Abstract
Background
Virtual histology intravascular ultrasound (VH-IVUS) imaging is an innovative tool for the morphological evaluation of coronary atherosclerosis. Evidence for the effects of statin therapy on VH-IVUS parameters have been inconclusive. Consequently, we performed a systematic review and meta-analysis to investigate the impact of statin therapy on plaque volume and its composition using VH-IVUS.
Methods
The search included PubMed, Cochrane Library, Scopus and Embase (through 30 November 2014) to identify prospective studies investigating the effects of statin therapy on plaque volume and its composition using VH-IVUS.
Results
We identified nine studies with 16 statin treatment arms and 830 participants. There was a significant effect of statin therapy in reducing plaque volume (standardized mean difference (SMD): −0.137, 95 % confidence interval (CI): −0.255, −0.019; P = 0.023), external elastic membrane volume (SMD: −0.097, 95 % CI: −0.183, −0.011; P = 0.027) but not lumen volume (SMD: −0.025, 95 % CI: −0.110, +0.061; P = 0.574). There was a significant reduction in fibrous plaque volume (SMD: −0.129, 95 % CI: −0.255, −0.003; P = 0.045) and an increase of dense calcium volume (SMD: +0.229, 95 % CI: +0.008, +0.450; P = 0.043), while changes in fibro-fatty (SMD: −0.247, 95 % CI: −0.592, +0.098; P = 0.16) and necrotic core (SMD: +0.011, 95 % CI: −0.144, +0.165; P = 0.892) tissue volumes were not statistically significant.
Conclusions
This meta-analysis indicates a significant effect of statin therapy on plaque and external elastic membrane volumes and fibrous and dense calcium volumes. There was no effect on lumen volume, fibro-fatty and necrotic tissue volumes.
Keywords: Virtual histology intravascular ultrasound, VH-IVUS, Statins, Statin therapy
Background
Despite continuously improving therapies used for acute coronary syndromes (ACS), cardiovascular disease (CVD) and its complications remain the leading causes of mortality and morbidity [1]. The most important mechanism leading to ACS is the rupture of a vulnerable plaque and subsequent thrombus formation [2–4]. The lesion most frequently prone to rupture is represented by the thin-cap fibroatheroma (TCFA), which contains a large necrotic core with an overlying thin fibrous cap [5]. The recently introduced technique of virtual histology intravascular ultrasound (VH-IVUS) utilizes spectral analysis of the radiofrequency ultrasound backscatter signals, which allows in vivo differentiation of four distinct atherosclerotic plaque phenotypes: fibrous; fibro-fatty; dense calcium; and necrotic core [6]. In vivo studies of coronary [7] and carotid plaques [8] have demonstrated the accuracy of VH-IVUS for histological characterization of atherosclerotic plaques.
The Providing Regional Observations to Study Predictors of Events in the Coronary Tree (PROSPECT), the VH-IVUS in Vulnerable Atherosclerosis (VIVA) and the European Collaborative Project on Inflammation and Vascular Wall Remodeling in Atherosclerosis (ATHEROREMO-IVUS) substudy are three important prospective studies that have demonstrated that the presence of VH-IVUS-derived TCFA lesions is strongly and independently predictive for the occurrence of major adverse cardiovascular events (MACE) [9–11]. Extensive research has focused on preventing CVD events, including therapies that may stabilize atherosclerotic plaques [12]. There is a well-established association between therapy with high doses of statins and regression of coronary atherosclerosis [13]. Also, there have been studies that have investigated the efficiency of statin therapy on coronary plaque composition evaluated with the VH-IVUS method [14, 15]. However, these studies were conducted in relatively small study cohorts and are not conclusive. It is not established whether and to what extent statins have an effect on coronary plaque composition. The purpose of this meta-analysis was therefore to investigate the impact of statin therapy on coronary plaque composition.
Methods
Data sources
This study was designed according to the guidelines of the 2009 Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement [16]. Our search included Scopus, Medline, Web of Science and Cochrane Library databases. It was limited to prospective studies carried out up to 30 November 2014, investigating the potential effects of statin therapy on plaque volume and its composition. The databases were searched using the following search terms in titles and abstracts (also in combination with Medical Subject Headings (MeSH) terms): ‘virtual histology intravascular ultrasound’ OR ‘virtual histology IVUS’ OR ‘VH IVUS’ OR ‘VH-IVUS’ AND ‘statins’ (all fields) OR ‘statin’ (all fields) OR ‘statin therapy’ (all fields) OR ‘rosuvastatin’ OR ‘pravastatin’ OR ‘fluvastatin’ OR ‘simvastatin’ OR ‘atorvastatin’ OR ‘pitavastatin’ OR ‘lovastatin’ OR ‘cerivastatin’ AND ‘virtual histology intravascular ultrasound’ (all fields) OR ‘virtual histology IVUS’ (all fields) OR ‘VH IVUS’ (all fields) OR ‘VH-IVUS’ (all fields). The wild-card term ‘*’ was used to increase the sensitivity of the search strategy. No language restriction was used in the literature search. The search was limited to studies in humans. References of all obtained articles were additionally explored for supplemental publications. Two reviewers (CS and AS) examined every article separately to minimize the possibility of duplication, investigating reviews, case studies and experimental studies. Disagreements were managed by discussion with a third party (MB).
Study selection
Inclusion criteria
Original studies were included if they met the following inclusion criteria: a) being a prospective clinical study; b) investigating the impact of statin therapy on plaque volume and/or its composition using VH-IVUS (in comparison to placebo group or high-intensity versus moderate/low-intensity statin therapy); c) presentation of sufficient information on VH-IVUS findings at baseline and at the end of study; and d) statin therapy for at least 2 weeks.
Exclusion criteria
Exclusion criteria were: a) non-clinical studies (experimental and basic studies); b) observational or retrospective studies; c) duplicate reports or secondary or post hoc analyses of the same study population; and d) lack of sufficient information on baseline or follow-up VH-IVUS data. Exclusion of an article for this reason was also done if no feedback was received after contacting the author(s).
Data extraction
Eligible studies were reviewed and the following data were abstracted: 1) first author’s name; 2) year of publication; 3) study location; 4) number of participants; 5) age, gender and body mass index (BMI) of study participants; 6) baseline levels of total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides (TG), high-sensitivity C-reactive protein (hs-CRP) and glucose; 7) systolic (SBP) and diastolic blood pressure (DBP); 8) statin type, statin dose and duration of treatment (both in research and control groups); and 9) data regarding baseline and follow-up VH-IVUS findings including plaque volume (PV), lumen volume (LV), external elastic membrane volume (EEMV), as well as atheroma compositional data (comprising volumes of fibrous, fibro-fatty, dense calcium and necrotic core tissues).
Quality assessment and quantitative data synthesis
The quality of included studies was assessed using the Cochrane scale. Meta-analysis was conducted using Review Manager, version 5.2 (Cochrane Collaboration, Oxford, UK), and Comprehensive Meta-Analysis (CMA) V2 software (Biostat, NJ, USA) [17]. Standard deviations (SD) of the mean difference were calculated using the following formula: SD = square root ((SDpre-treatment)2 + (SDpost-treatment)2 − (2R × SDpre-treatment × SDpost-treatment)), assuming a correlation coefficient (R) = 0.5. In case of reporting SEM, SD was estimated using the following formula: SD = SEM × sqrt (n), where n is the number of subjects. In case levels were reported as the median and interquartile range, the mean and SD were estimated using the recommendations of Hozo et al. [18].
Net changes in measurements (change scores) were calculated for parallel and crossover trials, as follows: measure at end of follow-up − measure at baseline. A random-effects model (using DerSimonian–Laird method) and the generic inverse variance method were used to compensate for the heterogeneity of studies in terms of statin type, statin dose, study design, treatment duration and the characteristics of populations being studied [19]. Effect sizes were expressed as weighed standardized mean difference (SMD) and 95 % confidence intervals (CI). In order to evaluate the influence of each study on the overall effect size, sensitivity analysis was conducted using the one-study remove (leave-one-out) approach.
