Abstract
Repetitive transcranial magnetic stimulation (rTMS) is a noninvasive neuromodulation technique that has been used to treat neurological and psychiatric conditions. Although results of rTMS intervention are promising, so far, little is known about the rTMS effect on brain functional networks in clinical populations. In this study, we used a whole-brain connectivity analysis of resting-state functional magnetic resonance imaging data to uncover changes in functional connectivity following rTMS intervention and their association with motor symptoms in patients with multiple system atrophy (MSA). Patients were randomized to active rTMS or sham rTMS groups and completed a 10-session 5-Hz rTMS treatment over the left primary motor area. The results showed significant rTMS-related changes in motor symptoms and functional connectivity. Specifically, (1) significant improvement of motor symptoms was observed in the active rTMS group, but not in the sham rTMS group; and (2) several functional links involving the default mode, cerebellar, and limbic networks exhibited positive changes in functional connectivity in the active rTMS group. Moreover, the positive changes in functional connectivity were associated with improvement in motor symptoms for the active rTMS group. The present findings suggest that rTMS may improve motor symptoms by modulating functional links connecting to the default mode, cerebellar, and limbic networks, inferring a future therapeutic candidate for patients with MSA.
Key words: : cerebellar network, default mode network, limbic network, Monte-Carlo simulation, multiple system atrophy, repetitive transcranial magnetic stimulation, resting-state functional connectivity, Unified Multiple System Atrophy Rating Scale
Introduction
Repetitive transcranial magnetic stimulation (rTMS) is a noninvasive neuromodulation technique that has been closely examined as a possible treatment for neurodegenerative diseases (Chou et al., 2015; Nardone et al., 2012). It delivers repeated magnetic pulses through a stimulation coil placed over the scalp to generate a relatively focal electromagnetic field capable of triggering action potentials in neurons (Barker et al., 1985; Rothwell, 1991). Although accumulating evidence suggests that rTMS can be utilized to enhance motor or cognitive function in patients with neurodegenerative diseases (Chou et al., 2015; Nardone et al., 2012), little is known about how the rTMS modulates deeper brain regions that are functionally connected to the stimulation site and how these changes correlate with improvement of symptoms.
Resting-state functional connectivity measured by functional magnetic resonance imaging (fMRI) has played an essential role in understanding brain functional networks and diseases (Fox and Greicius, 2010). Measures of resting-state functional connectivity refer to temporal correlations of fMRI signals between spatially distinct brain regions when participants are not performing a perceptual or behavioral task (Biswal et al., 1995). Neuroimaging studies have identified functional networks, such as the default mode, cerebellar, limbic, visual, attention, and executive control networks, among others (Biswal et al., 1995, 2010; Buckner et al., 2008; Greicius et al., 2003; Raichle et al., 2001).
In this study, we investigated the relationship between rTMS intervention, resting-state functional connectivity, and motor symptoms in patients with multiple system atrophy (MSA). MSA is an adult-onset, sporadic, progressive neurodegenerative disease characterized by a combination of symptoms that affect both the autonomic nervous system and movement (Gilman et al., 2008; Wenning et al., 2013). The defining neuropathology of MSA consists of degenerative lesions of the central autonomic, striatonigral, and olivopontocerebellar structures with glial cytoplasmic inclusions comprising filamentous α-synuclein proteins (Ozawa et al., 2006; Papp et al., 1989; Spillantini et al., 1998). Some of the motor symptoms, such as bradykinesia, rigidity, gait instability, and tremor, are similar to those of Parkinson's disease (PD) (Wenning et al., 1997). However, patients with MSA usually respond poorly to dopamine replacement therapy (Gilman et al., 2008). Given the limited efficacy of pharmacological treatment in improving motor symptoms of MSA (Gilman et al., 2008), there is a clinical need to determine whether and how rTMS could benefit this population.
The first aim of the study was to examine the effect of high-frequency rTMS over the primary motor cortex (M1) on motor symptoms in MSA patients, inspired by previous findings that the high-frequency rTMS to the M1 of PD patients could alleviate their motor symptoms (see Chou et al., 2015, for a review). The second aim was to use a whole-brain functional connectivity analysis to (1) identify neuronal networks that were modulated by the rTMS intervention and (2) assess whether the rTMS-induced functional connectivity modulation was associated with changes in motor symptoms.
Materials and Methods
Patients
Twenty-one right-handed MSA patients with predominantly parkinsonian features (i.e., bradykinesia, rigidity, and gait instability) were prospectively enrolled in this study. All patients fulfilled the diagnosis of probable MSA with predominant parkinsonism according to the established consensus criteria (Gilman et al., 2008). All of our patients did not respond well to the levodopa treatment. Exclusion criteria included significant medical or psychiatric illness, history of epilepsy or seizure, pregnancy, and mental diseases. Two patients were excluded due to uncertainty of the MSA diagnosis during follow-up. Characteristics of the study population are summarized in Table 1.
Table 1.
Baseline Characteristics of the Patients with MSA
| All | Active rTMS (n=9) | Sham rTMS (n=10) | p value | |
|---|---|---|---|---|
| Age (years) | 55±5 | 54±7 | 55±2 | 0.91 |
| Gender (M/F) | 10/9 | 6/3 | 4/6 | 0.24 |
| Disease duration (months) | 27±16 | 30±19 | 24±12 | 0.44 |
| UMSARS-II | 20±7 | 19±7 | 22±8 | 0.37 |
| Levodopa dosage (mg) | 195±186 | 278±221 | 120±111 | 0.06 |
| Hoehn–Yahr stage | 3.2±0.8 | 3.2±0.9 | 3.2±0.7 | 0.93 |
p-Value=significance level of difference between active rTMS and sham rTMS groups.
