Abstract
The damage of vascular endothelial cells has been speculated to be involved in the pathogenesis of dengue virus (DENV) infection. However, little is known about the role of soluble vascular cell adhesion molecule-1 (sVCAM-1) in predicting the severity of dengue infection in adults. In this study, 51 adults with DENV-1 infection (21 with severe dengue and 30 with dengue fever (DF) were included, and their serum levels of sVCAM-1 and other parameters were determined. The results indicated that the levels of sVCAM-1 were elevated on days 1–3 to 16.75 (11.55–34.74) ng/mL in the severe dengue patients. These levels increased rapidly to peak values of 43.53 (37.15–47.02) ng/mL on days 10–12 and then declined; however, the values were maintained at a high level (38.07 (26.06–39.63) ng/mL). Other parameters, including reduced platelet (PLT) counts, neutrophil (NEU) counts and increased levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), and creatine kinase (CK), were also observed in the severe dengue group but not in the DF group. The levels of cytokines, such as interleukin (IL)-6, IL-10, interferon γ (IFNγ), and tumor necrosis factor α (TNF-α), were transiently increased in the severe dengue patients. Among the aforementioned parameters, only sVCAM-1 levels were significantly elevated earlier and more persistently in the severe dengue patients than in the DF patients. sVCAM-1 positively correlated with the levels of ALT, AST, LDH, TNF-α, and IL-6 and negatively correlated with the levels of PLT, NEU, and viremia. Notably, the high levels of sVCAM-1 were closely associated with the severe dengue patients. In conclusion, sVCAM-1 may be a superior indicator for monitoring the severity of dengue.
Keywords: dengue virus, severe dengue, sVCAM-1, cytokines, vascular endothelial cells
INTRODUCTION
Dengue virus (DENV), which belongs to the Flaviviridae family, is a widespread mosquito-borne human pathogen with a worldwide prevalence. There are four antigenically distinct serotypes (DENV-1-4) that are based on differences in the envelope protein. DENV is the causative agent of dengue fever (DF). It is estimated that 50–100 million cases of dengue occur annually in tropical and subtropical regions, with more than 20 000 deaths.1 Thus, DF has reemerged as an important public health concern in endemic areas.
Because unusual clinical signs such as altered consciousness and hepatitis are often observed in DF patients,2,3,4,5 the 2009 World Health Organization guidelines suggested classifying dengue infection into DF and severe dengue according to the clinical severity.6 Severe dengue is defined by the occurrence of severe plasma leakage, severe bleeding, and/or severe organ impairment, whereas DF patients should be further divided into subgroups according to how the symptoms are manifested; DF with warning signs will likely progress to severe dengue. Because there are currently no licensed vaccines or antiviral drugs to prevent or treat dengue, an early diagnosis of severe dengue could improve diagnostic and therapeutic strategies, thereby limiting morbidity and mortality.
The pathogenesis of severe dengue is not completely understood; however, vascular leakage and hemorrhage in these patients are obvious hallmark features of increased vascular permeability, which implies vascular endothelial cell (VEC) damage during DENV infection.7 In fact, as a result of infection, endothelial cells respond to and elicit a myriad of cellular, platelet (PLT)-associated and secreted factors that affect vascular permeability, which is likely correlated with disease severity.8,9,10 Thus, there might be a soluble protein that is intimately associated with the impairment of VECs and could act as a novel biomarker to predict the severity of dengue. Vascular cell adhesion molecule-1 (VCAM-1) belongs to the immunoglobulin superfamily and binds to lymphocytes and monocytes; it is also found in cytokine-activated endothelial cells and dendritic cells. An increase in the expression of soluble VCAM-1 (sVCAM-1) can result in enhanced binding of leukocytes and PLTs and subsequent local inflammatory responses, which in turn lead to VEC damage and plasma leakage. Previous studies have implied that sVCAM-1 levels are significantly elevated in children infected with either DENV-2 or DENV-3.11,12 Whether this knowledge could be applied to adults infected with DENV-1 and its association with disease severity remains unknown. It has been reported that a variety of cytokines, such as interleukin (IL), interferon γ (IFNγ), and tumor necrosis factor α (TNF-α), are markedly elevated in DENV infection; some of these cytokines are considered to be indictors of a severe outcome.13,14,15,16,17,18,19,20,21 Therefore, in this study, we investigated dynamic changes of sVCAM-1 levels, a variety of inflammatory cytokines and viremia in adults with DENV-1 infection.
MATERIALS AND METHODS
Ethics statement
This study was performed in strict accordance with the institutional review board approval of the Guangzhou 8th People's Hospital, China. Written informed consent was obtained from all of the patients. The Chinese rules and regulations for human subject protection were strictly followed.
