Abstract
Introduction
The differentiation of viral from bacterial pneumonia is important in determining whether antibiotics are appropriate for treatment of these infections. Advances in diagnostic technologies such as respiratory panels (RP) utilizing polymerase chain reactions to detect viruses and determination of procalcitonin (PCT) concentrations may aid in this differentiation. However, some studies have shown limited impact for this purpose and thus continuation of antibiotics despite results suggesting viral infection. Our objective was to characterize clinician-prescribing behavior at our institution once RP and/or PCT results were known and suggestive of a viral respiratory infection.
Methods
This retrospective analysis was based upon records of hospitalized patients in whom proven or possible respiratory infections as indicated by RP testing, respiratory bacterial culture or International Statistical Classification of Diseases and Related Health Problems 9th revision codes for acute infectious respiratory illness was documented. Patients evaluated were required to have a RP or PCT within the first 72 h of presentation. Drug orders were evaluated for discontinuation of antibiotic therapy within 48 h of a procalcitonin of <0.25 μg/mL, a positive viral RP result, or both.
Results
Of 4869 patients with PCT and/or RP results, 2031 were included. PCT and RP testing were obtained in 503 and 1823 patients, respectively, with 295 patients having both. Results of these tests suggested 789 patients were potential candidates for antibiotic avoidance. These included 219 with a PCT <0.25 μg/mL, 601 with a positive viral RP result, and 31 with both. Antibiotics were administered to 307 patients (39%) within the first 72 h. In these, antibiotics were discontinued within 48 h of laboratory results availability.
Conclusion
These results suggest that positive viral RP and low PCT results are infrequently associated with discontinuation of antibiotic therapy in proven or possible respiratory infections at our institution. Direct interventions with clinicians are likely needed to correct this behavior and decrease unnecessary antibiotic use.
Electronic supplementary material
The online version of this article (doi:10.1007/s40121-015-0087-5) contains supplementary material, which is available to authorized users.
Keywords: Antibiotic prescribing, Antimicrobial stewardship, Pneumonia, Procalcitonin, Rapid diagnostics, Respiratory panel, Viral infection
Introduction
Pneumonia remains among the top 10 most common causes of mortality for all age groups in the United States and the most common infectious cause of mortality [1]. In the United States in 2013, pneumonia was responsible for 1.1 million hospital admissions and 53,282 deaths [2]. Pneumonia is commonly caused by viral or bacterial pathogens with the former being responsible for 29–39% of cases [3, 4]. Clinical signs and symptoms associated with the two etiologies are often indistinguishable [5]. Physical examination, routine microbiological testing of respiratory samples and radiologic findings are not routinely reliable in establishing an accurate diagnosis of bacterial pneumonia [6–8].
Developments in diagnostics that may assist in alleviating this situation have arisen. Polymerase chain reaction (PCR) technology has been adopted into clinical use for the rapid identification of various pathogens. Multiplex PCR technology has recently been added to allow the identification of a panel of potential respiratory pathogens in contrast to older PCR technologies that only identified a single target [8]. Biomarkers have been examined for their diagnostic utility as another approach to differentiate bacterial infections from other types of inflammatory states. While biomarkers such as C-reactive protein and erythrocyte sedimentation rate are often elevated in both infectious and noninfectious conditions, procalcitonin (PCT) levels are routinely elevated in the presence of bacterial infections while remaining low in the setting of other types of inflammatory processes [9–12]. The use of this test as a decision tool has been established as a safe method for ruling out bacterial infections and thus reducing antibiotic use [13]. However, while low serum levels of PCT are suggestive of the absence of bacterial infection, it does not exclude it, and therefore results should always be used in conjunction with clinical judgment with particular caution given in interpreting these results in seriously ill patients.
Despite the advances in and clinical application of such diagnostic technologies, it has been reported that many patients nonetheless continue to receive antibiotic therapy without regard to positive viral PCR and/or low PCT results [14–16]. It was our objective to determine the frequency of changes in empiric antibiotic therapy for known or proven cases of pneumonia once results of respiratory panels (RP) and/or PCT testing were known to the clinician and thus determine the need for an active stewardship intervention at our own institution.
