Abstract
Introduction
The efficacy and safety of canagliflozin, a sodium glucose co-transporter 2 (SGLT2) inhibitor, was evaluated in patients with type 2 diabetes mellitus (T2DM) inadequately controlled on sulfonylurea monotherapy.
Methods
The CANagliflozin cardioVascular Assessment Study (CANVAS) is a double-blind, placebo-controlled cardiovascular outcomes study that randomized participants to placebo or canagliflozin 100 or 300 mg once daily in addition to routine therapy. Participants in the CANVAS trial are men and women aged ≥30 years with T2DM and a history or high risk of cardiovascular disease, and inadequate glycemic control (glycated hemoglobin [HbA1c] ≥7.0% and ≤10.5%) on current antihyperglycemic therapies. The primary objective of this prespecified substudy was to assess change from baseline to 18 weeks in HbA1c among patients on sulfonylurea monotherapy.
Results
Of the 4330 patients enrolled in CANVAS, 127 met the entry criteria for the sulfonylurea monotherapy substudy (placebo, n = 45; canagliflozin 100 mg, n = 42; canagliflozin 300 mg, n = 40). At 18 weeks, placebo-subtracted changes (95% confidence interval) in HbA1c were −0.74% (−1.15, −0.33; P < 0.001) and −0.83% (−1.24, −0.42; P < 0.001) with canagliflozin 100 and 300 mg, respectively. Relative to placebo, canagliflozin 100 and 300 mg also decreased fasting plasma glucose (FPG; −2.1 mmol/L [−3.0, −1.2] and −2.7 mmol/L [−3.6, −1.7], respectively). Body weight was lower with canagliflozin 300 mg (–1.8% [−3.2, −0.4]; P = 0.014) but unchanged with canagliflozin 100 mg (−0.4% [−1.8, 1.0]; P = 0.557). Canagliflozin 300 mg increased hypoglycemia episodes compared to canagliflozin 100 mg and placebo (15%, 0%, and 4.4%, respectively). Adverse events (AEs) of male and female genital mycotic infections, pollakiuria, and thirst were more common with canagliflozin.
Conclusions
Canagliflozin added to ongoing sulfonylurea monotherapy produced improvements in HbA1c, FPG, and body weight, with an increased incidence of AEs consistent with the mechanism of action of SGLT2 inhibition.
Funding
Janssen Research & Development, LLC.
Clinical trial registration: ClinicalTrials.gov NCT01032629.
Electronic supplementary material
The online version of this article (doi:10.1007/s13300-015-0117-z) contains supplementary material, which is available to authorized users.
Keywords: Canagliflozin, Cardiovascular disease, SGLT2 inhibitor, Sulfonylureas, Type 2 diabetes
Introduction
Type 2 diabetes mellitus (T2DM) is a progressive disease that often requires combination therapy with antihyperglycemic agents (AHAs) to achieve and maintain glycemic control [1]. Metformin is the most widely recommended initial monotherapy approach, but some patients are started first with sulfonylureas either for intolerance to metformin or because of physician and/or patient preferences despite the known adverse effects, such as hypoglycemia and weight gain [1]. As the sulfonylurea glucose-lowering effects are not sustained, many patients fail to achieve individualized glycemic targets and will need additional therapy [2, 3]. Accordingly, the availability of new agents that can lower blood glucose levels with good safety and tolerability, without increasing hypoglycemia risk and ideally neutralizing the sulfonylurea-induced weight gain, may have significant potential in the future management of the condition.
Canagliflozin is a sodium glucose co-transporter 2 (SGLT2) inhibitor approved in the United States and elsewhere as an adjunct to diet and exercise to improve glycemic control in adults with T2DM [4–17]. Treatment produces significant urinary glucose loss with beneficial effects on glycemic control, body weight, and blood pressure (BP) [5–17]. Small increases in low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) have been observed, with the ratio remaining unchanged [5–17].
Canagliflozin is not associated with hypoglycemia when used in isolation, although rates may be increased when used in conjunction with insulin or insulin secretagogues [5–17]. The risks of genital mycotic infections and lower urinary tract infections, but not upper urinary tract infections, are elevated with canagliflozin [18, 19].
This report defines the effects of canagliflozin on indicators of glycemia, safety, and tolerability compared to placebo in a subset of patients who were on background sulfonylurea monotherapy in a prespecified substudy of the CANagliflozin cardioVascular Assessment Study (CANVAS).
Methods
Overall Design of the CANVAS Trial
CANVAS is a randomized, double-blind, placebo-controlled, parallel-group, multi-center trial. A total of 4330 individuals have been randomized to placebo, canagliflozin 100 mg or canagliflozin 300 mg (Janssen Pharmaceuticals, Inc.; Titusville, NJ, USA) [20].
Objectives and Specific Hypotheses for the Sulfonylurea Substudy
The prespecified CANVAS sulfonylurea substudy was designed to determine the effects of canagliflozin when used in addition to sulfonylurea monotherapy on efficacy, safety, and tolerability in patients with T2DM with inadequate glycemic control at 18 weeks without compromising the masked study design of the entire study cohort. The objectives of the substudy were to assess the changes in glycated hemoglobin (HbA1c) and effects on safety and tolerability with canagliflozin 100 and 300 mg compared to placebo at 18 weeks. A greater reduction in HbA1c with each dose of canagliflozin compared to placebo was the primary hypothesis to be tested.