Study subjects
During the dengue epidemic in 2013, 2894 dengue patients were identified in Guangdong, China. Among the hospitalized patients, serum samples were collected to test for the presence of hepatitis C virus, hepatitis B virus, human immunodeficiency virus, cytomegalovirus, rubella, measles, and chikungunya virus. Based on the clinical observations, a diagnosis of dengue was further confirmed by either anti-dengue antibody tests (Panbio, Brisbane, Queensland, Australia) or virus isolation in C6/36 mosquito cells. Fifty-one dengue patients tested negative for the above pathogens and, with serial serum stored at −80 °C, were enrolled in this study. According to the World Health Organization criteria,6 they were divided into severe dengue (n = 21) and DF (n = 30). The serum specimens were divided into the following time points for further analysis: days 1–3, days 4–6, days 7–9, days 10–12, and >day 12 post-fever onset. Generally, the three periods of <day 6, days 7–9, and >day 10 are clinically considered to be the acute phase, the middle phase, and the convalescence phase, respectively. The patients were not further divided by primary and secondary infections because we did not find a significant difference in the viral load between them in our previous study.22
Clinical monitoring and laboratory tests
Signs and symptoms, including diarrhea, nausea, hepatomegaly, gum bleeding, epistaxis, petechiae, positive tourniquet test, and internal bleeding, were recorded daily by a clinician. Routine laboratory tests, including blood cell counts and coagulation tests, were performed in the hospital laboratories according to standard protocols. The parameters that reflect tissue and organ injuries, including serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels as well as creatine kinase (CK) and lactate dehydrogenase (LDH) levels, were measured periodically.
Determination of cytokine and sVCAM-1 levels
Serum levels of IL-6, IL-10, IL-17A, IFNγ, TNF-α, soluble TNF receptor 1 (sTNFR1), and sVCAM-1 were measured using enzyme-linked immunosorbent assay kits (sTNFR1 Kit from R&D Systems, Minneapolis, MN, USA, and others from Diaclone, Besancon Cedex, France) according to the manufacturers' instructions.
Quantification of viral RNA by real-time polymerase chain reaction (PCR)
Viral RNA levels were measured by real-time PCR. Viral RNA was extracted from the serum samples using a QIAamp Viral RNA Mini Kit (Qiagen) and were transcribed into cDNA using a TaKaRa Prime Script TM RT Kit (TakaRa Biotech Ltd., Dalian, China). Real-time PCR assays were then performed using TaKaRa Premix Ex TaqTM (TaKaRa Biotech Ltd., Dalian, China) on an ABI 7300 real-time PCR system (ABI, Foster City, CA). The primer and probe sequences were designed based on DENV-1 isolated in Guangzhou (GenBank Accession NO EU280167). The following primers were used: forward primer, 5′-AAG GAC TAG AGG TTA GAG GAG ACC C-3′ (nucleotides 10 589 to 10 613); reverse primer, 5′-CGT TCT GTG CCT GGA ATG ATG-3′ (nucleotides 10 697 to 10 677); and probe, FAM-5′-TCT GGT CTC TCC CAG CGT CAA TAT GCT GTT-3′-TAMRA (nucleotides 10 657 to 10 628) (where FAM is the reporter and TAMRA is the quencher). A standard curve was established using a 10-fold serial dilution of plasmid DNA containing 103 to 109 copies of the DENV-1 genome. The lower limit of detection was 100 copies/mL. The viremia level of each serum sample was calculated according to the standard curve.
Statistical analysis
Statistical analysis was performed with SPSS 13.0. The results are given as the mean ± SD for the clinical parameters and as the median (25%–75% percentile) for the levels of viremia and cytokines. The chi-square test was used for group comparisons among clinical presentations. Nonparametric tests for two independent samples were performed for the comparison of nonparametric data. A univariate analysis was first performed to determine if the levels of sVCAM-1 or cytokines were associated with severe dengue. A multivariate logistic regression was then used to determine whether the identified variables from above were independent risk factors associated with severe dengue. The associations between cytokines and laboratory measurements were analyzed by the Spearman's rank correlation test. A two tailed P-value of <0.05 was considered statistically significant.
RESULTS
Clinical characteristics of severe dengue and DF patients
As shown in Table 1, a total of 198 serum samples from 21 severe dengue patients and 30 DF patients were analyzed. The severe dengue patients consisted of 12 males (57.1%) with a mean age of 41 years, whereas the DF patients consisted of 16 males (53.3%) with a mean age of 38 years. The sex ratios and the ages were comparable between the two groups (P > 0.05 for both). In the acute phase, the patients in both of the groups showed a wide range of clinical features, including fatigue, headache, muscle pain, and diarrhea. However, the severe dengue patients had significantly higher frequencies of nausea (42.9% vs. 13.3%, P = 0.017), petechiae (38.1% vs. 13.3%, P = 0.040), and positive tourniquet tests (47.6% vs. 0%, P < 0.001) compared with the DF patients. All of these symptoms gradually disappeared upon recovery from the disease, and no patients died.