Methods
Patient Population
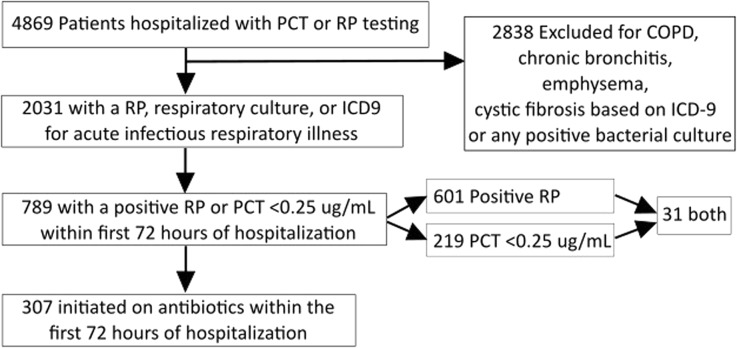
This retrospective, single-center study was performed at the Medical University of South Carolina Hospital, a 709 bed tertiary and quaternary academic medical center. The average daily census rate is 627, with over 36,000 annual admissions. Average adult inpatient length of stay, age and case-mix index are 6.2 days, 52 years, and 1.9642, respectively. A hospital’s case-mix index represents the average diagnosis-related group (DRG) relative weight for that hospital. It is calculated by summing the DRG weights for all Medicare discharges and dividing by the number of discharges. It is used in determining the allocation of resources to care for and/or treat the patients in the group. This study was reviewed and approved by the institutional review board at our institution. This article does not contain any new studies with human or animal subjects performed by any of the authors. Records accessed were from patients 18 years of age or older with proven or suspected respiratory infections as indicated by PCR, respiratory bacterial culture, or the International Statistical Classification of Diseases and Related Health Problems 9th revision (ICD-9) code for acute infectious respiratory illness (ICD-9 codes 460–466 and 480–488) associated with a hospitalization between January 01, 2012 and January 01, 2014. All patient records evaluated were required to have a RP or PCT within the first 72 h of presentation. Patients with cystic fibrosis based on ICD-9 or patients with positive bacterial cultures were excluded. Additionally, patients with COPD, chronic bronchitis, or emphysema were excluded as a lack of utility of PCT in distinguishing viral from bacterial respiratory illness in COPD has been described [17]. Basic study/design flow is illustrated in Fig. 1.
Fig. 1.
Flow diagram for study design and data collection/analysis. COPD chronic obstructive pulmonary disease, ICD-9 International Classification of Diseases 9th Revision, PCT procalcitonin, RP respiratory panel
Diagnostic Tests
Procalcitonin concentrations were determined with the VIDAS BRAHMS PCT automated test (bioMérieux, Marcy, France). PCT has a reported sensitivity of 88% and specificity of 81% in distinguishing a bacterial infection from other inflammatory processes [18]. Routine laboratory reporting denotes values of <0.25 μg/mL as suggestive of a bacterial infection being unlikely. PCT testing was generally available within two hours once the specimen was obtained. The presence of respiratory viruses was determined with the FilmArray Respiratory Panel (BioFire Diagnostics, Inc., Salt Lake City, UT, USA), a multiplex PCR test. This test detects 17 viruses including Adenovirus, Coronavirus 229E, Coronavirus HKU1, Coronavirus NL63, Coronavirus OC43, Human Metapneumovirus, Influenza A, Influenza A subtype H1, Influenza A subtype H3, Influenza A subtype 2009 H1, Influenza B, Parainfluenza Virus 1, Parainfluenza Virus 2, Parainfluenza Virus 3, Parainfluenza Virus 4, Rhinovirus/Enterovirus, and Respiratory Syncytial Virus. Results were available in one hour once specimen was obtained with a sensitivity and specificity that are 80–100% and 100%, respectively. No institutional protocol or guidance on the use/interpretation of PCT was in place at the time of the study. Neither test had education provided on their use during their implementation or during our analysis period. No specific antimicrobial stewardship activities or initiatives were in place specific for these tests during the study.
