SUMMARY
Visceral leishmaniasis (VL) caused by Leishmania spp. is an important vector-borne and largely zoonotic disease. In China, three epidemiological types of VL have been described: anthroponotic VL (AVL), mountain-type zoonotic VL (MT-ZVL), and desert-type ZVL (DT-ZVL). These are transmitted by four different sand fly species: Phlebotomus chinensis, P. longiductus, P. wui, and P. alexandri. In 1951, a detailed survey of VL showed that it was rampant in the vast rural areas west, northwest, and north of the Yangtze River. Control programs were designed and implemented stringently by the government at all administrative levels, resulting in elimination of the disease from most areas of endemicity, except the western and northwestern regions. The control programs consisted of (i) diagnosis and chemotherapy of patients, (ii) identification, isolation, and disposal of infected dogs, and (iii) residual insecticide indoor spraying for vector control. The success of the control programs is attributable to massive and effective mobilization of the general public and health workers to the cause. Nationally, the annual incidence is now very low, i.e., only 0.03/100,000 according to the available 2011 official record. The overwhelming majority of cases are reported from sites of endemicity in the western and northwestern regions. Here, we describe in some depth and breadth the current status of epidemiology, diagnosis, treatment, and prevention of the disease, with particular reference to the control programs. Pertinent information has been assembled from scattered literature of the past decades in different languages that are not readily accessible to the scientific community. The information provided constitutes an integral part of our knowledge on leishmaniasis in the global context and will be of special value to those interested in control programs.
INTRODUCTION
This article summarizes the current status of visceral leishmaniasis (VL), with particular reference to the impact of past accomplishments, especially the control programs undertaken more than 50 years ago in China. Visceral leishmaniasis, also known as kala-azar, is an important vector-borne and largely zoonotic disease (1). VL is endemic in 66 countries, with ∼500,000 cases, resulting in an estimated >50,000 deaths, per year worldwide (2, 3).
The disease is caused by trypanosomatid protozoa in the genus Leishmania and is transmitted naturally by phlebotomine sand flies as the vector. While the clinical manifestations of the disease vary widely, from innocuous self-healing skin lesions (e.g., simple cutaneous leishmaniasis [CL]) to the often fatal VL, the transmission cycles are similar. Leishmania exists extracellularly in the gut of the blood-feeding female vector as motile flagellated promastigotes. They develop into infective forms as they move forward toward the anterior region close to the mouth part of the sand fly. When such infected females take blood meals, the parasites are delivered into the skin, where they infect mononuclear phagocytes and differentiate into small nonmotile forms called amastigotes. While the infection remains localized in cutaneous leishmaniasis, it is metastasized to the spleen, liver, and bone marrow of the reticuloendothelial system in VL. The parasites, along with vector and reservoir species, have been examined but not completely characterized in most areas of endemicity, including China.
Leishmaniasis is very widespread in many temperate, subtropical, and tropical areas of the world. The sites of endemicity for leishmaniasis are numerous, but few have been studied for elucidation of their clinical epidemiology. This is true for both anthroponotic visceral leishmaniasis (AVL) and zoonotic visceral leishmaniasis (ZVL). Domestic dogs and wild canines are the confirmed reservoirs for ZVL, which is most prevalent in the Mediterranean basin and Brazil. AVL is also epidemic in the northern part of the Indian subcontinent, e.g., Nepal, Bihar state of India, and in the northeastern countries of Africa. Epidemiological congruity of the AVL and ZVL in these areas of endemicity with those reported in China has not been established.
The control programs for VL in practice are similar to those for other vector-borne diseases, consisting of the following components: (i) diagnosis of patients for treatment; (ii) reduction of vector populations by indoor residual spraying (IRS) of households with insecticide, and (iii) prevention of transmission by using insecticide/insect repellant-impregnated bed nets in the case of ZVL. Implementation of these control measures, in part or in whole, has not eliminated VL in any place except for the bulk of the areas of endemicity in China (to be discussed in this review). Stringent implementation of the programs may overcome obstacles that are seemingly unsurmountable, as demonstrated by results from China.
Cutaneous leishmaniasis (CL) has not been reported in China except in a single isolated site of endemicity, i.e., Karamay in the Xinjiang Autonomous Region in the northwest. It is caused by a variant of Leishmania infantum transmitted by Phlebotomus wui as the vector. The great gerbil is the suspected but unconfirmed reservoir. Available data from a 3-year survey between 1992 and 1994 indicated that the local incidence of CL in Karamay is 1.43% (74/5,186). The lesions are innocuous and self-healing. None of the known CL cases had clinical manifestations or any history of VL (4). Unlike VL, CL is not a nationally notifiable disease and thus is excluded from the database available from the Ministry of Health in China. The CL in Karamay is of epidemiological interest but in the context of public health is not significant enough to warrant further extensive discussion. In this review, mentioned is made in passing where appropriate (see Table 3 and Fig. 3).
TABLE 3.
Cutaneous leishmaniasis in Karamay and Leishmania spp. other than L. donovani and L. infantum in Chinaa
| Leishmania species | Disease | Vector(s) | Host | Region(s) of endemicity |
|---|---|---|---|---|
| L. infantum | CL | P. wui | Human | Karamay, Xinjiang |
| L. turanica | CL | P. mongolensis, P. andrejevi | Great gerbil (Rhombomys opimus) | Karamay, Xinjiang |
| L. gerbilli | None | P. wui | Great gerbil (R. opimus) | Karamay, Xinjiang |
| L. gerbilli | None | P. mongolensis, P. andrejevi | Great gerbil (R. opimus) | Jiayuguan, Zhangye, and Wuwei, Gansu |
| Urad Houqi, Ejina Banner, Inner Mongolia |
Cutaneous leishmaniasis (CL) was reported only in Karamay, Xinjiang Autonomous Region, and was caused by a variant of L. infantum via transmission by P. wui (4, 30, 31). L. turanica and L. gerbilli were discovered in great gerbil (Rhombomys opimus) and were also isolated in Karamay (32–36). L. turanica can cause skin infection when it is inoculated into humans, but it is not considered an epidemiologically relevant pathogen. This is presumably due to the behavior of its known vectors, P. mongolensis and P. andrejevi, which exist only in rodent burrows (32–34). L. gerbilli is also widely distributed in Gansu and Inner Mongolia, as indicated. The vectors include P. wui in Karamay and P. mongolensis and P. andrejevi in other regions of endemicity (35, 36).
FIG 3.
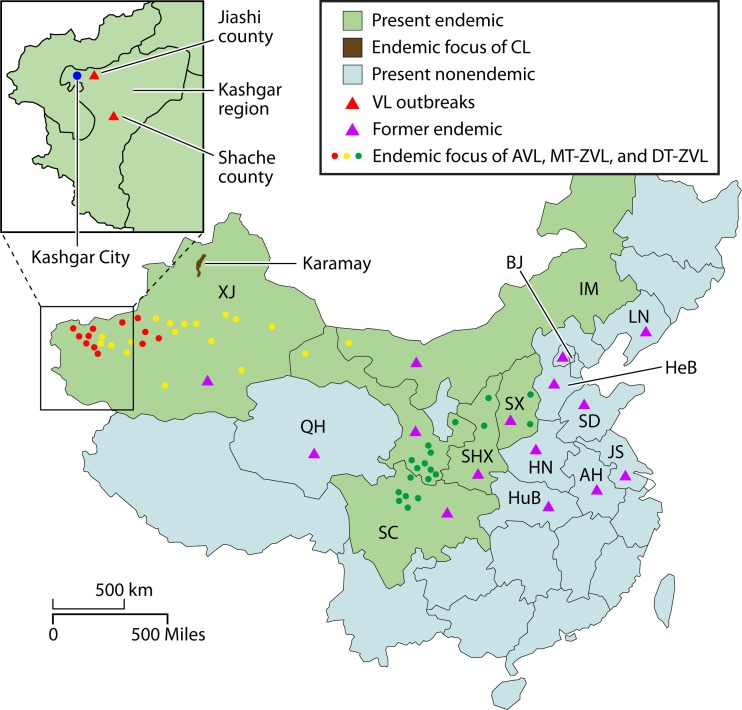
Distribution of foci of endemicity and outbreaks of visceral leishmaniasis (VL). Violet triangle, provinces of VL endemicity reported in 1951 (67). Circles, current foci of endemicity (red, anthroponotic visceral leishmaniasis [AVL]; green, mountain-type zoonotic visceral leishmaniasis [MT-ZVL]; yellow, desert-type zoonotic visceral leishmaniasis [DT-ZVL]). Green and pale blue, provinces where VL presently is and is not endemic, respectively (14, 71, 75). Brown area, a focus of cutaneous leishmaniasis (CL) endemicity at Karamay, Xinjiang (4). Inset, expanded Kashgar region showing the sites of recent outbreaks (red triangles) (76, 77). For abbreviations for provinces, see the legend to Fig. 2.
TYPES, DISTRIBUTION, AND CURRENT STATUS OF VISCERAL LEISHMANIASIS IN CHINA
Visceral leishmaniasis (VL) has been epidemiologically characterized as having three different forms in China: the anthroponotic type (AVL), the zoonotic mountain type (MT-ZVL), and the zoonotic desert type (DT-ZVL). They exist in specific areas with various landscapes (Fig. 1) and with different, albeit incompletely studied, parasites, vectors, and reservoirs (Table 1) (5–10).
FIG 1.
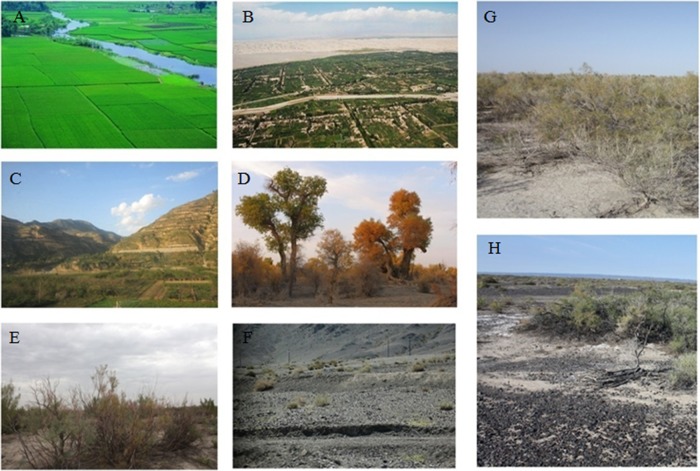
Typical topography of sites of endemicity for leishmaniasis in western and northwestern China. (A) Areas of flat plains, a site with AVL (Ma'anshan City, Anhui); (B) Kashgar alluvial plain, a site of AVL (Kashgar City, Xinjiang); (C) mountainous area with MT-ZVL (Lanzhou City, Gansu); (D) diversifolious poplar tree (Populus diversifolia) in desert with DT-ZVL (Korla City, Xinjiang); (E) branchy tamarisk bush (Tamarix ramosissima Ledeb) in desert with DT-ZVL (Changji City, Xinjiang); (F) pebble desert with DT-ZVL (Turpan, Xinjiang); (G) sacsaoul bush (Haloxylon ammodendron) in desert, Karamay, Xinjiang; (H) pebble desert (Karamay, Xinjiang). Original pictures were contributed by M.-S.W.
TABLE 1.
Epidemiological types of visceral leishmaniasis in China
| Disease | Leishmania species | Vector |
Reservoir(s) | |
|---|---|---|---|---|
| Species | Habitat | |||
| AVL | L. donovani | P. chinensis | Domiciliary | Patient (other reservoir not found) |
| P. longiductus | Domiciliary/peridomiciliary | Patient (other reservoir not found) | ||
| MT-ZVL | L. infantum | P. chinensis | Wild/peridomestic | Dog (Canis familiaris), raccoon dog (Nyctereutes procyonoides gray) |
| DT-ZVL | L. infantum | P. wui | Wild | Tarim hare (Lepus yarkandensis)a |
| P. alexandri | Wild | Unknown | ||
Based on preliminary incomplete evidence.
AVL has been reported in two geographically different regions of China: (i) the great northern plain and the eastern seaboard, where the AVL has been eliminated, as indicated by surveillance for incidence after completion of the control programs in 1958 (5–8, 11–13), and (ii) the Kashgar alluvial plain and the Aksu oasis of the Xinjiang Autonomous Region in northwestern China, where the AVL still persists (14, 15). AVL is defined as such because no reservoir animals have been found, despite repeated investigations over the decades. The causative agents belong to the Leishmania donovani/Leishmania infantum species complex, while the disease is transmitted by Phlebotomus longiductus in Xinjiang and by Phlebotomus chinensis elsewhere (5–8).
MT-ZVL is at present restricted to mountainous areas of the western region, primarily in the border region between southern Gansu and northern Sichuan but also occasionally in Qinghai, Shaanxi, and Shanxi provinces (5–8). L. infantum is the causative agent, which is transmitted by wild or peridomestic variants of P. chinensis, with dogs and apparently wild canines as the reservoirs. The principal reservoirs are domestic dogs, which are thought to acquire infection originally from canids of the sylvatic cycle (16, 17). Canine leishmaniasis has been reported at high rates in the western region, e.g., 59.43% (63/106) in Sichuan province and 77.21% (61/79) in Gansu province when surveyed in 2011 and 2006, respectively (18, 19).
