Abstract
Alzheimer's disease (AD) is a scourge of longevity that will drain enormous resources from public health budgets in the future. Currently, there is no diagnostic biomarker and/or treatment for this most common form of dementia in humans. AD can be of early familial-onset or sporadic with a late-onset. Apart from the two main hallmarks, amyloid-beta and neurofibrillary tangles, inflammation is a characteristic feature of AD neuropathology. Inflammation may be caused by a local central nervous system insult and/or by peripheral infections. Numerous microorganisms are suspected in AD brains ranging from bacteria (mainly oral and non-oral Treponema species), viruses (herpes simplex type I), and yeasts (Candida species). A causal relationship between periodontal pathogens and non-oral Treponema species of bacteria has been proposed via the amyloid-beta and inflammatory links. Periodontitis constitutes a peripheral oral infection that can provide the brain with intact bacteria and virulence factors and inflammatory mediators due to daily, transient bacteremias. If and when genetic risk factors meet environmental risk factors in the brain, disease is expressed, in which neurocognition may be impacted, leading to the development of dementia. To achieve the goal of finding a diagnostic biomarker and possible prophylactic treatment for AD, there is an initial need to solve the etiological puzzle contributing to its pathogenesis. This review therefore addresses oral infection as the plausible etiology of late-onset AD (LOAD).
Keywords: Alzheimer's disease, pathogenesis, microorganisms, oral bacteria, direct cause
Alzheimer's disease (AD) is a neurodegenerative disease and the most common example of a group of diseases that manifest as dementia. It is associated with atrophy and specific neuronal death particularly in the hippocampal region of the brain (1). Research into AD pathogenesis has flagged two main categories of the disease: the familial-onset presentation accounts for around 2% of all AD cases and the sporadic form of late-onset AD also referred to as LOAD constitutes approximately 98% of the cases. LOAD displays genetic susceptibility traits of which the well-known risk factor is inheritance of the apolipoprotein (APOEɛ4) gene allele (2) and, it appears to require an environmental factor for disease expression. For example, a pathogen–host interaction can exacerbate neurocognition in some elderly individuals who if in their 80+ years likely become diagnosed with LOAD (3, 4). The rationale for this review therefore is to try to explain the etiology in the vast proportion of LOAD cases that relies on common risk factors. Several scientists have proposed one of these to be peripheral infections (5–11) and the accompanying systemic and local inflammatory mediators (11–13). Of these, the plausible risk from oral infection is the main focus of this review.
Prevalence of AD
AD is a burden of longevity resulting from the superior quality of healthcare provision for all. This factor is likely to contribute to quadrupling of AD subjects living in our society during the next 40 years (14). It is estimated that by 2050 about 13–14 million people are likely to suffer from AD in the United States with a rise in the total costs estimated to be more than $1 trillion. The odds of having a diagnosis of AD when over 85 years of age exceed 1:3 (15). One in six people over 80 years in the United Kingdom has dementia (16). Estimates for the prevalence of AD in the United States indicate that more than 5 million individuals who are 65 years or older currently suffer from AD (1, 15). About 200,000 subjects have been diagnosed with the early-onset familial AD form and healthcare costs for this disease are about $200 billion per year (1). It is clear that AD is fast becoming a major health challenge in the United States and around the globe that will financially drain public health budgets and caregiver services.
Neuropathological characteristics of the AD brain
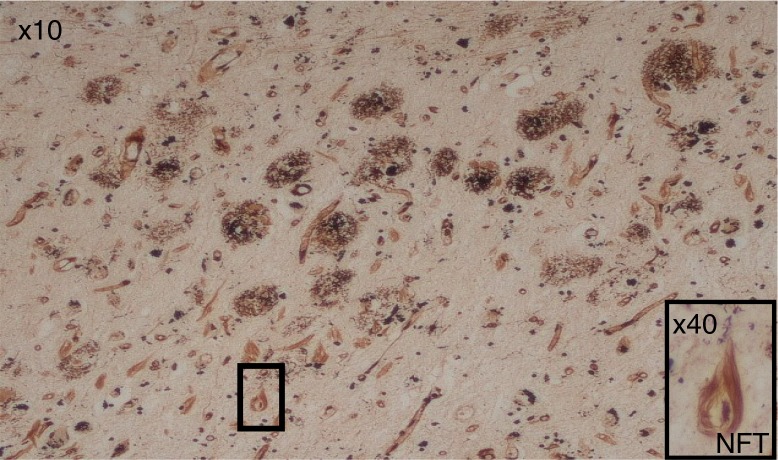
The AD brain is characterized by several neuropathological features of which two seminal hallmarks (Fig. 1) arise from proteostasis of the ongoing neurodegenerative processes and are essential for a definitive diagnosis of the disease post mortem (17). One of the hallmark proteins is made up of fibrils in the form of extracellular, insoluble plaques and consists primarily of amyloid-beta (Aβ) (18). These peptide deposits in variable sizes depend on the secretase enzymes (α-, β-, and Υ-secretases) that cleave it from the longer amyloid precursor protein (APP). Initial reports suggested fibrillar Aβ to be neurotoxic (19) as it has been shown to kill all types of cells by apoptosis induction (20). However, there are two known insoluble fibrillar Aβ amyloid peptides composed of Aβ40 and Aβ42 amino-acid residues which exhibit distinct physiological states within the human brain. There is a general consensus among scientists that the larger (Aβ42) peptide is the neurotoxic form as the aging brain of cognitive intact individuals also displays Aβ plaques. However, in the cognitively intact brain they are fewer in number and usually of the diffuse Aβ40 type that appears not to bear any, as yet known, pathological significance. In addition, there are the soluble monomeric, dimeric, and the multimeric forms of Aβ (21). The relative neurotoxicity of these isoforms remains unclear (22).
Fig. 1.
The pathological hallmarks of AD, numerous extracellular amyloid-Aβ plaques and intra-neuronal neurofibrillary tangles (NFTs). Although there are several NFTs, only one is picked out in boxes at 10× and 40× objective lens magnification.
More recently, the fibrillary forms of the Aβ(40/42) peptides released in the AD brain were also recognized as ‘defensin’ or innate immune defense molecules that act to protect the host against infection (23). For example, both of the aforementioned amyloidogenic peptides can bind to bacterial membranes and in that way lyse bacterial cells. Although Aβ is acting as an antimicrobial peptide (AMP), it may be a part of the brain's ancient/modern innate immune defense mechanism. AMPs are potent, broad-spectrum, pore-forming agents targeting Gram-negative and Gram-positive bacteria, enveloped viruses and protozoans (23), thereby supporting the hypothesis that AD has an infectious origin.
Furthermore, the senile plaques (Aβ42) are recognized as triggers that stimulate activation of microglial cells and initiate local immune responses (24). Activated microglia are the most important contributors of inflammation in the central nervous system (CNS) (25). They secrete a number of pro-inflammatory cytokines (24–26) and recognize pattern-associated molecular patterns (PAMPs) on bacteria and their cellular debris (27–30) in response to CNS infection.
The other pathological characteristic of AD is an accumulation of intracellular hyperphosphorylated tau and heat shock proteins constituting the neurofibrillary tangles (NFTs). Hyperphosphorylated tau protein alters the polymerization and stability of microtubules compromising their function (31). NFTs in AD reflect the severity of disease; however, the significance of pathogen–host interaction to the occurrence of NFTs in the AD brain is poorly understood. Current genetic evidence is pointing to aberrant innate immune responses (32, 33) and cholesterol lipid genes (34) having greater significance in AD pathogenesis. A dysfunctional immune system and predisposition to hyperlipidemia also support the role of reduced blood flow due to the vascular lesions and inflammation, Aβ deposition and microorganisms in AD.
In advanced AD pathology, synaptic dysfunction is another structural defect associated with a decline in memory (35–37). Although a circular argument, malnutrition plays a role in the gradual loss of synapses and fewer teeth during life is a known risk factor for AD (38). Neurons are capable of responding to injury by expressing multiple neurotransmitters. In AD, selective loss of cholinergic neurons in the basal forebrain (39) also correlates with the loss of cognitive function (18, 35).
The amyloid cascade hypothesis
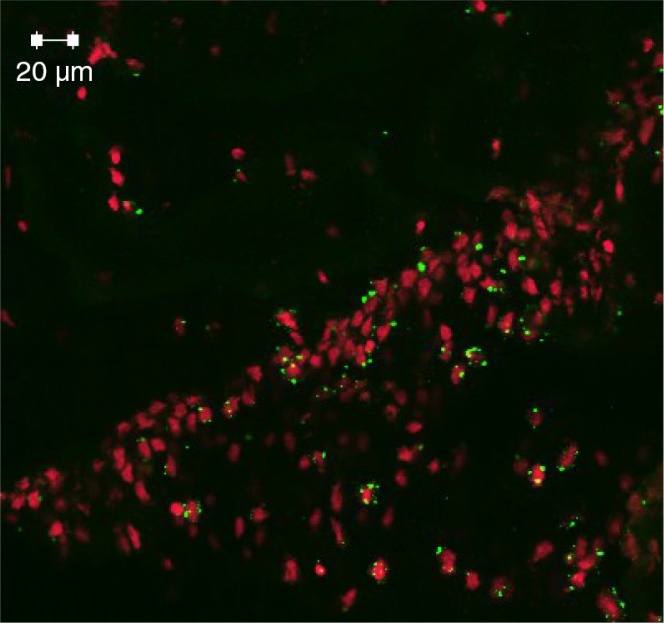
Several hypotheses have been advanced regarding the development of AD. The amyloid cascade hypothesis serves as a model particularly for the familial form of AD (40) which is a disease caused by mutations involving the amyloid-β protein precursor, located on chromosome 21 and presenilins 1 and 2 on chromosomes 14 and 1, respectively, that enhance the APP gene processing toward Aβ deposition (41, 42). The model, which was first proposed by Glenner and Wong (43), maintains that the neurodegenerative disease is due to an imbalance between the generation and clearance of Aβ. Genome-wide association studies (GWAS) highlighted the complement receptor 1 (CR1) gene playing a role in AD pathogenesis (44). One recognized role of CR1, a membrane-bound regulatory protein, is its ability to bind C3b opsonins (Fig. 2). It is abundantly expressed especially on erythrocyte membranes and as such participates in immune complex clearance by transporting waste to the liver and the spleen. As the CR1 gene is a risk factor for LOAD, this suggests loss of function as a possibility for the defective clearance of Aβ in the brain. Other tentative explanations suggest variation in CR1 protein isoforms (longer and shorter forms) (45), whereby the longer form is less involved in the disease process via its ability to bind more C3b and facilitate more effective clearance of Aβ in the brain (46). This is a process that inevitably fails favoring disease expression with more Aβ proteostasis buildup and complement pathway activation. The amyloid hypothesis has been modified several times, particularly due to the finding that soluble oligomers of Aβ may contribute to early preclinical stages of the disease that initiate the cascade leading to synaptic dysfunction, atrophy, and neuronal loss (47).
Fig. 2.
Immunofluorescence labeling (green dots) of hippocampal CA neurons opsonized by iC3b following monoinfection with P. gingivalis at 24 weeks of APOɛ gene knockout (ApoE−/ −) mice. This is indirect evidence of an oral infection having affected the host's brain.
The inflammatory hypothesis
The intrinsic model
Currently, there are two models of the inflammatory hypothesis of AD, an intrinsic and an extrinsic. The intrinsic inflammation model accounts for the intact ‘blood–brain barrier’ (BBB) restricting entry of neurotoxic immune molecules and systemic lymphocytes to the brain. As a consequence, the brain glial cells are able to generate a local and complete innate immune system when challenged by foreign agents (26, 48–50). Historically, neuroinflammation has largely been viewed as being a downstream consequence of the amyloid hypothesis, whereby the presence of amyloidogenic peptides results in the activation of microglia initiating pro-inflammatory cascades and the release of potentially neurotoxic substances resulting in degenerative changes in neurons. GWAS now implicates innate immune genes (44, 51) as being a risk factor and supports a primary role for the inflammatory elements of AD pathology via inappropriate activation of the complement system (52–54) in association with Aβ plaques and NFTs (55).
The extrinsic model
The extrinsic model accounts for communication of the glial cells with the immune challenges presented via the blood vascular system using the circumventricular organs and the choroid plexus that are devoid of the BBB (56). The cells from this region of the brain are fully equipped with the CD14 receptor and the toll-like receptor 4 (TLR 4) to recognize LPS from the peripheral blood circulation (27, 28). Hence, elements of systemic infections such as those originating from Gram-negative, highly virulent oral pathogens, bronchopneumonia and urinary tract infections (3, 4, 7, 57, 58) reach all organs including the CNS. Bacterial products entering the bloodstream trigger the innate immune responses of host cells via pattern recognition receptors (PPR) and TLRs that alert local and distant cells to the infectious threat by secreting immune mediators (cytokines) to confine and defeat the foreign agents. Increased risk of dementia in the elderly following multiple infectious episodes has been reported (4). In addition, systemic infections appear to contribute toward delirium in some clinically diagnosed AD patients and such episodes can exacerbate a premorbid cognitive status (3). Holmes et al. (3, 57) proposed that since cytokines are primary mediators released by the host to defend against infection, such secondary stimuli (IL-1β and TNF-α) may mediate their effect on the brain and indirectly contribute to cognitive decline.