Meta-regression
Meta-regression was performed using a random-effects model (using unrestricted maximum likelihood method) to evaluate the association between calculated SMD in plaque volume with duration of statin therapy and changes in LDL-C concentrations.
Publication bias
Potential publication bias was explored using visual inspection of Begg’s funnel plot asymmetry, and Begg’s rank correlation and Egger’s weighted regression tests. The Duval and Tweedie ‘trim and fill’ and ‘fail-safe N’ methods were used to adjust the analysis for the effects of publication bias [20].
Results
Search results and trial flow
A total of nine eligible studies comprising 16 treatment arms met the inclusion criteria and were included for the final meta-analysis [14, 21–28]. An overview of the study selection process is presented in Fig. 1.
Fig. 1.

Flow diagram for study selection. VH-IVUS, virtual histology intravascular ultrasound
Characteristics of included studies
Among 830 participants in the included studies, 737 were allocated to statin intervention groups (with different statin preparations and different doses) and 93 to placebo group. The number of participants in these studies ranged from 20 to 228. The studies were published between 2009 and 2014, and were conducted in USA (two studies), South Korea (two studies), China, Hong Kong and Japan (three studies). The following statin doses were administered in the included trials: 10 to 80 mg/day atorvastatin; 10 to 40 mg/day pravastatin; 20 mg/day simvastatin; 10 to 40 mg/day rosuvastatin; 60 mg/day fluvastatin; and 2 to 4 mg/day pitavastatin. One study did not mention statin preparation or dosage [24]. Duration of statin intervention ranged from 6 to 24 months. Only two studies were placebo-controlled, the other seven included only statin intervention groups. Demographic and baseline parameters of the included studies are shown in Table 1.
Table 1.
Demographic characteristics of the included studies
| Study | Eshtehardi et al. [21] | Guo et al. [22] | Hong et al. [23] | Hwang et al. [24] | Lee et al. [14] | Nasu et al. [25] | Nozue et al. [26] | Puri et al. [27] | Taguchi et al. [28] | |
|---|---|---|---|---|---|---|---|---|---|---|
| Year | 2012 | 2012 | 2009 | 2013 | 2012 | 2009 | 2012 | 2014 | 2013 | |
| Location | USA | China | Korea | Korea | Hong Kong | Japan | Japan | USA | Japan | |
| Design | Pilot study on consecutive patients treated with atorvastatin | Randomized placebo-controlled parallel group trial | Randomized parallel group trial | Prospective study on patients treated with statin | Prospective randomized double-blind parallel group trial | Prospective and multicenter study with non-randomized and no blinded design | Prospective, open-labeled, randomized, multicenter study | Randomized parallel-group trial | Prospective, non-randomized, non-controlled and open-label trial | |
| Duration of study | 6 months | 6 months | 12 months | 6 months | 6 months | 12 months | 8 months | 24 months | 8–10 months | |
| Inclusion criteria | Patients with an abnormal non-invasive stress test, stable angina or stabilized acute coronary syndrome who were found to have moderate lesions requiring invasive physiologic evaluation | Coronary heart disease patients with stable atherosclerotic plaques | Patients with de novo non-culprit/non-target lesions without significant stenosis by coronary angiogram (diameter stenosis <50 %), lesions with a plaque burden <0.75 by gray-scale IVUS, and lesions located in 1 of 3 major epicardial arteries in which stent implantation was not performed | Patients with acute coronary syndrome | Statin-naive patients free from unstable angina >8 weeks before intervention or acute coronary syndrome and with angiographic critical coronary stenosis requiring percutaneous coronary intervention | Patients older than 30 years of age with symptomatic stable angina pectoris. Angiographic inclusion criteria: 1) target vessel for VH-IVUS interrogation must not have undergone angioplasty or have more than 50 % luminal narrowing throughout a target segment with a minimum length of 30 mm; 2) target vessel for VH-IVUS interrogation had mild-to-moderate vessel tortuosity and calcification for safe and accurate examination; and 3) left ventricular ejection fraction >30 % | Patients with stable and unstable angina after successful percutaneous coronary intervention | Patients with angiographically demonstrable coronary disease and LDL-C <116 mg/dL, following a 2-week treatment period with atorvastatin (40 mg) or rosuvastatin (20 mg) daily | Patients with acute coronary syndrome defined as unstable angina of Braunwald class IIIB (angina at rest without increased levels of the creatine kinase-MB fraction within 24 hours before coronary angiography), non-ST-segment elevation myocardial infarction, or ST-segment elevation myocardial infarction | |
| Statin form | Atorvastatin | Atorvastatin | Simvastatin or rosuvastatin | NS | Atorvastatin | Fluvastatin | Pitavastatin or pravastatin | Rosuvastatin or atorvastatin | Atorvastatin or pitavastatin | |
| Statin intervention | 80 mg/day | 10–80 mg/day | 20 mg/day or 10 mg/day | NS | 10–40 mg/day | 60 mg/day | 4 mg/day or 20 mg/day | 40 mg/day or 80 mg/day | 10 mg/day or 2 mg/day | |
| Participants | Intervention | 20 | 47a | 50e | 54 | 19a | 40 | 58g | 36i | 60a |
| 45b | ||||||||||
| 43c | 50f | 20c | 61h | 35d | 60j | |||||
| 39d | ||||||||||
| Control | - | 54 | - | - | - | 39 | - | - | ||
| Age (years) | Intervention | 54 (46–68) | 62.64 ± 12.0a | 58 ± 10e | 59 ± 10 | 65.05 ± 9.99a | 63 ± 10 | 66 ± 9g | 57.6 ± 9.0** | 65.8 ± 16.2# |
| 59.18 ± 8.48b | ||||||||||
| 58.91 ± 12.90c | 59 ± 9f | 63.70 ± 9.80c | 67 ± 11h | 63.7 ± 16.5## | ||||||
| 58.95 ± 9.68d | ||||||||||
| Control | - | 62.07 ± 8.51 | - | - | - | 62 ± 12 | - | - | - | |
| Male (%) | Intervention | 65.0 | 88.88a | 80.0e | 70.37 | 73.68a | 80.0 | 89.65g | 80.3** | 76.6# |
| 85.10b | ||||||||||
| 80.0c | 74.0f | 90.0c | 77.05h | 69.2## | ||||||
| 95.35d | ||||||||||
| Control | - | 87.18 | - | - | - | 77.5 | - | - | - | |
| BMI (kg/m2) | Intervention | 30 (27–36) | NSa | NSe | NS | 26.83 ± 6.85a | NS | 24.4 ± 3.5g | 28.6 ± 4.5** | 24.0 ± 2.5# |
| NSb | ||||||||||
| NSc | NSf | 26.58 ± 5.44c | 24.5 ± 3.3h | 24.2 ± 2.7## | ||||||
| NSd | ||||||||||
| Control | - | NS | - | - | - | NS | - | - | - | |
| hs-CRP (mg/L) | Intervention | NS | 6.04 ± 2.52a | 0.17 ± 0.22e | 3.18 ± 5.29 | NSa | 2.05 ± 2.20 | 3.76 (1.22–9.22)g | 1.4 (0.7–2.7)** | NS# |
| 5.09 ± 1.94b | ||||||||||