Research design
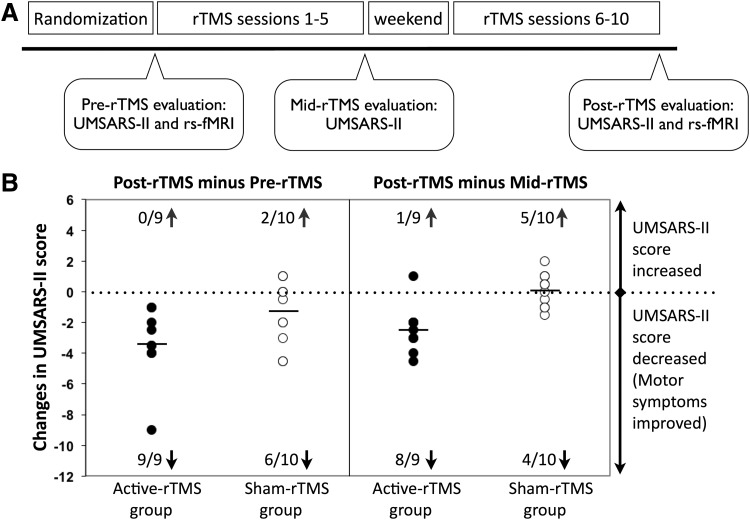
This was a randomized, double-blind sham rTMS-controlled study. All patients were randomly assigned into either an active rTMS group or a sham rTMS group according to a computer-generated randomization list. The rTMS procedure was performed by an experienced technician who did not participate in the evaluations. Both patients and clinical investigators were blind to the rTMS group assignment. Each patient completed a protocol comprising pre-rTMS evaluation (including motor examination of the Unified Multiple System Atrophy Rating Scale [UMSARS-II] and resting-state fMRI), rTMS intervention (sessions 1–5, one session per day), mid-rTMS evaluation (UMSARS-II only), rTMS intervention (sessions 6–10, one session per day), and post-rTMS evaluation (both UMSARS-II and resting-state fMRI), as illustrated in Figure 1A. The study protocol was approved by the Institutional Review Board of Peking Union Medical College Hospital, and all patients gave written informed consent before participation.
FIG. 1.
(A) A randomized, double-blind sham rTMS-controlled research design. All patients were randomly assigned into either an active rTMS or a sham rTMS group. Each patient completed a 10-session rTMS treatment consisting of pre-rTMS evaluation, rTMS intervention (sessions 1–5), mid-rTMS evaluation, rTMS intervention (sessions 6–10), and post-rTMS evaluation. (B) Changes in the UMSARS-II score between different time points for the active rTMS and sham rTMS groups. The active rTMS group (filled circles) exhibited a significant improvement in motor symptoms (i.e., decreased UMSARS-II score or negative values) between the post-rTMS and pre-rTMS and between the post-rTMS and mid-rTMS evaluations. The time effects were not significant for the sham rTMS group (open circles). n/Ngroup↑and n/Ngroup↓denotes the number of participants (out of the total number of participants within a group) that exhibited increased and decreased UMSARS-II scores, respectively, between different time points.
Repetitive transcranial magnetic stimulation
A 65-mm figure eight-shaped coil (MCF-B65) and a MagPro Compact stimulator (Dantec Company, Copenhagen, Denmark) were used in the rTMS sessions. We first determined the optimal scalp location of the left M1 for rTMS. The resting motor threshold (RMT) of the right abductor digiti minimi (ADM) muscle was measured for each patient. RMT was defined as the lowest intensity required to elicit at least five motor-evoked potentials of 50 μV peak-to-peak amplitude in 10 consecutive stimulations when single-pulse TMS was delivered to the left M1. Electromyographic recordings from the right ADM were acquired with surface electrodes using a Viking IV electromyography machine (Nicolet Biomedical, Madison, WI). Bandpass filters were set at 20–2000 Hz.
The rTMS protocol was based on published studies, which demonstrated that 5-Hz rTMS therapies improved motor symptoms in patients with PD (Khedr et al., 2003; Pascual-Leone et al., 1994; Siebner et al., 1999, 2000). We performed rTMS in 10 sessions over 2 weeks, one session per day for 5 consecutive weekdays in each week. Each session consisted of 10 trains of 100 pulses at 5 Hz with an intertrain interval of 40 sec. The intensity was set to 110% of the RMT. The coil was positioned over the left M1 corresponding to the hot spot of right ADM and fixed to a coil holder.
Patients in the sham rTMS group received the same rTMS procedure targeting the left M1, except that the coil was positioned with its back (inactive) surface touching the scalp (Lomarev et al., 2006). In our pilot study, we measured electric field power induced by the figure eight-shaped coil with the front and the back surfaces using a testing coil (MagProbe) and the electromyography machine. The data showed a reduced power of electric field by 92% when the coil was placed with its back surface touching the scalp (1.0 mV) relative to the active stimulation (11.9 mV). A certified neurologist observed the procedure to ensure an optimal conduction for each patient and for safety monitoring. Each patient received the rTMS intervention at the same time every morning (11:00 AM). Patients could take their usual antiparkinsonian medications, if any, after the rTMS stimulation or evaluations.
