Although conventional thinking suggests patients with cancer might be less willing to share their health information, the authors found that participants with cancer were more willing to share their inherited genetic information.
Abstract
Purpose:
Growing use of electronic health information increases opportunities to build population cancer databases for research and care delivery. Understanding patient views on reuse of health information is essential to shape privacy policies and build trust in these initiatives.
Methods:
We randomly assigned nationally representative participants (N = 3,336) with and without prior cancer to six of 18 scenarios describing different uses of electronic health information. The scenarios varied the user, use, and sensitivity of the information. Participants rated each scenario on a scale of 1 to 10 assessing their willingness to share their electronic health information. We used conjoint analysis to measure the relative importance of each attribute (ie, use, user, and sensitivity).
Results:
Participants with and without a prior diagnosis of cancer had a similar willingness to share health information (0.27; P = .42). Both cancer and noncancer participants rated the purpose of information use as the most important factor (importance weights, 67.1% and 45.6%, respectively). For cancer participants, the sensitivity of the information was more important (importance weights, 29.8% v 1.2%). However, cancer participants were more willing to share their health information when the information included more sensitive genetic information (0.48; P = .015). Cancer and noncancer respondents rated uses and users similarly.
Conclusion:
The information sharing preferences of participants with and without a prior diagnosis of cancer were driven mainly by the purpose of information reuse. Although conventional thinking suggests patients with cancer might be less willing to share their health information, we found participants with cancer were more willing to share their inherited genetic information.
Introduction
As electronic sources of health information become more prevalent,1 so does the opportunity to create large, detailed population-based health databases. These data create unique opportunities to advance population health through research (eg, comparative-effectiveness studies), quality improvement (eg, measurement of physician performance), and public health activities (eg, disease surveillance).2–4 Enthusiasm for the concept of rapid learning health systems is substantial within the oncology community, driven by the desire to optimize clinical practice and advance research in a field with a particularly complex evidence base and heterogeneous disease.5 The American Society of Clinical Oncology aims to launch CancerLinQ,6 which will collect data from a wide range of sources, including patient records, to support clinical cancer care and conduct cancer research. Commercial ventures like Flatiron7 are also beginning their efforts in cancer care because of the particular promise of big data in this context.
Understanding how patients view secondary uses of electronic health information is essential for developing trusted approaches to these big data initiatives.8–12 Several high-profile marketing cases involving national retailers13 and national security measures by the US government14 have heightened public sensitivity about electronic privacy in other settings.
Patients with a history of cancer, or specific risk factors, may have unique concerns about reuse of their health information; for example, they may fear that broader knowledge of their diagnoses or risks will lead to discrimination or economic harm.15 Prior studies examining whether individuals with worse health status are more reluctant to share their health information for research have produced mixed results.16,17 We are not aware of any studies that have examined information reuse preferences among individuals with a history of cancer. Patients with a history of cancer may also have additional concerns about the confidentiality of genetic test results, because the information may have meaning for others and because they might question whether genetic information is truly deidentifiable.18 These concerns have contributed to the long tradition within bioethics of treating genetic information as uniquely sensitive.19
In prior work,20 we used conjoint analysis in a nationally representative sample of Americans and found that the sensitivity of health information was, surprisingly, the least important factor in individuals' willingness to share their electronic health information compared with the purpose of using their data and who was using it. In this study, we extended our prior work to determine if individuals with a history of cancer are less willing to share their health information and weigh the sensitivity of health information more (and are particularly less inclined to share inherited genetic information) compared with individuals without a history of cancer. By obtaining information on the cancer history of the survey participants from our original study, we were able to compare these two populations.
Methods
Study Participants
We recruited study participants through an online research panel assembled and administered by GfK Knowledge Networks (Nuremburg, Germany). The details of the survey design and administration have been previously described.20 In brief, GfK Knowledge Networks uses a combination of probability-based address sampling and random-digit dialing to create a nationally representative panel with coverage of 97% of US households.21 Panelists are invited to participate in four to six Internet-based surveys per month and on average complete three per month. In exchange for participating, panelists receive financial compensation or Internet access and computer hardware if they do not already have it. In our study, we oversampled Latinos and African Americans as part of a larger study intended to study racial and ethnic differences in information-sharing preferences. We administered the survey from November 9, 2012, through December 2, 2012. We excluded speeders from the final study sample; these are individuals who completed the survey in less than half the median completion time (< 5 minutes) and therefore were unlikely to have read the questions before responding. In pretesting, speeders tended to have no variation in their responses (ie, they selected the same numeric answer to all questions). The institutional review board at the University of Pennsylvania (Philadelphia, PA) approved the study protocol.
We obtained information about whether study participants had a personal history of cancer (excluding skin cancer) from GfK Knowledge Networks. GfK Knowledge Networks routinely collects information on panelists, including the presence of certain health conditions. Specifically, panelists are asked: “Have you been diagnosed with any of the following conditions?” Panelists are then asked to respond yes or no to a series of options, including cancer. GfK Knowledge Networks does not collect information on the specific type of cancer or date of diagnosis.