| 5.67 ± 2.22c | 0.21 ± 0.20f | NSc | 4.23 (1.21–9.26)h | NS## | ||||||
| 6.10 ± 2.12d | ||||||||||
| Control | - | 5.07 ± 1.80 | - | - | - | 1.19 ± 1.03 | - | - | - | |
| Total cholesterol (mg/dL) | Intervention | 186.0 (168.0–212.5) | NSa | 191 ± 34e | 195.0 ± 35.9 | 200.58 ± 41.54a | 239.1 ± 32.8 | 199 ± 34g | 203.1 ± 38** | NS# |
| NSb | ||||||||||
| NSc | 189 ± 27f | 184.17 ± 29.27c | 210 ± 38h | NS## | ||||||
| NSd | ||||||||||
| Control | - | NS | - | - | - | 199.5 ± 22.8 | - | - | - | |
| LDL-C (mg/dL) | Intervention | 118.5 (105.3–140.5) | 116.96 ± 27.02a | 119 ± 30e | 119.7 ± 31.4 | 122.39 ± 39.54a | 144.9 ± 31.5 | 126 ± 28g | 128.6 ± 30.7** | 117.3 ± 34.7# |
| 112.71 ± 23.93b | ||||||||||
| 111.94 ± 13.12c | 116 ± 28f | 112.35 ± 27.14c | 137 ± 35h | 116.2 ± 26.7## | ||||||
| 109.24 ± 25.48d | ||||||||||
| Control | - | 113.48 ± 27.79 | - | - | - | 122.3 ± 18.9 | - | - | - | |
| HDL-C (mg/dL) | Intervention | 39.5 (33.3–52.8) | 34.74 ± 6.56a | 43 ± 10e | 38.9 ± 8.5 | 41.47 ± 9.46a | 52.7 ± 12.4 | 46 ± 11g | 44.7 ± 11.0** | 46.8 ± 10.9# |
| 35.90 ± 7.72b | ||||||||||
| 37.44 ± 9.26c | 43 ± 11f | 42.82 ± 17.45c | 47 ± 11h | 46.5 ± 11.4## | ||||||
| 34.74 ± 5.02d | ||||||||||
| Control | - | 37.06 ± 6.95 | - | - | - | 54.3 ± 17.8 | - | - | - | |
| Triglycerides (mg/dL) | Intervention | 115.5 (83.5–158.8) | NSa | 149 ± 69e | 178.5 ± 126.1 | 168.58 ± 96.19a | 200.6 ± 125.4 | 129 ± 73g | 130 (99–191)** | 115.6 ± 22.6# |
| NSb | ||||||||||
| NSc | 152 ± 75f | 154.42 ± 1.02c | 134 ± 58h | 119.9 ± 35.2## | ||||||
| NSd | ||||||||||
| Control | - | NS | - | - | - | 122.8 ± 50.1 | - | - | ||
| Glucose (mg/dL) | Intervention | NS | 103.14 ± 18.0a | NSe | NS | NSa | NS | NSg | NS** | NS# |
| 102.96 ± 14.76b | ||||||||||
| 90.0 ± 14.94c | NSf | NSc | NSh | NS## | ||||||
| 101.34 ± 17.46d | ||||||||||
| Control | - | 94.68 ± 17.64 | - | NS | - | NS | - | - | - | |
| SBP (mmHg) | Intervention | 129 (114–145) | NSa | NSe | NS | NSa | NS | NSg | NS** | NS# |
| NSb | ||||||||||
| NSc | NSf | NSc | NSh | NS## | ||||||
| NSd | ||||||||||
| Control | - | NS | - | - | - | NS | - | - | - | |
| DBP (mmHg) | Intervention | 72 (68–83) | NSa | NSe | NS | NSa | NS | NSg | NS** | NS# |
| NSb | ||||||||||
| NSc | NSf | NSc | NSh | NS## | ||||||
| NSd | ||||||||||
| Control | - | NS | - | - | - | NS | - | - | - | |
| Plaque volume (mm3) | Intervention | 308.8 (236.8–432.6) | 38.07 ± 13.94a | 88.3 ± 26.9e | 76.1 ± 32.1 | 98.47 ± 70.84a | 440.2 ± 220.3 | 9.06 ± 2.90g* | 146.0 ± 55.6** | 10.2 ± 3.0#* |
| 33.83 ± 10.56b | ||||||||||
| 37.06 ± 12.01c | 91.5 ± 27.5f | 144.17 ± 154.46c | 8.83 ± 3.67h* | 9.9 ± 2.9##* | ||||||
| 36.47 ± 14.68d | ||||||||||
| Control | - | 34.83 ± 13.76 | - | - | - | 432.9 ± 247.5 | - | - | - | |
| Lumen volume (mm3) | Intervention | 427.3 (310.9–703.7) | NSa | 85.2 ± 20.4e | 70.5 ± 24.1 | NSa | 373.7 ± 188.4 | 7.40 ± 2.55g* | 214.9 ± 71.5** | 6.6#*§ |
| NSb | ||||||||||
| NSc | 87.6 ± 26.2f | NSc | 7.42 ± 2.66h* | 8.0 ± 2.8##* | ||||||
| NSd | ||||||||||
| Control | - | NS | - | - | - | 444.7 ± 233.5 | - | - | - | |
| External elastic membrane volume (mm3) | Intervention | 830.9 (606.8–1,080.1) | NSa | 173.5 ± 37.1e | 146.6 ± 52.3 | NSa | 813.9 ± 398.5 | 16.46 ± 4.98g* | 360.9 ± 108.8** | 16.8 ± 4.6#* |
| NSb | ||||||||||
| NSc | 179.1 ± 46.6f | NSc | 16.25 ± 5.63h* | 17.9 ± 5.0##* | ||||||
| NSd | ||||||||||
| Control | - | NS | - | - | - | 877.6 ± 458.3 | - | - | - | |
| Fibrous volume (mm3) | Intervention | 89.9 (67.1–123.9) | NSa | 25.6 ± 12.7e | 27.7 ± 15.6 | 37.04 ± 30.41a | 146.5 ± 85.6 | 3.46 ± 1.65g* | 18.5 (9.8–29.3)** | 5.9 ± 2.6#* |
| NSb | ||||||||||
| NSc | 28.2 ± 14.4f | 54.90 ± 58.05c | 3.13 ± 1.98h* | 5.8 ± 2.3##* | ||||||
| NSd | ||||||||||
| Control | - | NS | - | - | - | 142.9 ± 113.3 | - | - | - | |
| Fibro-fatty volume (mm3) | Intervention | 10.6 (6.4–27.9) | NSa | 4.1 ± 2.9e | 4.5 ± 3.9 | 9.76 ± 9.80a | 80.1 ± 57.9 | 1.09 ± 0.88g* | 23.1 (8.8–36.3)** | 1.5 ± 1.1#* |
| NSb | ||||||||||
| NSc | 4.5 ± 4.0f | 19.39 ± 36.04c | 1.05 ± 1.03h* | 0.7 ± 0.6##* | ||||||
| NSd | ||||||||||
| Control | - | NS | - | - | - | 50.7 ± 32.9 | - | - | - | |
| Dense calcium volume (mm3) | Intervention | 10.5 (4.0–20.9) | NSa | 6.5 ± 6.3e | 4.2 ± 3.2 | 3.18 ± 3.44a | 9.4 ± 9.9 | 0.42 ± 0.35g* | 1.2 (0.2–3.8)** | 0.6#*§ |
| NSb | ||||||||||
| NSc | 6.8 ± 6.4f | 4.85 ± 7.68c | 0.44 ± 0.47h* | 0.6##*§ | ||||||
| NSd | ||||||||||
| Control | - | NS | - | - | - | 13.7 ± 12.7 | - | - | - | |
| Necrotic core volume (mm3) | Intervention | 30.8 (13.9–48.2) | NSa | 15.8 ± 11.3e | 8.7 ± 6.4 | 7.91 ± 7.47a | 21.4 ± 24.9 | 0.68 ± 0.42g* | 5.9 (2.6–12.3)** | 1.6 ± 0.9#* |
| NSb | ||||||||||
| NSc | 15.5 ± 8.4f | 11.89 ± 18.72c | 0.80 ± 0.66h* | 2.1 ± 1.4##* | ||||||
| NSd | ||||||||||
| Control | - | NS | - | - | - | 22.1 ± 17.4 | - | - | - |
Values are expressed as mean ± SD or median (25–75 percentiles). a10 mg/day atorvastatin arm; b20 mg/day atorvastatin arm; c40 mg/day atorvastatin arm; d80 mg/day atorvastatin arm; e20 mg/day simvastatin arm; f10 mg/day rosuvastatin arm; g4 mg/day pitavastatin arm; h20 mg/day pravastatin arm; i40 mg/day rosuvastatin arm; j2 mg/day pitavastatin arm; *the value was provided as volume index defined as the volume divided by the segment length (mm3/mm); **the value was provided for rosuvastatin and atorvastatin arms together; #patients belonging to plaque regression group (n = 94); ##patients belonging to plaque progression (n = 26) group; §SD not shown. BMI, body mass index; DBP, diastolic blood pressure; HDL-C, high-density lipoprotein cholesterol; hs-CRP, high-sensitivity C-reactive protein; IVUS, intravascular ultrasound; LDL-C, low-density lipoprotein cholesterol; MB, myocardial band; NS, not stated; SBP, systolic blood pressure; VH-IVUS, virtual histology intravascular ultrasound
Risk of bias assessment
According to the Cochrane Collaboration [29], a specific tool for assessing risk of bias in every study involved consists of selection of particular characteristics of the study. This involves assessing the risk of bias as ‘low risk’, ‘high risk or ‘unclear risk’. The last category reveals either lack of detail or concern over the potential for bias. There are seven examined fields including: sequence generation (selection bias); allocation sequence concealment (selection bias); blinding of participants and personnel (performance bias); blinding of outcome assessment (detection bias); incomplete outcome data (attrition bias); selective outcome reporting (reporting bias); and other potential sources of bias (Table 2).