Canine leishmaniasis was previously known to exist elsewhere, e.g., the Beijing area, where its epidemiological significance was first established experimentally. The key findings can be summarized as follows: (i) the development of typical VL via experimental transmission of Leishmania from infected dogs to laboratory animals by P. chinensis; (ii) the development of typical VL after inoculation of a human volunteer with Leishmania from the same source (20); and (iii) the finding of L. infantum in a wild raccoon dog (Nyctereutes procyonoides) in the hilly area north of Beijing (21). Based on extensive surveys from 1951 to 1959 (22), canine leishmaniasis of domestic dogs was reported in the adjacent regions of Beijing with landscapes similar to those in Liaoning, Hebei, Shandong, and other adjacent provinces in northeastern China (5–8, 21) (Table 2). The rate of incidence in dogs expressed as percentage of infection was modest, but it was higher in the north, e.g., northern Hebei (19/146 = 13%) and Liaoning (11/539 = 2%), and very low in the south, e.g., Shandong (34/3,100 = 0.1%) and southern Hebei (0/1,125 = 0%) (18, 19, 22). In these regions, there has since been no report of either human VL or canine VL in domestic dogs.
TABLE 2.
Canine leishmaniasis in representative foci of endemicity in Chinaa
| Province | No. of townships | Yr | No. of positive dogs/total | Rate of positivity (%) |
|---|---|---|---|---|
| Gansu | NAb | 2006 | 61/79 | 77 |
| Sichuan | 3 | 1956–1957 | 2/1,320 | 0.2 |
| Sichuan | NA | 2011 | 63/106 | 59 |
| Shanxi | 12 | 1959 | 12/8,048 | 0.01 |
| Liaoning | 11 | 1955–1958 | 11/539 | 2 |
| Hebei | 1 | 1959 | 19/146 | 13 |
| Qinghai | 4 | 1954–1958 | 10/1,135 | 0.8 |
DT-ZVL is found largely in the ancient oases and deserts reclaimed by settlers for agricultural development in the northwest, i.e., the southern and eastern regions of Xinjiang, the very western part of the Inner Mongolia Autonomous Region, and northern Gansu province (5–8). DT-ZVL in the Xinjiang Autonomous Region is further subdivided into sandy- and pebble-desert types, involving different vector species. The former exists near the oases at the foot of the southern slope of the Tianshan mountains along the north rim of the Taklamakan Desert in the Tarim basin, while the latter is located in the mountain gorges of the Tianshan further north and east, e.g., the Turpan and Hami basins (14, 23). In Inner Mongolia, DT-ZVL is of the pebble-desert type and has been reported only from Ejina Banner of the Alxa league or prefecture (24).The causative agent is L. infantum, which is transmitted by P. wui and P. alexandri.
A potential reservoir of DT-ZVL was identified in 2006, when L. infantum was isolated for the first time from ear lesions of the Tarim hare (Lepus yarkandensis), which is unique to the Taklamakan Desert of the Tarim basin (25). Isolation of Leishmania from older hares in the winter months was successful. They were presumably infected during the sand fly season from June to September of the previous year, in keeping with the generally known incubation period of 6 to 9 months. The harsh desert winter is thought to aggravate infection, rendering infected hares weak and thus more susceptible to capture. The seropositive rate for Leishmania is indeed much higher for hares caught in the winter than for those caught in the summer. The latter are largely young hares, which are mostly not infected or infected too recently to be positive serologically and microscopically for Leishmania, as noted in all the previous studies (15, 26). As a reservoir for DT-ZVL, however, infected hares must be demonstrated to exist during the sand fly season. The potential of the Tarim hare as a reservoir is suggested by the findings that L. infantum isolated from the hare was of the same strain as those from patients and from the expected vector, P. wui, in Bachu county (26). In addition, the isolates from all these sources produced visceral leishmaniasis experimentally in a susceptible animal, Lagurus lagurus (27). The potential of a lagomorph such as the Tarim hare as a reservoir for L. infantum is reminiscent of using rabbits for zooprophylaxis in other areas of ZVL endemicity of similar landscapes, e.g., the Sahara desert in north Africa (28). Recently, L. infantum was also isolated from the Iberian hare, a different species (Lepus granentansis), in the area of VL endemicity in Spain (29).Work is still under way to demonstrate experimental transmission of the L. infantum strain isolated from the specific species of the sand fly vector for DT-ZVL.
LEISHMANIA SPECIES COMPLEXITY
The Leishmania species described include L. donovani, L. infantum, L. gerbilli, L. turanica, and L. torentolae (Sauroleishmania). The last three species will not be further discussed, as they are well separated from human pathogens phylogenetically as discrete groups and are epidemiologically recognized as parasites of the great gerbil (Rhombomys opimus) in the northwestern desert and of lizards (Table 3 and its footnote give additional information for L. gerbilli and L. turanica) (4, 30–36).
The pathogenic species were named initially according to the epidemiology of the VL types they cause. Thus, isolates from AVL in the plains are referred to as L. donovani and those from ZVL as L. infantum. Initial analyses of some Leishmania isolates showed that they are more heterogeneous than previously thought. This was noted first by isoenzyme analysis of several isolates versus the “type species” (37) and then by kinetoplast DNA (kDNA) and nuclear DNA hybridization of ∼20 isolates (38). Primer sequence-specific PCR was subsequently used in attempts to discriminate isolates specific to different disease types, e.g., small-subunit ribosomal DNA (rDNA) and kinetoplast or mitochondrial DNA (kDNA) (39).
More recently, Leishmania sequence data were obtained for phylogenetic analysis. Up to 17 isolates were assessed by analyses of the rDNA intergenic spacer region (ITS-1) (40) and mitochondrial cytochrome b (cyt b) genes (41). In these studies, most sequences segregated into a clade, independent of known Leishmania complexes, while the remainder fell into the clades of L. donovani and L. tropica. The sequences of nuclear protein-coding genes were similarly analyzed in two additional studies. Dozens of isolates from China together with several hundred from elsewhere were examined in one study for five different single-copy genes (42). All canine and human VL isolates fell into the same clade of the L. infantum/L. donovani complex with the exception of three, which showed sequence homology to L. major or L. amazonensis. The majority of 15 isolates were identical to L. infantum from the Mediterranean region and the remainder to those from eastern Africa, but none were identical to L. donovani from India. Another study included two dozen isolates from China together with reference Leishmania isolates from elsewhere for similar analyses of seven concatenated protein-coding genes (43). All 10 of the canine and human VL isolates examined fell into the L. infantum/L. donovani complex but segregated into two clades: one closer to L. infantum and the other to Indian L. donovani. While more work is clearly needed to reconcile the data from these studies, they provide further evidence for the heterogeneity and complexity of Leishmania as promastigotes grown from infected tissues. The new approach is to construct a whole-genome-sequence database for phylogenetic analysis of individual Leishmania isolates from tissues of infected patients, reservoirs, and vectors.
SAND FLY VECTORS
Sand flies are widely distributed in temperate, subtropical, and tropical areas of the world. Only a few species serve as vectors to transmit leishmaniasis. These dipteran flies are fragile and smaller than mosquitoes. Female flies lay eggs in hidden moist locations rich in organic matter, e.g., rodent burrows. Hatchlings metamorphose into larval and pupal stages before emergence as adults. Their hairy wings rest in a V position, differentiating them from mosquitoes.
In China, the four species of sand flies mentioned in the previous section have been extensively studied to establish their vectorial capacity. P. chinensis was incriminated as a vector in the early 1920s, while the other three species, i.e., P. alexandri, P. longiductus and P. wui, were subsequently identified as such in the 1960s (22, 44, 45). Collection of sand flies and their identification as vectors are described in the literature cited in the previous sections. In short, sand flies were collected by using a suction pump, CDC light trap, and/or sticky paper and keyed for species identification. Vector species were identified by a combination of microscopy of dissected guts for the presence of promastigotes and the distribution and abundance of the identified species in given areas of endemicity. P. chinensis is a good example to provide specific details. It has long been accepted as the vector of VL in China based on the following evidence. (i) It is the most abundant and most widely distributed sand fly species correlating with the distribution of VL in areas of endemicity (12, 22) (Fig. 2). Data from a 1951survey of P. chinensis in Taian, Shandong province, showed the typical sand fly season, which begins in late May, peak in June, and gradually wanes from July to mid-September (12). (ii) Humans and dogs are the preferred feeding hosts of this sand fly species. One example to illustrate this is the analysis of fresh blood meals from P. chinensis in a 1953 survey conducted in Taian. The results showed that the blood meals originated largely from human and dog and to a lesser extent from other domestic animals, e.g., cow, donkey, and pig (12). (iii) Engorged P. chinensis flies caught in the field were found to harbor promastigotes, which were seen to migrate from the gut to the pharynx and mouth part (20, 46–49). A survey in Huai Yin, Jiangsu province, in 1935 showed that the natural infection rates for P. chinensis collected from the houses of VL patients were 1.66% (7/421) and 2.05% (11/537) in two adjacent villages (50, 51). (iv) Naturally infected P. chinensis was shown to transmit VL to hamsters experimentally in the laboratory (52, 53). (v) Finally, Leishmania isolates from P. chinensis are identical to those from VL patients in given regions of endemicity (37, 40, 48). A very large body of literature has provided overwhelming evidence for P. chinensis as the principal vector of VL in China. This is well recognized by not only national but also international authorities. Evidence is equally robust, albeit less well publicized, for the remaining three vector species, P. longiductus, P. alexandri, and P. wui, which are distributed in the northwestern part of China, including Xinjiang, western Gansu, and western Inner Mongolia (54–56). Sand flies have been exhaustively studied for decades as vectors of leishmaniasis in China, providing the database that is a necessary foundation for considering vector controls (57, 58), the epidemiology of DT-ZVL (59, 60), and the taxonomy of vectors and nonvector species in China (55, 61).
FIG 2.
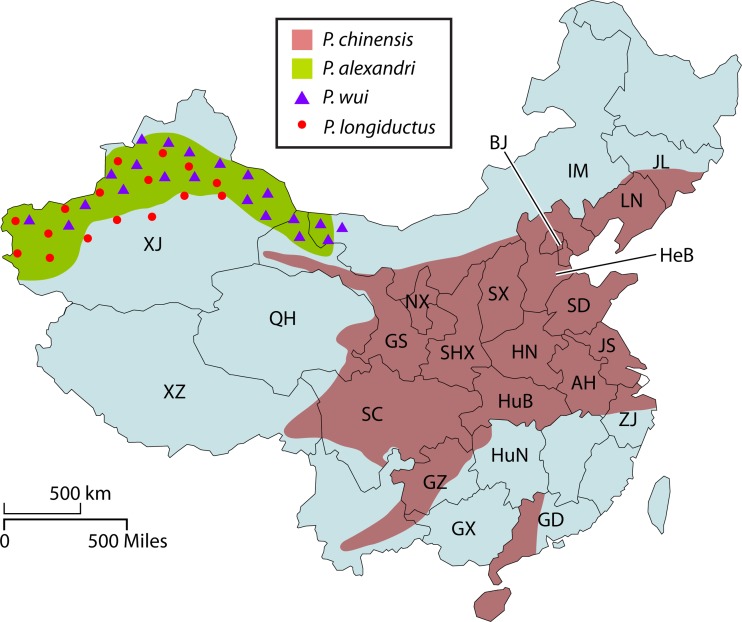
Distribution of sand fly vectors in China reported between 1916 and 1983. The distribution of the four sand fly vectors is colored-coded in the map based on data from references 54, 55, and 56. Abbreviations for provinces: AH, Anhui; BJ, Beijing; GS, Gansu; GZ, Guizhou; HN, Henan; HeB, Hebei; HuB, Hubei; HuN, Hunan; IM, Inner Mongolia; JL, Jilin; JS, Jiangsu; LN, Liaoning; NX, Ningxia; QH, Qinghai; SC, Sichuan; SD, Shandong; SHX, Shaanxi; SX, Shanxi; XJ, Xinjiang; XZ, Xizang (Tibet); ZJ, Zhejiang.
More recently, molecular approaches have been applied to Leishmania identification in infected vectors (62) and vector species differentiation (63, 64).
INCIDENCE OF REPORTED CASES BEFORE 2000
VL has been reported to exist in China for at least 120 years, going back to the late period of the Qing dynasty (1644 to 1911) and perhaps earlier (12). The first VL case was formally reported in 1904, and additional cases were reported subsequently in the 1910s (12). VL was then reported in 312 counties/cities nationwide, most of which (∼97%, 303/312) were distributed in eight provinces of China (9,672,018 km2): Hebei (190,000 km2), Shandong (157,126 km2), Jiangsu (102,658 km2), Anhui (139,427 km2), Henan (167,000 km2), Shaanxi (205,800 km2), Gansu (454,430 km2), and Liaoning (148,000 km2) (13, 65, 66) (Fig. 3). During the subsequent period of internal upheavals and civil war from ∼1920 to ∼1940, VL, along with other parasitic and infectious diseases, was rampant (12, 66).The problem was worsened with the outbreak of World War II and its aftermath during the next decade.