Experimental Instrument
We administered an online survey with an embedded conjoint experiment. Conjoint experiments are frequently used in marketing to identify how individuals value individual attributes and characteristics of products. It is an experimental method in which consumers are asked to rate or choose from different combinations of attributes and can reveal consumers' preferences and the relative importance of individual product attributes.22–24 The conjoint design in our study focused on secondary uses of electronic health information for cancer prevention activities. We compared three attributes describing secondary uses of electronic health information: user (who would be using your health information), use (the purpose of use), and sensitivity of the information. In our conjoint experiment, we investigated three possible users: university hospitals, public health departments, and drug companies. In addition, we tested three possible uses: research (“research new ways to prevent cancer”), quality improvement (“evaluate how well your doctor provides preventive cancer care”), and marketing (“identify what kinds of patients will be interested in buying their cancer prevention product”). Finally, we assessed two separate levels of information sensitivity: lower (“your medical history”) and higher (“your medical history and the results of a personal genetic test that predicts your chance of getting cancer”).
We presented the following introduction to study participants before the conjoint experiment:
“Many doctors and hospitals are starting to use electronic medical records instead of paper charts when they provide care. Electronic medical records can also be used for other health care and public health reasons. You will be shown some possible uses of your electronic health information. In each case, you will be shown what information will be used, who will use it, and what they will use it for. Please indicate how willing you would be to share your health information for each situation. Your name would not be released.”
Our 3 × 3 × 2 conjoint design yielded 18 possible scenarios. Each participant was randomly assigned to receive six of the 18 possible scenarios, and for each combination, the participant was asked to rate his or her willingness to share personal health information on a 1-to-10 scale (1 = not at all; 10 = very willing).
The survey instrument also measured health status (single item from the Behavioral Risk Factor Surveillance System 2010 questionnaire [short form 1]),25 health care access (insurance status, usual source of care, and financial barriers to care), and health care distrust.26 GfK Knowledge Networks provided us with previously collected demographic information (age, sex, race, ethnicity, income, educational attainment, and metropolitan or rural residential status) on all study participants. Finally, using a question adapted from the General Social Survey,27 we asked each participant to rate his or her confidence in various public and private organizations to protect personal health information; respondents chose from three categories: hardly any confidence at all, only some confidence, and very high confidence.
Statistical Analysis