Data Collection
Patient data collected included age, sex, co-morbid conditions, diagnoses, intensive care unit admission, antibiotic use and duration, and the results of bacterial cultures if obtained, PCT, and RP. Drug orders were evaluated for discontinuation of therapy within 48 h of a procalcitonin of <0.25 μg/mL, a positive viral result, or both. Data were analyzed descriptively. Analysis was performed in Excel 2013 (Microsoft, Redmond, WA, USA). Figures were created using Excel 2013 and Inkscape 0.91 (Free Software Foundation, Inc., Boston, MA, USA).
Results
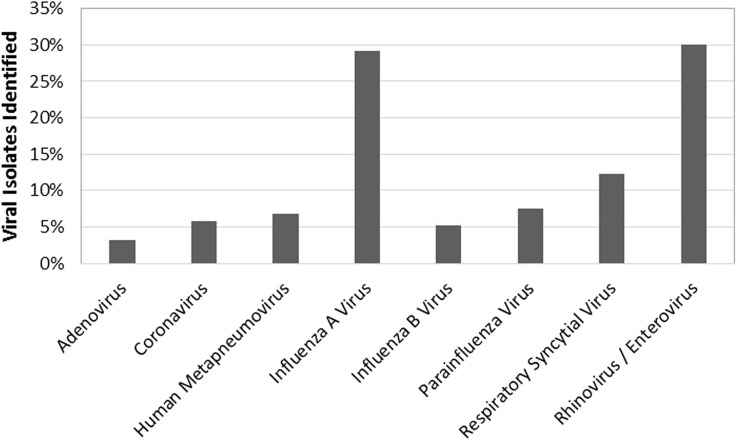
Of 4869 individual patient records with PCT and/or RP results, 2031 met inclusion for the study (Fig. 1). Results of PCT and RP testing were available in 503 and 1823 patients, respectively, with 295 patients having both. Of tests performed in the first 72 h, the results suggested that 789 (38.8%) patients may have had opportunities for antibiotic avoidance. In total, 219 patients with a PCT <0.25 μg/mL, 601 with a positive PCR, and 31 with both were identified. Of patients with testing results in the first 72 h, antibiotics were administered to 307 patients (38.9%) during the same period (Table 1). The most frequently detected respiratory viruses were influenza A/B (34% of total) and rhinovirus/enterovirus (30%; Fig. 2). Of the 307 patients prescribed antibiotics within the first 72 h, antibiotics were discontinued within 48 h of laboratory results in 60 (19.5%) patients with either a PCT <0.25 μg/mL, a positive PCR, or both (Table 2). The median duration of antibiotics was 3 days for all of these groups and ranged from 1 to 19 days, 1 to 27 days, and 1 to 13 days for a PCT <0.25 μg/mL, a positive RP, or both, respectively.
Table 1.
Patient characteristics
| PCT <0.25 μg/L (n = 219) | Positive viral RP (n = 601) | PCT <0.25 μg/L and positive viral RP (n = 31) | |
|---|---|---|---|
| Median age, years (IQR) | 58 (44–69) | 47 (33–60) | 55 (41–67) |
| Male sex, n (%) | 94 (42.0) | 256 (42.6) | 15 (48.4) |
| Charlson Score, median (IQR) | 1 (1–2) | 1 (0–1) | 1 (1–2) |
| ICU admission during hospitalization, n (%) | 89 (40.6) | 53 (8.8) | 13 (41.9) |
| Median length of stay, days (IQR) | 4 (2–6) | 1 (0–3) | 3 (2–6) |
| Antibiotic prescribed within first 72 h | 156 (71.2) | 170 (28.2) | 19 (61.3) |
ICU intensive care unit, IQR interquartile range, PCT procalcitonin, RP respiratory panel
Fig. 2.
Viruses identified by polymerase chain reaction (percent of total)
Table 2.