Table 1. Classification and characteristics of the severe dengue and DF patients.
| Classification | ||
|---|---|---|
| Severe dengue | DF | |
| Patients (n=51) | 21 | 30 |
| Male (%) | 12 (57.1%) | 16 (53.3%) |
| Duration of fever (day) | 6±2 | 5±2 |
| Age (year) | 41±13 | 38±17 |
| Serum samples (n=198) | 97 | 101 |
| Days 1–3 | 12 | 25 |
| Days 4–6 | 38 | 49 |
| Days 7–9 | 28 | 23 |
| Days 10–12 | 10 | 4 |
| >Day 12 | 9 | 0 |
DF, dengue fever.
The parameters are expressed as the mean ± SD.
Changes in routine clinical parameters in severe dengue and DF patients
A panel of routine clinical examinations was performed. As shown in Table 2, the white blood cell (WBC) counts, PLT counts, and neutrophil (NEU) counts decreased significantly during the acute phase in both of the groups. Moreover, in the acute phase, the levels of PLT and NEU were markedly reduced to 63.7 ± 28.3 × 109/L and 1.33 ± 0.68 × 109/L, respectively, in the severe dengue patients; these levels were significantly lower than the levels in the DF patients (P < 0.001 and P = 0.022, respectively). However, the high levels were followed by a rapid recovery in the middle phase (days 7–9) and a return to normal after day 10. There were no obvious changes in the hematocrit (HCT) levels.
Table 2. Changes in routine parameters and enzymes in the severe dengue and DF patients in the acute phase.
| Severe dengue | DF | |||
|---|---|---|---|---|
| Parameters | n=21 | n=30 | Normal range | P value |
| WBC (×109/L) | 2.66±1.46 | 2.94±1.51 | 4–10 | 0.296 |
| NEU (×109/L) | 1.33±0.68 | 2.01±1.37 | 2–7.5 | 0.022 |
| PLT (×109/L) | 63.7±28.3 | 111.7±33.3 | 100–300 | <0.001 |
| HCT (%) | 35.7±5.4 | 36.9±4.3 | 40–50 | 0.452 |
| ALT (U/L) | 101.97±55.51 | 32.95±15.88 | 5–40 | <0.001 |
| AST (U/L) | 162.03±90.21 | 44.36±18.63 | 5–40 | <0.001 |
| CK (U/L) | 395.66±213.29 | 124.55±57.25 | 26–174 | 0.003 |
| LDH (IU/L) | 448.17±245.6 | 213.68±44.87 | 120–230 | <0.001 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CK, creatine kinase; DF, dengue fever; HCT, hematocrit; LDH, lactate dehydrogenase; NEU, neutrophil; PLT, platelet; WBC, white blood cell.
The parameters are expressed as the mean ± SD.
Another set of parameters reflecting tissue and organ injuries was also measured (Table 2). In severe dengue patients in the acute phase, there were markedly increased levels of ALT, with 14.3% (3/21) of the values above 200 U/L. Similarly, AST levels were also increased, with 33.3% (7/21) of the values above 200 U/L. Elevated CK and LDH levels were also observed in the severe dengue patients, of whom 42.9% (9/21) had CK levels as high as 717.92 ± 234.06 U/L, and 42.9% (9/21) had LDH levels as high as 664.58 ± 247.65 U/L. Their levels recovered gradually and returned to normal in the convalescence phase. These levels did not show a clear change in the DF patients and were much lower (P < 0.001 for ALT, AST, and LDH; P = 0.003 for CK) than the levels in the severe dengue patients. This finding indicates that there were severe tissue and organ (liver) injuries in the severe dengue patients.
Viral kinetics in severe dengue and DF patients
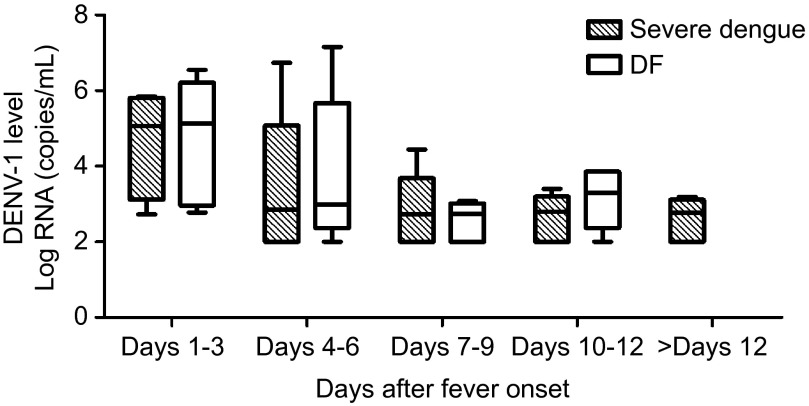
DENV-1 replication kinetics in the patients were determined by real-time PCR. The patients in both of the groups showed similar viral replication kinetics, with the highest levels occurring in the acute phase (Figure 1). On days 1–3, the values of the viral loads in the patients with severe dengue and DF were 5.06 (3.52–5.76) log copies/mL and 5.13 (3.15–5.87) log copies/mL, respectively, and both of the values rapidly decreased to the detection limit (100 copies/mL) on days 7–9 and remained at this low level during the observed period. Nevertheless, there were no differences in the viral loads of the severe dengue and DF patients at any time point.