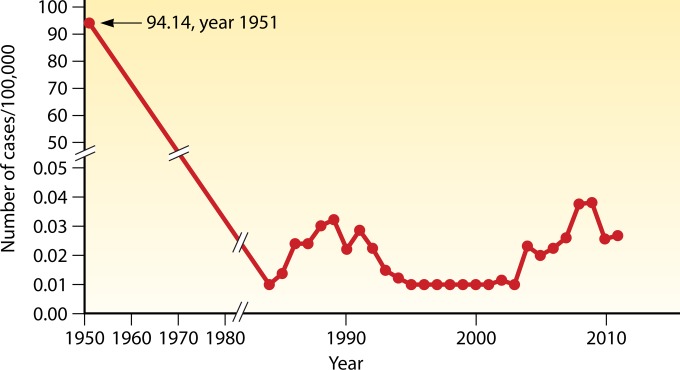
An exhaustive survey of VL, completed nationwide in 1951, showed a high rate of incidence, at 94/100,000, for a population of 563 million at that time (Fig. 4) (12, 67–69). There were thus ∼530,000 cases, which were spread far more widely than before in >650 counties, mainly in 16 provinces north of the Yangtze River (12). Elimination of VL in this area by the national campaign in the 1950s is indicated by a steady decline in the number of reported cases during the subsequent decades (Fig. 4) and the absence of any new cases there since 1983 (13, 22).
FIG 4.
National total incidence of visceral leishmaniasis (1951 and 1984 to 2011). From 1980 to 2011, detailed data are available and thus are graphed on an expanded scale. The broken line from 1950 to ∼1980 indicates the trend of decrease in incidence, since the data for this period are incomplete or not available (12, 67–69).
Transmission of VL, however, has never been interrupted in the western and northwestern provinces of Xinjiang (1,664,897 km2), Gansu, Sichuan (488,000 km2), Shaanxi, Shanxi (156,000 km2), and Inner Mongolia (1,183,000 km2) (6–8). A resurgence of VL began in these regions in the late 1980s (Fig. 4), coinciding with the national programs to develop western and northwestern China (8). Accelerated deforestation and an increase in the dog population ensued, conceivably providing habitats for the sand fly vectors and reservoirs favorable for the transmission of VL. As a result, the number of new cases officially reported jumped from 1,925 in the 1980s (1980 to 1989) to 2,629 in the 1990s (1990 to 1999), i.e., an ∼37% (704/1,925) increase (6–8), accounting for the bulk of the national VL endemicity.
INCIDENCE OF REPORTED CASES IN THE LAST DECADE
Since 2000, VL has remained endemic in the same six western and northwestern provinces (6–8), i.e., Xinjiang, Gansu, and Sichuan (Fig. 3), where the official rates of incidence are estimated to be ∼1 (14), ∼0.5 (70, 71), and ∼0.7 (72, 73) per 100,000, respectively (Table 4) (68, 69). The rate of national incidence was 0.03/100,000 in 2011 (69) due to the absence of reported cases in any significant number from the rest of the heavily populated provinces. The actual incidence may well be higher due to underreporting of the annual cases, especially in the remote areas of Xinjiang, even though VL is officially a notifiable disease.
TABLE 4.
Total incidence of visceral leishmaniasis nationally and in the three major provinces in which it is endemic for the period from 2002 to 2011a
| Area | Period | Total no. of cases | Incidence/100,000b |
|---|---|---|---|
| Nationwide | 2002–2011 | 3,169 | 0.0241 (0.0108–0.0381) |
| Xinjiang | 2005–2010 | 1,208 | 0.9568 (0.5919–1.4500) |
| Gansu | 2004–2010 | 748 | 0.4765 (0.2940–0.6114) |
| Sichuan | 2003–2010 | 436 | 0.0658 (0.0253–0.0960) |
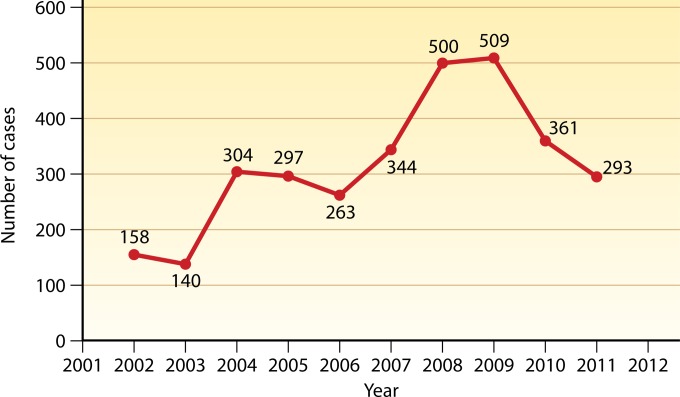
From 2002 to 2011, a total of 3,169 cases were officially reported nationwide, varying from 140 to 509 cases per year (Fig. 5). No epidemiological data are available to account for the annual fluctuation of VL incidence seen during this period, except for 2008 and 2009. The increase in the incidence in 2008 might be attributable to the spread of MT-ZVL in Sichuan, resulting from the large earthquake that year in the areas of endemicity in the province. The outbreak of DT-ZVL of unknown causes in Jiashi county (6,601 km2), Xinjiang, accounts for the increase in the total incidence there in 2009.
FIG 5.
National incidence of visceral leishmaniasis in total number per year reported from 2002 to 2011. The data shown are from the Ministry of Health of the People's Republic of China (2002 to 2011) (69).
There is evidence from recent data indicating that areas of endemicity have expanded and that the officially reported cases have increased in number in the areas where VL is not endemic. During the period from 2005 to 2010, a total of 2,450 VL cases were reported from 179 counties/cities in 18 provinces (74). Of these cases, ∼91% (2,224/2,450) were from 61 counties/cities instead of the 43 counties/cities previously known as sites of endemicity in the six western and northwestern provinces. The emergence or reemergence of 18 new sites of endemicity in these provinces is thus suggested. The remaining 226 (∼9%) cases were found scattered widely in 118 counties/cities considered not to be areas of endemicity, apparently resulting from the acquisition of VL by their residents while working as migrant laborers in the areas of endemicity. The potential contribution of such cases to the spread of VL from areas where it is endemic to those where it is not endemic or where it formerly was endemic is a cause of concern and requires follow-up investigation.
The incidence of VL is localized and varies considerably with different sites in the six provinces where it currently is endemic (Fig. 3). Of all the cases officially reported from 2005 to 2010, ∼98% (2,394/2,450) were distributed in the following provinces: Xinjiang (∼50%, 1,218/2,450), Gansu (∼34%, 825/2,450), and Sichuan (∼14%, 351/2,450) (74). Of the 1,218 cases reported in Xinjiang, 973 (∼80%) were from the 12 counties/cities in the Kashgar region (162,000 km2) out of the >100 counties in the entire Xinjiang Autonomous Region (14). In Gansu province, ∼360 cases were reported from 2005 to 2007 (71), of which ∼95% (341/360) occurred in five of the ∼90 counties, i.e., Wudu (4,683 km2), Wenxian (5,000 km2), Zhouqu (3,010 km2), Diebu (5,108 km2), and Dangchang (3,331 km2). During the period of 2004 to 2008, 177 of the 284 cases (∼62%) (72, 73) were reported from three of the ∼180 counties in Sichuan province, i.e., Jiuzhaigou (5,290 km2), Heishui (4,165 km2), and Maoxian (3,903 km2). Shaanxi, Shanxi, and Inner Mongolia are provinces of very low endemicity, having an average of only 1 to 3 new cases per year today (75).
Worthy of note are the two recent outbreaks of VL reported from the Kashgar region, Xinjiang (Fig. 3, inset). One occurred in Shache county (8,196 km2) from July 2004 to May 2006, resulting in 9 new cases (8 cases in the same village and one case in a location 2 km away). The first case appeared to be a patient who acquired VL in Kashgar city, a well-known focus of AVL endemicity. That was the apparent source of the infection that spread to other residents in Shache, where VL has not been reported for years (76). From January 2008 to May 2009, ∼96% (258/268) of the VL cases reported in Xinjiang occurred in the eastern part of Jiashi county, a well-known site of endemicity of DT-ZVL (77).
DEMOGRAPHIC PROFILES
The most current demographic information for the VL in the western and northwestern areas of endemicity for the years of 2002 to 2011 has recently been published (74). The essential points with additional details are briefly summarized below.
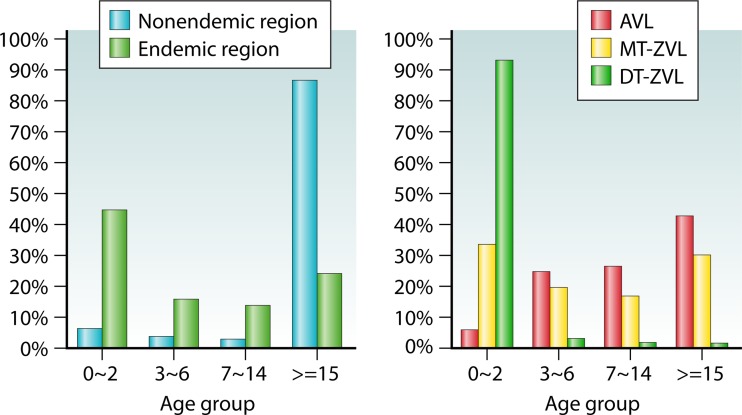
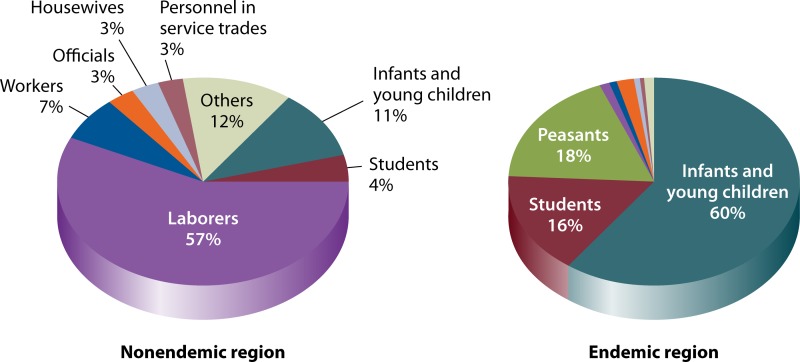
Of interest to note is the difference in the average age of the patients between the areas where VL is not endemic (∼87% are ≥15 years old) and the areas of endemicity (∼45% are 0 to 2 years old) (Fig. 6). This is consistent with the finding that patients in the areas where VL is not endemic are predominantly temporary workers in the areas of endemicity. Also of note are the marked differences in the average age of the patients for the three VL types: ∼43% of AVL patients are in the ≥15-year-old group, ∼53% of MT-ZVL patients are in the 3- to 6-year-old group, and ∼94% of DT-ZVL patients are in the 0- to 2-year-old group. The data for the age groups correlate with those of their occupations (Fig. 7): The majority of the VL patients are reported to be laborers in areas where VL is not endemic (∼57%) and infants or toddlers in the areas of endemicity (∼60%). The latter group increases to a very high level of ∼98% in the areas of DT-ZVL endemicity. These observations are attributable in part to the differences in the development of acquired immunity to VL between populations in areas where VL is and is not endemic and to age-related maturation of immunity to VL in the areas of endemicity. Both of these factors may contribute to the large share of VL in the “student” category, which spans the age groups from children and teens in elementary and high schools to young adults from areas where VL is not endemic or other areas who study in colleges/universities in the areas of endemicity.
FIG 6.
Age distribution (in years) of visceral leishmaniasis reported between 2005 and 2010. The data shown are from reference 74. Abbreviations: AVL, anthroponotic visceral leishmaniasis; MT-ZVL, mountain-type zoonotic visceral leishmaniasis; DT-ZVL, desert-type zoonotic visceral leishmaniasis. Age distributions for all VL types (left panel) and for specific VL types (right panel) are shown.
FIG 7.
Occupation distribution of visceral leishmaniasis cases reported between 2005 and 2010. The data shown are from reference 74. Distributions for all VL types in regions where VL is not endemic (left panel) and in regions where it is endemic (right panel) are shown.
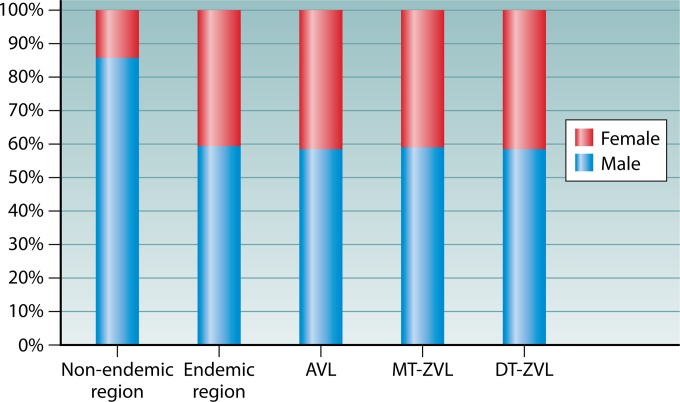
The gender of the patients is biased toward male (Fig. 8). This bias is noticeable in the regions of endemicity for all three VL types to similar extents (male-to-female ratio = 1.5:1). This ratio is higher than the national gender imbalance known to exist in favor of males (currently at a ratio of 1.27:1) due to the policy of one child per family for the past decades in China. This policy is expected to contribute very little, if any, to the male gender bias seen in the VL in the Xinjiang Autonomous Region, as it has not been enforced, at least not for the minority ethnic groups, which constitute ∼50% of the total population in this region. Expectedly, the gender-related occupational differences play a larger role: males work outdoors more frequently than females, thereby increasing their chance of exposure to the sand fly vectors known to transmit the disease in these areas of endemicity. The gender bias skews to a significant male-to-female ratio of 6.3:1 in the areas where VL is not endemic. This is also expected, since laborers who work short-term in the areas of endemicity are overwhelmingly male.