Table 2.
Assessment of risk of bias in the included studies using Cochrane criteria
| Study | Reference | Sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective outcome reporting | Other potential threats to validity |
|---|---|---|---|---|---|---|---|---|
| Eshtehardi et al. 2012 | [21] | H | H | H | H | L | L | L |
| Guo et al. 2012 | [22] | U | U | H | H | L | L | L |
| Hong et al. 2009 | [23] | U | U | H | H | L | L | L |
| Hwang et al. 2013 | [24] | H | H | H | L | L | L | H |
| Lee et al. 2012 | [14] | L | L | L | L | L | L | L |
| Nasu et al. 2009 | [25] | H | H | H | H | L | L | L |
| Nozue et al. 2012 | [26] | L | L | H | L | L | L | L |
| Puri et al. 2014 | [27] | U | U | H | H | L | L | L |
| Taguchi et al. 2013 | [28] | H | H | H | H | L | L | L |
H, high risk of bias; L, low risk of bias; U, unclear risk of bias
Quantitative data synthesis
Meta-analysis of data from 16 statin-treated arms showed a significant effect of statin therapy in reducing plaque volume (SMD: −0.137, 95 % CI: −0.255, −0.019; P = 0.023) (Fig. 2). This effect size was robust in the sensitivity analysis and remained at a significant or borderline significant levels following omission of each single study (Fig. 3). Statin therapy was also associated with a significant decrease in EEMV (SMD: −0.097, 95 % CI: −0.183, −0.011; P = 0.027) but not LV (SMD: −0.025, 95 % CI: −0.110, +0.061; P = 0.574) (Fig. 2).
Fig. 2.
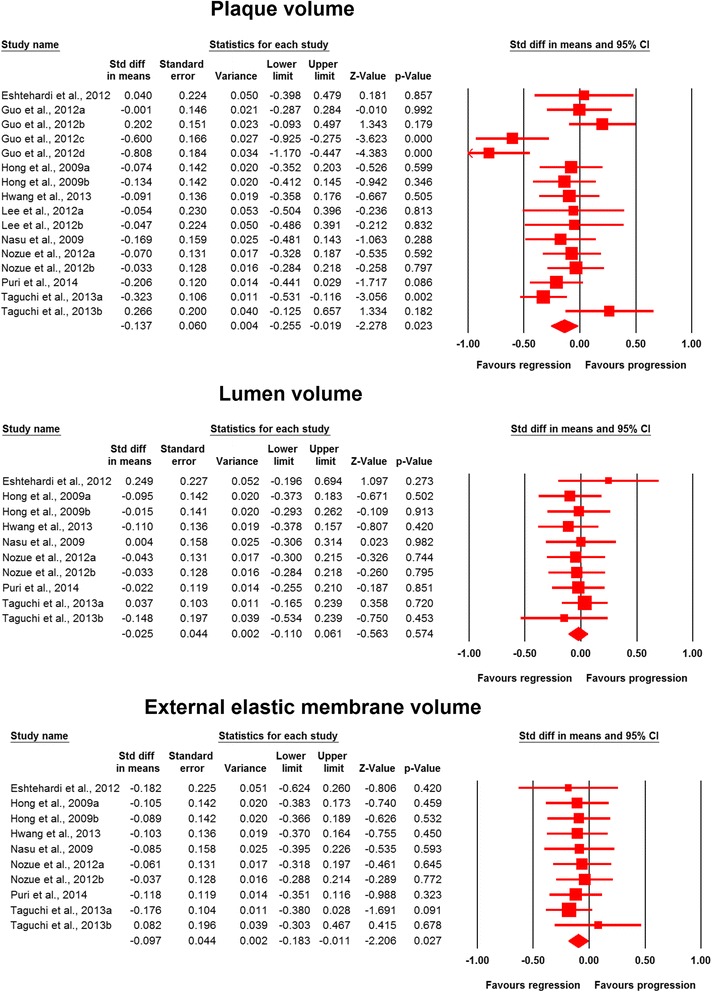
Forest plot detailing weighted mean difference and 95 % confidence intervals for the impact of statin therapy on plaque, lumen and external elastic membrane volumes according to virtual histology intravascular ultrasound (VH-IVUS). Meta-analysis was performed using a random-effects model with inverse variance weighting
Fig. 3.

Leave-one-out sensitivity analysis of the impact of statin therapy on plaque volume
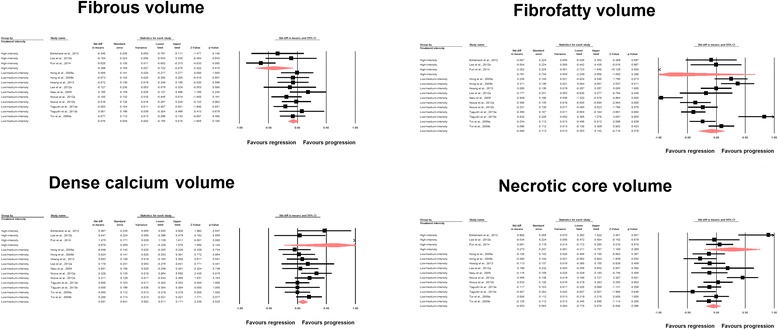
The analysis of plaque composition data indicated significant reduction in fibrous (SMD: −0.129, 95 % CI: −0.255, −0.003; P = 0.045) and increase in dense calcium (SMD: 0.229, 95 % CI: 0.008, 0.450; P = 0.043) volumes, while fibro-fatty (SMD: −0.247, 95 % CI: −0.592, +0.098; P = 0.160) and necrotic core (SMD: 0.011, 95 % CI: −0.144, +0.165; P = 0.892) tissue volumes remained statistically unaltered (Fig. 4).
Fig. 4.

