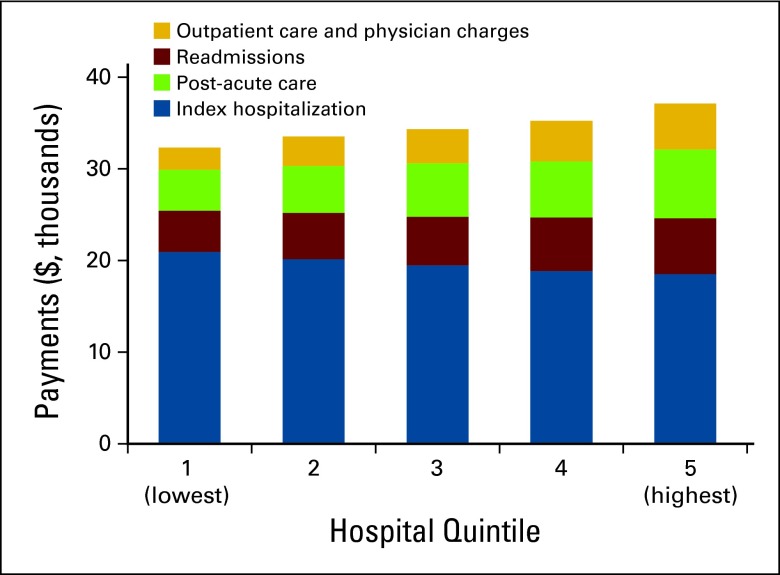
Medicare spending in the first year after colorectal cancer surgery varies across hospitals even after case-mix adjustment and price standardization.
Abstract
Purpose:
Colorectal cancer (CRC) is the second most expensive cancer in the United States. Episode-based bundled payments may be a strategy to decrease costs. However, it is unknown how payments are distributed across hospitals and different perioperative services.
Methods:
We extracted actual Medicare payments for patients in the fee-for-service Medicare population who underwent CRC surgery between January 2004 and Decembe 2006 (N = 105,016 patients). Payments included all service types from the date of hospitalization up to 1 year later. Hospitals were ranked from least to most expensive and grouped into quintiles. Results were case-mix adjusted and price standardized using empirical Bayes methods. We assessed the contributions of index hospitalization, physician services, readmissions, and postacute care to the overall variation in payment.
Results:
There is wide variation in total payments for CRC care within the first year after CRC surgery. Actual Medicare payments were $51,345 per patient in the highest quintile and $26,441 per patient in the lowest quintile, representing a difference of Δ = $24,902. Differences were persistent after price standardization (Δ = $17,184 per patient) and case-mix adjustment (Δ = $4,790 per patient). Payments for the index surgical hospitalization accounted for the largest share (65%) of payments but only minimally varied (11.6%) across quintiles. However, readmissions and postacute care services accounted for substantial variations in total payments.
Conclusion:
Medicare spending in the first year after CRC surgery varies across hospitals even after case-mix adjustment and price standardization. Variation is largely driven by postacute care and not the index surgical hospitalization. This has significant implications for policy decisions on how to bundle payments and define episodes of surgical CRC care.