Secondary objectives of the substudy were to assess the effects of canagliflozin 100 and 300 mg compared to placebo on body weight, fasting plasma glucose (FPG), proportion of participants reaching HbA1c <7.0%, systolic and diastolic BP, fasting plasma lipids (i.e., triglycerides, HDL-C, LDL-C, total cholesterol, and LDL-C to HDL-C ratio) at 18 weeks. Prespecified hypotheses were evaluated for effects on body weight, FPG, proportion of participants reaching HbA1c <7.0%, systolic BP, triglycerides, and HDL-C.
Recruitment
Patient recruitment methods for CANVAS (ClinicalTrials.gov Identifier: NCT01032629) have been previously described [20].
Participant Inclusion and Exclusion Criteria
Participants in the CANVAS trial are men and women aged ≥30 years with T2DM with inadequate glycemic control (HbA1c ≥7.0% and ≤10.5%) on current antihyperglycemic therapies and at increased risk of cardiovascular disease [20]. The specific inclusion and exclusion criteria and the overall CANVAS trial design (including screening and run-in procedures, randomization, and follow-up procedures) have been previously published [20].
The subset included in the sulfonylurea substudy are the participants who were taking minimum or above specified doses of sulfonylurea monotherapy at baseline, specifically glipizide 20 mg, glipizide extended release 10 mg, glyburide/glibenclamide 10 mg, glimepiride 4 mg, gliclazide 160 mg, or gliclazide modified release (MR) 60 mg (i.e., at least half the maximum labeled dose of sulfonylurea).
Background Drug Treatments
Participants were required to have stable background sulfonylurea monotherapy for 8 weeks prior to screening and to continue on the same sulphonylurea dose if at all possible for 18 weeks to allow for the evaluation of short-term effects of canagliflozin on biomarkers while participants were on stable background therapy. Criteria for the initiation of glycemic rescue therapy have been published [20]. In summary, glycemic rescue therapy was either up-titration of current sulfonylurea or the stepwise addition of non-insulin AHA(s), and then insulin therapies, instituted by investigators using local guidelines for glycemic targets.
Outcomes
The primary efficacy outcome for this substudy was change in HbA1c from baseline to week 18. The secondary efficacy outcomes evaluated at week 18 were body weight, FPG, proportion of participants reaching HbA1c <7.0%, systolic BP, triglycerides, and HDL-C.
Adverse events (AEs), including preidentified AEs of interest (i.e., genital mycotic infections, urinary tract infections, and AEs related to osmotic diuresis and reduced intravascular volume) were recorded. Hypoglycemia episodes were also reported and were defined as biochemically documented (concurrent finger-stick or plasma glucose ≤3.9 mmol/L, irrespective of symptoms) and severe (i.e., requiring the assistance of another individual or resulting in seizure or loss of consciousness).
Statistical Analyses
Efficacy and safety analyses were performed using the modified intent-to-treat population, consisting of all randomized patients who received ≥1 dose of study drug. The last observation carried forward approach was used to impute missing efficacy data. An analysis of covariance model including treatment as a fixed effect and corresponding baseline value as a covariate was used for primary and continuous secondary endpoints. Least squares means and 2-sided 95% confidence intervals (CIs) were calculated for the comparison of each canagliflozin dose versus placebo. A logistic regression model with treatment as a factor and baseline HbA1c as a covariate was used for the analysis of the proportion of patients reaching HbA1c <7.0%. A prespecified, hierarchical testing sequence was used to evaluate the prespecified 18-week hypotheses and estimate P values. For endpoints that were not prespecified for hypothesis testing, point estimates and 95% CIs are provided in lieu of P values. For patients who received rescue therapy, the last post-baseline value prior to the initiation of rescue therapy was used for analysis. Finally, the efficacy analyses were repeated for all CANVAS trial participants who recorded use of any sulfonylurea dose in monotherapy at baseline (data not shown, but conclusions not different). Data for other outcomes remain blinded. Statistical analyses were performed using SAS, version 9.2 (Cary, NC, USA).
Compliance with Ethics
The study is being conducted in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013, and is consistent with Good Clinical Practice. Regulatory approval for the conduct of the trial was obtained in each country, and ethics approval was received for every site prior to initiation. Informed consent was obtained from all patients included in the CANVAS trial.