Non-oral bacteria related to AD
Honjo et al. (59) using Bradford Hill's criteria for assessing the relationship between bacteria and disease found Chlamydophila pneumoniae to be a likely infectious agent related to the pathogenesis of AD. Maheshwari and Eslick (60) reported a strong correlation between C. pneumoniae and AD, and according to Shima et al. (61) C. pneumoniae is currently the most plausible of all infectious agents proposed to be involved in AD. Lim et al. (62) suggested that the pro- and chronic inflammatory states in AD pathogenesis may in part be due to C. pneumoniae infection of monocytes. C. pneumoniae antibodies from typical intracellular and atypical C. pneumoniae antigens have been identified both in the frontal and temporal cortices of brains from AD patients (63). Amyloid deposit and NFTs were detected in the same regions in apposition to one another suggesting that C. pneumoniae infection is involved in the development of AD pathology.
Using various techniques, Balin et al. (9) found C. pneumoniae in 80–90% of LOAD brain tissue specimens. C. pneumoniae infection was correlated with the APOEɛ4 allele expression. The same researchers subsequently demonstrated that astroglia, microglia, neurons, endothelial cells, and monocytes in the LOAD brain are permissive to this bacterium. The mechanisms of pathogenesis differ between actively and persistently infecting chlamydiae and it is in the persistent state that these organisms cause chronic disease (64, 65). C. pneumoniae was cultured from two AD brain samples after one or two passages in HEp-2 cells (66). Interestingly, the study indicated that brain isolates were more related to respiratory than to vascular/atheroma strains of C. pneumoniae. This suggested that C. pneumoniae infection of the brain was secondary to bronchopneumonia and at the end stages of LOAD.
It has been suggested that the phages phiCPAR39 and phiCPG1, associated with C. pneumoniae, may enter mitochondria of the bacterial host and work as slow viruses initiating AD (67). These authors hypothesized that mitochondrial recruitment by C. pneumoniae phages may be the primary initiating event in the pathogenesis of neurodegenerative disorders.
In a meta-analysis based on 25 relevant, primarily case-control studies, Maheshwari and Eslick (60) found a statistically significant association between AD and detectable evidence of infection caused by C. pneumoniae or spirochetes. They reported over a 10-fold increased occurrence of AD when there was evidence of spirochetal infection (OR: 10.61; 95% CI: 3.38–33.29) and over a fourfold increased occurrence of AD with a conservative risk estimate (OR: 4.45; 95% CI: 2.33–8.52). There was a fivefold increase in occurrence of AD with C. pneumoniae infection (OR: 5.66; 95% CI: 1.83–17.51). Accordingly, a strongly positive association between bacterial infection and AD was shown for both types of bacteria, but it was strongest for spirochetes.
It is generally accepted that the syphilis spirochete Treponema pallidum can cause chronic neuropsychiatric disorders including dementia as well as other neurodegenerative disorders (11). T. pallidum causes brain atrophy and Aβ deposition in the atrophic form of general paresis (68, 69) and is a strong indication for involvement of spirochetes in AD pathogenesis. Chronic diseases such as syphilis are frequently associated with deposition of amyloid (68, 69). Amyloid is an integral component of spirochetes which may contribute to amyloid deposition in AD (70). Spirochete accumulation in the cerebral cortex in the context of syphilis will also lead to formation of senile plaques, NFTs, and granulovacuolar degeneration (71).
Miklossy (68, 69) analyzed data on the ability of spirochetes to induce pathological and biological hallmarks of AD in vitro following Koch's and Hill's postulates and demonstrated a plausible causal relationship between neurospirochetosis and AD. The data revealed a statistically significant association between spirochetes and AD (p=1.5×1,017, OR=20, 95% CI=8–60, N=247). When mammalian cells were exposed to spirochetes, the pathological and biological hallmarks of AD were reproduced in vitro (68, 69). Historical observations supported the conclusion that chronic spirochetal infections can cause dementia and reproduce the neuropathological hallmarks of AD (72). According to Miklossy (72), these observations represent further evidence in support of a causal relationship between various spirochetal infections and AD.
Another spirochete also implicated in AD is Borrelia burgdorferi, the causative agent of Lyme disease which is transfected to humans via tick vectors. There are great similarities in the clinical and pathological manifestations of syphilis and Lyme disease (72, 73). The occurrence of B. burgdorferi in the brains of AD patients was first reported by MacDonald and Miranda (74) and was confirmed later by MacDonald (75, 76), Riviere et al. (5), and Miklossy et al. (77). Interestingly, Bu et al. (78) found that the infectious burden consisting of B. burgdorferi, C. pneumoniae, Helicobacter pylori, cytomegalovirus,and herpes simplex type-1 (HSV-1) is associated with AD. In contrast, Gutacker et al. (79) and Pappolla et al. (80) found no evidence of an association between B. burgdorferi and AD.
Among other bacterial species, H. pylori (monoinfection) has been found to be related to AD (59). These authors suggested that AD pathology can be initiated and exacerbated by some microorganisms with inflammatory and oxidative responses which may affect the brain continuously and gradually over time. However, the H. pylori status was not associated with AD in a study from Japan, probably due to the high prevalence of the organism in controls (81). This was refuted by Kountouras et al. (82) who had previously found that successful eradication of H. pylori infection was associated with significantly lower mortality risk in AD patients [HR (95% Cl) = 0.287 (0.114–0.725), p=0.008] (83).
Oral bacteria related to AD
The oral cavity harbors an impressive range of bacterial phylotypes (84). Molecular identification methods have detected close to 900 different predominant bacterial species of which 35% cannot yet be cultured (85). The oral microbiome profiles appear to be individualized (86), meaning that bacterial microbiomes can vary both qualitatively and quantitatively between individuals, although there are also significant overlaps. Each individual can harbor up to 200 different bacterial taxa in their mouth and there is a large variation in the microbiota in different oral sites (84, 87). Furthermore, the composition of the oral microbiota irrespective of being indigenous or pathogenic in the oral cavity keeps changing in view of major oral diseases (caries, gingivitis, aggressive and chronic periodontitis, periodontal-endodontic lesions, peri-implantitis, and mucositis) (88–94). Particularly, plaque-induced oral diseases such as periodontitis are associated with a change in the oral microbiota. There is a predominance of anaerobic bacteria in the oral cavity. Many of the major periodontal microorganisms are anaerobic, e.g. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia. The abundance of anaerobes tends to increase with the development of plaque-induced oral diseases.
Periodontal bacterial pathogens are related to AD
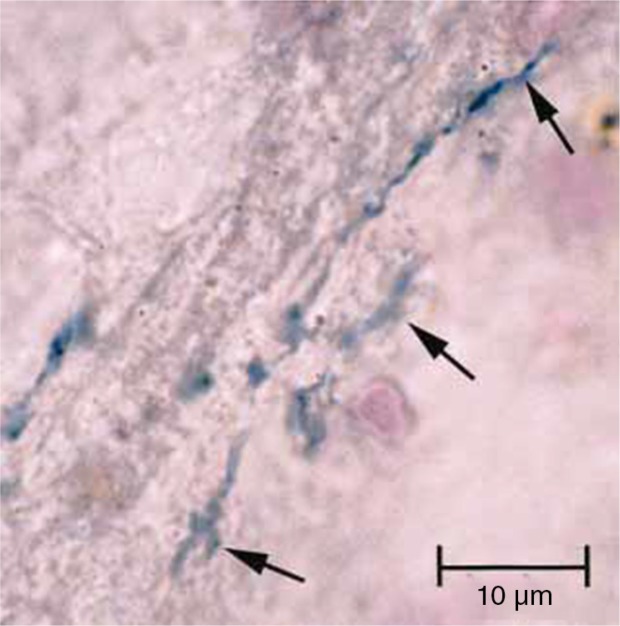
Major pathogens of chronic periodontitis such as P. gingivalis, T. forsythia, and T. denticola are implicated in the development of several inflammatory diseases at remote organ sites. Except for T. forsythia, all three of the above-named organisms of which T. denticola represents a spirochete, have been found in the AD brain (5, 8). Spirochetes are strongly neurotropic. They can spread along nerve fibers and via lymphatics (67, 68) and have been detected in the trigeminal nerve and trigeminal ganglia (95). Spirochetes and their antigens as well as DNA have been found associated with AD and are strongly implicated as the causative agents leading to dementia (68, 69). In 14 studies, spirochetes were detected in AD by different authors in different laboratories and countries by means of different techniques (for reviews see Miklossy (68, 69)). Riviere et al. (5) demonstrated the presence of seven different oral Treponema species in 14 out of 16 AD brain specimens (Fig. 3). Spirochetes were even cultivated from the brains of AD patients indicating that they were viable in the brain (67, 68, 77). Miklossy suggested a co-infection by several spirochetes in AD including the oral varieties (T. socranskii, T. pectinovorum, T. denticola, T. medium, T. amylovorum, and T. maltophilum) as demonstrated by Riviere et al. (5). Spirochetes reproduced the biological and pathological hallmarks of AD after exposure of mammalian neuronal and glial cells in organotypic cultures (68, 69).
Fig. 3.
Section of pons area of Alzheimer's disease brain from an 84-year-old female subject (from Ref. (5) with permission), demonstrates metabolically active Treponema pectinovorum oral bacteria (arrows) stained dark blue following immunostaining with anti-T. pectinovorum using the avidin–biotin peroxidase method.
It was demonstrated that LPS from periodontal bacteria can access the AD brain during life while detection in corresponding controls, with equivalent or longer postmortem interval was absent (8). This study supports the literature on elevated antibodies to periodontal disease-associated bacteria such as P. gingivalis, being found in AD patients (7). Furthermore, in 2,355 people 60 years and over, the third NHANES study found associations between periodontitis and cognitive impairment and between measures of immunoglobulin to P. gingivalis and cognitive test performance (96, 97). In this study, all participants were cognitively intact at baseline. Those who went on to develop AD had higher levels of serum antibodies to periodontal pathogens at baseline. The study suggested a temporal relationship in that the periodontal disease came before AD.
Other important periodontal pathogens related to AD are Fusobacterium nucleatum and Prevotella intermedia. In the NHANES study, antibody levels to these organisms were significantly increased (α=0.05) at baseline serum in patients with AD compared to that in controls (97). The results were significant after controlling for baseline age, Mini-Mental State Examination score, and allele APOEɛ4 status. Noble et al. (98) found that a high anti-Actinomyces naeslundii titer (>640 ng/ml, present in 10% of the subjects) was associated with increased risk of AD (HR = 2.0, 95% CI: 1.1–3.8). This association was stronger after adjusting for other significant titers (HR = 3.1, 95% CI: 1.5–6.4) and confirmed that periodontal pathogens may be associated with AD.
Possible consequences to the brain carrying oral bacterial pathogens
The fact that inflammation is sustained in the AD brain suggests that local immunogenic hallmark proteins and/or peripheral infections are key perpetrators. This is supported by reports highlighting microorganisms and their toxic products as well as DNA in brain tissue of AD patients and experimental animals (see below). Bacteria activate pathways that include the integrin receptor CR3 (CD11b/CD18) and TLR signaling (99) and the complement cascade (100). The NF-κB signaling pathway for cyto/chemokine release (TNF-α, IL-8) (101) produces free radicals, triggers nitric oxide and apoptosis (102). The oral cavity, lungs, and gastrointestinal and urinary tracts are plausible sources of brain microorganisms. The likely passage of the microorganisms of interest from their original sites to the brain is described below.
Infections with spirochetes can cause cerebral hypoperfusion (103), cerebrovascular lesions, and a severely disturbed capillary network (68, 69). Chronic spirochetal infections can also induce slowly progressive dementia, cortical atrophy, chronic inflammation, and Aβ deposition, indistinguishable from that occurring in AD brains (for reviews see Refs. 68, 69, 72). Furthermore, cultured neuronal cells exposed to spirochetes produce Aβ (104). Spirochetes are also able to form plaque-, tangle-, and curly fiber-like lesions (72, 105). They induce a latent and slowly progressive infection by evading host defenses. This promotes their survival and proliferation in the brain by blocking the complement cascade. Spirochetes may even survive and proliferate in hosts that are immune-competent. Interestingly, the remarkable ability of T. pallidum to evade clearance from the immune system has earned it the designation ‘stealth pathogen’ (106). The activated complement cascade following spirochete infections (11) may be used as a non-specific marker of CNS inflammation. Spirochete–host interactions initiate and sustain chronic inflammation triggering various immune responses that activate the innate and adaptive immune system, free radical production, apoptosis, and amyloid deposition typically seen in AD brains (107).
P. gingivalis has been designated as one of the ‘keystone’ periodontal pathogens because it is able to establish and maintain the periodontal disease-associated ‘inflammophilic’ microbiota (108). It is able to perform this task as it possesses an awesome variety of virulence factors, recently reviewed by Singhrao et al. (109), to evade the host immune defenses, thus serving two major functions: initial survival of P. gingivalis itself via a sustainable inflammatory milieu and sustainment of nutritional sources by eliminating microbial competitors (108).
The P. gingivalis endotoxin LPS demonstrates differences in the number of phosphate groups together with both the amount of lipid A fatty acids and their specific position. The presence of multiple lipid A structures makes it more difficult for the innate host responses to recognize the molecule thereby aiding the virulence of P. gingivalis (110). The consequences of finding P. gingivalis LPS in the host's body, e.g. the brain (8), include priming of immune cells for differential activation of the TLR-mediated NF-κB signaling pathway (111) leading to cytokine liberation, complement activation, and maintenance of intracerebral inflammation.