FIG 8.
Gender distribution of visceral leishmaniasis reported between 2005 and 2010. The data shown are from reference 74.
VL cases are reported throughout the year in both regions where VL is endemic and regions where it is not. Monthly distributions of reported cases vary with different types of VL in different areas of endemicity. Disease emergence is subsequent to the sand fly season, which normally spans the period from May to September (78). While AVL and MT-ZVL emerge all year round, they tend to peak in April and June, respectively. DT-ZVL occurs annually from September to February, peaking in December. The short incubation period is presumably due to the fact that the susceptible population is largely infants with less well-developed immunity.
DIAGNOSIS
Procedures for diagnosis of VL have followed the standard protocols: examinations of patients' records of clinical symptoms and of history of residency/travel in areas of endemicity and laboratory examinations of biopsied samples for the presence of Leishmania. Individuals suspected of having VL are those who live in, work in, or have a history of travel to areas of endemicity and recall experiences of insect bites, which are suggestive of sand fly exposure. A tentative diagnosis is often made in areas of endemicity when abdominal palpation is positive for splenomegaly. Hematological disorders, such as pancytopenia and hyperimmunoglobulinemia, provide further confirmation, but the blood tests needed to obtain such data are not always available, except in the cities. Additional clinical signs and symptoms of VL include irregular fevers, fatigue, lethargy, and weight loss, which are less reliable, since they are all shared with those of other endemic diseases, such as typhoid fever, disseminated histoplasmosis, malaria, and tuberculosis (79).
The gold standard for diagnosis of VL has been microscopic visualization of L. donovani bodies (amastigotes) in Giemsa-stained smears of infected tissues and/or of motile promastigotes after cultivation of the samples in NNN blood agar. Both methods have long been practiced using bone marrow samples collected by sternum or iliac punctures in China (12). In recent years, however, many clinics and hospitals have experienced technical difficulties with bone marrow puncture and microscopic examination of samples for Leishmania identification due to the lack of experienced personnel. This is clearly illustrated in recent studies of juvenile VL from 2006 to 2012 (80). Of the 1,093 confirmed VL cases, direct visualization of L. donovani bodies by microscopy was accomplished for only eight samples. Retraining of personnel for such parasitological skills is crucial not only for diagnosis but also for the sample preservation needed for further clinical and laboratory investigation.
Technical advances have significantly improved the sensitivity, specificity, and field deployability of the methodology for serodiagnosis of VL. The choice of antigens is important, as shown by studying the recombinant products of the 39-amino-acid repeats in the kinesin protein of L. donovani (referred to as rK39). Using the sera of VL patients from Gansu and Xinjiang provinces, high sensitivity and specificity for rK39 was first demonstrated with the soluble form of this recombinant antigen via endpoint titration by enzyme-linked immunosorbent assay (ELISA) (81), and its field deployability was demonstrated in a dipstick format by reaction with drops of blood from patients' fingers or earlobes (82). Subsequent commercialization of rK39 dipsticks greatly facilitated further evaluation of this method for diagnosis of VL. Extensive field tests in China confirmed its high sensitivity (97% to 100%) and specificity (83–85), which are comparable to those of the standard diagnostic methods by microscopy (96%–100%) (82, 86). Thus, the rK39 dipstick test is now the principal, if not the only, method of diagnosis for VL adopted by local Centers for Disease Control and Prevention (CDCs) in areas of endemicity in China.
Other serological tests have been developed to detect not only Leishmania-specific antibodies but also circulating antigens (87–89). One is the double-antigen sandwich ELISA (DAgS-ELISA), with 100% specificity at a sensitivity of ∼69% (46/67) for serodiagnosis of VL (87). Another is the monoclonal antibody-antigen spot test (McAb-AST) for detecting circulating antigens, with the sensitivity and specificity being ∼97% and ∼100%, respectively (90, 91). The McAb-AST has the advantages of differentiating active disease from asymptomatic infection and for monitoring treatment efficacy.
Molecular biotechnology also has been evaluated for diagnosis of VL in China, especially by targeting Leishmania-specific repetitive sequences, e.g., miniexon genes (92), and kinetoplast DNA minicircles (kDNAs) (89, 93–96). PCR amplification of patients' bone marrow and buffy coat samples for miniexon gave 100% sensitivity, while PCR of the kDNAs produced a positive rate of ∼31% (83/269), which is higher than the ∼24% (65/269) for ELISA of the sera with Leishmania soluble antigens from the same set of patients. PCR has been useful, with its greater sensitivity for detection of low parasite loads, as often seen in cases of asymptomatic infection known to exist in China (89). Parasite DNA/antigen detection is more discriminatory than detection of anti-Leishmania antibodies for current infection. While these assays still await commercialization as cost-effective products for diagnosis, their use in combination will be of value for studying the seroepidemiology of VL.
TREATMENT: CHEMOTHERAPY
Treatment of all VL cases has been based on chemotherapy, except for rare occasions when splenectomy was necessary for patients at very advanced stages (12). Sodium stibogluconate (SSG), manufactured in Shandong, was provided for free during the national campaign in the 1950s (12, 13).The drug from this source has remained in use and effective. It is recommended by the Ministry of Health as the first-line treatment for all types of VL in China today (Table 5) (15, 22, 97–99). Independent clinical trials of SSG involving a total of 3,897 VL patients have been carried out in the past decades. The curative rate was reported as 92% after a single 6-day course with total dosages of 120 to 140 mg/kg for children and 60 to 90 mg/kg (May 1950 to June 1951) or 96 to 108 mg/kg (after June 1951) for adults. A second course increased the curative rate to >97% (including relapsed cases) (12, 22, 99). In addition, it has been repeatedly confirmed that the SSG of the regimens used produces few serious side effects and is thus relatively safe (12). The side effects of the treatment are limited to very few patients and are considered insignificant, e.g., fever, cough, nausea, epistaxis, abdominal pain, and diarrhea (12).
TABLE 5.
Regimens and outcomes of clinical trials of antileishmanial drugs for visceral leishmaniasis at different sites of endemicity in China
| No. of patients (yr, region) | Drug | Dosage, mg/kg (total) | Regimen | Outcome | Reference |
|---|---|---|---|---|---|
| 73 (1987–1996, Gansu) | SSGa | 90–110 (adults), 150–220 (children) | 1 dose/day, 6 days | 72 patients cured, no serious side effectsb | 97 |
| 423 (1985–1991 Sichuan) | SSG | 90–130 (adults), 150–200 (children) | 1 dose/day, 6–10 days | 361 patients cured, 24 recrudesced, no serious side effects | 98 |
| 638 (1992–2009, Sichuan) | SSG | 220–240 (<20 kg), 200–220 (20–40 kg), 180–200 (>40 kg) | 6 doses daily, 8–15 days | 596 patients cured, 12 recrudesced, no serious side effects | 98 |
| 117 (1955, Beijing) | SSG | 200 (children), 140 (adults) | 2 doses/wk, 3 wk | All patients cured, no serious side effects | 99 |
| 90 (1954–1957, Shandong) | SSG | 200 (children), 140 (adults) | 2 doses/wk, 3 wk | All patients cured, 7 recrudesced, no serious side effects | 22 |
| 265 (1956, Shandong) | SSG | 90–110 (adults), 150–220 (children) | 6 doses daily, 3 days | 114 patients recovered, slight side effects | 22 |
| 10 (1951, Beijing) | Stilbamidine | 1–2 | 1 dose/day, 10–20 days | 9 patients cured, side effects detected | 99 |
| 70 (1958, Shandong) | Pantamidine | 3–5 | 1 dose/day, 10–15 days | 48 patients cured, side effects detected | 22 |
SSG, sodium stibogluconate.
See the text for a description of the side effects.
The “six-day therapy” is the most conventional regimen for VL treatment today, which rapidly alleviates clinical symptoms and shortens the duration of the disease; there is a significant reduction of splenomegaly and return of the body temperature to normal in 86 to 95% of the patients after four injections. About 2 weeks after the completion of a single course, L. donovani bodies became microscopically undetectable in the bone marrow in all but 0.4% of the treated patients (12). Regimens of “three-week therapy” and “three-day therapy,” although no longer in use, are equally effective. The three-week therapy was originally designed for treating VL at advanced stages and the three-day therapy to accommodate patients' needs for a shortened period of the treatment at the same total dosages. A slight increase in the side effects was noted with the latter treatment (12).
Aromatic diamidines, e.g., stilbamidine and pentamidine, have been used as second-line drugs for the rare SSG-resistant cases, although the outcomes are inconsistent in China. Serious side effects were often noted immediately or shortly after administration, e.g., severe lowering of blood pressure, rapid breathing, palpitations, and abdominal pain (12). Amphotericin B alone or in combination with SSG to increase efficacy with reduced side effects has been successful in treating VL. Three cases were cured by using domestically produced liposomal amphotericin B and two cases by a combination of SSG and amphotericin B (100–102). New compounds with low toxicity for the experimental treatment of Leishmania spp. have been reported, and some of these compounds showed high efficacy in killing amastigotes and promastigotes of various Leishmania spp. both in vivo and in vitro (103–107).
PREVENTION AND CONTROL
Administrative Framework
The administrative framework of the control programs is outlined briefly to facilitate further discussion in greater detail. The national programs for control of kala-azar were officially initiated by an executive order from the central government in 1951. The program was administered via the existing system of civil service for health, from national to provincial to county, city, township (people's communes), or village level. A national strategic plan was issued by the Ministry of Health for implementation aiming at elimination of VL nationwide before 1962. This executive order included four general directives. (i) The Ministry of Health is delegated full responsibilities at all administrative levels to execute the control programs according to the guidelines summarized in the following points: (ii) to thoroughly investigate VL nationwide, including surveys for the prevalence and distribution of the disease and discovery of vectors and reservoirs involved; (iii) to prevent and control VL in the regions of endemicity, including the elimination of sand flies and reservoirs (mainly domestic dogs) and treatment of patients (in regional and provincial hospitals for early- and late-stage cases, respectively); and (iv) to educate the general public about VL and train the professional personnel needed (12, 108).
Control Programs in Detail
In 1951, the Ministry of Health declared VL one of the nine notifiable infectious diseases targeted with urgency for elimination. Significant resources were allocated by the government to swiftly set up multiple task forces with the sole responsibility of VL elimination at the regional and provincial levels in areas of endemicity. Before then, investigation of leishmaniasis and sand fly vectors had already been well under way, providing a sound foundation of clinical and epidemiological knowledge for launching the control programs. The early work was initiated by foreign investigators from 1910 to 1920 and then mainly by Chinese physician scientists since the 1920s. Individuals in the latter group and their associates provided the leadership instrumental to the successful establishment of the task forces for the control programs. The East China Institute of Prevention and Control for Visceral Leishmaniasis coordinated the provincial institutes with the same designation to embark on 3- to 6-month multidisciplinary training courses for health personnel. Via such courses, thousands were trained to become proficient in dealing with patient diagnosis, clinical symptoms/management, and chemotherapy, as well as collection, identification, and control of vectors and reservoirs. Standard manuals were written for the course and provided to the trainees. Trained persons were organized into units to work in the local health stations throughout the cities and rural villages in the areas of endemicity. The programs included massive mobilization of the general public by a top-down approach from the provincial to the village level for education of the population about VL by trained personnel. No less significant was the action of the Ministry of Health to commission Shandong Xinhua Pharmaceutical Company to produce the high-quality SSG needed for treating patients. The control strategies placed great emphasis on persistent efforts over the years to carry out massive and thorough screening of populations in areas of endemicity to identify VL patients for treatment. Simultaneous efforts were devoted to collection, identification, and control of suspected reservoir animals and vectors. The information obtained led to the implementation of vector control by indoor dichlorodiphenyltrichloroethane (DDT) spraying of human dwellings and animal shelters (5, 6, 12, 22, 107).
As a result of these massive endeavors, a total of more than half a million patients were identified and treated for free in the 16 provinces where VL was endemic from 1951 to 1958 (12). Indoor residual insecticide spraying campaigns using DDT to control vector sand flies had been initiated and completed in all the regions of endemicity by 1955. Tens of thousands of rodents, dogs, and other animals were trapped and examined as potential reservoirs of VL, leading to programs for elimination of infected and/or noninfected dog populations, depending on the infection rate in different places. As a result, the number of patients, density of vector sand flies, and dog populations all decreased precipitously, bringing VL under control in all areas of endemicity, especially in the eastern part of China proper, by the end of the 1950s (5, 12).
Postcontrol Surveillance
The control programs were followed up by yearly surveillance of all areas of endemicity during the subsequent decades (12). Measures were taken by local health stations (now CDCs) to control recurrent patient cases, vector density, and, where applicable, canine leishmaniasis. Large-scale surveys of sand fly densities, canine leishmaniasis, and populations in areas of endemicity by skin test for delayed-type hypersensitivity (DTH) had been periodically undertaken in selected places. The results of surveillance from the last several decades show the current status of VL, as described in the previous sections. Some details are summarized below for specific sites of endemicity.
(i) In the major areas of endemicity of eastern and northern China, both AVL and MT-ZVL have been reduced to undetectable levels, as indicated by the absence of infected vectors, patients, and dogs. Human VL has not been reported for decades in the following provinces where VL had been endemic (the year of the last reported human case is given): Hubei (1963), Shaanxi (1965), Anhui (1972), Liaoning (1975), Jiangsu (1976), Henan (1983), Shandong (1991), and Hebei (2002) (22).
