Abstract
Introduction
The European Cubicin® Outcomes Registry and Experience (EU-CORESM) was a retrospective, non-interventional, multicenter study which evaluated the safety and effectiveness of daptomycin therapy in patients with Gram-positive infections including infective endocarditis (IE).
Methods
Data from the EU-CORE registry were collected for patients with IE who had received at least one dose of daptomycin between January 2006 and April 2012, across 18 countries in Europe (12), Latin America (5) and Asia (1). Clinical outcomes were assessed as success (cured or improved), failure or non-evaluable. Adverse events (AEs) were recorded during treatment and for up to 30 days post-treatment; follow-up data were collected for 2 years.
Results
Of 6075 patients included in the EU-CORE registry, 610 were diagnosed with IE as primary infection; 149 (24.4%) right-sided IE (RIE), 414 (67.9%) left-sided IE (LIE), and 47 (7.7%) with both right- and left-sided IE (BRLIE). Overall clinical success was achieved in 80.0% of patients (RIE 88.6%, LIE 76.6% and BRLIE 82.9%). Success rates for methicillin-resistant Staphylococcus aureus (MRSA) infections were 90.9%, 71.7% and 66.6% in patients with RIE, LIE and BRLIE, respectively. The overall sustained clinical success rate in patients followed for up to 2 years was 86.7% (RIE 93.5%, LIE 88.3% and BRLIE 77.8%). AEs deemed possibly related to daptomycin in the investigator’s opinion were reported in 2 (1.3%) RIE, 18 (4.3%) LIE and 1 (2.1%) BRLIE patients. There were 11 (1.8%) patients (2 with RIE, 8 with LIE and 1 with BRLIE) with AEs of creatine phosphokinase elevation reported as possibly related to daptomycin.
Conclusion
Data from this real-world clinical setting showed that daptomycin was well tolerated and effective for the treatment of LIE and BRLIE in addition to RIE caused by Gram-positive bacteria, including MRSA. Two-year follow-up data showed that a high proportion of patients had a sustained response.
Electronic supplementary material
The online version of this article (doi:10.1007/s40121-015-0075-9) contains supplementary material, which is available to authorized users.
Keywords: Daptomycin, Endocarditis, EU-CORE, Left-sided endocarditis, Right-sided endocarditis
Introduction
Infective endocarditis (IE), primarily caused by Gram-positive bacteria, is associated with a high rate of morbidity and mortality, which represents a large burden to the healthcare system [1]. Hospitalizations due to IE rose from 25,511 to 38,976 between 1998 and 2009 in the United States, with increase in serious neurologic and cardiac complications [2]. Mortality associated with IE ranges between 15% and 20% [2–5].
Although IE is associated with a variety of microorganisms, staphylococci, streptococci and enterococci account for the majority of cases [6]. Staphylococcus aureus is the most commonly detected causative agent [4, 5]. A cohort study showed that among 2781 patients with IE, the most common pathogens were S. aureus (31%), viridans group streptococci (17%), enterococci (10%), coagulase-negative staphylococci (11%), Streptococcus bovis (6%), and other streptococci (6%) [5].
Methicillin-resistant S. aureus (MRSA) has emerged as a common pathogen in both healthcare and community-acquired infections [7, 8]. Community-acquired MRSA has been found to be particularly responsible for causing IE in patients with human immunodeficiency virus [9]. Another multinational study reported an increase in the relative proportion of both hospital- and community-onset of MRSA bloodstream infections [10]. MRSA infections, including IE, are associated with higher levels of mortality compared to methicillin-susceptible S. aureus (MSSA) [11, 12]. The resistance of pathogens to commonly used antibiotics is one of the major public health problems, and the successful treatment of IE remains challenging. Patients with IE require an aggressive treatment approach with effective antibiotics or a combination of effective antibiotics and surgery [13–15].
Daptomycin is a bactericidal, cyclic lipopeptide that is active against Gram-positive bacteria. The mechanism of action involves binding (in the presence of calcium ions) to bacterial membranes of both growing and stationary phase cells causing depolarization and leading to a rapid inhibition of protein, deoxyribonucleic acid and ribonucleic acid synthesis. This results in bacterial cell death with negligible cell lysis [16]. Daptomycin is associated with concentration-dependent activity, hence a high dose of daptomycin has the ability to penetrate bacterial biofilm and may help to prevent the emergence of bacterial resistance [1]. Daptomycin is approved for the treatment of complicated skin and skin-structure infections caused by Gram-positive pathogens, bacteremia and right-sided IE caused by S. aureus [17, 18].
Infective endocarditis can affect the right side (RIE), left side (LIE) or both sides (BRLIE) of the heart. Reports from earlier studies suggest that daptomycin can be useful in the treatment of both LIE as well as RIE [18–21], although the drug is only indicated for use in patients with RIE.
The objective of this analysis from the European Cubicin® Outcomes Registry and Experience (EU-CORESM) study was to acquire real-world data on the use and clinical outcomes of patients who received daptomycin treatment for IE.
Methods
Patients and Data Collection
This analysis includes patients enrolled in EU-CORE, a non-interventional, multicenter, retrospective, patient registry designed to collect real-world outcome data on patients who had received at least one dose of daptomycin for the treatment of a serious Gram-positive bacterial infection. The protocol was approved by the health authority and the Institutional Review Board (IRB) or Ethics Committee (EC) in each country and written informed consent was obtained according to the requirements of the IRB or EC and/or the local data privacy regulations. Patients who might have received daptomycin as part of a controlled clinical trial were excluded from retrospective collection of data. Details of the EU-CORE registry have been published previously [11, 22–24].