Forest plot detailing weighted mean difference and 95 % confidence intervals for the impact of statin therapy on plaque composition parameters according to virtual histology intravascular ultrasound (VH-IVUS). Meta-analysis was performed using a random-effects model with inverse variance weighting
A subgroup analysis was performed to compare the impact of high-intensity versus moderate/low-intensity statin therapy on coronary atherosclerosis according to American College of Cardiology (ACC)/American Heart Association (AHA) lipid guidelines [30]. High-intensity statin therapy had a greater effect in reducing plaque volume (SMD: −0.338, 95 % CI: −0.637, −0.040; P = 0.026) compared with moderate/low-intensity treatment (SMD: −0.071, 95 % CI: −0.167, +0.026; P = 0.152) (Fig. 5). However, no significant difference between the subgroups was observed in terms of effects on LV and EEMV (Fig. 5). With respect to plaque composition parameters, significant changes in dense calcium (SMD: 0.091, 95 % CI: 0.011, 0.171; P = 0.025) and fibrous (SMD: −0.399, 95 % CI: −0.722, −0.076; P = 0.015) volumes were observed in the moderate/low-intensity and high-intensity subgroups, respectively (Fig. 6). The effects of both treatment regimens on fibro-fatty and necrotic core tissue volumes were statistically comparable (Fig. 6).
Fig. 5.

Forest plot detailing weighted mean difference and 95 % confidence intervals for the impact of high-intensity versus moderate/low-intensity statin therapy on plaque, lumen and external elastic membrane volumes according to virtual histology intravascular ultrasound (VH-IVUS). Meta-analysis was performed using a random-effects model with inverse variance weighting
Fig. 6.
Forest plot detailing weighted mean difference and 95 % confidence intervals for the impact of high-intensity versus moderate/low-intensity statin therapy on plaque composition parameters according to virtual histology intravascular ultrasound (VH-IVUS). Meta-analysis was performed using a random-effects model with inverse variance weighting
Another subgroup analysis was performed to compare the effects of statin therapy on coronary atherosclerosis in the subgroups of trials with and without ACS patients. PV was reduced only in the subset of trials not recruiting ACS patients (SMD: −0.175, 95 % CI: −0.334, −0.015; P = 0.032). The impact of statin therapy on other indices in ACS+ and ACS− subgroups are summarized in Table 3.
Table 3.
Comparison of the effects of statin therapy on coronary atherosclerosis indices in subgroups of trials recruiting subjects with and without ACS
| Without ACS | With ACS | |||||
|---|---|---|---|---|---|---|
| SMD | 95 % CI | P value | SMD | 95 % CI | P value | |
| Plaque volume | −0.175 | −0.334, −0.015 | 0.032 | −0.080 | −0.258, 0.099 | 0.382 |
| Lumen volume | −0.033 | −0.121, 0.056 | 0.469 | −0.007 | −0.148, 0.134 | 0.919 |
| External elastic membrane volume (mm3) | −0.065 | −0.154, 0.024 | 0.150 | −0.121 | −0.263, 0.020 | 0.093 |
| Fibrous volume (mm3) | −0.010 | −0.053, 0.133 | 0.888 | 0.027 | −0.243, 0.297 | 0.844 |
| Fibro-fatty volume | −0.395 | −0.824, 0.034 | 0.071 | 0.008 | −0.312, 0.328 | 0.961 |
| Dense calcium volume | −0.119 | −0.304, 0.065 | 0.206 | −0.137 | −0.266, −0.007 | 0.038 |
| Necrotic core volume | 0.271 | −0.013, 0.555 | 0.062 | 0.074 | −0.055, 0.203 | 0.261 |
ACS, acute coronary syndrome; CI, confidence interval; SMD, standardized mean difference
Meta-regression
Meta-regression analysis was conducted to assess the association between statin-induced changes in PV with duration of statin therapy and respective changes in plasma LDL-C concentrations as potential confounders. In meta-regression analysis, the impact of statins on PV was found to be independent of treatment duration (slope: 0.00007; 95 % CI: −0.006, +0.006; P = 0.980). Likewise, statin-induced reduction in PV was not found to be significantly associated with LDL-C reductions (slope: −0.002; 95 % CI: −0.015, +0.011; P = 0.788) (Fig. 7). Further analyses did not reveal any significant association between statin-induced changes in PV and other potential confounders including age, dose (atorvastatin), age, proportion of males, proportion of diabetics, proportion of smokers and baseline LDL-C (Table 4).
Fig. 7.

Random effects meta-regression plots of the association between mean changes in plaque volume with treatment duration, and changes in plasma low-density lipoprotein cholesterol (LDL-C) concentrations. The size of each circle is inversely proportional to the variance of change. Meta-regression was performed using unrestricted maximum likelihood method
Table 4.
Impact of potential confounders on changes in plaque volume following statin therapy in random-effects meta-regression
| Confounder | Slope | 95 % CI | P value |
|---|---|---|---|
| Age (years) | 0.009 | −0.020, 0.039 | 0.537 |
| % Males | −0.011 | −0.024, 0.002 | 0.106 |
| % Diabetics | 0.003 | −0.002, 0.008 | 0.255 |
| % Smokers | −0.004 | −0.009, 0.0004 | 0.075 |
| Dose (mg/day)a | −0.007 | −0.015, 0.001 | 0.091 |
| Baseline LDL-C (mg/dL) | 0.004 | −0.007, 0.016 | 0.435 |
aRestricted to atorvastatin trials. CI, confidence interval; LDL-C, low-density lipoprotein cholesterol
Publication bias
The results of Egger’s linear regression (intercept = 0.860, standard error = 1.866; 95 % CI: −3.142, +4.861, t = 0.461, df = 14.00; two-tailed P = 0.652) and Begg’s rank correlation (Kendall’s tau with continuity correction = 0.025, Z = 0.135; two-tailed P = 0.893) tests did not provide any proof of significant publication bias for the decreasing effect of statin therapy on PV. However, the funnel plot of precision (1/standard error) by effect size (SMD) was found to be asymmetric and suggestive of potential publication bias. The observed publication bias was imputed using trim-and-fill correction. This correction suggested no asymmetry on the right of the mean, while five potentially missing studies were imputed on the left of the mean leading to a corrected effect size that was significant: SMD: −0.232 (95 % CI: −0.351, −0.114). The ‘fail-safe N’ method indicated that 38 theoretically missing studies would need to be added to the analysis before the overall effect size becomes trivial. Funnel plot of the impact of statins on plaque volume is illustrated in Fig. 8.
Fig. 8.
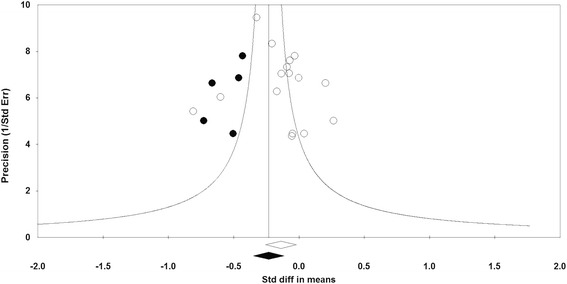
Funnel plot detailing publication bias in the studies reporting the impact of statin therapy on plaque volume. Open circles represent observed published studies; closed circles represent imputed unpublished studies
Discussion
The present systematic review and meta-analysis provides a comprehensive assessment of the impact of statin therapy on coronary plaque composition assessed with VH-IVUS. We observed a significant effect of statin therapy on plaque volume (however with no significant changes in lumen volume), external elastic membrane, fibrous and dense calcium volumes, while fibro-fatty and necrotic core tissue volumes remained statistically unchanged.
The potential reason for obtaining these results may lie in the fact that foam cells function as a substrate for the progression of necrosis [31]. The existence of foam cells and non-load-bearing lipid pools enzymatic together with destruction of collagen by matrix metalloproteinases, and microcalcifications might produce a TCFA, increasing the risk of plaque rupture and MACE [32]. However, statins have been associated with increase in fibrous cap thickness in optical coherence tomography (OCT) studies [33]. In these OCT studies, only assessment of the near field can be achieved due to the poor penetration of the technology and therefore the quantification of fibrous tissue in the total plaque cannot be obtained. In our meta-analysis that included only VH-IVUS studies, we observed a global decrease in fibrous tissue associated with statin treatment. In other words, there may be two differential effects of statin treatment, on the one hand a focal increase in cap thickness and on the other hand a global decrease in fibrous tissue. This hypothesis needs further investigation.