Figure 1.
Serum levels of DENV-1 RNA in patients with severe dengue and DF. The bottom and top of the boxes are the first and third quartiles, respectively. The length of the box, thus, represents the interquartile range within which 50% of the values were located. The line through the middle of each box represents the median. There are no differences between the two groups.
sVCAM-1 and cytokine kinetics in patients with severe dengue and DF
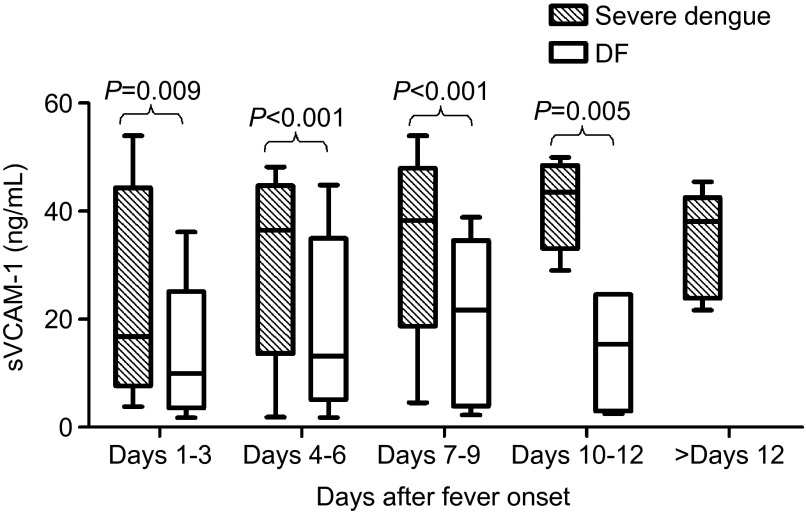
In the severe dengue patients, the levels of sVCAM-1 were clearly elevated on days 1–3 to 16.75 (11.55–34.74) ng/mL. The levels increased rapidly to peak values of 43.53 (37.15–47.02) ng/mL on days 10–12 and then declined but were still maintained at a high level (38.07 (26.06–39.63) ng/mL) during the convalescence phase. The changes in the sVCAM-1 levels in the DF patients showed a similar trend; however, the amplitude was much lower. The levels increased slightly after days 1–3 from 9.99 (5.38–14.17) ng/mL to the highest levels at 21.68 (5.59–30.3) ng/mL on days 7–9 and then declined. These results indicate that the severe dengue patients had significantly higher sVCAM-1 levels than the DF patients at almost all of the time points (Figure 2).
Figure 2.
Serum levels of sVCAM-1 in patients with severe dengue and DF. sVCAM-1 levels were compared between the two groups, and the significant differences are marked by their respective P values. The bottom and top of the boxes are the first and third quartiles, respectively. The length of the box, thus, represents the interquartile range within which 50% of the values were located. The line through the middle of each box represents the median.
As shown in Figure 3, the levels of cytokines had complex patterns of change. The kinetics of IL-6 and TNF-α in both of the groups were similar: the levels increased from days 1–3 and gradually reached peak values on days 10–12 and days 7–9, respectively. The levels in the severe dengue patients were much higher than the levels in the DF patients from days 1–3 to days 7–9. The sTNFR1 levels in both of the groups increased early after fever onset and then gradually declined to low levels on days 10–12; however, there were no significant differences between the two groups (data not shown). The IL-10 levels in the severe dengue patients were higher than the levels in the DF patients in the acute phase, and then they gradually declined to similar levels to those of the DF patients. IFNγ showed increased expression in both of the groups from the beginning; however, the severe dengue patients had higher levels on days 4–6. Moreover, there were no obvious changes in the levels of IL-17A after the infection (data not shown).
Figure 3.
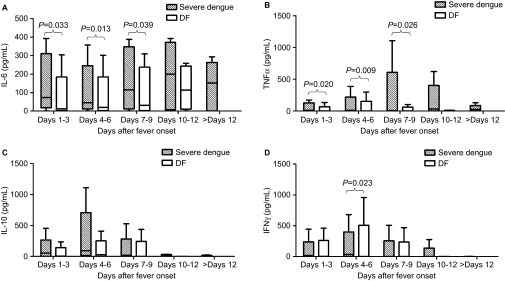
Serum levels of cytokines in patients with severe dengue and DF. Cytokine expression was compared between the two groups, and the significant differences are marked by their respective P values. The bottom and top of the boxes are the first and third quartiles, respectively. The length of the box, thus, represents the interquartile range within which 50% of the values were located. The line through the middle of each box represents the median. A, B, C, and D present the serum levels of IL-6, TNF-α, IL-10, and IFNγ, respectively.