P. gingivalis evades circulating phagocytes by adhering to erythrocytes (112). An active invasion of P. gingivalis and infection-induced complement activation with bystander neural injury was detected in the brains of ApoE−/ − mice (113). This supported previous notions that bacterial infections can contribute to the development of AD pathology via mechanisms involving acute-phase proteins such as cytokines and the complement cascade where neurons would be attacked.
Oral virus related to AD
Herpes simplex virus (HSV) is present in more than 70% of the population after 50 years of age (114–116). It persists latently in the peripheral nervous system and is periodically reactivated. Characteristically, HSV-1 has been designated as the enemy within (10). Herpes viruses, including Epstein-Barr virus and cytomegalovirus, are found in high copy counts in aggressive periodontitis, and may interact synergistically with periodontopathic bacteria in the pathogenesis of this disease (117). Periodontal infections activated by Herpes virus may impair local host defenses and thus increase the aggressiveness of resident periodontopathic bacteria. The bacteria, in turn, may augment the virulence of the herpes viruses.
High proportions of viral-associated proteins in amyloid-containing plaques and/or NFTs corroborate with the involvement of HSV-1 in AD pathology (118). Notably, De Chiara et al. (119) reported an association between Aβ accumulation in the brain and HSV infection. Itzhaki et al. (120) suggested that not only does HSV-1 produce the main components of amyloid plaques and NFTs (i.e. Aβ and hyperphosphorylated tau), but it also interferes with the autophagic events that prevent degradation of these proteins and eventually leading to their accumulation in the AD brain. Furthermore, in vitro and in vivo investigations in murine models following HSV-1 infections demonstrated Aβ accumulation (121).
A number of scientists have suggested that there is an imbalance between production and clearance of β-amyloid in the brain, a premise first proposed by Wisniewski et al. (122) based on the discovery of soluble species of this protein and later confirmed by Zlokovic et al. (123). It is now widely accepted that defective clearance of this protein is a hallmark of AD brains leading to its accumulation in the form of insoluble Aβ40/42 plaques. Although HSV and cytomegalovirus have been detected in the brains of older adults with and without AD (124–126), HSV-1 viral DNA is present in a higher proportion of AD patients (127). It is particularly seen in the temporal and frontal cortices which are the brain regions that are most damaged in AD (128, 129). The relevance of this association is still under investigation; however, a plausible role for the HSV-1 viral DNA could be associated with the plaque maturation process. Jamieson et al. (127) found that the virus was absent in the brains of most young people, probably because it enters the brain during old age either with immune senescence (130) or the virus itself is initially responsible for weakening host's immune defenses. This latter explanation is likely and is supported by us and others (131).
HSV-1 is a strong risk factor for AD in the brains of those with the APOEɛ4 allele (125, 132). This virus is not only a dormant passenger but can also persist in the latent form in neurons or replicate at a very low level in neuroglia (133). During persistence, it may release toxic products continuously and induce pro-inflammatory cytokines at low levels which become an additional burden to a host already challenged by age, poor diet, restricted exercise as well as any genetic susceptibilities. Itzaki and Wozniak (10) suggested that stress or peripheral infection can reactivate the virus periodically from latency in the brain. This may cause an acute but presumably localized infection, and subsequent damage modulated by the APOɛ gene can lead to formation of Aβ plaques and NFTs.
The presence of anti-HSV IgM, a sign of reactivated infection, almost doubled the risk for AD while anti-HSV IgG did not influence the risk (134). Kobayashi et al. (135) suggested that the anti-HSV-1 Ig antibody avidity index could be a useful biomarker for early diagnosis of amnestic mild cognitive impairment, which is prodromal to AD, as well as for AD sufferers.
Reactivation of HSV seropositivity is highly correlated with incident-AD (136). Letenneur et al. (136) speculated that AD pathology starts many years before frank dementia and recurrent reactivation of HSV can act as a potent stimulus to brain microglia, increasing cytokine levels, and triggering a positive feedback cycle leading to increasing accumulation of neurohistopathological changes. In other words, infection, followed by local CNS inflammatory reaction is the likely primary stimulus whereas proteostasis is a consequence of the primary event leading to the development of AD.
Hill et al. (137) suggested a role for HSV-1-induced miRNA-146a in the evasion of HSV-1 from the complement system which is a major first-line host defense mechanism, and the activation of key elements in the arachidonic acid cascade known to contribute to AD-type neuropathological changes.
Oral yeasts related to AD
Oral yeast infection represents a secondary opportunistic infection particularly involving Candida albicans, but increasingly non-albicans species, e.g. Candida glabrata. With a growing population of elderly, severe systemic fungal infections have increased dramatically in this age group during the last 30 years (138, 139). Oral yeasts can be found in periodontal pockets, in root canals, on the mucosae and underneath dentures (denture stomatitis) (140–142). Denture stomatitis is prevalent in elderly wearing dentures that are heavily contaminated with yeasts which can be a source of systemic mycosis. Disseminated mycoses have recently been reported in AD patients (143, 144). Fungal molecules including proteins and polysaccharides [(1,3)-β-glucan] were detected in peripheral blood serum, and fungal proteins and DNA were demonstrated by PCR in brain tissue of AD patients. Chitin-like fungal structures have also been found in the AD brain (145) and chitinase activity has been proposed as a powerful biomarker of AD (146). In AD brains, cytoplasmic material in a small number of cells was targeted by antibodies with immunoreactivity to yeast cells (147). These findings were consistent with the idea that neurons can be infected by fungi. Interestingly, antifungal treatment reversed the clinical symptoms of some AD patients (148, 149).
How do oral microorganisms reach the brain?
Blood stream dissemination
The most likely pathway for dissemination of oral microorganisms to the brain is through the blood stream (150). Dental treatment as well as brushing, flossing, chewing, and use of tooth picks in a patient with periodontitis will release a bacteremia (151). This can occur several times during the day and has been estimated to last for up to 3 hours for oral bacteria (152). The bacteremia is usually contained by immune cells of the body. However, in people with reduced immune defense, e.g. older individuals, bacteria may localize to crevices of the oral cavity and vascular channels (150).
The blood–brain barrier
An intact BBB prevents microorganisms in the blood from accessing the brain. However, aging favors overgrowth of oral microorganisms, particularly anaerobic bacteria and facultative yeasts that established earlier in life and provoked pro-inflammatory responses that weakened the BBB (16). Notably, magnetic resonance imaging (MRI) confirmed loss of BBB integrity in a mouse model of disseminated candidosis (153). Loss of integrity allows microorganisms to spread through the blood stream and quietly contribute in the pathogenesis of AD. During immunosenescence, the innate immune system gradually takes over for the acquired immune system. This contributes to a rise in circulating pro-inflammatory cytokines such as TNF-α (16). Indeed, pro-inflammatory mediators can cross the BBB (3, 7, 154). APOEɛ4, TNF-α and perhaps Ephrin Type-A Receptor 1 (EphA1) may influence BBB integrity and thus be important for penetration of bacteria, LPS, and other toxic bacterial products as well as yeasts into the brains of AD patients (16). APOEɛ4 affects the integrity of the BBB by activating the cyclophilin A matrix metalloproteinase MMP-9 pathway (155).
It is also plausible to suggest that the permeability of the BBB increases with age and thus promotes AD pathogenesis making the brain accessible to microorganisms. Mice with a mutation in the APP gene which is related to early-onset AD in humans, showed increased permeability of the BBB and increased formation of senile plaque as compared to that in control mice (156). The changes increased with age.
Circumventricular organs and perivascular spaces
Circumventricular organs (permit polypeptide hypothalamic hormones to leave the brain without disrupting the BBB) are not dependent on the BBB (56) and may act as another entry portal to the brain for bacteria (157). Poole et al. (8) postulated that bacteria and their products may also directly access the brain via the systemic circulation through the perivascular spaces.
The olfactory hypothesis
The ‘olfactory hypothesis’ suggests the olfactory tract as a potential route for pathogenic bacteria to enter the brain and thereby trigger the production of Aβ and NFTs (158). The olfactory and trigeminal nerves are known to be used by periodontal pathogens to bypass the BBB for direct passage to the CNS (5, 150, 159, 160). Identification of oral treponemes in the trigeminal ganglia supports such a route of dissemination (5). Furthermore, spirochetes may spread along the fila olfactoria and tractus olfactorius (68, 69).
Olfactory unsheathing cells (OECs) engulf bacteria and migrate toward TNF-α released by activated astrocytes (161). Therefore, OECs could be a vehicle for transporting live bacteria into the brain (i.e. Trojan horse). The olfactory bulb was the first area where NFTs and Aβ deposition were detected in the neuropathological trajectory of AD in humans (162) and in mouse models of AD (163).
Genetic, nutritional, and environmental factors promoting AD
While early-onset AD is genetically determined, LOAD is thought to result from interaction between genetic and environmental factors (12). Several mutated genes are associated with the familial AD, such as the amyloid beta (Aβ) precursor protein (AβPP) gene and the presenelin-1 (PSEN-1) and PSEN-2 gene (164–166). A major risk factor for LOAD is polymorphism in the APOɛ4 allele (2). Also cytokine-related genes seem to be involved in the susceptibility to inflammation in both LOAD (167, 168) and periodontitis (169–171). Thus, polymorphisms that increase TNF-α also increase the risk of both AD and periodontitis (172, 173). Lambert et al. (174) found that 20 different loci can increase host susceptibility to AD including polymorphisms in genes associated with interleukin-1 (IL-1) (71, 175–178) and TNFα (71, 172, 179–181). The APOɛ4 gene, which is one of these 20 loci, is highly correlated with AD (182) but it is also a risk factor for infection and increases the expression of inflammatory mediators (11). Recently, genetic overlap between AD, C-reactive protein (CRP) and plasma lipids was demonstrated by using summary statistics from GWAS of over 200,000 individuals (183). There may also be interplay between genetic risk and environmental risk factors such as toxins and or bacterial, viral and fungal pathogens in LOAD reflecting its complex and multifactorial etiology (1).
Diet with its content of essential B-vitamins, phospholipids, and other micronutrients is important for forming new nerve synapses (184). Nutritional deficiencies are common both in elderly and in dementia subjects as briefly discussed by Singhrao et al. (150).
Association between chronic periodontal disease and AD
There is increasing evidence for an association between chronic periodontitis and LOAD (185). Cross-sectional and longitudinal studies have demonstrated that gingival bleeding, loss of periodontal attachment, periodontal probing depth, alveolar bone loss, and antibodies to periodontal pathogens are significantly associated with lower cognitive function and decline after adjustment for co-variates (for a review see (12)). Acute-phase proteins, including cytokines are possible indirect links between periodontal pathogens and/or their virulence factors (12, 13). Elderly often show neglect of oral hygiene which can stimulate recurrent chronic oral infection (150). This again promotes inflammation which can lead to confusion and dementia (3, 4, 154). In 152 subjects 50–70 years of age who were followed for 20 years, greater levels of periodontal inflammation correlated with lower cognitive levels (186). Furthermore, gingival bleeding and loss of periodontal attachment were significantly associated with cognitive impairment in a cohort of 5,138 people aged 20–59 years (187). In 144 nuns, those encoding APOEɛ4 and who had fewer teeth experienced more rapid cognitive decline than those with neither or either of these risk factors (188). Clinical and epidemiological studies showed that loss of teeth is associated with poor memory (6, 96, 187, 189). In another study of 597 community dwelling men followed for 32 years, tooth loss, increasing periodontal pocket depths, and progression of alveolar bone loss were associated with impaired cognition particularly in those over 45 years of age (190). Recently, de Souza Rolim et al. (191) found that periodontal infections were more frequent in patients with mild AD than in healthy subjects. Another interesting feature related to the pathogenesis of AD is the low level of infection by ‘commensals on the loose’ (16). These ‘immuno-tolerated’ bacteria may silently multiply in sites outside of their primary niche and an ongoing infection at their secondary location may have significant deleterious effects upon the health of the elderly or demented host with an existing immunocompromised status.
Putative treatment and prophylaxis of AD
There is no effective treatment or prophylaxis yet for AD, but several approaches have been proposed. Efforts in this respect are important. If we could delay onset of dementia by only 2 years we might lower the prevalence of AD by more than 22 million cases over the next 40 years (14). Notably, the inheritance of the APOEɛ4 allele in the very old (90 + ) age group appears to confer protection (192), having bypassed a period of being at risk around 85+ years of age.
If periodontal disease is implicated in AD, periodontitis prophylaxis could be of help. It would be interesting to see if this has any effect on the initiation and aggravation of AD but an observation period of decennia is probably needed.
In a study of subjects with mild-to-moderate AD, a 3-month course of doxycycline and rifampicin reduced cognitive deterioration during a 6 months’ follow-up interval (193). It was concluded that use of antibacterial compounds may not have had any effect on the treatment of C. pneumoniae but had a beneficial effect on cognitive decline in AD (193). This might be related to prevention or attenuation of a number of peripheral infections or dampening down the pro-inflammatory cytokine response. Minocycline was found to correct early, pre-plaque neuroinflammation and inhibit the APP cleaving enzyme 1 (BACE-1) in a transgenic model of AD-like amyloid pathology (194). It was suggested that interfering with inflammation could be a useful therapeutic approach in early, pre-plaque stages of AD-like amyloid pathology.