The data from the registry were collected using standardized case report forms for patients with IE who had received at least one dose of daptomycin between January 2006 and April 2012. Supplementary and 2-year follow-up (until 2014) data were collected for patients with IE. Patients were included from sites across 18 countries: Argentina, Austria, Brazil, Bulgaria, Colombia, France, Germany, Greece, India, Italy, Mexico, Romania, Russia, Slovenia, Spain, Turkey, United Kingdom, and Venezuela. Data were collected from the registry for those patients who received daptomycin treatment for IE as a primary infection and for whom this treatment was initiated and completed within the course of the registry reporting period.
Clinical Outcomes and Safety
Clinical outcomes were assessed by investigators at the end of therapy as cured, improved, failed, or non-evaluable according to the following protocol-defined criteria: cured, clinical signs and symptoms resolved, no additional antibiotic therapy was necessary, or infection cleared with a negative culture reported; improved, partial resolution of clinical signs and symptoms and/or additional antibiotic therapy was warranted; failed, inadequate response to daptomycin therapy, worsening or new/recurrent signs and symptoms, need for a change in antibiotic therapy, or a positive culture reported at the end of therapy; and non-evaluable, unable to determine response due to insufficient information. Clinical success was defined as outcomes cured or improved. Time to improvement was also recorded. The reasons for stopping daptomycin therapy and other antibiotics prescribed following daptomycin were also collected [11].
The diagnosis of IE was done according to modified Duke criteria [25]. The duration of treatment was estimated as the number of inpatient and outpatient days the patient received daptomycin therapy, even if the treatment was non-consecutive. Long-term assessments were done after completion of daptomycin therapy at different follow-up visits: 1, 3, 6, 9,12,15,18, 21, and 24 months and during final follow-up visit. The time to recurrence or relapse was analyzed using Kaplan–Meier method for all patients who had clinical outcomes cured or improved at the end of daptomycin treatment.
All patients who received at least one dose of daptomycin were eligible for safety analysis. Adverse events (AEs) and serious AEs (SAEs) were recorded during daptomycin treatment and for 30 days post-treatment. Patients with IE were further followed for an additional period of up to 2 years. All reported AEs, regardless of their relationship to daptomycin, were recorded and their severity was determined by the local investigators.
Statistical Analysis
Statistical analysis was performed using statistical analysis system (SAS) version 9.3 (SAS Institute Inc., Cary, NC, USA). Inferential analyses were not conducted because of the nature of the study and no formal statistical methodology other than simple descriptive statistics was used. All analyses were considered to be explanatory.
Numerical variables were summarized as arithmetic mean, standard deviation, median, minimum, first quartile, third quartile, and maximum for continuous variables. Categorical variables were summarized by absolute and relative frequencies.
Results
Patient Demographic and Clinical Characteristics
Out of 6075 patients included in the EU-CORE registry, 610 (10.0%) were diagnosed with IE as primary infection, of whom 149 (24.4%) patients had RIE, 414 (67.9%) patients had LIE and 47 (7.7%) patients had BRLIE (Table 1).
Table 1.
Patient disposition and analysis sets in the EU-CORE study (safety population)
| Patient disposition | RIE n (%) |
LIE n (%) |
BRLIE n (%) |
|---|---|---|---|
| Infective endocarditis patients in EU-CORE | 149 (100) | 414 (100) | 47 (100) |
| Completed daptomycin therapy | 94 (63.1) | 244 (58.9) | 29 (61.7) |
| Primary reason for stopping daptomycin therapy | |||
| Switched therapy | 36 (24.2) | 59 (14.3) | 9 (19.1) |
| Adverse event | 4 (2.7) | 30 (7.2) | 2 (4.3) |
| Failure | 4 (2.7) | 17 (4.1) | 2 (4.3) |
| Unable to determine | 1 (0.7) | 17 (4.1) | 2 (4.3) |
| Other | 10 (6.7) | 46 (11.1) | 3 (6.4) |
| Unknown | – | 1 (0.2) | – |
| Entered safety population | 149 (100) | 414 (100) | 47 (100) |
| Entered efficacy population | 149 (100) | 414 (100) | 47 (100) |
BRLIE both right- and left-sided infective endocarditis, EU-CORE European Cubicin® Outcomes Registry and Experience, LIE left-sided infective endocarditis, RIE right-sided infective endocarditis
Supplementary data collected from 272 patients showed the following major and minor criteria used in the diagnosis of IE: positive blood culture, 191 (70.2%); transthoracic echocardiogram, 192 (70.6%), transesophageal echocardiogram, 158 (58.1%); predisposing heart condition or intravenous drug use, 152 (55.9%); fever, 198 (72.8%); vascular phenomena, 52 (19.1%); immunologic phenomena, 21 (7.7%); and serologic evidence, 20 (7.4%). Risk factors for IE other than predisposing heart condition or intravenous drug use (55.9%) included vascular catheter (11.8%) and dental disease or treatment (7.0%).
Of the total 610 patients, 367 (60.2%) completed daptomycin therapy without further antibiotic treatment and 104 (17.0%) switched to another antibiotic after the end of daptomycin therapy (e.g., step-down to oral antibiotic therapy). Patient disposition and analysis sets are described in Table 1. The demographics, baseline characteristics and significant underlying diseases of patients with IE are summarized in Table 2. The majority of patients had a significant underlying disease: 133 (89.3%) RIE, 378 (91.3%) LIE and 43 (91.5%) BRLIE. The most common underlying diseases were cardiovascular, gastrointestinal, pulmonary, and renal. The majority of patients with IE were hospitalized prior to receiving daptomycin treatment (Table 2). The concomitant use of statins with daptomycin was reported in 19 (12.8%) RIE, 59 (14.3%) LIE and 3 (6.4%) BRLIE patients, respectively.
