Abstract
Heart failure induced by myocardial infarct (MI) attenuates the heart rate variability (HRV) and baroreflex sensitivity, which are important risk factors for life-threatening cardiovascular events. Therapies with mesenchymal stem cells (MSCs) have shown promising results after MI. However, the effects of MSCs on hemodynamic (heart rate and arterial pressure) variability and baroreflex sensitivity in chronic heart failure (CHF) following MI have not been evaluated thus far. Male Wistar rats received MSCs or saline solution intravenously 1 week after ligation of the left coronary artery. Control (noninfarcted) rats were also evaluated. MI size was assessed using single-photon emission computed tomography (SPECT). The left ventricular ejection fraction (LVEF) was evaluated using radionuclide ventriculography. Four weeks after MSC injection, the animals were anesthetized and instrumented for chronic ECG recording and catheters were implanted in the femoral artery to record arterial pressure. Arterial pressure and HRVs were determined in time and frequency domain (spectral analysis) while HRV was also examined using nonlinear methods: DFA (detrended fluctuation analysis) and sample entropy. The initial MI size was the same among all infarcted rats but was reduced by MSCs. CHF rats exhibited increased myocardial interstitial collagen and sample entropy combined with the attenuation of the following cardiocirculatory parameters: DFA indices, LVEF, baroreflex sensitivity, and HRV. Nevertheless, MSCs hampered all these alterations, except the LVEF reduction. Therefore, 4 weeks after MSC therapy was applied to CHF rats, MI size and myocardial interstitial fibrosis decreased, while baroreflex sensitivity and HRV improved.
Introduction
Chronic heart failure (CHF) is characterized by ventricular dysfunction with decreased myocardial contractility and cardiac output, increased end-diastolic volume of the ventricles and reduced functional cardiac reserve [1]. Thus, cardiocirculatory adjustments are required to maintain adequate tissue perfusion; one adjustment involves baroreflex function, which plays an important role in modulating heart rate (HR), cardiac output, myocardial contractility, and regional distribution of blood flow [2].
However, patients with CHF induced by myocardial infarction (MI) have impaired baroreflex function [3,4]. Moreover, one of the major changes that occurs beginning in the early stages of heart failure is an autonomic imbalance that is characterized by sympathetic hyperactivity and reduced vagal tone [5–7]. These outcomes emphasize the importance of the baroreflex control on cardiovascular autonomic modulation in CHF.
Adult mesenchymal stem cells (MSCs) are stem cells with high potential for therapeutic applications [8]. MSCs therapy is an ongoing approach to repair the heart in preclinical and clinical studies after cardiac events and has brought positive results [8–10]. Despite the evidences that MSCs reduce left ventricular dilatation [11], increase vascular density [12,13], decrease the extent of the infarcted area [9,11], reduce the apoptosis, and necrosis of cardiomyocytes [11,14], more consistent results from stem cell therapy in heart diseases may, indeed, be achieved through rigorous clinical trials, amount of cells providing maximal efficiency, optimal time frame for transplant, best procedures to manipulate the cells, and via of administration [8,9].
Pak et al. [15] demonstrated an increased magnitude of cardiac nerve sprouting in both atria and ventricles and an increased magnitude of atrial sympathetic hyperinnervation 2 months after the transplantation of MSCs into swine post-MI. Another study showed that human MSC transplantation increased cardiac sympathetic innervation after transplanted in the canine heart [16]. Despite the benefits of MSC transplantation after MI, the evidence of increased sympathetic innervation indicates the need for more accurate studies, particularly those related to the sympathovagal balance in the heart. Accordingly, Wang et al. [17] have demonstrated that MSC transplantation promotes cardiac nerve sprouting and modifies the autonomic neural balance in diabetic rats.
Studies of cardiac autonomic modulation involving MSC transplantation in models of CHF are beneficial because several clinical studies are currently in progress. Nevertheless, no study has evaluated the cardiac sympathovagal modulation, the heart rate variability (HRV), and baroreflex sensitivity in either patients or experimental models of CHF receiving MSCs.
Therefore, the aim of this study was to evaluate the effect of MSCs on hemodynamic (heart rate and arterial pressure) variability and baroreflex sensitivity in conscious CHF rats following MI.
Materials and Methods
Experiments were performed on male Wistar rats (240–320 g); the animals were housed individually with free access to food and water and maintained on a 12:12 h light-dark cycle. Young male rats (150–200 g) were used as MSCs donors. The experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals [Dept. of Health, Education and Welfare, Publication No. (NIH) 85–23, Revised 1985; Office of Science and Health Reports, DRR/NIH, Bethesda, MD]. The experimental protocols were approved by the Ethics Committee on Animal Research of the Medical School of Ribeirao Preto (Protocol # 034/2012).
Isolation and characterization of MSCs
Bone marrow was harvested from the femora, tibiae, and humeri of donor rats. The cells were fractionated by centrifugation on a Ficoll-Paque gradient (Sigma-Aldrich Corporation). After centrifugation, mononuclear cells were collected and dispensed into culture-treated polystyrene flasks (Sarstedt, Inc.) at a density of 4×105 cells/cm2 in complete medium [low-glucose Dulbecco's modified Eagle's medium (DMEM; Life Technologies) supplemented with 10% fetal bovine serum (Life Technologies) and 1% penicillin–streptomycin (Life Technologies)]. Primary cultures were placed in an incubator with a humidified atmosphere containing 5% CO2 at 37°C. Nonadherent cells were removed by changing the medium after 3 days of culture, and medium changes were performed twice a week. When the cultures attained 80–90% confluence, the cells were detached with 0.25% trypsin-ethylenediaminetetraacetic acid (trypsin-EDTA; Life Technologies) and seeded in new culture flasks in complete culture medium at a density of 8×103 cells/cm2. After three passages, adherent cells were characterized as MSCs according to criteria established by the International Society for Cellular Therapy [18], as follows.
