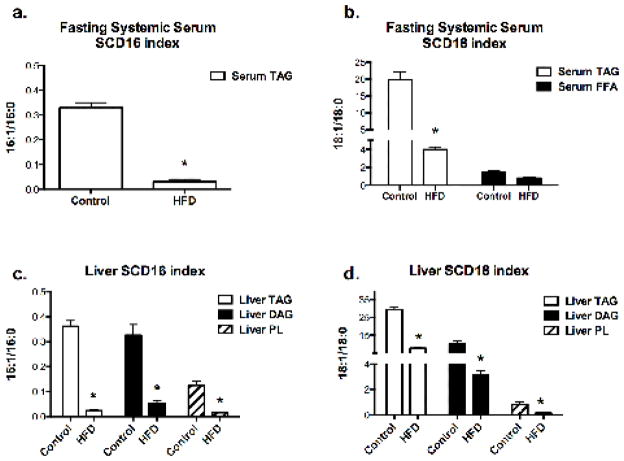
SCD1 indices in the serum and liver
SCD-1, also called delta-9 desaturase, is an enzyme that converts SFA (16:0 and 18:0) to MUFA (16:1 and 18:1), and thus modulates the percentages of SFA and MUFA. Previous studies have reported that liver SCD1 mRNA expression is positively correlated with SCD1 desaturase indices in the liver and the plasma triglyceride fractions, and the elevated SCD-18 index is associated with increased plasma TAG levels [25, 26]. SCD1 desaturase indices are good biomarkers for actual enzyme activity and have also been proposed as a good clinical indicator of SCD1 gene expression. Hence, to assess SCD1 activity we calculated two different SCD1 indices by dividing the total concentration of 16:1 with 16:0 (SCD-16) and 18:1 with 18:0 (SCD-18) in each lipid fraction from serum and liver extracts [25, 27]. In the HFD group, the SCD-16 and SCD-18 indices were decreased in the liver TAG, DAG, and PL fractions as well as fasting systemic serum TAG (Fig. 7). SCD-16 in the systemic serum FFA fraction was not calculable because 16:1 was not detectable in most of the samples. Fasting systemic serum of HFD fed rats had significantly lower (p<0.05) SCD-16 and SCD-18 indices compared to the control (Fig. 7a&b). SCD-18 in fasting systemic serum FFA fraction did not change with HFD feeding (Fig. 7b). SCD-16 and 18 in the liver TAG, DAG and PL were all significantly lower (p<0.05) with HFD feeding under fasted state (Fig. 7c&d).
Figure 7. SCD16 and SCD18 indices in (a, b) fasting systemic serum and (c, d) liver.
Data are shown as mean ± SEM, n=4 per group. * indicated a significant difference in the HFD versus control diet, p<0.05
Discussion
Overconsumption of dietary fat is widely used to induce obesity and insulin resistance in in-vivo rodent models, whereas excessive fatty acids are commonly used in cell culture to mimic the in-vivo environment of HFD-induced lipotoxicity. The impact of a HFD on fatty acids in the circulation is unclear; therefore, the fatty acid species currently used for creating lipotoxicity in cell culture models potentially fail to imitate what organs are exposed to in-vivo. Here, we report that three weeks of HFD feeding in obesity-prone Sprague-Dawley rats led to a greater proportion of circulating PUFA compared to MUFA in both TAG and FFA fractions compared to the control diet fed animals. The increased proportion of PUFA was mainly attributed to increases in linoleic and arachidonic acids. Overall, these data suggest that in-vitro experiments solely using palmitic or oleic acids are not adequately mimicking circulating lipids in HFD fed rodents.
The short three-week HFD used in the current study was chosen to examine the effect of the HFD and to avoid the abnormal metabolism that may be induced by a more chronic HFD. Obesity-prone rats have a thrifty phenotype marked by a consistent, elevated storage of fat into adipose tissue and reduced oxidation in skeletal muscle [28]. The larger fat pad masses and 2-fold higher liver TAG content in the HFD group support this theory. Under fasted conditions, total serum FFA level was no different between control diet and HFD, conflicting with the elevated FFA levels previously reported in the serum of obese human subjects [6]. However, the animals in the current study received HFD over a relatively short duration and the resulting obesity might not have been severe enough to display abnormal FFA levels under a fasted state. Moreover, some data suggest that obesity is not always linked with elevated serum FFA [29]. In contrast, the HFD led to greater circulating serum FFA levels in the fed state, likely a result of both increased dietary intake of lipids and an impaired ability of postprandial insulin to effectively inhibit lipolysis.