Antibiotics discontinued within 48 h of PCT and RP results
| PCT <0.25 μg/mL (n = 156) | Positive viral RP (n = 170) | PCT <0.25 μg/mL and positive viral RP (n = 31) | |
|---|---|---|---|
| Antibiotics discontinued, n (%) | 32 (20.5) | 30 (17.6) | 2 (10.5) |
| Median length of stay, days (IQR) | 4 (3–7) | 4 (2–6) | 5 (3–7) |
IQR interquartile range, PCT procalcitonin, RP respiratory panel
Discussion
The problem of inappropriate use of antibiotics leading to antibiotic-resistant organisms cannot be overstated. This issue has escalated to being nationally recognized and addressed in the White House’s National Action Plan for Combating Antibiotic-Resistant Bacteria, which among other initiatives, calls for increased antimicrobial stewardship activities for the judicious use of antimicrobials and for innovation in rapid diagnostics to facilitate that effort [19]. In the outpatient setting including the emergency department, the majority of antibiotic prescribing has been for patients presenting with respiratory conditions [20]. Many such infections are of viral origin including nearly 30% of pneumonias [3, 4]. In turn, much antibiotic prescribing for respiratory infections in outpatients is unwarranted [21, 22]. Unnecessary antibiotic use in the hospitalized patients with apparent respiratory tract infections also occurs [23]. This overuse can be explained to a large extent by the inherent difficulty in clinically differentiating bacterial from viral infection. At the same time, it appears that the treatment of respiratory tract infections represents a major area of opportunity for more judicious and limited use of antibiotics. The utilization of rapid diagnostic tests may help with this differential.
The use of PCT and RP alone, or in combination, has been successfully and safely used to guide diagnosis and therapy [13, 24–27]. A number of studies have shown PCT to be an effective test to utilize in altering antibiotic prescribing patterns without adversely affecting patient outcomes [13, 24, 25]. In these studies, empiric antibiotics therapy was strongly discouraged and/or recommended for discontinuation in patients with low PCT levels. Similar to the use of PCT to rule out bacterial infections, positive results of RP have been shown to be useful in efforts to reduce antibiotic use as they provide direct proof of viral respiratory infections. Afzal’s and colleagues’ use of RP results led to a reduction in antibiotic duration when that information was made available to the primary care provider [26]. Patients in whom the positive viral RP was disclosed to the primary care provider (n = 11) had a median duration of antibiotics of 7 days as opposed to 12 days (P = 0.05) in patients with proven bacterial infection (n = 28). Furthermore, when a positive viral PCR was not disclosed (n = 19), the median duration of antibiotics was 12 days—similar to the bacterial infection group. These findings of decreased antibiotic exposure based on viral PCR were also reported by Oosterheert and colleagues [27]. In their study, positive PR results led to a discontinuation of antibiotics in 11% of patients.
One potential challenge of the reliance on these rapid tests to rule out bacterial infection is co-infection with viral and bacterial pathogens, which has been reported in 14–40% of cases of infectious pneumonia [14, 28, 29]. Co-infection has recently been explored by Musher and colleagues in a study analyzing the etiology of community-acquired pneumonia (CAP) in a cohort of hospitalized CAP patients in a US Veterans Administration hospital [16]. Of 259, 4.6% were found to be co-infected with a virus and a bacterium or fungus. However, in the 42 cases of viral infection, 12 (28.5%) had a documented bacterial co-infection. In patients with severe CAP requiring mechanical ventilation, co-infection may occur more often. In a study by Karhu and colleagues, bacterial–viral co-infections were found in 39% of patients [30]. Importantly, these investigators reported that PCT concentrations actually helped distinguish bacterial from non-bacterial infections. Within their bacterial/viral co-infection patients, median admission and peak PCT concentrations were 20.1 and 24.3 μg/L, respectively, while those values in the viral infection group were 1.4 and 1.7 μg/L, respectively. These data are consistent with previous PCT data in suggesting that such results are effective in discriminating between viral and bacterial infections. However, it should be noted that while many of the aforementioned studies establish PCT as a safe and effective way of ruling out bacterial infections, others have cast doubt on sensitivity [16, 31] with one group reporting a sensitivity of approximately 40% [31]. Other biomarkers may have a role in improving the use of this test [32, 33].