sVCAM-1 and cytokines associated with the severity of dengue diseases
Because the biomarkers sVCAM-1 and cytokines are involved in the pathogenesis of severe dengue, as mentioned above, we applied a logistic regression to investigate whether sVCAM-1 is superior to the cytokines associated with severe dengue in the acute phase. A univariate analysis of the clinical features indicated that the levels of sVCAM-1 (P < 0.001), IL-6 (P = 0.008), IL-10 (P = 0.003), and TNF-α (P = 0.031) were associated with severe dengue, whereas the levels of IFNγ, sTNFR1, and IL-17A were not associated with severe dengue (all P > 0.05). Therefore, the markers that associated with severe dengue in the univariate analysis were included in the multivariate analysis. As shown in Table 3, the higher levels of sVCAM-1 (P < 0.001) and IL-10 (P = 0.031) were closely associated with the severe dengue patients.
Table 3. Multivariate analysis of risk factors for severe dengue patients.
| Parameter | OR | 95% CI | P value |
|---|---|---|---|
| IL-6 | 1.005 | 0.999–1.010 | 0.103 |
| IL-10 | 1.004 | 1.000–1.008 | 0.031 |
| TNF-α | 1.002 | 0.994–1.009 | 0.681 |
| sVCAM-1 | 1.082 | 1.040–1.124 | <0.001 |
CI, confidence interval; OR, odds ratio.
Relationships between sVCAM-1 and injuries in the patients
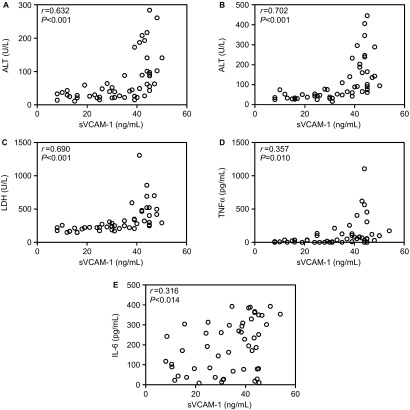
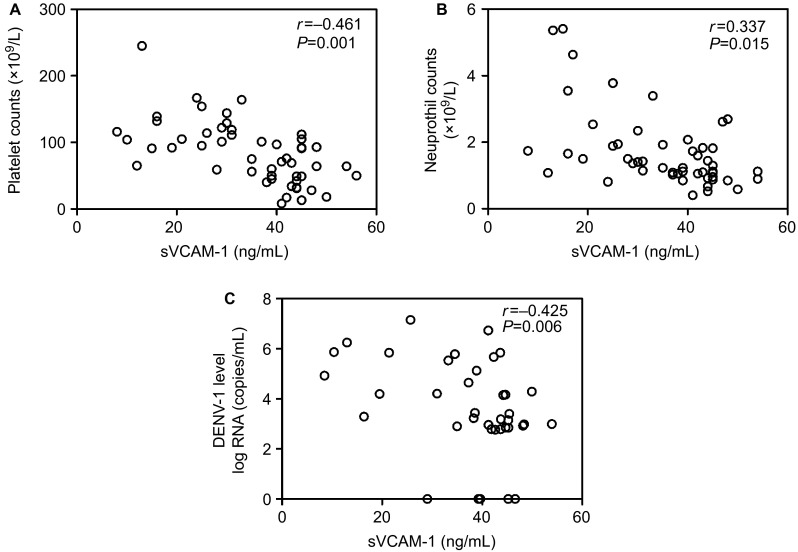
To further assess the potential value of sVCAM-1 in evaluating the severity of dengue, we analyzed the association between sVCAM-1 and the other parameters measured in the present study. As shown in Figures 4 and 5, sVCAM-1 levels were positively correlated with serum levels of ALT (r = 0.632), AST (r = 0.702), LDH (r = 0.690), TNF-α (r = 0.357), and IL-6 (r = 0.316) and negatively correlated with PLT counts (r = −0.461), NEU counts (r = −0.337) and viral loads (r = −0.425). These results indicated that sVCAM-1 correlated with inflammation and the tissue and organ injuries induced by DENV-1 infection.
Figure 4.
The serum levels of sVCAM-1 positively correlated with the levels of ALT (A), AST (B), LDH (C), TNF-α (D), and IL-6 (E).
Figure 5.
The serum levels of sVCAM-1 negatively correlated with PLT counts (A), NEU counts (B), and viral load (C).