We compared participants with and without a history of cancer using descriptive statistics (frequencies and proportions) and χ2 tests. We conducted a conjoint analysis based on a main-effects analysis-of-variance model. To compare the relative importance of the three attributes (ie, use, user, and sensitivity) between those with and without a cancer history, we first estimated a part-worth utility value for each level of each attribute. We considered the attributes with the largest part-worth utility range to be the most important in explaining the variability in outcome. The differences in importance weights between the comparison groups were assessed by including all pairwise cancer status × attribute interaction terms in the linear regression models. Models also included the three test attributes, patient race/ethnicity, a measure of high health care distrust (top quartile), health status, measures of access to care, and other sociodemographic variables. We specified a baseline scenario in the models where we hypothesized support would be the highest (ie, sensitivity, low; user, university hospital; use, research).
We used generalized estimating equations with a working independence correlation structure and robust (Huber-White) variance estimation28 to account for the correlation of vignettes nested within respondent induced by the study design. To account for survey nonresponse and the planned oversampling scheme, we used post-stratification weights provided by GfK Knowledge Networks. The weights in our analysis are calculated based on the differential probability of inclusion in the online panel as a result of oversampling and undersampling of certain populations (compared with an equal probability sample) and also planned oversampling and nonresponse among those sampled for this study from the online panel. There were few to no missing data (< 0.5% on all covariates); hence, analyses were conducted on observed data only. We used Wald tests to compare parameters. All tests were two sided, with a type I error rate of 0.05. We conducted all analyses using SAS software (version 9.3; SAS Institute, Cary, NC).
Results
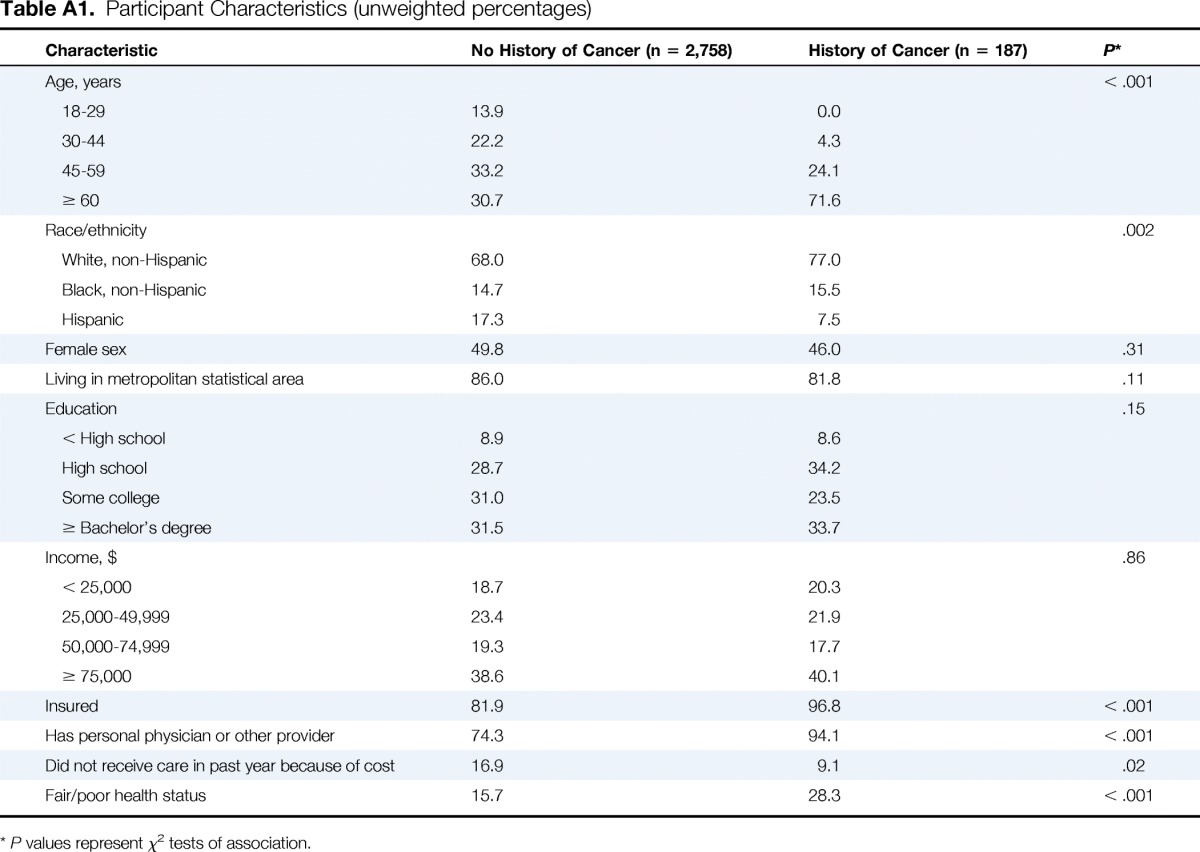
We received responses from 3,336 of 5,119 invited panel members, reflecting a response rate of 65.2%. We excluded 272 speeders and 119 individuals for whom data were missing on whether they had a personal history of cancer, resulting in a final sample size of 2,945. The characteristics of the study population are summarized in Appendix Table A1 (online only). Of the 2,945 individuals in this sample, 187 (6.3%) had a personal history of cancer (ie, cancer group), which is similar to national estimates among adults (6.2%).29 Those with a history of cancer were more likely to be older and white, have worse health status, and report better access to care (ie, lower rates of uninsurance, more likely to have a usual source of care, and less likely to report cost barriers). Nonresponders were more likely to be female and have lower income and less education. We applied post-stratification weights to our analyses to account for nonresponse bias.
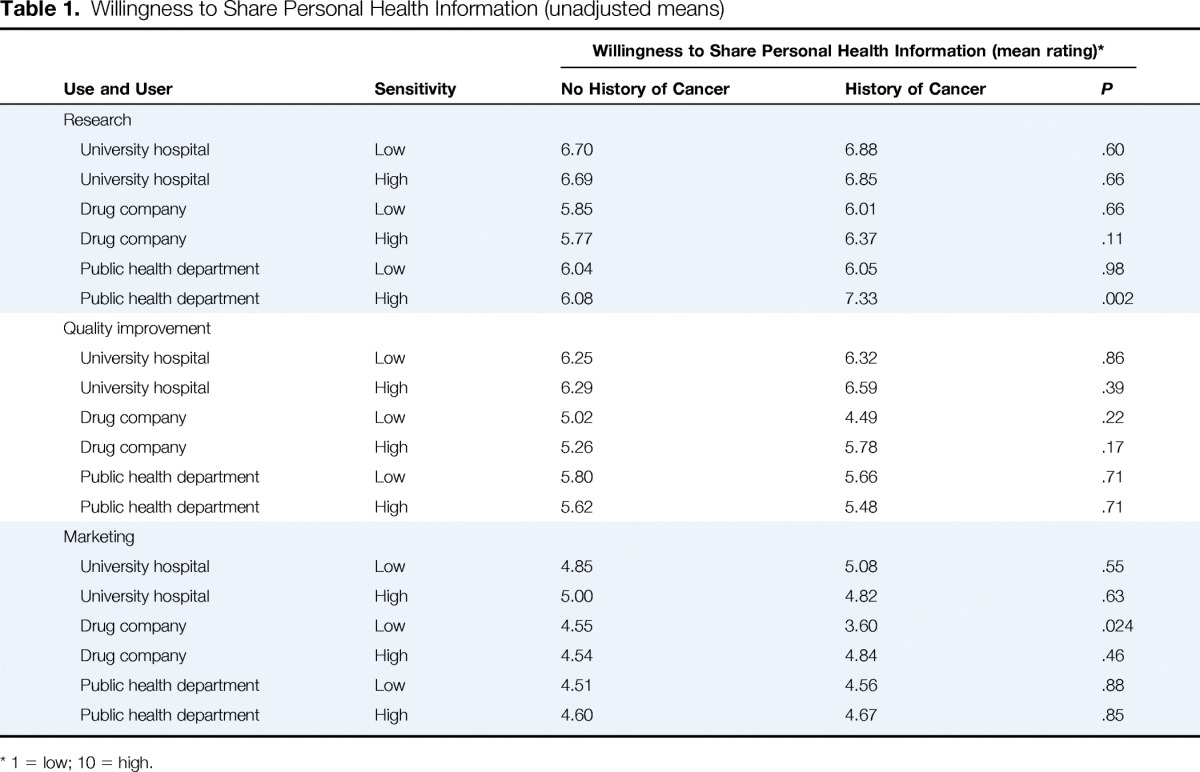
Table 1 lists unadjusted means for each of the 18 health information use scenarios. The highest rated scenario was the same for cancer and noncancer respondents: a university hospital using less sensitive electronic health information for research (6.88 and 6.70, respectively). Both cancer and noncancer participants rated this scenario as the one for which they were most willing to share their own electronic health information. Marketing uses were generally rated lower than other uses by both cancer and noncancer participants.
