Although HIV infection rates among patients with cancer were higher than the prevalence threshold above which national guidelines recommend routine opt-out testing, the overall HIV testing rate was low.
Abstract
Purpose:
To determine the rates of HIV testing and infection among patients with cancer at initiation of systemic cancer therapy.
Methods:
We conducted a retrospective cohort study of adults with cancer who registered at a comprehensive cancer center from January 2004 through April 2011 and received systemic cancer therapy. We determined rates of HIV-1/2 and/or Western blot testing and HIV positivity at initiation of systemic cancer therapy. Multivariable logistic regression was used to determine predictors of HIV testing.
Results:
Of 18,874 patients with cancer who received systemic cancer therapy during the study period, 3,514 (18.6%) were tested for HIV at initiation of cancer therapy. The prevalence of positive HIV test results was 1.2% (41 of 3,514), and the prevalence of newly diagnosed HIV was 0.3% (12 of 3,514). The HIV testing rate was lower in black than in white patients (13.7% v 19.2%), but the prevalence of positive test results was higher in black patients (4.5%) than in any other racial/ethnic group. Among patients with AIDS-defining cancers (eg, non-Hodgkin lymphoma and cervical cancer), predictors of HIV testing were history of non-Hodgkin lymphoma, younger age, and registration after 2006. Among patients with non–AIDS-defining cancers, predictors of HIV testing were younger age, registration after 2006, male sex, history of illicit drug use or sexually transmitted disease, having a hematologic malignancy, and black race.
Conclusion:
The prevalence of HIV infection among patients with cancer was 1.2%, higher than the 0.1% prevalence threshold above which national guidelines recommend routine opt-out testing; however, the overall HIV testing rate was low.
Introduction
In the United States, more than 1.2 million individuals are infected with HIV, of whom approximately 16% are unaware of their infection status.1 Thus, the Centers for Disease Control and Prevention (CDC) and the US Preventive Services Task Force (USPSTF)2,3 recommend routine opt-out HIV testing in areas where the prevalence of HIV infection exceeds 0.1% and for persons age 13 to 65 years to expeditiously identify and treat persons with HIV, reduce transmission to uninfected persons, and reduce the risk of AIDS-related events, such as development of AIDS-defining cancers. According to these recommendations, HIV testing should be performed as part of routine medical care unless the patient declines. Of note, to increase the efficiency of HIV testing, the CDC advises that separate written informed consent and pretest HIV prevention counseling should not be required before HIV testing.2 Despite these recommendations, relatively few institutions have successfully implemented routine HIV testing, in many cases because of system- and physician-related barriers.2,4,5
Because of immune deficiency, patients with HIV infection have a high risk of developing not only cancers considered to be AIDS defining (eg, non-Hodgkin lymphoma [NHL], cervical cancer, and Kaposi sarcoma) but also non–AIDS-defining cancers (eg, Hodgkin lymphoma and anal, liver, lung, and oropharyngeal cancers).4 HIV-infected patients with non–AIDS-defining cancers have been reported to have overall worse cancer outcomes than HIV-uninfected patients with the same cancers; however, some studies have reported substantial improvement in outcomes in HIV-infected patients in the current era of highly active antiretroviral therapy (HAART).4 Systemic cancer therapy may cause prolonged immunosuppression in patients with HIV,6,7 and the concomitant administration of cancer therapy and antiretroviral therapy can cause serious drug interactions through cytochrome P450 3A4 and other inducers and inhibitors central to chemotherapy metabolism.8 Thus, at the initiation of cancer therapy, testing patients with cancer for HIV infection could facilitate selection of appropriate cancer and antiretroviral therapies, which could improve patients' clinical outcomes.9
Rates of HIV testing have been reported to be low among cancer survivors (41%)10 and in studies with small cohorts of patients with AIDS-defining cancers (11% to 60%).11,12 However, little is known about rates of HIV testing and infection among patients with cancer before initiation of systemic cancer therapy. In this study, we aimed to determine the rates of HIV testing and positivity at the initiation of systemic cancer therapy among patients with AIDS-defining and non–AIDS-defining cancers at a large US comprehensive cancer center.
Methods
Data Sources
For this retrospective cohort study, we searched institutional tumor registry and informatics databases to identify adults with all cancer types who registered at The University of Texas MD Anderson Cancer Center (Houston, TX) from January 1, 2004, through April 30, 2011. We identified patients who received systemic cancer therapy, defined as cytotoxic chemotherapy and other therapies administered through intravenous or other nonoral routes, in outpatient treatment centers. We excluded oral chemotherapy because we could not validate medication dispensing dates. We excluded patients in clinical trials because some clinical trials excluded patients with HIV and because HIV testing in a clinical trial might have been dictated by the clinical trial and not reflective of the investigators' decisions. There was no official policy recommending prechemotherapy HIV testing for all patients at MD Anderson during the study period. This study was approved by the institutional review board of MD Anderson Cancer Center, which waived the requirement for informed consent.
Through institutional clinical databases, we determined patients' demographic information, cancer types, cancer chemotherapy drugs and dates administered, and information about whether HIV testing was performed. Through institutional administrative databases, we determined whether patients had selected risk factors for HIV infection, such as history of a sexually transmitted disease or history of illicit drug use, by International Classification of Diseases (ninth revision; ICD-9) codes for these conditions recorded before the second cancer therapy administration date.