Clinical rating scale
The UMSARS-II (i.e., total motor score) was used to assess the severity of signs and symptoms of MSA (Wenning et al., 2004). The UMSARS has been validated to assess rates of progression and is sensitive to change over time (Geser et al., 2006). The UMSARS-II contains 14 questions with higher scores representing more severe signs or symptoms. The UMSARS-II measures were obtained from patients at baseline and within 1 h following the 5th and the 10th sessions of rTMS intervention. The overall score of each patient was log transformed to improve the normality for statistical analyses.
Imaging protocol
Each patient was scanned on a 3T Signa Excite II VHi MR scanner (GE Healthcare, Milwaukee, Wisconsin). Foam pads and ear plugs were used to reduce head motion and scan noise. The imaging protocol consisted of the axial plane gradient-echo echo-planar imaging sequence sensitive to the blood oxygen level-dependent (BOLD) contrast (repetition time=2000 msec, echo time=30 msec, flip angle=90°, field of view=24×24 cm, in-plane matrix size=64×64, slice thickness=5 mm with 1-mm gap, 20 slices with whole-brain coverage, scan time=8 min, 240 volumes), whole-brain three-dimensional T1-weighted spoiled gradient-recalled sequence, and other routine structural MRI sequences. The patients were instructed to remain still, keep their eyes closed, and not to think of anything particular during fMRI data acquisition.
fMRI data analysis
The preprocessing of fMRI data was conducted through the Duke Brain Imaging and Analysis Center preprocessing pipelines based on the tools from the Oxford Centre for Functional MRI of the Brain Software Library (FSL version 5.0.1, www.fmrib.ox.ac.uk/fsl) and locally developed Matlab codes (MathWorks, Natick, MA). The first four volumes were discarded to reach the T1 steady state. The data were corrected for slice-timing differences and motion (six parameters: three translations and three rotations). The data were then registered to the Montreal Neurological Institute (MNI) 152 template using a 12 degrees of freedom affine transformation implemented in the FSL Linear Image Registration Tool. All subsequent analyses were conducted in the MNI standard space. We regressed out the six-parameter rigid body head motion (obtained from motion correction), the averaged time course profiles in the white matter, and the averaged time course profiles in the cerebrospinal fluid regions to reduce non-neuronal contributions to BOLD correlations (Van Dijk et al., 2010). We also removed constant offsets and linear drift. Time domain signals with their frequencies less than 0.08 Hz were retained.
For each participant, the preprocessed low-frequency fMRI data were parceled into a set of 116 brain regions (90 within the cerebral cortex and 26 within the cerebellum) using an Automated Anatomical Labeling template (AAL, Tzourio-Mazoyer et al., 2002). Each participant's BOLD time series was averaged within each brain region. We used Pearson correlation as the metric of association between the time series for each pair of the 116 brain regions. This resulted in a 116×116 correlation matrix with 6670 ([116×115]/2) unique inter-regional correlation coefficients (r). These inter-regional r values were transformed to Zr values with Fisher's r-to-z transformation (Fisher, 1921).
Statistical analysis
First, to examine whether active rTMS was more effective relative to the sham rTMS on changes in the UMSARS-II score, we performed a two-way analysis of variance (ANOVA) with rTMS groups (active rTMS vs. sham rTMS) as an independent factor and time (pre-rTMS vs. mid-rTMS vs. post-rTMS) as a repeated factor. Second, to identify the functional links that were significantly influenced by rTMS, we performed a two-way ANOVA on each functional connection of the whole brain with rTMS groups (active rTMS vs. sham rTMS) as an independent factor and time (pre-rTMS vs. post-rTMS) as a repeated factor. Third, we examined whether changes in functional connectivity of links identified by the whole-brain two-way ANOVA were significantly associated with changes in motor symptoms in the active rTMS group.
For the whole-brain two-way ANOVA, we performed 6670 separate statistical tests. Multiple comparisons were corrected using a degree-based correction (Supplementary Data; Supplementary Data are available online at www.liebertpub.com/brain). Briefly, methodologically similar to the network-based statistics (Zalesky et al., 2010) and cluster correction in voxel-based task fMRI analysis (Forman et al., 1995), our multiple comparisons were corrected based on nonrandom data distribution patterns. Based on our 10,000 Monte Carlo simulations, the number of functional links connected to a single region (i.e., the degree) should be at least 15 for matrix-based fMRI analysis of a 116×116 matrix size (i.e., with 6670 separate statistical tests) to correct for multiple comparisons at p<0.05.
Results
rTMS effect on UMSARS-II score
All patients tolerated rTMS well without adverse effects. Before participation, all patients were informed orally and in a written form that they would be randomly assigned into either the active or sham rTMS group. At the end of the study, participants were interviewed regarding their expectation of benefits and group assignment. All of the patients thought they had received the active rTMS treatment. The UMSARS-II score at baseline, age, gender, disease duration, estimated levodopa dosage, and Hoehn–Yahr stage was not significantly different between the active rTMS and the sham rTMS groups (Table 1).