In the current study, 3 weeks of HFD feeding altered both serum TAG and FFA composition from predominantly MUFA to predominantly PUFA. Linoleic acid and arachidonic acid, largely accounted for the increased percentage of PUFA in the serum TAG (72% and 15% of the PUFA were linoleic acid and arachidonic acid, respectively) and serum FFA (83% and 4%, respectively). Although we can only speculate, it is possible that the increased percentage of PUFA in circulating FFA pools result from an increased flux of SFA and MUFA to storage or oxidative pathways. But this is purely speculation. The mechanisms by which PUFA increased in circulating FFA and TAG following a HFD are beyond the scope of this investigation but clearly deserve further study.
Linoleic acid (18:2, n-6) is an essential dietary fatty acid that serves as a precursor for in-vivo arachidonic acids synthesis. The HFD used in the current study had more linoleic acid compared to the control diet, and linoleic acid is present in relatively high amounts in the portal and systemic serum of the HFD-fed rats. This has particular implications for in-vitro FFA incubations studies. Serum utilized to culture cells typically has linoleic acid, an important component given that it is an essential fatty acid. However, in-vitro fatty acid exposure studies are usually performed using serum-free media, and thus media free of linoleic acid. This is a major concern and could negatively impact a number of outcome measures and further highlights that current methods for in-vitro fatty acid exposure studies largely ignore physiological relevance.
n-6 PUFA have been long suggested as pro-inflammatory due to their downstream pathways that produce pro-inflammatory eicosanoids, however, this view has been challenged by a human randomized clinical trial showing that serum inflammatory markers were not affected with increased n-6 PUFA consumption [30]. At the same time, there are numerous reports showing that linoleic acid and arachidonic derived metabolites have potent and substantial effects on the nuclear receptors LXR and PPARs that subsequently can alter lipid metabolism, insulin signaling, and adipogenesis [31–33]. Overall, our data show that the percentage of fatty acids comprised of n-6 PUFA do increase with HFD feeding and subsequently that in-vitro studies should not ignore the important role that these fatty acids may have on outcome measures.
The changes observed in percentage of SFA and MUFA due to HFD could also be modulated by the enzyme SCD1, predominantly expressed in the liver, which converts saturated fatty acids (16:0 and 18:0) derived from dietary fatty acids or from de novo lipogenesis into MUFA (16:1 and 18:1). As stated above, previous studies have reported that liver SCD1 mRNA expression is positively correlated with SCD1 desaturase indices in the liver and the plasma triglyceride fractions, and the elevated SCD-18 index is associated with increased plasma TAG levels [25, 26]. Therefore, we used SCD-1 indices to assess serum liver SCD-1 activity. Numerous studies have suggested that SCD-1 indices have been found to correlate positively with obesity and the development of metabolic syndrome [34, 35]. Moreover, high SCD-1 activity is associated with decreased fat oxidation and increased fatty acid synthesis [36, 37]. Hence, we were surprised to find that both of the SCD-1 indices, SCD-16 (16:1/16:0) and SCD-18 (18:1/18:0), in the serum and liver samples of current study were all decreased in the HFD group. It is possible that the decreased SCD-1 indices found in the current study were associated with the lower serum triglyceride level in the HFD fed animals. Additionally, a previous study showed that a 3-day high carbohydrate and low fat diet (75%kcal carbohydrate, 10%kcal fat) feeding increased the SCD-16 index in the VLDL triglycerides in human subjects compared to a diet with 40%kcal fat and 45%kcal carbohydrate [38]. The control diet used in this study had a similar high percentage of carbohydrates (70%kcal carbohydrates), which possibly explains the SCD-1 indices being higher in the control versus the HFD group. Furthermore, a recent study conducted by Li et al. [39] suggested that elevated SFA following decreased SCD-1 activity in the liver results in hepatocellular apoptosis, steatohepatitis and fibrosis. In contrast, increased SCD-1 activity in the liver partitioned excess SFA into MUFA and a benign hepatic steatosis [39]. Overall, these findings suggest that suppression of SCD-1 may lead to greater hepatic lipotoxicity.
Even though the overall profile in the portal serum was identical with systemic serum and the HFD group had a similar degree of shifting from MUFA to PUFA in both portal serum and systemic serum TAG, percentages of certain fatty acid species shifted dramatically when compared the portal serum vs. systemic serum. For example, percentage of 16:0 in the portal serum was significantly higher in both control and HFD when compared to systemic serum. Because liver is exposed to fatty acids from the blood stream of both systemic circulation and the portal vein, the differences of fatty acid profile in the systemic circulation and portal vein should be taken into consideration when treating hepatocytes with FFA in-vitro. On the other hand, the systemic fatty acid profile observed in this study may be used in cell culture studies involving other cell types such as adipocytes, myocytes, endothelial cells…etc.
