Abstract
Most research on unburned tobacco has focused on the harmful chemicals associated with the tobacco itself. However, certain flavor additives in tobacco products can pose additional health risks. Flavors like camphor, coumarin, pulegone, eugenol, methyl salicylate, menthol and diphenyl ether have exhibited biological activity and/or toxicity in both lab animals and humans. This publication presents a new GC/MS method for the quantitation of ten flavor compounds (eucalyptol, camphor, menthol, pulegone, ethyl salicylate, methyl salicylate, cinnamaldehyde, eugenol, diphenyl ether and coumarin) in a variety of tobacco products, including smokeless products and cigar filler. Excellent linearity (>0.997), accuracy (93.9% - 106.6%) and precision (C.V., 0.5% - 3.0%) were achieved for all flavor analytes measured. A summary of the concentrations of these flavors in selected international smokeless tobacco (SLT) products including zarda, quiwam, gutkha, and khaini varieties from Southeast Asia and snuff, clove cigarette filler and flavored cigar filler from the United States is reported. High concentrations of eugenol (2110 μg/g), coumarin (439 μg/g), camphor (1060 μg/g) and diphenyl ether (4840 μg/g) were found in selected products. Accurate identification and quantitation of potentially hazardous flavor compounds is important because they can exist in relatively high levels in some tobacco products, including international SLT products. We outline a versatile method which can be used to quantitate flavor compounds in multiple types of tobacco products.
1. Introduction
Flavor additives are often an important part of tobacco products because they provide a product its signature or characteristic taste and appeal. Hundreds of synthetic and natural sources of flavors are used in tobacco products.1-5 A large portion of US tobacco products contain significant amounts of flavor additives.6 Flavorings for US products include spice powders, extracts, tinctures, oleoresins, essential oils and individual flavor chemicals.7 In the United States, approximately 31% of the cigarettes and 75% of smokeless tobacco (SLT) products are advertised as “flavored,” with menthol and wintergreen being the most popular flavor for cigarettes and SLT products, respectively.8,9 Flavored little cigars have also gained increased attention due to the recent ban on cigarettes marketed with a “characterizing” flavor, excluding menthol, under the Family Smoking Prevention and Tobacco Control Act of 2009.10
In Southeast Asian populations, the use levels of SLT products and custom-made preparations are relatively high.11 Many SLT products contain a diverse mixture of spices and additives for flavor enhancement that can include hazardous constituents. Key Southeast Asian SLT products include zarda, quiwam, khaini and gutkha. For example, zarda typically contains a mixture of tobacco, lime, spices and occasionally silver flakes as well as other flavoring agents. Quiwam is a paste-like preparation containing tobacco extract, spices and additives. Preparations of khaini typically involve the use of sun-dried tobacco and slaked-lime; gutkha usually contains areca nut, slaked lime, catechu and flavoring agents to improve appeal.12
A number of flavor chemicals commonly found in select SLT products potentially have harmful health effects. Eugenol, the main flavor chemical of cloves, can cause respiratory infection, aspiration pneumonitis, hemoptysis, and hemorrhagic pulmonary edema in some individuals.13 Camphor is toxic at large doses and can cause disorientation, muscle spasms, abdominal cramps, lethargy, irritability, vomiting, seizures, and convulsions.14-17 Coumarin can be found in tonka bean, vanilla grass and sweet woodruff, and was shown in the mid-1950s to cause liver toxicity in laboratory animals following oral administration.18,19 Subsequently, coumarin and tonka bean were eliminated as flavoring agents in the United States.20 Diphenyl ether is a synthetic compound used in a variety of applications, including a heat transfer medium component, and as a soap perfume.21 At large doses, diphenyl ether has also been shown to cause severe, irreversible degenerative lesions on the liver and kidneys of humans.22 As a tobacco flavoring agent, menthol is the most widely used additive. Menthol ingestion has been shown to cause vertigo or ataxia in some individuals and menthol can potentially act as a nicotine delivery enhancement agent in tobacco products as well as a reinforcer of smoking behavior.23-26
In comparison to cigarette smoke, relatively little data has been reported on quantitative analysis of flavor additives in tobacco products. Solid-phase microextraction (SPME) coupled with gas chromatography/ mass spectrometry (GC/MS) methods are a commonly used technique for quantitating flavor chemicals in both whole tobacco product as well as the smoked products.27-30, Limitations for many conventional analytical methods is that the concentration ranges of the analytes are relatively low and the precision (C.V.%) can be rather poor (~15% for some analytes). Other methods of quantitation utilize solid-phase extraction followed by liquid-liquid extraction before GC-MS analysis, or extraction followed by gas chromatography-time of flight (GC-TOF) analysis.23,31-32 HPLC-MS analysis has also been done and provides results comparable to those of the same flavor analytes under GC-MS conditions.33
SLT products inherently contain many harmful constituents that are related to the tobacco itself. Additives, such as flavors, could pose additional potential health risks. Some international SLT products contain high levels of harmful flavor chemicals that are currently not found in US products. The aim of this research was to develop a versatile method to measure the concentrations of ten common flavor chemicals found in various tobacco products (eucalyptol, camphor, menthol, pulegone, ethyl salicylate, methyl salicylate, cinnamaldehyde, eugenol, diphenyl ether and coumarin) in any whole tobacco product (smokeless or filler). Southeast Asian SLTs were included because of their chemical complexity, diverse nature and potential for high exposure to harmful additives. We quantitate and present results for potentially harmful flavor chemicals found in international SLT varieties like zarda, quiwam, gutkha, and khaini, as well as US snuff, cigarette filler and cigar filler.