Table 2.
Demographic and clinical characteristics (safety population)
| Characteristics | Patients treated with daptomycin | ||
|---|---|---|---|
| RIE N = 149 |
LIE N = 414 |
BRLIE N = 47 |
|
| Age (years) | |||
| Median (range) | 58.0 (1–90) | 62.0 (10–91) | 63.0 (24–87) |
| <65, n (%) | 93 (62.4) | 227 (54.8) | 25 (53.2) |
| ≥65, n (%) | 56 (37.6) | 187 (45.2) | 22 (46.8) |
| ≥75, n (%) | 28 (18.8) | 82 (19.8) | 16 (34.0) |
| Sex, n (%) | |||
| Female | 45 (30.2) | 154 (37.2) | 17 (36.2) |
| Male | 104 (69.8) | 260 (62.8) | 30 (63.8) |
| Race, n (%) | |||
| Caucasian | 124 (83.2) | 355 (85.7) | 40 (85.1) |
| Othera | 14 (9.4) | 28 (6.8) | 2 (4.3) |
| Unknown | 11 (7.4) | 31 (7.5) | 5 (10.6) |
| Body weight (kg) | |||
| Median (range) | 68.0 (6–98) | 73.0 (25–120) | 70.0 (43–93) |
| Setting prior to daptomycin therapy, n (%) | |||
| Hospital | 133 (89.3) | 389 (94.0) | 43 (91.5) |
| Nursing home/extended care | – | – | 1 (2.1) |
| Community | 14 (9.4) | 24 (5.8) | 2 (4.3) |
| Unknown | – | – | 1 (2.1) |
| Other | 2 (1.3) | 1 (0.2) | – |
| Received HMG-CoA reductase inhibitor (statin) with daptomycin, n (%) | |||
| Yes | 19 (12.8) | 59 (14.3) | 3 (6.4) |
| No | 129 (86.6) | 354 (85.5) | 44 (93.6) |
| Unknown | 1 (0.7) | 1 (0.2) | – |
| Severe renal impairment (CrCl <30 mL/min) at initiation of daptomycin therapy, n (%) | 18 (12.1) | 76 (18.4) | 8 (17.0) |
| Patients on dialysis at daptomycin initiation, n (%) | 13 (8.7) | 51 (12.3) | 6 (12.8) |
| Any significant underlying diseases (>10% of patients in every group), n (%) | 133 (89.3) | 378 (91.3) | 43 (91.5) |
| Cardiovascular disease | 93 (62.4) | 322 (77.8) | 34 (72.3) |
| Gastrointestinal disease | 24 (16.1) | 55 (13.3) | 7 (14.9) |
| Pulmonary disease | 21 (14.1) | 51 (12.3) | 9 (19.1) |
| Renal disease | 25 (16.8) | 83 (20.0) | 12 (25.5) |
BRLIE both right- and left-sided infective endocarditis, CrCl creatinine clearance, HMG-CoA 3-hydroxy-3-methylglutaryl-coenzyme A, LIE left-sided infective endocarditis, RIE right-sided infective endocarditis, SD standard deviation
aAsian, Black and missing
Microbiology
Microbiologic data consisted of culture results obtained from the study. In total 110 (73.8%) RIE, 274 (66.2%) LIE and 29 (61.7%) BRLIE patients were reported to have positive cultures (Table 3). MSSA was most commonly identified in 39 (35.5%), 50 (18.2%) and 7 (24.1%) patients (RIE, LIE and BRLIE, respectively). MRSA was identified in 11 (10.0%), 39 (14.2%) and 3 (10.3%) patients (RIE, LIE and BRLIE, respectively). Of patients with infections caused by enterococci, vancomycin-susceptible Enterococcus faecalis was identified in 5 (4.5%), 30 (10.9%), and 2 (6.9%) patients (RIE, LIE and BRLIE, respectively). Vancomycin-resistant Enterococcus faecium was identified in 2 (0.7%) and 1 (3.4%) patients with LIE and BRLIE, respectively.
Table 3.