It is important to mention that ceramide species and other sphingolipids were not measured in this work, some of which have been shown to contribute to insulin resistance and often associated with multiple obesity related diseases that deserve further study [40]. Another important question is whether the fatty acid profile measured in this study is human relevant. Certainly, the percent energy coming from fatty acids in the rodent diet (60%) is much higher than what humans typically consume. However, NHANES data would suggest that the relative percentage of total fat that comes from SFA, MUFA, and PUFA sources are quite similar to the rodent HFD utilized here[41]. Thus, there is the possibility that dietary similarities may lead to similar results in circulating lipids. However, digestive, and metabolic differences between humans and rodents may ultimately lead to different circulating fatty acids. We would posit that the fatty acid profile described here, while not confirmed in humans, would still be a more physiologically relevant method than using single fatty acids for in-vitro studies.
In conclusion, 3 weeks of HFD alters serum fatty acid composition of TAG and FFA from predominantly MUFA to predominantly n-6 PUFA. The increased linoleic acid and arachidonic acid contributed to the majority of the elevation of n-6 PUFA. Therefore, a more physiologically relevant in-vitro fatty acid mixture to mimic the initial stage of HFD induced obesity should include increased linoleic acids and arachidonic acids relative to SFA and MUFA. Further studies are needed to determine if in-vitro studies using these FFA would induce meaningful alterations in inflammation, ectopic lipid storage, and insulin signaling.
Supplementary Material
Acknowledgments
This work was funded by National Institutes of Health (NIH R01DK088940 (JPT)) and 5T32 AR-48523-8 (EMM). This work was also completed in part with resources of the Research Services Department of the Harry S Truman Memorial Veterans Hospital. We would like to thank Abiezer Blandon, Grace M. Meers, Melissa A. Linden and Justin A. Fletcher for their help and technical assistance towards this study.
List of Abbreviations
- DAG
diacylglycerol(s)
- FFA
non-esterified fatty acids
- MAG
monoacylglycerol(s)
- MUFA
monounsaturated fatty acid(s)
- PL
phospholipids
- PUFA
polyunsaturated fatty acid(s)
- SFA
saturated fatty acid(s)
- TAG
triacylglycerol(s)
Footnotes
Conflict of Interest
The authors have no conflicts of interest, financial or otherwise, to disclose for this research.
Contributor Information
Tzu-Wen Liu, Email: tliu42@illinois.edu.
Timothy D. Heden, Email: hedent14@ecu.edu.
E. Matthew Morris, Email: morrisem@health.missouri.edu.
Kevin L. Fritsche, Email: FritscheK@missouri.edu.
Victoria J. Vieira-Potter, Email: vieirapotterv@missouri.edu.
John P. Thyfault, Email: jthyfault@kumc.edu.
References
- 1.Kewalramani G, Bilan PJ, Klip A. Muscle insulin resistance: assault by lipids, cytokines and local macrophages. Current opinion in clinical nutrition and metabolic care. 2010;13:382–390. doi: 10.1097/MCO.0b013e32833aabd9. [DOI] [PubMed] [Google Scholar]
- 2.Szendroedi J, Roden M. Ectopic lipids and organ function. Current opinion in lipidology. 2009;20:50–56. doi: 10.1097/mol.0b013e328321b3a8. [DOI] [PubMed] [Google Scholar]
- 3.O’Rourke AT, Peacock M, Samuel JE, Frazier ME, Natvig DO, Mallavia LP, Baca O. Genomic analysis of phase I and II Coxiella burnetii with restriction endonucleases. Journal of general microbiology. 1985;131:1543–1546. doi: 10.1099/00221287-131-6-1543. [DOI] [PubMed] [Google Scholar]
- 4.Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology. 2012;142:711–725. e716. doi: 10.1053/j.gastro.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Mortiaux A, Dawson AM. Plasma free fatty acid in liver disease. Gut. 1961;2:304–309. doi: 10.1136/gut.2.4.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bjorntorp P, Bergman H, Varnauskas E. Plasma free fatty acid turnover rate in obesity. Acta medica Scandinavica. 1969;185:351–356. doi: 10.1111/j.0954-6820.1969.tb07347.x. [DOI] [PubMed] [Google Scholar]
- 7.Yuan G, Al-Shali KZ, Hegele RA. Hypertriglyceridemia: its etiology, effects and treatment. Cmaj. 2007;176:1113–1120. doi: 10.1503/cmaj.060963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunzell JD. Clinical practice. Hypertriglyceridemia. N Engl J Med. 2007;357:1009–1017. doi: 10.1056/NEJMcp070061. [DOI] [PubMed] [Google Scholar]