2. Experimental Section
2.1 Samples
Southeast Asian products were purchased and provided by Dr. Ray Croucher (Queen Mary’s School of Medicine and Dentistry, London, England) through collaboration with the Centers for Disease Control and Prevention for the analysis of international SLT. Domestic products were purchased at local retail or wholesale locations through The Lab Depot (Dawsonville, GA, USA). Upon receipt, samples were logged into a custom database, assigned barcodes with unique ID, and stored in their original containers until analyzed.
2.2 Reagents and materials
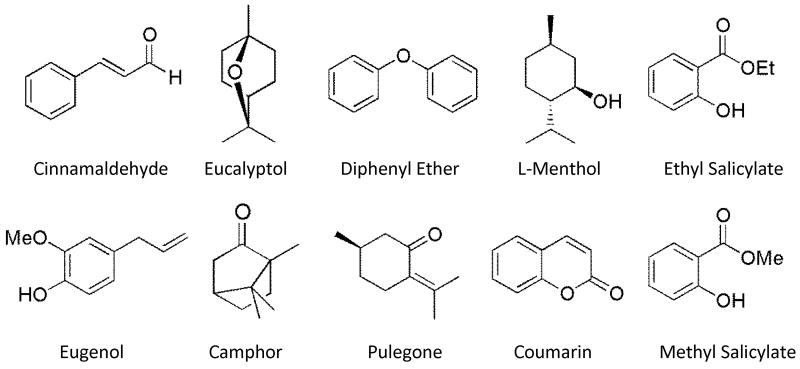
Flavor standards (eucalyptol, camphor, menthol, methyl salicylate, pulegone, ethyl salicylate, cinnamaldehyde, eugenol, diphenyl ether and coumarin) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Structural information can be found in Figure 1. 3’,4’-(methylenedioxy)-acetophenone (MDA) was also purchased from Sigma-Aldrich and was used as an internal standard for quantitation of flavor analytes. Research cigarette, 3R4F, was obtained from the University of Kentucky and was used as matrix blank for the addition of calibration standards (Lexington, KY, USA). All other chemicals were of analytical grade and were purchased through Fisher Scientific unless otherwise indicated (Pittsburgh, PA, USA).
Figure 1.
Structures of the ten flavor compounds found in various tobacco products that can be measured using the presented method.
2.3 Sample Preparation and Analysis Procedure
A 400-mg sample of blank matrix or tobacco product was placed into a 15-mL amber vial and the product weight recorded. 50 μL of MDA internal standard solution was added to the tobacco and allowed to stand for 15 min to allow for absorption into the matrix. The sample was then extracted with a 10-mL of methyl tert-butyl ether (MTBE). MTBE was chosen as an extraction solvent due to its polar property and extraction efficiency for the desired analytes. Vials were capped and placed on a Rugged Rotator (Glas-Col; Terre Haute, IN, USA) to tumble at 70 revolutions/min for 1 hour. After agitating, 1 mL aliquots of the sample extract were expressed through a 0.45 μm syringe filter directly into individual GC vials. Samples were then analyzed by GC/MS in triplicate (n=3). Note: if concentrations of any flavor analytes fell outside the upper calibration range, the samples were re-run with a smaller sample mass to ensure accurate quantitation. Reported analyte concentrations were corrected for sample mass variation.