We studied clinician-prescribing behavior at our institution once RP and/or PCT laboratory results were available to the prescribing physician and were suggestive of a pure viral respiratory infection. We observed antibiotic discontinuation rates of only 17–20% in patients with positive viral RP results or PCT <0.25 μg/mL. In patients with both test results available (n = 31), and highly suggestive of a pure viral infection, antibiotic discontinuation rates were even lower (10%). One possible explanation for the low rates of antibiotic discontinuation could relate to the relatively low prescribing of antibiotics (38.9%) to patients with proven or suspected respiratory infections which had either a low PCT, a positive viral RP result, or both. We believe the relatively low incidence of prescribing in this context suggests many prescribers avoided antibiotic therapy based on other clinical factors suggesting a non-bacterial illness causing respiratory symptoms. An earlier study by Falsey and colleagues of respiratory illness reflected similar results to ours substantiating the opportunity for more judicious antimicrobial use based on the results of RP and PCT [14]. In this single-center study, patients were deemed to have a pure viral infection if a viral test was positive, all bacterial tests were negative, and serum PCT levels were <0.25 μg/mL on admission and day two of hospitalization. Duration of inpatient antibiotics was found to be significantly less in the viral alone group as compared to the mixed viral–bacterial group with means of 4.2 vs 6.2 days, respectively. These data suggest an opportunity in the use of combining RP and PCT results to narrow the etiologic diagnosis and perhaps decrease unnecessary antibiotic exposure. While mixed viral–bacterial infection was noted in 40% of patients, the utilized diagnostic battery appeared to distinguish these from pure viral infections. It is also notable, however, that while RP and PCT results suggesting pure viral infections resulted in shorter durations of antibiotic therapy, the average duration of therapy extended more than 2 days beyond the availability results of RP and PCT testing. Recently, in a follow-up study, antibiotic prescribing patterns associated with low PCT and positive viral RP results in patients with non-pneumonic lower respiratory tract infections were characterized into two groups of patients, one receiving standard of care and the second receiving care specified by use of PCT algorithms and viral testing [15]. The investigators observed antibiotic discontinuation rates of 57% when PCT was <0.25 μg/mL and a positive viral RP was reported. This was substantially different than the 10% antibiotic discontinuation rate we observed. Several differences in study methods likely explain these disparate results. Importantly, “nonpneumonic” was apparently defined as those with clinically suggestive symptoms of lower respiratory tract infection with no clear evidence of or risk factors for bacterial pneumonia. Further, as the previous study [14] was conducted at the same institution as this more recent study and the methods and results of that study were shared with medical staff prior to commencing the latter study, a source of internal bias was likely introduced that would have influenced antibiotic usage/discontinuation rates. The observation that usage/discontinuation rates did not differ in the comparator groups may substantiate that impression. At the same time, these studies and the proactive intervention in the second provides further evidence that some proactive intervention, beyond the availability of rapid diagnostic test results, can influence prescribing behavior.
Our study is not without limitations. As it was a retrospective, single-center study, the prescribing behavior observed may very well differ from that at other institutions. As we excluded patients with chronic obstructive pulmonary disease, chronic bronchitis, emphysema, and cystic fibrosis, our results cannot be generalized as relevant to respiratory tract infections in these patient populations. Our criteria for antibiotic prescribing were based solely upon availability of and values for three specific tests (PCT, RP, bacterial culture). Some patients may have had other indicators suggestive of bacterial pulmonary infection such as severity of illness, indicative signs or symptoms, imaging studies, elevated white cells, or urinary bacterial antigens and so on which would have likely influenced decisions to use/continue antibiotic therapy. We did not account for the influence of such evidence on prescribing patterns in our analysis and their potential impact is of obvious importance. Additionally, we did not evaluate the timing of antibiotic administration in relation to time of availability of PCT or RP test results. Early testing is optimal for ensuring appropriate therapy decisions. However, studies such as PRORATA [24] have used higher PCT levels to determine appropriate antibiotic discontinuation. Those data suggest that regardless of the timing of administration of antibiotics, our more conservative PCT level would have suggested an opportunity for appropriate antibiotic discontinuation. Moreover, the PCT level used here for evaluation of antibiotic avoidance or discontinuation is consistent with other studies involving PCT guidance of therapy in possible or proven respiratory tract infections [15, 25, 34, 35]. Finally, to fully characterize the true impact of PCT and PCR on antibiotic prescribing, comparisons would need to include all patients with PCR and PCT results, not only those patients with results suggestive of mono-microbial viral respiratory infections.