Results
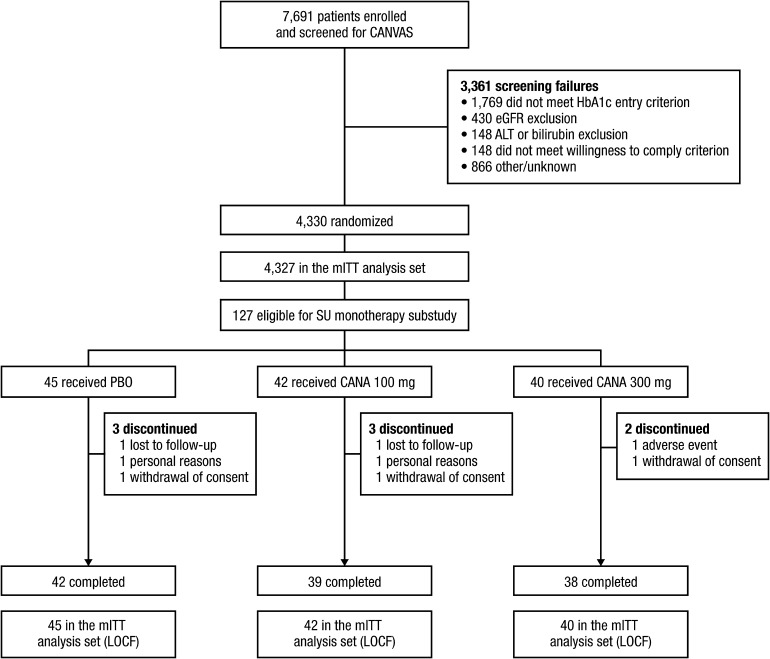
During a recruitment period of 15 months, 7691 individuals were screened and 4330 were randomized (Fig. 1). The CANVAS trial participants who met the inclusion criteria for this sulfonylurea substudy (sulfonylurea monotherapy at the prespecified minimum doses) were 127 individuals, of whom 119 (93.7%) completed the 18-week treatment period. A further 88 patients at baseline were receiving sulfonylurea monotherapy at less than the prespecified doses; when the total sulfonylurea-taking population was analyzed, the conclusions were the same as from the predefined analysis (data not shown). Amongst the 127 patients in the primary analysis, 45 were assigned to placebo, 42 to canagliflozin 100 mg, and 40 to canagliflozin 300 mg. No patients in the canagliflozin 300 mg group required rescue therapy in the first 18 weeks, while 4.8% (2 patients) of the canagliflozin 100 mg group and 17.8% (8 patients) of the placebo group did.
Fig. 1.
Study flow diagram. ALT alanine aminotransferase, CANA canagliflozin, CANVAS CANagliflozin cardioVascular Assessment Study, eGFR estimated glomerular filtration rate, LOCF last observation carried forward, mITT modified intent-to-treat, PBO placebo, SU sulfonylurea
Baseline Characteristics of Participants
Baseline demographic and disease characteristics were generally similar across treatment groups (Table 1). At entry to the study, mean age was 64.8 years, HbA1c was 8.4%, body mass index was 29.9 kg/m2, and the median duration of diabetes was 10.2 years. The estimated glomerular filtration rate (eGFR) was 69.3 mL/min/1.73 m2 and FPG was 10.0 mmol/L. The most common sulfonylurea therapies were glimepiride (35%), glyburide/glibenclamide (29%), and gliclazide MR (27%).
Table 1.
Baseline demographic and disease characteristics
| Characteristic | Study population | |||
|---|---|---|---|---|
| PBO (n = 45) | CANA 100 mg (n = 42) | CANA 300 mg (n = 40) | Total (n = 127) | |
| Sex, n (%) | ||||
| Male | 26 (58) | 24 (57) | 22 (55) | 72 (57) |
| Female | 19 (42) | 18 (43) | 18 (45) | 55 (43) |
| Mean ± SD age, years | 64.8 ± 7.8 | 64.1 ± 7.5 | 65.5 ± 7.8 | 64.8 ± 7.7 |