Table 1.
Willingness to Share Personal Health Information (unadjusted means)
| Use and User | Sensitivity | Willingness to Share Personal Health Information (mean rating)* |
||
|---|---|---|---|---|
| No History of Cancer | History of Cancer | P | ||
| Research | ||||
| University hospital | Low | 6.70 | 6.88 | .60 |
| University hospital | High | 6.69 | 6.85 | .66 |
| Drug company | Low | 5.85 | 6.01 | .66 |
| Drug company | High | 5.77 | 6.37 | .11 |
| Public health department | Low | 6.04 | 6.05 | .98 |
| Public health department | High | 6.08 | 7.33 | .002 |
| Quality improvement | ||||
| University hospital | Low | 6.25 | 6.32 | .86 |
| University hospital | High | 6.29 | 6.59 | .39 |
| Drug company | Low | 5.02 | 4.49 | .22 |
| Drug company | High | 5.26 | 5.78 | .17 |
| Public health department | Low | 5.80 | 5.66 | .71 |
| Public health department | High | 5.62 | 5.48 | .71 |
| Marketing | ||||
| University hospital | Low | 4.85 | 5.08 | .55 |
| University hospital | High | 5.00 | 4.82 | .63 |
| Drug company | Low | 4.55 | 3.60 | .024 |
| Drug company | High | 4.54 | 4.84 | .46 |
| Public health department | Low | 4.51 | 4.56 | .88 |
| Public health department | High | 4.60 | 4.67 | .85 |
1 = low; 10 = high.
Using conjoint analysis, we determined the importance weights of the three factors in the experiment to participants: use, user, and sensitivity of the information. For both cancer and noncancer participants, use was the most important factor (45.6 and 67.1, respectively). However, for patients with cancer, sensitivity was a more important factor compared with noncancer participants (importance weights, 29.8% v 1.2%).
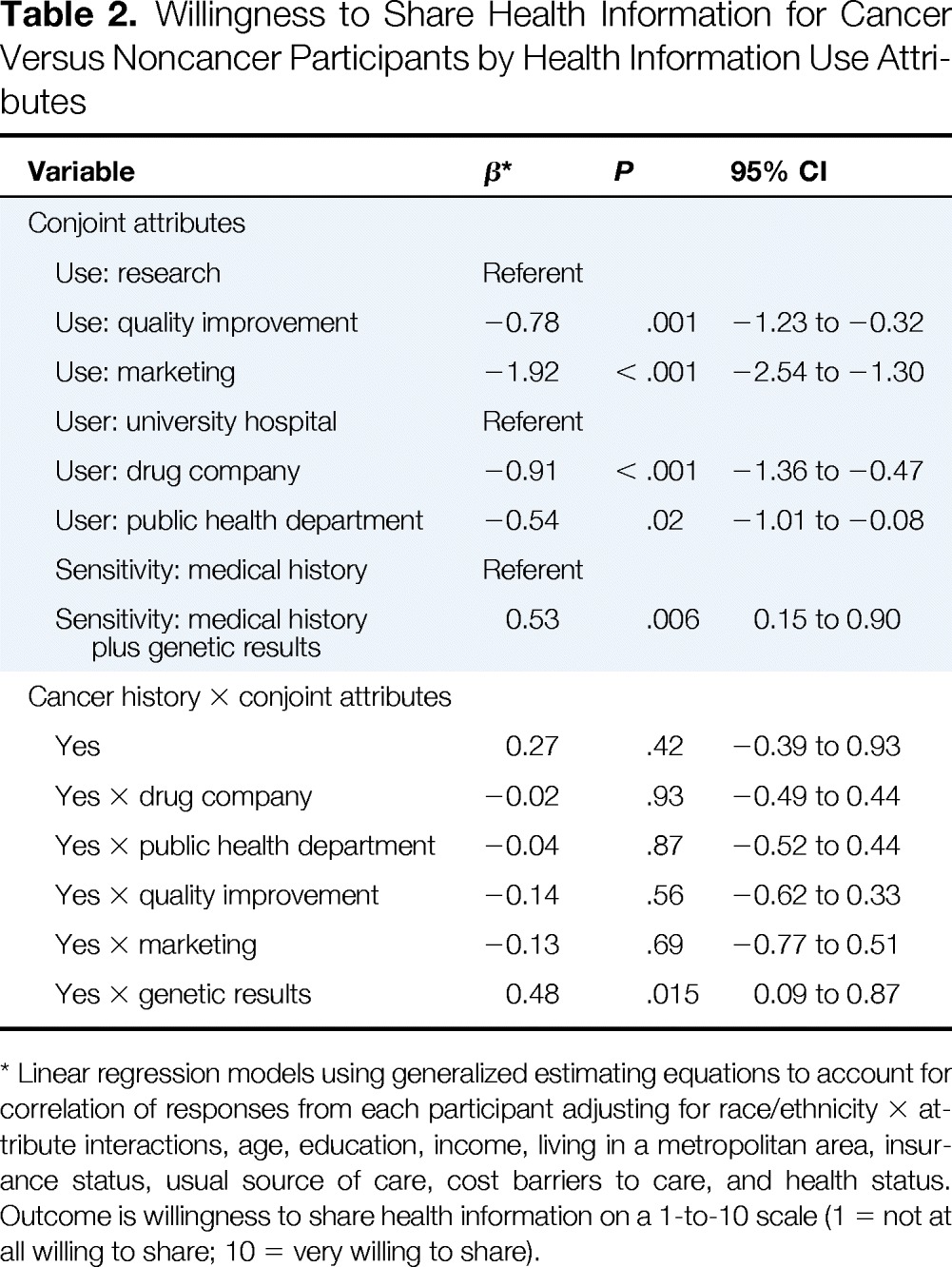
Table 2 summarizes differences in participants' willingness to share health information for different uses, users, and sensitivity of health information after adjusting for demographic characteristics, health status, and health care access measures. Overall, cancer and noncancer participants had a similar willingness to share electronic health information (0.27; P = .42). However, cancer participants were more willing to share than noncancer participants when the information was more sensitive (ie, inherited genetic information; 0.48; P = .015). Cancer and noncancer respondents rated different uses (quality improvement, −0.14; P = .56; marketing, −0.13; P = .69) and users (drug company, −0.02; P = .93; public health department, −0.04; P = .87) similarly.