Primary pathogens in patients with positive cultures
| Primary pathogens | RIE N = 110, n (%) |
LIE N = 274, n (%) |
BRLIE N = 29, n (%) |
|---|---|---|---|
| Staphylococcus aureus | 53 (48.2) | 101 (36.9) | 11 (37.9) |
| Methicillin susceptible | 39 (35.5) | 50 (18.2) | 7 (24.1) |
| Methicillin resistant | 11 (10.0) | 39 (14.2) | 3 (10.3) |
| Methicillin susceptibility unknown | 3 (2.7) | 12 (4.4) | 1 (3.4) |
| Coagulase-negative staphylococci | |||
| Staphylococcus epidermidis | 30 (27.3) | 49 (17.9) | 2 (6.9) |
| Methicillin susceptible | 5 (4.5) | 5 (1.8) | – |
| Methicillin resistant | 22 (20.0) | 37 (13.5) | 1 (3.4) |
| Methicillin susceptibility unknown | 3 (2.7) | 7 (2.6) | 1 (3.4) |
| Other | 13 (11.8) | 32 (11.7) | 6 (20.7) |
| Methicillin susceptible | 3 (2.7) | 5 (1.8) | 1 (3.4) |
| Methicillin resistant | 10 (9.1) | 25 (9.1) | 2 (6.9) |
| Methicillin susceptibility unknown | – | 2 (0.7) | 3 (10.3) |
| Staphylococcus species—coagulase not specified | 1 (0.9) | 2 (0.7) | – |
| Enterococcus faecium | – | 7 (2.6) | 1 (3.4) |
| Vancomycin susceptible | – | 3 (1.1) | – |
| Vancomycin resistant | – | 2 (0.7) | 1 (3.4) |
| Vancomycin susceptibility unknown | – | 2 (0.7) | – |
| Enterococcus faecalis | 6 (5.5) | 34 (12.4) | 3 (10.3) |
| Vancomycin susceptible | 5 (4.5) | 30 (10.9) | 2 (6.9) |
| Vancomycin susceptibility unknown | 1 (0.9) | 4 (1.5) | 1 (3.4) |
| Other Enterococcus species | – | 7 (2.6) | – |
| Streptococcus agalactiae or group B streptococci | 1 (0.9) | 2 (0.7) | – |
| Streptococcus dysgalactiae | – | 1 (0.4) | – |
| Streptococcus dysgalactiae equisimilis | – | 1 (0.4) | – |
| Streptococcus pneumonia | – | 1 (0.4) | – |
| Streptococcus pyogenes or group A streptococci | – | 2 (0.7) | – |
| Streptococcus species | 3 (2.7) | 9 (3.3) | 1 (3.4) |
| Viridians streptococci group | 3 (2.7) | 18 (6.6) | 2 (6.9) |
| Gram-negative bacilli | – | 2 (0.7) | 1 (3.4) |
| Gram-positive cocci | – | 2 (0.7) | 1 (3.4) |
| Othera | – | 4 (1.5) | 1 (3.4) |
BRLIE both right- and left- sided infective endocarditis, LIE left-sided infective endocarditis, RIE right-sided infective endocarditis
aIncludes Corynebacterium species
Daptomycin Prescribing Patterns
Among the 610 patients with IE, the most commonly prescribed dose of daptomycin was 6 mg/kg/day given to 342 (56.1%) patients: 84 (56.4%), 231 (55.8%) and 27 (57.4%) patients with RIE, LIE and BRLIE, respectively. However, 49 (8%) patients received >6 to <8 mg/kg/day, 109 (17.9%) received ≥8 to ≤10 mg/kg/day and 7 (1.1%) patients received >10 mg/kg/day dose of daptomycin (maximum dose was 12 mg/kg/day in the study), with similar proportions of RIE, LIE and BRLIE patients receiving daptomycin dose ranges >6 mg/kg/day. The median duration for inpatient therapy was 19 (range 1–81) days, 18 (range 1–112) days and 14 (range 5–72) days for RIE, LIE and BRLIE, respectively. Of those treated as inpatients, the median duration of outpatient follow-up therapy with daptomycin was 21 (range 7–50) days, 21 (range 5–85) days and 30 (range 22–75) days for RIE, LIE and BRLIE, respectively.
Clinical Outcomes
In this retrospective study, data from 610 patients with IE as well as 185 patients with foreign body intracardiac and 154 patients with foreign body intravascular device infections were collected and analyzed.
Of the 610 patients with IE who were treated with daptomycin, the overall clinical success rate was achieved in 488 (80.0%) patients. Only 47 (7.7%) patients had a treatment failure outcome and 75 (12.3%) patients were non-evaluable. The clinical success rate was achieved in 142/185 (76.8%) patients with foreign body intracardiac device infection and 120/154 (77.9%) patients with foreign body intravascular device infection.
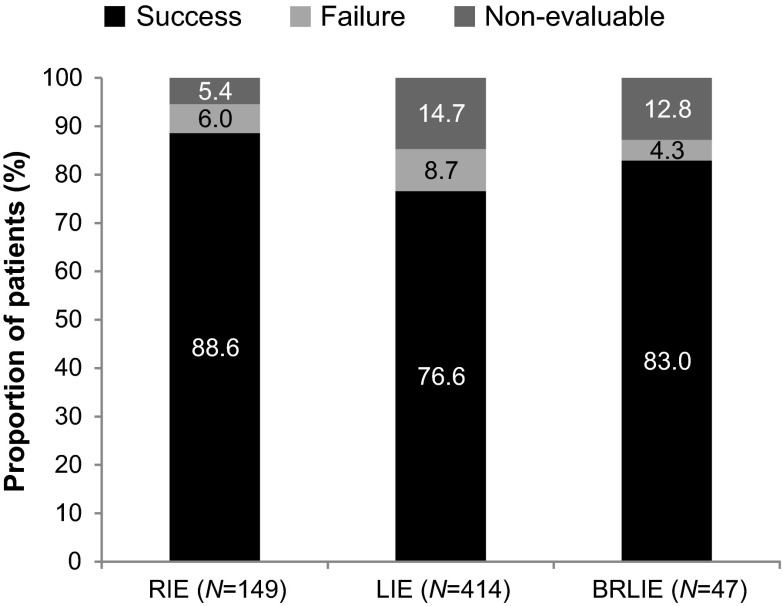
Clinical success rate was achieved in 132 (88.6%), 317 (76.6%) and 39 (83.0%) patients with RIE, LIE and BRLIE infections, respectively (Fig. 1). Treatment failure rates were low (4.3–8.7%) across all types of IE. Clinical success rate in 107 (92.2%) patients treated with daptomycin doses ≥8 mg/kg/day was higher compared with lower doses. There was a trend towards higher rates of success as daptomycin doses increased (Table 4). Patients with RIE, LIE and BRLIE who had received a daptomycin dose of 6 mg/kg/day had a median of 5 (range 1–46) days, 4 (range 1–41) days and 4.5 (range 0–16) days of time to improvement.
Fig. 1.
Clinical outcomes in patients with infective endocarditis. BRLIE both right- and left-sided infective endocarditis, LIE left-sided infective endocarditis, RIE right-sided infective endocarditis
Table 4.