Figure 1B illustrates score changes in the UMSARS-II between different time points for each patient. All patients in the active rTMS group exhibited a decreased UMSARS-II score after the 10-session rTMS intervention (mean change=−3.39±2.41, mean percentage change=21%±15%), while only 60% of patients in the sham rTMS group showed a decreased UMSARS-II score (mean change=−1.45±2.07, range=7%±12%). An ANOVA of the UMSARS-II score yielded a significant main effect of time, F(2,34)=11.41, p=0.0002, and a significant rTMS group×time interaction, F(2,34)=4.04, p=0.03. The rTMS group×time interaction occurred because the time effect was significant for the active rTMS group, F(2,16)=8.84, p=0.003, but not for the sham rTMS group, F(2,18)=3.03, p=0.07.
The time effect observed in the active rTMS group represented a significant improvement in the motor symptom score in the post-rTMS condition (i.e., after the 10th session) relative to the pre-rTMS condition (i.e., the baseline), F(1,8)=11.84, p=0.009, and the mid-rTMS condition (i.e., after the 5th session), F(1,8)=16.12, p=0.004. The effect size (Cohen's d) for difference in score change between groups was 0.92, indicating a large effect favoring active rTMS over sham rTMS after the 10-session rTMS intervention. Given the effect size estimated and a type I error of 5%, our sample size provided enough power (97%, type II error=3%) to detect the rTMS effect.
The active rTMS group had a relatively higher dose of dopaminergic medication at baseline than the sham rTMS group (p=0.06). To examine whether the dose of dopaminergic medication at baseline could be a confounding factor for the observed effects, we conducted two additional analyses. First, we included the estimated levodopa dosage as a covariate to the ANOVA. The results of the ANOVA showed that inclusion of the estimated levodopa dosage as a covariate did not change the findings; in other words, the effects of time, F(2,32)=6.49, p=0.004, and rTMS group×time interaction, F(2,32)=3.66, p=0.04, remained significant.
Second, we split the patients into two groups based on the levodopa dosage (high L-dopa vs. low L-dopa group). An ANOVA of the UMSARS-II score with dosage (high L-dopa group vs. low L-dopa group) as an independent factor and time (pre-rTMS vs. mid-rTMS vs. post-rTMS) as a repeated factor yielded a significant main effect of time, F(2,34)=9.53, p=0.0005. The main effect of dosage, F(2,34)=0.36, p=0.5544, and the interaction effect of dosage×time, F(2,34)=2.19, p=0.1271, were not significant. The nonsignificant main effect of dosage and nonsignificant dosage×time interaction effect indicated that (1) motor symptoms were not significantly different between the high L-dopa and low L-dopa groups across time points and (2) the time effect did not significantly differ between the high L-dopa and low L-dopa groups. The additional analyses excluded the potential confounding effect of L-dopa dosage and provide further support of the rTMS intervention effect.
rTMS-induced changes in functional connectivity
For the resting-state fMRI data, no excessive head motion during fMRI scans was found for any patient (rotation <1°; translation <1.5 mm), and the head motion did not significantly differ between the two rTMS groups or between pre-rTMS and post-rTMS conditions. Although there were no significant differences in head motion and dopaminergic medication between the active and sham rTMS groups, we controlled for these two factors while performing the ANOVA of functional connectivity.
The two-way analysis of covariance (ANCOVA) with rTMS (active rTMS vs. sham rTMS) as an independent factor and time (pre-rTMS vs. post-rTMS) as a repeated factor on each functional link of the whole brain produced a significant interaction effect between rTMS group and time for 47 functional links (Fig. 2). The 47 functional links were identified because their change in functional connectivity (post-rTMS minus pre-rTMS) was significantly different between the active rTMS and the sham rTMS groups at p<0.05, and the number of significant links connected to a single region was equal to or greater than 15, corrected for multiple comparisons at p<0.05 based on 10,000 Monte Carlo simulations of a degree-based correction (Supplementary Data).
FIG. 2.
Functional links that exhibited a significant rTMS group×time interaction effect. The majority of the links were connected between regions within the default mode (red color) and the cerebellar (yellow color) networks and between regions within the default mode (red color) and the limbic (blue color) networks.
Among the 47 functional links, 15 links were connected to the left parahippocampal gyrus (PHG cluster), 16 (including one link connected to the L-PHG) to the right medial prefrontal cortex (MPFC cluster), and 18 (including one link connected to the L-PHG) to the left angular gyrus (ANG cluster). Figure 2 illustrates the set of 47 functional connections that exhibited a significant interaction effect between rTMS group and time. The three regions (L-PHG, R-MPFC, and L-ANG) are typically categorized as parts of the default mode network (DMN, red color).
Among these identified links, first, we found a link connecting between the L-M1 (rTMS site, green color) and the L-ANG. The L-ANG might be an important relay center that transmitted the influence of rTMS from the rTMS site to other brain regions. Second, the majority of the links were connected between the DMN (red color) and the cerebellar regions (yellow color) and between the DMN (red color) and the limbic areas (blue color). Third, the links connecting the DMN and few other brain regions (e.g., dorsolateral prefrontal cortex or visual areas, gray color) were also identified.