Table 2.
Willingness to Share Health Information for Cancer Versus Noncancer Participants by Health Information Use Attributes
| Variable | β* | P | 95% CI |
|---|---|---|---|
| Conjoint attributes | |||
| Use: research | Referent | ||
| Use: quality improvement | −0.78 | .001 | −1.23 to −0.32 |
| Use: marketing | −1.92 | < .001 | −2.54 to −1.30 |
| User: university hospital | Referent | ||
| User: drug company | −0.91 | < .001 | −1.36 to −0.47 |
| User: public health department | −0.54 | .02 | −1.01 to −0.08 |
| Sensitivity: medical history | Referent | ||
| Sensitivity: medical history plus genetic results | 0.53 | .006 | 0.15 to 0.90 |
| Cancer history × conjoint attributes | |||
| Yes | 0.27 | .42 | −0.39 to 0.93 |
| Yes × drug company | −0.02 | .93 | −0.49 to 0.44 |
| Yes × public health department | −0.04 | .87 | −0.52 to 0.44 |
| Yes × quality improvement | −0.14 | .56 | −0.62 to 0.33 |
| Yes × marketing | −0.13 | .69 | −0.77 to 0.51 |
| Yes × genetic results | 0.48 | .015 | 0.09 to 0.87 |
Linear regression models using generalized estimating equations to account for correlation of responses from each participant adjusting for race/ethnicity × attribute interactions, age, education, income, living in a metropolitan area, insurance status, usual source of care, cost barriers to care, and health status. Outcome is willingness to share health information on a 1-to-10 scale (1 = not at all willing to share; 10 = very willing to share).
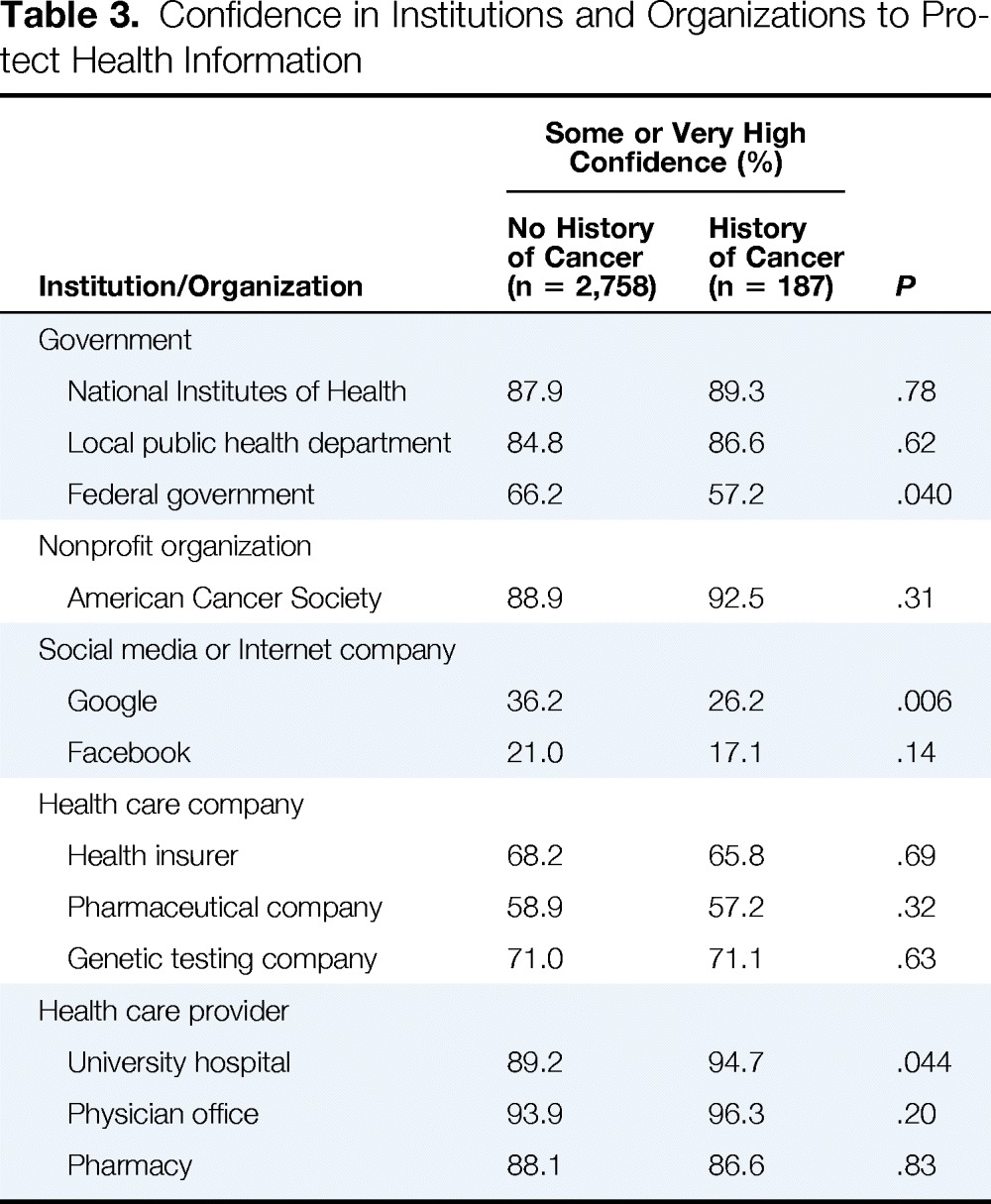
We also asked participants to rate their confidence in various public and private institutions and organizations to secure their health information (Table 3). Cancer and noncancer participants generally rated organizations similarly, expressing the highest level of confidence in health care providers (ie, university hospitals, physician offices, and pharmacies) and the least amount of confidence in social media and Internet companies (ie, Google and Facebook). However, cancer participants rated the federal government and Google somewhat lower than noncancer participants and rated university hospitals somewhat higher, although all of the differences were modest.
Table 3.
Confidence in Institutions and Organizations to Protect Health Information
| Institution/Organization | Some or Very High Confidence (%) |
P | |
|---|---|---|---|
| No History of Cancer (n = 2,758) | History of Cancer (n = 187) | ||
| Government | |||
| National Institutes of Health | 87.9 | 89.3 | .78 |
| Local public health department | 84.8 | 86.6 | .62 |
| Federal government | 66.2 | 57.2 | .040 |
| Nonprofit organization | |||
| American Cancer Society | 88.9 | 92.5 | .31 |
| Social media or Internet company | |||
| 36.2 | 26.2 | .006 | |
| 21.0 | 17.1 | .14 | |
| Health care company | |||
| Health insurer | 68.2 | 65.8 | .69 |
| Pharmaceutical company | 58.9 | 57.2 | .32 |
| Genetic testing company | 71.0 | 71.1 | .63 |
| Health care provider | |||
| University hospital | 89.2 | 94.7 | .044 |
| Physician office | 93.9 | 96.3 | .20 |
| Pharmacy | 88.1 | 86.6 | .83 |
Discussion
As efforts grow to collect, store, and analyze big data in oncology,6,7 understanding patient preferences regarding reuse of electronic health information is essential to developing good public policy. This study has two key findings. First, individuals with a prior diagnosis of cancer are as willing to share their health information as those without cancer. Their cancer history does not seem to make them more reticent to supporting reuse of their health information. Second, the sensitivity of health information is more important to individuals with a prior diagnosis of cancer compared with individuals without a prior diagnosis of cancer. For participants without cancer, sensitivity was not at all important; for participants with a history of cancer, sensitivity was the second most important factor (second to the actual use). However, the direction of this effect was opposite from what we hypothesized. It was the inherited genetic information that made cancer participants more willing to share. Perhaps cancer participants are eager to have their genetic information used in an altruistic manner to benefit society.