Introduction
Colorectal cancer (CRC) is the third most common cancer in the United States and the second leading cause of cancer-related deaths.1 On the basis of current trends toward an aging population, the prevalence of CRC is projected to increase rapidly by the year 2020, to more than 1.5 million men and women.2,3 Likewise, the cost associated with CRC care for the Medicare population is growing. This increase is driven, in part, by the rising median age of the population and the increasing use of chemoradiation, surgical therapy, and postacute care. In fact, CRC is the second most expensive malignancy in the United States, with an estimated cost of $17 billion for the year 2020, second only to breast cancer.2 Most of this expense is concentrated in the first year after diagnosis, as a result of multimodality treatment costs.4,5 Traditionally, Medicare makes separate payments to these multidisciplinary providers for each service they perform for the beneficiaries (ie, fee-for-service payments). This approach results in fragmented care and a compartmentalized delivery system, with multiple providers across many health care settings. In additional, it does nothing to reward efforts at coordinated care.6
To this end, episode-based bundled payments (such as the Center for Medicare and Medicaid Innovation's Oncology Care Model) are being evaluated in order to align incentives for hospitals, health care providers, and other practitioners, allowing them to work closely together across all specialties and settings.7,8 The central issue in any proposal for aggregating payments is determining how the various services should be bundled together. For CRC, this is especially challenging. First, the current distribution of payments in 1 year after surgical care is unknown, thus the potential savings from bundling cannot be fully realized. In addition, accepting a global payment for all of a CRC patient's therapy in 1 year requires a high degree of integration among multiple physicians, which may be difficult to implement. On the other hand, splitting the services into multiple shorter episodes (eg, surgical services or medical oncology services alone) may limit the potential savings by eliminating the need for coordination between providers, which is the purpose behind bundling in the first place. Last, the relationship between payments and outcomes is unclear.
In this context, we investigated how 1-year global payments are currently distributed to different perioperative services and how the breakdown of these payments after 1 year of CRC surgery varies across hospitals. Should there be substantial variation in payments, the findings of this study will better inform stakeholders on the potential savings that can be achieved by moving from a fee-for-service to an episode-based bundled payment model for CRC. To better understand whether more efficient care is associated with better outcomes, we also examined the relationship between the payments, readmissions, and mortality.
Methods
Data Sources and Population
This study was based on complete national Medicare claims data for 105,016 patients who underwent surgical resection for colorectal cancer, based on International Classification of Diseases ninth edition (ICD-9) codes (153.xx, 154.xx) between January 2004 and December 2006. We excluded patients who were less than 65 or more than 99 years of age, and those who were not continuously enrolled in Medicare parts A and B at the time of their surgery. Analysis was limited to hospitals with at least 10 colon and three rectal cases to minimize chance variation.
Patients undergoing surgery were identified from the inpatient file based on the appropriate ICD-9 procedure codes. We linked these patients' records to other Medicare files containing claims relevant to their surgical episode, including the durable medical equipment, home health, long-stay hospital, outpatient, and skilled nursing facility files.
Determination of Payments
For each patient, we analyzed actual Medicare payments, not submitted charges. Actual payments reflect the payer's perspective, and as reported here, reflect what Medicare actually spends. We extracted payment information for all service types from the date of hospitalization for the index operation and up to 1 year later. This payment window would capture postoperative cancer-related care and is reasonable on empiric grounds. All payments were then price standardized using previously described methods.9 Medicare payment policies explicitly recognize geographic variation in spending power by varying payments to health care providers on the basis of local differences in prevailing wages. For example, Medicare reimburses physicians in New York more for an office visit than for an identical office visit in Wisconsin by paying more per relative value unit. In brief, price standardization “undoes” this differential by re-creating spending using the same dollar payment per relative value unit whether the physician is in Wisconsin or New York. Price standardization allows for evaluation of services provided, not just payments. Thus, if spending after price standardization is higher for a physician visit in New York, it is because more services are provided, and not because prices are higher.
All payments were also adjusted for reliability using hierarchical regression models and empirical Bayes methods to generate predicted payments for each hospital. Reliability is a measure of precision and is a function of both hospital sample size (which determines ‘‘noise” variation) and the amount of true variation across hospitals (‘‘signal”). Reliability adjustment shrinks the point estimates back toward the national average payment and minimizes chance variation in payments. For example, for hospitals with small sample sizes, payments are more variable and tend to have lower reliability, so they are “shrunk” more toward the national average, which produces a more conservative estimate of variation in payments.10,11
Last, we performed case-mix adjustment using multiple linear regression, accounting for patients' age, sex, race, admission acuity, the primary procedure code, and individual Elixhauser comorbidities.12 To further minimize confounding by unmeasured differences in disease severity and baseline costliness, we adjusted for expenditures occurring in the 6 months before surgery.10 All estimates also accounted for clustering of patients within hospitals.
