Abstract
Pediatric Vogt-Koyanagi-Harada Syndrome (VKH) is rare with limited cases of corticosteroid-sparing immunosuppression use reported. A 15-year-old Hispanic female was referred for bilateral intraocular inflammation. Her initial visual acuity (VA) was 20/30 OD and 20/200 OS with granulomatous keratic precipitates, anterior chamber and vitreous cell, optic disc edema, and nummular depigmented chorioretinal lesions on exam consistent with VKH after an unrevealing workup. Inflammation was recurrent despite oral prednisone and methotrexate. Adalimumab, a TNF-α inhibitor, led to rapid resolution of inflammation, successful dose reduction of prednisone and methotrexate, and final VA of 20/25 OD and 20/40 OS at 26-months follow up.
Introduction
Vogt-Koyanagi-Harada Syndrome (VKH) is a chronic, bilateral granulomatous panuveitis of autoimmune etiology with neurologic and integumentary findings.1 It predominantly occurs in the third to fourth decades of life. While corticosteroids are effective for the treatment of acute inflammation in VKH, corticosteroid-sparing immunosuppression is associated with reduced risk of visual loss.2 Pediatric-aged VKH is rare and primarily confined to case reports in the literature. In children, chronic systemic steroids can have serious side effects making corticosteroid-sparing immunosuppression important for long-term management. Methotrexate and infliximab have been reported for corticosteroid-sparing immunosuppression in pediatric VKH.3,4 Herein, we report the successful use of adalimumab for refractory pediatric VKH.
Case Report
A 15-year-old Hispanic female with insulin-dependent diabetes mellitus presented with bilateral vision loss, photophobia, headaches, and mild neck stiffness of three months duration. She denied any skin changes or ocular trauma. Her referring ophthalmologist documented visual acuities (VA) of 20/400 OD and light perception OS with severe anterior chamber and vitreous inflammation OU. Bilateral orbital corticosteroid injections were performed, and the patient was referred to our service one month later for further management.
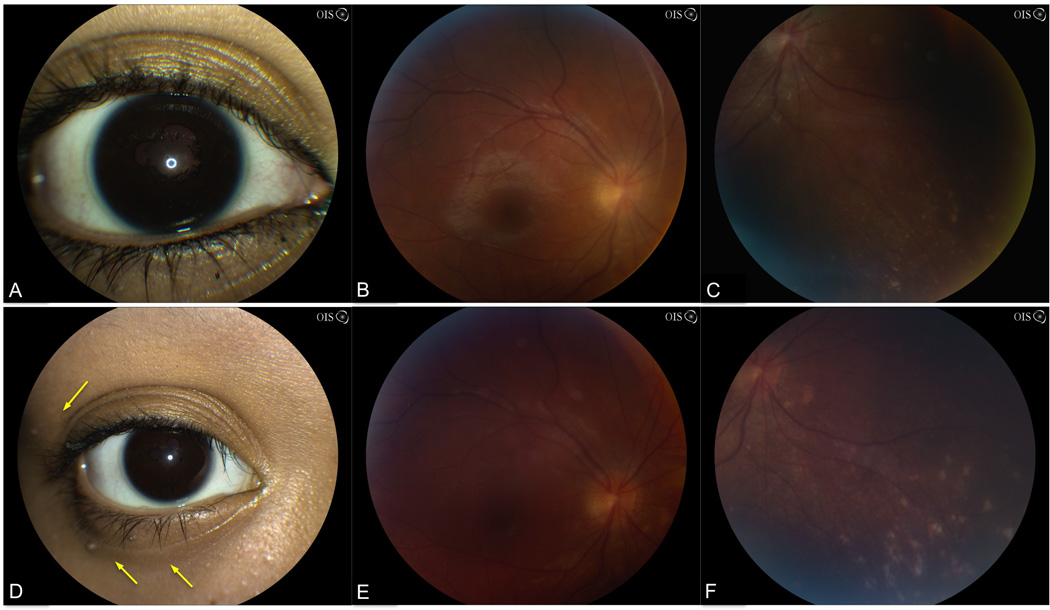
On our initial examination, VA was 20/30 OD and 20/200 OS. Slit lamp examination showed granulomatous keratic precipitates, 3+ anterior chamber cell, posterior synechiae, and mild cataracts OU. Ophthalmoscopic exam was notable for 2+ vitreous cell, mild optic disc edema, and nummular depigmented chorioretinal lesions inferiorly OD and 3+ vitreous cell with no view OS (Figure 1). B-scan ultrasound showed bilateral optic disc elevation, attached retinae, and vitreous opacities. ACE, RPR, MHA-TP, HIV, and PPD testing were negative. The patient’s presentation of bilateral granulomatous panuveitis, headaches, and neck stiffness was consistent with incomplete VKH syndrome.
Figure 1.
Slit lamp photograph and fundus photographs. At presentation, 3 months after the development of symptoms, slit lamp examination showed posterior synechiae and active inflammation (A). Fundus photograph showed blurred disc margins and optic disc edema (B) and depigmented chorioretinal scars inferiorly OD (C). At 18-months follow-up, external examination showed madarosis and poliosis at the eyelash tips (D, yellow arrows). Fundus photograph showed a hazy view due to media opacity but the optic disc edema has resolved (E). Increasing areas of nummular chorioretinal depigmentation are observed (F).
Oral prednisone 60 mg daily was started with topical prednisolone acetate 1% and atropine 1% BID. VA improved to 20/25 OD and 20/60 OS. Once the media cleared, fluorescein angiography showed mild optic disc leakage OU. Optical coherence tomography showed no evidence of cystoid macular edema. Within two months, poliosis, madarosis, and alopecia developed, meeting criteria for complete VKH (Figure 1).