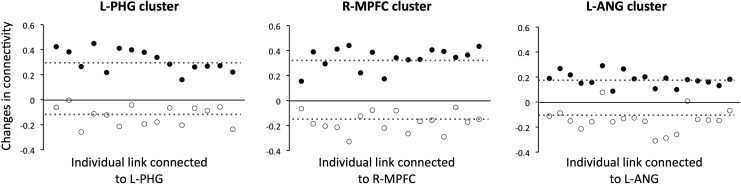
Changes in functional connectivity of each individual link following rTMS intervention for each rTMS group and each cluster are illustrated in Figure 3. For the active rTMS group, the changes in functional connectivity (i.e., post-rTMS minus pre-rTMS) were positive for all links, indicating that (1) positive connections became even more positive, (2) negative connections became positive, or (3) negative connections became less negative. For the sham rTMS group, the changes in functional connectivity were negative for 96% of the links, indicating that (1) negative connections became even more negative, (2) positive connections became negative, or (3) positive connections became less positive.
FIG. 3.
Changes in functional connectivity of each individual link between post-rTMS and pre-rTMS evaluations for the active rTMS (filled circles) and sham rTMS groups (open circles).
Correlation between changes in connectivity and improvement of motor symptoms
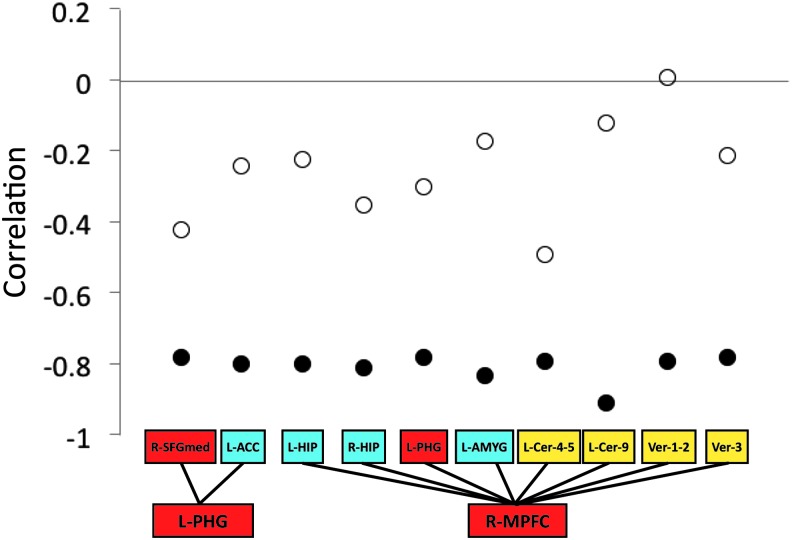
We next examined the relationship between functional connectivity changes of each link and improvement of motor symptoms, with the effects of head motion and dopaminergic medication removed, for each rTMS group. For the active rTMS group, the correlation was significant for 10 functional links (Fig. 4). None of the correlations were significant for the sham rTMS group. The 10 links represent functional connections between regions in the DMN and those in the cerebellar and limbic networks. For the active rTMS group, these correlations were negative (r ≤ −0.78, p<0.05, uncorrected), representing positive changes in functional connectivity associated with greater improvement of motor symptoms following rTMS intervention.
FIG. 4.
Correlations between changes in UMSARS-II score and changes in functional connectivity. For the active rTMS group (filled circles), the correlation was significant for the 10 functional links (red color: default mode regions; blue color: limbic regions; yellow color: cerebellar regions). None of the correlations were significant for the sham rTMS group (open circles).
Discussion
The present study investigated whether rTMS could modulate brain functional networks in patients with MSA, and whether the modulated functional connectivity was associated with changes in motor symptoms. First, the rTMS intervention improved motor function for the active rTMS group, but not for the sham rTMS group, in patients with MSA. Second, three clusters of functional links (mainly connecting the DMN, the cerebellar network, and the limbic network) exhibited rTMS-related effects (Fig. 2). A functional link connecting the rTMS site (i.e., left M1) and a region within the DMN (i.e., left ANG) was also identified in our analyses. Third, the improvement of motor symptoms in the active rTMS group was associated with positive changes in functional connectivity following the rTMS intervention.
Our whole-brain ANOVA of functional connectivity based on the rTMS (active rTMS vs. sham rTMS)×time (pre-rTMS vs. post-rTMS) interaction is a less biased approach relative to seed-based connectivity analysis or other statistical analyses (e.g., paired sample t-test) constrained by nonexperimental designs. This approach enables identification of links that exhibited a significant difference in connectivity change between the active rTMS and the sham rTMS groups without a priori knowledge. All the ANOVA-identified functional links, corrected for multiple comparisons, were connected to brain regions that are typically categorized within the DMN. This result is consistent with a number of recent findings applying noninvasive brain stimulations (e.g., rTMS, transcranial direct current stimulation, or theta burst stimulation) to healthy adults (Amadi et al., 2013; Chen et al., 2013; Gratton et al., 2013; Keeser et al., 2011, Polania et al., 2011, 2012; van der Werf et al., 2010) and patients with depression (Liston et al., 2014). Despite variability in individual protocols (e.g., stimulation site, excitatory or inhibitory stimulation) and connectivity analysis methods (e.g., independent component analysis or seed-based analysis), all these studies have identified regions associated with the DMN, among others, in response to noninvasive brain stimulations.