The postcontrol surveillance in Shandong offers the best example for specific information, since it was one of the provinces where VL was highly endemic. Implementation of the control programs had brought the VL incidence there to a very low level in 1958 (3/100,000) (12, 22, 109). Continuous surveillance since then included the following actions prescribed in the guidelines. (i) Investigation by a team of kala-azar specialists was initiated when suspected cases were reported throughout the province. (ii) The finding of any positive case triggered the immediate actions of treating the patient and monitoring the vector density in the vicinity. The guidelines of the control programs were strictly followed to ascertain cure of the patients and control of the vector, if found at a high density. (iii) Monitoring of the patients and vector populations was continued during the subsequent years. Under such stringent surveillance measures, the VL incidence was found to further decline continuously to a very low level of 0.01/100,000 in 1970 (22). Surveillance data from 1972 to 1987 showed no new VL patient and no P. chinensis in 84.5% of the villages in the entire province (110). A large-scale immunological survey of the population was carried out by leishmanin skin test for DTH in 1989 (111). The DTH positive rate was zero for the population (0/8,020) under 30 years old and 4.4% (98/2,219) for those over 30 years old. This is the case for other areas of endemicity of northern and eastern China based on similar data collected from postcontrol surveillance. There is thus clear evidence indicating that the transmission of kala-azar had been interrupted in all these regions where VL was formally endemic. After 1990, reports of kala-azar have been extremely rare and invariably turned out to be recrudescent or imported cases.
(ii) The persistence of AVL is evident in the Xinjiang Autonomous Region and that of MT-ZVL in the western provinces, mainly Sichuan and Gansu provinces. Given below are some examples of control measures and the possible causes for the persistence of VL (112–117).
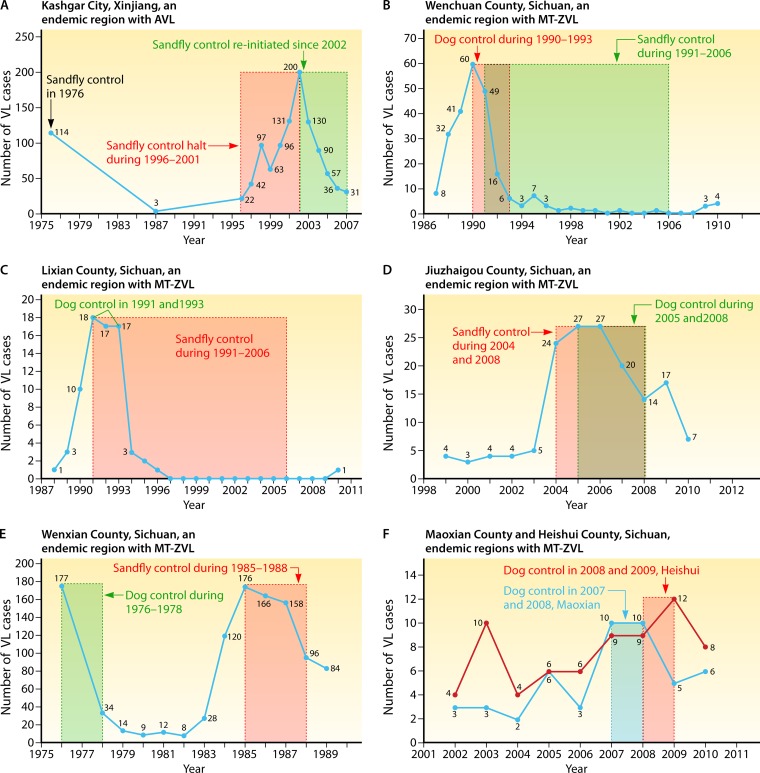
A significant risk factor of AVL in Xinjiang is the natural infection of the vector, P. wui, at relatively high rates from 0.8% (4/453) to 5.7% (17/300) (118, 119). In the late 1970s, a large-scale campaign, consisting of intensive insecticide spraying and identification of patients for treatment, was undertaken in the areas of endemicity of Kashgar and Aksu, Xinjiang Autonomous Region, resulting in a dramatic decline of AVL (Fig. 9A). However, AVL reemerged with a vengeance from 1996 to 2002 when the campaign was discontinued. It was brought under control again only after reinstallation of the discontinued programs (112).
FIG 9.
Relative efficacy of insecticide spray for sand fly control and dog control programs against visceral leishmaniasis at various sites of endemicity in Xinjiang, Sichuan, and Gansu. See references 112 to 117, 120, and 121 for details of the control programs. Shown are the total number of cases of visceral leishmaniasis per year in relation to the use of different control measures, as indicated, in the following sites of endemicity: Kashgar City, Xinjiang (A); Wenchuan county, Sichuan (B); Lixian county, Sichuan (C); Jiuzhaigou county, Sichuan (D); Wenxian county, Gansu (E); and Heishui county and Maoxian county, Sichuan (F). Vertical axes, number of VL cases; horizontal axes, year; areas framed by dotted lines, duration of sand fly or dog control programs and their outcomes, as indicated in the line graph for the changes of VL; numbers along line graph, number of VL cases recorded in the specific year.
In the areas of MT-ZVL endemicity of Gansu and Sichuan provinces (Table 4), vector and dog control programs had been implemented, resulting in a decrease in the human VL incidence. Dog control measure consisted of (i) identification of infected dogs followed by their elimination, (ii) prohibition of dog keeping by decree, and (iii) delta-methrin bathing of dogs. These measures in combination were found most effective (120–122). Some specific data are given below.
Insecticide spray to control sand fly vectors was found to be effective to some extent when implemented in 1985 (Fig. 9E). Sand fly control was further implemented in the counties of Wenchuan and Lixian during the period from 1991 to 2006. A survey by using human baits in Wenchuan showed that the sand fly densities were reduced by >4-fold, from 18 (1991) to 4 (2005) per bait per hour, after implementing the sand fly control program (113, 120–122).
Implementation of strict dog control programs was found to lower the number of human VL cases more significantly in specific locations (Fig. 9B to D). The rate of Leishmania infection in dogs was quite high, ranging from 12.2% (9/74) to 36.8% (46/125), as noted, for example, in Wenchuan county, Sichuan province, in 1990 (123). In the areas of endemicity in this province, the effective element of the control programs was thus periodic elimination of dogs on a large scale, amounting to totals of 4,482 (∼98%) (1991 to 1993), 5,194 (1990 to 1993), and 4,966 (2005 to 2008) dogs culled in the counties of Lixian, Wenchuan, and Jiuzhaigou, respectively. In Wenxian county, Gansu province, when the domestic dog population was reduced to ∼58% of the total (4,806/8,258), the human VL cases decreased sharply in number from 177 to 34 during a 3-year period from 1976 to 1978 (113). Discontinuation of this program in the following years resulted in the return of the dog population and, concomitantly, an increase in the incidence of human VL to the precontrol level.
After the natural disaster of the large earthquake in Heishui and Maoxian counties of Sichuan province, however, strict enforcement of the dog control policy did not prevent the emergence of new human VL cases (Fig. 9F) (117). The earthquake-related environmental changes may have inadvertently exposed the local population to the sylvatic cycle of transmission involving a hitherto-unidentified canid reservoir (124). The raccoon dog (Nyctereutes procyonoides), which is the apparent reservoir in the Beijing area, is excluded, since it is absent in the western regions (21).
(iii) DT-ZVL in Xinjiang has not been brought under control, in view of its persistence with sporadic outbreaks. Only diagnosis to identify patients for treatment has shown any practical value. Indoor residual insecticide spray was ineffective in controlling the exophilic vectors (125, 126) which transmit VL among the potential wild reservoirs, e.g., the Tarim hares living in the desert. Insecticide-impregnated sheets, curtains, and bed nets and insect repellents for personal protection were recommended for use to avoid sand fly bites during the transmission season. These recommendations have not yet been widely adopted, pending improvement of the housing conditions and education of the population in the areas of endemicity.
GB 15986-1995: Kala-Azar Diagnosis Standard and Management Principles
The Ministry of Health issued document GB 15986-1995, Diagnostic Criteria and Principles of Management of Kala-Azar, in 1995 as the last national guidelines available for the management of leishmaniasis. It is still posted, along with guidelines for managing other diseases, on the official website of China CDC, indicating that it is still in force today (http://www.chinacdc.cn/n272442/n272530/n272967/n272997/) (127). The six-page document, authored by Qu Jin-qi and Guan Li-ren of the Institute of Parasitic Diseases of the National Diseases Control of China, Shanghai, summarized the standard methodology recommended for diagnosis, treatments of patients, and vector/reservoir controls of all disease types. The bulk of the methodology follows the blueprint previously established for the control programs, but new methodology is also included, e.g., indirect fluorescent-antibody assay (IFA), ELISA, and McAb-AST (as mentioned in Diagnosis section above). Methods for diagnosis, chemotherapy, and vector/reservoir controls are described step by step, with uneven details, in Appendices A, B, and C. Specific values are given in some cases, such as the total dosages of SSG for the standard “six-day therapy” at 120 to 150 mg/kg and 200 to 240 mg/kg for adults and children, respectively. Shortly after the issue of this document, the Ministry of Health decentralized the public health operation by delegating responsibilities for the management of endemic diseases, including leishmaniasis, to the regional CDC. GB 15986-1995 thus continues to serve as the nationwide general guidelines, leaving revisions and updates to the local authorities according to the needs of specific sites of endemicity. There have thus been no new national guidelines other than GB 15986-1995.
KNOWLEDGE GAPS THAT REQUIRE FURTHER STUDY
While the past accomplishment is considerable, our knowledge on all areas of VL in China remains fragmentary, as pointed out in the sections above. Attempts to adopt contemporary approaches in biotechnology to fill in the knowledge gaps have been made but have been insufficient. In parasitology, more attention is necessary to sort out the Leishmania strains and species related to different VL types, which remain unclear. The clinical epidemiology of MT-ZVL and DT-ZVL is of considerable interest but has not been thoroughly studied. For example, none of the vector species has been successfully colonized for laboratory breeding, as is needed for laboratory transmission and other studies. More work on the reservoirs is also clearly needed. While the role of the domestic dog as a reservoir of MT-ZVL is clear, very little is known about the role of wild canids serving in that significant capacity in epidemiology. Infection of the Tarim hare by L. infantum is a significant discovery. There are stringent criteria, which remain unmet, to verify its status as a reservoir of DT-ZVL. It is a challenging endeavor to fulfill these criteria, considering that the Tarim hare is a protected wild animal species, refractory to laboratory maintenance and domestication. Advances are also surprisingly limited in the areas of diagnosis, clinical science, and managements of kala-azar, which still rely largely on dated products, methodology, and information. There is a paucity of information, for example, on asymptomatic infection. The national guidelines for management of leishmaniasis are decades old, and updated versions unavailable on the CDC websites of the provinces where VL is endemic. The persistence of MT-ZVL and DT-ZVL provides no excuse for this lack of attention, as a victim of the success of the control programs. All these gaps present obstacles to effectively integrate the knowledge of Leishmania and leishmaniasis in China and other countries where they are endemic.
CONCLUDING REMARKS
The extremely low incidence of VL today in China is undoubtedly attributable to the control programs that were launched over half a century ago as a national priority at an unprecedented scale of historical proportion (12, 22). The lack of similar success elsewhere underscores the significance of this achievement. The success was made possible by what now appears to be a sustained effort for a considerable number of years to thoroughly implement the otherwise basic elements for any control programs for vector-borne zoonotic diseases. The feasibility of duplicating such a program to control similar diseases elsewhere cannot be assessed by anyone but the authority in the areas of endemicity of concern.
By any measure, the control programs represent a spectacular success in reducing VL to a negligible level today in China. Due to the great success in the control of VL, it is now limited to only some regions in China. As such, VL is understandably no longer considered a priority for allocation of resources. Adequate support is, however, still very necessary, not only to make advances but also to maintain the past success of keeping VL under control. Surveillance of the disease in the areas where VL was formerly endemic is as necessary as new laboratory and field investigation toward better control of VL in the western regions. This is especially necessary considering the need for managing VL cases imported from overseas and the potential spread of VL domestically from areas where it is endemic to those where it is not, resulting from increasing national and international traffic related to economic development. For the same reason, it is very necessary to continue the programs of public health education, modernization of rural health clinics, and regular training of personnel for parasitological skills and knowledge relevant to leishmaniasis under the framework of One Health (128, 129). This approach is particularly suitable domestically for the needs of a country as large as China. It is also needed for the health of its citizens who, in increasing numbers, travel to other countries and stay overseas (130). As a country of increasing significance, there is a further need for China to join the international efforts to assist developing and underdeveloped nations in their struggles with the plight of zoonotic diseases. Leishmaniasis is very widespread in Asia, the Middle East, Africa, and Central and South America, offering fertile grounds for China to project its soft power of expertise in the control of this and other zoonotic diseases.
LITERATURE COLLECTION
References for this review were collected by searching CNKI (http://www.cnki.com.cn), Wanfang Data (http://www.wanfangdata.com.cn/), PubMed, and the ISI Web of Knowledge. Search terms used included “leishmaniasis,” “visceral leishmaniasis,” “neizanglishimanbing” (visceral leishmaniasis), “kala-azar,” and “heirebing” (kala-azar). Relevant articles or book chapters in English and Chinese were also consulted. Epidemiological data were obtained from the articles cited, the Yearbook of Heath Statistics, and reports released by the Ministry of Health of the People's Republic of China and the Chinese Center for Disease Control and Prevention (China CDC). The search of these databases was completed in 2012, when this review was prepared. During the revision of the manuscript, additional citations brought to our attention were added.