We determined the test dates and results for HIV testing, defined as ordering of an HIV-1/2 antibody test by enzyme-linked immunosorbent assay and/or confirmatory Western blot testing after registration at MD Anderson. We defined the period of initiation of cancer therapy as the period from 2 months before the first systemic cancer therapy administration until receipt of the second systemic cancer therapy administration. Positive results on HIV-1/2 antibody tests and associated Western blot tests defined HIV infection. Until recently, at MD Anderson, Western blot confirmatory testing was not reflexively performed among patients with positive HIV-1/2 test results; instead, confirmatory testing had to be ordered separately by the treating medical provider. Thus, not all study patients who had a positive HIV-1/2 test result had subsequent confirmatory testing. If only one test (HIV-1/2 or Western blot) was performed, the result of that test was used to determine the patient's HIV test status. Patients with a positive HIV-1/2 test result but a negative Western blot test result were considered to be without HIV infection. After we identified patients with positive HIV test results through database review, we reviewed the medical records of those patients to determine whether they were known to be infected with HIV before they underwent HIV testing at the initiation of cancer therapy.
Statistical Analyses
Primary outcomes were prevalence of HIV testing and prevalence of positive HIV test results. We used Fisher's exact, χ2, and t tests to compare characteristics between patients who were tested and patients who were not tested for HIV and between tested patients who were HIV positive and tested patients who were HIV negative. A multivariable logistic regression model using Firth's penalized maximum likelihood estimation was used to determine predictors of HIV testing. Potential predictors included age, sex, race/ethnicity, history of sexually transmitted disease, history of illicit drug use, and cancer type. Year of registration at MD Anderson was used to examine potential changes in HIV testing rates after publication of the 2006 CDC HIV screening recommendations.2 Modeling was performed separately for patients with AIDS-defining and patients with non–AIDS-defining cancers. For statistical analysis, we used SAS software (version 9.3; SAS Institute, Cary, NC).
Results
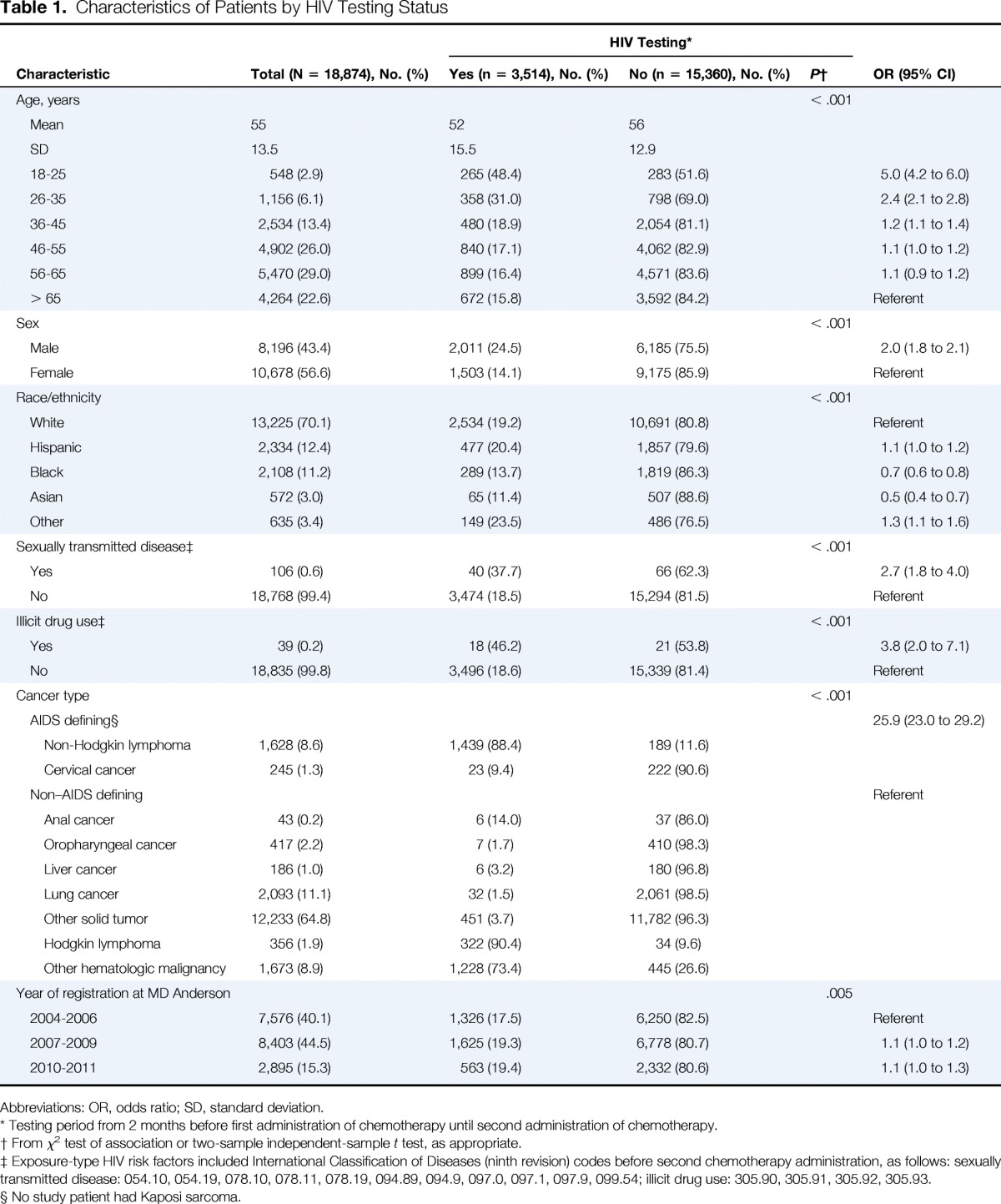
A total of 18,874 patients with cancer received systemic cancer therapy during the study period and met study criteria. Of these, 3,514 (18.6%) were tested for HIV at the initiation of systemic cancer therapy. Patient characteristics are summarized in Table 1. The median age was 56 years. The rate of HIV testing among patients younger than 65 years was 19.5%, and the rate of HIV testing in this age group ranged from 16.4% in the subset of patients age 56 to 65 years to 48.4% in the subset of patients age 18 to 25 years. A total of 3,479 patients had HIV-1/2 testing but no Western blot confirmatory testing, 31 patients had HIV-1/2 testing and Western blot confirmatory testing, and four patients did not have HIV-1/2 testing but did have Western blot testing.