| Race, n (%)a | ||||
| White | 34 (76) | 30 (71) | 31 (78) | 95 (75) |
| Black or African American | 1 (2) | 0 | 0 | 1 (1) |
| Asian | 9 (20) | 12 (29) | 8 (20) | 29 (23) |
| Otherb | 1 (2) | 0 | 1 (3) | 2 (2) |
| Mean ± SD body weight, kg | 85.2 ± 19.3 | 83.7 ± 17.4 | 79.9 ± 19.5 | 83.0 ± 18.7 |
| Mean ± SD BMI, kg/m2 | 30.7 ± 6.1 | 30.2 ± 5.0 | 28.7 ± 6.2 | 29.9 ± 5.8 |
| Mean ± SD eGFR, mL/min/1.73 m2 | 68.8 ± 18.8 | 71.5 ± 18.4 | 67.7 ± 18.7 | 69.3 ± 18.6 |
| Mean ± SD duration of T2DM, years | 11.4 ± 6.7 | 10.6 ± 5.9 | 8.4 ± 6.2 | 10.2 ± 6.4 |
| Mean ± SD HbA1c, % | 8.5 ± 1.13 | 8.3 ± 0.82 | 8.2 ± 1.01 | 8.4 ± 1.00 |
| Mean ± SD FPG, mmol/L | 10.3 ± 2.68 | 10.1 ± 2.67 | 9.7 ± 2.28 | 10.0 ± 2.55 |
| Microvascular complications, n (%) | 18 (40) | 15 (36) | 22 (55) | 55 (43) |
BMI body mass index, CANA canagliflozin, eGFR estimated glomerular filtration rate, FPG fasting plasma glucose, HbA1c glycated hemoglobin, PBO placebo, SD standard deviation, T2DM type 2 diabetes mellitus
aPercentages may not total 100% due to rounding
bIncluding other
Effects of Canagliflozin on Efficacy Outcomes
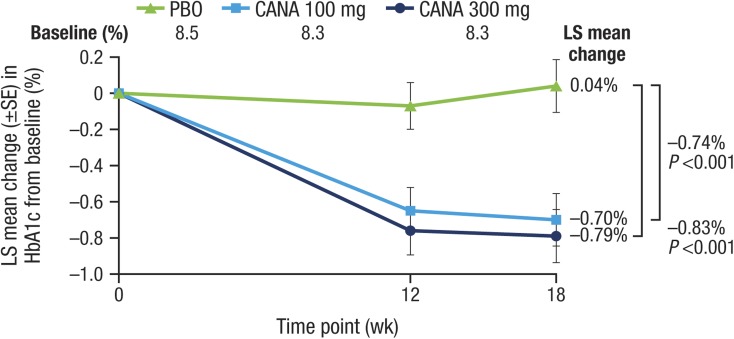
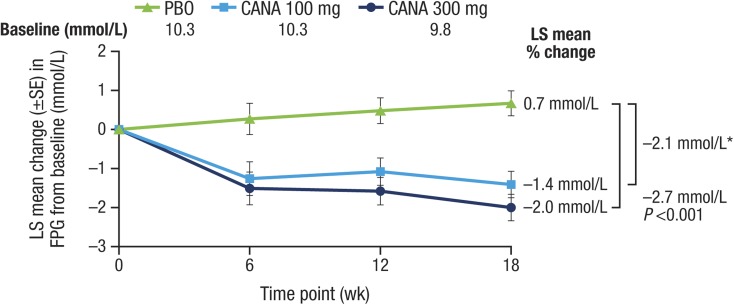
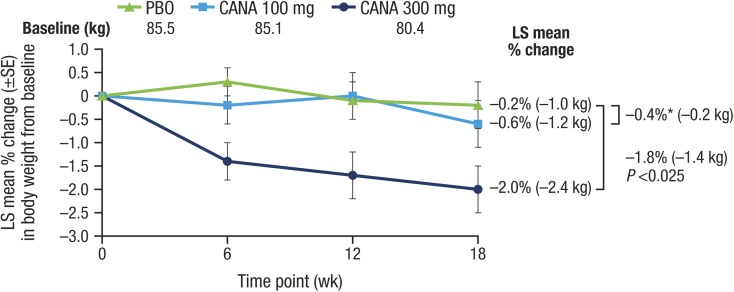
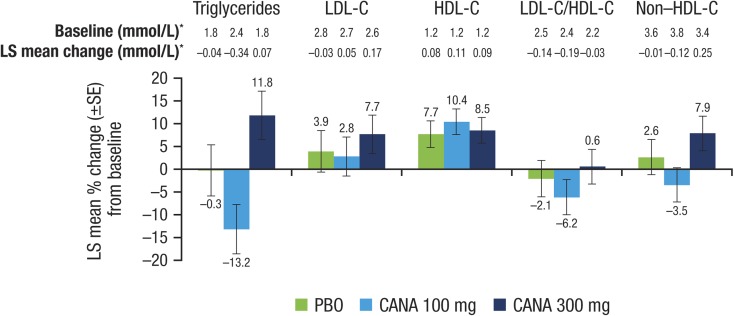
Both doses of canagliflozin significantly reduced the primary outcome of HbA1c relative to placebo at week 18 (placebo-subtracted changes [95% CI] of −0.74% [−1.15, −0.33; P < 0.001] and −0.83% [−1.24, −0.42; P < 0.001] with canagliflozin 100 and 300 mg, respectively; Table 2; Fig. 2) and a higher proportion of patients treated with canagliflozin 100 and 300 mg achieved HbA1c <7.0% versus placebo (25.0% and 33.3% vs 5.0%, respectively). FPG was also lower with both doses (Fig. 3; Table 2). There was also a statistically significant reduction in the secondary outcome of body weight with canagliflozin 300 mg but not canagliflozin 100 mg (Fig. 4; Table 2). There were no notable differences detected in systolic BP with canagliflozin 100 or 300 mg (Table 2). Clear effects on blood lipids were not apparent, with large CIs about most estimates (Fig. 5).
Table 2.