Immunophenotyping–Third-passage MSCs were subjected to cell surface antigen phenotyping using flow cytometry. Cells were incubated with phycoerythrin (PE)- or fluorescein isothiocyanate (FITC)-conjugated mouse anti-rat monoclonal antibodies (BD Bioscience) raised against the molecules CD11b (PE), CD31 (PE), CD44 (FITC), CD45 (PE), CD29 (FITC), and CD90 (FITC). Cells stained with PE- or FITC-conjugated mouse isotype-matched antibodies served as control. Fluorescence was read using a FACSCalibur 4C flow cytometer (Becton Dickinson and Company). A total of 20,000 events, as defined by size (FSC) and granularity (SSC) parameters, were acquired per sample. Data analysis was performed using CellQuest software (BD).
Osteogenic differentiation–Third-passage MSCs were plated in six-well plates at a density of 3×103 cells per well. After 3 days, complete medium was replaced by osteogenic induction medium [complete medium augmented with 100 nM dexamethasone (Sigma), 50 μM ascorbate-2-phosphate (Sigma), and 10 mM beta-glycerol phosphate (Sigma)]. The medium was changed every 3–4 days. After 20 days of culture in osteogenic medium, the cultures were fixed with 4% paraformaldehyde (Sigma) and stained with Alizarin Red S (Sigma) at a pH of 4.1 to reveal calcium-rich mineralized extracellular matrix. MSCs cultured in complete medium with no osteogenic supplements served as controls.
Adipogenic differentiation–MSCs at passage 3 were plated in six-well plates at 2×104 cells per well in complete culture medium. Three days after plating, the medium was replaced by adipogenic induction medium [complete medium augmented with 1 μM dexamethasone (Sigma), 100 μM indomethacin (Sigma), 0.5 mM IBMX (Sigma), and 10 μg/mL insulin (Sigma)], which was changed every 3–4 days. Cells bearing cytoplasmic lipid droplets could be detected by observation under a phase contrast microscope after 1 week in culture. After 3 weeks under adipogenic conditions, the cultures were fixed in 4% paraformaldehyde (Sigma), and lipid vacuoles were stained with Oil Red O (Sigma). Harris hematoxylin (Sigma) was used to stain the cell nuclei. MSCs cultured in complete medium with no adipogenic supplements served as controls.
MI model
CHF was induced by MI according to the technique described by Pfeffer et al. [19]. Briefly, rats were anesthetized with ketamine (50 mg/kg of body weight (b.w.), i.p.; União Química Farmacêutica Nacional S/A) and xylazine (10 mg/kg b.w., i.p.; União Química Farmacêutica Nacional S/A), endotracheally intubated, and mechanically ventilated with room air. A left thoracotomy was performed, the pericardium was opened, and the heart was exteriorized. The left anterior descending coronary artery was ligated between the pulmonary artery and the left atrium with polyester sutures (4-0; Ethicon). Control rats underwent a similar surgical procedure without coronary ligation. After the ligature, the animals were implanted with polyethylene catheters (PE-10/PE-50; Intramedic, BD) into the left jugular vein for the administration of MSCs and drugs. Next, the animals received 0.7 mL/kg b.w. of 2% lidocaine hydrochloride subcutaneously (Hipolabor Famacêutica Ltda, Sabará, MG, Brazil) for its antiarrhythmic effects and the same dose of polyvalent antibiotic (Pentabiotic Veterinary) for small animals (Fort Dodge Animal Health), i.m, to prevent postsurgical infections.
Intravenous administration of MSCs
Seven days after the MI, 2×106 third-passage bone marrow MSCs were administered through the jugular vein in the CHF+MSC group, dissolved in 250 μL of saline solution; the same volume of saline was administered to the CHF group. The control rats received 250 μL of the saline solution alone, at the same time of the other groups, and represented the CONTROL group.
Myocardial perfusion scintigraphy with 99mTc-sestamibi - SPECT
Two to three days after MI, the extent of the infarcted area was determined using SPECT according to Oliveira et al. [20]. Briefly, under inhaled isoflurane (Isothane; Baxter Healthcare Corporation, Guayama, Puerto Rico: 5% for induction and 1.5–2% for maintenance), the animals received a dose of 99mTc-sestamibi (555 MBq; Piro-Tec; IPEN; CardioliteR, Lantheus Medical Imaging) into the penile vein; after 90 min, the animals were placed in a motorized cylindrical apparatus coupled to a “pinhole” collimator adapted to a clinical gamma-camera (DST; SMV America). Forty projections, whose image elapsed time was 30 s for each projection, equally spaced within 360°, were obtained. The projections were recorded in 128×128 matrix, providing a 3.4×3.4 mm pixel size. A 6.3× magnification factor was adopted for all experiments, with a corresponding voxel size of 0.54 mm. After acquisition, the images were exported in DICOM format and processed. The myocardial perfusion analysis was based on the construction of polar maps for quantitative evaluation of the areas of severe defects (scores below 50% of the maximum pixel value). Subsequently, the perfusion polar map of the left ventricle (LV) was divided into 16 segments (6 basal, 6 mid-ventricular, and 4 apical). Only infarcted hearts showing perfusion defects larger than 40% of the LV circumference were taken into account in the study.
Radionuclide ventriculography
After 72 h of SPECT and 4 weeks after MSC therapy, all animals were anesthetized with isoflurane for a radionuclide ventriculography analysis of the ventricular function using a gamma-camera designed for clinical use (DST, SMV America) equipped with a pinhole collimator with a 1.5 mm aperture. The animals received 75 mg of stannous agent (PIRO-TEC, IPEN) into the penile vein. After 15 min, they also received an injection of 555 MBq of technetium-99m pertechnetate (IPEN) into the same vein. Then, the animals were taken to the gamma-camera and placed in supine position below the detector, and four electrodes were subcutaneously implanted in the limbs for ECG monitoring. The images were acquired as a matrix (word mode) of 64×64 pixels and were synchronized with ECG with an acceptance window of 20% around the mean value of the QRS duration, with 32 frames per cardiac cycle, and 200 kilocounts per frame. The symmetric energy window was centered on the photopeak at 20% of the Tc energy peak of 140 KeV. Commercially available software (planar gated blood pool, SMV America) was used for image processing. Parametric images of phase and amplitude, detected semi-automatically, were taken from the LV border and a time versus activity curve was obtained. From the curve, the left ventricular ejection fraction (LVEF) was calculated, expressed as a percentage (%), defined as the difference between the diastolic and end-systolic counts corrected for background radiation, and then divided by the end-diastolic count.