2.4 Instrumentation and Apparatus
The GC/MS analysis was performed using an Agilent 7890 GC coupled with a 5975 MSD (Agilent Technologies; Newark, DE, USA). The GC/MS system was equipped with a CTC autosampler (LEAP Technologies; Carrboro, NC, USA), which injects 1 μL of the extract from each vial into the GC inlet. The GC injector was maintained at 250°C with a helium split flow rate of 70 ml/min. All injections were made in split mode with a split ratio of 40:1 and a solvent delay of 2.0 min. The chromatographic separation was accomplished using an Ultra-2 capillary column (25m × 0.32mm × 0.25μm) (Agilent Technologies; Andover, MA, USA) with research grade helium (>99.9999% purity) used as the carrier gas and a sample chromatogram is shown in Figure 2. GC ramp conditions were as follows: 35°C, hold 0.75 min; ramp at 80°C/min to 170°C; ramp 1°C/min to 172°C; lastly ramp at 80°C/min to 280°C, no hold. Total GC run time was 5.8 min. The transfer line temperature was maintained at 285°C. Compounds were ionized with electron ionization energy of 70eV and ionized in positive ion mode. The MS ion source and quadrupole were maintained at 230°C and 150°C, respectively. Mass to charge measurements were made using selected ion monitoring (SIM). The compound retention times and quantitation/confirmation ions are recorded in Table 1.
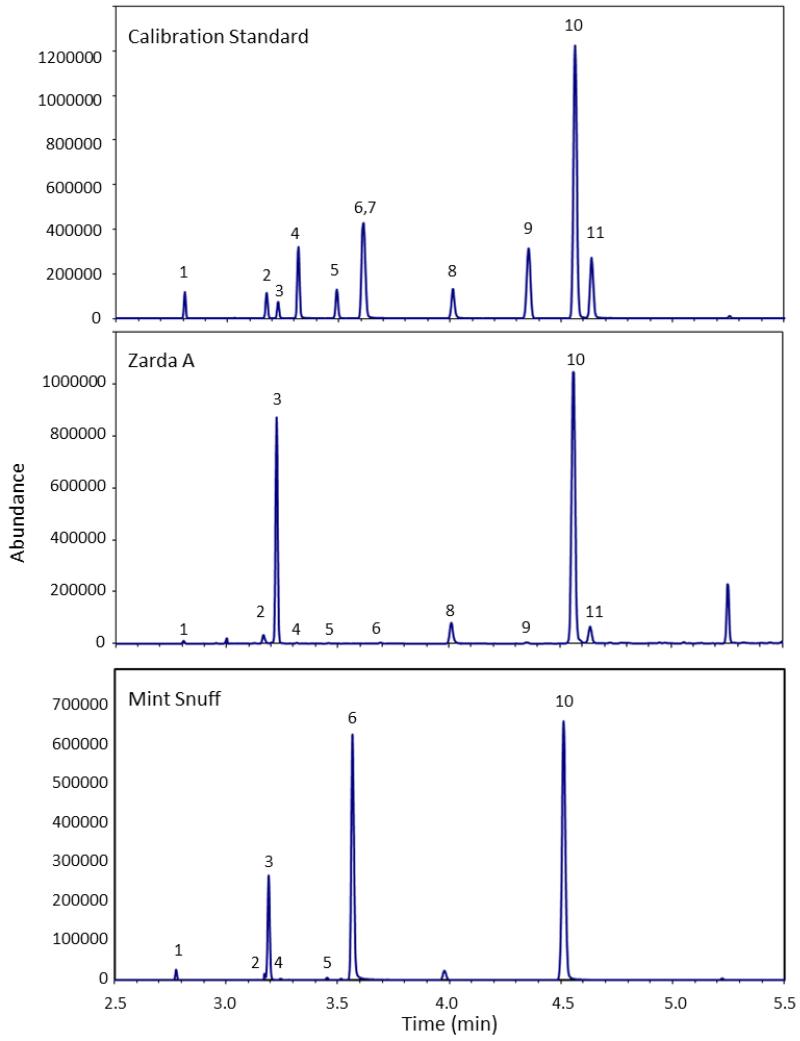
Figure 2.
Selected Ion Monitoring (SIM) mode GC/MS chromatogram of a Calibration Standard, Zarda A and Mint Snuff. 1: Eucalyptol, 2: Camphor, 3: Menthol, 4: Methyl Salicylate, 5: Pulegone, 6: Ethyl Salicylate, 7: Cinnamaldehyde, 8: Eugenol, 9: Diphenyl Ether, 10: MDA (ISTD), 11: Coumarin.
Table 1.