Effects of canagliflozin on primary and secondary outcomes
| Treatment difference, 95% CI | ||||
|---|---|---|---|---|
| CANA 100 mg vs PBO | CANA 300 mg vs PBO | |||
| HbA1c, %a | –0.74 | –1.15 to −0.33 | –0.83 | –1.24 to –0.42 |
| % change in body weightb | –0.4 | –1.8 to 1.0 | –1.8 | –3.2 to –0.4 |
| FPG, mmol/L | –2.1 | –3.0 to −1.2 | –2.7 | –3.6 to –1.7 |
| Proportion with HbA1c <7.0%, % | 20.0 | 2.5 to 37.5 | 28.3 | 9.5 to 47.1 |
| Systolic BP, mmHg | –0.10 | –6.45 to 6.25 | –1.77 | –8.21 to 4.67 |
| % change in HDL-C | 2.7 | –5.3 to 10.7 | 0.9 | –7.1 to 8.8 |
| % change in triglycerides | –13.0 | –28.5 to 2.6 | 12.0 | –3.0 to 27.1 |
| % change in LDL-C | –1.1 | –13.3 to 11.1 | 3.7 | –8.5 to 15.9 |
BP blood pressure, CANA canagliflozin, CI confidence interval, FPG fasting plasma glucose, HbA1c glycated hemoglobin, PBO placebo, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol
aBoth doses vs PBO, P < 0.001
bCANA 100 mg vs PBO, P = 0.557; CANA 300 mg vs PBO, P = 0.014
Fig. 2.
Effects of canagliflozin on HbA1c (LOCF). CANA canagliflozin, HbA1c glycated hemoglobin, LOCF last observation carried forward, LS least squares, PBO placebo, SE standard error, wk week
Fig. 3.
Effects of canagliflozin on FPG (LOCF). CANA canagliflozin, FPG fasting plasma glucose, LOCF last observation carried forward, LS least squares, PBO placebo, SE standard error, wk week. Asterisk Not statistically significant vs PBO based on the hypothesis testing sequence (nominal P < 0.001)
Fig. 4.
Effects of canagliflozin on body weight (LOCF). CANA canagliflozin, LOCF last observation carried forward, LS least squares, PBO placebo, SE standard error, wk week. Asterisk Not statistically significant vs PBO
Fig. 5.
Effects of canagliflozin on fasting plasma lipids (LOCF). CANA canagliflozin, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, LOCF last observation carried forward, LS least squares, PBO placebo, SE standard error. Asterisk Units of mol/mol for LDL-C/HDL-C
Effects of Canagliflozin on Safety and Tolerability Outcomes
AEs were reported for 66.7%, 26.2%, and 45.0% of participants treated with placebo, canagliflozin 100 mg, and canagliflozin 300 mg, respectively (Table 3). The corresponding figures for serious AEs were 8.9%, 0%, and 7.5%, respectively, with no specific serious AE terms reported in more than 1 patient in any group. AEs leading to discontinuation of treatment were numerically similar with canagliflozin 300 mg compared to canagliflozin 100 mg and placebo. Genital mycotic infections were more common with canagliflozin compared with placebo for women (5.6% [1/18], 5.6% [1/18], and 0% [0/19] with canagliflozin 100 and 300 mg and placebo, respectively), and no genital mycotic infections were reported in men across groups; there was no evidence of an increased rate of upper or lower urinary tract infection. AEs attributable to volume depletion, such as postural hypotension and dizziness, were more common with active treatment compared to placebo. The rates of documented hypoglycemia were greater with canagliflozin than placebo, and there were no cases defined as severe hypoglycemia reported across treatment groups (Table 3). Small to moderate mean percent changes from baseline in serum creatinine were observed with canagliflozin 100 and 300 mg and placebo (4.1%, 9.9%, and 5.7%, respectively). The largest increase in serum creatinine occurred by week 6 in both the canagliflozin 100 and 300 mg groups, and the levels were trending toward baseline by week 18. Similar but reciprocal differences in the mean percent change from baseline in eGFR were observed with canagliflozin 100 and 300 mg and placebo (−2.5%, −9.6%, and –4.7%, respectively).
Table 3.