Increased quantities of calcium in coronary plaques have been linked to negative remodeling [34, 35], in contrast to increased lipid and fibro-fatty elements usually seen in positively remodeled lesions [36, 37]. Moreover, ACS and histological features of plaque vulnerability such as a large lipid core and high macrophage content seems to be associated with a positive coronary arterial remodeling [38].
Many studies such as the Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) [39] and the Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombolysis in Myocardial Infarction 22 (PROVE IT-TIMI 22) [40] have reported that intensive statin therapy reduces MACE in patients with coronary heart disease. Significant plaque burden, extensive remodeling and calcification have been regarded as fundamental morphologies of high-risk plaques leading to MACE [41]. It has been shown that statin therapy improves plaque hyperechogenicity without a considerable decrease in plaque volume, suggesting that statins might influence coronary artery plaque composition [42]. Moreover, in non-culprit, high-risk coronary lesions after the onset of ACS, statins proved to be beneficial for regression and stabilization of vulnerable plaques [41]. However, the effect of statin therapy on plaque volume and composition might essentially differ by statin preparations, doses, duration of therapy, methods of imaging, as well as plaque localization. In the Reversal of Atherosclerosis with Aggressive Lipid Lowering (REVERSAL) trial [43], moderate lipid-lowering therapy with 40 mg of pravastatin did not stop plaque progression, while treatment with 80 mg of atorvastatin did. The first study showing reduction on plaque size was the a Study to Evaluate the Effect of Rosuvastatin on Intravascular Ultrasound-Derived Coronary Atheroma Burden (ASTEROID) trial with 40 mg of rosuvastatin [44]. However, these trials have only evaluated quantitative changes of coronary artery plaque using gray-scale IVUS and did not study plaque composition changes. Our meta-analysis showed that statin therapy reduces atheroma plaque volume, however with no significant changes in lumen volume. It also influences plaque composition reducing fibrous volume, however with no significant changes in fibro-fatty and necrotic core tissue volumes. Although these results differed between available studies [14, 21–28], these observations confirm the changes in plaque composition affecting lesion size and plaque stability (changes the composition of plaques from fatty to fibrous). On the other hand, the lack of effect on necrotic material is highly concerning for the field, given that the outcome studies in this field have largely supported the findings that TCFA is associated with adverse outcomes [45].
Statin therapy induced a significant regression of IVUS-measured coronary plaque volume, especially when reaching the target LDL-C level, as shown in a meta-analysis of gray-scale IVUS studies investigating temporal modifications in coronary plaque volume [46]. However, conventional gray-scale IVUS compared with VH-IVUS method has many limitations in the evaluation of atheromatous plaque composition and identification of a vulnerable plaque prior to rupture [47–49]. Another study indicated that VH-IVUS may potentially allow the best detection of features associated with future plaque rupture, increasing the probability of superior risk stratification at the moment of percutaneous coronary intervention [50].
The present meta-analysis has several limitations. Most importantly, there were few eligible prospective trials, and most had small numbers of patients. Furthermore, the included studies were heterogeneous regarding factors such as population characteristics (different statins, doses and duration of treatment), study design and VH-IVUS methodology (for example, in some of the included studies VH-IVUS was not performed in all patients and there were different IVUS catheters used in the included studies). There were only two studies controlled with placebo, and others compared high-intensity versus moderate/low-intensity statin therapy. Furthermore, VH-IVUS was only performed in one coronary vessel, which might not reflect changes in plaque features sampled from other regions of the coronary tree. Plaque volume might be also very variable when measured in mm3 across studies. Finally, the use of serial VH-IVUS imaging might be problematic, as it is ECG gated, so there is limited ability to precisely match segments.
Conclusions
In conclusion, this meta-analysis of nine prospective studies comprising 16 statin-treated arms indicates a significant effect of statin therapy on plaque, external elastic membrane, fibrous and dense calcium volumes, while fibro-fatty and necrotic core tissue volumes remained statistically unchanged. Further large-scale, well-designed head-to-head trials are warranted to fully address the differential effects on these parameters with different statins.
Abbreviations
- ACC
American College of Cardiology
- ACS
Acute coronary syndrome
- AHA
American Heart Association
- ASTEROID
A Study to Evaluate the Effect of Rosuvastatin on Intravascular Ultrasound-Derived Coronary Atheroma Burden
- ATHEROREMO-IVUS
European Collaborative Project on Inflammation and Vascular Wall Remodeling in Atherosclerosis
- BMI
Body mass index
- CI
Confidence interval
- CMA
Comprehensive Meta-Analysis
- CVD
Cardiovascular disease
- DBP
Diastolic blood pressure
- EEMV
External elastic membrane volume
- HDL-C
High-density lipoprotein cholesterol
- hs-CRP
high-sensitivity C-reactive protein
- LDL-C
Low-density lipoprotein cholesterol
- LV
Lumen volume
- MACE
Major adverse cardiovascular event
- MeSH
Medical Subject Headings
- MIRACL
Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering
- OCT
Optical coherence tomography
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- PROSPECT
Providing Regional Observations to Study Predictors of Events in the Coronary Tree
- PROVE IT-TIMI 22
Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombolysis in Myocardial Infarction 22
- PV
Plaque volume
- REVERSAL
Reversal of Atherosclerosis with Aggressive Lipid Lowering
- SBP
Systolic blood pressure
- SD
Standard deviation
- SEM
Standard error of the mean
- SMD
Standardized mean difference
- TC
Total cholesterol
- TCFA
Thin-cap fibroatheroma
- TG
Triglyceride
- VH-IVUS
Virtual histology intravascular ultrasound
- VIVA
VH-IVUS in Vulnerable Atherosclerosis
Footnotes
Maciej Banach and Corina Serban contributed equally to this work.
Competing interests
MB reports honoraria for lectures from: Amgen, Sanofi, Abbott, MSD/Merck, he is consultant/member of an international advisory board in Amgen, Sanofi, Daiichi Sankyo, Resverlogix and Abbott Vascular, reports grants from VALLEANT; DPM has given talks and attended conferences sponsored by MSD, AstraZeneca and Libytec; KKR reports honoraria for lectures from: Novo Nordisk, Roche, Novartis, Pfizer, AstraZeneca, Daiichi Sankyo, Lilly, he is consultant/member of advisory board in: Novo Nordisk, Roche, Novartis, Pfizer, AstraZeneca, Daiichi Sankyo, Lilly, Merck; PPT reports honoraria for lectures from: Amgen, Amarin, AstraZeneca, GSK, Kowa, Merck, he is consultant/member of advisory board in: Amgen, AstraZeneca, Kowa, Merck, Novartis; PM reports grants, personal fees and other from Amgen; GKH reports that his institution receives funding from Dezima, Amgen, Pfizer, Sanofi, Regeneron, AstraZeneca, Genzyme, Cerenis, Synageva, Roche, ISIS pharmaceuticals, Kowa, and Merck for undertaking clinical trials related to various forms of lipid-lowering medication and he reports consulting fees from: Amgen, Pfizer, Roche, and Sanofi; JJP reports personal fees from Dezima Pharmaceuticals, Cerenis, The Medicines Company, CSL Behring, Amgen, Sanofi, Regeneron, Eli Lilly, Genzyme, Aegerion, Esperion, AstraZeneca, Omthera, Pronova, Vascular Biogenics, Boehringer Ingelheim, Catabasis, AtheroNova, UniQure, Novartis, Merck, Isis Pharmaceuticals, and Kowa; PWS is a member of the international advisory board of Abbott Vascular; CS, AS, SU, JR, SM and HMGG have nothing to declare.