- 9.Lee H, Lee H, Cho J, Stampfer M, Willett W, Kim C, Cho E. Overall and abdominal adiposity and hypertriglyceridemia among Korean adults: the Korea National Health and Nutrition Examination Survey 2007–2008. Eur J Clin Nutr. 2013;67:83–90. doi: 10.1038/ejcn.2012.181. [DOI] [PubMed] [Google Scholar]
- 10.Reaven GM. The insulin resistance syndrome: definition and dietary approaches to treatment. Annu Rev Nutr. 2005;25:391–406. doi: 10.1146/annurev.nutr.24.012003.132155. [DOI] [PubMed] [Google Scholar]
- 11.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. The Journal of clinical investigation. 2011;121:2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ontko JA. Metabolism of free fatty acids in isolated liver cells. Factors affecting the partition between esterification and oxidation. The Journal of biological chemistry. 1972;247:1788–1800. [PubMed] [Google Scholar]
- 13.Patsch W, Tamai T, Schonfeld G. Effect of fatty acids on lipid and apoprotein secretion and association in hepatocyte cultures. The Journal of clinical investigation. 1983;72:371–378. doi: 10.1172/JCI110977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Almeida IT, Cortez-Pinto H, Fidalgo G, Rodrigues D, Camilo ME. Plasma total and free fatty acids composition in human non-alcoholic steatohepatitis. Clin Nutr. 2002;21:219–223. doi: 10.1054/clnu.2001.0529. [DOI] [PubMed] [Google Scholar]
- 15.Bysted A, Holmer G, Lund P, Sandstrom B, Tholstrup T. Effect of dietary fatty acids on the postprandial fatty acid composition of triacylglycerol-rich lipoproteins in healthy male subjects. Eur J Clin Nutr. 2005;59:24–34. doi: 10.1038/sj.ejcn.1602028. [DOI] [PubMed] [Google Scholar]
- 16.Firl N, Kienberger H, Hauser T, Rychlik M. Determination of the fatty acid profile of neutral lipids, free fatty acids and phospholipids in human plasma. Clin Chem Lab Med. 2012;0:1–12. doi: 10.1515/cclm-2012-0203. [DOI] [PubMed] [Google Scholar]
- 17.Chavez JA, Summers SA. Characterizing the effects of saturated fatty acids on insulin signaling and ceramide and diacylglycerol accumulation in 3T3-L1 adipocytes and C2C12 myotubes. Arch Biochem Biophys. 2003;419:101–109. doi: 10.1016/j.abb.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 18.Gorgani-Firuzjaee S, Bakhtiyari S, Golestani A, Meshkani R. Leukocyte antigen-related inhibition attenuates palmitate-induced insulin resistance in muscle cells. J Endocrinol. 2012;215:71–77. doi: 10.1530/JOE-12-0160. [DOI] [PubMed] [Google Scholar]
- 19.Jung TW, Lee KT, Lee MW, Ka KH. SIRT1 attenuates palmitate-induced endoplasmic reticulum stress and insulin resistance in HepG2 cells via induction of oxygen-regulated protein 150. Biochem Biophys Res Commun. 2012;422:229–232. doi: 10.1016/j.bbrc.2012.04.129. [DOI] [PubMed] [Google Scholar]
- 20.Savary S, Trompier D, Andreoletti P, Le Borgne F, Demarquoy J, Lizard G. Fatty acids - induced lipotoxicity and inflammation. Current drug metabolism. 2012;13:1358–1370. doi: 10.2174/138920012803762729. [DOI] [PubMed] [Google Scholar]
- 21.Lima TM, Kanunfre CC, Pompeia C, Verlengia R, Curi R. Ranking the toxicity of fatty acids on Jurkat and Raji cells by flow cytometric analysis. Toxicology in vitro: an international journal published in association with BIBRA. 2002;16:741–747. doi: 10.1016/s0887-2333(02)00095-4. [DOI] [PubMed] [Google Scholar]
- 22.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. The Journal of biological chemistry. 1957;226:497–509. [PubMed] [Google Scholar]
- 23.Borengasser SJ, Rector RS, Uptergrove GM, Morris EM, Perfield JW, 2nd, Booth FW, Fritsche KL, Ibdah JA, Thyfault JP. Exercise and Omega-3 Polyunsaturated Fatty Acid Supplementation for the Treatment of Hepatic Steatosis in Hyperphagic OLETF Rats. Journal of nutrition and metabolism. 2012;2012:268680. doi: 10.1155/2012/268680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heden TD, Morris EM, Kearney ML, Liu TW, Park YM, Kanaley JA, Thyfault JP. Differential effects of low-fat and high-fat diets on fed-state hepatic triacylglycerol secretion, hepatic fatty acid profiles, and DGAT-1 protein expression in obese-prone Sprague-Dawley rats. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2014;39:472–479. doi: 10.1139/apnm-2013-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paton CM, Ntambi JM. Biochemical and physiological function of stearoyl-CoA desaturase. American journal of physiology Endocrinology and metabolism. 2009;297:E28–37. doi: 10.1152/ajpendo.90897.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hofacer R, Magrisso IJ, Jandacek R, Rider T, Tso P, Benoit SC, McNamara RK. Omega-3 fatty acid deficiency increases stearoyl-CoA desaturase expression and activity indices in rat liver: positive association with non-fasting plasma triglyceride levels. Prostaglandins, leukotrienes, and essential fatty acids. 2012;86:71–77. doi: 10.1016/j.plefa.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stamatikos AD, Paton CM. Role of stearoyl-CoA desaturase-1 in skeletal muscle function and metabolism. American journal of physiology Endocrinology and metabolism. 2013;305:E767–775. doi: 10.1152/ajpendo.00268.2013. [DOI] [PubMed] [Google Scholar]
- 28.Jackman MR, Kramer RE, MacLean PS, Bessesen DH. Trafficking of dietary fat in obesity-prone and obesity-resistant rats. American journal of physiology Endocrinology and metabolism. 2006;291:E1083–1091. doi: 10.1152/ajpendo.00159.2006. [DOI] [PubMed] [Google Scholar]
- 29.Karpe F, Dickmann JR, Frayn KN. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes. 2011;60:2441–2449. doi: 10.2337/db11-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson GH, Fritsche K. Effect of dietary linoleic acid on markers of inflammation in healthy persons: a systematic review of randomized controlled trials. Journal of the Academy of Nutrition and Dietetics. 2012;112:1029–1041. 1041 e1021–1015. doi: 10.1016/j.jand.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 31.Hihi AK, Michalik L, Wahli W. PPARs: transcriptional effectors of fatty acids and their derivatives. Cellular and molecular life sciences: CMLS. 2002;59:790–798. doi: 10.1007/s00018-002-8467-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varga T, Czimmerer Z, Nagy L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochimica et biophysica acta. 2011;1812:1007–1022. doi: 10.1016/j.bbadis.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Georgiadi A, Kersten S. Mechanisms of gene regulation by fatty acids. Advances in nutrition. 2012;3:127–134. doi: 10.3945/an.111.001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ntambi JM, Miyazaki M, Stoehr JP, Lan H, Kendziorski CM, Yandell BS, Song Y, Cohen P, Friedman JM, Attie AD. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11482–11486. doi: 10.1073/pnas.132384699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Warensjo E, Riserus U, Vessby B. Fatty acid composition of serum lipids predicts the development of the metabolic syndrome in men. Diabetologia. 2005;48:1999–2005. doi: 10.1007/s00125-005-1897-x. [DOI] [PubMed] [Google Scholar]
- 36.Bjermo H, Riserus U. Role of hepatic desaturases in obesity-related metabolic disorders. Current opinion in clinical nutrition and metabolic care. 2010;13:703–708. doi: 10.1097/MCO.0b013e32833ec41b. [DOI] [PubMed] [Google Scholar]
- 37.Flowers MT, Ntambi JM. Stearoyl-CoA desaturase and its relation to high-carbohydrate diets and obesity. Biochimica et biophysica acta. 2009;1791:85–91. doi: 10.1016/j.bbalip.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chong MF, Hodson L, Bickerton AS, Roberts R, Neville M, Karpe F, Frayn KN, Fielding BA. Parallel activation of de novo lipogenesis and stearoyl-CoA desaturase activity after 3 d of high-carbohydrate feeding. The American journal of clinical nutrition. 2008;87:817–823. doi: 10.1093/ajcn/87.4.817. [DOI] [PubMed] [Google Scholar]
- 39.Li ZZ, Berk M, McIntyre TM, Feldstein AE. Hepatic lipid partitioning and liver damage in nonalcoholic fatty liver disease: role of stearoyl-CoA desaturase. The Journal of biological chemistry. 2009;284:5637–5644. doi: 10.1074/jbc.M807616200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holland WL, Summers SA. Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism. Endocrine reviews. 2008;29:381–402. doi: 10.1210/er.2007-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ervin RB, Wright JD, Wang CY, Kennedy-Stephenson J. Dietary intake of fats and fatty acids for the United States population: 1999–2000. Advance data. 2004:1–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.