Overall safety and selected adverse events
| Patients, n (%) | |||
|---|---|---|---|
| PBO (n = 45) | CANA 100 mg (n = 42) | CANA 300 mg (n = 40) | |
| Any AEs | 30 (66.7) | 11 (26.2) | 18 (45.0) |
| AEs causing discontinuation | 0 | 1 (2.4) | 1 (2.5) |
| AEs related to study druga | 8 (17.8) | 3 (7.1) | 6 (15.0) |
| Serious AEs | 4 (8.9)b | 0 | 3 (7.5)c |
| Deaths | 0 | 0 | 0 |
| AEs of special interest | |||
| Genital mycotic infections | |||
| Male | 0 | 0 | 0 |
| Femaled,e | 0 | 1 (5.6) | 1 (5.6) |
| Urinary tract infections | 1 (2.2) | 1 (2.4) | 1 (2.5) |
| Osmotic diuresis-related events | |||
| Pollakiuria | 0 | 1 (2.4) | 2 (5.0) |
| Polyuria | 1 (2.2) | 1 (2.4) | 0 |
| Volume-related events | |||
| Postural dizziness | 0 | 0 | 0 |
| Orthostatic hypotension | 0 | 0 | 0 |
| Documented hypoglycemiaf,g | 2 (4.4) | 0 | 6 (15.0) |
| Severe hypoglycemia | 0 | 0 | 0 |
AE adverse event, CANA canagliflozin, PBO placebo
aPossibly, probably, or very likely related to study drug, as assessed by investigators
bIncluding asthma, atrioventricular block second degree, blood creatinine increased, diabetes mellitus, flank pain, and hyperglycemia
cIncluding angina pectoris, ankle fracture, colon cancer metastatic, and coronary artery disease
dThe proportions of female genital mycotic infections were calculated using the number of female patients in each treatment group, as follows: PBO, n = 19; CANA 100 mg, n = 18; CANA 300 mg, n = 18
eIncluding vaginal infection and vulvovaginitis
fAll documented hypoglycemia episodes are reported for prior to rescue therapy
gDocumented hypoglycemia included episodes that were biochemically documented (≤3.9 mmol/L) or severe (i.e., requiring the assistance of another individual or resulting in seizure or loss of consciousness)
Discussion
The addition of canagliflozin to background sulfonylurea monotherapy was efficacious, with further placebo-adjusted decreases of HbA1c of −0.74% and −0.83% for canagliflozin 100 and 300 mg, respectively, at 18 weeks. Furthermore, the reductions in HbA1c were accompanied by a significant decrease in body weight for the 300-mg dose (−1.8%) although not for the 100-mg dose. Canagliflozin 100 mg has been associated with consistent weight loss in other Phase 3 studies [5–17], with significant weight loss observed with canagliflozin 100 mg versus placebo (–1.4%) in the 26-week study as add-on to metformin plus sulfonylurea [6]. Thus, it seems unlikely that the addition of canagliflozin to the background of a sulfonylurea alone would diminish the extent of weight loss and suggests that the modest reduction in body weight with canagliflozin 100 mg in this study is likely an outlying estimate. Changes in BP, while not significant, were in a similar direction to those observed in other reports [5–17]. Effects on lipid metabolism were also inconsistent and nonsignificant, but the overall pattern appeared to be similar to that reported previously in larger, better powered studies with small increases in LDL-C [5–17]. Importantly, there was no change in the LDL-C/HDL-C ratio with either canagliflozin 300 or 100 mg.
The observed additive glycemic effects of canagliflozin on top of sulfonylurea are anticipated on the basis of its complementary mechanism of action, and while the efficacy of sulfonylurea is dependent on adequate pancreatic insulin-secretory capacity, this is not the case with the SGLT2 inhibitors. For this reason, it is hypothesized that canagliflozin will be an effective treatment choice at most stages of the disease, and in combination with other glucose-lowering therapies. The 300-mg dose of canagliflozin was associated with numerically greater effects on several parameters compared with the 100-mg dose, including a modest increase in the percentage of patients achieving a target HbA1c <7.0% (placebo-subtracted differences of 28.3% vs 20.0%, respectively).
We and others have previously reported that the additional efficacy effects of the 300-mg over the 100-mg dose were achieved at the expense of an increased risk of drug-related AEs [5–17]. By contrast, (almost certainly as the result of the much smaller study numbers), osmotic diuresis-related (e.g., polyuria, pollakiuria, thirst) and volume-related AEs (e.g., postural dizziness, orthostatic hypotension, hypotension, syncope, presyncope) were similar in all treatment groups, with no difference between the 2 canagliflozin doses. We should not, however, conclude that the combination of canagliflozin with a sulfonylurea provides a protective effect against these side effects, and identifying patients potentially susceptible to AEs will be an important component of a patient-centered approach to diabetes management. At the same time, it reinforces the impression that serious adverse effects are relatively uncommon with this compound.
The other AEs observed with canagliflozin were those generally recognized for SGLT2 inhibitors [21]. Genital mycotic infections were more common with canagliflozin than placebo. As has been reported, they were generally mild or moderate in intensity, were managed with usual therapies, and treatment was continued [19]. There was no evidence of an increased rate of either upper or lower urinary tract infections, although this is a recognized potential complication with this drug class in larger datasets [21]. The observed decline in eGFR is likely to be hemodynamic in origin and was not associated with an excess of renal AEs. The small size of the decline in eGFR and the other favorable metabolic effects suggest that the net impact of canagliflozin on renal outcomes is unlikely to be harmful.
The primary weakness of this study is the relatively small sample size. This almost certainly reflects a decrease in the use of sulfonylureas as initial therapy in general, and the small proportion of diabetic patients managed on sulfonylurea monotherapy. As such, the confidence intervals about many estimates are wide, and, while the point estimates of effects sometimes appear different to those reported in prior studies, it is difficult to know whether this reflects real differences in efficacy and safety or chance. In this context, these substudy findings are best interpreted in the context of the broader experience with canagliflozin in this and other patient groups. The conduct of the analyses at 18 weeks provides estimates of short-term effects only, with the long-term impact of canagliflozin in this group remaining to be established.