ACKNOWLEDGMENTS
Z.-R.L. is supported by research grants from the National Science Foundation of China (31272305 and 31472058) and the National Basic Research Program (973 project, 2010CB530000). K.P.C. is currently supported by U.S. NIH grant AI097830 and other sources.
We thank members of our laboratories and Geoff Hide of the School of Environment and Life Sciences, University of Salford, United Kingdom, for their assistance during the preparation of the manuscript. Special thanks are due to John Keller of the Chicago Medical School, North Chicago, IL, USA, for reviewing the manuscript.
Biographies

Zhao-Rong Lun received his M.Sc. and Ph.D. in zoology/parasitology from Sun Yat-Sen University, Guangzhou, China, and had his postdoctoral training at the Swiss Tropical Institute, Basel, Switzerland, and the University of Toronto, Canada. Dr. Lun has been appointed Research Scientist at the Canadian Food Inspection Agency and Assistant Professor, Lecturer, Associate Professor, and Professor at Sun Yat-Sen University. Dr. Lun is currently Professor and Director of the Center for Parasitic Organisms in the School of Life Sciences, Sun Yat-Sen University. He has been well supported by research grants and has published extensively in international journals. He is most interested in the epidemiology of zoonotic pathogens, the innate immune response of the host to parasitic infection, and drug resistance, gene function, and evolution of parasitic protozoans, mainly animal trypanosomes but also Toxoplasma gondii, Leishmania, and Giardia lamblia.

Ming-Shui Wu received his B.Sc. from South China Agriculture University, Guangzhou, China, and is now a Ph.D. candidate at the Center for Parasitic Organisms, School of Life Sciences, Sun Yat-Sen University, Guangzhou, China. He is interested in the epidemiology, phylogeny, and gene function of parasitic protozoa, mainly the kinetoplastid species, in animal and human infections.

Yun-Fu Chen received his B.Sc. from Sun Yat-Sen University, Guangzhou, China, and is now a Ph.D. candidate at the Center for Parasitic Organisms, School of Life Sciences, Sun Yat-Sen University. He is most interested in the epidemiology and phylogeny of parasitic protozoans, the innate immune reaction of the host to Leishmania infection, and chemotherapy of experimental leishmaniasis.

Jun-Yun Wang has been a member of the National Institute of Parasitic Diseases (NIPD), Chinese Center for Disease Control and Prevention (CCDC), Shanghai, China, since the 1980s. He held the position of Associate Head/Head of the Laboratories of Leishmaniasis, Filariasis, and Echinococcosis. He currently holds the position of Head, Laboratory of Biotechnology, NIPD. Dr. Wang has published his research work extensively, including several recent articles in international journals on the control and diagnosis of visceral leishmaniasis, identification of Leishmania, development of diagnostic products, and epidemiology of visceral leishmaniasis.

Xiao-Nong Zhou received his Ph.D. in biology from Copenhagen University, Denmark, and now is Director of the National Institute of Parasitic Diseases at the Chinese CDC in Shanghai. He works across the fields of ecology, population biology, epidemiology, and malacology. Dr. Zhou chairs the National Expert Advisory Committee on schistosomiasis and other parasitic diseases for China's National Health and Family Planning Commission. He is serving as the Chair for the WHO/WPRO Regional Programme Review Group on Neglected Tropical Diseases (NTD) and as a member of the WHO Science and Technology Advisory Committee on NTD and the WHO Foodborne Burden Epidemiology Reference Group. Since 2000, he has contributed to the Regional Network on Asian Schistosomiasis and Other Important Zoonoses, including service as its former President. He is the editor-in-chief for Infectious Diseases of Poverty (published by BioMed Central) and for the Chinese Journal of Schistosomiasis Control (a Chinese national journal).

Li-Fu Liao received his B.Sc. from the Department of Biology, Xinjiang University, in 1982. Since graduation, he has been Assistant Research Scientist and Research Scientist at the Institute of Endemic Diseases of Xinjiang (now Xinjiang CDC), Urumqi, Xinjiang, China. His work has been focused on the survey of zoonotic diseases and control of harmful animals. He is particularly interested in the reservoir host of Leishmania spp. in Xinjiang and is now working on the isolation and cultivation of Leishmania. He is the holder of patents. He has published >60 articles, and his work is supported by the Natural Science Foundation of China.

Jian-Ping Chen received his M.B. from Sichuan Medical College, China, in 1984 and his M.D. in Molecular Parasitology from West China University of Medical Sciences in 1995. He completed postdoctoral training in molecular microbiology at the National Institute of Infectious Diseases, Chinese Center for Disease Control and Prevention (CCDC), Beijing, in 1997. Dr. Chen thereafter held the position of Assistant/Associate Professor at CCDC and West China Center of Medical Sciences, Sichuan University. He has been Professor of Microbiology and Parasitology in the same institution since 2000. Dr. Chen is interested in molecular and cellular mechanisms of pathogenesis of parasites and bacteria, including Leishmania, Mycobacterium tuberculosis, Legionella pneumophila, Vibrio cholerae, Schistosoma japonicum, and Echinococcus multilocularis. He has published >100 journal articles. His work has been supported by research grants from the Chinese Ministry of Health and Natural Science Foundation.

Larry M. C. Chow received his B.Sc. from the Imperial College London in 1990 and his D.Sc. from the Harvard School of Public Health in 1996. He was Lecturer/Associate Professor and is now Professor at the Department of Applied Biology and Chemical Technology, Hong Kong Polytechnic University, Hong Kong. He is currently also the Associate Head of the department. Dr. Chow has published extensively on the subject of Leishmania drug resistance mechanisms since receiving his doctoral degree, focusing on the role of membrane transporters in mediating drug resistance. Recently, he has developed a novel class of antileishmanial compounds based on synthetic flavonoids. His research has been supported by granting agencies in Hong Kong.

Kwang Poo Chang has been Professor of Microbiology/Immunology, Chicago Medical School/RFUMS, North Chicago, IL, since 1983. He received his B.Sc. in entomology from National Taiwan University, Taipei, in 1965 and his M.Sc./Ph.D. in endosymbiosis in microbiology/cell biology, OAC, Guelph, Canada, in 1972. He had postdoctoral training in parasitology at Rockefeller University, New York, NY, where he remained as Assistant/Associate Professor until 1983. He has published >100 journal articles, edited books, and contributed to an encyclopedia on Leishmania and leishmaniasis. His work has been supported by U.S. NIH and other sources since 1974. He has contributed to the promotion of international collaboration, including research with colleagues in China from the 1980s to the 2000s. He has been the organizer of the Scientific Committee for the quadrennial International Conference of Leishmaniasis since 1997. His current work includes the development of transgenic Leishmania as novel vaccine/vaccine carrier for photodynamic vaccination against leishmaniasis and other disease.
REFERENCES
- 1.Herwaldt BL. 1999. Leishmaniasis Lancet 354:1191–1199. [DOI] [PubMed] [Google Scholar]
- 2.Desjeux P. 2004. Leishmaniasis: current situation and new perspectives. Comp Immunol Microb 27:305–318. doi: 10.1016/j.cimid.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization Expert Committee. 2010. Control of the leishmaniases. Tech Rep Ser 949:104. [PubMed] [Google Scholar]
- 4.Guan LR, Yang YQ, Qu JQ, Ren HY, Chai JJ. 2013. Discovery and study of cutaneous leishmaniasis in Karamay of Xinjiang, West China. Infect Dis Poverty 2:20. doi: 10.1186/2049-9957-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang ZJ, Xiong GH, Guan LR. 2000. Achievement on the epidemiology and control of kala-azar in China. Chin J Epidemiol 21:51–54. (In Chinese.) [PubMed] [Google Scholar]
- 6.Guan LR, Qu JQ, Chai JJ. 2000. Leishmaniasis in China–present status of prevalence and suggestions on its control. Endem Dis Bull 15:49–52. (In Chinese.) [Google Scholar]
- 7.Xiong GH. 1992. Present epidemiological situation of visceral leishmaniasis in China. Endem Dis Bull 7:113–125. (In Chinese.) [Google Scholar]
- 8.Li YF, Zhong WX, Zhao GH, Wang HF. 2011. Prevalence and control of kala-azar in China. J Pathog Biol 6:629–631. (In Chinese.) [Google Scholar]
- 9.Guan LR, Wang J, Hu YD. 1976. Epidemiological patterns of kala-azar in China and their relationship to control program. J Control Res Epidemiol Dis 3:225–232. (In Chinese.) [Google Scholar]
- 10.Guan LR. 1991. Current status of kala-azar and vector control in China. Bull World Health Organ 69:595–601. [PMC free article] [PubMed] [Google Scholar]
- 11.Guan LR, Wu ZX. 2014. Historical experience in the elimination of visceral leishmaniasis in the plain region of Eastern and Central China. Infect Dis Poverty 3:10. doi: 10.1186/2049-9957-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang CT, Wu CC. 1956. Kala-azar. People's Health Publisher, Beijing, China: (In Chinese.) [Google Scholar]
- 13.Wang CT. 1986. Leishmaniasis in China: epidemiology and control program, p 469–478. In Chang KP, Bray RS (ed), Leishmaniasis, part II. Elsevier, Amsterdam, The Netherlands. [Google Scholar]
- 14.Osman Y, Hou YY. 2011. Retrospective analysis of prevalence of visceral leishmaniasis in Xinjiang from 2005 to 2010. Bull Dis Control Prev 26:3–6. (In Chinese.) [Google Scholar]
- 15.Chai JJ, Guan LR. 2006. Leishmaniasis and sand flies in Xinjiang Uyghur Autonomous Region, p 114–165. People's Publisher of Xinjiang, Urumqi, China: (In Chinese.) [Google Scholar]
- 16.Li YM, Guan LR. 1990. Investigations of kala-azar in the mountainous regions of Gansu and Sichuan provinces. Chin J Parasit Dis Control 3:13–16. (In Chinese.) [Google Scholar]
- 17.Xiong GH, Jin CF. 1989. Studies on the longitudinal distribution of sandfly Phlebotomus chinensis and its relation to kala-azar in southern Gansu and northern Sichuan. Endem Dis Bull 4:15–21. (In Chinese.) [Google Scholar]
- 18.Wang JY, Ha Y, Gao CH, Wang Y, Yang YT, Chen HT. 2011. The prevalence of canine Leishmania infantum infection in western China detected by PCR and serological tests. Parasites Vectors 4:69–76. doi: 10.1186/1756-3305-4-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang JY, Chen SB, Gao CH, Jin CF, Feng Y, Zhang CJ, He HX, Yang CM, Yang YT, Bao YF. 2006. Survey on the Leishmania infantum asymptomatic infection in dogs in Wenxian county of Gansu province. Chin J Zoonoses 22:734–737. (In Chinese.) [Google Scholar]
- 20.Chung HL. 1953. A resume of kala-azar work in China. Chin Med J 71:421–464. [PubMed] [Google Scholar]
- 21.Xu CB, Deng ZC, Chen WK, Chung HL, You JY, Liu ZT, Ling Y. 1982. Discovery of naturally infected raccoon dog (Nyctereutes procyonoides Gray), wild animal reservoir host of leishmaniasis in China. Chin Med J 95:329–330. [PubMed] [Google Scholar]
- 22.Guan LR, Yang YQ. 2012. Leishmaniasis, p 161–193. In Tang LW, Xu LQ, Chen YD (ed), Parasitic disease control and research in China, vol 1 Beijing Science and Technology Publishing, Beijing, China: (In Chinese.) [Google Scholar]
- 23.Chai JJ, Zuo XP, Zhang S. 1997. The desert-type kala-azar in Xinjiang, China. Endem Dis Bull 12:27–32. [Google Scholar]