We believe that there are several potential clinical implications of our findings. While 2031 patients had proven or presumed respiratory tract infections, only 295 patients had both RP and PCT testing. The challenge of co-infections with viruses and bacteria denotes the need for respiratory infection protocols including both PCT and RP tests ordered in the emergency department or upon admission to ensure prompt results for assistance in the clinical decision-making process and the appropriate use of antibiotics. The combined use of these tests upon admission could have decreased antibiotic use by an average of 2 days in most patients in our study with a positive viral RP, a PCT <0.25 μg/L, or both. Given issues with PCT sensitivity, it would appear prudent that both tests be employed. With the low rate of antibiotic discontinuation in the setting of positive viral RP and <0.25 μg/mL PCT results, there appears to be a need for education of providers. This education should include reviewing the etiologies of respiratory tract infections, providing PCT testing and interpretation information, and the need for judicious use of antibiotics related to potential complications and resistance development. Finally, there is an opportunity for antimicrobial stewardship programs (ASP). ASPs can provide education for practitioners, develop antibiotic guidelines, PCT/RP algorithms, and proactively recommend antibiotic discontinuation based upon proper interpretation of PCT and RP test results. Proactive intervention, such as that provided by ASPs is important in ensuring meaningful use of rapid diagnostics as opposed to a lack of clinical benefit in the absence of such ASP-facilitated interventions [36, 37].
Conclusion
Our findings suggest that PCT and RP results, alone or in combination, when indicative of a diagnosis of pure viral respiratory infections are infrequently associated with discontinuation of antibiotic therapy at our institution. Demonstration of this prescribing behavior suggests a possible opportunity for antimicrobial stewardship in the development of a clinical pathway or decision tool and the provision of relevant education on the importance of these tests as well as direct intervention with clinicians with the goal of lessening unnecessary antibiotic use. Additional studies should evaluate variations in antibiotic prescribing through the combination of RP and PCT protocols in respiratory tract infections along with stewardship activities of provider education and response to RP and PCT results.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
No funding or sponsorship was received for this study or publication of this article.
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Conflict of interest
T. Timbrook, M. Maxam and J. Bosso declare that they have no conflicts of interest.
Compliance with ethics guidelines
This study was reviewed and approved by the institutional review board at our institution. This article does not contain any new studies with human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
References
- 1.Miniño AM, Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2008. Natl Vital Stat Rep. 2011;59:1–126. [PubMed] [Google Scholar]
- 2.FastStats—Pneumonia. http://www.cdc.gov/nchs/fastats/pneumonia.htm. Accessed July 22, 2015.
- 3.Johansson N, Kalin M, Tiveljung-Lindell A, Giske CG, Hedlund J. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin Infect Dis. 2010;50:202–209. doi: 10.1086/648678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnstone J, Majumdar SR, Fox JD, Marrie TJ. Viral infection in adults hospitalized with community-acquired pneumonia: prevalence, pathogens, and presentation. Chest. 2008;134:1141–1148. doi: 10.1378/chest.08-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juvén T, Mertsola J, Waris M, et al. Etiology of community-acquired pneumonia in 254 hospitalized children. Pediatr Infect Dis J. 2000;19:293–298. doi: 10.1097/00006454-200004000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Rosón B, Carratalà J, Verdaguer R, Dorca J, Manresa F, Gudiol F. Prospective study of the usefulness of sputum Gram stain in the initial approach to community-acquired pneumonia requiring hospitalization. Clin Infect Dis. 2000;31:869–874. doi: 10.1086/318151. [DOI] [PubMed] [Google Scholar]