Brain regions that are typically categorized within the DMN include the medial temporal lobe/PHG, MPFC, lateral parietal cortex/angular gyrus, and posterior cingulate cortex/precuneus (Greicius et al., 2003; Raichle et al., 2001). These brain regions show the highest activation during the resting state and deactivate during the performance of externally oriented tasks (Shulman et al., 1997). Relative to other brain regions, the DMN shows disproportionately high glucose metabolism (Minoshima et al., 1997) and regional blood flow during rest (Raichle et al., 2001) and is considered to be involved in a high degree of neuroplasticity (Fjell et al., 2014). Although the mechanisms underlying modulations of the DMN are not yet clear, it seems plausible that the DMN plasticity might be sensitive to the rTMS treatment effects, and the consolidation and maintenance of brain function might be facilitated through the DMN plasticity.
The rTMS-related functional links identified in our study were mainly connected to the cerebellar and limbic networks from the DMN. Recent studies have found close relationships between these two networks and the DMN (Catani et al., 2013, Halko et al., 2014). For example, a few studies have defined the cerebellar DMN within the Crus I and Crus II of the cerebellar hemispheres (Buckner et al., 2011, Halko et al., 2014). Likewise, many regions within the limbic system (e.g., medial temporal regions and cingulate gyrus) exhibit similar function and structurally overlap with the DMN (Catani et al., 2013).
In addition to the close relationships with the DMN, these two networks are highly relevant to the symptoms observed in MSA. First, it is well known that a major function of the cerebellum is related to motor coordination, and our analysis showed that motor symptom improvement in the active rTMS group was associated with positive changes in cerebellar connectivity following the rTMS intervention. Second, as has been reported in the literature (Christopher et al., 2014; Herman et al., 2005), the limbic network, including hippocampus, amygdala, olfactory bulbs, cingulate gyrus, and other nearby regions, regulates autonomic function (e.g., basic metabolism, respiration, and circulation) by sending direct or indirect projections to the hypothalamic paraventricular nucleus within the autonomic system. It is likely that the positive changes in limbic network functional connectivity observed in our study might contribute to improvement in some nonmotor symptoms (e.g., orthostatic hypotension or urinary and bowel dysfunction), although this needs to be verified by future studies that measure changes in both motor and nonmotor symptoms in response to rTMS intervention.
Several issues should be acknowledged while interpreting our results. First, it is important to note that the mean score change in the UMSARS-II following rTMS intervention was 3.39±2.41 for the active rTMS group. Although the minimal clinically important difference (CID) for the UMSARS-II score has not been documented, the rTMS-induced change in the UMSARS-II observed in our study was greater than the minimal CID (2.3–2.7 points) for the Unified Parkinson's Disease Rating Scale motor score (UPDRS-III, which has identical number of test items and the same range of scores relative to the UMSARS-II) (Shulman et al., 2010). We believe that a change of the UMSARS-II score by 3.39±2.41 is meaningful for patients with MSA and is clinically significant based on the minimal CID for the UPDRS-III (Shulman et al., 2010).
Second, although the rTMS intervention modulated functional connectivity between the rTMS site (i.e., left M1) and a region within the DMN (i.e., left ANG), most of the brain regions within the cortical motor network were not significantly influenced by the rTMS intervention. One possible reason was that we used the AAL template to parcel the brain and some of the AAL regions within the cortical motor network were relatively large. Different brain parcellation techniques and analytic methods (e.g., finer scale seed ROI-based connectivity analysis, amplitude of low-frequency fluctuation, regional homogeneity, or independent component analysis) should be applied for future studies. Third, our study is the first rTMS intervention study using both behavioral data and functional connectivity as outcome measures for MSA. Although we developed a new technique (i.e., degree-based correction) to correct for multiple comparisons, and our findings suggested that changes in UMSARS-II score were strongly associated with changes in functional connectivity (r ≤ −0.78), future studies with a larger sample size will be required to validate our findings.
Fourth, patients with MSA in our study did not respond well to the L-dopa, but did respond to the rTMS intervention. It is possible that the mechanism of rTMS was different from that of L-dopa. Alternatively, the rTMS could prime the brain to bolster the effectiveness of other treatments (e.g., L-dopa or physical therapy) (Avenanti et al., 2012, Cassidy et al., 2014). This issue warrants further investigation to elucidate the underlying mechanisms of rTMS effects and the interaction between rTMS and other treatment effects. Finally, MSA is relentlessly progressive and therefore a short-term rTMS intervention may only improve the symptoms transiently. Future research is needed to better define the duration of the rTMS effects in MSA patients and further optimize the rTMS treatment for long-term benefit.
Conclusion
In this randomized, double-blind sham rTMS-controlled study, we used a whole-brain connectivity analysis to uncover associations between rTMS treatment, brain functional connectivity, and changes of motor symptoms in patients with MSA. The present results showed a significant improvement of motor symptoms and positive changes in connectivity of functional links connecting to the DMN, cerebellar network, and limbic network in patients with MSA. Furthermore, the positive changes in functional connectivity in response to the rTMS treatment were significantly associated with improvement in motor symptoms. Our findings suggest that a 10-session 5-Hz rTMS targeting the left M1 may improve motor symptoms by modulating functional links connecting to the DMN, cerebellar network, and limbic network for patients with MSA.
Supplementary Material
Acknowledgments
This research was supported, in part, by the Natural Science Foundation of China, Grant No. 30670608 (H.Y. and F.F.); the Natural Science Foundation of China, Grant No. 30800352 (H.W.); and the National Institutes of Health research grant R01-NS074045 (N.-K.C.).
Author Disclosure Statement
No competing financial interests exist.