All payment types (hospital, physician, postacute care) were included. Hospital payments included those related to the index hospitalization (diagnosis-related group [DRG] payment plus outlier payments when present) as well as those for readmissions. Readmissions did not include transfers to other facilities. Rehabilitation costs include payments made for patients admitted to inpatient rehabilitation facilities. Skilled nursing costs include payments made for skilled nursing care, and nursing home costs include payments made for medically necessary services billed while the patient was in a nursing home. Hospitals were ranked from lowest to highest in price-standardized payments for index hospitalization payments. We then divided them into quintiles to facilitate average comparisons.
Data analyses for this study were generated using STATA special edition (version 13; StataCorp, College Station, TX). The institutional review board at the University of Michigan judged this study exempt from human subject review.
Results
Payments
We observed wide variation in total payments for colorectal cancer care within the first year after CRC surgery (Table 1). Actual Medicare payments were $51,345 per patient in the highest quintile and $26,441 per patient in the lowest quintile, representing a difference of $24,902 per patient (94.2%). Although price and case-mix adjustments had the net effect of narrowing payment differences across hospital quintiles, differences were still significant. After price, case-mix, and reliability adjustment, average total payments differed by $4,790 per patient between the lowest ($32,341) and highest ($37,131) hospital payment quintiles. The price, case-mix, and reliability adjusted payments will be reported hereafter as the variation in these payments most closely represents the differences in services provided for the same patient.
Table 1.
Average Total Medicare Payments in the First Year After Colorectal Cancer Surgery
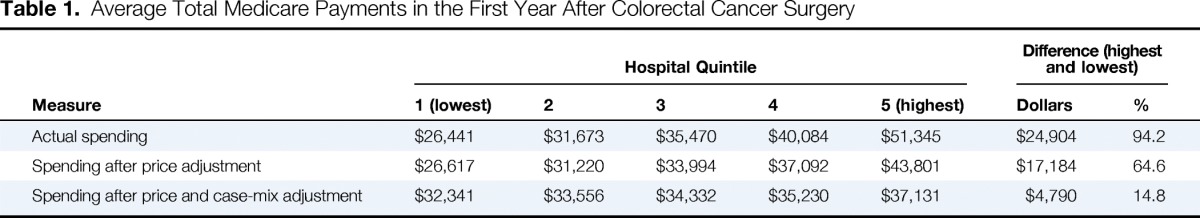
| Measure | Hospital Quintile |
Difference (highest and lowest) |
|||||
|---|---|---|---|---|---|---|---|
| 1 (lowest) | 2 | 3 | 4 | 5 (highest) | Dollars | % | |
| Actual spending | $26,441 | $31,673 | $35,470 | $40,084 | $51,345 | $24,904 | 94.2 |
| Spending after price adjustment | $26,617 | $31,220 | $33,994 | $37,092 | $43,801 | $17,184 | 64.6 |
| Spending after price and case-mix adjustment | $32,341 | $33,556 | $34,332 | $35,230 | $37,131 | $4,790 | 14.8 |
Although the index hospitalization payments accounted for the largest share of total payments, they were not the driving force behind the variation in payments across quintiles (Table 2). Index hospitalization payments were actually $2,419 (11.6%) lower in the highest quintile compared with hospitals in the lowest quintile for total payments. On the other hand, readmissions and postdischarge ancillary care services accounted for large variations in total payments (Figure 1). The biggest contributors were payments for medical services at nursing homes (approximately 13 times higher) and rehabilitation (4 times higher). Physician payments also varied across quintiles, ranging from $2,272 to $4,957 per patient. Chemotherapy-associated payments, when present (26.9% of patients), averaged $18,369 per patient.
Table 2.