Anti-inflammatory drugs given for at least 2 years before the onset of dementia delayed the disease process (195–197). It may also be beneficial to combine anti-inflammatory agents with antibacterials (193). Examination of several available non-steroidal anti-inflammatory drugs (NSAIDs) showed that only a few of them had any useful Aβ-modifying or other activity of therapeutic use in LOAD (for a review see (1)).
Itzhaki and Wozniak (10, 198) suggested that antiviral therapy and perhaps vaccination against HSV-1 in early life could be useful. If HSV-1 is implicated in AD, vaccination could prevent the excessive accumulation of Aβ in the brain. Vaccination with mixed HSV glycoproteins prior to HSV infection protected against viral latency in mouse brains (199). Also Mori (200) maintained that antiviral approaches including chemotherapy and vaccination are promising for prevention and treatment of AD and remaining to be validated. Furthermore, Carter (118) suggested that vaccination or antiviral agents and immune suppressants may be considered as therapeutic options before or during the early stages of AD. Interestingly, exposure of HSV-1-infected cell cultures to intravenous immunoglobulin acting via anti-β-amyloid antibodies reduced the accumulation of Aβ and phosphorylated tau (201).
Angiotensin-converting enzyme (ACE) from Stigmatella aurantiaca may cleave the Aβ peptide similar to human ACE and may be used as a novel form of treatment against AD (202). Furthermore, Chiarini et al. (203) maintained that calcilytics could halt AD progression and preserve the patients’ cortical neurons, cognitive abilities, and eventually life if given at minimal cognitive impairment or at earlier stages. Studies using mice suggested the use of tau aggregation inhibitors as potential drugs for the treatment of AD and other tauopathies (204).
Resveratrol is a polyphenol present in red wine. Its capability of directly interfering with the toxic β-amyloid protein aggregation in AD has recently been shown (205). Resveratrol was found to reduce Aβ-induced toxicity in a Caenorhabditis elegans model of AD by targeting specific proteins involved in proteostasis and thereby reducing the amount of aggregated Aβ (206). This is in concert with our previous finding that the effect of a drinking pattern of 2–7 times per week reduced the risk of myocardial infarction among men who had a history of tooth extractions due to periodontal/dental infection (207).
Potent inhibitors of Aβ oligomer formation or Aβ-induced cell toxicity have proven to be attractive means for therapeutic intervention of AD. Song et al. (208) found that the anti-Alzheimer effects of centipedegrass, which contains several C-glycosyl flavone constituents, occurred through inhibition of neuronal cell death by intervening with oligomeric Aβ formation and reducing beta-site APP cleaving enzyme 1 activity. The authors suggested that maysin, a major flavonoid of corn silk, in centipedegrass could be an excellent therapeutic candidate for the prevention of AD.
Active immunization against important domains of Alzheimer tau eliminated tau aggregation and neurofibrillary pathology (209). The AD type of tau hyperphosphorylation was abolished in transgenic mice by vaccination across a wide range of AD phospho-epitopes. Kontsekova et al. (209) demonstrated that active immunization of rats with a tau peptide encompassing the epitope revealed by monoclonal antibody DC8E8 led to elimination of all major hallmarks of neurofibrillary pathology involving a 95% reduction in the AD-type hyperphosphorylation of tau.
Conclusions
LOAD, which is the predominant form of AD, does not seem to have a single cause. On the contrary, a multitude of factors may be involved and they may act in concert. Among others, both genetic and environmental factors may be involved. Even among microorganisms, cooperation may occur since the brain can hardly differentiate between different microbial insults which collectively contribute capacity for enhancing inflammation. Irrespective of the cause, systemic inflammation may predict the onset of dementia. Organisms such as spirochetes, P. gingivalis, C. pneumoniae, H. pylori, Herpes simplex type I virus, and Candida are among the prime candidate pathogens in AD brains. In the cascade of events causing AD, oral microorganisms may play a role, particularly anaerobic bacteria such as treponemes, P. gingivalis, Prevotella spp., Fusobacterium and Actinomyces, but also facultative anaerobic Candida species. It is important to recognize that infection can occur decades before the manifestation of dementia. The most convincing evidence for a causal relationship between oral bacteria and AD is noted for spirochetes which are both neurotropic and motile. It is likely that oral infection can be a risk factor for AD but it is not the only one. Experiments in humans may require long exposure time to disclose key events and mechanisms of AD. There is, as yet, no cure for AD despite concerted efforts and investment by industry. Prevention of AD through long-term use of antibiotics may be impractical and could select for resistant bacteria. This is worrisome as the prevalence of AD and the public expenses related to its management are expected to increase greatly in the next decade.
If anaerobes of periodontitis play a major role in AD, dental hygiene and treatment will provide the AD prophylaxis from an early age as periodontitis is modifiable. However, improving oral hygiene and treating periodontal disease in the AD patient can be challenging since patients are often uncooperative. There is also need for training caregivers to assist with oral care in such patients.
Vaccination against key organisms and important domains of AD has had some beneficial effect. Also several agents interfering directly with the pathogenesis of AD have been tested. In order to find a cure, there is a need for clinical diagnostic information and knowledge of the causal agents for AD so that specific treatment options targeting these organisms can be developed. As for diagnostic biomarkers, increased antibody levels to specific oral pathogens in particular to P. gingivalis may be used as a monitoring tool years before clinical manifestation of AD. This is important because treatment will probably have to start early.
Acknowledgement
IO acknowledges funding through the European Commission (FP7-HEALTH-306029 ‘TRIGGER’).
Conflict of interest and funding
There is no conflict of interest in the present study for any of the authors.
References
- 1.Balin BJ, Hudson AP. Etiology and pathogenesis of late-onset Alzheimer's disease. Curr Allergy Asthma Rep. 2014;14:417. doi: 10.1007/s11882-013-0417-1. doi: http://dx.doi.org/10.1007/s11882-013-0417-1. [DOI] [PubMed] [Google Scholar]
- 2.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–3. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 3.Holmes C, El-Okl M, Williams AL, Cunningham C, Wilcockson D, Perry VH. Systemic infection, interleukin 1 beta, and cognitive decline in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2003;74:788–9. doi: 10.1136/jnnp.74.6.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunn N, Mullee M, Perry VH, Holmes C. Association between dementia and infectious disease: evidence from a case-control study. Alzheimer Dis Assoc Discord. 2005;19:91–4. doi: 10.1097/01.wad.0000165511.52746.1f. [DOI] [PubMed] [Google Scholar]
- 5.Riviere GR, Riviere KH, Smith KS. Molecular and immunological evidence of oral Treponema in the human brain and their association with Alzheimer's disease. Oral Microbiol Immunol. 2002;17:113–18. doi: 10.1046/j.0902-0055.2001.00100.x. [DOI] [PubMed] [Google Scholar]
- 6.Stein PS, Desrosiers M, Donegan SJ, Yepes JF, Kryscio RJ. Tooth loss, dementia and neuropathy in the NUN study. J Am Dent Assoc. 2007;138:1314–22. doi: 10.14219/jada.archive.2007.0046. quiz 1381–2. [DOI] [PubMed] [Google Scholar]
- 7.Kamer AR, Craig RG, Pirraglia E, Dasanayake AP, Norman RG, Boylan RJ, et al. TNF-α and antibodies to periodontal bacteria discriminate between Alzheimer's disease patients and normal subjects. J Neuroimmunol. 2009;216:92–7. doi: 10.1016/j.jneuroim.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poole S, Singhrao SK, Kesavalu L, Curtis MA, Crean S. Determining the presence of periodontopathic virulence factors in short-term postmortem Alzheimer's disease brain tissue. J Alzheimers Dis. 2013;36:665–77. doi: 10.3233/JAD-121918. doi: http://dx.doi.org/10.3233/JAD-121918. [DOI] [PubMed] [Google Scholar]
- 9.Balin BJ, Little CS, Hammond CJ, Appelt DM, Whittum-Hudson JA, Gérard HC, et al. Chlamydophila penumoniae and the etiology of late-onset Alzheimer's disease. J Alzheimers Dis. 2008;13:371–80. doi: 10.3233/jad-2008-13403. [DOI] [PubMed] [Google Scholar]
- 10.Itzhaki RF, Wozniak MA. Herpes simplex virus type 1 in Alzheimer's disease: the enemy within. J Alzheimers Dis. 2008;13:393–405. doi: 10.3233/jad-2008-13405. [DOI] [PubMed] [Google Scholar]
- 11.Miklossy J. Chronic inflammation and amyloidogenesis in Alzheimer's disease – role of spirochetes. J Alzheimers Dis. 2008;13:381–91. doi: 10.3233/jad-2008-13404. [DOI] [PubMed] [Google Scholar]
- 12.Kamer AR, Dasanayake AP, Craig RG, Glodzik-Sobanska L, Bry M, de Leon MJ. Alzheimer's disease and peripheral infections: the possible contribution from periodontal infections, model and hypothesis. J Alzheimers Dis. 2008;13:437–49. doi: 10.3233/jad-2008-13408. [DOI] [PubMed] [Google Scholar]
- 13.Watts A, Crimmins EM, Gatz M. Inflammation as a potential mediator for the association between periodontal disease and Alzheimer's disease. Neuropsychiatr Dis Treat. 2008;4:865–76. doi: 10.2147/ndt.s3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement. 2007;3:186–91. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 15.Ouerfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362:329–44. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 16.Shoemark DK, Allen SJ. The microbiome and disease: reviewing the links between the oral microbiome, aging and Alzheimer's disease. J Alzheimers Dis. 2015;43:725–38. doi: 10.3233/JAD-141170. [DOI] [PubMed] [Google Scholar]
- 17.Alzheimer A. Über eine eigenartige Erkrankung der Hirnrinde. All Z Psychiat. 1907;64:146–8. doi: 10.1002/ca.980080612. [DOI] [PubMed] [Google Scholar]
- 18.Selkoe DJ. Alzheimer's disease. Cold Spring Harb Perspect Biol. 2011;2011:3. doi: 10.1101/cshperspect.a004457. doi: http://dx.doi.org/10.1101/cshperspect.a004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yankner BA, Dawes LR, Fisher S, Villa-Komaroff L, Oster-Granite ML, Neve RL. Neurotoxicity of a fragment of the amyloid precursor associated with Alzheimer's disease. Science. 1989;245:417–20. doi: 10.1126/science.2474201. [DOI] [PubMed] [Google Scholar]
- 20.Deshpande A, Mina E, Glabe C, Busciglio J. Different conformations of amyloid beta induce neurotoxicity by distinct mechanisms in human cortical neurons. J Neurosci. 2006;26:6011–18. doi: 10.1523/JNEUROSCI.1189-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glabe CC. Amyloid accumulation and pathogenesis of Alzheimer's disease: significance of monomeric, oligomeric and fibrillar Abeta. Subcell Biochem. 2005;38:167–77. doi: 10.1007/0-387-23226-5_8. [DOI] [PubMed] [Google Scholar]
