Abstract
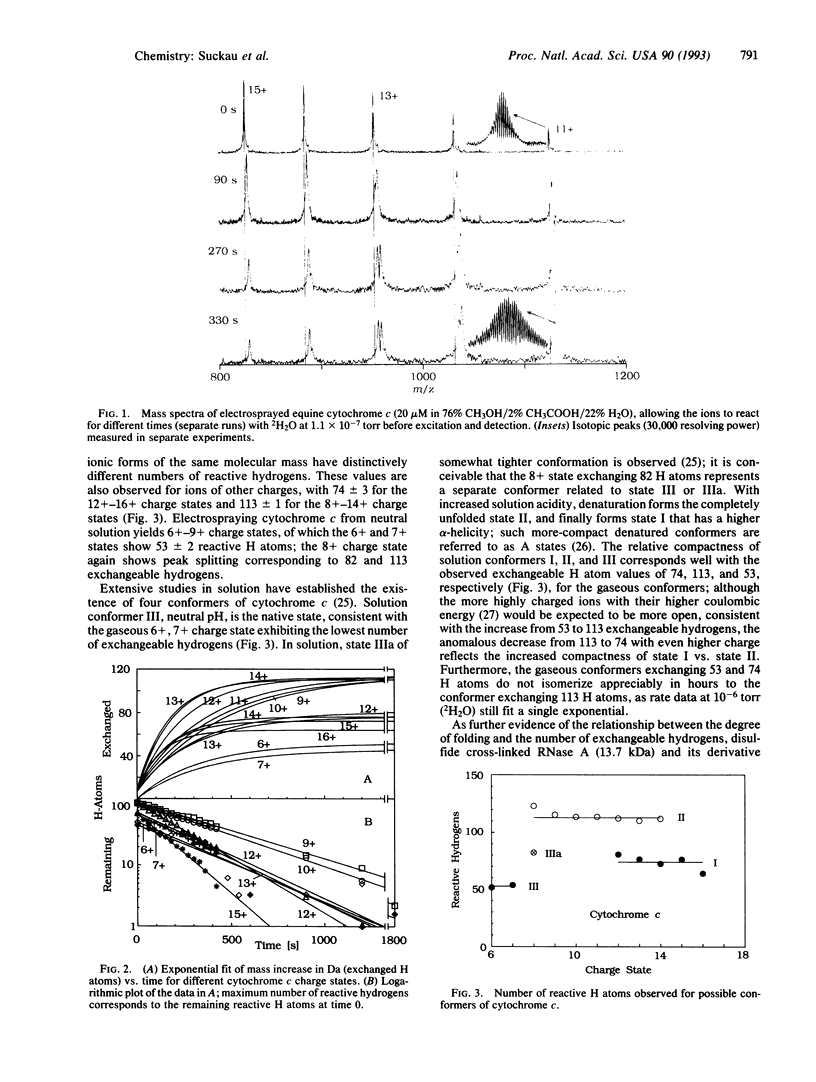
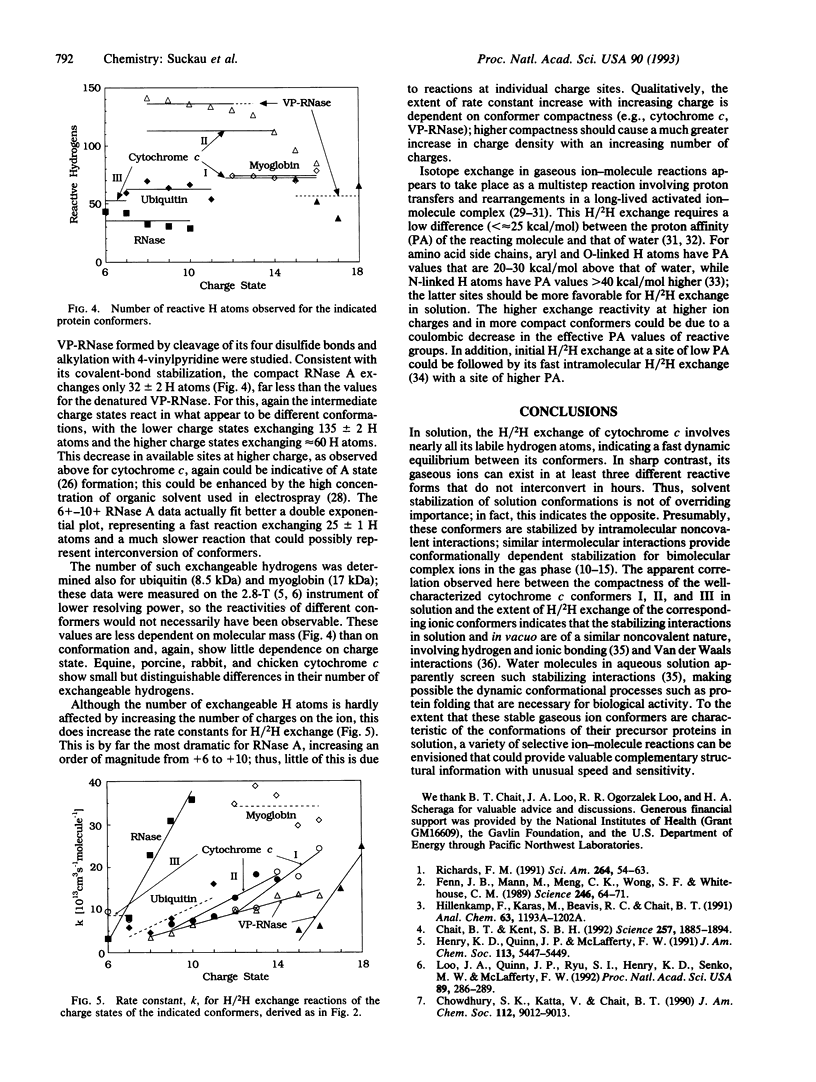
For further insight into the role of solvent in protein conformer stabilization, the structural and dynamic properties of protein ions in vacuo have been probed by hydrogen-deuterium exchange in a Fourier-transform mass spectrometer. Multiply charged ions generated by electrospray ionization of five proteins show exchange reactions with 2H2O at 10(-7) torr (1 torr = 133.3 Pa) exhibiting pseudo-first-order kinetics. Gas-phase compactness of the S-S cross-linked RNase A relative to denatured S-derivatized RNase A is indicated by exchange of 35 and 135 hydrogen atoms, respectively. For pure cytochrome c ions, the existence of at least three distinct gaseous conformers is indicated by the substantially different values--52, 113, and 74--of reactive H atoms; the observation of these same values for ions of a number--2, 7, and 5, respectively--of different charge states indicates conformational insensitivity to coulombic forces. For each of these conformers, the compactness in vacuo indicated by these values corresponds directly to that of a known conformer structure in the solution from which the conformer ions are produced by electrospray. S-derivatized RNase A ions also exist as at least two gaseous conformers exchanging 50-140 H atoms. Gaseous conformer ions are isometrically stable for hours; removal of solvent greatly increases conformational rigidity. More specific ion-molecule reactions could provide further details of conformer structures.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Affleck R., Xu Z. F., Suzawa V., Focht K., Clark D. S., Dordick J. S. Enzymatic catalysis and dynamics in low-water environments. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):1100–1104. doi: 10.1073/pnas.89.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chait B. T., Kent S. B. Weighing naked proteins: practical, high-accuracy mass measurement of peptides and proteins. Science. 1992 Sep 25;257(5078):1885–1894. doi: 10.1126/science.1411504. [DOI] [PubMed] [Google Scholar]
