Abstract
Objective: To clarify the characteristics of ankle-brachial index (ABI), toe-brachial index (TBI), and pulse volume recording (PVR) of the ankle with brachial-ankle pulse wave velocity (baPWV) in healthy young adults.
Material and Methods: We analyzed ABI, TBI, baPWV, and PVR in the ankle of healthy adults aged 20 to 25 years (median, 20 years) using an automatic oscillometric device between 2002 and 2013. The ABI, baPWV, and PVR in 1282 legs of 641 subjects (301 men and 340 women) and the TBI in 474 toes of 237 subjects (117 men and 120 women) were evaluated.
Results: The measured values showed no bilateral differences. ABI and baPWV were higher in men than in women, but TBI was similar in both sexes. ABI <1.0 was observed in 18.1% of the legs in men and in 25.6% in women. TBI <0.7 was observed in 16.2% of the toes in men and 19.1% in women. For ankle PVR, the % mean arterial pressure was higher in women than in men. The upstroke time was <180 ms in most subjects.
Conclusions: For young people, ABI <1.0 or TBI <0.7 may not always indicate vascular abnormalities. When evaluating circulatory indexes, age and sex should be considered.
Keywords: ABI, TBI, baPWV, PVR, healthy young adults
Introduction
Measurements of the ankle-brachial index (ABI) and toe-brachial index (TBI) are the most common primary methods for assessing peripheral artery diseases (PAD).1) There have been many lines of evidence regarding the indications of ABI. The cut-off value of ABI for detecting PAD has been confirmed as 0.9 with high sensitivity and specificity.2) Moreover, ABI is a strong predictor of cardiovascular events: the normal ABI range was revised as 1.00 to 1.40, because patients with ABI ranging from 0.91 to 0.99 have been shown to be at the borderline of cardiovascular risk.2) On the other hand, TBI measurements are more cumbersome and are limited to special institutions (e.g., vascular laboratories) using different techniques including laser Doppler flowmetry, photoplethysmography, oscillometric plethysmography, and mercury strain gauge plethysmography. Thus, basic data on a uniform technique for a large population remain limited. Although the cut-off value of TBI for detecting PAD is set at 0.6 or 0.7, there has been no global consensus.1,3–5)
Although the normal standard ranges of ABI and TBI are based on data obtained mostly from middle and senior adults,3–6) young patients with lower limb complaints such as coldness, pain, or claudication are also primarily examined by ABI and TBI measurements because PAD including Buerger disease, Raynaud disease, Takayasu arteritis, and even arteriosclerotic diseases can occur in young patients, as the prevalence of PAD is estimated to be 1.2% to 4% of people in their late 20s.7) However, they occasionally show ABI <1.0 or TBI <0.7 despite their normal physical findings, making the diagnosis confusing.
The gold standard method for ABI measurement has been the Doppler method since the 1980s,2) but on the other hand, automated oscillometric sphygmomanometers have come into use recently. Moreover, full-automated ABI/TBI measuring devices have been developed since the late 1990s.8,9) We have used the automated ABI/TBI measuring devices in our clinical work. The form PWV/ABI® (Colin Co., Aichi, Japan) device is equipped with an oscillometric pressure sensor and an air plethysmographic sensor connected to the cuffs, performs simultaneous pressure measurement in the brachia and ankles, and routinely records the pulse volume waveforms of the brachia and ankles following pressure measurements. This device also calculates the following circulatory indexes: (1) brachial-ankle pulse wave velocity (baPWV), which is a potentially powerful predictor of arteriosclerotic organ damage and cardiovascular events, as well as carotid-femoral PWV (cfPWV);10) (2) % mean arterial pressure (%MAP) and upstroke time (UT) as the indexes of pulse volume recording (PVR).11,12) Oscillometric blood pressure measurement is equally as reliable as Doppler pressure measurement at the ankles13–16) and photoplethysmography at the toes.17) Automated devices are expected to be fast, simple, and accurate vascular screening tools.9,18,19)
We had lectures and practicum for our medical students regarding these instruments. The students measured ABI or TBI among themselves using form PWV/ABI®. We noted that the ABI they obtained was occasionally <1.0. The mechanism underlying ABI ≥1.0 is not yet fully clarified;2) however, not only the difference in the distance from the heart to the brachium and ankle, but also some differences in arterial characteristics between the arm and the leg including vascular resistance and arterial stiffness possibly play certain roles. Some studies have shown that ABI is higher in men than in women2,20) and suggested that ABI may increase with age in childhood,20,21) likely similar to PWV which increases with age and the increase is more rapid in men than in women.18,22) However, data regarding age-related ABI characteristics of young people are limited. baPWV data of people in their early 20s are also lacking. Furthermore, the effects of age and sex on TBI are almost unknown.4,23)
In this study, we clarified the characteristics of ABI, TBI, and PVR of healthy people in their early 20s together with baPWV using data measured from our medical students. We also discussed their ankle %MAP and UT, and the implications of these measurements.