DISCUSSION
The classifications of dengue, based on clinical signs, are subjective, and the varied mechanisms responsible for each detected complication are not completely understood. Thus, many previous studies have attempted to identify the specific factors that are involved in the pathogenesis of severe dengue, including antibody-dependent enhancement, the cytokine storm phenomenon, an individual's genetic background, the virulence of the virus strains, viremia levels in the acute phase, and nutritional status.8 Here, we describe the dynamic changes in the levels of sVCAM-1, cytokines, viremia, and other parameters reflecting tissue and organ injuries in DENV-1-infected adults over time. We found that the levels of sVCAM-1 and cytokines, including IL-6, TNF-α, and IL-10, were markedly increased in both the severe dengue and DF groups. However, significantly higher levels of the aforementioned parameters were observed in the severe dengue group than in the DF group. Moreover, elevated levels of ALT, AST, LDH, and CK and drastically decreased PLT and NEU counts were also observed in the severe dengue patients, indicating that there were severe organ and tissue injuries. Surprisingly, among all of the parameters measured, only sVCAM-1 levels were persistently high from the beginning of fever onset to the convalescence phase and were closely associated with the clinical parameters reflecting the severity of the disease; sVCAM-1 levels correlated positively with ALT, AST, LDH, TNF-α, and IL-6 levels and negatively with PLT and NEU counts and viral loads. Taken together, these results suggest that sVCAM-1 might be a strongly associated biomarker for the severity of adults with DENV-1 infection.
There are limited studies on the role of sVCAM-1 in DENV infection. Murgue et al. reported that sVCAM-1, but not sP- or sE-selectin, was significantly associated with children with dengue hemorrhagic fever (DHF), and no association was found between sVCAM-1 levels and primary versus secondary infections.11 Koraka et al. also demonstrated that an elevated amplitude of sVCAM-1 levels was closely linked to the severity of the disease in children; however, this relationship was insufficient to differentiate dengue shock syndrome (DSS) from DHF, thus having a limited prognostic value in children with dengue.12 Moreover, in patients with Crimean-Congo hemorrhagic fever and hemorrhagic fever with renal syndrome induced by Hantaan virus infection,23 increased sVCAM-1 levels were also found to be positively correlated with liver enzyme levels and showed a close association with disease severity. Our results further indicate that sVCAM-1 levels are a potential biomarker that could be used, instead of cytokines, to predict the severity DENV-1 infection in adults.
VCAM-1 is an adhesion molecule that is mainly expressed on activated VECs. VCAM-1 plays an important role in leukocyte rolling, adhesion, transmigration, and inflammation.24 High levels of adhesion molecules such as VCAM and the intercellular adhesion molecule (ICAM) in the blood have been demonstrated to be associated with plasma leakage and hemorrhage in children with dengue, indicating VEC damage during the course of the disease.25 Accordingly, the increased levels of sVCAM-1 that were observed in this study also indicated that there was VEC damage in the DENV-1-infected adults. This finding was partially consistent with a recent report by Djamiatun,26 who found that there were high circulating levels of von Willebrand factor (VWF) in DHF/DSS patients, indicating that the injury or activation of VECs leads to the secretion of VWF during the course of the disease. However, the mechanisms underlying VEC damage are not clear, and several factors were demonstrated to be involved in this process. Previous studies reported that the roles of cytokines were very compelling in the pathogenesis of DHF/DSS.27,28 In our study, drastically elevated levels of IL-6 and TNF-α were observed in the severe dengue patients but not in the DF patients. Interestingly, sVCAM-1 levels positively correlated with TNF-α and IL-6 levels and negatively correlated with viral load. It is known that TNF-α and IL-6 are critically early cytokines that mediate inflammatory signaling and amplify the host inflammatory response to infection. In vitro and in vivo studies have clearly demonstrated that the secretion of VCAM-1 could be induced by the interaction of VECs with TNF-α and/or DENV infection of VECs,29,30,31 which is considered to be an important cause of VEC damage and sVCAM-1 overexpression. Damaged VECs could directly alter the permeability of the VEC barrier; permit immune cell targeting of DENV expressed by VECs; elicit immune cell-enhancing cytokine responses; and affect the rolling, adhesion, and transmigration of leukocytes and PLTs. These phenomena may further result in hemorrhagic signs such as petechiae, a positive tourniquet test, thrombocytopenia and neutropenia, as were observed in our study. Additionally, Vaughn et al. reported that high viremia, DENV-2 infection and antibody response patterns were considered to be important causes of the pathogenesis of severe dengue.32 Therefore, a cytokine storm may be induced by high viremia, which causes VEC damage and ultimately results in severe dengue. However, there were no obvious differences in the DENV-1 loads between the two groups of patients in our study. This finding is consistent with the above report, in which greater DENV-2 virus replication in primed hosts confers an enhanced pathogenicity compared with the pathogenicity observed in DENV-1 secondary or primary infections. We also previously reported that a weak DENV-1 pathogenicity was isolated from adult dengue patients.4 In combination with other reports, this finding indicates that TNF-α and IL-6 are involved in VEC damage that is induced by DENV-1 infection and further suggests that immune factors might be the predominant contributors to the damage of endothelial cells and the pathogenesis of severe dengue. Although TNF-α and IL-6 play an important role in pathogenesis, the multivariate analysis showed their levels were more inferior than sVCAM-1 in predicting the severity of dengue.