Table 1.
Characteristics of Patients by HIV Testing Status
| Characteristic | Total (N = 18,874), No. (%) | HIV Testing* |
OR (95% CI) | ||
|---|---|---|---|---|---|
| Yes (n = 3,514), No. (%) | No (n = 15,360), No. (%) | P† | |||
| Age, years | < .001 | ||||
| Mean | 55 | 52 | 56 | ||
| SD | 13.5 | 15.5 | 12.9 | ||
| 18-25 | 548 (2.9) | 265 (48.4) | 283 (51.6) | 5.0 (4.2 to 6.0) | |
| 26-35 | 1,156 (6.1) | 358 (31.0) | 798 (69.0) | 2.4 (2.1 to 2.8) | |
| 36-45 | 2,534 (13.4) | 480 (18.9) | 2,054 (81.1) | 1.2 (1.1 to 1.4) | |
| 46-55 | 4,902 (26.0) | 840 (17.1) | 4,062 (82.9) | 1.1 (1.0 to 1.2) | |
| 56-65 | 5,470 (29.0) | 899 (16.4) | 4,571 (83.6) | 1.1 (0.9 to 1.2) | |
| > 65 | 4,264 (22.6) | 672 (15.8) | 3,592 (84.2) | Referent | |
| Sex | < .001 | ||||
| Male | 8,196 (43.4) | 2,011 (24.5) | 6,185 (75.5) | 2.0 (1.8 to 2.1) | |
| Female | 10,678 (56.6) | 1,503 (14.1) | 9,175 (85.9) | Referent | |
| Race/ethnicity | < .001 | ||||
| White | 13,225 (70.1) | 2,534 (19.2) | 10,691 (80.8) | Referent | |
| Hispanic | 2,334 (12.4) | 477 (20.4) | 1,857 (79.6) | 1.1 (1.0 to 1.2) | |
| Black | 2,108 (11.2) | 289 (13.7) | 1,819 (86.3) | 0.7 (0.6 to 0.8) | |
| Asian | 572 (3.0) | 65 (11.4) | 507 (88.6) | 0.5 (0.4 to 0.7) | |
| Other | 635 (3.4) | 149 (23.5) | 486 (76.5) | 1.3 (1.1 to 1.6) | |
| Sexually transmitted disease‡ | < .001 | ||||
| Yes | 106 (0.6) | 40 (37.7) | 66 (62.3) | 2.7 (1.8 to 4.0) | |
| No | 18,768 (99.4) | 3,474 (18.5) | 15,294 (81.5) | Referent | |
| Illicit drug use‡ | < .001 | ||||
| Yes | 39 (0.2) | 18 (46.2) | 21 (53.8) | 3.8 (2.0 to 7.1) | |
| No | 18,835 (99.8) | 3,496 (18.6) | 15,339 (81.4) | Referent | |
| Cancer type | < .001 | ||||
| AIDS defining§ | 25.9 (23.0 to 29.2) | ||||
| Non-Hodgkin lymphoma | 1,628 (8.6) | 1,439 (88.4) | 189 (11.6) | ||
| Cervical cancer | 245 (1.3) | 23 (9.4) | 222 (90.6) | ||
| Non–AIDS defining | Referent | ||||
| Anal cancer | 43 (0.2) | 6 (14.0) | 37 (86.0) | ||
| Oropharyngeal cancer | 417 (2.2) | 7 (1.7) | 410 (98.3) | ||
| Liver cancer | 186 (1.0) | 6 (3.2) | 180 (96.8) | ||
| Lung cancer | 2,093 (11.1) | 32 (1.5) | 2,061 (98.5) | ||
| Other solid tumor | 12,233 (64.8) | 451 (3.7) | 11,782 (96.3) | ||
| Hodgkin lymphoma | 356 (1.9) | 322 (90.4) | 34 (9.6) | ||
| Other hematologic malignancy | 1,673 (8.9) | 1,228 (73.4) | 445 (26.6) | ||
| Year of registration at MD Anderson | .005 | ||||
| 2004-2006 | 7,576 (40.1) | 1,326 (17.5) | 6,250 (82.5) | Referent | |
| 2007-2009 | 8,403 (44.5) | 1,625 (19.3) | 6,778 (80.7) | 1.1 (1.0 to 1.2) | |
| 2010-2011 | 2,895 (15.3) | 563 (19.4) | 2,332 (80.6) | 1.1 (1.0 to 1.3) | |
Abbreviations: OR, odds ratio; SD, standard deviation.
Testing period from 2 months before first administration of chemotherapy until second administration of chemotherapy.
From χ2 test of association or two-sample independent-sample t test, as appropriate.
Exposure-type HIV risk factors included International Classification of Diseases (ninth revision) codes before second chemotherapy administration, as follows: sexually transmitted disease: 054.10, 054.19, 078.10, 078.11, 078.19, 094.89, 094.9, 097.0, 097.1, 097.9, 099.54; illicit drug use: 305.90, 305.91, 305.92, 305.93.
No study patient had Kaposi sarcoma.
As expected, the odds of HIV testing were significantly higher among patients with a history of a sexually transmitted disease (37.7% v 18.5%) or a history of illicit drug use (46.2% v 18.6%) than among patients without these characteristics (P < .001 for each), although the majority of patients with either of these characteristics were not tested. HIV testing rates were lowest among black (13.7%) and Asian patients (11.4%). Among patients with AIDS-defining cancers, HIV testing rates were relatively high for patients with NHL (88.4%) but low for patients with cervical cancer (9.4%). None of the study patients had Kaposi sarcoma. Only 12.1% of patients with non–AIDS-defining cancers had HIV testing, although rates were higher among patients with Hodgkin lymphoma and other hematologic malignancies. The overall HIV testing rate increased only incrementally after publication of the CDC recommendations, from 17.5% in the period of 2004 to 2006 to 19.3% in the period of 2007 to 2009.