Materials and Methods
Healthy medical students of Kawasaki Medical School and Kawasaki College of Allied Health Profession had a lecture and practicum of ABI and TBI measurements using form PWV/ABI® between May 2002 and January 2013. The measurement principals of this device were previously described.19,24)
The students measured ABI and TBI among themselves under the appropriate supervision of skilled doctors at room temperature (25 °C). Each subject was examined in the supine position with the pressure cuffs wrapped on the bilateral brachia and ankles when measuring ABI. Age, sex, and height were inputted into the device for the calculation of baPWV, which is measured together with PVR following pressure measurement in the series of programs. The name and identity number of the subjects were not inputted. Weight was inputted optionally. Ankle %MAP and UT were routinely calculated from the PVR. %MAP was calculated using the following formula:
UT indicates the time interval from the onset to the peak of a pulse volume wave.25) For TBI measurement, 2.5-cm-wide pressure cuffs were connected to the device in place of the ankle cuffs and were wrapped on the bilateral first toes without pretest heating. Brachial pressures were also measured simultaneously. The higher value of either the left or right brachial pressure was used for calculating ABI and TBI.
We extracted and retrospectively analyzed the data of 937 students whose measurements were recorded in a computer system of form PWV/ABI®. The study protocol was approved by the Research Ethics Committee of Kawasaki Medical School and Hospital.
Statistical analyses were performed using JMP statistical software (version 10.0.2; SAS Institute, Cary, NC, USA). Normally distributed values identified using the Shapiro-Wink test were presented as means ± standard deviation (SD). Values with non-normal distribution were presented as medians (quartile 1–3 [Q1–Q3]). The means were compared using the two-sided t-test. The medians were compared using the Wilcoxon rank-sum test. The following associations were calculated using linear regression analysis: between height or heart rate (HR) and ABI or TBI, and between baPWV and ABI. The correlations between baPWV, limb blood pressures, ankle %MAP, and ankle UT were also analyzed. A P-value of >0.01 indicated a statistically significant difference in all the analyses.
Results
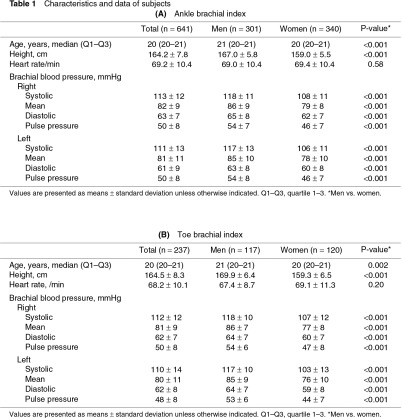
Of the 937 subjects, data from those who had complete bilateral ABI or TBI measurements were studied. ABI, baPWV, and PVR were therefore analyzed in 1282 legs of 641 subjects (301 men and 340 women, Table 1A). TBI was analyzed in 474 toes of 237 subjects (117 men and 120 women, Table 1B). The subjects were 20–25 years old, and their median age was 21 (Q1–Q3: 20–21) years for men and 20 (20–21) years for women. The heights and brachial blood pressures were significantly higher in men than in women.