ECG recordings and HRV
Four weeks after MSC administration, the animals were anesthetized with isoflurane and subjected to a 12-lead ECG recording (CL-6 615422-1, Gould Instruments Systems, Inc.). The conventional bipolar limb leads (D1, D2, D3), the unipolar limb leads (aVR, aVL, and aVF), and the unipolar precordial (chest) leads (V1, V2, V3, V4, V5, and V6) were continuously recorded with the ECG waves sampled at 2 kHz. While still under anesthesia, the rats were implanted with subcutaneous electrodes for chronic ECG recording.
To determine the HRV, 48h after the electrodes implantation, conscious animals were connected to an ECG amplifier (Model 8811A, Bioelectric Amplifier; Hewlett Packard) attached to an analog/digital interface (DI-220 Dataq Instruments), and basal ECG was recorded for 2 h from freely moving rats. Next day, ECG was recorded for another 2 h.
Arterial pressure recordings and baroreflex sensitivity
After the second day of ECG recording, rats were anesthetized with isoflurane and a polyethylene catheter (Intramedic, Becton Dickinson and Company) was implanted into the femoral artery for arterial pressure (AP) recording. After 24 h, the arterial catheter was attached to a pressure transducer (Statham P23XL model, Grass, MA) connected to an amplifier (CL-6 615422-1; Gould Instruments Systems, Inc.), and the AP was recorded (1 kHz) using an IBM/PC equipped with an analog/digital interface (DI-220; Dataq Instruments). After recording the basal AP for 1h, phenylephrine (2 mg/kg; Sigma) and sodium nitroprusside (16 mg/kg; Sigma) were intravenously administered to measure the baroreflex sensitivity.
Cardiac weight
After the hemodynamic recordings, the rats were killed using an overdose of anesthetic, and the still-beating hearts were rapidly removed, rinsed in 0.9% NaCl solution, weighed, and kept overnight in formaldehyde (10%) for histological analysis.
Histological analysis
The hearts were sectioned, and the middle-ventricular portions were embedded in paraffin and serially cut into 7 μm thick sections. The sections were stained with hematoxylin and eosin or picrosirius red. Hematoxylin and eosin staining was used for infarct size analysis. The infarct size was measured using the public-domain software ImageJ (developed by National Institutes of Health and available on the internet site http://rsb.info.nih.gov/nih-image/). The infarct size was calculated by dividing the length of the infarcted area by the total circumference of the LV and was expressed as percentage. To estimate the volume fraction (%) of collagen in picrosirius red-stained sections, the surviving LV was quantitatively examined using a medium-power light-microscopy field. For each heart, 15 fields/rat were randomly selected and analyzed using Leica Qwin software (Leica Imaging Systems Ltd.). The mean value was subsequently calculated.
ECG and arterial pressure data analysis
Segments of ECG recordings from animals anesthetized with isoflurane were processed using a specific computer program (LabChart 7 Pro, ADInstruments, Bella Vista, Australia) module for ECG analysis to measure the PRi, Pd, QRSi, and QT length. QT segment was normalized by the RR interval (QTc, Bazett's formula: QT interval/ interval). The basal ECG and AP recordings from conscious rats were also processed using LabChart. Series, beat to beat, of RR interval and mean and systolic AP values were generated. The bradycardia induced by increasing the AP with phenylephrine injection and the tachycardia induced by the decrease in AP produced by injection of sodium nitroprusside were used to calculate the index of cardiac baroreflex sensitivity, which was calculated as the ratio between the reflex change in HR and the change in AP (ΔHR/ΔMAP). The time- and frequency-domain analysis of RR and SAP variability was performed using custom software (CardioSeries v2.4, available on the internet www.danielpenteado.com). The overall RR variability was evaluated using the standard deviation of the normal-to-normal RR interval (SDNN), and the square root of the mean of the sum of the squares of differences between adjacent NN intervals (RMSSD) was calculated and taken as an index of cardiac parasympathetic modulation.
interval). The basal ECG and AP recordings from conscious rats were also processed using LabChart. Series, beat to beat, of RR interval and mean and systolic AP values were generated. The bradycardia induced by increasing the AP with phenylephrine injection and the tachycardia induced by the decrease in AP produced by injection of sodium nitroprusside were used to calculate the index of cardiac baroreflex sensitivity, which was calculated as the ratio between the reflex change in HR and the change in AP (ΔHR/ΔMAP). The time- and frequency-domain analysis of RR and SAP variability was performed using custom software (CardioSeries v2.4, available on the internet www.danielpenteado.com). The overall RR variability was evaluated using the standard deviation of the normal-to-normal RR interval (SDNN), and the square root of the mean of the sum of the squares of differences between adjacent NN intervals (RMSSD) was calculated and taken as an index of cardiac parasympathetic modulation.
For power spectral analysis of the RR and SAP variability, the beat-by-beat series of RR and SAP values were converted to data points every 100 ms using cubic spline interpolation (10 Hz). Next, the interpolated series were divided into half-overlapping segments of 512 points. A Hanning window was used to attenuate the side effects, and the spectrum was calculated for all segments using a fast Fourier transform algorithm for discrete time series. All segments were visually inspected, and segments with artifacts or nonstationary data were excluded from analysis. Finally, the spectra were integrated in low (LF: 0.2–0.75 Hz) and high-frequency (HF: 0.75–3.0 Hz) bands.
For classical nonlinear measures, the sample entropy [21] and stochastically detrended fluctuation analysis (DFA) [22] was applied. For the DFA, one scaling exponent α was calculated for all window sizes “n”. For the sample entropy, the parameters were set to m=2 and r=15% of the standard deviation in the series. All indices for the time-domain and nonlinear methods were calculated using specific plugins for the customized computer software JBio [23].