- 24.Guan LR. 1997. Studies on Phlebotomus (Paraphlebotomus) alexandri (Sinton, 1928) in China. Endem Dis Bull 12:109–115. [Google Scholar]
- 25.Liao LF, Yan SS, Wuso B, Wu M, Xu B, Zhang Y, Hou YY, Lei G. 2009. Leishmania infantum firstly isolated from Yarkand hare (Lepus yarkandensis). Chin J Vector Biol Control 20:45–47. (In Chinese.) [Google Scholar]
- 26.Zhao EM, Chai JJ, Li BS, Li J S, Yi MM, Jiao LE. 1985. Epidemic survey of kala-azar in Bachu reclamation area, Xinjiang. Proc Xinjiang Med College. 8:259–262. (In Chinese.) [Google Scholar]
- 27.Hou YY, Zuo XP, Jiang W. 1999. The effects of different doses of Leishmania infantum on the experimental infection of Lagurus lagurus. Endem Dis Bull 14:74–76. (In Chinese.) [Google Scholar]
- 28.Chelbi I, Kaabi B, Derbali M, Ahmed SB, Dellagi K, Zhioua E. 2008. Zooprophylaxis: impact of breeding rabbits around houses on reducing the indoor abundance of Phlebotomus papatasi. Vector Borne Zoonotic Dis 8:741–747. doi: 10.1089/vbz.2007.0265. [DOI] [PubMed] [Google Scholar]
- 29.Molina R, Jiménez MI, Cruz I, Iriso A, Martín-Martín I, Sevillano O, Melero S, Bernal J. 2012. The hare (Lepus granatensis) as potential sylvatic reservoir of Leishmania infantum in Spain. Vet Parasitol 190:268–271. doi: 10.1016/j.vetpar.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Wang JY, Qu JQ, Wang RQ, Guan LR, Ren HY, Chang KP. 1996. Analysis on homology in several isolates of Leishmania from Karamay, Xinjiang. Chin J Parasitol Parasit Dis 14:266–269. (In Chinese.) [Google Scholar]
- 31.Guan LR, Xu YX, Zuo XP, Wang G. 1996. Study on the vector transmitting cutaneous leishmaniasis in Karamay, Xinjiang Uygur Autonomous Region of China. Endem Dis Bull 11:38–41. (In Chinese.) [Google Scholar]
- 32.Guan LR, Yang YQ, Qu JQ, Shen WX. 1995. Discovery and study of Leishmania turanica for the first time in China. Bull World Health Organ 73:667–672. [PMC free article] [PubMed] [Google Scholar]
- 33.Guan LR, Yang YQ, Xu YX, Wu JT. 1992. Leishmaniasis in Karamay. XI. The development of cutaneous leishmaniasis in monkey and man experimentally infected with Leishmania from Karamay great gerbil. Chin J Parasitol Parasit Dis 10:263–267. (In Chinese.) [PubMed] [Google Scholar]
- 34.Guan LR, Xu YX, Yang YQ, Zuo XP, Wang G, Chai JJ, Qu JQ, Bao YF, Wu JT, Yang YT. 1992. Leishmaniasis in Karamay. IX. Identification of Leishmania parasite isolates from naturally infected sand flies. Endem Dis Bull 7:9–16. (In Chinese.) [Google Scholar]
- 35.Wang J, Xiong GH, Guan LR, Qu JQ. 1985. Studies on Leishmania gerbilli in China. Chin J Parasitol Parasit Dis 3:133–135. (In Chinese.) [PubMed] [Google Scholar]
- 36.Guan LR, Xu YX, Zuo XP, Zhang S, Chai JJ. 1994. Studies on the living environment of great gerbil and its natural infection of Leishmania and sand fly vectors in north Xinjiang, China. Endem Dis Bull 9:7–10. (In Chinese.) [Google Scholar]
- 37.Xu CB, Le Blancq S, Evans DA, Peters W. 1984. The characterization by isoenzyme electrophoresis of Leishmania isolated in the People's Republic of China. Trans R Soc Trop Med Hyg 78:689–693. doi: 10.1016/0035-9203(84)90243-8. [DOI] [PubMed] [Google Scholar]
- 38.Lu HG, Zhong L, Guan LR, Qu J Q, Hu XS, Chai JJ, Xu ZB, Wang CT, Chang KP. 1994. Separation of Chinese Leishmania isolates into five genotypes by kinetoplast and chromosomal DNA heterogeneity. Am J Trop Med Hyg 50:763–770. [DOI] [PubMed] [Google Scholar]
- 39.Ma Y, Bu L, Hua X. 2011. 20-year search on molecular markers of Leishmania isolates from different Kala-azar foci in China to confirm whether genetic fingerprints of Kala-azar pathogens correlate with disease types. J Biomed Eng 28:997–1000. (In Chinese.) [PubMed] [Google Scholar]
- 40.Yang BB, Guo XG, Hu XS, Zhang JG, Liao L, Chen DL, Chen JP. 2010. Species discrimination and phylogenetic inference of 17 Chinese Leishmania isolates based on internal transcribed spacer 1 (ITS1) sequences. Parasitol Res 107:1049–1065. doi: 10.1007/s00436-010-1969-9. [DOI] [PubMed] [Google Scholar]
- 41.Yang BB, Chen DL, Chen JP, Liao L, Hu XS, Xu JN. 2013. Analysis of kinetoplast cytochrome b gene of 16 Leishmania isolates from different foci of China: different species of Leishmania in China and their phylogenetic inference. Parasites Vectors 6:32. doi: 10.1186/1756-3305-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Waki K, Dutta S, Ray D, Kolli BK, Akman L, Kawazu S, Lin CP, Chang KP. 2007. Transmembrane molecules for phylogenetic analyses of pathogenic protists: Leishmania-specific informative sites in hydrophilic loops of trans-endoplasmic reticulum N-acetylglucosamine-1-phosphate transferase. Eukaryot Cell 6:198–210. doi: 10.1128/EC.00282-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang CY, Lu XJ, Du XQ, Jian J, Shu L, Ma Y. 2013. Phylogenetic and evolutionary analysis of Chinese Leishmania isolates based on multilocus sequence typing. PLoS One 8:e63124. doi: 10.1371/journal.pone.0063124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gu DA, Jin CF, Zhang Y. 2006. Present situation and perspective of leishmaniasis and its vector sand fly control. Int J Med Parasit Dis 33:236–238. (In Chinese.) [Google Scholar]
- 45.Guan LR. 2013. Prospect on the investigation of sand flies (Diptera: Psychodidae) in China. Chin J Parasitol Parasit Dis 31:310–314. (In Chinese.) [PubMed] [Google Scholar]
- 46.Feng LC, Chung HL. 1939. The development of Leishmania in Chinese sand flies fed on dogs with canine leishmaniasis. Chin Med J 56:35–46. (In Chinese.) [Google Scholar]
- 47.Patton WS, Hindle E. 1927. The development of Chinese Leishmania in Phlebotomus major var. chinensis and P sergenti var. Proc R Soc Ser B 101:390. [Google Scholar]
- 48.He KZ, Wang Z, Huang CM. 1959. Compilation of data from investigations of kala-azar. Shanghai Scientific and Technical Publishers, Shanghai, China: (In Chinese.) [Google Scholar]
- 49.Chung HL, Feng LC. 1939. Natural infection of Phlebotomus chinensis in Peiping with Leishmania flagellates. Chin Med J 56:47–51. (In Chinese.) [Google Scholar]
- 50.Sun CT, Yao YT, Chu HJ, Wu CC. 1936. Natural infection of Phlebotomus chinensis with flagellates morphologically indistinguishable from those of L. donovani. Chin Med J 50:911 (In Chinese.) [Google Scholar]
- 51.Sun CT, Wu CC. 1937. Notes on the study of kala-azar transmission. II. Further observations on the natural infection of Phlebotomus chinensis with Leptomonas donovani. Chin Med J 52:665 (In Chinese.) [Google Scholar]
- 52.Feng LC, Chung HL. 1941. Experiment on the transmission of kala-azar from dogs to hamsters by Chinese sand flies. Chin Med J 60:489–496. (In Chinese.) [Google Scholar]
- 53.Chung HL, Feng LC, Feng SL. 1951. Observations concerning the successful transmission of kala-azar in North China by the bite of naturally infected P. chinensis. Peking Nat Hist Bull 19(2–3): 301–326. (In Chinese.) [Google Scholar]
- 54.Leng YJ. 1988. A review of phlebotomine sand flies and their transmission of leishmaniasis in China. Jpn J Sanit Zool 39:323–337. [Google Scholar]
- 55.Zhang LM, Leng YJ. 1997. Eighty-year research of phlebotomine sand flies (Diptera: Psychodidae) in China (1915-1995). II. Phlebotomine vectors of leishmaniasis in China. Parasite 4:299–306. [DOI] [PubMed] [Google Scholar]
- 56.Leng YJ, Zhang LM. 1993. Check list and geographical distribution of phlebotomine sand flies in China. Ann Trop Med Parasitol 87:83–94. [DOI] [PubMed] [Google Scholar]
- 57.Jin CF, He SQ, Hong YM, Li GR. 2004. The effect of sand fly control on the transmission of visceral leishmaniasis. Chin J Parasitol Parasit Dis 22:338–339. (In Chinese.) [PubMed] [Google Scholar]
- 58.Xiong GH, Jin CF. 2003. The impact of sand flies and leishmaniasis research on the development of western China. Chin J Parasitol Parasit Dis 21:119–122. (In Chinese.) [PubMed] [Google Scholar]
- 59.Guan LR, Xu YX, Li BS, Dong JA. 1985. The role of Phlebotomus alexandri in the transmission of kala-azar. Chin J Parasitol Parasit Dis 3:85–88. (In Chinese.) [PubMed] [Google Scholar]
- 60.Guan LR, Xu YX, Mao YD, Wang W. 1986. The sand fly fauna and its role in transmission of kala-azar in four landscape zones of the Aksu region, Xinjiang. Chin J Parasitol Parasit Dis 4:169–172. (In Chinese.) [PubMed] [Google Scholar]
- 61.Leng YJ. 1987. A preliminary survey of phlebotomine sand flies in limestone caves of Sichuan and Guizhou Provinces, southwest China, and description and discussion of a primitive new genus Chinius. Ann Trop Med Parasitol 81:311–317. [DOI] [PubMed] [Google Scholar]
- 62.Wei F, Shang L, Jin H, Lian H, Liu W, Gao H, Liu Q. 2011. Molecular detection and genetic diversity of Leishmania donovani in naturally infected Phlebotomus chinensis from southwestern China. Vector Borne Zoonotic Dis 11:849–852. doi: 10.1089/vbz.2010.0148. [DOI] [PubMed] [Google Scholar]
- 63.Gu DA, Zhang Y, Lan QX. 2012. Analysis of polymorphism on ribosomal internal transcribed spacer 2 in four species of sand flies with PCR-RFLP. Chin J Parasitol Parasit Dis 30:100–108. (In Chinese.) [PubMed] [Google Scholar]
- 64.Zhang L, Ma Y, Xu J. 2013. Genetic differentiation between sandfly populations of Phlebotomus chinensis and Phlebotomus sichuanensis (Diptera: Psychodidae) in China inferred by microsatellites. Parasites Vectors 6:115. doi: 10.1186/1756-3305-6-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cochran S. 1914. Distribution of kala-azar in China and Korea. China Med J 28:274–276. [Google Scholar]
- 66.Young CW. 1923. Kala-azar in China. China Med J 37:797–822. [Google Scholar]
- 67.Chinese Medical Association. 1958. Studies on kala-azar in new China. Scientific Medical Publishing House, Beijing, China: (In Chinese.) [Google Scholar]
- 68.Anonymous. 1985–2002. Yearbook of public health in the People's Republic of China. People's Medical Publishing House, Beijing, China. (In Chinese.) [Google Scholar]
- 69.Anonymous. 2002–2011. National epidemic situation of notifiable diseases. Ministry of Health of the People's Republic of China, Beijing, China: (In Chinese.) [Google Scholar]
- 70.Zheng CJ, Wang LY, Xu X, Zhu XH, Wu WP. 2009. Visceral leishmaniasis in China during 2004-2007. Chin J Parasitol Parasit Dis 27:344–346. (In Chinese.) [PubMed] [Google Scholar]
- 71.Li F, Chen SB, Feng Y, Yang CM. 2009. Analysis of prevalence factors of kala-azar in Gansu province during 2005 to 2007. Endem Dis Bull 24:37–38. (In Chinese.) [Google Scholar]
- 72.Zhang FN, Zhang LP, Zhang JG, Hu YJ. 2011. Epidemic trends and prevention and control of leishmaniasis in Sichuan province after the earthquake. Parasit Infect Dis 9:59–63. (In Chinese.) [Google Scholar]
- 73.Zhang FN. 2006. The present epidemic situation and control of kala-azar in Sichuan province. Parasit Infect Dis 4:126–128. (In Chinese.) [Google Scholar]
- 74.Wang JY, Cui G, Chen HT, Zhou XN, Gao CH, Yang YT. 2012. Current epidemiological profile and features of visceral leishmaniasis in People's Republic of China. Parasites Vectors 5:31–41. doi: 10.1186/1756-3305-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guan LR. 2009. Present situation of visceral leishmaniasis and prospect for its control in China. Chin J Parasitol Parasit Dis 27:394–397. (In Chinese.) [PubMed] [Google Scholar]
- 76.Osman Y, Zhang CW, Keyoumu K, Zhang S, Jumahun R, Wayit S, Wu WP. 2007. Investigation on the kala-azar outbreak in a new epidemic place in Shache County, Xinjiang. Endem Dis Bull 22:27–29. (In Chinese.) [Google Scholar]