Selected Ion Monitoring (SIM) parameters, limit of detection (LOD), and calibration curve range/linearity for the quantitation of ten flavor analytes.
| SIM Ions, m/z | ||||||
|---|---|---|---|---|---|---|
| Compound | Retention Time (min) |
Dwell time (ms) | LOD (μg/g) |
Calibration Range (μg/g) |
Linearity, R2 (Average) |
|
| Quant. Ion | Conf. Ion | |||||
| Eucalyptol | 2.79 | 154.2 (75) | 139.1 (75) | 5.69 | 5.02 – 10041 | 0.998 |
| Camphor | 3.16 | 152.1 (50) | 108.1 (85) | 3.69 | 4.86 – 9725 | 0.997 |
| Menthol | 3.20 | 138.2 (65) | 123.1 (65) | 5.07 | 5.04 – 10090 | 0.998 |
| Methyl Salicylate | 3.30 | 120.1 (65) | 152.1 (65) | 0.95 | 5.18 – 10356 | 0.999 |
| Pulegone | 3.47 | 152.1 (75) | 137.1 (100) | 3.12 | 4.91 – 9813 | 0.998 |
| Ethyl Salicylate | 3.58 | 166.1 (65) | 120.0 (50) | 0.44 | 5.02 – 10042 | 0.998 |
| Cinnamaldehyde | 3.59 | 131.1 (40) | 103.1 (65) | 1.08 | 5.07 – 10136 | 0.997 |
| Eugenol | 3.98 | 164.1 (55) | 131.1 (75) | 0.75 | 4.91 – 9822 | 0.999 |
| Diphenyl Ether | 4.32 | 170.1 (75) | 141.1 (90) | 0.28 | 5.03 – 10056 | 0.999 |
| Coumarin | 4.61 | 149.0 (50) | 118.1 (50) | 0.38 | 5.08 – 10160 | 0.999 |
| MDA (ISTD) | 4.53 | 164.1 (50) | 146.0 (50) | – | – | – |
ISTD = Internal Standard
R2 = Coefficient of Determination, Linearity
A standard stock solution was prepared by weighing each flavor standard and diluting it with acetonitrile to a volume of 50 mL. Acetonitrile was chosen as solvent to preserve the stability of the aldehyde and ester flavor standards. Known volumes of the stock solution were further diluted to provide the desired calibration standards. Standard curves (9-points) were then constructed by spiking approximately 400 mg of the 3R4F research cigarette filler with 200 μL of each calibration standard and 50 μL of the MDA internal standard. Calibration curves were examined using 1/x weighting, and all analytes exhibited linearity (R2) greater than 0.997. An initial LOD for each analyte was estimated as 3s0 where s0 is the estimate of the standard deviation at zero analyte concentration. The value of s0 was taken as the y-intercept of a linear regression of standard deviation versus concentration as specified by Taylor et al.34 A summary of the linearity, LOD, calibration range and retention time for each flavor analyte are available in Table 1.
In order to validate the method, the method precision and accuracy of each analyte at three concentration levels was determined. Precision/accuracy data was obtained by adding flavor standards to a blank 3R4F matrix at low, medium and high concentration levels of flavor analytes. A synthetic standard had to be used in order to assess the precision and accuracy of the ten flavor analytes due to the unavailability of flavored tobacco standards. A blank control was prepared by assessing five 3R4F reference cigarette filler samples with only the MDA internal standard. The recovery range spanned 94% to 107% for all three addition levels, and precision was excellent (Table 2). Note: the extraction time of 1 hour was found to be optimal. Samples were prepared as described above and analyzed at 30 minutes, 1 hour and 2 hours. After 1 hour, extraction was found to be complete. In general, interferences from the tobacco matrix were minor but in order to confirm the presence of each analyte of interest, confirmation ion ratios for each analyte were calculated and used to confirm the presence of each analyte of interest rather than matrix interferences. If observed confirmation ion ratios were ≥10% different than found in the standard, the concentration of that sample was not reported. Relative retention time (analyte vs. MDA internal standard) was also used to confirm analyte presence. The robustness of the extraction solvent, MTBE, was also tested by extracting QC samples with 7.5, 10 and 12.5 mL of MTBE. It was found that observed concentrations of spiked analytes onto a 3R4F blank matrix remained constant despite differences in extraction volume due to the presence of internal standard in the sample.
Table 2.