- 22.Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, et al. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–42. doi: 10.1038/nm1782. doi: http://dx.doi.org/10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soscia SJ, Kirby JE, Washicosky KJ, Tucker SM, Ingelsson M, Hyman B, et al. The Alzheimer's disease-associated amyloid beta-protein is an antimicrobial peptide. PLoS One. 2010:5, e9505. doi: 10.1371/journal.pone.0009505. doi: http://dx.doi.org/10.1371/journal.pone.0009505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, et al. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nat Rev Neurol. 2010;6:193–201. doi: 10.1038/nrneurol.2010.17. http://dx.doi.org/10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- 26.Hanisch UK. Microglia as a source and target of cytokines. Glia. 2002;40:140–55. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- 27.Lacroix S, Feinstein D, Rivest S. The bacterial endotoxin lipopolysaccharide has the ability to target the brain in upregulating its membrane CD14 receptor within specific cellular populations. Brain Pathol. 1998;8:625–40. doi: 10.1111/j.1750-3639.1998.tb00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laflamme N, Rivest S. Toll-like receptor 4: the missing link of the cerebral innate immune response triggered by circulating Gram-negative bacterial cell wall components. FASEB J. 2001;15:155–63. doi: 10.1096/fj.00-0339com. [DOI] [PubMed] [Google Scholar]
- 29.Beutler B, Hoebe K, Du X, Ulevitch RJ. How we detect microbes and respond to them: the toll-like receptors and their transducers. J Leukoc Biol. 2003;74:479–85. doi: 10.1189/jlb.0203082. [DOI] [PubMed] [Google Scholar]
- 30.Rivest S. Regulation of innate immune responses in the brain. Nat Rev Immunol. 2009;9:429–39. doi: 10.1038/nri2565. doi: http://dx.doi.org/10.1038/nri2565. [DOI] [PubMed] [Google Scholar]
- 31.Iqbal K, Grue-Iqbal I. Ubiquitination and abnormal phosphorylation of paired helical filaments in Alzheimer's disease. Mol Neurobiol. 1991;5:399–410. doi: 10.1007/BF02935561. [DOI] [PubMed] [Google Scholar]
- 32.Malpass K. Alzheimer disease: functional dissection of CD33 locus implicates innate immune response in Alzheimer disease pathology. Nat Rev Neurol. 2013;9:360. doi: 10.1038/nrneurol.2013.119. doi: http://dx.doi.org/10.1038/nrneurol.2013.119. [DOI] [PubMed] [Google Scholar]
- 33.Shulman JM, Chen K, Keenan BT, Chibnik LB, Fleisher A, Thiyyagura P, et al. Genetic susceptibility for Alzheimer disease neuritic plaque pathology. JAMA Neurol. 2013;70:1150–7. doi: 10.1001/jamaneurol.2013.2815. doi: http://dx.doi.org/10.1001/jamaneurol.2013.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guerreiro RJ, Hardy J. Alzheimer's disease genetics: lessons to improve disease modelling. Biochem Soc Trans. 2011;39:910–16. doi: 10.1042/BST0390910. doi: http://dx.doi.org/10.1042/BST0390910. [DOI] [PubMed] [Google Scholar]
- 35.Terry RD. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–80. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 36.Masliah E, Mallory M, Hansen L, Alford M, Albright T, DeTeresa R, et al. Patterns of aberrant sprouting in Alzheimer's disease. Neuron. 1991;6:729–39. doi: 10.1016/0896-6273(91)90170-5. [DOI] [PubMed] [Google Scholar]
- 37.Masliah E, Mallory M, Hansen L, DeTeresa R, Terry RD. Quantitative synaptic alterations in the human neocortex during normal aging. Neurology. 1993;43:192–7. doi: 10.1212/wnl.43.1_part_1.192. [DOI] [PubMed] [Google Scholar]
- 38.Kondo K, Niino M, Shido K. A case-control study of Alzheimer's disease in Japan – significance of life-styles. Dementia. 1994;5:314–26. doi: 10.1159/000106741. [DOI] [PubMed] [Google Scholar]
- 39.Bartus RT, Dean RL, 3rd, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–14. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 40.Demetrius LA, Magistretti PJ, Pellerin L. Alzheimer's disease: the amyloid hypothesis and the Inverse Warburg effect. Front Physiol. 2015;5:522. doi: 10.3389/fphys.2014.00522. doi: http://dx.doi.org/10.3389/fphys.2014.00522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanzi RE, Watkins PC, Stewart GD, Wexler NS, Gusella JF, Haines JL. A genetic linkage map of human chromosome 21: analysis of recombination as a function of sex and age. Am J Hum Genet. 1992;50:551–8. [PMC free article] [PubMed] [Google Scholar]
- 42.Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, et al. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer's disease. Nat Med. 1996;2:864–70. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- 43.Glenner GG, Wong CW. Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984;122:1131–5. doi: 10.1016/0006-291x(84)91209-9. [DOI] [PubMed] [Google Scholar]
- 44.Lambert JC, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer's disease. Nat Genet. 2009;41:1094–9. doi: 10.1038/ng.439. doi: http://dx.doi.org/10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- 45.Thambisetty M, An Y, Nalls M, Sojkova J, Swaminathan S, Zhou Y, et al. Effect of complement CR1 on brain amyloid burden during aging and its modification by APOE genotype. Biol Psychiatry. 2013;73:422–8. doi: 10.1016/j.biopsych.2012.08.015. doi: http://dx.doi.org/10.1016/j.biopsych.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Killick R, Hughes TR, Morgan BP, Lovestone S. Deletion of Crry, the murine ortholog of the sporadic Alzheimer's disease risk gene CR1, impacts tau phosphorylation and brain CFH. Neurosci Lett. 2013;533:96–9. doi: 10.1016/j.neulet.2012.11.008. doi: http://dx.doi.org/10.1016/j.neulet.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119–28. doi: 10.1016/S1474-4422(09)70299-6. doi: http://dx.doi.org/10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morgan BP, Gasque P. Expression of complement in the brain: role in health and disease. Immunol Today. 1996;17:461–6. doi: 10.1016/0167-5699(96)20028-f. [DOI] [PubMed] [Google Scholar]
- 49.Benveniste EN. Cytokine actions in the central nervous system. Cytokine Growth Factor Rev. 1998;9:259–75. doi: 10.1016/s1359-6101(98)00015-x. [DOI] [PubMed] [Google Scholar]
- 50.Gasque P. Complement: a unique innate immune sensor for danger signals. Mol Immunol. 2004;41:1089–98. doi: 10.1016/j.molimm.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 51.Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer's disease. Nat Genet. 2009;41:1088–93. doi: 10.1038/ng.440. doi: http://dx.doi.org/10.1038/ng.440. Erratum in: Nat Genet 2009; 41: 1156. Nat Genet 2013; 45: 712. Haun, Reinhard [added] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eikelenboom P, Stam FC. Immunoglobulins and complement factors in senile plaques. An immunoperoxidase study. Acta Neuropathol. 1982;57:239–42. doi: 10.1007/BF00685397. [DOI] [PubMed] [Google Scholar]
- 53.McGeer PL, Akiyama H, Itagaki S, McGeer EG. Activation of the classical complement pathway in brain tissue of Alzheimer patients. Neurosci Lett. 1989;107:341–6. doi: 10.1016/0304-3940(89)90843-4. [DOI] [PubMed] [Google Scholar]
- 54.Rogers J, Cooper NR, Webster S, Schultz J, McGeer PL, Styren SD, et al. Complement activation by beta-amyloid in Alzheimer disease. Proc Natl Acad Sci USA. 1992;89:10016–20. doi: 10.1073/pnas.89.21.10016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shen Y, Lue L, Yang L, Roher A, Kuo Y, Strohmeyer R, et al. Complement activation by neurofibrillary tangles in Alzheimer's disease. Neurosci Lett. 2001;305:165–8. doi: 10.1016/s0304-3940(01)01842-0. [DOI] [PubMed] [Google Scholar]
- 56.Oldfield BJ, Mckinley MJ. Circumventricular organs. In: Paxinos G, editor. The rat nervous system. San Diego: Academic Press; 1995. pp. 391–403. [Google Scholar]
- 57.Holmes C, Cunningham C, Zotova E, Woolford J, Dean C, Kerr S, et al. Systemic inflammation and disease progression in Alzheimer disease. Neurology. 2009;73:768–74. doi: 10.1212/WNL.0b013e3181b6bb95. doi: http://dx.doi.org/10.1212/WNL.0b013e3181b6bb95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Oliveira JM, Lisboa Lde B. Hospital-acquired infections due to Gram-negative bacteria. N Engl J Med. 2010;363:1482–3. doi: 10.1056/NEJMc1006641. author reply 1483–4. [DOI] [PubMed] [Google Scholar]
- 59.Honjo K, van Reekum R, Verhoeff NPLG. Alzheimer's disease and infection: do infectious agents contribute to progression of Alzheimer's disease? Alzheimers Dement. 2009;5:348–60. doi: 10.1016/j.jalz.2008.12.001. doi: http://dx.doi.org/10.1016/j.jalz.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 60.Maheshwari P, Eslick GD. Bacterial infection and Alzheimer's disease: a meta-analysis. J Alzheimers Dis. 2015;43:957–66. doi: 10.3233/JAD-140621. [DOI] [PubMed] [Google Scholar]
- 61.Shima K, Kuhlenbäumer G, Rupp J. Chlamydia pneumoniae-infection and Alzheimer's disease: a connection to remember? Med Microbiol Immunol. 2010;199:283–9. doi: 10.1007/s00430-010-0162-1. doi: http://dx.doi.org/10.1007/s00430-010-0162-1. [DOI] [PubMed] [Google Scholar]
- 62.Lim C, Hammond CJ, Hingley ST, Balin BJ. Chlamydia pneumoniae infection of monocytes in vitro stimulates innate and adaptive immune responses relevant to those in Alzheimer's disease. J Neuroinflammation. 2014;11:217. doi: 10.1186/s12974-014-0217-0. doi: http://dx.doi.org/10.1186/s12974-014-0217-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hammond CJ, Hallock LR, Howanski RJ, Appelt DM, Little CS. Immunohistological detection of Chlamydia pneumoniae in the Alzheimer's disease brain. Neuroscience. 2010;11:121. doi: 10.1186/1471-2202-11-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hogan RJ, Mathews SA, Mukhopadhyay S, Summersgill JT, Timms P. Chlamydial persistence: beyond the biphasic paradigm. Infect Immun. 2004;72:1843–55. doi: 10.1128/IAI.72.4.1843-1855.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Whittum-Hudson JA, Schumacher HR, Hudson AP. Chlamydia pneumoniae and inflammatory arthritis. In: Yamamoto Y, Friedman H, Bendinelli M, editors. Chlamydia pneumoniae infection and diseases. New York: Kluwer/Academic Press; 2004. pp. 227–38. [Google Scholar]
- 66.Dreses-Werringloer U, Bhuiyan M, Zhao Y, Gérard HC, Whittum-Hudson JA, Hudson AP. Initial characterization of Chlamydophila (Chlamydia) pneumoniae cultured from the late-onset Alzheimer brain. Int J Med Microbiol. 2009;299:187–201. doi: 10.1016/j.ijmm.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dezfulian M, Shokrgozar MA, Sardari S, Parivar K, Javadi G. Can phages cause Alzheimer's disease? Med Hypotheses. 2008;71:651–6. doi: 10.1016/j.mehy.2008.07.005. doi: http://dx.doi.org/10.1016/j.mehy.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 68.Miklossy J. Alzheimer's disease – a neurospirochetosis. Analysis of the evidence following Koch's and Hill's criteria. J Neuroinflammation. 2011;8:90. doi: 10.1186/1742-2094-8-90. doi: http://dx.doi.org/10.1186/1742-2094-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miklossy J. Emerging roles of pathogens in Alzheimer disease. Expert Rev Mol Med. 2011;13:e30. doi: 10.1017/S1462399411002006. doi: http://dx.doi.org/10.1017/S1462399411002006. [DOI] [PubMed] [Google Scholar]
- 70.Ohnishi S, Koide A, Koide S. Solution conformation and amyloid-like fibril formation of a polar peptide derived from a beta-hairpin in the OspA single-layer beta-sheet. J Mol Biol. 2000;301:477–89. doi: 10.1006/jmbi.2000.3980. [DOI] [PubMed] [Google Scholar]
- 71.McGeer PL, McGeer EG. Polymorphisms in inflammatory genes and the risk of Alzheimer disease. Arch Neurol. 2001;58:1790–2. doi: 10.1001/archneur.58.11.1790. [DOI] [PubMed] [Google Scholar]
- 72.Miklossy J. Historic evidence to support a causal relationship between spirochetal infections and Alzheimer's disease. Front Aging Neurosci. 2015;7:46. doi: 10.3389/fnagi.2015.00046. doi: http://dx.doi.org/10.3389/fnagi.2015.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fallon BA, Nields JA. Lyme disease: a neuropsychiatric illness. Am J Psychiatry. 1994;151:1571–83. doi: 10.1176/ajp.151.11.1571. [DOI] [PubMed] [Google Scholar]
- 74.MacDonald AB, Miranda JM. Concurrent neocortical borreliosis and Alzheimer's disease. Hum Pathol. 1987;18:759–61. doi: 10.1016/s0046-8177(87)80252-6. [DOI] [PubMed] [Google Scholar]
- 75.MacDonald AB. Concurrent neocortical borreliosis and Alzheimer's disease. Demonstration of a spirochetal cyst form. Ann N Y Acad Sci. 1988;539:468–70. doi: http://dx.doi.org/10.1111/j.1749-6632.1988.tb31909.x. [Google Scholar]