- Conio G., Patrone E., Brighetti S. The effect of aliphatic alcohols on the helix-coil transition of poly-L-ornithine and poly-L-glutamic acid. J Biol Chem. 1970 Jul 10;245(13):3335–3340. [PubMed] [Google Scholar]
- Delepierre M., Dobson C. M., Karplus M., Poulsen F. M., States D. J., Wedin R. E. Electrostatic effects and hydrogen exchange behaviour in proteins. The pH dependence of exchange rates in lysozyme. J Mol Biol. 1987 Sep 5;197(1):111–130. doi: 10.1016/0022-2836(87)90613-9. [DOI] [PubMed] [Google Scholar]
- Dill K. A., Shortle D. Denatured states of proteins. Annu Rev Biochem. 1991;60:795–825. doi: 10.1146/annurev.bi.60.070191.004051. [DOI] [PubMed] [Google Scholar]
- Englander S. W., Englander J. J., McKinnie R. E., Ackers G. K., Turner G. J., Westrick J. A., Gill S. J. Hydrogen exchange measurement of the free energy of structural and allosteric change in hemoglobin. Science. 1992 Jun 19;256(5064):1684–1687. doi: 10.1126/science.256.5064.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson A. E., Baase W. A., Zhang X. J., Heinz D. W., Blaber M., Baldwin E. P., Matthews B. W. Response of a protein structure to cavity-creating mutations and its relation to the hydrophobic effect. Science. 1992 Jan 10;255(5041):178–183. doi: 10.1126/science.1553543. [DOI] [PubMed] [Google Scholar]
- Fenn J. B., Mann M., Meng C. K., Wong S. F., Whitehouse C. M. Electrospray ionization for mass spectrometry of large biomolecules. Science. 1989 Oct 6;246(4926):64–71. doi: 10.1126/science.2675315. [DOI] [PubMed] [Google Scholar]
- Hillenkamp F., Karas M., Beavis R. C., Chait B. T. Matrix-assisted laser desorption/ionization mass spectrometry of biopolymers. Anal Chem. 1991 Dec 15;63(24):1193A–1203A. doi: 10.1021/ac00024a002. [DOI] [PubMed] [Google Scholar]