- 7.Miyashita N, Shimizu H, Ouchi K, et al. Assessment of the usefulness of sputum Gram stain and culture for diagnosis of community-acquired pneumonia requiring hospitalization. Med Sci Monit. 2008;14:CR171–6. [PubMed]
- 8.Nolte FS. Molecular diagnostics for detection of bacterial and viral pathogens in community-acquired pneumonia. Clin Infect Dis. 2008;47:S123–S126. doi: 10.1086/591392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Müller B, Harbarth S, Stolz D, et al. Diagnostic and prognostic accuracy of clinical and laboratory parameters in community-acquired pneumonia. BMC Infect Dis. 2007;7:10. doi: 10.1186/1471-2334-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Müller B, Becker KL, Schächinger H, et al. Calcitonin precursors are reliable markers of sepsis in a medical intensive care unit. Crit Care Med. 2000;28:977–983. doi: 10.1097/00003246-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Müller B, Becker KL. Procalcitonin: how a hormone became a marker and mediator of sepsis. Swiss Med Wkly. 2001;131:595–602. doi: 10.4414/smw.2001.09751. [DOI] [PubMed] [Google Scholar]
- 12.Müller F, Christ-Crain M, Bregenzer T, et al. Procalcitonin levels predict bacteremia in patients with community-acquired pneumonia: a prospective cohort trial. Chest. 2010;138:121–129. doi: 10.1378/chest.09-2920. [DOI] [PubMed] [Google Scholar]
- 13.Schuetz P, Müller B, Christ-Crain M, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2012;9:CD007498. [DOI] [PMC free article] [PubMed]
- 14.Falsey AR, Becker KL, Swinburne AJ, et al. Bacterial complications of respiratory tract viral illness: a comprehensive evaluation. J Infect Dis. 2013;208:432–441. doi: 10.1093/infdis/jit190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Branche AR, Walsh EE, Vargas R, et al. Serum procalcitonin measurement and viral testing to guide antibiotic use for respiratory infections in hospitalized adults: a randomized controlled trial. J Infect Dis. 2015. doi:10.1093/infdis/jiv252 [DOI] [PMC free article] [PubMed]
- 16.Musher DM, Roig IL, Cazares G, Stager CE, Logan N, Safar H. Can an etiologic agent be identified in adults who are hospitalized for community-acquired pneumonia: results of a one-year study. J Infect. 2013;67:11–18. doi: 10.1016/j.jinf.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falsey AR, Becker KL, Swinburne AJ, et al. Utility of serum procalcitonin values in patients with acute exacerbations of chronic obstructive pulmonary disease: a cautionary note. Int J Chron Obstruct Pulmon Dis. 2012;7:127–135. doi: 10.2147/COPD.S29149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simon L, Gauvin F, Amre DK, Saint-Louis P, Lacroix J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis. 2004;39:206–217. doi: 10.1086/421997. [DOI] [PubMed] [Google Scholar]
- 19.FACT SHEET: Over 150 Animal and Health Stakeholders Join White House Effort to Combat Antibiotic Resistance|whitehouse.gov. 2015. https://www.whitehouse.gov/the-press-office/2015/06/02/fact-sheet-over-150-animal-and-health-stakeholders-join-white-house-effo. Accessed June 28, 2015.
- 20.Shapiro DJ, Hicks LA, Pavia AT, Hersh AL. Antibiotic prescribing for adults in ambulatory care in the USA, 2007–09. J Antimicrob Chemother. 2014;69:234–240. doi: 10.1093/jac/dkt301. [DOI] [PubMed] [Google Scholar]
- 21.Donnelly JP, Baddley JW, Wang HE. Antibiotic utilization for acute respiratory tract infections in U.S. emergency departments. Antimicrob Agents Chemother. 2014;58:1451–1457. doi: 10.1128/AAC.02039-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzales R, Malone DC, Maselli JH, Sande MA. Excessive antibiotic use for acute respiratory infections in the United States. Clin Infect Dis. 2001;33:757–762. doi: 10.1086/322627. [DOI] [PubMed] [Google Scholar]