- 76.MacDonald AB. Transfection “Junk” DNA – a link to the pathogenesis of Alzheimer's disease? Med Hypotheses. 2006;66:1140–1. doi: 10.1016/j.mehy.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 77.Miklossy J, Khalili K, Gern L, Ericson RL, Darekar P, Bolle L, et al. Borrelia burgdorferi persists in the brain in chronic lyme neuroborreliosis and may be associated with Alzheimer disease. J Alzheimers Dis. 2004;6:639–49. doi: 10.3233/jad-2004-6608. discussion 673–81. [DOI] [PubMed] [Google Scholar]
- 78.Bu XL, Yao XQ, Jiao SS, Zeng F, Liu YH, Xiang Y, et al. A study on the association between infectious burden and Alzheimer's disease. Eur J Neurol. 2014 doi: 10.1111/ene.12477. doi: http://dx.doi.org/10.1111/ene.12477 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 79.Gutacker M, Valsangiacomo C, Balmelli T, Bernasconi MV, Bouras C, Piffaretti JC. Arguments against the involvement of Borrelia burgdorferi sensu lato in Alzheimer's disease. Res Microbiol. 1998;149:31–7. doi: 10.1016/s0923-2508(97)83621-2. [DOI] [PubMed] [Google Scholar]
- 80.Pappolla MA, Omar R, Saran B, Andorn A, Suarez M, Pavia C, et al. Concurrent neuroborreliosis and Alzheimer's disease: analysis of the evidence. Hum Pathol. 1989;20:753–7. doi: 10.1016/0046-8177(89)90068-3. [DOI] [PubMed] [Google Scholar]
- 81.Shiota S, Murakami K, Yoshiiwa A, Yamamoto K, Ohno S, Kuroda A, et al. The relationship between Helicobacter pylori infection and Alzheimer's disease in Japan. J Neurol. 2011;258:1460–3. doi: 10.1007/s00415-011-5957-5. doi: http://dx.doi.org/10.1007/s00415-011-5957-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kountouras J, Zavos C, Boziki M, Gavalas E, Kyriakou P, Deretzi G, et al. Association between Helicobacter pylori infection and Alzheimer's disease in Japan. J Neurol. 2011;258:2086. doi: 10.1007/s00415-011-6054-5. http://dx.doi.org/10.1007/s00415-011-6054-5. [DOI] [PubMed] [Google Scholar]
- 83.Kountouras J, Boziki M, Gavalas E, Zavos C, Deretzi G, Chatzigeorgiou S, et al. Five-year survival after Helicobacter pylori eradication in Alzheimer disease patients. Cogn Behav Neurol. 2010;23:199–204. doi: 10.1097/WNN.0b013e3181df3034. http://dx.doi.org/10.1097/WNN.0b013e3181df3034. [DOI] [PubMed] [Google Scholar]
- 84.Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43:5721–32. doi: 10.1128/JCM.43.11.5721-5732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, et al. The human microbiome. J Bacteriol. 2010;192:5002–17. doi: 10.1128/JB.00542-10. doi: http://dx.doi.org/10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Imangaliyev S, Keijser B, Crielaard W, Tsivtsivadze E. Personalized microbial network inference via co-regularized spectral clustering. Methods. 2015;83:28–35. doi: 10.1016/j.ymeth.2015.03.017. doi: http://dx.doi.org/10.1016/j.ymeth.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 87.Segata N, Haake SK, Mannon P, Lemon KP, Waldron L, Gevers D, et al. Composition of the adult digestive tract bacterial microbiome based on seven mouth surfaces, tonsils, throat and stool samples. Genome Biol. 2012;13:R42. doi: 10.1186/gb-2012-13-6-r42. doi: http://dx.doi.org/10.1186/gb-2012-13-6-r42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Axelsson P, Lindhe J, Nyström B. On the prevention of caries and periodontal disease. Results of a 15-year longitudinal study in adults. J Clin Periodontol. 1991;18:182–9. doi: 10.1111/j.1600-051x.1991.tb01131.x. [DOI] [PubMed] [Google Scholar]
- 89.Flemmig TF. Periodontitis. Ann Periodontol. 1999;4:32–8. doi: 10.1902/annals.1999.4.1.32. [DOI] [PubMed] [Google Scholar]
- 90.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 91.Holt SC, Ebersole JL. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: the “red complex,” a prototype polybacterial pathogenic consortium in periodontitis. Periodontol 2000. 2005;38:72–122. doi: 10.1111/j.1600-0757.2005.00113.x. [DOI] [PubMed] [Google Scholar]
- 92.Colombo AP, Boches SK, Cotton SL, Goodson JM, Kent R, Haffajee AD, et al. Comparisons of subgingival microbial profiles of refractory periodontitis, severe periodontitis, and periodontal health using the human oral microbe identification microarray. J Periodontol. 2009;80:1421–32. doi: 10.1902/jop.2009.090185. doi: http://dx.doi.org/10.1902/jop.2009.090185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Preza D, Olsen I, Willumsen T, Boches SK, Cotton SL, Grinde B, et al. Microarray analysis of the microflora of root caries in elderly. Eur J Clin Microbiol Infect Dis. 2009;28:509–17. doi: 10.1007/s10096-008-0662-8. doi: http://dx.doi.org/10.1007/s10096-008-0662-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Torlakovic L, Klepac-Ceraj V, Ogaard B, Cotton SL, Paster BJ, Olsen I. Microbial community succession on developing lesions on human enamel. J Oral Microbiol. 2012;4:16125. doi: 10.3402/jom.v4i0.16125. doi: http://dx.doi.org/10.3402/jom.v4i0.16125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hardy JA, Mann DM, Wester P, Winblad B. An integrative hypothesis concerning the pathogenesis and progression of Alzheimer's disease. Neurobiol Aging. 1986;7:489–502. doi: 10.1016/0197-4580(86)90086-2. [DOI] [PubMed] [Google Scholar]
- 96.Noble JM, Borrell LN, Papapanou PN, Elkind MSV, Scarmeas N, Wright CB. Periodontitis is associated with cognitive impairment among older adults: Analysis of NHANES-III. J Neurol Neurosurg Psychiatry. 2009;80:1206–11. doi: 10.1136/jnnp.2009.174029. doi: http://dx.doi.org/10.1136/jnp.2009.174029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sparks Stein P, Steffen MJ, Smith C, Jicha G, Ebersole JL, Abner E, et al. Serum antibodies to periodontal pathogens are a risk factor for Alzheimer's disease. Alzheimers Dement. 2012;8:196–203. doi: 10.1016/j.jalz.2011.04.006. doi: http://dx.doi.org/10.1016/j.jalz.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Noble JM, Scarmeas N, Celenti RS, Elkind MSV, Wright CB, Schupf N, et al. Serum IgG antibody levels to periodontal microbiota are associated with incident Alzheimer disease. PLoS One. 2014;9:e114959. doi: 10.1371/journal.pone.0114959. doi: http://dx.doi.org/10.1371/journal.pone.0114959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hajishengallis G. Too old to fight? Aging and its toll on innate immunity. Mol Oral Microbiol. 2010;25:25–37. doi: 10.1111/j.2041-1014.2009.00562.x. doi: http://dx.doi.org/10.1111/j.2041-1014.2009.00562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–97. doi: 10.1038/ni.1923. doi: http://dx.doi.org/10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fong ON, Chan KY, Leung KT, Lam HS, Cheung HM, Leung TY, et al. Expression profile of cord blood neutrophils and dysregulation of HSPA1A and OLR1 upon challenge by bacterial peptidoglycan. J Leukoc Biol. 2014;95:169–78. doi: 10.1189/jlb.0413219. doi: http://dx.doi.org/10.1189/jlb.0413219. [DOI] [PubMed] [Google Scholar]
- 102.Bibi F, Yasir M, Sohrab SS, Azhar EI, Al-Qahtani MH, Abuzenadah AM, et al. Link between chronic bacterial inflammation and Alzheimer disease. CNS Neurol Disord Drug Targets. 2014;13:1140–7. doi: 10.2174/1871527313666140917115741. [DOI] [PubMed] [Google Scholar]
- 103.Miklossy J, Kraftsik R, Pillevuit O, Lepori D, Genton C, Bosman FT. Curly fiber and tangle-like inclusions in the ependyma and choroid plexus – a pathogenetic relationship with the cortical Alzheimer-type changes? J Neuropathol Exp Neurol. 1998;7:1202–12. doi: 10.1097/00005072-199812000-00012. [DOI] [PubMed] [Google Scholar]
- 104.Miklossy J, Kis A, Radenovic A, Miller L, Forro L, Martins R, et al. Beta-amyloid deposition and Alzheimer's type changes induced by Borrelia spirochetes. Neurobiol Aging. 2006;27:228–36. doi: 10.1016/j.neurobiolaging.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 105.Hachinsky V, Munoz DG. Cerebrovascular pathology in Alzheimer's disease: cause, effect or epiphenomenon? Ann N Y Acad Sci. 1997;826:1–6. doi: 10.1111/j.1749-6632.1997.tb48456.x. [DOI] [PubMed] [Google Scholar]
- 106.Radolf JD, Desroisers DC. Treponema pallidum, the stealth pathogen, doth change, but how? Mol Microbiol. 2009;72:1081–6. doi: 10.1111/j.1365-2958.2009.06711.x. doi: http://dx.doi.org/10.1111/j.1365-2958.2009.06711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA. 1997;277:813–7. [PubMed] [Google Scholar]
- 108.Hajishengallis G. The inflammophilic character of the periodontitis-associated microbiota. Mol Oral Microbiol. 2014;29:248–57. doi: 10.1111/omi.12065. doi: http://dx.doi.org/10.1111/omi.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Singhrao SK, Harding A, Poole S, Kesavalu L, Crean S. Porphyromonas gingivalis periodontal infection and its putative links with Alzheimer's disease. Mediators Inflamm. 2015;2015:137357. doi: 10.1155/2015/137357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Reife RA, Coats SR, Al-Qutub M, Dixon DM, Braham PA, Billharz RJ, et al. Porphyromonas gingivalis lipopolysaccharide lipid A heterogeneity: differential activities of tetra- and penta-acylated lipid A structures on E-selectin expression and TLR4 recognition. Cell Microbiol. 2006;8:857–68. doi: 10.1111/j.1462-5822.2005.00672.x. [DOI] [PubMed] [Google Scholar]
- 111.Kocgozlu L, Elkaim R, Tenenbaum H, Werner S. Variable cell responses to P. gingivalis lipopolysaccharide. J Dent Res. 2009;88:741–5. doi: 10.1177/0022034509341166. doi: http://dx.doi.org/10.1177/0022034509341166. [DOI] [PubMed] [Google Scholar]
- 112.Belstrøm D, Holmstrup P, Damgaard C, Borch TS, Skjødt MO, Bendtzen K, et al. The atherogenic bacterium Porphyromonas gingivalis evades circulating phagocytes by adhering to erythrocytes. Infect Immun. 2011;79:1559–65. doi: 10.1128/IAI.01036-10. doi: http://dx.doi.org/10.1128/IAI.01036-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Poole S, Singhrao SK, Chukkapalli S, Rivera M, Velsko I, Kesavalu L, et al. Active invasion of Porphyromonas gingivalis and infection-induced complement activation in ApoE−/− mice brains. J Alzheimers Dis. 2015;43:67–80. doi: 10.3233/JAD-140315. [DOI] [PubMed] [Google Scholar]
- 114.Xu F, Sternberg MR, Kottiri BJ, McQuillan GM, Lee FK, Nahmias AJ, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA. 2006;296:964–73. doi: 10.1001/jama.296.8.964. [DOI] [PubMed] [Google Scholar]
- 115.Bünzli D, Wietlisbach V, Barazzoni F, Sahli R, Meylan PR. Seroepidemiology of Herpes simplex virus type 1 and 2 in Western and Southern Switzerland in adults aged 25–74 in 1992–93: a population-based study. BMC Infect Dis. 2004;17:10. doi: 10.1186/1471-2334-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Malkin JE, Morand P, Malvy D, Ly TD, Chanzy B, de Labareyre C, et al. Seroprevalence of HSV-1 and HSV-2 infection in the general French population. Sex Transm Infect. 2002;78:201–3. doi: 10.1136/sti.78.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Slots J. Herpesvirus periodontitis: infection beyond biofilm. J Calif Dent Assoc. 2011;39:393–9. [PubMed] [Google Scholar]
- 118.Carter CJ. Alzheimer's disease plaques and tangles: cemeteries of a Pyrrhic victory of the immune defense network against herpes simplex infection at the expense of complement and inflammation-mediated neuronal destruction. Neurochem Int. 2011;58:301–20. doi: 10.1016/j.neuint.2010.12.003. doi: http://dx.doi.org/10.1016/j.neuint.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 119.De Chiara G, Marcocci ME, Civitelli L, Argnani R, Piacentini R, Ripoli C, et al. APP processing induced by herpes simplex virus type 1 (HSV-1) yields several APP fragments in human and rat neuronal cells. PLoS One. 2010;5:e13989. doi: 10.1371/journal.pone.0013989. doi: http://dx.doi.org/10.1371/journal.pone.0013989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Itzhaki RF, Cosby SL, Wozniak MA. Herpes simplex virus type 1 and Alzheimer's disease: the autophagy connection. J Neurovirol. 2008;14:1–4. doi: 10.1080/13550280701802543. doi: http://dx.doi.org/10.1080/13550280701802543. [DOI] [PubMed] [Google Scholar]
- 121.Wozniak MA, Mee AP, Itzaki RF. Herpes simplex virus type I DNA is located within Alzheimer's disease amyloid plaques. J Pathol. 2009;217:131–8. doi: 10.1002/path.2449. [DOI] [PubMed] [Google Scholar]