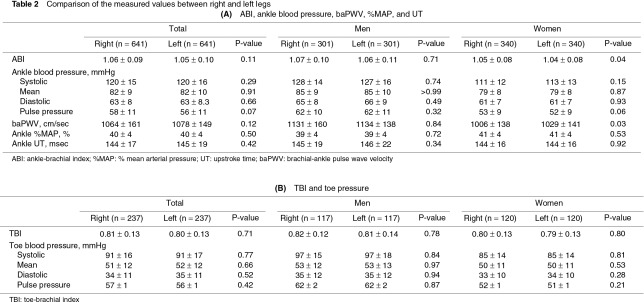
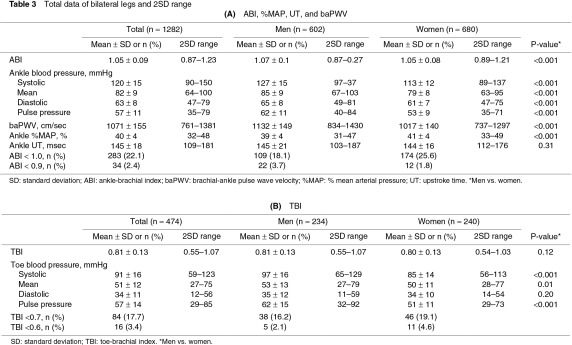
The measured values showed no significant bilateral differences (Table 2). Therefore, the total data for the bilateral legs or toes were compared between men and women and were expressed with a 2SD range. ABI, ankle pressures, and baPWV were significantly higher in men than in women (Table 3A). ABI <1.0 was observed in 18.1% of the legs in men and in 25.6% of the legs in women. Ankle %MAP was higher in women than in men. Ankle UT was <180 ms in most subjects in both sexes (Figs. 1a, 1b). TBI was not significantly different between men and women, despite the higher systolic pressure and pulse pressure of the toes in men than in women (Table 3B). TBI <0.7 was observed in 16.2% of the toes in men and in 19.1% of the toes in women.
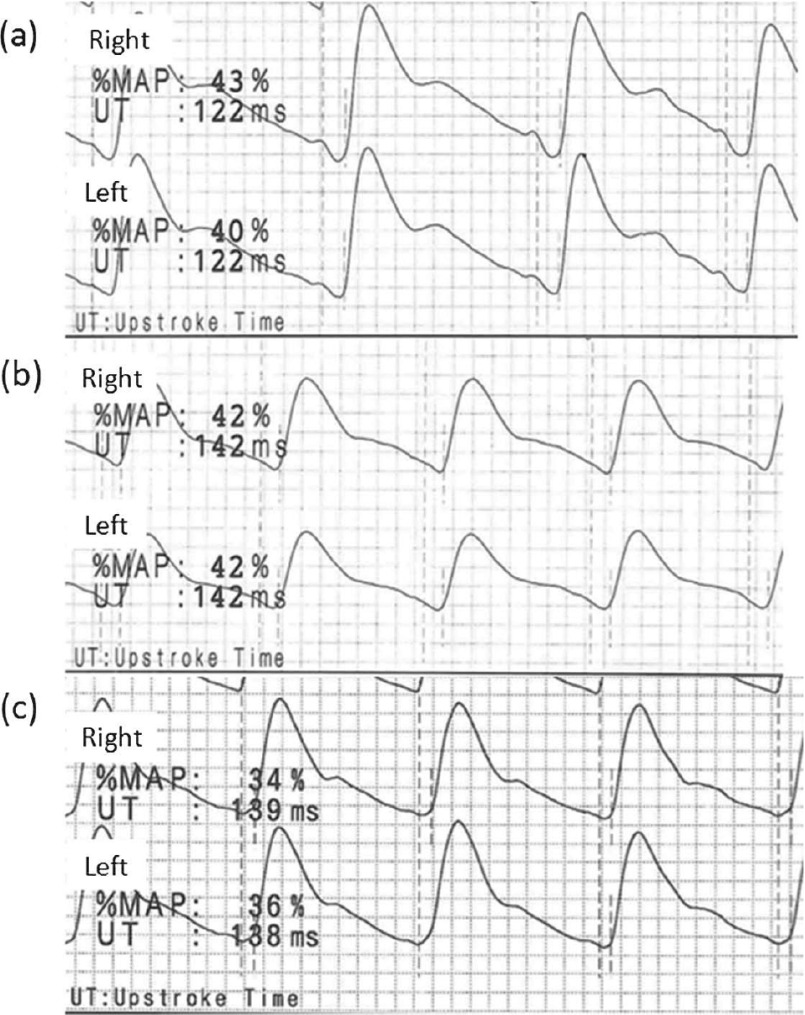
Fig. 1.