Our study has several implications. First, similar to individuals without cancer, individuals with a prior diagnosis of cancer consider the purpose of health information reuse as the most important determinant of their willingness to share. In contrast, current policies emphasize the sensitivity of the information, often defining sensitivity by whether information is identifiable and whether it was collected in an encounter with the health care system. If information is not considered sensitive, nearly all uses are permitted. Our results suggest a need to revisit policies on reusing health information if the goal is to align these policies with patient preferences (ie, promote research uses and limit marketing uses). Second, although policymakers, clinicians, and ethicists tend to add extra protections to genetic information because of concerns over reidentification, discrimination, and the unknown significance of certain findings based on current knowledge,30 the cancer participants in our study were more willing to share their information when inherited genetic results were included. These ethical concerns are important, and security and safeguards are essential to increasing public trust in research and protecting patients, particularly when involving genetic information. However, our findings suggest a need to ensure that policies balance these privacy considerations with the desire of patients to have their information used for altruistic, socially beneficial purposes. Our policies on information sharing have largely been determined by our intuitions, which may be faulty, rather than empirically observed public opinion; one prior study found that linking health information to biologic samples did not influence patients' consent preferences, whereas inclusion of socioeconomic information significantly reduced the proportion willing to forgo consent.17 In a survey of patients at an academic medical center, 89% approved of the university using anonymized genetic information for research.31 Nonetheless, that does not mean patients do not worry about their information being used against them. In a study of individuals with a family history of colorectal cancer, half were concerned about genetic discrimination if they were to undergo genetic testing or if others found out about their family health history.18
Our study has several important limitations. First, the study was not originally powered to detect differences between cancer versus noncancer participants. This limits our ability to test for more complex interactions. Second, we do not know when the respondents were diagnosed with cancer or the type of cancer. Patients with a current or recent diagnosis of cancer or patients with specific types of cancer with different levels of heritability might have different preferences regarding reuse of their health information. For example, cancers that are familial, more consequential, or occur at a younger age might yield different views about information sharing. Third, we tested only two different levels of sensitivity in our conjoint experiment. Had we included a broader range of possibilities, we might have found that sensitivity was more or less important. Nevertheless, the finding that cancer participants were more favorable toward reuse of their health information when genetic information is included is a novel finding. Fourth, we presented participants with hypothetical scenarios rather than observing real-world decisions. Therefore, we were unable to measure how actual changes in behavior would be correlated with effect sizes in our experiment. However, responses to hypothetical scenarios have been shown to be highly predictive of behavior.32 In addition, we used a controlled experimental design that is more likely to reveal individual preferences than static survey questions.