Breakdown of Payments, Price and Case-Mix Adjusted
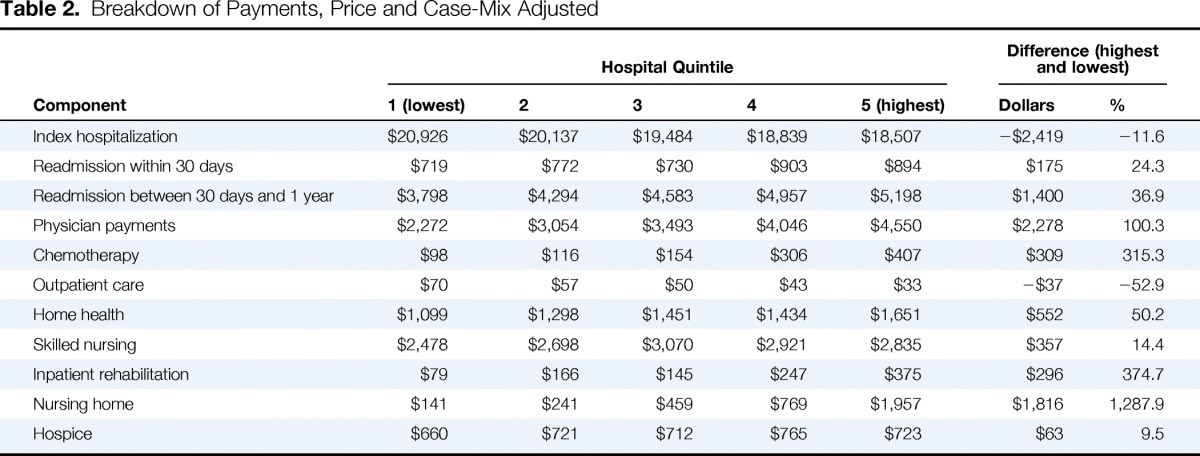
| Component | Hospital Quintile |
Difference (highest and lowest) |
|||||
|---|---|---|---|---|---|---|---|
| 1 (lowest) | 2 | 3 | 4 | 5 (highest) | Dollars | % | |
| Index hospitalization | $20,926 | $20,137 | $19,484 | $18,839 | $18,507 | −$2,419 | −11.6 |
| Readmission within 30 days | $719 | $772 | $730 | $903 | $894 | $175 | 24.3 |
| Readmission between 30 days and 1 year | $3,798 | $4,294 | $4,583 | $4,957 | $5,198 | $1,400 | 36.9 |
| Physician payments | $2,272 | $3,054 | $3,493 | $4,046 | $4,550 | $2,278 | 100.3 |
| Chemotherapy | $98 | $116 | $154 | $306 | $407 | $309 | 315.3 |
| Outpatient care | $70 | $57 | $50 | $43 | $33 | −$37 | −52.9 |
| Home health | $1,099 | $1,298 | $1,451 | $1,434 | $1,651 | $552 | 50.2 |
| Skilled nursing | $2,478 | $2,698 | $3,070 | $2,921 | $2,835 | $357 | 14.4 |
| Inpatient rehabilitation | $79 | $166 | $145 | $247 | $375 | $296 | 374.7 |
| Nursing home | $141 | $241 | $459 | $769 | $1,957 | $1,816 | 1,287.9 |
| Hospice | $660 | $721 | $712 | $765 | $723 | $63 | 9.5 |
Figure 1.
Case-mix and price adjusted Medicare payments in the first year after surgery for colorectal cancer.
Outcomes
Overall, 8.7% of patients required a hospital readmission within 30 days of discharge, which accounted for average payments of $9,014 (when present). Of patients who required readmission within 30-days, 46.1% were subsequently readmitted within 1 year, and 20.2% had multiple readmissions. Patients who required readmission within 30 days had a 1-year mortality rate of 24%, compared with 13.9% for those patients discharged and not readmitted. Hospital 30-day mortality rates were 6.3% and 5.5% at the lowest and highest hospital payment quintiles, respectively, and 1-year mortality rates were 17.4% and 18.5%, respectively.