Statistical analysis
All data were expressed as the mean±SEM. Student's t-test was used for comparisons between groups. For multiple comparisons, the data were analyzed using multivariate analysis of variance using the GLM Procedure of SAS software (SAS Institute, Inc.). The level of significance was p<0.05.
Results
Characterization of MSCs
To characterize the MSCs according to International Society for Cellular Therapy [18], the immunophenotyping, differentiation potential, and morphology were analyzed as follows:
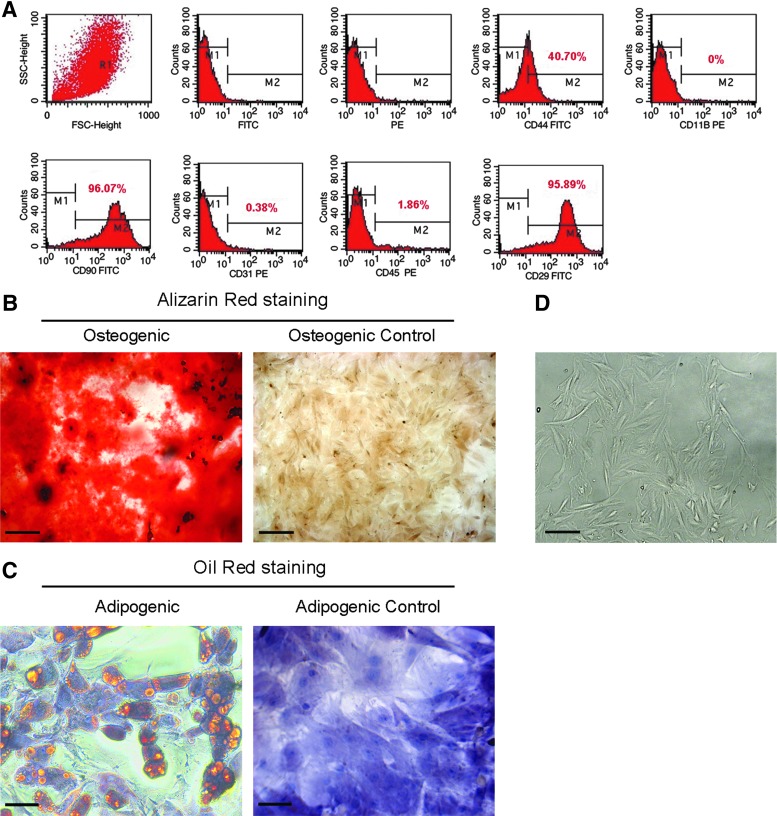
Immunophenotyping–Immunophenotypic characterization of MSCs by flow cytometry demonstrated the expression of the characteristic cell surface antigens of these cells, that is, CD90 (96.07%), CD29 (95.89%), and CD44 (40.7%). A negligible number of cells in MSC culture expressed the pan-hematopoietic marker CD45 (1.86%), the macrophage marker CD11b (0%), or the endothelial cell marker CD31 (0.38%); these findings indicate that these cultures were free from significant contamination by hematopoietic or endothelial cells (Fig. 1A).
FIG. 1.
Immunophenotypic profile and differentiation potential of the mesenchymal stem cells (MSCs). (A) Flow cytometric analysis of MSCs showed that these cells were positive for the cell surface markers CD29, CD44, and CD90, which are typically expressed in mesenchymal stem cells. (B) Photomicrographs of the in vitro differentiation into osteoblasts of MSCs cultured in osteogenic inducing media (Osteogenic), and undifferentiated MSCs cultured in complete media, without osteogenic inductors (Osteogenic Control). Alizarin Red staining showed the presence of calcium deposits (red regions), indicating osteoblast differentiation. (C) Photomicrographs of the in vitro differentiation into adipocytes of MSCs cultured in adipogenic inducing media (Adipogenic), and undifferentiated MSCs cultured in complete media, without adipogenic inductors (Adipogenic Control). Oil Red O staining showed the presence of prominent lipid deposits in the cytoplasm of cells (orange regions), indicating the differentiation of MSCs into adipocytes. (D) MSCs cultured in complete medium showed a homogeneous spindle-shaped population during subsequent passages. Representative photomicrograph of MSCs at passage 5 is shown. Magnifications of 100× (B and D; bar=200 μm) and 400× (C; bar=50 μm).
Differentiation–To evaluate their differentiation potential, the MSCs were differentiated into osteoblasts and adipocytes, after cultivation in a differentiation inducing media. The differentiation into osteoblasts in vitro was confirmed by observation of calcium-rich extracellular matrix after Alizarin Red staining (Fig. 1B). No calcified extracellular matrix was observed in osteogenic control cultures (Fig. 1B). The in vitro differentiation of MSCs into adipocytes was confirmed by showing stained lipid-laden vacuoles in the cytoplasm of the MSCs with Oil Red O (Fig. 1C). Adipogenic control cultures did not develop lipid vacuoles (Fig. 1C). Cultured MSCs demonstrated the ability to undergo osteogenic and adipogenic differentiation.
Morphology–MSCs displayed a homogeneous spindle-shaped morphology that was maintained during subsequent passages (Fig. 1D). Collectively, the results suggest that the cells used in this study showed the characteristics determined for MSCs.
Extent of MI as determined by SPECT and cardiac function
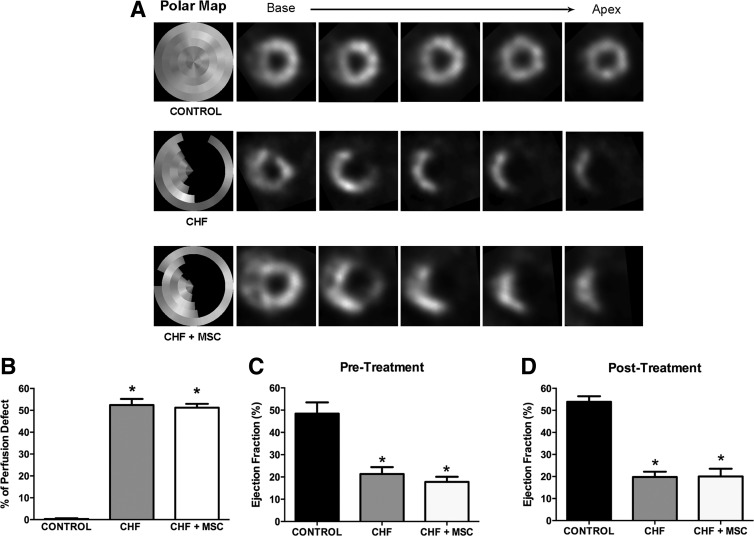
The SPECT was used to match the infarct size of CHF rats before the treatment with MSCs or saline. Both groups, that is, CHF and CHF+MSC, displayed a similar extent of MI before receiving saline (52%±2.8%) or MSCs (51%±1.7%) (Fig. 2A, B). Therefore, CHF and CHF+MSC rats exhibited, approximately, the same infarct size before treatment. The myocardial perfusion was preserved in CONTROL rats (Fig. 2A, B).