Pulse volume recordings (PVR) of the ankles obtained from healthy young adults (a, b) and an aged adult (c). (a) Ankle PVRs of a 21-year-old man (166 cm tall) expressed with % mean arterial pressure (%MAP) and upstroke time (UT). His right ankle-brachial index (ABI) was 1.30 and left ABI was 1.28. (b) Ankle PVRs of a 21-year-old woman (155 cm tall) expressed with %MAP and UT. Her right ABI was 1.04 and left ABI was 0.95. (c) Ankle PVRs of a 65-year-old man (156 cm tall) with hypertension, dyslipidemia, and diabetes mellitus, but without significant arterial disease in the lower extremities expressed with %MAP and UT. His right ABI was 1.10 and left ABI was 1.08.
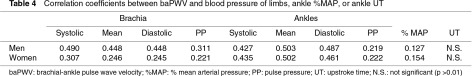
A moderate negative relationship was observed between HR and ABI in both sexes (men: regression coefficient [CR] = –0.0039, determination coefficient [r2] = 0.15; women: CR = –0.0027, r2 = 0.12), and between HR and TBI only in women (men: CR = –0.0026, r2 = 0.03; women: CR = –0.0038, r2 = 0.11) by simple linear regression analysis. Neither ABI nor TBI was associated with height. ABI was also not associated with baPWV; the factor with the strongest correlation with baPWV was ankle mean pressure (Table 4). A moderate correlation was observed between ankle %MAP and UT only in men (r2 = 0.39 in men and 0.11 in women).
Discussion
We clarified the distribution of ABI, TBI, and indexes of ankle PVR among healthy young people in their early 20s, including sex-related differences. To the best of our knowledge, this study involves the analysis of the ABI, TBI, and PVR features of the largest series of healthy young adults in Japan.
ABI and TBI in young men and women
Previous studies have shown a higher ABI in men than in women.2,18,20) On the other hand, as for age-related ABI characteristics, although some large studies among people after middle age noted that ABI decreases with age, there are as yet only a few studies regarding young people. Kats et al. reported a mean ABI of 0.88 in normal infants younger than 2.5 years using the Doppler method, and ABI <0.9 was observed in 58% of these infants.21) Niboshi et al. reported an ABI of 1.04 for healthy boys and 1.03 for healthy girls of 15–17 years of age using an oscillometric device similar to the device we used in our lectures.18) Using a new version of the same device, Ishida et al. described that the ABI of people aged 20–29 years was higher by 0.02 than that of the subjects reported in the study of Niboshi et al. for each gender. Moreover, ABI <1.0 was observed in 8% of the men and in 19% of the women aged 20–39 years.20) Our results obtained from people who were in their early 20s corresponded well to these previous studies; even so, ABI <0.9 appeared not to be within the standard as the lower 2SD value showed.
Height increases with age and this possibly affects the proportion of the distance from the heart to the pressure measurement site of the brachium and that of the ankle. However, it was also found that height did not have a significant effect on ABI similarly to previous reports.2,20) Although HR showed a moderate negative relationship with ABI, the CRs were very small to have a significant effect on ABI clinically. The same things held true for TBI.
As for TBI in young people, Carter et al. alone studied people aged 21 ± 4 years (n = 16) using strain-gauge plethysmography and reported a mean TBI of 0.86 (2SD range, 0.62–1.10),6) which was slightly higher than our results. Carter et al. heated the toes before their examinations but this was not performed in our study because of time constraints. As TBI is certainly affected by toe temperature, the difference in the results between the study of Carter et al. and ours may be due to not only the measuring method but also the pretest heating.4) However, under natural temperature, the toe systolic pressure in our study was sufficiently higher than 50 mmHg, suggesting clinical limb ischemia in patients with foot ulcer.1) Our study also indicated that TBI <0.6 was also observed in only a small proportion even in young people.
No measured values showed significant bilateral differences in our study. According to some previous reports, ABI of the right leg could be slightly higher than that of the left leg; however, these results were possibly affected by the order of measurements using Doppler methods (usually the right leg first).2) Our data suggested that the circulatory indexes including ABI would be equal between the bilateral legs when those were measured without time difference.
baPWV and ABI
PWV is strongly associated with age and sex, and sex hormones have been shown to cause a slower arteriosclerosis progression and PWV increase in young women.26) Niboshi et al. showed that baPWV increases with age even in childhood and reported a higher baPWV in boys (1041 cm/sec) than in girls (952 cm/sec) aged 15–17 years.18) Tomiyama et al. examined 326 people aged 25–30 years and reported a baPWV of 1150 cm/sec in men and 990 cm/sec in women.8) Although our present results showed slightly higher values for women than the values of previous reports, our overall data corresponded well to the results of these previous studies.