The prevalence of positive HIV test results among tested patients was 1.2% (41 of 3,514). Characteristics of tested patients by HIV status are summarized in Table 2. Rates of HIV positivity were highest among patients age 26 to 55 years, male patients, and black patients. Among the 41 HIV-positive patients, 23 had NHL, and 18 had a non–AIDS-defining cancer. A review of medical records for the 41 patients with positive HIV test results showed that 29 patients were known to have HIV infection before HIV testing at the initiation of cancer therapy, whereas 12 patients, or 0.3% of all patients tested for HIV, were newly diagnosed with HIV.
Table 2.
Characteristics of Patients by HIV Test Result

| Characteristic | Total (N = 3,514), No. (%) | Result |
OR (95% CI) | ||
|---|---|---|---|---|---|
| Positive (N = 41), No. (%) | Negative (N = 3,473), No. (%) | P* | |||
| Age, years | < .001 | ||||
| Mean | 52 | 44 | 52 | ||
| SD | 15.5 | 10.6 | 15.5 | ||
| 18-25 | 265 (7.5) | 2 (0.8) | 263 (99.2) | 5.1 (0.5 to 56.5) | |
| 26-35 | 358 (10.2) | 7 (2.0) | 351 (98.0) | 13.4 (1.6 to 109.2) | |
| 36-45 | 480 (13.7) | 13 (2.7) | 467 (97.3) | 18.7 (2.4 to 143.3) | |
| 46-55 | 840 (23.9) | 14 (1.7) | 826 (98.3) | 11.4 (1.5 to 86.7) | |
| 56-65 | 899 (25.6) | 4 (0.4) | 895 (99.6) | 3.0 (0.3 to 26.9) | |
| > 65 | 672 (19.1) | 1 (0.1) | 671 (99.9) | Referent | |
| Sex | .002 | ||||
| Male | 2,011 (57.2) | 33 (1.6) | 1,978 (98.4) | 3.1 (1.4 to 6.8) | |
| Female | 1,503 (42.8) | 8 (0.5) | 1,495 (99.5) | Referent | |
| Race/ethnicity | < .001 | ||||
| White | 2,534 (72.1) | 23 (0.9) | 2,511 (99.1) | Referent | |
| Hispanic | 477 (13.6) | 4 (0.8) | 473 (99.2) | 1.0 (0.4 to 2.8) | |
| Black | 289 (8.2) | 13 (4.5) | 276 (95.5) | 5.2 (2.6 to 10.3) | |
| Asian | 65 (1.8) | 0 (0.0) | 65 (100.0) | 0.8 (0.0 to 13.9) | |
| Other | 149 (4.2) | 1 (0.7) | 148 (99.3) | 1.1 (0.2 to 5.7) | |
| Sexually transmitted disease† | .078 | ||||
| Yes | 40 (1.1) | 2 (5.0) | 38 (95.0) | 4.6 (1.1 to 19.9) | |
| No | 3,474 (98.9) | 39 (1.1) | 3,435 (98.9) | Referent | |
| Illicit drug use† | .191 | ||||
| Yes | 18 (0.5) | 1 (5.6) | 17 (94.4) | 5.1 (0.7 to 39.1) | |
| No | 3,496 (99.5) | 40 (1.1) | 3,456 (98.9) | Referent | |
| Cancer type | .058 | ||||
| AIDS defining‡ | 1.8 (1.0 to 3.4) | ||||
| Non-Hodgkin lymphoma | 1,439 (41.0) | 23 (1.6) | 1,416 (98.4) | ||
| Cervical cancer | 23 (0.7) | 0 (0.0) | 23 (100.0) | ||
| Non–AIDS defining | Referent | ||||
| Anal cancer | 6 (0.2) | 1 (16.7) | 5 (83.3) | ||
| Oropharyngeal cancer | 7 (0.2) | 0 (0.0) | 7 (100.0) | ||
| Liver cancer | 6 (0.2) | 2 (33.3) | 4 (66.7) | ||
| Lung cancer | 32 (0.9) | 0 (0.0) | 32 (100.0) | ||
| Other solid tumor | 451 (12.8) | 7 (1.6) | 444 (98.4) | ||
| Hodgkin lymphoma | 322 (9.2) | 2 (0.6) | 320 (99.4) | ||
| Other hematologic malignancy | 1,228 (34.9) | 6 (0.5) | 1,222 (99.5) | ||
| Year of registration at MD Anderson | .577 | ||||
| 2004-2006 | 1,326 (37.7) | 14 (1.1) | 1,312 (98.9) | Referent | |
| 2007-2009 | 1,625 (46.2) | 18 (1.1) | 1,607 (98.9) | 1.1 (0.5 to 2.1) | |
| 2010-2011 | 563 (16.0) | 9 (1.6) | 554 (98.4) | 1.5 (0.7 to 3.5) | |
Abbreviations: OR, odds ratio; SD, standard deviation.
From χ2 test of association, Fisher's exact test, or two-sample independent-sample t test, as appropriate.
Exposure-type HIV risk factors included International Classification of Diseases (ninth revision) codes before second chemotherapy administration, as follows: sexually transmitted disease: 054.10, 054.19, 078.10, 078.11, 078.19, 094.89, 094.9, 097.0, 097.1, 097.9, 099.54; illicit drug use: 305.90, 305.91, 305.92, 305.93.
No study patient had Kaposi sarcoma.