FIG. 2.
Quantification of myocardial infarcted area and measure of left ventricular function in chronic heart failure (CHF) rats. (A) Illustration of myocardial perfusion SPECT tomographic slices. The Polar Map represents the heart as a whole in the left column. Targeted cuts from base to apex of the heart are represented in the contiguous columns. The dark area enclosed in the silver circle denotes cardiac injury. (B) Quantitative data from SPECT showing similar extent of MI (% of Perfusion Defect), before treatment, in CHF (N=15) and CHF+MSC (N=9), and preserved myocardial perfusion in CONTROL (N=9). (C, D) Bar graphs showing LVEF (%), assessed by radionuclide ventriculography, before (C) and after 1 month of (D) treatment in CHF (N=9), CHF+MSC (N=7), and CONTROL (N=9). SPECT, single photon emission computed tomography; CONTROL, fictitious ligature of the left coronary artery; LVEF, left ventricular ejection fraction. The asterisk indicates a statistically significant difference between CHF and CONTROL, *p<0.05. Data are expressed as mean±standard error of mean (SEM).
Ventricular function was assessed by radionuclide ventriculography before and 1 month after the administration of MSCs or saline. A remarkable reduction in the LVEF, after MI, was observed in not treated group, that is, CHF (21%±3.1%), and treated with MSC, that is, CHF+MSC (18%±2.3%), compared with CONTROL (48%±5%) (Fig. 2C). One month after the treatment, the LVEF was still reduced in the CHF rats, that is, CHF (20%±3.5%) and CHF+MSC (20%±2.3%) groups, compared with CONTROL group (54%±2.5%) (Fig. D). This finding suggests that MI reduced ejection fraction, which was not improved by the administration of MSCs.
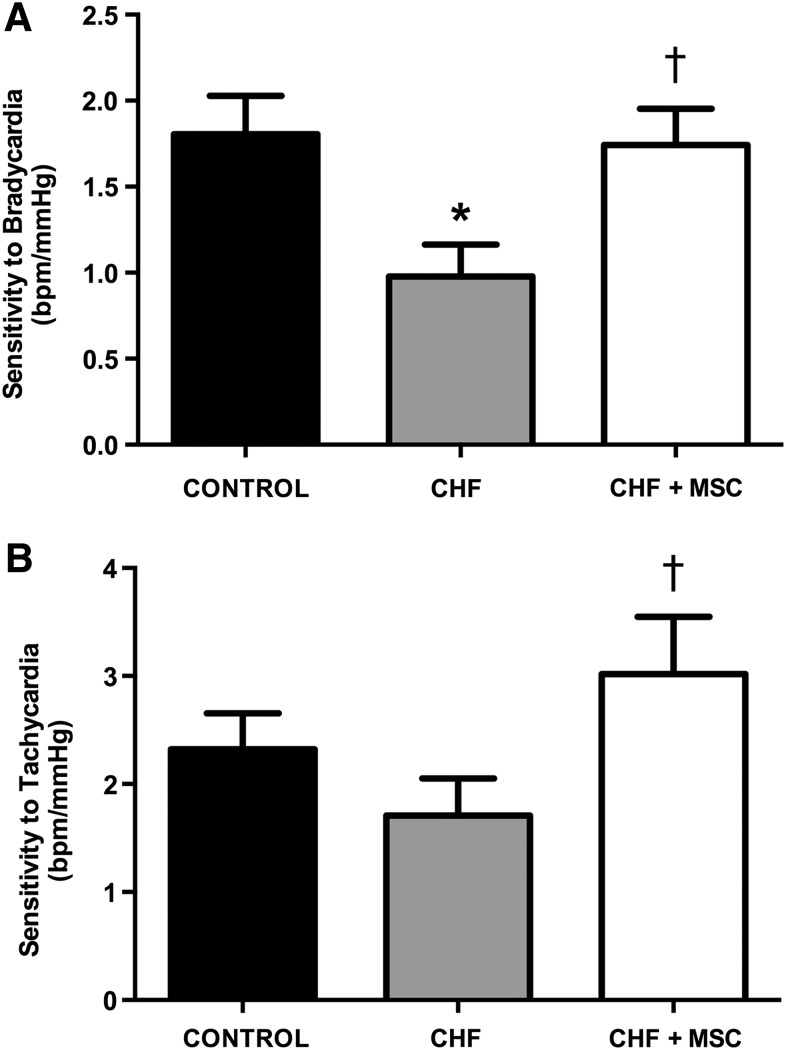
FIG. 4.
Baroreflex sensitivity from control (CONTROL) and CHF rats treated or not with MSC after 1 month of the treatment. (A) Bar graphs showing baroreflex sensitivity to bradycardia evoked by phenylephrine injection from CONTROL (N=5), CHF (N=7), and CHF+MSC (N=4); (B) Bar graphs showing baroreflex sensitivity to tachycardia evoked by sodium nitroprusside injection of CONTROL (N=6), CHF (N=5), and CHF+MSC (N=4). The asterisk indicates a statistically significant difference between CHF and CONTROL, *p<0.05; the dagger indicates a statistically significant difference between CHF+MSC and CHF, †p<0.05. Data are expressed as mean±standard error of mean (SEM).