Authors’ contributions
MB designed the study, made the literature search, drafted the manuscript, prepared the final version and submitted the paper. CS designed the study, made the literature search and drafted the manuscript. AS designed the study, made the statistical analysis and corrected the draft of the paper. SU made the literature search. DPM, KKR, JR, PPT, PM, SM, HMG-G, GKH, JJPK and PWS corrected the draft of the paper and prepared the final version of the manuscript. All authors read and approved the final manuscript.
Contributor Information
Maciej Banach, Phone: +48 42 639 37 71, Email: maciejbanach@aol.co.uk.
Corina Serban, Email: dr.corinaserban@yahoo.com.
Amirhossein Sahebkar, Email: amir_saheb2000@yahoo.com.
Dimitri P. Mikhailidis, Email: mikhailidis@aol.com
Sorin Ursoniu, Email: sursoniu@yahoo.com.
Kausik K. Ray, Email: koshray@gmail.com
Jacek Rysz, Email: jacek.rysz@umed.lodz.pl.
Peter P. Toth, Email: Peter.Toth@cghmc.com
Paul Muntner, Email: pmuntner@uab.edu.
Svetlana Mosteoru, Email: tanaliliana@yahoo.co.uk.
Hector M. García-García, Email: hgarcia@cardialysis.nl
G. Kees Hovingh, Email: g.k.hovingh@amc.uva.nl.
John JP Kastelein, Email: j.j.kastelein@amc.uva.nl.
Patrick W. Serruys, Email: patrick.w.j.c.serruys@gmail.com
References
- 1.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, et al. Heart disease and stroke statistics−2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, et al. From vulnerable plaque to vulnerable patient a call for new definitions and risk assessment strategies: part I. Circulation. 2003;108:1664–72. doi: 10.1161/01.CIR.0000087480.94275.97. [DOI] [PubMed] [Google Scholar]
- 3.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47:C13–8. doi: 10.1016/j.jacc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 4.Silva Marques J, Pinto FJ. The vulnerable plaque: Current concepts and future perspectives on coronary morphology, composition and wall stress imaging. Rev Port Cardiol. 2014;33:101–10. doi: 10.1016/j.repc.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Pedrigi RM, de Silva R, Bovens SM, Mehta VV, Petretto E, Krams R. Thin-cap fibroatheroma rupture is associated with a fine interplay of sand wall stress. Arterioscler Thromb Vasc Biol. 2014;34:2224–31. doi: 10.1161/ATVBAHA.114.303426. [DOI] [PubMed] [Google Scholar]
- 6.Chiocchi M, Chiaravalloti A, Morosetti D, Loreni G, Gandini R, Mancino S, et al. Virtual histology-intravascular ultrasound as a diagnostic alternative for morphological characterization of carotid plaque: comparison with histology and high-resolution magnetic resonance findings. J Cardiovasc Med. 2014; doi: 10.2459/JCM.0b013e328356a5d2 [DOI] [PubMed]
- 7.Zamani P, Ganz P, Libby P, Sutradhar SC, Rifai N, Nicholls SJ, et al. Relationship of antihypertensive treatment to plasma markers of vascular inflammation and remodeling in the Comparison of Amlodipine versus Enalapril to Limit Occurrences of Thrombosis study. Am Heart J. 2012;163:735–40. doi: 10.1016/j.ahj.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Diethrich EB, Margolis MP, Reid DB, Burke A, Ramaiah V, Rodriguez-Lopez JA, et al. Virtual histology intravascular ultrasound assessment of carotid artery disease: the Carotid Artery Plaque Virtual Histology Evaluation (CAPITAL) study. J Endovasc Ther. 2007;14:676–86. doi: 10.1177/152660280701400512. [DOI] [PubMed] [Google Scholar]
- 9.Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–35. doi: 10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]
- 10.Calvert PA, Obaid DR, O'Sullivan M, Shapiro LM, McNab D, Densem CG, et al. Association between IVUS findings and adverse outcomes in patients with coronary artery disease: the VIVA (VH-IVUS in Vulnerable Atherosclerosis) Study. JACC Cardiovasc Imaging. 2011;4:894–901. doi: 10.1016/j.jcmg.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Cheng JM, Garcia-Garcia HM, de Boer SP, Kardys I, Heo JH, Akkerhuis KM, et al. In vivo detection of high-risk coronary plaques by radiofrequency intravascular ultrasound and cardiovascular outcome: results of the ATHEROREMO-IVUS study. Eur Heart J. 2014;35:639–47. doi: 10.1093/eurheartj/eht484. [DOI] [PubMed] [Google Scholar]
- 12.Nakano M, Ladich E, Virmani R. Multi-modality atherosclerosis imaging and diagnosis. New York, NY: Springer; 2014. Histopathology of atherosclerosis progression: what imagers need to know; pp. 15–24. [Google Scholar]
- 13.Hou J, Jia H, Hu S, Xing L, Yang S, Zhang S, et al. Effect of intensive versus moderate lipid-lowering therapy on the progression of coronary lipid-rich plaque: a prospective, randomized, serial combined optical coherence tomography and intravascular ultrasound study. Circulation. 2014;130:A16064–4. [Google Scholar]
- 14.Lee SW, Hau WK, Kong SL, Chan KK, Chan PH, Lam SC, et al. Virtual histology findings and effects of varying doses of atorvastatin on coronary plaque volume and composition in statin-naive patients: the VENUS study. Circ J. 2012;76:2662–72. doi: 10.1253/circj.CJ-12-0325. [DOI] [PubMed] [Google Scholar]
- 15.Tian J, Gu X, Sun Y, Ban X, Xiao Y, Hu S, et al. Effect of statin therapy on the progression of coronary atherosclerosis. BMC Cardiovasc Disord. 2012;12:70. doi: 10.1186/1471-2261-12-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 17.Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive Meta-Analysis. Version 2. Englewood, NJ: Biostat; 2005. pp. 104–4. [Google Scholar]
- 18.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sutton AJ, Abrams KR, Jones DR, Jones DR, Sheldon TA, Song F. Methods for meta-analysis in medical research. Chichester: John Wiley & Sons; 2000. [Google Scholar]
- 20.Duval S, Tweedie R. Trim and fill: a simple funnel‐plot–based method of testing and adjusting for publication bias in meta‐analysis. Biometrics. 2000;56:455–63. doi: 10.1111/j.0006-341X.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 21.Eshtehardi P, McDaniel MC, Dhawan SS, Binongo J, Krishnan SK, Golub L, et al. Effect of intensive atorvastatin therapy on coronary atherosclerosis progression, composition, arterial remodeling, and microvascular function. J Invasive Cardiol. 2012;24:522–9. [PubMed] [Google Scholar]