Method precision and accuracy for flavors standards added onto a blank 3R4F tobacco matrix at three concentrations (approx. 250, 750, and 5000 μg/g).
| Compound | Level | Standard Level (μg/g) |
Accuracy (Recovery,%) |
Precision (CV,%) |
|---|---|---|---|---|
| Eucalyptol | Low | 251 | 103.6 | 0.7 |
| Medium | 753 | 105.0 | 1.7 | |
| High | 5020 | 101.5 | 1.5 | |
|
| ||||
| Camphor | Low | 243 | 106.3 | 0.9 |
| Medium | 729 | 105.4 | 2.4 | |
| High | 4860 | 101.6 | 1.5 | |
|
| ||||
| Menthol | Low | 252 | 106.6 | 0.5 |
| Medium | 757 | 104.3 | 3.0 | |
| High | 5040 | 101.6 | 1.4 | |
|
| ||||
| Methyl Salicylate | Low | 259 | 103.0 | 1.6 |
| Medium | 777 | 102.3 | 2.7 | |
| High | 5180 | 101.5 | 1.4 | |
|
| ||||
| Pulegone | Low | 245 | 103.5 | 0.7 |
| Medium | 736 | 103.9 | 2.7 | |
| High | 4910 | 102.0 | 1.3 | |
|
| ||||
| Cinnamaldehyde | Low | 251 | 100.6 | 0.7 |
| Medium | 753 | 102.6 | 2.4 | |
| High | 5020 | 101.9 | 1.1 | |
|
| ||||
| Ethyl Salicylate | Low | 253 | 101.6 | 0.8 |
| Medium | 760 | 103.1 | 2.7 | |
| High | 5070 | 101.9 | 1.2 | |
|
| ||||
| Eugenol | Low | 246 | 93.9 | 0.7 |
| Medium | 737 | 97.9 | 2.0 | |
| High | 4910 | 101.7 | 1.0 | |
|
| ||||
| Diphenyl Ether | Low | 251 | 105.5 | 1.1 |
| Medium | 754 | 105.1 | 2.3 | |
| High | 5028 | 101.2 | 1.1 | |
|
| ||||
| Coumarin | Low | 254 | 101.6 | 1.0 |
| Medium | 762 | 101.5 | 1.0 | |
| High | 5080 | 101.0 | 1.2 | |
|
| ||||
| Average | 102.4 | 1.5 | ||
3. Results and Discussion
This method allows for quick and rapid quantitation of selected flavor compounds in any whole tobacco product, smoked or smokeless, with the same sample preparation procedure. Excellent linearity (>0.997), accuracy (93.9% - 106.6%) and precision (C.V., 0.5% - 3.0%) were achieved for all flavor analytes measured. A larger calibration range (5 μg/g – 10,000 μg/g) allowed for convenient quantitation of a wide range of products without further sample dilution. This is particularly important when analyzing SLT products with extremely high levels of flavor analytes such as methyl salicylate and diphenyl ether. The highest prevalence for the ten flavor compounds in SLT was in products from Southeast Asia (Table 3). With the exception of mint snuff, the prevalence in domestic tobacco tested was much lower.
Table 3.
Mean concentrations (±Standard Deviation) of flavor analytes found in selected international SLT products (n=3).
| Brand | EUC | CAM | MEN | MSAL | PUL | CINN | ESAL | EUG | DPE | COUM |
|---|---|---|---|---|---|---|---|---|---|---|
| Southeast Asian Products | ||||||||||
| Zarda A | 187±8.3 | 1060±54 | 21700±979 | 17.9±1.2 | 11.6±7.6 | 28.9±1.6 | – | 1010±48 | 27.2±1.5 | 439±12 |
| Zarda B | – | 34.1±2.9 | 5400±502 | – | – | 8.5±3.8 | 14.7±4.5 | 193±16 | 4840±581 | 383±38 |
| Qiwam | 69.2±25.6 | 96.2±14.1 | 12300±2620 | – | – | 11.3±3.5 | – | 863±197 | – | 188±36.8 |
| Gutkha | – | – | 1080±112 | – | – | – | – | 25.4±3.7 | – | – |
| Khaini | 123±2.6 | 6.9±1.4 | 7000±198 | – | – | 13.9±1.3 | – | – | – | – |
| US Cigar Filler | ||||||||||
| Product A-Strawberry | – | 34.0±6.0 | – | – | – | – | – | – | – | – |
| Product B-Wild Cherry | – | – | – | – | – | – | – | – | – | – |
| US Snuff Products | ||||||||||
| Mint Snuff | 218±3.0 | 9.9±0.4 | 3240±140 | 10.0±1.0 | 48.8±1.3 | – | 1770±45 | – | – | – |
| Wintergreen Snuff | – | – | – | 9860±488 | – | – | – | – | – | – |
| Clove Cigarette Filler | ||||||||||
| Clove Cigarette A | – | – | – | – | – | – | – | 2110±15.0 | – | 4.6±0.1 |
| Clove Cigarette B | – | – | – | – | – | – | – | 69.4±8.6 | – | – |