Clinical success by type of endocarditis and dose groups (efficacy population)
| Dose (mg/kg/day) | 4 n/N (%) |
>4 and <6 n/N (%) |
6 n/N (%) |
>6 and <8 n/N (%) |
≥8 n/N (%) |
|---|---|---|---|---|---|
| Infective endocarditis | |||||
| All | 23/38 (60.5) | 32/46 (69.6) | 280/342 (81.9) | 37/49 (75.5) | 107/116 (92.2) |
| Right sided | 5/7 (71.4) | 11/11 (100) | 73/84 (86.9) | 11/11 (100) | 32/34 (94.1) |
| Left sided | 15/28 (53.6) | 18/31 (58.1) | 185/231 (80.1) | 23/35 (65.7) | 68/74 (91.9) |
| Both right and left sided | 3/3 (100) | 3/4 (75.0) | 22/27 (81.5) | 3/3 (100) | 7/8 (87.5) |
Clinical success rate was achieved in 46/53 (86.8%) RIE, 79/101 (78.2%) LIE and 10/11 (90.9%) BRLIE patients with infections caused by S. aureus. High rates of clinical success were reported in patients who had MSSA and MRSA infections. Patients with RIE achieved 87.1% (n = 34) and 90.9% (n = 10) success, patients with LIE 84.0% (n = 42) and 71.8% (n = 28) success, and patients with BRLIE 100% (n = 7) and 66.7% (n = 2) success against MSSA and MRSA infections, respectively. Clinical success was achieved in 96.7% (n = 30) RIE, 79.6% (n = 49) LIE and 100% (n = 2) BRLIE patients with infections caused by Staphylococcus epidermidis. Surgical interventions such as replacement of heart valves and removal of foreign devices were also required for the management of patients with IE. In 21 (14.1%) RIE, 166 (40.1%) LIE and 11 (23.4%) BRLIE patients heart valves were replaced; whereas in 37 (24.8%) RIE and 14 (3.4%) LIE patients foreign devices were surgically removed.
Of 272 patients, who had supplementary data collected, the most common surgical procedures during daptomycin treatment were heart valves replaced in 57 (21.0%), foreign intracardiac device removed in 14 (5.1%), tissue debridement in 6 (2.2%), vascular graft removed/replaced in 3 (1.1%), and incision and drainage in 2 patients (0.7%). Surgical procedures performed within 60 days after stopping treatment with daptomycin were: heart valves replaced in 11 (4.0%), foreign intracardiac device removed in 3 (1.1%) and tissue debridement in 1 (0.4%) patient. Of the patients who underwent heart valve replacement during or after stopping daptomycin therapy, 65.9% had this procedure done by day 14 from start of daptomycin, 81.8% by day 30 and 100% by day 90.
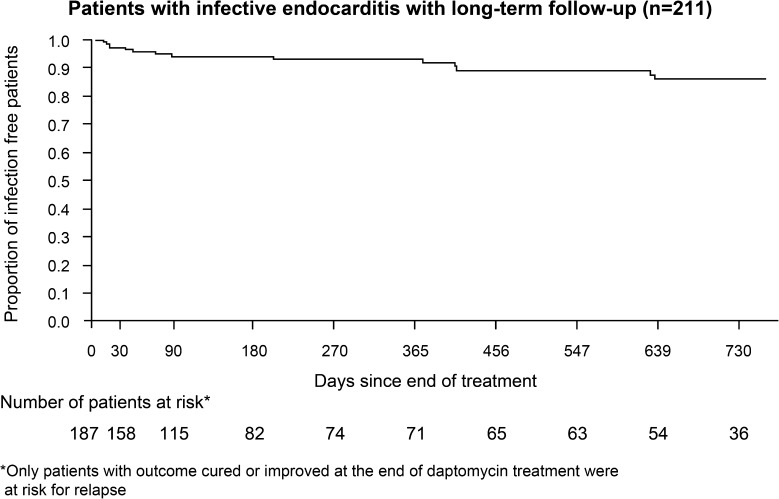
The sustained clinical success rate in 211 patients with IE and/or foreign body intracardiac/intravascular device infection who were followed for up to 2 years was 86.7% (93.5% for RIE, 88.3% for LIE and 77.8% for BRLIE). The majority (86.2%) of these patients remained relapse free at the end of the 2-year follow-up period (Fig. 2).
Fig. 2.
Kaplan–Meier survival curve for patients with infective endocarditis and/or foreign body intracardiac/intravascular device infection with long-term follow-up
Safety
An overview of AEs is presented in Table 5. Overall, 5 (3.4%), 30 (7.2%) and 2 (4.3%) patients with RIE, LIE and BRLIE discontinued daptomycin therapy due to AEs, respectively. The numbers of reported AEs which were related to daptomycin were low: 2 (1.3%) in patients with RIE, 18 (4.3%) in patients with LIE, and 1 (2.1%) patients with BRLIE. Among AEs reported as related to daptomycin, creatine phosphokinase (CPK) elevation was observed in 2 (1.3%), 8 (1.9%) and 1 (2.1%) patients, with RIE, LIE and BRLIE, respectively. Two patients (LIE) developed eosinophilic pneumonia. Death was reported in 5 (3.4%), 53 (12.8%) and 7 (14.9%) patients with RIE, LIE and BRLIE, respectively; none of the SAEs associated with deaths were considered to be related to daptomycin.
Table 5.