- 22.Guo S, Wang R, Yang Z, Li K, Wang Q. Effects of atorvastatin on serum lipids, serum inflammation and plaque morphology in patients with stable atherosclerotic plaques. Exp Ther Med. 2012;4:1069–74. doi: 10.3892/etm.2012.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hong MK, Park DW, Lee CW, Lee SW, Kim YH, Kang DH, et al. Effects of statin treatments on coronary plaques assessed by volumetric virtual histology intravascular ultrasound analysis. JACC Cardiovasc Interv. 2009;2:679–88. doi: 10.1016/j.jcin.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 24.Hwang DS, Shin ES, Kim SJ, Lee JH, Kim JM, Lee SG. Early differential changes in coronary plaque composition according to plaque stability following statin initiation in acute coronary syndrome: classification and analysis by intravascular ultrasound-virtual histology. Yonsei Med J. 2013;54:336–44. doi: 10.3349/ymj.2013.54.2.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nasu K, Tsuchikane E, Katoh O, Tanaka N, Kimura M, Ehara M, et al. Effect of fluvastatin on progression of coronary atherosclerotic plaque evaluated by virtual histology intravascular ultrasound. JACC Cardiovasc Interv. 2009;2:689–96. doi: 10.1016/j.jcin.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 26.Nozue T, Yamamoto S, Tohyama S, Umezawa S, Kunishima T, Sato A, et al. Statin treatment for coronary artery plaque composition based on intravascular ultrasound radiofrequency data analysis. Am Heart J. 2012;163:191–9. doi: 10.1016/j.ahj.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Puri R, Libby P, Nissen SE, Wolski K, Ballantyne CM, Barter PJ, et al. Long-term effects of maximally intensive statin therapy on changes in coronary atheroma composition: insights from SATURN. Eur Heart J Cardiovasc Imaging. 2014;15:380–8. doi: 10.1093/ehjci/jet251. [DOI] [PubMed] [Google Scholar]
- 28.Taguchi I, Oda K, Yoneda S, Kageyama M, Kanaya T, Toyoda S, et al. Evaluation of serial changes in tissue characteristics during statin-induced plaque regression using virtual histology-intravascular ultrasound studies. Am J Cardiol. 2013;111:1246–52. doi: 10.1016/j.amjcard.2013.01.265. [DOI] [PubMed] [Google Scholar]
- 29.Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. Oxford: The Cochrane Collaboration; 2011. [Google Scholar]
- 30.Stone N, Robinson J, Lichtenstein A, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;129:S1–45. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–75. doi: 10.1161/01.ATV.20.5.1262. [DOI] [PubMed] [Google Scholar]
- 32.Garcia-Garcia HM, Jang I-K, Serruys PW, Kovacic JC, Narula J, Fayad ZA. Imaging plaques to predict and better manage patients with acute coronary events. Circ Res. 2014;114:1904–17. doi: 10.1161/CIRCRESAHA.114.302745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takarada S, Imanishi T, Kubo T, Tanimoto T, Kitabata H, Nakamura N, et al. Effect of statin therapy on coronary fibrous-cap thickness in patients with acute coronary syndrome: assessment by optical coherence tomography study. Atherosclerosis. 2009;202:491–7. doi: 10.1016/j.atherosclerosis.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 34.Garcia-Garcia HM, Mintz GS, Lerman A, Vince DG, Margolis MP, van Es GA, et al. Tissue characterisation using intravascular radiofrequency data analysis: recommendations for acquisition, analysis, interpretation and reporting. EuroIntervention. 2009;5:177–89. doi: 10.4244/EIJV5I2A29. [DOI] [PubMed] [Google Scholar]
- 35.Mintz GS, Kent KM, Pichard AD, Satler LF, Popma JJ, Leon MB. Contribution of inadequate arterial remodeling to the development of focal coronary artery stenoses an intravascular ultrasound study. Circulation. 1997;95:1791–8. doi: 10.1161/01.CIR.95.7.1791. [DOI] [PubMed] [Google Scholar]
- 36.Burke AP, Kolodgie FD, Farb A, Weber D, Virmani R. Morphological predictors of arterial remodeling in coronary atherosclerosis. Circulation. 2002;105:297–303. doi: 10.1161/hc0302.102610. [DOI] [PubMed] [Google Scholar]
- 37.Fujii K, Carlier SG, Mintz GS, Wijns W, Colombo A, Böse D, et al. Association of plaque characterization by intravascular ultrasound virtual histology and arterial remodeling. Am J Cardiol. 2005;96:1476–83. doi: 10.1016/j.amjcard.2005.07.054. [DOI] [PubMed] [Google Scholar]
- 38.Vancraeynest D, Pasquet A, Roelants V, Gerber BL, Vanoverschelde JLJ. Imaging the vulnerable plaque. J Am Coll Cardiol. 2011;57:1961–79. doi: 10.1016/j.jacc.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz GG, Olsson AG, Ezekowitz MD, Ganz P, Oliver MF, Waters D, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA. 2001;285:1711–8. doi: 10.1001/jama.285.13.1711. [DOI] [PubMed] [Google Scholar]
- 40.Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 41.Okazaki S, Yokoyama T, Miyauchi K, Shimada K, Kurata T, Sato H, et al. Early statin treatment in patients with acute coronary syndrome demonstration of the beneficial effect on atherosclerotic lesions by serial volumetric intravascular ultrasound analysis during half a year after coronary event: The ESTABLISH Study. Circulation. 2004;110:1061–8. doi: 10.1161/01.CIR.0000140261.58966.A4. [DOI] [PubMed] [Google Scholar]
- 42.Schartl M, Bocksch W, Koschyk DH, Voelker W, Karsch KR, Kreuzer J, et al. Use of intravascular ultrasound to compare effects of different strategies of lipid-lowering therapy on plaque volume and composition in patients with coronary artery disease. Circulation. 2001;104:387–92. doi: 10.1161/hc2901.093188. [DOI] [PubMed] [Google Scholar]
- 43.Nissen SE, Tuzcu EM, Schoenhagen P, Brown B, Ganz P, Vogel R, et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA. 2004;291:1071–80. doi: 10.1001/jama.291.9.1071. [DOI] [PubMed] [Google Scholar]
- 44.Nissen SE, Nicholls SJ, Sipahi I, Libby P, Raichlen JS, Ballantyne CM, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA. 2006;295:1556–65. doi: 10.1001/jama.295.13.jpc60002. [DOI] [PubMed] [Google Scholar]
- 45.Ueda T, Uemura S, Watanabe M, Sugawara Y, Soeda T, Okayama S, et al. Colocalization of thin-cap fibroatheroma and spotty calcification is a powerful predictor of procedure-related myocardial injury after elective coronary stent implantation. Coron Artery Dis. 2014;25:384–91. doi: 10.1097/MCA.0000000000000114. [DOI] [PubMed] [Google Scholar]
- 46.Rodriguez-Granillo GA, Agostoni P, Garcia-Garcia HM, Biondi-Zoccai GG, McFadden E, Amoroso G, et al. Meta-analysis of the studies assessing temporal changes in coronary plaque volume using intravascular ultrasound. Am J Cardiol. 2007;99:5–10. doi: 10.1016/j.amjcard.2006.07.054. [DOI] [PubMed] [Google Scholar]
- 47.Nicholls SJ, Hsu A, Wolski K, Hu B, Bayturan O, Lavoie A, et al. Intravascular ultrasound-derived measures of coronary atherosclerotic plaque burden and clinical outcome. J Am Coll Cardiol. 2010;55:2399–407. doi: 10.1016/j.jacc.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 48.Puri R, Nissen SE, Shao M, Ballantyne CM, Barter PJ, Chapman MJ, et al. Antiatherosclerotic effects of long-term maximally intensive statin therapy after acute coronary syndrome: insights from Study of Coronary Atheroma by Intravascular Ultrasound: Effect of Rosuvastatin Versus Atorvastatin. Arterioscler Thromb Vasc Biol. 2014;34:2465–72. doi: 10.1161/ATVBAHA.114.303932. [DOI] [PubMed] [Google Scholar]
- 49.Nair A, Kuban BD, Tuzcu EM, Schoenhagen P, Nissen SE, Vince DG. Coronary plaque classification with intravascular ultrasound radiofrequency data analysis. Circulation. 2002;106:2200–6. doi: 10.1161/01.CIR.0000035654.18341.5E. [DOI] [PubMed] [Google Scholar]
- 50.Pundziute G, Schuijf JD, Jukema JW, Decramer I, Sarno G, Vanhoenacker PK, et al. Evaluation of plaque characteristics in acute coronary syndromes: non-invasive assessment with multi-slice computed tomography and invasive evaluation with intravascular ultrasound radiofrequency data analysis. Eur Heart J. 2008;29:2373–81. doi: 10.1093/eurheartj/ehn356. [DOI] [PubMed] [Google Scholar]