- 77.Wang JY, Gao CH, Yang YT, Chen HT, Zhu XH, Lv S, Chen SB, Tong SX, Steinmann P, Ziegelbauer K, Zhou XN. 2010. An outbreak of the desert sub-type of zoonotic visceral leishmaniasis in Jiashi, Xinjiang Uygur Autonomous Region, People's Republic of China. Parasitol Int 59:331–337. doi: 10.1016/j.parint.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 78.Guan LR, Chai JJ, Zuo XP. 1999. Progress of biological study on sandflies in Xinjiang Uygur Autonomous Region, China. End. Dis Bull 14:87–91. (In Chinese.) [Google Scholar]
- 79.Su GH, Li XP. 2010. Analysis of the cause for the misdiagnosis of 31 VL cases. Clin Misdiagn Misther 23:959–960. (In Chinese.) [Google Scholar]
- 80.Fu Q, Li SZ, Wu WP, Hou YY, Zhang S, Feng Y, Zhang LP, Tang LH. 2013. Endemic characteristics of infantile visceral leishmaniasis in the People's Republic of China. Parasites Vectors 6:143–150. doi: 10.1186/1756-3305-6-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Qu JQ, Zhong L, Masoom-Yasinza M, Abdur-Rab M, Aksu HS, Reed SG, Chang KP, Gilman-Sachs A. 1994. Serodiagnosis of Asian leishmaniasis with a recombinant antigen from the repetitive domain of a Leishmania kinesin. Trans R Soc Trop Med Hyg 88:543–545. doi: 10.1016/0035-9203(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 82.Qu JQ, Guan LR, Imamu S, Zuo XP, Chai JJ, Chen SB, Shinichiro K, Katakura K, Matsumoto Y, Reed SG. 2000. Rapid screening with a recombinant antigen (rK39) for diagnosis of visceral leishmaniasis using dipstick. Chin J Parasitol Parasit Dis 18:155–158. (In Chinese.) [PubMed] [Google Scholar]
- 83.Chen SB, Li F, Chen XW, He J P, Guan LR. 2000. Application of rK39 immunochromatographic assay strip for human and canine visceral leishmaniasis diagnosis in Gansu province. Endem Dis Bull 15:91–92. (In Chinese.) [Google Scholar]
- 84.Zhang FN, Xiao N, Chen YL. 2008. Assessment of results of detection of leishmaniasis patients with rk39 dipstick. Chin Trop Med 18:710–711. (In Chinese.) [Google Scholar]
- 85.Li GR, Duan M, Jiang CD, Li L, Li Y, Zhang LZ, Lan YH, Zhang FN. 2005. Recombinant antigen (rK39) strip for kala-azar diagnosis. Parasit Infect Dis 3:14–15. (In Chinese.) [Google Scholar]
- 86.Zuo XP, Yimam S, Kaiser K, Zhang S, Jiao W, Qu JQ, Chang KP, Chai JJ. 2007. Application of rK39 dipstick on diagnosis and epidemiological survey of kala-azar in Xinjiang, China. J Trop Dis Parasitol 5:19–22. (In Chinese.) [Google Scholar]
- 87.Lei G, Xu EJ, Lv TY, Xu YM, Chang KP, Rena T, Dou HP, Kaiser K, Xu BC, Liao LF. 2010. Study on the use of a double-antigen sandwich enzyme-linked immunosorbent assay (DAgS-ELISA) to detect antibodies to visceral leishmaniasis. J Pathog Biol 5:743–745. (In Chinese.) [Google Scholar]
- 88.Qu JQ, Bao YF, Xu YX, Jia JX, Liu PZ, Chen SB, Wang KQ. 1987. Studies on the dot enzyme-linked immunosorbent assay (dot-ELISA) for diagnosis of kala-azar. Endem Dis Bull 2:66–68. (In Chinese.) [Google Scholar]
- 89.Wang JY, Feng Y, Gao CH, Jin CF, Chen SB, Zhang CJ, He JP, Yang CM, Yang YT, Bao YF. 2007. Asymptomatic Leishmania infection in human population of Wenxian County, Gansu province. Chin J Parasitol Parasit Dis 25:62–64. (In Chinese.) [PubMed] [Google Scholar]
- 90.Qu JQ, Xu YX, Bao YF, Yang YT, Wang JY. 1991. Application of monoclonal antibodies against Leishmania donovani. II. Detection of circulating antigen in sera of visceral leishmaniasis before and after treatment. Chin J Parasitol Parasit Dis 9:21–23. (In Chinese.) [PubMed] [Google Scholar]
- 91.Hu XS, Lin FQ, Liu Q, Kan B, Liu PN, Wang ZL. 1989. Further study on the application of McAb-AST to the detection of antigens in serum for diagnosis of kala-azar. Chin J Parasit Dis Control 2:69–74. (In Chinese.) [Google Scholar]
- 92.Katakura K, Kawazu S, Naya T, Nagakura K, Ito M, Aikawa M, Qu J Q, Guan LR, Zuo XP, Chai JJ, Chang KP, Matsumoto Y. 1998. Diagnosis of kala-azar by nested PCR based on amplification of the Leishmania mini-exon gene. J Clin Microbiol 36:2173–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hu XS, Yang WT, Lu HG, Yan HP, Chen JP, Ma Y, Jin BQ, Zhang T. 2000. Sequencing a specific kinetoplast DNA fragment of Leishmania donovani for PCR amplification in diagnosis of leishmaniasis in bone marrow and blood samples. J Parasitol 86:822–826. doi: 10.1645/0022-3395(2000)086[0822:SASKDF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 94.Qu JH, Wang HF, Zhao GH, Zhong WX, Kasaier K, Dou HP, Cui Y, Li J, Kong FH. 2010. Survey of Leishmania donovani infection in humans in Kashi city of Xinjiang Uygur Autonomous Region. Chin Trop Med 10:1324–1327. (In Chinese.) [Google Scholar]
- 95.Gao CH, Wang JY, Yang YT, Bao YF. 2006. Study on PCR method for detecting the asymptomatic Infection of Leishmania infantum. Chin J Parasitol Parasit Dis 24:92–96. (In Chinese.) [PubMed] [Google Scholar]
- 96.Lu DM, Hu XS, Qiao ZD. 2001. Analysis of Leishmania species and strains from China by RAPD technique. Chin J Parasitol Parasit Dis 19:290–293. (In Chinese.) [PubMed] [Google Scholar]
- 97.Yao ZQ. 2001. Clinical analysis of 73 kala-azar cases in Longnan area. Endem Dis Bull 16:67–68. (In Chinese.) [Google Scholar]
- 98.Zhang FN, Zhang LP. 2010. Analysis on recurrence and death of leishmaniasis cases in Sichuan province. J Prev Med Infect 26:961–965. (In Chinese.) [Google Scholar]
- 99.Wang CT, Wu CC. 1958. Review of investigation and research on kala-azar of the People's Republic of China. Science and Technology Press, Beijing, China: (In Chinese.) [Google Scholar]
- 100.Chen SB, Yang CM, Zhang CJ, Du GB. 2007. Case report: one kala-azar case resistant to antimony cured by domestic amphotericin B. Chin J Parasitol Parasit Dis 25:cover 2 (In Chinese.) [PubMed] [Google Scholar]
- 101.Yuan QR. 2010. Case report: two kala-azar cases resistant to antimony cured by domestic amphotericin B. Chin J Parasitol Parasit Dis 28:209–210. (In Chinese.) [Google Scholar]
- 102.Liu XL, He SQ. 2007. Case report: two kala-azar cases cured by combination of sodium stibogluconate and amphotericin B. Endem Dis Bull 22:113 (In Chinese.) [Google Scholar]
- 103.Wong IL, Chan KF, Burkett BA, Zhao Y, Chai Y, Sun H, Chan TH, Chow LM. 2007. Flavonoid dimers as bivalent modulators for pentamidine and sodium stiboglucanate resistance in Leishmania. Antimicrob Agents Chemother 51:930–940. doi: 10.1128/AAC.00998-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wong IL, Chan KF, Zhao Y, Chan TH, Chow LM. 2009. Quinacrine and a novel apigenin dimer can synergistically increase the pentamidine susceptibility of the protozoan parasite Leishmania. J Antimicrob Agents Chemother 63:1179–1190. doi: 10.1093/jac/dkp130. [DOI] [PubMed] [Google Scholar]
- 105.Wong IL, Chan KF, Chan TH, Chow LM. 2012. Flavonoid dimers as novel, potent antileishmanial agents. J Med Chem 55:8891–8902. doi: 10.1021/jm301172v. [DOI] [PubMed] [Google Scholar]
- 106.Wong IL, Chan KF, Chen YF, Lun ZR, Chan TH, Chow LM. 2014. In vitro and in vivo efficacy of novel flavonoid dimers against cutaneous leishmaniasis. Antimicrob Agents Chemother 58:3379–3388. doi: 10.1128/AAC.02425-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wu CC, Wang CT, He KZ, Wang J. 1955. Observations on the bionomics of sand flies (genus Phlebotomus) in East China from 1951 to 1953. Acta Entomol Sinica 5:393–414. (In Chinese.) [Google Scholar]
- 108.Ministry of Health of the People's Republic of China. 1957. Handbook for the control and prevention of kala-azar. People's Health Publisher, Beijing, China: (In Chinese.) [Google Scholar]
- 109.Wang CT. 1959. Major achievements in prevention and control of kala-azar in Shandong. Shandong Med J 10:8–13. [Google Scholar]
- 110.Shan QF, Wang TC. 1989. Surveillance of kala-azar for 30 years after basic elimination of the disease in Shandong province. J Pathog Biol. 2(Suppl 1):1–4. [Google Scholar]
- 111.Wang CT, Bao Y, Shao QF, Gao JT. 1991. Immunological evaluation of kala-azar control measures in Shandong province. Chin J Parasit Dis Control 2(4):81–85. [Google Scholar]
- 112.Osman Y, Tong ST, Zhang Y, Li X, Zuo XP, Sultan Y, Zhang S, Keyoumu K, Gu DA, Lan QX. 2008. Endemic situation of visceral leishmaniasis at Kashi of Xinjiang Uygur Autonomous Region during 1996 to 2007. J Pathog Biol 3:940–942. (In Chinese.) [Google Scholar]
- 113.Zhang FN, Kou MJ, Ji JJ, Deng L, Zhang N. 2008. Long-term effect on leishmaniasis control by large-scale killing and complete prohibition on keeping dogs in endemic areas. J Pathog Biol 3:720–721. (In Chinese.) [Google Scholar]
- 114.Ji JJ, Li GR. 2005. The prevalence of VL and its control in Lixian county of Sichuan province. Parasit Infect Dis 3:44–45. (In Chinese.) [Google Scholar]
- 115.Zhang FN, Li GR, Lei Y, Chen YL. 2007. Analysis on leishmaniasis in Sichuan province from 1984 to 2005. J Pathog Biol 2:79–80. (In Chinese.) [Google Scholar]
- 116.Chen SB. 1996. Current status of visceral leishmaniasis (kala-azar) in Gansu province. Endem Dis Bull 11:114–116. (In Chinese.) [Google Scholar]
- 117.Zhang FN, Xiao N, Chen YL. 2009. Prevalent trends and control strategy of leishmaniasis in Sichuan province in 2004-2008. Chin. Trop Med 9:1836–1838. (In Chinese.) [Google Scholar]
- 118.Xiong GH, Guan LR, Guo YK. 1974. Studies on vectors of transmission of kala-azar in Xinjiang, China. Prophylactico-Ther Res Epidem 4:327–434. (In Chinese.) [Google Scholar]
- 119.Zhao EM, Chai JJ, Li BS, Li JS, Yi MM, Jiao LE. 1985. Epidemiologic survey of kala-azar in Bachu reclamation area of Xinjiang. J Xinjiang Med Col 8(3):259–262. (In Chinese.) [Google Scholar]
- 120.Chen SB, Li F, He JP, Chen XW, Wang DG, Wei LS, Yang HP, Guan LR. 2001. Experimental study on prevention of dog-sand fly contact by deltamethrin collar. Endem Dis Bull 16:17–20. (In Chinese.) [Google Scholar]
- 121.Xiong GH, Jin CF, Cheng XZZWSU, Hong YM. 1994. Deltamethrin bath of domestic dog in the prevention of sand fly bite. Endem Dis Bull 9:32–34. (In Chinese.) [Google Scholar]
- 122.Xiong GH, Jin CF, Hong YM, Su ZW, Xu PZ, Xie WK, Zhang AZ, Li GR, Gao B. 1995. Studies on the deltamethrin-medicated bath of domestic dogs for interrupting visceral leishmaniasis transmission. Chin J Parasitol Parasit Dis 13:178–181. (In Chinese.) [PubMed] [Google Scholar]
- 123.Wu YX, Li GR, Gao B. 1994. Exploration about epidemiological factors of visceral leishmaniasis in Sichuan province. Endem Dis Bull 9(2):55–57. (In Chinese.) [Google Scholar]
- 124.Sun K, Guan W, Zhang JG, Wang YJ, Tian Y, Liao L, Yang BB, Chen DL, Chen JP. 2012. Prevalence of canine leishmaniasis in Beichuan County, Sichuan, China and phylogenetic evidence for an undescribed Leishmania sp. in China based on 7SL RNA. Parasites Vectors 5:75. doi: 10.1186/1756-3305-5-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jin CF, Xiong GH. 1991. Evaluation of deltamethrin and DDT in the control of periwild sand fly Phlebotomus chinensis. Endem Dis Bull 6:113–116. (In Chinese.) [Google Scholar]
- 126.Xiong GH, Jin CF. 1987. Studies on deltamethrin in the control of periwild Phlebotomus chinensis. Chin J Parasitol Parasit Dis 5:176–179. (In Chinese.) [PubMed] [Google Scholar]
- 127.Qu JQ, Guan LR. 1995. Diagnostic criteria and principles of management of kala-azar. GB 15986-1995. Standardization Administration of China, Beijing, China: (In Chinese.) [Google Scholar]
- 128.Zhou XN. 2012. Prioritizing research for “One Health—One World.” Infect Dis Poverty 1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gibbs EP. 2014. The evolution of One Health: a decade of progress and challenges for the future. Vet Rec 174:85–91. doi: 10.1136/vr.g143. [DOI] [PubMed] [Google Scholar]
- 130.Zhou XN, Bergquist R, Tanner M. 2013. Elimination of tropical disease through surveillance and response. Infect Dis Poverty 2:1. doi: 10.1186/2049-9957-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]