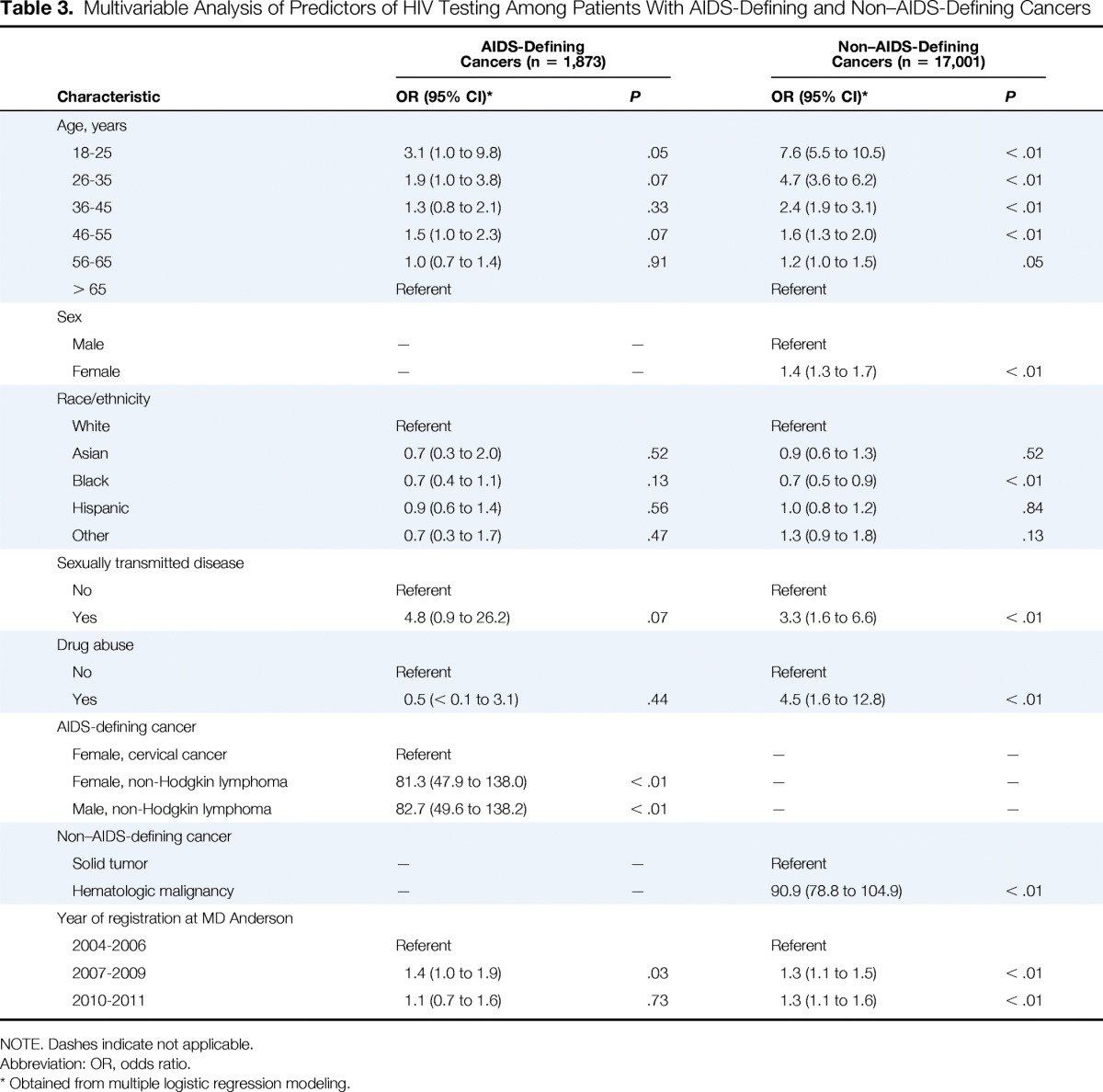
Among the 1,873 patients with AIDS-defining cancers, 1,462 (78.1%) were tested for HIV infection at the initiation of systemic cancer therapy. A multivariable logistic regression analysis (Table 3) showed that younger age, history of NHL, and registration after publication of the 2006 CDC recommendations were significant predictors of HIV testing at the initiation of cancer therapy. Conversely, predictors of not having HIV testing were older age, history of cervical cancer, and registration in the period of 2004 to 2006. Women with NHL had higher odds of being screened than women with cervical cancer.
Table 3.
Multivariable Analysis of Predictors of HIV Testing Among Patients With AIDS-Defining and Non–AIDS-Defining Cancers
| Characteristic | AIDS-Defining Cancers (n = 1,873) |
Non–AIDS-Defining Cancers (n = 17,001) |
||
|---|---|---|---|---|
| OR (95% CI)* | P | OR (95% CI)* | P | |
| Age, years | ||||
| 18-25 | 3.1 (1.0 to 9.8) | .05 | 7.6 (5.5 to 10.5) | < .01 |
| 26-35 | 1.9 (1.0 to 3.8) | .07 | 4.7 (3.6 to 6.2) | < .01 |
| 36-45 | 1.3 (0.8 to 2.1) | .33 | 2.4 (1.9 to 3.1) | < .01 |
| 46-55 | 1.5 (1.0 to 2.3) | .07 | 1.6 (1.3 to 2.0) | < .01 |
| 56-65 | 1.0 (0.7 to 1.4) | .91 | 1.2 (1.0 to 1.5) | .05 |
| > 65 | Referent | Referent | ||
| Sex | ||||
| Male | — | — | Referent | |
| Female | — | — | 1.4 (1.3 to 1.7) | < .01 |
| Race/ethnicity | ||||
| White | Referent | Referent | ||
| Asian | 0.7 (0.3 to 2.0) | .52 | 0.9 (0.6 to 1.3) | .52 |
| Black | 0.7 (0.4 to 1.1) | .13 | 0.7 (0.5 to 0.9) | < .01 |
| Hispanic | 0.9 (0.6 to 1.4) | .56 | 1.0 (0.8 to 1.2) | .84 |
| Other | 0.7 (0.3 to 1.7) | .47 | 1.3 (0.9 to 1.8) | .13 |
| Sexually transmitted disease | ||||
| No | Referent | Referent | ||
| Yes | 4.8 (0.9 to 26.2) | .07 | 3.3 (1.6 to 6.6) | < .01 |
| Drug abuse | ||||
| No | Referent | Referent | ||
| Yes | 0.5 (< 0.1 to 3.1) | .44 | 4.5 (1.6 to 12.8) | < .01 |
| AIDS-defining cancer | ||||
| Female, cervical cancer | Referent | — | — | |
| Female, non-Hodgkin lymphoma | 81.3 (47.9 to 138.0) | < .01 | — | — |
| Male, non-Hodgkin lymphoma | 82.7 (49.6 to 138.2) | < .01 | — | — |
| Non–AIDS-defining cancer | ||||
| Solid tumor | — | — | Referent | |
| Hematologic malignancy | — | — | 90.9 (78.8 to 104.9) | < .01 |
| Year of registration at MD Anderson | ||||
| 2004-2006 | Referent | Referent | ||
| 2007-2009 | 1.4 (1.0 to 1.9) | .03 | 1.3 (1.1 to 1.5) | < .01 |
| 2010-2011 | 1.1 (0.7 to 1.6) | .73 | 1.3 (1.1 to 1.6) | < .01 |
NOTE. Dashes indicate not applicable.