References
- Amadi U, Ilie A, Johansen-Berg H, Stagg CJ. 2013. Polarity-specific effects of motor transcranial direct current stimulation on fMRI resting state networks. Neuroimage 88C:155–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avenanti A, Coccia M, Ladavas E, Provinciali L, Ceravolo MG. 2012. Low-frequency rTMS promotes use-dependent motor plasticity in chronic stroke: a randomized trial. Neurology 78:256–264 [DOI] [PubMed] [Google Scholar]
- Barker AT, Jalinous RI, Freeston L. 1985. Non-invasive magnetic stimulation of human motor cortex. Lancet 1:1106–1107 [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. 1995. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34:537–541 [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith S. M, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski AM, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kötter R, Li SJ, Lin CP, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts SA, Rypma B, Schlaggar BL, Schmidt S, Seidler RD, Siegle GJ, Sorg C, Teng GJ, Veijola J, Villringer A, Walter M, Wang L, Weng XC, Whitfield-Gabrieli S, Williamson P, Windischberger C, Zang YF, Zhang H. Y, Castellanos FX, Milham MP. 2010. Toward discovery science of human brain function. Proc Natl Acad Sci U S A 107:4734–4739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. 2008. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124:1–38 [DOI] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT. 2011. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol 106:2322–2345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy JM, Gillick BT, Carey JR. 2014. Priming the brain to capitalize on metaplasticity in stroke rehabilitation. Phys Ther 94:139–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Dell'acqua F, Thiebaut de Schotten M. 2013. A revised limbic system model for memory, emotion and behaviour. Neurosci Biobehav Rev 37:1724–1737 [DOI] [PubMed] [Google Scholar]
- Chen AC, Oathes DJ, Chang C, Bradley T, Zhou ZW, Williams LM, Glover GH, Deisseroth K, Etkin A. 2013. Causal interactions between fronto-parietal central executive and default-mode networks in humans. Proc Natl Acad Sci U S A 110:19944–19949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou YH, Hickey PT, Sundman M, Song AW, Chen NK. 2015. Effects of repetitive transcranial magnetic stimulation on motor symptoms in Parkinson disease: A systematic review and meta-analysis. JAMA Neurol [Epub ahead of print]; doi: 10.1001/jamaneurol.2014.4380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher L, Koshimori Y, Lang AE, Criaud M, Strafella AP. 2014. Uncovering the role of the insula in non-motor symptoms of Parkinson's disease. Brain 137(Pt 8):2143–2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RA. 1921. On the probable error of a coefficient of correlation deduced from a small sample. Metron 1:3–32 [Google Scholar]
- Fjell AM, McEvoy L, Holland D, Dale AM, Walhovd KB, Alzheimer's Disease Neuroimaging I. 2014. What is normal in normal aging? Effects of aging, amyloid and Alzheimer's disease on the cerebral cortex and the hippocampus. Prog Neurobiol 117:20–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. 1995. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med 33:636–647 [DOI] [PubMed] [Google Scholar]
- Fox MD, Greicius M. 2010. Clinical applications of resting state functional connectivity. Front Syst Neurosci 4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geser F, Wenning GK, Seppi K, Stampfer-Kountchev M, Scherfler C, Sawires M, Frick C, Ndayisaba JP, Ulmer H, Pellecchia MT, Barone P, Kim HT, Hooker J, Quinn NP, Cardozo A, Tolosa E, Abele M, Klockgether T, Ostergaard K, Dupont E, Schimke N, Eggert KM, Oertel W, Djaldetti R, Poewe W. 2006. Progression of multiple system atrophy (MSA): a prospective natural history study by the European MSA Study Group (EMSA SG). Mov Disord 21:179–186 [DOI] [PubMed] [Google Scholar]
- Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, Wood NW, Colosimo C, Durr A, Fowler CJ, Kaufmann H, Klockgether T, Lees A, Poewe W, Quinn N, Revesz T, Robertson D, Sandroni P, Seppi K, Vidailhet M. 2008. Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71:670–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton C, Lee TG, Nomura EM, D'Esposito M. 2013. The effect of theta-burst TMS on cognitive control networks measured with resting state fMRI. Front Syst Neurosci 7:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. 2003. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A 100:253–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halko MA, Farzan F, Eldaief MC, Schmahmann JD, Pascual-Leone A. 2014. Intermittent theta-burst stimulation of the lateral cerebellum increases functional connectivity of the default network. J Neurosci 34:12049–12056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. 2005. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry 29:1201–1213 [DOI] [PubMed] [Google Scholar]
- Keeser D, Meindl T, Bor J, Palm U, Pogarell O, Mulert C, Brunelin J, Moller HJ, Reiser M, Padberg F. 2011. Prefrontal transcranial direct current stimulation changes connectivity of resting-state networks during fMRI. J Neurosci 31:15284–15293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khedr EM, Farweez HM, Islam H. 2003. Therapeutic effect of repetitive transcranial magnetic stimulation on motor function in Parkinson's disease patients. Eur J Neurol 10:567–572 [DOI] [PubMed] [Google Scholar]