Electrocardiographic recording
To assess the electrocardiographic consequences of the MI and the effect of MSCs, a 12-lead ECG was recorded, in anesthetized animals, 5 weeks after MI including the time frame established 1 month after administration of MSCs. The ECG parameters are shown in Table 1. A lengthening of the QRS interval was observed in CHF as compared with the CONTROL group; but not in the MSC-treated (CHF+MSC) group. The QT and QTc intervals from CHF rats not treated were also increased. Treatment with MSCs did not affect the QT intervals. All other ECG parameters were similar among groups (Table 1).
Table 1.
Electrocardiographic Parameters from Control (CONTROL) and Chronic Heart Failure Rats Treated or Not with Mesenchymal Stem Cells
| CONTROL (n=7) | CHF (n=7) | CHF+MSC (n=8) | |
|---|---|---|---|
| PRi (ms) | 58±1.4 | 61±1.9 | 63±2.5 |
| Pd (ms) | 30±1.2 | 29±2.5 | 29±3 |
| QRS (ms) | 25±1 | 30±1.7* | 29±1.7 |
| QTi (ms) | 85±2.4 | 96±1.3* | 99±2.8* |
| QTc (ms) | 197±3.2 | 228±4.9* | 227±5.6* |
The ECG parameters, from 12-lead ECG recording in anesthetized animals are the following: PRi=PR interval, Pd=duration of P wave, QRS=duration of QRS complex, QTi=QT interval, QTc=QT interval corrected by heart rate (Bazzet's formula). CONTROL=fictitious ligature of the left coronary artery. The asterisk indicates a statistically significant difference between CHF and CONTROL, *p<0.05. Data are expressed as mean±standard error of mean (SEM).
CHF, chronic heart failure; MSC, mesenchymal stem cells.
Heart rate variability
HRV allows evaluating the autonomic—sympathetic and parasympathetic—modulation of cardiac activity [24]. Therefore, chronic ECG recording was performed 5 weeks after MI including a time frame established 1 month after administration of MSCs. The basal RR interval (RRi) did not differ among groups (Table 2). The analysis of HRV in the time domain showed a reduction in SDNN and RMSSD in the CHF group; however, this reduction was not observed in the group treated with MSCs, that is, CHF+MSC group (Table 2). Frequency domain analysis of the HRV showed that the RRi spectra from CHF group presented a lower power for the LF and HF bands than those in CONTROL group; conversely, CHF+MSC exhibited an increase in the LF and HF band powers compared with those in the nontreated, CHF group (Table 2 and Fig. 3). The nonlinear analysis (Table 2) revealed that DFA was increased in the CHF group, but not in the group treated with MSC. Similarly, sample entropy of RRi was reduced only in CHF but not in the CHF+MSC group. These data indicate that MSCs improved the HRV, which was deranged by the MI.
Table 2.
Heart Rate Variability from Control (CONTROL) and Chronic Heart Failure Rats Treated or Not with Mesenchymal Stem Cells
| CONTROL (n=9) | CHF (n=10) | CHF+MSC (n=6) | |
|---|---|---|---|
| Time domain | |||
| RR (ms) | 190±4.4 | 187±4 | 195±4.3 |
| SDNN (ms) | 7.7±0.7 | 4.9±0.4* | 6.8±1.3 |
| RMSSD (ms) | 5±0.4 | 3.4±0.4* | 4.7±1 |
| Frequency domain | |||
| LF abs (ms2) | 1.6±0.3 | 0.4±0.1* | 1.6±0.7† |
| HF abs (ms2) | 6.5±1 | 2.3±0.6* | 5.3±1.3† |
| LF/HF | 0.29±0.06 | 0.23±0.03 | 0.32±0.06 |
| Nonlinear methods | |||
| DFA | 1.12±0.01 | 1.18±0.01* | 1.12±0.03† |
| Entropy | 1.32±0.06 | 0.98±0.08* | 1.08±0.15 |
Heart rate variability was examined using linear (time and frequency domain) and nonlinear (DFA and Entropy) methods. ECG recording during 4 h (2 h each day), in conscious animals, provided the data for the analysis of heart rate variability using customized computer software, CardioSeries for linear methods and JBio for nonlinear methods. RR=RR interval, SDNN=standard deviation of normal-to-normal RR intervals, RMSSD, square root of the mean of the squares of differences between adjacent RR intervals; LF and HF=power of the RR spectra in low- and high-frequency bands, DFA=stochastically detrended fluctuation analysis. CONTROL=fictitious ligature of the left coronary artery. The asterisk indicates a statistically significant difference between CHF and CONTROL, *p<0.05; the dagger indicates a statistically significant difference between CHF+MSC and CHF, †p<0.05. Data are expressed as mean±standard error of mean (SEM).
FIG. 3.
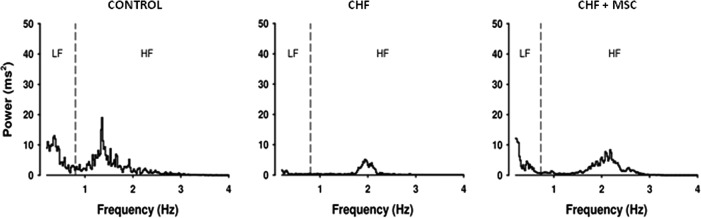
Representative spectra of heart rate variability, in the frequency domain, from control (CONTROL) and CHF rats treated or not with MSC. The individual spectra show the LF and HF bands of CONTROL, CHF, and CHF+MSC groups. LF, low frequency; HF, high frequency.
Arterial pressure variability
The assessment of arterial pressure variability also allowed quantifying the contribution of sympathetic and parasympathetic modulation of vasomotor activity [24]. Basal levels of the mean (MAP) and systolic (SAP) arterial pressure 5 weeks after MI, including a time frame established 1 month after administration of MSCs, are shown in Table 3. These parameters were reduced in CHF rats, whether treated with MSCs or not, as compared with animals in the CONTROL group. Time domain analysis of the SAP variability revealed decreased SDNN in CHF rats, that is, CHF treated or not, as compared with the CONTROL group. Finally, when the arterial pressure variability was analyzed in the frequency domain, the SAP spectra from CHF and CHF+MSC groups exhibited a lower power for the LF band when compared with the CONTROL group (Table 3). Therefore, these data indicate that MSCs did not improve the arterial pressure variability, which was disturbed by the MI, as observed in the CHF rats.