- Katta V., Chait B. T. Conformational changes in proteins probed by hydrogen-exchange electrospray-ionization mass spectrometry. Rapid Commun Mass Spectrom. 1991 Apr;5(4):214–217. doi: 10.1002/rcm.1290050415. [DOI] [PubMed] [Google Scholar]
- Loo J. A., Loo R. R., Udseth H. R., Edmonds C. G., Smith R. D. Solvent-induced conformational changes of polypeptides probed by electrospray-ionization mass spectrometry. Rapid Commun Mass Spectrom. 1991 Mar;5(3):101–105. doi: 10.1002/rcm.1290050303. [DOI] [PubMed] [Google Scholar]
- Loo J. A., Quinn J. P., Ryu S. I., Henry K. D., Senko M. W., McLafferty F. W. High-resolution tandem mass spectrometry of large biomolecules. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):286–289. doi: 10.1073/pnas.89.1.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer Y. P., Saturno A. F. Horse heart ferricytochrome c: conformation and heme configuration of low ionic strength acidic forms. J Protein Chem. 1990 Aug;9(4):379–387. doi: 10.1007/BF01024613. [DOI] [PubMed] [Google Scholar]
- Otting G., Liepinsh E., Wüthrich K. Protein hydration in aqueous solution. Science. 1991 Nov 15;254(5034):974–980. doi: 10.1126/science.1948083. [DOI] [PubMed] [Google Scholar]
- Richards F. M. The protein folding problem. Sci Am. 1991 Jan;264(1):54-7, 60-3. doi: 10.1038/scientificamerican0191-54. [DOI] [PubMed] [Google Scholar]
- Wagner G., Wüthrich K. Amide protein exchange and surface conformation of the basic pancreatic trypsin inhibitor in solution. Studies with two-dimensional nuclear magnetic resonance. J Mol Biol. 1982 Sep 15;160(2):343–361. doi: 10.1016/0022-2836(82)90180-2. [DOI] [PubMed] [Google Scholar]
- Wendoloski J. J., Matthew J. B. Molecular dynamics effects on protein electrostatics. Proteins. 1989;5(4):313–321. doi: 10.1002/prot.340050407. [DOI] [PubMed] [Google Scholar]