- Liston C, Chen AC, Zebley BD, Drysdale AT, Gordon R, Leuchter B, Voss HU, Casey BJ, Etkin A, Dubin MJ. 2014. Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol Psychiatry 76:517–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomarev MP, Kanchana S, Bara-Jimenez W, Iyer M, Wassermann EM, Hallett M. 2006. Placebo-controlled study of rTMS for the treatment of Parkinson's disease. Mov Disord 21:325–331 [DOI] [PubMed] [Google Scholar]
- Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. 1997. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer's disease. Ann Neurol 42:85–94 [DOI] [PubMed] [Google Scholar]
- Nardone R, Bergmann J, Christova M, Caleri F, Tezzon F, Ladurner G, Trinka E, Golaszewski S. 2012. Effect of transcranial brain stimulation for the treatment of Alzheimer disease: a review. Int J Alzheimers Dis 2012:687909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa T, Healy DG, Abou-Sleiman PM, Ahmadi KR, Quinn N, Lees AJ, Shaw K, Wullner U, Berciano J, Moller JC, Kamm C, Burk K, Josephs KA, Barone P, Tolosa E, Goldstein DB, Wenning G, Geser F, Holton JL, Gasser T, Revesz T, Wood NW. 2006. The alpha-synuclein gene in multiple system atrophy. J Neurol Neurosurg Psychiatry 77:464–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp MI, Kahn JE, Lantos PL. 1989. Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome). J Neurol Sci 94:79–100 [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Sole J, Brasil-Neto JP, Cammarota A, Grafman J, Hallett M. 1994. Akinesia in Parkinson's disease. II. Effects of subthreshold repetitive transcranial motor cortex stimulation. Neurology 44:892–898 [DOI] [PubMed] [Google Scholar]
- Polania R, Paulus W, Antal A, Nitsche MA. 2011. Introducing graph theory to track for neuroplastic alterations in the resting human brain: a transcranial direct current stimulation study. Neuroimage 54:2287–2296 [DOI] [PubMed] [Google Scholar]
- Polania R, Paulus W, Nitsche MA. 2012. Modulating cortico-striatal and thalamo-cortical functional connectivity with transcranial direct current stimulation. Hum Brain Mapp 33:2499–2508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. 2001. A default mode of brain function. Proc Natl Acad Sci U S A 98:676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell R. 1991. Physiological studies on electric and magnetic stimulation of the human brain. In Levy WJ, Cracco RQ, Barker AT. and Rothwell JC. Magnetic Motor Stimulation: Basic Principles and Clinical Experience. Amsterdam, Elsevier; pp. 29–35 [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE. 1997. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cogn Neurosci 9:648–663 [DOI] [PubMed] [Google Scholar]
- Shulman LM, Gruber-Baldini AL, Anderson KE, Fishman PS, Reich SG, Weiner WJ. 2010. The clinically important difference on the unified Parkinson's disease rating scale. Arch Neurol 67:64–70 [DOI] [PubMed] [Google Scholar]
- Siebner HR, Mentschel C, Auer C, Conrad B. 1999. Repetitive transcranial magnetic stimulation has a beneficial effect on bradykinesia in Parkinson's disease. Neuroreport 10:589–594 [DOI] [PubMed] [Google Scholar]
- Siebner HR, Rossmeier C, Mentschel C, Peinemann A, Conrad B. 2000. Short-term motor improvement after sub-threshold 5-Hz repetitive transcranial magnetic stimulation of the primary motor hand area in Parkinson's disease. J Neurol Sci 178:91–94 [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Crowther RA, Jakes R, Cairns NJ, Lantos PL, Goedert M. 1998. Filamentous alpha-synuclein inclusions link multiple system atrophy with Parkinson's disease and dementia with Lewy bodies. Neurosci Lett 251:205–208 [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. 2002. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15:273–289 [DOI] [PubMed] [Google Scholar]
- van der Werf YD, Sanz-Arigita EJ, Menning S, van den Heuvel OA. 2010. Modulating spontaneous brain activity using repetitive transcranial magnetic stimulation. BMC Neurosci 11:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol 103:297–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenning GK, Geser F, Krismer F, Seppi K, Duerr S, Boesch S, Kollensperger M, Goebel G, Pfeiffer KP, Barone P, Pellecchia MT, Quinn NP, Koukouni V, Fowler CJ, Schrag A, Mathias CJ, Giladi N, Gurevich T, Dupont E, Ostergaard K, Nilsson CF, Widner H, Oertel W, Eggert KM, Albanese A, del Sorbo F, Tolosa E, Cardozo A, Deuschl G, Hellriegel H, Klockgether T, Dodel R, Sampaio C, Coelho M, Djaldetti R, Melamed E, Gasser T, Kamm C, Meco G, Colosimo C, Rascol O, Meissner WG, Tison F, Poewe W. 2013. The natural history of multiple system atrophy: a prospective European cohort study. Lancet Neurol 12:264–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenning GK, Tison F, Ben Shlomo Y, Daniel SE, Quinn NP. 1997. Multiple system atrophy: a review of 203 pathologically proven cases. Mov Disord 12:133–147 [DOI] [PubMed] [Google Scholar]
- Wenning GK, Tison F, Seppi K, Sampaio C, Diem A, Yekhlef F, Ghorayeb I, Ory F, Galitzky M, Scaravilli T, Bozi M, Colosimo C, Gilman S, Shults CW, Quinn N. P, Rascol O, Poewe W. 2004. Development and validation of the Unified Multiple System Atrophy Rating Scale (UMSARS). Mov Disord 19:1391–1402 [DOI] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Bullmore ET. 2010. Network-based statistic: identifying differences in brain networks. Neuroimage 53:1197–1207 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.