all concentrations are reported in μg/g, (-) denotes <LOD
Key: EUC = Eucalyptol, CAM = Camphor, MEN = Menthol, MSAL = Methyl Salicylate, PUL = Pulegone, CINN = Cinnamaldehyde, ESAL = Ethyl Salicylate, EUG = Eugenol, DPE = Diphenyl Ether, COUM = Coumarin
A wide calibration range with good linearity is important for many analytes when examining diverse products. As previously noted, Southeast Asian products contained a wide range of flavor compounds with varying concentration ranges. For example, menthol was found in all the brands in a wide concentration range but at the relatively high concentrations of menthol, intentional inclusion in many product types is likely even though those products are not marketed as containing menthol. Cinnamaldehyde and camphor were found in all five SLT varieties, while eugenol was found in four of the five varieties tested. Zarda A contained the largest concentrations of these analytes, 1060 μg/g and 1010 μg/g for camphor and eugenol respectively. Also of interest, coumarin, which is banned in US products, was found in three Southeast Asian products at moderate levels (188 μg/g – 439 μg/g). Zarda B contains a high level of diphenyl ether (4840 μg/g). The single quiwam brand tested contained a diverse blend of flavor additives including eugenol (863 μg/g) and coumarin (188 μg/g). Khaini and gutkha products analyzed in this study did contain some measured amounts of flavor additives, but in much lower concentrations than their zarda and quiwam counterparts.
For US snuff products, results were within typical ranges. The mint flavored snuff contained appreciable levels of eucalyptol (218 μg/g), menthol (3240 μg/g) and ethyl salicylate (1770 μg/g), which is consistent with comparable products.19 Smaller, but measurable, levels of camphor, methyl salicylate and pulegone were also present in the mint product. The wintergreen snuff varieties exhibited high levels of methyl salicylate, (9860 μg/g). Although methyl salicylate is on the “Generally Regarded As Safe” (GRAS) list, toxic doses can easily be ingested (as little as 4 mL of the readily available oil of wintergreen has caused death in children).16
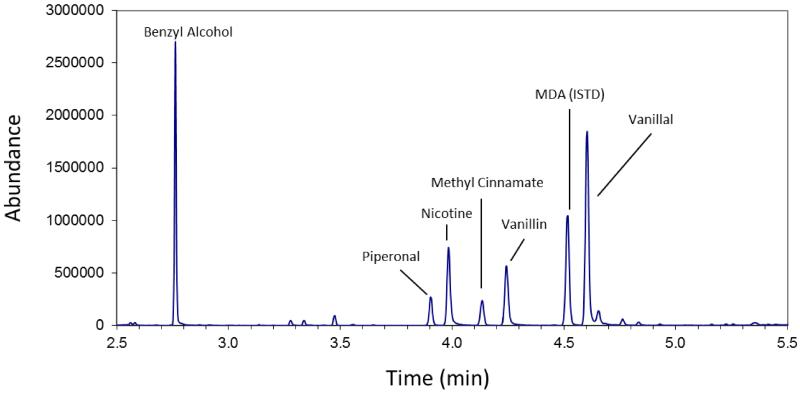
Generalizability of the current methodology is limited in that many of the flavor compounds found in domestic flavored tobacco products such as cigar filler are not included in the current analyte panel. Domestic cigar filler analyzed contained only a few of the analytes surveyed in this method. A strawberry flavored cigar “Product A” did contain a small, but measureable amount of camphor (34 μg/g). However, when examining the full scan data for cigar filler, benzyl alcohol and vanillal were found in 27% and 34.2% relative abundance for the Strawberry Product A. The wild cherry cigar filler (Product B) had measurable levels of benzaldehyde and piperonal. Sample full-scan chromatograms contain abundant flavor related information (Figure 3). Thus, flavor additives in cigar filler and SLT products can differ greatly. The full-scan data obtained reveals numerous flavor compounds that could potentially be added to the method if desired. Compounds such as benzaldehyde, piperonal, vanilla and others, which are extractable under the same conditions, could be readily included and validated as needed to cover a more diverse range of tobacco products.
Figure 3.
Full Scan GC/MS chromatogram of Strawberry cigar filler (ProductA). New compounds were identified using the Wiley Flavor and Fragrances of Natural Synthetic Compounds 2 (FFNSC 2) Mass Spec Library.