Summary of adverse events (safety population)
| Safety parameters | RIE N = 149, n (%) |
LIE N = 414, n (%) |
BRLIE N = 47, n (%) |
|---|---|---|---|
| AEs | 16 (10.7) | 98 (23.7) | 10 (21.3) |
| SAEs | 9 (6.0) | 70 (16.9) | 10 (21.3) |
| Discontinuations due to AEs | 5 (3.4)) | 30 (7.2) | 2 (4.3) |
| AEs possibly related to daptomycin, n (%) | 2 (1.3) | 18 (4.3) | 1 (2.1) |
| Blood CPK increased | 2 (1.3) | 8 (1.9) | 1 (2.1) |
| Myalgia | 1 (0.7) | – | – |
| Agranulocytosis | – | 1 (0.2) | – |
| Eosinophilia | – | 1 (0.2) | – |
| Eye pain | – | 1 (0.2) | – |
| Mouth ulceration | – | 1 (0.2) | – |
| Cholestasis | – | 1 (0.2) | – |
| Pneumonia | – | 1 (0.2) | – |
| Rhabdomyolysis | – | 1 (0.2) | – |
| Eosinophilic pneumonia | – | 2 (0.5) | – |
| Pulmonary interstitial emphysema syndrome | – | 1 (0.2) | – |
| Dermatitis allergic | – | 1 (0.2) | – |
| Rash | – | 1 (0.2) | – |
| Rash generalized | – | 1 (0.2) | – |
| SAEs possibly related to daptomycin, n (%) | – | 6 (1.4) | – |
| Agranulocytosis | – | 1 (0.2) | – |
| Cholestasis | – | 1 (0.2) | – |
| Blood CPK increased | – | 2 (0.5) | – |
| Rhabdomyolysis | – | 1 (0.2) | – |
| Eosinophilic pneumonia | – | 2 (0.5) | – |
| Pulmonary interstitial emphysema syndrome | – | 1 (0.2) | – |
| Rash generalized | – | 1 (0.2) | – |
AE adverse event, BRLIE both right- and left- sided infective endocarditis, CPK creatine phosphokinase, LIE left-sided infective endocarditis, RIE right-sided infective endocarditis, SAE serious AE
Discussion
Infective endocarditis is a complex infection which can present in different ways, which vary according to the initial clinical manifestation, underlying cardiac disease, associated pathogen, and the presence of any complications. The incidence of IE and its mortality have not decreased in the past 30 years. Although there were major advances in diagnostic and therapeutic procedures, the disease still carries a poor prognosis and a high mortality [26]. The present retrospective analysis from the EU-CORE patient registry provides valuable information on real-life experience of patients with IE who were treated with daptomycin across multiple centers from 18 countries, which may not otherwise be apparent from a randomized clinical trial.
Data from this analysis demonstrate that daptomycin was successful for the treatment of patients with IE caused by Gram-positive bacteria, including MRSA. This finding is consistent with the earlier published reports [11, 27]. Overall, clinical success rates were high, demonstrating effective use of daptomycin to treat IE.
In this analysis, there was a trend towards higher success rate with higher daptomycin doses ≥8 mg/kg/day (maximum dose was 12 mg/kg/day) compared with lower doses. This was consistent with a previous report [21]. Hence, the use of higher doses of daptomycin ≥8 mg/kg/day may result in better treatment outcome against IE. This supports recommendations from international treatment guidelines for the use of higher doses of daptomycin in the treatment of patients with IE [1, 28]. Further exploratory analyses will be required to assess outcome by patients underlying disease status, and whether treatment was administered as first or second line.
Data from 414 patients with LIE were collected and analyzed in this analysis. The results were similar to those of 149 patients with RIE and 47 patients with BRLIE. In an observational cohort study, out of 178 patients with LIE, 29 received daptomycin and 149 received standard-of-care (SOC) therapy (e.g., penicillins, vancomycin, ampicillin, aminoglycosides). There was a trend towards better outcome with higher doses of daptomycin compared to SOC therapy for patients with LIE [21], which is consistent with our findings. Although daptomycin is not currently approved for the treatment of LIE, data from the present analysis have shown that daptomycin was used successfully for the treatment of LIE and BRLIE with a trend towards higher clinical success rates with higher daptomycin doses. Therefore, data from this analysis suggest that daptomycin may be effective for the treatment of LIE and BRLIE.
The most commonly encountered causative agent was S. aureus, followed by S. epidermidis. There was a higher proportion of enterococcal infection in patients with LIE than in RIE and BRLIE. Also, there was a broader range of streptococcal infections in LIE than in RIE or BRLIE. In this study, MSSA was more commonly isolated than MRSA from patients with IE. Patients with IE who had complicated MSSA and MRSA infections and received daptomycin therapy had high success rates. These results are consistent with the findings from an earlier in vitro study on IE model which showed that daptomycin had better bactericidal activity against the biofilm-forming MRSA compared with vancomycin [29]. Data from a prospective cohort study reported that the mortality of patients with IE infections caused by MRSA isolates was high with vancomycin minimum inhibitory concentration of 2 mg/L (determined by Etest) [30]. Daptomycin at higher doses may be considered as first-line therapy to manage both MSSA and MRSA infections in patients with IE, which was also mentioned in an earlier published report [31]. In an in vitro study, it was observed that daptomycin in combination with β-lactams enhanced efficacy of anti-MRSA therapeutic options against daptomycin-resistant MRSA infections [32]. The results of another in vitro study showed that the combination of high doses of daptomycin with fosfomycin was effective in the treatment of left-sided endocarditis (both native and prosthetic valves) caused by MSSA or MRSA infections [33]. In the present study, the notable finding was that the clinical success rates remained high in patients with reported long-term follow-up results for up to 2 years.