Abbreviation: OR, odds ratio.
Obtained from multiple logistic regression modeling.
In contrast, only 2,052 (12.1%) of the 17,001 patients with non–AIDS-defining cancers were tested for HIV infection at the initiation of systemic cancer therapy. A multivariable logistic regression analysis (Table 3) showed that younger age, male sex, black race, history of sexually transmitted disease, history of illicit drug use, hematologic malignancy, and registration after publication of the 2006 CDC recommendations were significant predictors of HIV testing at the initiation of cancer therapy. Black patients had 30% lower odds of HIV testing than white patients.
Discussion
In this single-site study of HIV testing among patients initiating systemic cancer therapy, the overall rate of HIV testing at the initiation of cancer therapy was low (18.6%), and the rate of HIV testing increased marginally after publication of the 2006 CDC screening recommendations, from 17.5% in the period of 2004 to 2006 to 19.3% in the period of 2007 to 2009. Among tested patients, the prevalence of newly diagnosed HIV was 0.3%. The overall HIV positivity rate among all patients was 1.2%, higher than the prevalence above which CDC and USPSTF guidelines recommend routine opt-out HIV screening.2,3 The prevalence of HIV has been reported to be 0.6% in Houston and 0.3% in Texas.13 HIV prevalence was highest among black patients (4.5%). However, the rate of HIV screening among black patients was low (13.7%). Of the 41 patients with positive HIV test results at the initiation of cancer therapy, 12 (29%; 0.3% of all patients tested) were newly diagnosed with HIV at the initiation of cancer therapy.
In our study, rates of HIV testing among patients with NHL and Hodgkin lymphoma were relatively high (88% and 90%, respectively). In other studies, rates of HIV testing were previously reported to be approximately 60% among patients with NHL and 59% to 63% among patients with Hodgkin lymphoma.11,12 Although some of our institutional departments have developed clinical practices that include HIV testing, the actual decision of whether to order testing remains at the discretion of the physician or other medical provider. The high rates of HIV testing among patients with hematologic malignancies in our study likely reflected providers' heightened awareness of the link between HIV infection and NHL. Both the European Society for Medical Oncology and National Comprehensive Cancer Network (NCCN) guidelines recommend HIV testing in patients with NHL as well as those with Hodgkin lymphoma.14–16 In contrast, the NCCN cervical cancer guideline panel had major disagreements about HIV testing, and thus, routine screening is not supported by the NCCN.17 The lack of guidance from national oncology societies may have influenced the low rate of HIV testing among patients with cervical cancer in our study (9%), which was similar to that in another study of patients with cervical cancer (11%).11
The rates of HIV testing among patients being treated for solid tumors other than cervical cancer have not been well studied. One recent population-based study of survivors of solid tumors reported HIV testing rates of 35% to 62%.10 In comparison, our rate of HIV testing among patients with solid tumors was lower, which may indicate different screening practices for patients with cancer undergoing active cancer therapy versus cancer survivors who have completed therapy. To date, there are no clear recommendations from oncology societies regarding HIV testing at the initiation of cancer therapy for patients with solid tumors. The American Society of Clinical Oncology has not issued specific guidelines for HIV testing of patients cancer. The NCCN cancer-related infections guideline cites the 2006 CDC testing guidelines, but with the caveat that “the implementation of these guidelines is largely dependent on institutional practices and the prevalence of undiagnosed HIV infections in specific institutions.”18
In 2006, the CDC released revised recommendations for HIV testing in all health care settings, calling for routine, nontargeted opt-out screening for individuals age 13 to 64 years.2 Many physician groups support this approach, including the American Medical Association and the American College of Physicians.19,20 In 2013, the USPSTF echoed the 2006 CDC recommendations, citing several reasons for altering the previous risk-based testing guidelines.3 First, risk-based testing for HIV has proven ineffective; several studies have shown delayed HIV diagnosis in patients who have had multiple encounters with emergency rooms, hospitals, and clinics. Second, overwhelming evidence demonstrates that early diagnosis of HIV infection and treatment with combined antiretroviral therapy improve survival. Third, routine opt-out HIV testing of pregnant women has substantially decreased the rate of perinatal HIV infection. Despite these recommendations, many barriers have hindered routine implementation of opt-out HIV testing in the United States.19 Physician attitudes, logistic problems, and funding issues associated with the extra effort associated with opt-out testing continue to threaten the long-term sustainability of many opt-out HIV testing programs.5,21
However, opt-out HIV testing for all patients presenting for cancer care may be of particular importance because of the increased risk of morbidity and mortality associated with surgery, radiation therapy, and cytotoxic chemotherapy in patients with HIV-related immunosuppression.4 For these patients, preventing further immunosuppression with immediate initiation of HAART and commencement of appropriate prophylaxis seems to improve survival.22–24 Indeed, before widespread use of HAART, the outcomes of patients with HIV-related malignancies were extremely poor, with median survival times often less than 6 months.25 In contrast, in the HAART era, survival and outcomes of individuals with AIDS-related malignancies have greatly improved,23,26 and outcomes for HIV-infected patients with cancer are now comparable to those of HIV-uninfected patients with the same cancer.8,27,28 Thus, early identification and treatment of HIV are extremely important determinants of treatment outcomes for HIV-positive patients with cancer.