Conclusion
Canagliflozin appears to offer significant and clinically meaningful benefits when used in conjunction with sulfonylureas with a similar class-effect AE profile. Overall, findings from this study support the efficacy and safety of canagliflozin as add-on to sulfonylurea monotherapy in patients with T2DM and cardiovascular risk.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
Steering Committee: D. R. Matthews (Co-chair), B. Neal (Co-chair), G. Fulcher, K. Mahaffey, V. Perkovic, G. Meininger, D. de Zeeuw. Independent Data Monitoring Committee: P. Home (Chair), J. Anderson, I. Campbell, J. Lachin, D. Scharfstein, S. Solomon, R. Uzzo. Endpoint Adjudication Committee: G. Fulcher, J. Amerena, C. Chow, G. Figtree, J. French, G. Hillis, B. Jenkins, R. Lindley, B. McGrath, A. Street, J. Watson. The authors thank all investigators, study teams, and patients for participating in this study. The trial is funded by Janssen Research & Development, LLC (Raritan, NJ, USA) and article processing charges were supported by Janssen Global Services, LLC (Raritan, NJ, USA). Technical editorial assistance was provided by Alaina DeToma, PhD, of MedErgy, and was funded by Janssen Global Services, LLC. Canagliflozin has been developed by Janssen Research & Development, LLC, in collaboration with Mitsubishi Tanabe Pharma Corporation. Greg Fulcher contributed to the design and conduct of the study and the interpretation of the data and wrote the first draft of the paper. David R. Matthews, Vlado Perkovic, Dick de Zeeuw, Kenneth W. Mahaffey, Robert Weiss, Julio Rosenstock, and Bruce Neal contributed to the design and conduct of the study and the interpretation of data. George Capuano contributed to the analysis and interpretation of the data. Mehul Desai and Frank Vercruysse contributed to the conduct of the study, and the acquisition and interpretation of data. Wayne Shaw contributed to the design and conduct of the study, and the acquisition of data. Gary Meininger contributed to the design and conduct of the study, and the acquisition, analysis, and interpretation of the data. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. All named authors meet the ICMJE criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Conflict of interest
Greg Fulcher has served on advisory boards for Johnson & Johnson and as a consultant to Janssen.
David R. Matthews has served on advisory boards or as a consultant for Novo Nordisk, GlaxoSmithKline, Novartis, Eli Lilly, Sanofi Aventis, Janssen, and Servier; receives current research support from Janssen and NIHR; and has given lectures for Novo Nordisk, Servier, Sanofi Aventis, Eli Lilly, Novartis, Janssen, and Aché Laboratories.
Vlado Perkovic is supported by a Senior Research Fellowship from the Australian National Health and Medical Research Council; has served on advisory boards and/or spoken at scientific meetings sponsored by Janssen, Baxter, Abbvie, Astellas, Boehringer Ingelheim, AstraZeneca, Merck, and GlaxoSmithKline; and has a policy of honoraria going to his employer.
Dick de Zeeuw has served as a consultant to AbbVie, Astellas, Chemocentryx, Eli Lilly, Fresenius, Janssen, and Merck Darmstadt; all consultancy honoraria are paid to his institution.
Kenneth W. Mahaffey has provided continuing medical education on behalf of, and/or has served as a consultant to the American College of Cardiology, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Cubist, Eli Lilly, Elsevier, Forest, GlaxoSmithKline, Johnson & Johnson, Medtronic, Merck, Omthera, Portola Pharma, Spring Publishing, The Medicines Company, and WebMD; and has received research support from Medtronic and St. Jude.
Robert Weiss has received research grants from Johnson & Johnson, Boehringer Ingelheim, Sanofi, Amgen, and Pfizer.
Julio Rosenstock has served on scientific advisory boards and received honoraria or consulting fees from companies involved in the development of SGLT2 inhibitors, including Bristol-Myers Squibb, AstraZeneca, Merck, Janssen, Boehringer Ingelheim, and Lexicon; and has received grants/research support from Pfizer, Merck, Janssen, Bristol-Myers Squibb, AstraZeneca, Boehringer Ingelheim, and Lexicon.
Bruce Neal is supported by a National Health and Medical Research Council Senior Research Fellowship; holds a research grant for this study from Janssen and for other large-scale cardiovascular outcome trials from Roche, Servier, and Merck Schering Plough; and has received honoraria or travel support for contributions to the continuing medical education programs of Abbott, Novartis, Pfizer, Roche, and Servier.
George Capuano is a full-time employee of Janssen Research & Development, LLC and a shareholder of Johnson & Johnson.
Mehul Desai is a full-time employee of Janssen Research & Development, LLC.
Wayne Shaw is a full-time employee of Janssen Research & Development, LLC.
Frank Vercruysse is a full-time employee of Janssen Research & Development and a shareholder of Johnson & Johnson.
Gary Meininger is a full-time employee of Janssen Research & Development, LLC and a shareholder of Johnson & Johnson.
Compliance with ethics
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Footnotes
On behalf of the CANVAS trial collaborative group.