- 122.Wisniewski T, Ghiso J, Frangione B. Alzheimer's disease and soluble A beta. Neurobiol Aging. 1994;15:143–52. doi: 10.1016/0197-4580(94)90105-8. [DOI] [PubMed] [Google Scholar]
- 123.Zlokovic BV, Yamada S, Holtzman D, Ghiso J, Frangione B. Clearance of amyloid beta-peptide from brain: transport or metabolism? Nat Med. 2000;6:718–19. doi: 10.1038/77397. [DOI] [PubMed] [Google Scholar]
- 124.Aiello AE, Haan M, Blythe L, Moore K, Gonzalez JM, Jagust W. The influence of latent viral infection on rate of cognitive decline over 4 years. J Am Geriatr Soc. 2006;54:1046–54. doi: 10.1111/j.1532-5415.2006.00796.x. [DOI] [PubMed] [Google Scholar]
- 125.Itzhaki RF, Lin WR, Shang D, Wilcock GK, Faragher B, Jamieson GA. Herpes simplex virus type 1 in brain and risk of Alzheimer's disease. Lancet. 1997;349:241–4. doi: 10.1016/S0140-6736(96)10149-5. [DOI] [PubMed] [Google Scholar]
- 126.Lurain NS, Hanson BA, Martinson J, Leurgans SE, Landay AL, Bennett DA, et al. Virological and immunological characteristics of human cytomegalovirus infection associated with Alzheimer disease. J Infect Dis. 2013;208:564–72. doi: 10.1093/infdis/jit210. doi: http://dx.doi.org/10.1093/infdis/jit210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jamieson GA, Maitland NJ, Wilcock GK, Yates CM, Itzhaki RF. Herpes simplex virus type 1 DNA is present in specific regions of brain from aged people with and without senile dementia of the Alzheimer type. J Pathol. 1992;167:365–8. doi: 10.1002/path.1711670403. [DOI] [PubMed] [Google Scholar]
- 128.Ball MJ. “Limbic” predilection in Alzheimer dementia: is reactivated herpesvirus involved? Can J Neurol. 1982;9:303–6. doi: 10.1017/s0317167100044115. [DOI] [PubMed] [Google Scholar]
- 129.Ball MJ, Lukiw WJ, Kammermann EM, Hill JM. Intracerebral propagation of Alzheimer's disease: strengthening evidence of a herpes virus etiology. Alzheimers Dement. 2013;9:169–75. doi: 10.1016/j.jalz.2012.07.005. doi: http://dx.doi.org/10.1016/j.jalz.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gibson KL, Wu YC, Barnett Y, Duggan O, Vaughan R, Kondeatis E, et al. B-cell diversity decreases in old age and is correlated with poor health status. Aging Cell. 2009;8:18–25. doi: 10.1111/j.1474-9726.2008.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wozniak MA, Shipley SJ, Combrinck M, Wilcock GK, Itzhaki RF. Productive herpes simplex virus in brain of elderly normal subjects and Alzheimer's disease patients. J Med Virol. 2005;75:300–6. doi: 10.1002/jmv.20271. [DOI] [PubMed] [Google Scholar]
- 132.Lin WR, Graham J, MacGowan SM, Wilcock GK, Itzhaki RF. Alzheimer's disease, herpes virus in brain, apolipoprotein E4 and herpes labialis. Alzheimer's Rep. 1998;1:173–8. [Google Scholar]
- 133.Urovesic N, Martins RN. Infection and Alzheimer's disease: the APOE epsilon4 connection and lipid metabolism. J Alzheimers Dis. 2008;13:421–35. doi: 10.3233/jad-2008-13407. [DOI] [PubMed] [Google Scholar]
- 134.Lövheim H, Gilthorpe J, Adolfsson R, Nilsson LG, Elgh F. Reactivated herpes simplex infection increases the risk of Alzheimer's disease. Alzheimers Dement. 2015;11:593–9. doi: 10.1016/j.jalz.2014.04.522. doi: http://dx.doi.org/10.1016/j.jalz.2014.04.522. [DOI] [PubMed] [Google Scholar]
- 135.Kobayashi N, Nagata T, Shinagawa S, Oka N, Shimada K, Shimizu S, et al. Increase in the IgG activity index due to herpes simplex virus type 1 reactivation and its relationship with cognitive function in amnestic mild cognitive impairment and Alzheimer's disease. Biochem Biophys Res Commun. 2013;430:907–11. doi: 10.1016/j.bbrc.2012.12.054. doi: http://dx.doi.org/10.1016/j.bbrc.2012.12.054. [DOI] [PubMed] [Google Scholar]
- 136.Letenneur L, Pérès K, Fleury H, Garrigue I, Barberger-Gateau P, Helmer C, et al. Seropositivity to herpes simplex virus antibodies and risk of Alzheimer's disease: a population-based cohort study. PLoS One. 2008;3:e3637. doi: 10.1371/journal.pone.0003637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hill MJ, Zhao Y, Clement C, Neumann DM, Lukiw WJ. HSV-1 infection of human brain cells induces miRNA-146a and Alzheimer-type inflammatory signaling. Neuroreport. 2009;20:1500–5. doi: 10.1097/WNR.0b013e3283329c05. doi: http://dx.doi.org/10.1097/WNR.0b013e3283329c05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lewis RE. Overview of the changing epidemiology of candidemia. Curr Med Res Opin. 2009;25:1732–40. doi: 10.1185/03007990902990817. doi: http://dx.doi.org/10.1185/03007990902990817. [DOI] [PubMed] [Google Scholar]
- 139.Miceli MH, Diaz JA, Lee SA. Emerging opportunistic yeast infections. Lancet Infect Dis. 2011;11:142–51. doi: 10.1016/S1473-3099(10)70218-8. doi: http://dx.doi.org/10.1016/S1473-3099(10)70218-8. [DOI] [PubMed] [Google Scholar]
- 140.Song X, Eribe ER, Sun J, Hansen BF, Olsen I. Genetic relatedness of oral yeasts within and between patients with marginal periodontitis and subjects with oral health. J Periodontal Res. 2005;40:446–52. doi: 10.1111/j.1600-0765.2005.00816.x. [DOI] [PubMed] [Google Scholar]
- 141.Kumar J, Sharma R, Sharma M, Prabhavathi V, Paul J, Chowdary CD. Presence of Candida albicans in root canals of teeth with apical periodontitis and evaluation of their possible role in failure of endodontic treatment. J Int Oral Health. 2015;7:42–5. [PMC free article] [PubMed] [Google Scholar]
- 142.Olsen I. Denture stomatitis, Occurrence and distribution of fungi. Acta Odontol Scand. 1974;32:329–33. doi: 10.3109/00016357409002556. [DOI] [PubMed] [Google Scholar]
- 143.Alonso R, Pisa D, Marina AI, Morato E, Rábano A, Carrasco L. Fungal infections in patients with Alzheimer's disease. J Alzheimers Dis. 2014;41:301–11. doi: 10.3233/JAD-132681. doi: http://dx.doi.org/10.3233/JAD-132681. [DOI] [PubMed] [Google Scholar]
- 144.Alonso R, Pisa D, Rábano A, Carrasco L. Alzheimer's disease and disseminated mycoses. Eur J Clin Microbiol Infect Dis. 2014;33:1125–32. doi: 10.1007/s10096-013-2045-z. doi: http://dx.doi.org/10.1007/s10096-013-2045-z. [DOI] [PubMed] [Google Scholar]
- 145.Castellani RJ, Perry G, Smith MA. The role of novel chitin-like polysaccharides in Alzheimer disease. Neurotox Res. 2007;12:269–74. doi: 10.1007/BF03033910. [DOI] [PubMed] [Google Scholar]
- 146.Watabe-Rudolph M, Song Z, Lausser L, Schnack C, Begus-Nahrmann Y, Scheithauer MO, et al. Chitinase enzyme activity in CSF is a powerful biomarker of Alzheimer disease. Neurology. 2012;78:569–77. doi: 10.1212/WNL.0b013e318247caa1. doi: http://dx.doi.org/10.1212/WNL.0b013e318247caa1. [DOI] [PubMed] [Google Scholar]
- 147.Pisa D, Alonso R, Juarranz A, Rábano A, Carrasco L. Direct visualization of fungal infection in brains from patients with Alzheimer's disease. J Alzheimers Dis. 2015;43:613–24. doi: 10.3233/JAD-141386. doi: http://dx.doi.org/10.3233/JAD-141386. [DOI] [PubMed] [Google Scholar]
- 148.Ala TA, Doss RC, Sullivan CJ. Reversible dementia: a case of cryptococcal meningitis masquerading as Alzheimer's disease. J Alzheimers Dis. 2004;6:503–8. doi: 10.3233/jad-2004-6507. [DOI] [PubMed] [Google Scholar]
- 149.Hoffmann M, Muniz J, Carroll E, De Villasante J. Cryptococcal meningitis misdiagnosed as Alzheimer's disease: complete neurological and cognitive recovery with treatment. J Alzheimers Dis. 2009;16:517–20. doi: 10.3233/JAD-2009-0985. doi: http://dx.doi.org/10.3233/JAD-2009-0985. [DOI] [PubMed] [Google Scholar]
- 150.Singhrao SK, Harding A, Simmons T, Robinson S, Kesavalu L, Crean S. Oral inflammation, tooth loss, risk factors, and association with progression of Alzheimer's disease. J Alzheimers Dis. 2014;42:723–37. doi: 10.3233/JAD-140387. [DOI] [PubMed] [Google Scholar]
- 151.Olsen I. Update on bacteraemia related to dental procedures. Transfus Apher Sci. 2008;39:173–8. doi: 10.1016/j.transci.2008.06.008. doi: http://dx.doi.org/10.1016/j.transci.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 152.Tomas I, Diz P, Tobias A, Scully C, Donos N. Periodontal health status and bacteraemia from daily oral activities: systematic review/meta-analysis. J Clin Periodontol. 2012;39:213–28. doi: 10.1111/j.1600-051X.2011.01784.x. [DOI] [PubMed] [Google Scholar]
- 153.Navarathna DH, Munasinghe J, Lizak MJ, Nayak D, McGavern DB, Roberts DD. MRI confirms loss of blood–brain barrier integrity in a mouse model of disseminated candidiasis. NMR Biomed. 2013;26:1125–34. doi: 10.1002/nbm.2926. doi: http://dx.doi.org/10.1002/nbm.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Holmes C, Cotterell D. Role of infection in the pathogenesis of Alzheimer's disease. CNS Drugs. 2009;23:993–1002. doi: 10.2165/11310910-000000000-00000. doi: http://dx.doi.org/10.2165/11310910-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 155.Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, Wu Z, et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485:512–6. doi: 10.1038/nature11087. doi: http://dx.doi.org/10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ujiie M, Dickstein DL, Carlow DA, Jefferies WA. Blood–brain barrier permeability precedes senile plaque formation in an Alzheimer disease model. Microcirculation. 2003;10:463–70. doi: 10.1038/sj.mn.7800212. [DOI] [PubMed] [Google Scholar]
- 157.Fry M, Ferguson AV. The sensory circumventricular organs: brain targets for circulating signals controlling ingestive behavior. Physiol Behav. 2007;91:413–23. doi: 10.1016/j.physbeh.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 158.Mann DM, Tucker CM, Yates PO. Alzheimer's disease: an olfactory connection? Mech Ageing Dev. 1988;42:1–15. doi: 10.1016/0047-6374(88)90058-9. [DOI] [PubMed] [Google Scholar]
- 159.Danielyan L, Schäfer R, von Ameln-Mayerhofer A, Buadze M, Geisler J, Klopfer T, et al. Intranasal delivery of cells to the brain. Eur J Cell Biol. 2009;88:315–24. doi: 10.1016/j.ejcb.2009.02.001. doi: http://dx.doi.org/10.1016/j.ejcb.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 160.Johnson NJ, Hanson LR, Frey WH. Trigeminal pathways deliver a low molecular weight drug from the nose to the brain and orofacial structures. Mol Pharm. 2010;7:884–93. doi: 10.1021/mp100029t. doi: http://dx.doi.org/10.1021/mp100029t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Leung JY, Chapman JA, Harris JA, Hale D, Chung RS, West AK, et al. Olfactory ensheathing cells are attracted to, and can endocytose, bacteria. Cell Moll Life Sci. 2008;65:2732–9. doi: 10.1007/s00018-008-8184-1. doi: http://dx.doi.org/10.1007/s00018-008-8184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Kovács T, Cairns NJ, Lantos PL. Beta-amyloid deposition and neurofibrillary tangle formation in the olfactory bulb in ageing and Alzheimer's disease. Neuropathol Appl Neurobiol. 1999;25:481–91. doi: 10.1046/j.1365-2990.1999.00208.x. [DOI] [PubMed] [Google Scholar]
- 163.Wesson DW, Levy E, Nixon RA, Wilson DA. Olfactory dysfunction correlates with amyloid-beta burden in an Alzheimer's disease mouse model. J Neurosci. 2010;30:505–14. doi: 10.1523/JNEUROSCI.4622-09.2010. doi: http://dx.doi.org/10.1523/JNEUROSCI.4622-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Goate A, Chartier-Harlin MC, Mullan M, Brown J, Crawford F, Fidani L, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature. 1991;349:704–6. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- 165.Levy-Lahad E, Wasco W, Poorkaj P, Romano DM, Oshima J, Pettingell WH, et al. Candidate gene for the chromosome 1 familial Alzheimer's disease locus. Science. 1995;269:973–7. doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- 166.Rogaev EI, Sherrington R, Rogaeva EA, Levesque G, Ikeda M, Liang Y, et al. Familial Alzheimer's disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer's disease type 3 gene. Nature. 1995;376:775–8. doi: 10.1038/376775a0. [DOI] [PubMed] [Google Scholar]
- 167.Nicoll JA, Mrak RE, Graham DI, Stewart J, Wilcock G, MacGowan S, et al. Association of interleukin-1 gene polymorphisms with Alzheimer's disease. Ann Neurol. 2000;47:365–8. [PMC free article] [PubMed] [Google Scholar]