Discussion
In this national study examining the variation in Medicare payments in the first year after colorectal cancer we found the following: The index surgical hospitalization had the largest share of payments; however, payments around the index hospitalization for surgery were not the driving force behind the observed variation. On the contrary, the largest variations in payments were largely due to readmissions and discretionary services such as, postacute care utilization. Last, 30-day and 1-year mortality rates were similar between the highest and lowest hospital quintiles.
The variation in total Medicare payments in the first year after surgery for CRC presented herein implies sizable potential savings for payers. Before untangling the reasons for the observed variation, it is important to emphasize the importance of price standardization, which takes into account how much of this variation in payments is due to intentional differences in Medicare payments—based on regional wage disparities, and the importance of case-mix adjustments, which accounts for the high cost to hospitals caring for very sick patients. The persistent variation after accounting for these two factors most closely represents the potentially unwarranted differences in services provided for patients with similar risk and cost profiles.
This study provides insights on how payments are distributed to various services and providers after surgery for colorectal cancer. Interestingly, payments attributed to the index hospitalization were somewhat similar or even less than expected after correcting for case-mix and price standardization across quintiles. In other words, payments for the surgical hospitalization are not the driving force behind the higher costs. Therefore, savings from episode-based bundled payments may not be appreciated if the episodes of care are limited to the index hospitalization for the surgical procedure. Even though it seems that the index hospitalization is responsible for the largest share of the cost, the potential for savings is elsewhere.
The complex nature of this variation appears to be largely driven by differences in readmission and discretionary services, such as postacute care or physician services. Use of postacute care services, such as home health care or skilled nursing facilities, is largely discretionary after colorectal cancer surgery, and is responsible for a large share of this variation. Payments for postacute care were substantial and varied widely across hospitals. Such payments would be reduced by incentives to minimize postoperative complications, which are inherently associated with higher rates of postacute care use or readmissions, and efforts to coordinate care to limit postacute care to those patients who truly require further assistance. In addition, readmissions were not only independently associated with substantial financial costs, but were also correlated with worse outcomes. This echoes the findings of prior research on readmissions13 and aligns with the Affordable Care Act's Hospital Readmissions Reduction Program.14
It is clear from this study that defining an episode of care after CRC surgery to achieve financial savings is challenging. The 1-year global payment scheme used herein is informative and identifies areas where there is a need for coordination between services to obtain savings. For example, hospitals that were efficient in their index surgical hospitalization payments were not necessarily as efficient at preventing readmissions nor in their use of postacute care services, and vice versa. Therefore, limiting an episode's definition to include only specific services alone may not result in the desired savings or coordination of care on a large scale. For example, the newly proposed Oncology Care Model, which bundles all Medicare payments made after the initiation of chemotherapy to 6 months after, will hopefully enhance care coordination between oncologists and may decrease the variation in outpatient, physician, or chemotherapy services.7,8 However, it may not affect patient needs related to cancer surgery, readmissions, and postacute care, thus substantial savings may not be readily appreciable for some services. Therefore, it is readily apparent that for episode-based payment models to succeed, the developed bundles should be inclusive of potentially variable services in order to achieve the desired savings without inadvertently penalizing efficient providers.
The present study has several limitations. First, it is limited by the use of administrative date and unmeasured confounding. Specifically, tumor stage is not available in Medicare data. However, patients in this study were selected if they had undergone surgical resection, which may suggest that the cohort had relatively low rates of advanced-stage CRC. Second, this study specifically examines a 1-year episode after surgical resection. Such a strategy anticipates only the potential savings from a broad-based bundling approach. Additional work will be needed to examine the impact of bundling systemic therapy separately. Further, given that this study was performed using Medicare data, we realize that the findings may not be generalizable to the population younger than 65 or those using other types of health care insurance. Nonetheless, more than 50% of all CRC cases occur in patients who are 65 or older,1 and the single government payer design aids in payment standardization.