Table 3.
Arterial Pressure Variability from Control (CONTROL) and Chronic Heart Failure Rats Treated or Not with Mesenchymal Stem Cells
| CONTROL (n=9) | CHF (n=12) | CHF+MSC (n=8) | |
|---|---|---|---|
| MAP (mmHg) | 107±4.5 | 88±2.8* | 94±2* |
| SAP (mmHg) | 121±5.1 | 100±2.4* | 104±2.1* |
| SDNN (mmHg) | 4.6±0.2 | 3.3±0.1* | 3.5±0.2* |
| LF (mmHg2) | 4.3±0.9 | 1.0±0.2* | 1.5±0.3* |
Arterial pressure variability was examined in time (SDNN) and frequency (LF) domain. The AP recording of 1 h, in conscious animals, was used for AP variability analysis, using a customized computer software CardioSeries. MAP and SAP=mean and systolic arterial pressure, respectively, SDNN=standard deviation of normal-to-normal intervals between successive SAP, LF=power of the SAP spectra in low-frequency band. CONTROL=fictitious ligature of the left coronary artery. The asterisk indicates a statistically significant difference between CHF and CONTROL, *p<0.05. Data are expressed as mean±standard error of mean (SEM)
Baroreflex sensitivity
The arterial baroreflex plays an important role in cardiocirculatory control and is also impaired in CHF [3,4]. The index of baroreflex sensitivity, a hallmark of baroreflex integrity, is defined as the ratio between the reflex changes in HR and MAP and is shown in Fig. 4. Baroreflex sensitivity to bradycardia was reduced in the CHF group (0.98±0.2 bpm/mmHg) compared with the CONTROL group (1.80±0.2 bpm/mmHg). The MSC-treated group (CHF+MSC) exhibited higher baroreflex sensitivity to bradycardia (1.74±0.2 bpm/mmHg) compared with the nontreated CHF group (Fig. 4A). Baroreflex sensitivity in response to tachycardia was higher in the CHF+MSC group (3±0.5 bpm/mmHg) when compared with the nontreated CHF group (1.71±0.3 bpm/mmHg), but no difference was observed between the CONTROL (2.32±0.3 bpm/mmHg) and CHF rats, treated or not with MSCs (Fig. 4B). Therefore, this finding indicates that the therapy with MSC prevented in CHF rats only the attenuation of baroreflex sensitivity to bradycardic responses.
Histological analysis and weight of the heart
The extent of fibrosis and weight of the heart were examined to assess the benefit of MSCs upon CHF.
Extent of ischemic area
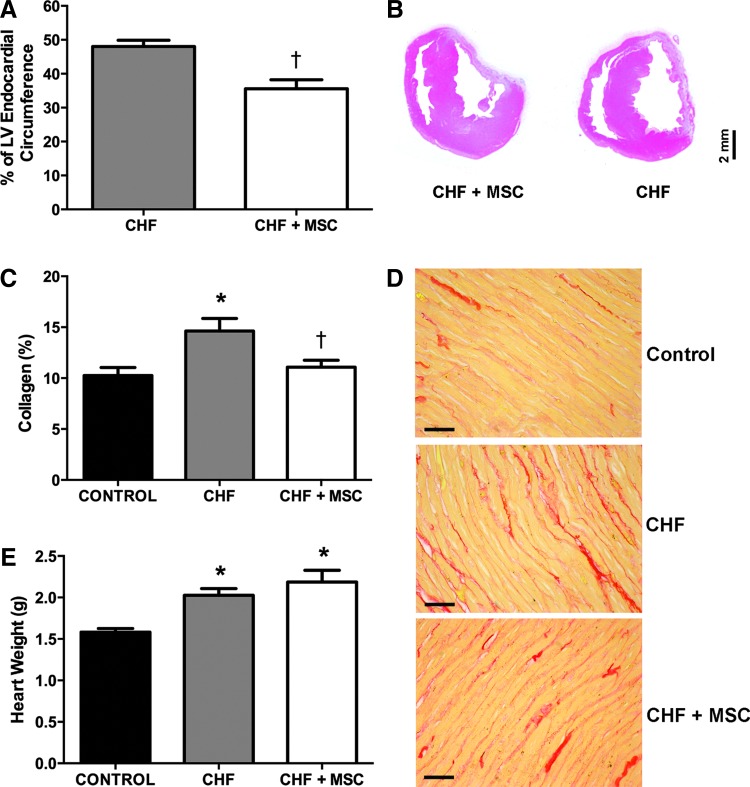
One month after administration of MSCs, the infarct size of the MSC-treated group, that is, CHF+MSC (36±2%), was significantly smaller than that of the nontreated animals, that is, the CHF group (48±2%) (Fig. 5A, B). Hence, the therapy with MSC reduced the infarct size.
FIG. 5.
Histopathological analysis and heart weight from control (CONTROL) and CHF rats treated or not with MSC. Histological assessment and heart weight measure was performed in a time frame established 1 month after the treatment with MSCs. (A) Bar graphs showing the infarct size (% of LV Endocardial Circumference) in CHF (N=11) and CHF+MSC (N=7). (B) Photographs of heart sections stained with hematoxylin and eosin in CHF and CHF+MSC group. (C) Bar graphs showing the percentage of interstitial collagen in surviving LV myocardium in CONTROL (N=9), CHF (N=9), and CHF+MSC (N=7). (D) Photomicrograph of hearts sections stained with picrosirius red in CONTROL, CHF, and CHF+MSC (Magnifications of 400×, bar=30 μm). (E) Bar graphs showing the heart weight of the CONTROL (N=8), CHF (N=8), and CHF+MSC (N=6) group. The asterisk indicates a statistically significant difference between CHF and CONTROL, *p<0.05; the dagger indicates a statistically significant difference between CHF+MSC and CHF, †p<0.05. Data are expressed as mean±standard error of mean (SEM).