This study has limitations due to its retrospective and non-comparative design. It was not as strictly controlled as a randomized controlled trial. Analysis was not conducted to provide statistical outcomes, although it is very difficult to conduct controlled trials in IE patients. All patients with at least one dose of daptomycin were included, however, it should be noted that the correct course of treatment for daptomycin lasts between 4 and 6 weeks. Some patients included in the registry might have received other antibiotics besides daptomycin.
Conclusions
These results of real-world experience from the EU-CORE registry showed that patients with IE were treated successfully with daptomycin. Daptomycin was well tolerated and effective for the treatment of LIE and BRLIE in addition to the approved indication of RIE, which provides evidence that daptomycin is a potential treatment option in the management of RIE, LIE and BRLIE. Data showed that daptomycin is effective for treating Gram-positive infections, including MRSA infections. The majority of patients with available long-term data remained relapse free during the 2-year follow-up period.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This study and article processing charges were sponsored by Novartis Pharma AG. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. Medical writing support was provided by Novartis Global Medical and Clinical Services medical writers Rajeeb Ghosh and Farid Khalfi. Funding for writing was provided by Novartis Pharm AG.
Conflict of interest
Achyut Guleri received fees for advisory boards and speakers panels from Novartis, and consultancy fees from Astellas, AstraZeneca, MSD, and Schering-Plough. He also received support to attend scientific conferences including accommodation and travel payments from BD, Carefusion UK, Janssen-Cilag, and MSD.
Riccardo Utili received from Novartis a research grant, fees for advisory boards and speakers panels, and support to attend meetings including accommodation and travel payments.
Pascal Dohmen received a research grant and fees for speakers panels from Novartis. He also received grants from other companies.
Nicola Petrosillo received speakers fees from Cepheid and Astellas. He is also a member of the board of Cilag and MSD.
Cornelia Piper has no conflicts of interest to declare.
Rashidkhan Pathan is an employee of Novartis Healthcare Pvt. Ltd.
Kamal Hamed is an employee of Novartis Pharmaceuticals Corporation.
Compliance with ethics guidelines
The study has been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The protocol was approved by the health authority and the Institutional Review Board (IRB) or Ethics Committee (EC) in each country and written informed consent was obtained according to the requirements of the IRB or EC and/or the local data privacy regulations.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
References
- 1.Smith JR, Claeys KC, Barber KE, Rybak MJ. High-dose daptomycin therapy for staphylococcal endocarditis and when to apply it. Curr Infect Dis Rep. 2014;16(10):429. doi: 10.1007/s11908-014-0429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bor DH, Woolhandler S, Nardin R, Brusch J, Himmelstein DU. Infective endocarditis in the U.S., 1998–2009: a nationwide study. PLoS One. 2013;8(3):e60033. doi: 10.1371/journal.pone.0060033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoen B, Alla F, Selton-Suty C, et al. Changing profile of infective endocarditis: results of a 1-year survey in France. JAMA. 2002;288(1):75–81. doi: 10.1001/jama.288.1.75. [DOI] [PubMed] [Google Scholar]
- 4.Cabell CH, Jollis JG, Peterson GE, et al. Changing patient characteristics and the effect on mortality in endocarditis. Arch Intern Med. 2002;162(1):90–94. doi: 10.1001/archinte.162.1.90. [DOI] [PubMed] [Google Scholar]
- 5.Murdoch DR, Corey GR, Hoen B, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med. 2009;169(5):463–473. doi: 10.1001/archinternmed.2008.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selton-Suty C, Celard M, Le Moing V, et al. Preeminence of Staphylococcus aureus in infective endocarditis: a 1-year population-based survey. Clin Infect Dis. 2012;54(9):1230–1239. doi: 10.1093/cid/cis199. [DOI] [PubMed] [Google Scholar]
- 7.Durand C, Brueckner A, Sampadian C, Willett KC, Belliveau P. Daptomycin use in pediatric patients. Am J Health Syst Pharm. 2014;71(14):1177–1182. doi: 10.2146/ajhp130601. [DOI] [PubMed] [Google Scholar]
- 8.Ardura MI, Mejias A, Katz KS, et al. Daptomycin therapy for invasive Gram-positive bacterial infections in children. Pediatr Infect Dis J. 2007;26(12):1128–1132. doi: 10.1097/INF.0b013e31814523f8. [DOI] [PubMed] [Google Scholar]
- 9.Furuno JP, Johnson JK, Schweizer ML, et al. Community-associated methicillin-resistant Staphylococcus aureus bacteremia and endocarditis among HIV patients: a cohort study. BMC Infect Dis. 2011;11:298. doi: 10.1186/1471-2334-11-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laupland KB, Lyytikainen O, Sogaard M, et al. The changing epidemiology of Staphylococcus aureus bloodstream infection: a multinational population-based surveillance study. Clin Microbiol Infect. 2013;19(5):465–471. doi: 10.1111/j.1469-0691.2012.03903.x. [DOI] [PubMed] [Google Scholar]
- 11.Dohmen PM, Guleri A, Capone A, et al. Daptomycin for the treatment of infective endocarditis: results from a European registry. J Antimicrob Chemother. 2013;68(4):936–942. doi: 10.1093/jac/dks467. [DOI] [PubMed] [Google Scholar]