Blood pressure is also a major determinant of PWV along with age.22) Our present study showed that mean blood pressure at the ankle rather than brachial pressure was the most highly correlated factor with baPWV. Naturally, baPWV reflects the condition of not only the aorta but also the peripheral arteries,19) and possibly more of the leg arteries than the arm arteries.
Although the mechanism underlying ABI ≥1.0 is unclear,2) the utility of ABI for detecting PAD is based on the actual premise that arteriosclerotic changes affect the arteries of the upper extremity less frequently than the arteries of the lower extremity. An age-related increase in PWV was also shown to be larger in the lower extremity than in the upper extremity.27) An increase in PWV increases the amplitudes of forward and reflected pulse waves. This is a major mechanism of age-related increases in both systolic and pulse pressures.28) Hosseini and Maleki reported an ABI of 1.05 in young people (mean age: 22 years) and an ABI range of 1.09 to 1.12 in older people (mean age: 59 years), because the ankle pulse pressure in the young people was lower than the brachial pulse pressure and ankle pulse pressure in the older people.29) Among people of the same age, an issue of interest is whether a person with a higher baPWV has a higher ABI owing to the larger pulse pressure differences between the brachium and the ankle. Indeed, in our present comparison with women, men had a significantly higher baPWV, a significantly larger pulse pressure difference between the brachium and the ankle (men: 9 mmHg, women: 6 mmHg, p <0.01), and a higher ABI. Notably, the pulse pressure difference between the brachium and the ankle strongly correlated with ABI. However, baPWV showed no direct relationship with the pulse pressure difference or ABI on overall analysis or upon the analysis of each sex.
Taken together, ABI was more likely associated with the overall features particularly sex and age and not only with the individual factors of baPWV, height, or HR.
Ankle %MAP and UT of PVR
%MAP and UT represent the values quantifying pulse waveform. Generally, a normal waveform of PVR shows a rapid upstroke with a high amplitude, a sharp systolic peak, a prominent dicrotic notch, and a gradual run-off. An example of ankle PVRs in an aged adult without PAD is shown in Fig. 1c. A decreasing blood flow results in an absence of a dicrotic notch and a prolonged systolic downslope in early disease. A rounded systolic peak is a suspect for a moderate disease. A severe occlusive pattern is characterized by a flattened wave with a delayed upstroke and run-off.30) Thus, %MAP increase and UT prolongation can potentially appear in an early disease.
Previous studies have provided a few more definitions and discussion points regarding the clinical significance of these PVR indexes.11,12) Some small-scale studies have suggested that ankle %MAP >43%–45% or ankle UT <160–180 ms may indicate PAD.11,12) In our study, the lower 2SD value of ankle UT was almost 180 ms in both sexes. This supports the suggestion that the normal limit of ankle UT is 180 ms. Ankle %MAP also showed a positive correlation with ankle UT as a theoretical pattern, but only in men. Our data revealed that high ankle %MAP does not always indicate abnormality in young adults, particularly in women. Previous studies of %MAP were performed in elderly PAD patients, and defined the patients’ opposite leg without a significant disease as the control.11,12) The young adults often showed ankle pulse waves with a dicrotic notch on a high position of the downslope (Fig. 1a) or with a normal waveform in a low amplitude (Fig. 1b), possibly accounting for the high %MAP. However, we could extract only the calculated values of %MAP and UT, and obtain the actual PVRs from limited subjects.
Ankle %MAP possibly varies with age, particularly in women; therefore, it may be difficult to indicate PAD itself in young patients. Moreover, UT is certainly affected by cardiac factors including aortic valve stenosis. However, %MAP and UT facilitate a more objective PVR reading and may help in making a more definitive diagnosis if used together with ABI measurement. PVR is a valuable assessment tool even for patients with non-compressive vessels.1) Further studies regarding the uses of PVR indexes in elderly people in combination with ABI and vascular imaging are warranted.