Our single-institution study has several limitations, including the retrospective study design and limited generalizability of the findings. Clinical risk factors for HIV infection may have been inconsistent because of errors or inaccurate use of ICD-9 codes. However, our study included a large and heterogeneous population of patients with hematologic malignancies and solid tumors who underwent HIV testing. Because not all positive HIV test results were confirmed by Western blot testing, the HIV test results in this study need to be interpreted with caution. Patients could have had HIV testing before our study testing period or outside of MD Anderson, and providers may have elected not to repeat HIV testing before initiating cancer therapy, even among patients with known HIV infection. There were no patients with Kaposi sarcoma, which likely reflects referral patterns to our institution during the study period.
In summary, in this study from a comprehensive cancer center, we found that the overall prevalence of HIV infection was 1.2%, and prevalence of newly diagnosed HIV was 0.3%. Both rates exceed the 0.1% prevalence threshold set by the CDC and USPSTF above which routine opt-out HIV screening is recommended.2,3 Thus, our study supports testing patients with cancer for HIV infection before cancer therapy, which will allow early identification of HIV-infected patients and personalized HIV and cancer therapies. We found that although the HIV testing rate was low among patients with non–AIDS-defining solid tumors, the HIV testing rate was high among patients with hematologic malignancies (both AIDS defining and non–AIDS defining), demonstrating that high rates of HIV testing for patients presenting for cancer care are feasible.
Acknowledgment
Supported by Grant No. K07CA132955 from the National Cancer Institute (principal investigator [PI], J.P.H.), Grant No. P30CA125123-04S1 from the AIDS Malignancy Consortium at Baylor College of Medicine Dan L. Duncan Cancer Center (PI, E.Y.C.), and Grant No. P30AI036211 from the Baylor College of Medicine–The University of Texas Houston Center for AIDS Research (PI, Janet Butel, PhD), and in part by resources and the use of facilities at the Center for Innovations in Quality, Effectiveness and Safety (Grant No. CIN 13-413), Michael E. DeBakey Veterans Affairs Medical Center.
Presented in abstract form at the Society of General Internal Medicine Annual Meeting, Toronto, Ontario, Canada, April 22-25, 2015, and the Multinational Association of Supportive Care in Cancer Annual Meeting, Copenhagen, Denmark, June 25-27, 2015.
We thank Stephanie Deming for editorial review; Laurissa Gann for assistance with references; Sanjivkumar Dave, Chun Feng, Geoffrey Giacco, Anne Park, and Weiming Shi for assistance with collection of institutional data; and Jessica T. Foreman for assistance with manuscript preparation.
Authors' Disclosures of Potential Conflicts of Interest
Disclosures provided by the authors are available with this article at jop.ascopubs.org.
Author Contributions
Conception and design: Jessica P. Hwang
Financial support: Jessica P. Hwang
Collection and assembly of data: Jessica P. Hwang
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
HIV Testing in Patients With Cancer at the Initiation of Therapy at a Large US Comprehensive Cancer Center
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jop.ascopubs.org/site//misc/ifc.xhtml.
Jessica P. Hwang
No relationship to disclose
Bruno P. Granwehr
Research Funding: Gilead Sciences (Inst)
Harrys A. Torres
Consulting or Advisory Role: Gilead Sciences, Merck, Janssen
Research Funding: Merck, Vertex, The University of Texas MD Anderson Cancer Center
Maria E. Suarez-Almazor
No relationship to disclose
Thomas P. Giordano
No relationship to disclose
Andrea G. Barbo
No relationship to disclose
Heather Y. Lin
No relationship to disclose
Michael J. Fisch
Employment: AIM Specialty Health
Stock or Other Ownership: Anthem
Consulting or Advisory Role: WellPoint
Elizabeth Y. Chiao
No relationship to disclose
References
- 1.Bradley H, Hall HI, Wolitski RJ, et al. Vital signs: HIV diagnosis, care, and treatment among persons living with HIV—United States, 2011. MMWR Morb Mortal Wkly Rep. 2014;63:1113–1117. [PMC free article] [PubMed] [Google Scholar]
- 2.Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55:1–17. quiz CE11-CE14. [PubMed] [Google Scholar]
- 3.Moyer VA. Screening for HIV: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:51–60. doi: 10.7326/0003-4819-159-1-201307020-00645. [DOI] [PubMed] [Google Scholar]
- 4.Chiao EY, Dezube BJ, Krown SE, et al. Time for oncologists to opt in for routine opt-out HIV testing? JAMA. 2010;304:334–339. doi: 10.1001/jama.2010.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burke RC, Sepkowitz KA, Bernstein KT, et al. Why don't physicians test for HIV? A review of the US literature. AIDS. 2007;21:1617–1624. doi: 10.1097/QAD.0b013e32823f91ff. [DOI] [PubMed] [Google Scholar]
- 6.Alfa-Wali M, Allen-Mersh T, Antoniou A, et al. Chemoradiotherapy for anal cancer in HIV patients causes prolonged CD4 cell count suppression. Ann Oncol. 2012;23:141–147. doi: 10.1093/annonc/mdr050. [DOI] [PubMed] [Google Scholar]