International clove flavored cigarette filler was also tested to demonstrate this method’s utility. The clove cigarette filler showed differing amounts of eugenol, which originates in clove buds. Clove Cigarette A showed concentrations considerably higher (~30×) than Clove Cigarette B. The difference is most likely due to manufacturing differences between the brands. Clove Cigarette B states that the clove flavoring is concentrated in the filter and only the tobacco filler was tested in these experiments. Similar analyte limitations for screening flavored cigars are found with clove cigarettes due to a different flavor additive profile for smoked products such as cigars and clove cigarettes compared to smokeless products. Also, a strategic decision was made to analyze only filler for cigar and cigarette products and not the wrappers. In general, the wrapper makes up a small percentage of the product mass and even if flavors were applied directly to the wrappers, diffusion throughout the product is expected. Despite these limitations, this approach is very applicable to diverse smokeless tobacco products and the analytes included are found in a wide variety of products from around the world. Also, the wide concentration range allows for the quantitation of all analytes without further sample manipulation (dilution). Any non-combusted tobacco product can be analyzed and additional analytes could be easily added in the future to cover more common flavor analytes in smoked products.
4. Conclusions
This work presents a versatile method for quantitating ten common flavor compounds (eucalyptol, camphor, menthol, pulegone, ethyl salicylate, methyl salicylate, cinnamaldehyde, eugenol, diphenyl ether and coumarin) in any smokeless tobacco products and select whole tobacco product (cigarette filler, cigar filler or non-combustible products). The method exhibits excellent precision, accuracy and curve linearity for each analyte. The method was applied to selected Southeast Asian SLT varieties (zarda, quiwam, gutkha, and khaini) as well as flavored US snuff, flavored cigar, and cigarette filler. High concentrations of selected flavor compounds were found in SLT products from Southeast Asia and the US smokeless products. US cigar filler and international clove cigarette filler also showed the presence of selected flavor analytes (camphor and eugenol), some at high concentrations (eugenol). The method also offers the opportunity to expand the analyte panel to include flavor additives more commonly used in US smoked products. Most notably, this method provides means to quantitate flavor additives found in a wide range of tobacco products that could pose additional health risks beyond the risks associated with tobacco itself.
Footnotes
Disclaimer: This information is distributed solely for the purpose of pre-dissemination peer review under applicable information quality guidelines. It has not been formally disseminated by the Centers for Disease Control and Prevention/the Agency for Toxic Substances and Disease Registry. It does not represent and should not be construed to represent any agency determination or policy.
References
- 1.Brown and Williamson Tobacco Corporation [Accessed April 24, 2014];1994 Bates number 608164265/608164314. Available at: http://legacy.library.ucsf.edu/tid/sjw19j00.
- 2.Brown and Williamson Tobacco Corporation. Byfield Snuff Co. Conwood Co. L.P. Helme Tobacco Co. House of Windsor Inc. National Tobacco Co. The PinkertonTobacco Co. R.C. Owen Co. Fred Stoker and Sons Inc. United States Tobacco Co. [Accessed December 15, 2013];1994 Bates Number 566415479/5524. Available at: http://legacy.library.ucsf.edu/tid/pac33f00.
- 3.Crouse WE, Miller SS, Connelly MW. 1983 Bates number 88323584/3605. Available at: http://legacy.library.ucsf.edu/tid/oel43c00.
- 4.Lorrilard Tobacco Company [Accessed December 15, 2013];1985 Bates Number 87471384/1396. Available at: http://legacy.library.ucsf.edu/tid/ujq30e00.
- 5.RJ Reynolds Tobacco Incorporated [Accessed December 15, 2013];1972 Bates Number 582103899/3970. Available at: http://legacy.library.ucsf.edu/tid/wrv41f00.
- 6.Brown and Williamson Tobacco Corporation [Accessed December 15, 2013];1994 Bates Number 566942632. Available at http://legacy.library.ucsf.edu/tid/opw11c00.
- 7.Penn RN. Perfum. Flavor. 1997;22:21–28. [Google Scholar]
- 8.U.S. Securities and Exchange Commission . Lorillard, Inc.; [Accessed April 24, 2014]. 2012. p. 40. Form 10-K. [Google Scholar]
- 9.Alpert HR, Koh H, Connolly GN. Tob. Control. 2008;17:332–338. doi: 10.1136/tc.2008.025247. [DOI] [PubMed] [Google Scholar]
- 10.Campaign for tobacco-free kids [Accessed November 12, 2013];The rise of cigars and cigar smoking harms. Available at: www.tobaccofreekids.org.