Exploring how individuals with a history of cancer define sensitivity and how important it is in shaping their preferences on information reuse will be essential for good policy in this area. This study suggests that similar to the general population, individuals with a prior diagnosis of cancer are less concerned about the sensitivity or user of their reused health information and most willing to share their health information when it is put to good use. Rather than focusing exclusively on the sensitivity of information, as is the case with current regulatory policies, future policies should more strongly consider social purpose and further explore what information patients consider sensitive.
Supplementary Material
Acknowledgment
Supported by Grant No. 5R21HG006047-02 from the National Human Genome Research Institute. The funder had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript. D.G. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Myriad Genetics provides research funding for genetic testing in a study led by A.R.B. A.R.B. and R.J. are members of the CancerLinQ Data Governance and Oversight Committee of the American Society of Clinical Oncology and leaders of its ethics committee.
Appendix
Table A1.
Participant Characteristics (unweighted percentages)
| Characteristic | No History of Cancer (n = 2,758) | History of Cancer (n = 187) | P* |
|---|---|---|---|
| Age, years | < .001 | ||
| 18-29 | 13.9 | 0.0 | |
| 30-44 | 22.2 | 4.3 | |
| 45-59 | 33.2 | 24.1 | |
| ≥ 60 | 30.7 | 71.6 | |
| Race/ethnicity | .002 | ||
| White, non-Hispanic | 68.0 | 77.0 | |
| Black, non-Hispanic | 14.7 | 15.5 | |
| Hispanic | 17.3 | 7.5 | |
| Female sex | 49.8 | 46.0 | .31 |
| Living in metropolitan statistical area | 86.0 | 81.8 | .11 |
| Education | .15 | ||
| < High school | 8.9 | 8.6 | |
| High school | 28.7 | 34.2 | |
| Some college | 31.0 | 23.5 | |
| ≥ Bachelor's degree | 31.5 | 33.7 | |
| Income, $ | .86 | ||
| < 25,000 | 18.7 | 20.3 | |
| 25,000-49,999 | 23.4 | 21.9 | |
| 50,000-74,999 | 19.3 | 17.7 | |
| ≥ 75,000 | 38.6 | 40.1 | |
| Insured | 81.9 | 96.8 | < .001 |
| Has personal physician or other provider | 74.3 | 94.1 | < .001 |
| Did not receive care in past year because of cost | 16.9 | 9.1 | .02 |
| Fair/poor health status | 15.7 | 28.3 | < .001 |
P values represent χ2 tests of association.
Authors' Disclosures of Potential Conflicts of Interest
Disclosures provided by the authors are available with this article at jop.ascopubs.org.
Author Contributions
Conception and design: David Grande, David A. Asch, Angela R. Bradbury, Reshma Jagsi, Nandita Mitra
Collection and assembly of data: David Grande, Nandita Mitra
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Are Patients With Cancer Less Willing to Share Their Health Information? Privacy, Sensitivity, and Social Purpose
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jop.ascopubs.org/site//misc/ifc.xhtml.