- 12.Hill EE, Peetermans WE, Vanderschueren S, et al. Methicillin-resistant versus methicillin-sensitive Staphylococcus aureus infective endocarditis. Eur J Clin Microbiol Infect Dis. 2008;27(6):445–450. doi: 10.1007/s10096-007-0458-2. [DOI] [PubMed] [Google Scholar]
- 13.Wu G, Abraham T, Rapp J, et al. Daptomycin: evaluation of a high-dose treatment strategy. Int J Antimicrob Agents. 2011;38(3):192–196. doi: 10.1016/j.ijantimicag.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Kullar R, McKinnell JA, Sakoulas G. Avoiding the perfect storm: the biologic and clinical case for reevaluating the 7-day expectation for methicillin-resistant Staphylococcus aureus bacteremia before switching therapy. Clin Infect Dis. 2014;59(10):1455–1461. doi: 10.1093/cid/ciu583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Que YA, Moreillon P. Infective endocarditis. Nat Rev Cardiol. 2011;8(6):322–336. doi: 10.1038/nrcardio.2011.43. [DOI] [PubMed] [Google Scholar]
- 16.Lee SY, Fan HW, Kuti JL, Nicolau DP. Update on daptomycin: the first approved lipopeptide antibiotic. Expert Opin Pharmacother. 2006;7(10):1381–1397. doi: 10.1517/14656566.7.10.1381. [DOI] [PubMed] [Google Scholar]
- 17.Fowler VG, Jr, Boucher HW, Corey GR, et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med. 2006;355(7):653–665. doi: 10.1056/NEJMoa053783. [DOI] [PubMed] [Google Scholar]
- 18.Levine DP, Lamp KC. Daptomycin in the treatment of patients with infective endocarditis: experience from a registry. Am J Med. 2007;120(10 Suppl 1):S28–S33. doi: 10.1016/j.amjmed.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 19.Das I, Saluja T, Steeds R. Use of daptomycin in complicated cases of infective endocarditis. Eur J Clin Microbiol Infect Dis. 2011;30(6):807–812. doi: 10.1007/s10096-011-1160-y. [DOI] [PubMed] [Google Scholar]
- 20.Kaya S, Yilmaz G, Kalkan A, Ertunc B, Koksal I. Treatment of Gram-positive left-sided infective endocarditis with daptomycin. J Infect Chemother. 2013;19(4):698–702. doi: 10.1007/s10156-012-0546-9. [DOI] [PubMed] [Google Scholar]
- 21.Carugati M, Bayer AS, Miro JM, et al. High-dose daptomycin therapy for left-sided infective endocarditis: a prospective study from the international collaboration on endocarditis. Antimicrob Agents Chemother. 2013;57(12):6213–6222. doi: 10.1128/AAC.01563-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez-Ruiz A, Beiras-Fernandez A, Lehmkuhl H, et al. Effectiveness and safety of daptomycin in complicated skin and soft-tissue infections and bacteraemia in clinical practice: results of a large non-interventional study. Int J Antimicrob Agents. 2013;41(4):372–378. doi: 10.1016/j.ijantimicag.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 23.Seaton RA, Gonzalez-Ramallo VJ, Prisco V, et al. Daptomycin for outpatient parenteral antibiotic therapy: a European registry experience. Int J Antimicrob Agents. 2013;41(5):468–472. doi: 10.1016/j.ijantimicag.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 24.Seaton RA, Malizos KN, Viale P, et al. Daptomycin use in patients with osteomyelitis: a preliminary report from the EU-CORE(SM) database. J Antimicrob Chemother. 2013;68(7):1642–1649. doi: 10.1093/jac/dkt067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Durack DT, Lukes AS, Bright DK, et al. New criteria for diagnosis of infective endocarditis—utilization of specific echocardiographic findings. Am J Med. 1994;96(3):200–209. doi: 10.1016/0002-9343(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 26.Habib G, Hoen B, Tornos P, et al. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009) Eur Heart J. 2009;30(19):2369–2413. doi: 10.1093/eurheartj/ehp285. [DOI] [PubMed] [Google Scholar]
- 27.Martone WJ, Lindfield KC, Katz DE. Outpatient parenteral antibiotic therapy with daptomycin: insights from a patient registry. Int J Clin Pract. 2008;62(8):1183–1187. doi: 10.1111/j.1742-1241.2008.01824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52(3):285–292. doi: 10.1093/cid/cir034. [DOI] [PubMed] [Google Scholar]
- 29.LaPlante KL, Woodmansee S. Activities of daptomycin and vancomycin alone and in combination with rifampin and gentamicin against biofilm-forming methicillin-resistant Staphylococcus aureus isolates in an experimental model of endocarditis. Antimicrob Agents Chemother. 2009;53(9):3880–3886. doi: 10.1128/AAC.00134-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bae IG, Federspiel JJ, Miro JM, et al. Heterogeneous vancomycin-intermediate susceptibility phenotype in bloodstream methicillin-resistant Staphylococcus aureus isolates from an international cohort of patients with infective endocarditis: prevalence, genotype, and clinical significance. J Infect Dis. 2009;200(9):1355–1366. doi: 10.1086/606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cervera C, Castaneda X, Pericas JM, et al. Clinical utility of daptomycin in infective endocarditis caused by Gram-positive cocci. Int J Antimicrob Agents. 2011;38(5):365–370. doi: 10.1016/j.ijantimicag.2010.11.038. [DOI] [PubMed] [Google Scholar]
- 32.Mehta S, Singh C, Plata KB, et al. beta-Lactams increase the antibacterial activity of daptomycin against clinical methicillin-resistant Staphylococcus aureus strains and prevent selection of daptomycin-resistant derivatives. Antimicrob Agents Chemother. 2012;56(12):6192–6200. doi: 10.1128/AAC.01525-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miro JM, Entenza JM, del Rio A, et al. High-dose daptomycin plus fosfomycin is safe and effective in treating methicillin-susceptible and methicillin-resistant Staphylococcus aureus endocarditis. Antimicrob Agents Chemother. 2012;56(8):4511–4515. doi: 10.1128/AAC.06449-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.