- 168.McGeer PL, McGeer EG. History of innate immunity in neurodegenerative disorders. Front Pharmacol. 2011;2:77. doi: 10.3389/fphar.2011.00077. doi: http://dx.doi.org/10.3389/fphar.2011.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kornman KS, Crane A, Wang HY, di Giovine FS, Newman MG, Pirk FW, et al. The interleukin-1 genotype as a severity factor in adult periodontal disease. J Clin Periodontol. 1997;24:72–7. doi: 10.1111/j.1600-051x.1997.tb01187.x. [DOI] [PubMed] [Google Scholar]
- 170.Galbraith GM, Hendley TM, Sanders JJ, Palesch Y, Pandey JP. Polymorphic cytokine genotypes as markers of disease severity in adult periodontitis. J Clin Periodontol. 1999;26:705–9. doi: 10.1034/j.1600-051x.1999.t01-1-261101.x. [DOI] [PubMed] [Google Scholar]
- 171.Shao MY, Huang P, Cheng R, Hu T. Interleukin-6 polymorphisms modify the risk of periodontitis: a systematic review and meta-analysis. J Zhejiang Univ Sci B. 2009;10:920–7. doi: 10.1631/jzus.B0920279. doi: http://dx.doi.org/10.1631/jzus.B0920279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Di Bona D, Candore G, Franceschi C, Licastro F, Colonna-Romano G, Cammà C, et al. Systematic review by meta-analyses on the possible role of TNF-alpha polymorphisms in association with Alzheimer's disease. Brain Res Rev. 2009;61:60–8. doi: 10.1016/j.brainresrev.2009.05.001. doi: http://dx.doi.org/10.1016/j.brainresrev.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 173.Yang W, Jia Y, Wu H. Four tumor necrosis factor alpha genes polymorphisms and periodontitis risk in a Chinese population. Hum Immunol. 2013;74:1684–7. doi: 10.1016/j.humimm.2013.08.009. doi: http://dx.doi.org/10.1016/j.humimm.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 174.Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease. Nat Genet. 2013;45:1452–8. doi: 10.1038/ng.2802. doi: http://dx.doi.org/10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yuan H, Xia Q, Ge P, Wu S. Genetic polymorphism of interleukin 1β-511C/T and susceptibility to sporadic Alzheimer's disease: a meta-analysis. Mol Biol Rep. 2013;40:1827–34. doi: 10.1007/s11033-012-2237-0. doi: http://dx.doi.org/10.1007/s11033-012-2237-0. [DOI] [PubMed] [Google Scholar]
- 176.Di Bona D, Plaia A, Vasto S, Cavallone L, Lescai F, Franceschi C, et al. Association between the interleukin-1beta polymorphisms and Alzheimer's disease: a systematic review and meta-analysis. Brain Res Rev. 2008;59:155–63. doi: 10.1016/j.brainresrev.2008.07.003. doi: http://dx.doi.org/10.1016/j.brainresrev.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 177.Zhu XC, Tan L, Jiang T, Tan MS, Zhang W, Yu JT. Association of IL-12A and IL-12B polymorphisms with Alzheimer's disease susceptibility in a Han Chinese population. J Neuroimmunol. 2014;274:180–4. doi: 10.1016/j.jneuroim.2014.06.026. doi: http://dx.doi.org/10.1016/j.jneuroim.2014.06.026. [DOI] [PubMed] [Google Scholar]
- 178.Payão SL, Gonçalves GM, de Labio RW, Horiguchi L, Mizumoto I, Rasmussen LT, et al. Association of interleukin 1β polymorphisms and halotypes with Alzheimer's disease. J Neuroimmnol. 2012;247:59–62. doi: 10.1016/j.jneuroim.2012.03.012. doi: http://dx.doi.org/10.1016/j.jneuroim.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 179.Wang B, Zhou S, Yang Z, Xie YC, Wang J, Zhang P, et al. Genetic analysis of tumor necrosis factor-alpha (TNF-alpha) G-308A and Saitohin Q7R polymorphisms with Alzheimer's disease. J Neurol Sci. 2008;270:148–51. doi: 10.1016/j.jns.2008.02.021. doi: http://dx.doi.org/10.1016/j.jns.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 180.Lio D, Annoni G, Licastro F, Crivello A, Forte GI, Scola L, et al. Tumor necrosis factor-alpha -308A/G polymorphism is associated with age at onset of Alzheimer's disease. Mech Ageing Dev. 2006;127:567–71. doi: 10.1016/j.mad.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 181.Kornman KS. Interleukin 1 genetics, inflammatory mechanisms, and nutrigenetic opportunities to modulate diseases of aging. Am J Clin Nutrit. 2006;83:475S–83S. doi: 10.1093/ajcn/83.2.475S. [DOI] [PubMed] [Google Scholar]
- 182.Sando SB, Melquist S, Cannon A, Hutton ML, Sletvold O, Saltvedt I, et al. APOE epsilon 4 lowers age onset and is a high risk factor for Alzheimer's disease: a case control study from central Norway. BMC Neurol. 2008;8:9. doi: 10.1186/1471-2377-8-9. doi: http://dx.doi.org/10.1186/1471-2377-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Desikan RS, Schork AJ, Wang Y, Thompson WK, Dehghan A, Ridker PM, et al. Polygenic overlap between C-reactive protein, plasma lipids and Alzheimer's disease. Circulation. 2015;131:2061–9. doi: 10.1161/CIRCULATIONAHA.115.015489. doi: http://dx.doi.org/10.1161/CIRCULATIONAHA.115.015489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Engelborghs S, Gilles C, Ivanoiu A, Vandewoude M. Rationale and clinical data supporting nutritional intervention in Alzheimer's disease. Acta Clin Belg. 2014;69:17–24. doi: 10.1179/0001551213Z.0000000006. doi: http://dx.doi.org/10.1179/0001551213Z.0000000006. [DOI] [PubMed] [Google Scholar]
- 185.Cerajewska TL, Davies M, West NX. Periodontitis: a potential risk factor for Alzheimer's disease. Brit Dent J. 2015;218:29–34. doi: 10.1038/sj.bdj.2014.1137. [DOI] [PubMed] [Google Scholar]
- 186.Cicciù M, Matacena G, Signorino F, Brugaletta A, Cicciù A, Bramanti E. Relationship between oral health and its impact on the quality life of Alzheimer's disease patients: a supportive care trial. Int J Clin Exp Med. 2013;6:766–72. [PMC free article] [PubMed] [Google Scholar]
- 187.Stewart R, Sabbah W, Tsakos G, D'Aiuto F, Watt RG. Oral health and cognitive function in the Third National Health and Nutrition Examination Survey (NHANES III) Psychosom Med. 2008;70:936–41. doi: 10.1097/PSY.0b013e3181870aec. doi: http://dx.doi.org/10.1097/PSY.0b013e3181870aec. [DOI] [PubMed] [Google Scholar]
- 188.Stein PS, Kryscio RJ, Desrosiers M, Donegan SJ, Gibbs MB. Tooth loss, apolipoprotein E, and decline in delayed word recall. J Dent Res. 2010;89:473–7. doi: 10.1177/0022034509357881. doi: http://dx.doi.org/10.1177/0022034509357881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gatz M, Mortimer JA, Fratiglioni L, Johansson B, Berg S, Reynolds CA, et al. Potentially modifiable risk factors for dementia in identical twins. Alzheimers Dement. 2006;2:110–7. doi: 10.1016/j.jalz.2006.01.002. doi: http://dx.doi.org/10.1016/j.jalz.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 190.Kaye EK, Valencia A, Baba N, Spiro A, 3rd, Dietrich T, Garcia RI. Tooth loss and periodontal disease predict poor cognitive function in older men. J Am Geriatr Soc. 2010;58:713–8. doi: 10.1111/j.1532-5415.2010.02788.x. doi: http://dx.doi.org/10.1111/j.1532-5415.2010.02788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.de Souza Rolim T, Fabri GM, Nitrini R, Anghinah R, Teixeira MJ, de Siqueira JT, et al. Oral infections and orofacial pain in Alzheimer's disease: a case-control study. J Alzheimers Dis. 2014;38:823–9. doi: 10.3233/JAD-131283. doi: http://dx.doi.org/10.3233/JAD-131283. [DOI] [PubMed] [Google Scholar]
- 192.Corrada MM, Paganini-Hill A, Berlau DJ, Kawas CH. Apolipoprotein E genotype, dementia, and mortality in the oldest old: the 90+ study. Alzheimers Dement. 2013;9:12–8. doi: 10.1016/j.jalz.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Loeb MB, Molloy DW, Smieja M, Standish T, Goldsmith CH, Mahony J, et al. A randomized, controlled trial of doxycycline and rifampin for patients with Alzheimer's disease. J Am Geriatr Soc. 2004;52:381–7. doi: 10.1111/j.1532-5415.2004.52109.x. [DOI] [PubMed] [Google Scholar]
- 194.Ferretti MT, Allard S, Partridge V, Ducatenzeiler A, Cuello AC. Minocycline corrects early, pre-plaque neuroinflammation and inhibits BACE-1 in a transgenic model of Alzheimer's disease-like amyloid pathology. J Neuroinflammation. 2012;9 doi: 10.1186/1742-2094-9-62. 62. doi: http://dx.doi.org/10.1186/1742-2094-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.in't Veld BA, Ruitenberg A, Hofman A, Stricker BH, Breteler MM. Antihypertensive drugs and incidence of dementia: the Rotterdam Study. Neurobiol Aging. 2001;22:407–12. doi: 10.1016/s0197-4580(00)00241-4. [DOI] [PubMed] [Google Scholar]
- 196.Stewart WF, Kawas C, Corrada M, Metter EJ. Risk of Alzheimer's disease and duration of NSAID use. Neurology. 1997;48:626–32. doi: 10.1212/wnl.48.3.626. [DOI] [PubMed] [Google Scholar]
- 197.McGeer PL, McGeer EG. Anti-inflammatory drugs in the fight against Alzheimer's disease. Ann N Y Acad Sci. 1996;777:213–20. doi: 10.1111/j.1749-6632.1996.tb34421.x. [DOI] [PubMed] [Google Scholar]
- 198.Itzhaki R, Wozniak MA. Could antivirals be used to treat Alzheimer's disease. Future Microbiol. 2012;7:307–9. doi: 10.2217/fmb.12.10. [DOI] [PubMed] [Google Scholar]
- 199.Lin WR, Jennings R, Smith TL, Wozniak MA, Itzhaki RF. Vaccination prevents latent HSV1 infection of mouse brain. Neurobiol Aging. 2001;22:699–703. doi: 10.1016/s0197-4580(01)00239-1. [DOI] [PubMed] [Google Scholar]
- 200.Mori I. “Spontaneous molecular reactivation” of herpes simplex virus type 1 in the brain as a pathogenic mechanism of Alzheimer's disease. Med Hypotheses. 2011;77:462. doi: 10.1016/j.mehy.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 201.Wozniak MA, Itzhaki RF. Intravenous immunoglobulin reduces β amyloid and abnormal tau formation caused by herpes simplex virus type 1. J Neuroimmunol. 2013;257:7–12. doi: 10.1016/j.jneuroim.2013.01.005. doi: http://dx.doi.org/10.1016/j.jneuroim.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 202.Jalkute CB, Sonawane KD. Evaluation of a possible role of Stigmatella aurantiaca ACE in Aβ peptide degradation: a molecular modeling approach. J Mol Microbiol Biotechnol. 2015;25:26–36. doi: 10.1159/000370114. doi: http://dx.doi.org/10.1159/000370114. [DOI] [PubMed] [Google Scholar]
- 203.Chiarini A, Gardenal E, Whitfield JF, Chakravarthy B, Armato U, Dal Pra I. Preventing the spread of Alzheimer's disease neuropathology: a role for calcilytics? Curr Pharm Biotechnol. 2015;16:696–706. doi: 10.2174/1389201016666150505123813. [DOI] [PubMed] [Google Scholar]
- 204.Hochgräfe K, Sydow A, Matenia D, Cadinu D, Könen S, Petrova O, et al. Preventive methylene blue treatment preserves cognition in mice expressing full-length pro- aggregant human Tau. Acta Neuropathol Commun. 2015;3:25. doi: 10.1186/s40478-015-0204-4. doi: http://dx.doi.org/10.1186/s40478-015-0204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Richard T, Pawlus AD, Iglésias ML, Pedrot E, Waffo-Teguo P, Mérillon JM, et al. Neuroprotective properties of resveratrol and derivatives. Ann N Y Acad Sci. 2011;1215:103–8. doi: 10.1111/j.1749-6632.2010.05865.x. doi: http://dx.doi.org/10.1111/j.1749-6632.2010.05865.x. [DOI] [PubMed] [Google Scholar]
- 206.Regitz C, Fitzenberger E, Mahn FL, Dußling LM, Wenzel U. Resveratrol reduces amyloid-beta (Aβ1-42)-induced paralysis through targeting proteostasis in an Alzheimer model of Caenorhabditis elegans . Eur J Nutr. 2015 doi: 10.1007/s00394-015-0894-1. [DOI] [PubMed] [Google Scholar]
- 207.Håheim LL, Olsen I, Rønningen KS. Oral infection, regular alcohol drinking pattern, and myocardial infarction. Med Hypotheses. 2012;79:725–30. doi: 10.1016/j.mehy.2012.08.010. doi: http://dx.doi.org/10.1016/j.mehy.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 208.Song Y, Kim HD, Lee MK, Kim MK, Kang SN, Ko YG, et al. Protective effect of centipedegrass against Aβ oligomerization and Aβ-mediated cell death in PC12 cells. Pharm Biol. 2015;8:1–7. doi: 10.3109/13880209.2014.974062. [DOI] [PubMed] [Google Scholar]
- 209.Kontsekova E, Zilka N, Kovacech B, Novak P, Novak M. First-in-man tau vaccine targeting structural determinants essential for pathological tau–tau interaction reduces tau oligomerisation and neurofibrillary degeneration in an Alzheimer's disease model. Alzheimers Res Ther. 2014;6:44. doi: 10.1186/alzrt278. doi: http://dx.doi.org/10.1186/alzrt278. [DOI] [PMC free article] [PubMed] [Google Scholar]