Strengths and limitations
Our study has some limitations. First, the health statuses of the subjects were not confirmed by vascular imaging or blood examinations. Weight, obesity, and smoking history were also not specifically considered. Smoking can temporarily increase systemic blood pressure and decrease digital pulse wave amplitude, and is also a strong risk factor of arteriosclerosis. However, all measurements were performed during the school lectures and thus the subjects could not smoke at the last minutes. Moreover, they did not have a long history of smoking because Japanese law prohibits people under the age of 20 from smoking. Second, although the oscillometric method has been shown to have good correlations with the Doppler method in ABI measurement,14,16) a rather high ABI value compared with the Doppler method might have been obtained particularly in the low range,13,15) despite the low ABI values obtained in our study. Third, different medical students performed each measurement. However, the obtained values showed clear normal distributions in all measurement items. Moreover, the results of ABI and baPWV corresponded well to previous reports of children and adults.2,8,18,20) Thus, we consider our ABI and baPWV data to be reliable results reflecting the characteristics of healthy young adults similarly to previous results. This may also hold true for TBI, %MAP, and UT. Additionally, our results add support to the advantages of an automated ABI/TBI measuring device, that is, even beginner technicians can use the device appropriately and with ease incurring only a small technical bias.19)
Conclusions
For young people, ABI <1.0, TBI <0.7, or high %MAP may not always indicate vascular abnormalities. Age and sex should be carefully considered when evaluating not only baPWV but also ABI, TBI, and PVR. When assessing lower limb ischemia by ABI or TBI measurement in young patients, holistic diagnosis with careful consideration of the other clinical findings of subjects and the actual pulse waveforms is needed. UT would be sufficiently useful for making a diagnosis even in young patients.
Acknowledgments
We thank Dr. Edward F. Barroga, Associate Professor and Senior Medical Editor of Tokyo Medical University, for editing the manuscript.
Disclosure Statement
The authors have no conflicts of interest.
References
- Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg 2007; 45 Suppl S: S5-67. [DOI] [PubMed] [Google Scholar]
- Aboyans V, Criqui MH, Abraham P, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation 2012; 126: 2890-909. [DOI] [PubMed] [Google Scholar]
- Bundó M, Urrea M, Muñoz L, et al. Correlation between toe-brachial index and ankle-brachial index in patients with diabetes mellitus type 2. Med Clin (Barc) 2013; 140: 390-4. [DOI] [PubMed] [Google Scholar]
- Høyer C, Sandermann J, Petersen LJ. The toe-brachial index in the diagnosis of peripheral arterial disease. J Vasc Surg 2013; 58: 231-8. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Oka M, Maesato K, et al. Peripheral arterial occlusive disease is more prevalent in patients with hemodialysis: comparison with the findings of multidetector-row computed tomography. Am J Kidney Dis 2006; 48: 269-76. [DOI] [PubMed] [Google Scholar]
- Carter SA, Lezack JD. Digital systolic pressures in the lower limb in arterial disease. Circulation 1971; 43: 905-14. [DOI] [PubMed] [Google Scholar]
- Fowkes FG, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet 2013; 382: 1329-40. [DOI] [PubMed] [Google Scholar]
- Tomiyama H, Yamashina A, Arai T, et al. Influences of age and gender on results of noninvasive brachial-ankle pulse wave velocity measurement—a survey of 12517 subjects. Atherosclerosis 2003; 166: 303-9. [DOI] [PubMed] [Google Scholar]
- Ohtake T, Oka M, Ikee R, et al. Impact of lower limbs’ arterial calcification on the prevalence and severity of PAD in patients on hemodialysis. J Vasc Surg 2011; 53: 676-83. [DOI] [PubMed] [Google Scholar]
- Munakata M, Konno S, Miura Y, et al. Prognostic significance of the brachial-ankle pulse wave velocity in patients with essential hypertension: final results of the J-TOPP study. Hypertens Res 2012; 35: 839-42. [DOI] [PubMed] [Google Scholar]