David Grande
No relationship to disclose
David A. Asch
Employment: VAL Health
Leadership: VAL Health
Stock or Other Ownership: VAL Health
Consulting or Advisory Role: VAL Health
Fei Wan
No relationship to disclose
Angela R. Bradbury
Research Funding: Myriad Genetics, Hill-Rom (I)
Travel, Accommodations, Expenses: Hill-Rom (I)
Reshma Jagsi
Employment: University of Michigan
Honoraria: International Journal of Radiation Oncology Biology Physics
Consulting or Advisory Role: Eviti
Research Funding: AbbVie (Inst)
Nandita Mitra
No relationship to disclose
References
- 1.Hsiao CJ, Hing E. Use and characteristics of electronic health record systems among office-based physician practices: United States, 2001-2013. www.cdc.gov/nchs/data/databriefs/db143.pdf. [PubMed]
- 2.Krumholz HM. Big data and new knowledge in medicine: The thinking, training, and tools needed for a learning health system. Health Aff (Millwood) 2014;33:1163–1170. doi: 10.1377/hlthaff.2014.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forrest CB, Margolis P, Seid M, et al. PEDSnet: How a prototype pediatric learning health system is being expanded into a national network. Health Aff (Millwood) 2014;33:1171–1177. doi: 10.1377/hlthaff.2014.0127. [DOI] [PubMed] [Google Scholar]
- 4.Wallace PJ, Shah ND, Dennen T, et al. Optum Labs: Building a novel node in the learning health care system. Health Aff (Millwood) 2014;33:1187–1194. doi: 10.1377/hlthaff.2014.0038. [DOI] [PubMed] [Google Scholar]
- 5.Abernethy AP, Etheredge LM, Ganz PA, et al. Rapid-learning system for cancer care. J Clin Oncol. 2010;20:4268–4274. doi: 10.1200/JCO.2010.28.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Society of Clinical Oncology. CancerLinq. http://cancerlinq.org/
- 7.Noyes K. Flatiron Health's bold proposition to fight cancer with big data. http://fortune.com/2014/06/12/flatiron-healths-bold-proposition-to-fight-cancer-with-big-data/
- 8.Safran C, Bloomrosen M, Hammond W, et al. Toward a national framework for the secondary use of health data: An American Medical Informatics Association white paper. J Am Med Inform Assoc. 2007;14:1–9. doi: 10.1197/jamia.M2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robling MR, Hood K, Houston H, et al. Public attitudes towards the use of primary care patient record data in medical research without consent: A qualitative study. J Med Ethics. 2004;30:104–109. doi: 10.1136/jme.2003.005157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whiddett R, Hunter I, Engelbrecht J, et al. Patients' attitudes towards sharing their health information. Int J Med Inform. 2006;75:530–541. doi: 10.1016/j.ijmedinf.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Trinidad SB, Fullerton SM, Bares JM, et al. Genomic research and wide data sharing: Views of prospective participants. Genet Med. 2010;12:486–495. doi: 10.1097/GIM.0b013e3181e38f9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erlich Y, Williams JB, Glazer D, et al. Redefining genomic privacy: Trust and empowerment. PLoS Biol. 2014;12:e1001983. doi: 10.1371/journal.pbio.1001983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duhigg C. How companies learn your secrets. New York Times Magazine. February 16, 2012. www.nytimes.com/2012/02/19/magazine/shopping-habits.html?_r=0.
- 14.Cassidy J. Why Edward Snowden is a hero. The New Yorker. June 10, 2013. www.newyorker.com/news/john-cassidy/why-edward-snowden-is-a-hero.
- 15.Peterson EA, Milliron KJ, Lewis KE, et al. Health insurance and discrimination concerns and BRCA1/2 testing in a clinic population. Cancer Epidemiol Biomarkers Prev. 2002;11:79–87. [PubMed] [Google Scholar]
- 16.Markle. Survey finds Americans want electronic personal health information to improve own health care. www.markle.org/downloadable_assets/research_doc_120706.pdf.
- 17.Willison DJ, Steeves V, Charles C, et al. Consent for use of personal information for health research: Do people with potentially stigmatizing health conditions and the general public differ in their opinions? BMC Med Ethics. 2009;10:10. doi: 10.1186/1472-6939-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Apse KA, Biesecker BB, Giardiello FM, et al. Perceptions of genetic discrimination among at-risk relatives of colorectal cancer patients. Genet Med. 2004;6:510–516. doi: 10.1097/01.gim.0000144013.96456.6c. [DOI] [PubMed] [Google Scholar]
- 19. Genetic Information Nondiscrimination Act of 2008, 122 Stat 881, 2008.
- 20.Grande D, Mitra N, Shah A, et al. Public preferences about secondary uses of electronic health information. JAMA Intern Med. 2013;173:1798–1806. doi: 10.1001/jamainternmed.2013.9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.GfK Knowledge Networks. KnowledgePanel demographic profile. www.knowledgenetworks.com/knpanel/docs/GfK-KnowledgePanel(R)-Demographic-Profile.pdf.
- 22.Green P, Srinivasan V. Conjoint analysis in marketing: New developments with implications for research and practice. J Mark. 1990;54:3–19. [Google Scholar]
- 23.Green PE, Rao VR. Conjoint measurement for quantifying judgmental data. J Mark Res. 1971;8:355–363. [Google Scholar]
- 24.Green P, Srinivasan V. Conjoint analysis in consumer research: Issues and outlook. J Consum Res. 1978;5:103–123. [Google Scholar]
- 25.Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System Survey Questionnaire. www.cdc.gov/brfss/questionnaires/
- 26.Shea J, Micco E, Dean L, et al. Development of a revised health care system distrust scale. J Gen Intern Med. 2008;23:727–732. doi: 10.1007/s11606-008-0575-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis J, Smith T, Marsden P. 1972-2008: Cumulative Codebook. Chicago, IL: National Opinion Research Center; 2009. General Social Surveys. [Google Scholar]
- 28.Liang KY, Zeger S. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 29.National Center for Health Statistics. Health United States, 2013: With Special Feature on Prescription Drugs. Hyattsville, MD: National Center for Health Statistics; 2014. [PubMed] [Google Scholar]
- 30.Andrews LB, Fullarton JE, Holtzman NA, Motulsky AG, editors. Assessing Genetic Risks: Implications for Health and Social Policy. Washington, DC: National Academy Press; 1994. [PubMed] [Google Scholar]
- 31.Pulley JM, Brace MM, Bernard GR, et al. Attitudes and perceptions of patients towards methods of establishing a DNA biobank. Cell Tissue Bank. 2008;9:55–65. doi: 10.1007/s10561-007-9051-2. [DOI] [PubMed] [Google Scholar]
- 32.Peabody J, Luck J, Glassman P, et al. Comparison of vignettes, standardized patients, and chart abstraction: A prospective validation study of 3 methods for measuring quality. JAMA. 2000;283:1715–1722. doi: 10.1001/jama.283.13.1715. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