- 23.Shiley KT, Lautenbach E, Lee I. The use of antimicrobial agents after diagnosis of viral respiratory tract infections in hospitalized adults: antibiotics or anxiolytics? Infect Control Hosp Epidemiol. 2010;31:1177–1183. doi: 10.1086/656596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouadma L, Luyt C-E, Tubach F, et al. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet. 2010;375:463–474. doi: 10.1016/S0140-6736(09)61879-1. [DOI] [PubMed] [Google Scholar]
- 25.Albrich WC, Dusemund F, Bucher B, et al. Effectiveness and safety of procalcitonin-guided antibiotic therapy in lower respiratory tract infections in “real life”: an International, Multicenter Poststudy Survey (ProREAL) Arch Intern Med. 2012;172:715–722. doi: 10.1001/archinternmed.2012.770. [DOI] [PubMed] [Google Scholar]
- 26.Afzal Z, Minard CG, Stager CE, Yu VL, Musher DM. Clinical diagnosis, viral pcr, and antibiotic utilization in community-acquired pneumonia. Am J Ther. 2013 [Epub ahead of print]. [DOI] [PubMed]
- 27.Oosterheert JJ, van Loon AM, Schuurman R, et al. Impact of rapid detection of viral and atypical bacterial pathogens by real-time polymerase chain reaction for patients with lower respiratory tract infection. Clin Infect Dis. 2005;41:1438–1444. doi: 10.1086/497134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jennings LC, Anderson TP, Beynon KA, et al. Incidence and characteristics of viral community-acquired pneumonia in adults. Thorax. 2008;63:42–48. doi: 10.1136/thx.2006.075077. [DOI] [PubMed] [Google Scholar]
- 29.Diederen BMW, Der Van, Eerden MM, Vlaspolder F, Boersma WG, Kluytmans JAJW, Peeters MF. Detection of respiratory viruses and Legionella spp. by real-time polymerase chain reaction in patients with community acquired pneumonia. Scand J Infect Dis. 2009;41:45–50. doi: 10.1080/00365540802448799. [DOI] [PubMed] [Google Scholar]
- 30.Karhu J, Ala-Kokko TI, Vuorinen T, Ohtonen P, Syrjälä H. Lower respiratory tract virus findings in mechanically ventilated patients with severe community-acquired pneumonia. Clin Infect Dis. 2014;59:62–70. doi: 10.1093/cid/ciu237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suarez NM, Bunsow E, Falsey AR, Walsh EE, Mejias A, Ramilo O. Superiority of transcriptional profiling over procalcitonin for distinguishing bacterial from viral lower respiratory tract infections in hospitalized adults. J Infect Dis. 2015;212:213–222. doi: 10.1093/infdis/jiv047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Angeletti S, Battistoni F, Fioravanti M, Bernardini S, Dicuonzo G. Procalcitonin and mid-regional pro-adrenomedullin test combination in sepsis diagnosis. Clin Chem Lab Med. 2013;51:1059–1067. doi: 10.1515/cclm-2012-0595. [DOI] [PubMed] [Google Scholar]
- 33.Angeletti S, Spoto S, Fogolari M, et al. Diagnostic and prognostic role of procalcitonin (PCT) and MR-pro-Adrenomedullin (MR-proADM) in bacterial infections. APMIS. 2015;123:740–748. doi: 10.1111/apm.12406. [DOI] [PubMed] [Google Scholar]
- 34.Christ-Crain M, Stolz D, Bingisser R, et al. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia. Am J Respir Crit Care Med. 2006;174:88–93. doi: 10.1164/rccm.200512-1922OC. [DOI] [PubMed] [Google Scholar]
- 35.Kristoffersen KB, Søgaarda OS, Wejse C, et al. Antibiotic treatment interruption of suspected lower respiratory tract infection based on a single procalcitonin measurement at hospital admission—a randomized trial. Clin Microbiol Infect. 2009;15:481–487. doi: 10.1111/j.1469-0691.2009.02709.x. [DOI] [PubMed] [Google Scholar]
- 36.Frye AM, Baker CA, Rustvold DL, et al. Clinical impact of a real-time PCR assay for rapid identification of staphylococcal bacteremia. J Clin Microbiol. 2011;50:127–133. doi: 10.1128/JCM.06169-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holtzman C, Whitney D, Barlam T, Miller NS. Assessment of impact of peptide nucleic acid fluorescence in situ hybridization for rapid identification of coagulase-negative staphylococci in the absence of antimicrobial stewardship intervention. J Clin Microbiol. 2011;49:1581–1582. doi: 10.1128/JCM.02461-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.