- 11.Eriksen M, Mackay J, Ross H. The Tobacco Atlas. Fourth Ed. American Cancer Society; New York, NY: [Accessed November 15, 2013]. 2012. World Lung Foundation. Also available at www.TobaccoAtlas.org. [Google Scholar]
- 12.Bhisey RA. Indian J. Cancer. 2012;49:364–372. doi: 10.4103/0019-509X.107735. [DOI] [PubMed] [Google Scholar]
- 13.Guidotti TL, Binder S, Stratton JW, Schecher FG, Jenkins RA. In: Current Topics in Pulmonary Pharmacology and Toxicology. Hollinger MA, editor. Vol. 2. Elsevier; New York: 1987. pp. 1–23. [Google Scholar]
- 14.Goldfrank LR, editor. Goldfrank’s Toxicologic Emergencies. 8th ed McGraw Hill; New York, NY: 2006. [Google Scholar]
- 15.American Association of Poison Control Centers Clin. Tox. 2006;44:357–370. [Google Scholar]
- 16.Martin D, Valdez J, Boren J, Mayersohn M. J. Clin. Pharmacol. 2004;44:1151–7. doi: 10.1177/0091270004268409. [DOI] [PubMed] [Google Scholar]
- 17.Uc A, Bishop WP, Sanders KD. South. Med. J. 2000;93:596–8. [PubMed] [Google Scholar]
- 18.Ehlers D, Pfister M, Bork WR, Toffel-Nadolny P. Z. Lebensm Unters Forsch. 1995;201:278–82. doi: 10.1007/BF01193004. [DOI] [PubMed] [Google Scholar]
- 19.Canuto KM, Silveira ER. Química Nova. 2006;29:1241–1243. [Google Scholar]
- 20.Lake BG. Food Chem. Toxicol. 1999;37:423–453. doi: 10.1016/s0278-6915(99)00010-1. [DOI] [PubMed] [Google Scholar]
- 21.Ungnade HE, Orwoll EF. Org. Synth. 1955;3:566. [Google Scholar]
- 22.International Labour Office . Encyclopedia of Occupational Health and Safety. I and II. McGraw Hill; New York, New York: 1971. p. 392. [Google Scholar]
- 23.Chen C, Isabelle LM, Pickworth WB, Pankow JF. Food Chem Tox. 2010;48:755–763. doi: 10.1016/j.fct.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 24.Leung AY, Foster S. Encyclopedia of Common Natural Ingredients Used in Food, Drugs, and Cosmetics. John Wiley & Sons, Inc; New York, New York: 1996. p. 370. [Google Scholar]
- 25.Gosselin RE, Hodge HC, Smith RP, Gleason MN. Clinical Toxicology of Commercial Products. 4th ed. Williams and Wilkins; Baltimore, Maryland: 1976. pp. II–168. [Google Scholar]
- 26.Ahijevych K, Garrett BE. Nicotine Tob. Res. 2010;12:S110–S116. doi: 10.1093/ntr/ntq203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stanfill SB, Ashley DB. J. Chrom. A. 1999;858:79–89. doi: 10.1016/s0021-9673(99)00796-7. [DOI] [PubMed] [Google Scholar]
- 28.Clark TJ, Bunch JE. J. Agric. Food Chem. 1997;45:844–849. [Google Scholar]
- 29.Stanfill SB, Calafat AM, Brown CR, Polzin GM, Chiang JM, Watson CH, Ashley DL. Food and Chemical Toxicology. 2003;41:303–317. doi: 10.1016/s0278-6915(02)00230-2. [DOI] [PubMed] [Google Scholar]
- 30.Polzin GM, Stanfill SB, Brown CR, Watson CH. Food Chem. Tox. 2007;45:1948–1953. doi: 10.1016/j.fct.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 31.Stanfill SB, Ashley DB. J. Agric. Food Chem. 2000;48:1298–1306. doi: 10.1021/jf990772i. [DOI] [PubMed] [Google Scholar]
- 32.Huang Lan-Fang, Wu Ming-Jian, Zhong Ke-Jun, Sun Xian-Jun, Liang Yi-Zeng, Dai Yun-Hui, Huang Ke-Long, Guo Fang-Qiu. Anal. Chim. Acta. 2007;588:216–223. doi: 10.1016/j.aca.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 33.De Jager LS, Perfetti GA, Diachenko GW. Food Chem. 2008;107:1701–1709. [Google Scholar]
- 34.Taylor JK. Quality Assurance of Chemical Measurements. Lewis Publishers; Chelsea, Michigan: 1987. [Google Scholar]