Several limitations in our study should be considered. First, other biomarkers of endothelial cell activation/dysfunction such as angiopoietins (Ang)-1 and -2 and VWF and other cytokines, such as IL-1β, monocyte chemoattractant protein-1, and IL-8, were not measured. The combined detection of a number of biomarkers is more helpful for the prediction of illness severity and for the evaluation of disease prognosis.26,33 Therefore, if we investigated more parameters, the study would have more scientific merit and assist in the clarification of the biomarkers debate in the field of dengue, and more parameters will be included in our future studies. Second, the sVCAM-1 levels in our study were much lower than the levels in previous reports.11,12 This difference may be due to the different detection levels of the kits made by different companies. Last, due to the retrospective nature and the small sample size of the study, we could not conclude that sVCAM-1 levels at baseline could predict the clinical outcome of dengue. Therefore, this issue may require a prospective study with a larger sample size.
In summary, we analyzed the dynamic changes of sVCAM-1, specific cytokines, and viremia in dengue patients. Our results revealed that sVCAM-1 levels were persistently higher in severe dengue throughout the course of the disease and were closely associated with disease severity. The results may suggest that sVCAM-1 is a potential biomarker for predicting the severity of dengue and could be useful in routine clinical diagnoses. Overall, further studies are necessary to investigate the mechanism and efficacy of sVCAM-1 in severe dengue.
Acknowledgments
We thank Jin An (Department of Microbiology, School of Basic Medical Sciences, Capital Medical University, Beijing, China) for the editorial assistance. This study was supported by grants from the Special Project to Benefit the People of Guangzhou Municipal Science and Technology Bureau (NO 2014Y2-00185) and the National Key Clinical Department of Infectious Disease (2013–2014).
References
- 1World Health Organization. Dengue haemorrhagic fever: diagnosis, treatment, prevention and control. 2nd ed. Geneva: WHO, 1997. Available at http://www.who.int/csr/resources/publications/dengue/Denguepublication/en/ (accessed 12 September 2009). [Google Scholar]
- 2Gutch M, Agarwal A, Amar A. Hypokalemic quadriparesis: an unusual manifestation of dengue fever. J Nat Sci Biol Med 2012; 3: 81–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3Misra UK, Kalita J, Syam UK, Dhole TN. Neurological manifestations of dengue virus infection. J Neurol Sci 2006; 244: 117–122. [DOI] [PubMed] [Google Scholar]
- 4Zhang FC, Tang XP, Hu XC et al. A clinical, epidemiological and virological study of a dengue fever outbreak in Guangzhou, China, 2002–2006. Dengue Bull 2007; 31: 10–18. [Google Scholar]
- 5Tang Y, Kou Z, Tang X et al. Unique impacts of HBV co-infection on clinical and laboratory findings in a recent dengue outbreak in China. Am J Trop Med Hyg 2008; 79: 154–158. [PubMed] [Google Scholar]
- 6World Health Organization. Dengue guidelines for diagnosis, treatment, prevention and control. Geneva: WHO, 2009. Available at http://www.who.int/csr/resources/publications/dengue_9789241547871/en/ (accessed 7 July 2009). [PubMed] [Google Scholar]
- 7Dalrymple N, Mackow ER. Productive dengue virus infection of human endothelial cells is directed by heparan sulfate-containing proteoglycan receptors. J Virol 2011; 85: 9478–9485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Noisakran S, Perng GC. Alternate hypothesis on the pathogenesis of dengue hemorrhagic fever (DHF)/dengue shock syndrome (DSS) in dengue virus infection. Exp Biol Med (Maywood) 2008; 233: 401–408. [DOI] [PubMed] [Google Scholar]
- 9Lei HY, Yeh TM, Liu HS, Lin YS, Chen SH, Liu CC. Immunopathogenesis of dengue virus infection. J Biomed Sci. 2001; 8: 377–388. [DOI] [PubMed] [Google Scholar]
- 10Kurane I. Dengue hemorrhagic fever with special emphasis on immunopathogenesis. Comp Immunol Microbiol Infect Dis 2007; 30: 329–340. [DOI] [PubMed] [Google Scholar]
- 11Murgue B, Cassar O, Deparis X. Plasma concentrations of sVCAM-1 and severity of dengue infections. J Med Virol 2001; 65: 97–104. [PubMed] [Google Scholar]
- 12Koraka P, Murgue B, Deparis X et al. Elevation of soluble VCAM-1 plasma levels in children with acute dengue virus infection of varying severity. J Med Virol 2004; 72: 445–450. [DOI] [PubMed] [Google Scholar]