Oral prednisone was tapered to 10 mg daily over a 10-week period with initiation of methotrexate 15 mg weekly via subcutaneous injection (SQ). Despite escalation of methotrexate to 25 mg weekly, 1+ anterior chamber inflammation recurred OU when prednisone was tapered below 10 mg daily over the course of six months.
Adalimumab 20 mg SQ every 2 weeks was initiated with complete resolution of anterior chamber and vitreous inflammation after 6 weeks of therapy. Once oral prednisone was tapered to discontinuation, methotrexate was then gradually decreased to and maintained at 15 mg/week. At 26-months follow-up, VA was 20/25 OD and 20/40 OS, and the examination remained stable.
Discussion
The pathogenesis of VKH has been attributed to T-cell-mediated, autoimmune targeting of melanocytic antigens.1 The exact trigger and target antigen remain unknown. VKH primarily affects persons of Hispanic, Native American, Middle Eastern, Indian, and Asian descent suggesting a genetic predisposition to developing VKH.1
The mainstay of therapy includes high dose oral corticosteroids (1 to 1.5 mg/kg/day) with a gradual taper over the course of 4 to 6 months with immunomodulatory therapy used for patients intolerant to corticosteroids and those with chronic, recurrent disease. The goals of long-term immunosuppression are to reduce ocular complications associated with recurrent and chronic VKH such as cataract, glaucoma, subretinal neovascularization, and subretinal fibrosis.2
Pediatric VKH is rare, and reports of immunosuppressive therapy use for acute and chronic VKH are limited. Soheilian et al first reported the use of oral methotrexate in six of 10 pediatric VKH patients with inflammation persistence or recurrence despite oral and periocular corticosteroids.3 In these patients, addition of methotrexate resulted in resolution of inflammation, sustained or improved final visual acuity, and allowed for successful tapering of systemic corticosteroids.3 In another report by Khalifa et al, two patients with pediatric VKH were treated with systemic corticosteroids supplemented with monthly intravenous infliximab, a chimeric murine/human monoclonal antibody targeting TNF-α. One patient experienced significant improvement in their exudative retinal detachments while another patient showed complete resolution with eventual discontinuation of systemic corticosteroids within four months.4
Adalimumab, a recombinant human monoclonal anti-TNF-α antibody, depresses TH1-cellmediated immunity by blocking TNF-α from binding to its cell surface receptors. Bimonthly dosed adalimumab may confer advantages compared to intravenous monthly infliximab due to its subcutaneous administration and decreased immunogenicity.5, 6 A recent non-comparative, prospective trial of 131 patients treated with adalimumab for refractory uveitis showed low incidence of adverse events as well as promising efficacy with 85% of individuals reducing their immunosuppressive load by at least 50%.6 Even in the 44 patients who had lost the efficacy initially experienced with TNF-α inhibitors such as etanercept and infliximab, adalimumab was successful in controlling inflammation in these patients.6
In our pediatric patient, addition of methotrexate was insufficient to control inflammation for safely tapering systemic corticosteroids. Addition of adalimumab was effective in eradication of chronic, recurrent inflammation within 6 weeks of use. Furthermore, addition of adalimumab led to a reduction in the patient’s immunosuppressive load. TNF-α inhibitors have demonstrated efficacy in pediatric rheumatologic and uveitic conditions.5,6 Although rare, it is important to discuss black box warning including tuberculosis reactivation and development of malignancies such as lymphoma reported in children and adolescents. Adalimumab should be considered for corticosteroid-sparing immunosuppression for pediatric VKH and ocular autoimmune diseases in which TNF-α is thought to play a role in their pathogenesis.
Acknowledgment
This work was supported in part by an unrestricted departmental grant from Research to Prevent Blindness (New York, NY) to the Emory Eye Center and an NEI Core Grant for Vision Research (P30 EY 006360), and the Knights Templar Educational Foundation of Georgia (SY, SAH). Dr. Angeles-Han was supported by the National Eye Institute of the National Institutes of Health under Award Number K23 EY021760. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Read RW, Holland GN, Rao NA, et al. Revised diagnostic criteria for Vogt-Koyanagi-Harada disease: report of an international committee on nomenclature. Am J Ophthalmol. 2001;131:747–752. doi: 10.1016/s0002-9394(01)00925-4. [DOI] [PubMed] [Google Scholar]
- 2.Bykhovskaya I, Thorne JE, Kempen JH. Vogt-Koyanagi-Harada disease: clinical outcomes. Am J Ophthalmol. 2005;140:674–678. doi: 10.1016/j.ajo.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 3.Soheilian M, Aletaha M, Yazdani S, Dehghan M. Management of pediatric Vogt-Koyanagi- Harada (VKH)-associated panuveitis. Ocul Immunol Inflamm. 2006;14:91–98. doi: 10.1080/09273940600557001. [DOI] [PubMed] [Google Scholar]
- 4.Khalifa Y, Bailony M, Acharya N. Treatment of pediatric Vogt-Koyanagi-Harada syndrome with infliximab. Ocul Immunol Inflamm. 2010;18:218–222. doi: 10.3109/09273941003739910. [DOI] [PubMed] [Google Scholar]
- 5.Yeh S, Faia LJ, Nussenblatt RB. Advances in the diagnosis and immunotherapy for ocular inflammatory disease. Semin Immunopathol. 2008;30:145–164. doi: 10.1007/s00281-008-0109-4. [DOI] [PubMed] [Google Scholar]
- 6.Diaz-Llopis M, Salom D, Garcia-de-Vicuna C, et al. Treatment of refractory uveitis with adalimumab: a prospective multicenter study of 131 patients. Ophthalmology. 2012;119(8):1575–1581. doi: 10.1016/j.ophtha.2012.02.018. [DOI] [PubMed] [Google Scholar]