- 7.Sankatsing SU, Hillebregt MM, Gras L, et al. Prolonged decrease of CD4+ T lymphocytes in HIV-1-infected patients after radiotherapy for a solid tumor. J Acquir Immune Defic Syndr. 2013;62:546–549. doi: 10.1097/QAI.0b013e318285d934. [DOI] [PubMed] [Google Scholar]
- 8.Spano JP, Costagliola D, Katlama C, et al. AIDS-related malignancies: State of the art and therapeutic challenges. J Clin Oncol. 2008;26:4834–4842. doi: 10.1200/JCO.2008.16.8252. [DOI] [PubMed] [Google Scholar]
- 9.Torres HA, Rallapalli V, Saxena A, et al. Efficacy and safety of antiretrovirals in HIV-infected patients with cancer. Clin Microbiol Infect. 2014;20:O672–O679. doi: 10.1111/1469-0691.12589. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Thompson TD, Tai E, et al. Testing for human immunodeficiency virus among cancer survivors under age 65 in the United States. Prev Chronic Dis. 2014;11:E200. doi: 10.5888/pcd11.140274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mosimann V, Cavassini M, Hugli O, et al. Patients with AIDS-defining cancers are not universally screened for HIV: A 10-year retrospective analysis of HIV-testing practices in a Swiss university hospital. HIV Med. 2014;15:631–634. doi: 10.1111/hiv.12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cave J, Edwards SG, Miller RF, et al. Should we implement ‘opt-out' HIV testing for patients with lymphoma? Clin Med. 2009;9:320–322. doi: 10.7861/clinmedicine.9-4-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.AIDSVu. Persons living with an HIV or AIDS diagnosis, 2011. http://aidsvu.org/map/
- 14.Tilly H, Vitolo U, Walewski J, et al. Diffuse large B-cell lymphoma (DLBCL): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(suppl 7):vii78–vii82. doi: 10.1093/annonc/mds273. [DOI] [PubMed] [Google Scholar]
- 15.Eichenauer DA, Engert A, André M, et al. Hodgkin's lymphoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(suppl 3):iii70–iii75. doi: 10.1093/annonc/mdu181. [DOI] [PubMed] [Google Scholar]
- 16.National Comprehensive Cancer Network. Non-Hodgkin's lymphomas (version 1.2015) www.nccn.org/professionals/physician_gls/f_guidelines.asp#nhl.
- 17.National Comprehensive Cancer Network. Cervical cancer (version 2. 1015) www.nccn.org/professionals/physician_gls/f_guidelines.asp#cervical.
- 18.National Comprehensive Cancer Network. Prevention and treatment of cancer-related infections (version 2.2014) www.nccn.org/professionals/physician_gls/f_guidelines.asp#infections.
- 19.Bartlett JG, Branson BM, Fenton K, et al. Opt-out testing for human immunodeficiency virus in the United States: Progress and challenges. JAMA. 2008;300:945–951. doi: 10.1001/jama.300.8.945. [DOI] [PubMed] [Google Scholar]
- 20.Qaseem A, Snow V, Shekelle P, et al. Screening for HIV in health care settings: A guidance statement from the American College of Physicians and HIV Medicine Association. Ann Intern Med. 2009;150:125–131. doi: 10.7326/0003-4819-150-2-200901200-00300. [DOI] [PubMed] [Google Scholar]
- 21.Zheng MY, Suneja A, Chou AL, et al. Physician barriers to successful implementation of US Preventive Services Task Force routine HIV testing recommendations. J Int Assoc Provid AIDS Care. 2014;13:200–205. doi: 10.1177/2325957413514276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stadler RF, Gregorcyk SG, Euhus DM, et al. Outcome of HIV-infected patients with invasive squamous-cell carcinoma of the anal canal in the era of highly active antiretroviral therapy. Dis Colon Rectum. 2004;47:1305–1309. doi: 10.1007/s10350-004-0584-1. [DOI] [PubMed] [Google Scholar]
- 23.Berenguer J, Miralles P, Ribera JM, et al. Characteristics and outcome of AIDS-related Hodgkin lymphoma before and after the introduction of highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2008;47:422–428. [PubMed] [Google Scholar]
- 24.Besson C, Goubar A, Gabarre J, et al. Changes in AIDS-related lymphoma since the era of highly active antiretroviral therapy. Blood. 2001;98:2339–2344. doi: 10.1182/blood.v98.8.2339. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan LD, Abrams DI, Feigal E, et al. AIDS-associated non-Hodgkin's lymphoma in San Francisco. JAMA. 1989;261:719–724. [PubMed] [Google Scholar]
- 26.Hoffmann C, Chow KU, Wolf E, et al. Strong impact of highly active antiretroviral therapy on survival in patients with human immunodeficiency virus-associated Hodgkin's disease. Br J Haematol. 2004;125:455–462. doi: 10.1111/j.1365-2141.2004.04934.x. [DOI] [PubMed] [Google Scholar]
- 27.Chiao EY, Giordano TP, Richardson P, et al. Human immunodeficiency virus-associated squamous cell cancer of the anus: Epidemiology and outcomes in the highly active antiretroviral therapy era. J Clin Oncol. 2008;26:474–479. doi: 10.1200/JCO.2007.14.2810. [DOI] [PubMed] [Google Scholar]
- 28.Powles T, Thirwell C, Newsom-Davis T, et al. Does HIV adversely influence the outcome in advanced non-small-cell lung cancer in the era of HAART? Br J Cancer. 2003;89:457–459. doi: 10.1038/sj.bjc.6601111. [DOI] [PMC free article] [PubMed] [Google Scholar]