- 13Green S, Pichyangkul S, Vaughn DW et al. Early CD69 expression on peripheral blood lymphocytes from children with dengue hemorrhagic fever. J Infect Dis 1999; 180: 1429–1435. [DOI] [PubMed] [Google Scholar]
- 14Kurane I, Innis BL, Nimmannitya et al. Activation of T lymphocytes in dengue virus infections. High levels of soluble interleukin 2 receptor, soluble CD4, soluble CD8, interleukin 2, and interferon-gamma in sera of children with dengue. J Clin Invest 1991; 88: 1473–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15Raghupathy R, Chaturvedi UC, Al-Sayer H et al. Elevated levels of IL-8 in dengue hemorrhagic fever. J Med Virol 1998; 56: 280–285. [DOI] [PubMed] [Google Scholar]
- 16Hober D, Poli L, Roblin B et al. Serum levels of tumor necrosis factor-alpha (TNF-alpha), interleukin-6 (IL-6), and interleukin-1 beta (IL-1 beta) in dengue-infected patients. Am J Trop Med Hyg 1993; 48: 324–331. [DOI] [PubMed] [Google Scholar]
- 17Juffrie M, van Der Meer GM, Hack CE et al. Inflammatory mediators in dengue virus infection in children: interleukin-8 and its relationship to neutrophil degranulation. Infect Immun 2000; 68: 702–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18Mustafa AS, Elbishbishi EA, Agarwal R, Chaturvedi UC. Elevated levels of interleukin-13 and IL-18 in patients with dengue hemorrhagic fever. FEMS Immunol Med Microbiol 2001; 30: 229–333. [DOI] [PubMed] [Google Scholar]
- 19Gagnon SJ, Mori M, Kurane I et al. Cytokine gene expression and protein production in peripheral blood mononuclear cells of children with acute dengue virus infections. J Med Virol 2002; 67: 41–46. [DOI] [PubMed] [Google Scholar]
- 20Nguyen TH, Lei HY, Nguyen TL et al. Dengue hemorrhagic fever in infants: a study of clinical and cytokine profiles. J Infect Dis 2004; 189: 221–232. [DOI] [PubMed] [Google Scholar]
- 21Lee YR, Liu MT, Lei HY et al. MCP-1, a highly expressed chemokine in dengue haemorrhagic fever/dengue shock syndrome patients, may cause permeability change, possibly through reduced tight junctions of vascular endothelium cells. J Gen Virol 2006; 87(Pt 12): 3623–3630. [DOI] [PubMed] [Google Scholar]
- 22Tang Y, Kou Z, Zhang F et al. Both viremia and cytokine levels associate with the lack of severe disease in secondary dengue 1 infection among adult Chinese patients. PLoS One 2010; 5: e15631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23Qi BT, Wang P, Li J, Ren HX, Xie M. Levels of soluble vascular cell adhesion molecule-1 and soluble intercellular adhesion molecule-2 in plasma of patients with hemorrhagic fever with renal syndrome, and significance of the changes in level. Viral Immunol 2006; 19: 565–569. [DOI] [PubMed] [Google Scholar]
- 24Rice GE, Munro JM, Corless C, Bevilacqua MP. Vascular and nonvascular expression of INCAM-110. A target for mononuclear leukocyte adhesion in normal and inflamed human tissues. Am J Pathol 1991; 138: 385–393. [PMC free article] [PubMed] [Google Scholar]
- 25van Gorp EC, Suharti C, Ten CH et al. Review: infectious diseases and coagulation disorders. J Infect Dis 1999; 180: 176–186. [DOI] [PubMed] [Google Scholar]
- 26Djamiatun K, van der Ven AJ, de Groot PG et al. Severe dengue is associated with consumption of von Willebrand factor and its cleaving enzyme ADAMTS-13. PLoS Negl Trop Dis 2012; 6: e1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27Rothman AL. Immunity to dengue virus: a tale of original antigenic sin and tropical cytokine storms. Nat Rev Immunol 2011; 11: 532–543. [DOI] [PubMed] [Google Scholar]
- 28Martina BE, Koraka P, Osterhaus AD. Dengue virus pathogenesis: an integrated view. Clin Microbiol Rev 2009; 22: 564–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29Basu A, Chaturvedi UC. Vascular endothelium: the battlefield of dengue viruses. FEMS Immunol Med Microbiol 2008; 53: 287–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30Oishi K, Saito M, Mapua CA, Natividad FF. Dengue illness: clinical features and pathogenesis. J Infect Chemother 2007; 13: 125–133. [DOI] [PubMed] [Google Scholar]
- 31Dalrymple NA, Mackow ER. Roles for endothelial cells in dengue virus infection. Adv Virol 2012; 2012: 840654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32Vaughn DW, Green S, Kalayanarooj S et al. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis 2000; 181: 2–9. [DOI] [PubMed] [Google Scholar]
- 33Michels M, van der Ven AJ, Djamiatun K et al. Imbalance of angiopoietin-1 and angiopoetin-2 in severe dengue and relationship with thrombocytopenia, endothelial activation, and vascular stability. Am J Trop Med Hyg 2012; 87: 943–946. [DOI] [PMC free article] [PubMed] [Google Scholar]