In conclusion, this study demonstrates that Medicare spending in the first year after surgery for colorectal cancer varies significantly across hospitals. This variation is persistent even after price and case-mix adjustment, and is largely driven by postacute care use and readmissions, and not by short-term payments related to the index surgical hospitalization. This has significant implications for payers and stakeholders with regard to policy decisions on how to bundle payments and define episodes of surgical CRC care.
Acknowledgment
Z.M.A. is supported by Agency for Healthcare Research and Quality (AHRQ) Grant No. T32 HS000053-22. J.D.B. is supported by National Institute on Aging Grant No. P01 AG019783-11. S.L.W. is supported by AHRQ Grant No. 1K08 HS20937-01 and American Cancer Society Grant No. RSG-12-269-01-CPHPS. ArborMetrix had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication or presentation.
Authors' Disclosures of Potential Conflicts of Interest
Disclosures provided by the authors are available with this article at jop.ascopubs.org.
Author Contributions
Conception and design: All authors
Collection and assembly of data: Zaid M. Abdelsattar, Sandra L. Wong
Data analysis and interpretation: Zaid M. Abdelsattar, Sandra L. Wong
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Variation in Medicare Payments for Colorectal Cancer Surgery
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jop.ascopubs.org/site//misc/ifc.xhtml.
Zaid M. Abdelsattar
No relationship to disclose
John D. Birkmeyer
Stock or Other Ownership: ArborMetrix
Consulting or Advisory Role: ArborMetrix
Sandra L. Wong
No relationship to disclose
References
- 1.American Cancer Society. Cancer Facts & Figures 2014. Atlanta, GA: American Cancer Society; 2014. [Google Scholar]
- 2.Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103:117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yabroff KR, Mariotto AB, Feuer E, et al. Projections of the costs associated with colorectal cancer care in the United States, 2000-2020. Health Econ. 2008;17:947–959. doi: 10.1002/hec.1307. [DOI] [PubMed] [Google Scholar]
- 4.Ross W, Lynch P, Raju G, et al. Biomarkers, bundled payments, and colorectal cancer care. Genes Cancer. 2012;3:16–22. doi: 10.1177/1947601912448958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lang K, Lines LM, Lee DW, et al. Lifetime and treatment-phase costs associated with colorectal cancer: Evidence from SEER-Medicare data. Clin Gastroenterol Hepatol. 2009;7:198–204. doi: 10.1016/j.cgh.2008.08.034. [DOI] [PubMed] [Google Scholar]
- 6.Birkmeyer JD, Gust C, Baser O, et al. Medicare payments for common inpatient procedures: Implications for episode-based payment bundling. Health Serv Res. 2010;45:1783–1795. doi: 10.1111/j.1475-6773.2010.01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kline RM, Bazell C, Smith E, et al. Centers for Medicare and Medicaid Services: Using an episode-based payment model to improve oncology care. J Oncol Pract. 2015;11:114–116. doi: 10.1200/JOP.2014.002337. [DOI] [PubMed] [Google Scholar]
- 8.Center for Medicare and Medicaid Innovation. Oncology Care Model. http://innovation.cms.gov/initiatives/Oncology-Care/
- 9.Gottlieb DJ, Zhou W, Song Y, et al. Prices don't drive regional Medicare spending variations. Health Aff (Millwood) 2010;29:537–543. doi: 10.1377/hlthaff.2009.0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller DC, Gust C, Dimick JB, et al. Large variations in Medicare payments for surgery highlight savings potential from bundled payment programs. Health Aff (Millwood) 2011;30:2107–2115. doi: 10.1377/hlthaff.2011.0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dimick JB, Staiger DO, Birkmeyer JD. Ranking hospitals on surgical mortality: The importance of reliability adjustment. Health Serv Res. 2010;45:1614–1629. doi: 10.1111/j.1475-6773.2010.01158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez A, Abdelsattar ZM, Dimick JB, et al. Time-to-readmission and mortality after high-risk surgery. Ann Surg. 2014;10:1–7. doi: 10.1097/SLA.0000000000000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hospital Readmissions Reduction Program. 42 USC § 1395ww(q)(1)