References
- 1.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association Standards of medical care in diabetes—2015. Diabetes Care. 2015;38(Suppl):S1–S93. [Google Scholar]
- 3.Neumiller JJ, White JR, Jr, Campbell RK. Sodium-glucose co-transport inhibitors: progress and therapeutic potential in type 2 diabetes mellitus. Drugs. 2010;70:377–385. doi: 10.2165/11318680-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.Rosenstock J, Aggarwal N, Polidori D, Zhao Y, Arbit D, Usiskin K, et al. Dose-ranging effects of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to metformin in subjects with type 2 diabetes. Diabetes Care. 2012;35:1232–1238. doi: 10.2337/dc11-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stenlöf K, Cefalu WT, Kim KA, Alba M, Usiskin K, Tong C, et al. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab. 2013;15:372–382. doi: 10.1111/dom.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilding JP, Charpentier G, Hollander P, González-Gálvez G, Mathieu C, Vercruysse F, et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sulphonylurea: a randomised trial. Int J Clin Pract. 2013;67:1267–1282. doi: 10.1111/ijcp.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lavalle-González FJ, Januszewicz A, Davidson J, Tong C, Qiu R, Canovatchel W, et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia. 2013;56:2582–2592. doi: 10.1007/s00125-013-3039-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forst T, Guthrie R, Goldenberg R, Yee J, Vijapurkar U, Meininger G, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes on background metformin and pioglitazone. Diabetes Obes Metab. 2014;16:467–477. doi: 10.1111/dom.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neal B, Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Ways K, et al. Efficacy and safety of canagliflozin, an inhibitor of sodium glucose co-transporter 2, when used in conjunction with insulin therapy in patients with type 2 diabetes. Diabetes Care. 2015;38:403–411. doi: 10.2337/dc14-1237. [DOI] [PubMed] [Google Scholar]
- 10.Cefalu WT, Leiter LA, Yoon KH, Arias P, Niskanen L, Xie J, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet. 2013;382:941–950. doi: 10.1016/S0140-6736(13)60683-2. [DOI] [PubMed] [Google Scholar]
- 11.Schernthaner G, Gross JL, Rosenstock J, Guarisco M, Fu M, Yee J, et al. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week, randomized trial. Diabetes Care. 2013;36:2508–2515. doi: 10.2337/dc12-2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bode B, Stenlöf K, Sullivan D, Fung A, Usiskin K. Efficacy and safety of canagliflozin treatment in older subjects with type 2 diabetes mellitus: a randomized trial. Hosp Pract. 2013;41:72–84. doi: 10.3810/hp.2013.04.1020. [DOI] [PubMed] [Google Scholar]
- 13.Yale JF, Bakris G, Cariou B, Yue D, David-Neto E, Xi L, et al. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab. 2013;15:463–473. doi: 10.1111/dom.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yale JF, Bakris G, Cariou B, Nieto J, David-Neto E, Yue D, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes mellitus and chronic kidney disease. Diabetes Obes Metab. 2014;16:1016–1027. doi: 10.1111/dom.12348. [DOI] [PubMed] [Google Scholar]
- 15.Stenlöf K, Cefalu WT, Kim KA, Jodar E, Alba M, Edwards R, et al. Long-term efficacy and safety of canagliflozin monotherapy in patients with type 2 diabetes inadequately controlled with diet and exercise: findings from the 52-week CANTATA-M study. Curr Med Res Opin. 2014;30:163–175. doi: 10.1185/03007995.2013.850066. [DOI] [PubMed] [Google Scholar]
- 16.Leiter LA, Yoon KH, Arias P, Langslet G, Xie J, Balis D, et al. Canagliflozin provides durable glycemic improvements and body weight reduction over 104 weeks versus glimepiride in patients with type 2 diabetes on metformin: a randomized, double-blind, Phase 3 study. Diabetes Care. 2015;38:355–364. doi: 10.2337/dc13-2762. [DOI] [PubMed] [Google Scholar]
- 17.Bode B, Stenlöf K, Harris S, Sullivan D, Fung A, Usiskin K, et al. Long-term efficacy and safety of canagliflozin over 104 weeks in patients aged 55 to 80 years with type 2 diabetes. Diabetes Obes Metab. 2015;17:294–303. doi: 10.1111/dom.12428. [DOI] [PubMed] [Google Scholar]
- 18.Nicolle LE, Capuano G, Fung A, Usiskin K. Urinary tract infection in randomized phase III studies of canagliflozin, a sodium glucose co-transporter 2 inhibitor. Postgrad Med. 2014;126:7–17. doi: 10.3810/pgm.2014.01.2720. [DOI] [PubMed] [Google Scholar]
- 19.Nyirjesy P, Sobel JD, Fung A, Mayer C, Capuano G, Ways K, et al. Genital mycotic infections with canagliflozin, a sodium glucose co-transporter 2 inhibitor, in patients with type 2 diabetes mellitus: a pooled analysis of clinical studies. Curr Med Res Opin. 2014;30:1109–1119. doi: 10.1185/03007995.2014.890925. [DOI] [PubMed] [Google Scholar]
- 20.Neal B, Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Stein P, et al. Rationale, design, and baseline characteristics of the canagliflozin cardiovascular assessment study (CANVAS)—a randomized placebo-controlled trial. Am Heart J. 2013;166:217–223. doi: 10.1016/j.ahj.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Vasilakou D, Karagiannis T, Athanasiadou E, Mainou M, Liakos A, Bekiari E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2013;159:262–274. doi: 10.7326/0003-4819-159-4-201308200-00007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.