- Goto M, Kimura Y, Ogawa M, et al. Assessment of below-knee artery lesions using an automatic device for blood pressure and pulse wave measurement. J Jpn Coll Angiol 2011; 51: 223-7. [Google Scholar]
- Nakashima R, Inoue Y, Sugano N, et al. Upstroke time and percentage of mean arterial pressure with ABI-form. J Jpn Coll Angiol 2005; 45: 7-10. [Google Scholar]
- Beckman JA, Higgins CO, Gerhard-Herman M. Automated oscillometric determination of the ankle-brachial index provides accuracy necessary for office practice. Hypertension 2006; 47: 35-8. [DOI] [PubMed] [Google Scholar]
- Koji Y, Tomiyama H, Ichihashi H, et al. Comparison of ankle-brachial pressure index and pulse wave velocity as markers of the presence of coronary artery disease in subjects with a high risk of atherosclerotic cardiovascular disease. Am J Cardiol 2004; 94: 868-72. [DOI] [PubMed] [Google Scholar]
- Masaki H, Morita I, Tabuchi A, et al. Evaluation of intermittent claudication by FormPWV/ABI®. J Jpn Coll Angiol 2003; 43: 303-6. [Google Scholar]
- Verberk WJ, Kollias A, Stergiou GS. Automated oscillometric determination of the ankle-brachial index: a systematic review and meta-analysis. Hypertens Res 2012; 35: 883-91. [DOI] [PubMed] [Google Scholar]
- Harrison ML, Lin HF, Blakely DW, et al. Preliminary assessment of an automatic screening device for peripheral arterial disease using ankle-brachial and toe-brachial indices. Blood Press Monit 2011; 16: 138-41. [DOI] [PubMed] [Google Scholar]
- Niboshi A, Hamaoka K, Sakata K, et al. Characteristics of brachial-ankle pulse wave velocity in Japanese children. Eur J Pediatr 2006; 165: 625-9. [DOI] [PubMed] [Google Scholar]
- Munakata M, Ito N, Nunokawa T, et al. Utility of automated brachial ankle pulse wave velocity measurements in hypertensive patients. Am J Hypertens 2003; 16: 653-7. [DOI] [PubMed] [Google Scholar]
- Ishida A, Miyagi M, Kinjo K, et al. Age- and sex-related effects on ankle-brachial index in a screened cohort of Japanese: the Okinawa Peripheral Arterial Disease Study (OPADS). Eur J Prev Cardiol 2014; 21: 712-8. [DOI] [PubMed] [Google Scholar]
- Katz S, Globerman A, Avitzour M, et al. The ankle-brachial index in normal neonates and infants is significantly lower than in older children and adults. J Pediatr Surg 1997; 32: 269-71. [DOI] [PubMed] [Google Scholar]
- Yamashina A, Tomiyama H, Arai T, et al. Nomogram of the relation of brachial-ankle pulse wave velocity with blood pressure. Hypertens Res 2003; 26: 801-6. [DOI] [PubMed] [Google Scholar]
- Spångéus A, Wijkman M, Lindström T, et al. Toe brachial index in middle aged patients with diabetes mellitus type 2: not just a peripheral issue. Diabetes Res Clin Pract 2013; 100: 195-202. [DOI] [PubMed] [Google Scholar]
- Yamashina A, Tomiyama H, Takeda K, et al. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res 2002; 25: 359-64. [DOI] [PubMed] [Google Scholar]
- Park JS, Choi UJ, Lim HS, et al. The Relationship between coronary artery calcification as assessed by multi-detector computed tomography and arterial stiffness. Clin Exp Hypertens 2011; 33: 501-5. [DOI] [PubMed] [Google Scholar]
- Creatsa M, Armeni E, Stamatelopoulos K, et al. Circulating androgen levels are associated with subclinical atherosclerosis and arterial stiffness in healthy recently menopausal women. Metab Clin Exp 2012; 61: 193-201. [DOI] [PubMed] [Google Scholar]
- Avolio AP, Deng FQ, Li WQ, et al. Effects of aging on arterial distensibility in populations with high and low prevalence of hypertension: comparison between urban and rural communities in China. Circulation 1985; 71: 202-10. [DOI] [PubMed] [Google Scholar]
- Remington JW, O’Brien LJ. Construction of aortic flow pulse from pressure pulse. Am J Physiol 1970; 218: 437-47. [DOI] [PubMed] [Google Scholar]
- Hosseini SM, Maleki AR. Ageing and ankle pulse pressure. Cardiol J 2010; 17: 163-5. [PubMed] [Google Scholar]
- Rutherford RB, Lowenstein DH, Klein MF. Combining segmental systolic pressures and plethysmography to diagnose arterial occlusive disease of the legs. Am J Surg 1979; 138: 211-8. [DOI] [PubMed] [Google Scholar]