Interstitial fibrosis
Left ventricular interstitial fibrosis was examined in a time frame established 1 month after administration of MSCs or saline. A larger accumulation of interstitial collagen (red color; Fig. 5C, D) was observed in the surviving LV myocardium in the CHF group (14.6%±1.5%) compared with the CONTROL group (10.3%±0.8%). This collage accumulation after MI was reduced by MSCs treatment (11%±0.7%) (Fig. 5C, D).
Cardiac weight
CHF in rats also leads to cardiac hypertrophy [19]. Therefore, the heart weight was measured to evaluate the degree of cardiac hypertrophy indirectly [19]. The hearts of the CHF rats, MSC-treated (CHF+MSC group, 2.18±0.14 g) or not (CHF, 2.11±0.11 g), exhibited higher weights compared with the CONTROL group (1.58±0.05 g) (Fig. 5E). Thus, these data indicate that the rats with CHF exhibited a heavier heart compared with the CONTROL group. This finding suggests that despite the treatment with MSCs improved the histological data, there was no effect on heart weight.
Discussion
This study is the first to evaluate the effect of MSC therapy on baroreflex sensitivity and HR and AP variability in CHF. This study showed that after 5 weeks of MI and 4 weeks after treatment, the MSCs improved the HRV, as indicated by analyses in time- and frequency-domain (spectral analysis), and nonlinear analysis (entropy and DFA). The baroreflex sensitivity, collagen density, and extent of ischemic area were also improved by the treatment with MSCs. However, the MSCs showed no effect on the LVEF, MAP, AP variability, and heart weight.
After MI the heart undergoes a process known as cardiac remodeling, which involves changes in the architecture of the ventricular chamber, such as the development of a collagen-based scar in the ischemic area and compensatory myocyte hypertrophy and interstitial fibrosis in the surviving myocardium combined with impairment in cardiac function [25,26]. MSC therapy reduced the extent of the MI and collagen in the surviving LV. The beneficial effect of MSCs on these variables might be explained by the MSC secretion of bioactive molecules, such as growth factors, cytokines, and chemokines that possess trophic (antiapoptotic, supportive, and angiogenic), immunomodulatory, anti-scarring, and chemoattractant paracrine effects, which constitute their most significant biological role under physiopathological conditions [27]. Therefore, MSCs helped to protect the myocardium from ischemic deterioration and improved the reparative process.
Cardiac hypertrophy was evaluated using an indirect method, that is, measurement of the heart weight. The heart weight in CHF rats increased, but MSCs did not affect this outcome. Nevertheless, despite their effect on morphological structure of the heart, MSCs did not improve the cardiac function, which still remained depressed in CHF rats treated with MSCs; this finding is consistent with other studies [28–30].
Morphological alteration could also be associated with changes in HR. Nevertheless, in this study, HR was not different among groups, which is consistent with a previous report [13]. However, the HR changes in CHF are still debated, for example, studies have shown increased [31,32], reduced [33], or unchanged [34,35] HR in this pathological condition.
Consistent with previous reports [19,35], it was shown that the AP was reduced without a change in HR after MI in CHF rats. MSC therapy did not reverse hypotension. Hypotension in MI could be related to lower cardiac output [36]. The LVEF was reduced in CHF rats despite the MSC treatment. Therefore, the observed hypotensive effect may be attributed to the decreased cardiac output presumably caused by the depressed cardiac function.
Baroreflex sensitivity is a marker of the integrity of short-term regulation of AP by the autonomic nervous system [37]. In heart diseases, such as CHF, a disorder of baroreflex function has been consistently documented [38,39]. This study demonstrated a reduction in the reflex bradycardia in the CHF rats, while the reflex tachycardia was not altered. These findings are consistent with previous observations [35,40]. Attenuation of baroreflex sensitivity can be an important factor in the prognosis of patients with heart failure [41]. MSCs increased the baroreflex sensitivity to bradycardia post-MI, showing a beneficial effect on the cardiovascular control of CHF rats.
HRV and AP variability are robust noninvasive tools for estimating the relative autonomic modulation of the cardiovascular system [24]. Additionally, it has been well documented that HRV is attenuated in CHF, and this change is a reliable predictor of cardiac mortality for diseases such as MI or CHF [4]. The absolute values of the indices of HRV in time- (RMSSD and SDNN) and frequency-domain (spectral analysis) were reduced in CHF rats, indicating autonomic imbalance in these animals. MSC therapy improved the reduced autonomic control triggered by ischemic event. Although MSCs improved the HRV, the AP variability was reduced in CHF rats, regardless of MSC treatment.
HRV was also examined using nonlinear methods (DFA and sample entropy). Although the absolute values of the time- and frequency-domain measures of HRV describe the overall magnitude of the HR variance, DFA and entropy describe the nonlinear dynamics of HR fluctuations [42]. Sample entropy measures the regularity (usually related to the complexity level) of RR interval time series [43], whereas DFA quantifies the fractal scaling properties of these time series over different time scales [44]. Decreases in entropy values are often linked to a loss of cardiovascular complexity [45]. The results show that sample entropy was reduced in CHF rats. Moreover, mean entropy tended to increase after MSC treatment. Otherwise, it has been widely reported that a reduction in short-term DFA index (α1) is the most powerful predictor of mortality in patients with heart failure [44,46,47]. Here, a single DFA index was extracted (α1). In this case, although there is no distinction between short- and long-term fractal properties, the single index shows better correlation with the long-range properties. More details can be found elsewhere [22]. Usually, healthy systems are characterized by a DFA index of approximately one. The results show that the DFA fractal index increases in CHF and recovers after MSC treatment, indicating that the fractal mechanisms involved in HRV are degraded by CHF and restored by MSC treatment.
HRV data, and the improvement in baroreflex sensitivity, are correlated with better cardiovascular autonomic control in the infarcted rats treated with MSCs; this finding suggests that the cells had a positive effect on the CHF rats and restored the autonomic control of HR.
Possible mechanisms for the beneficial effects of MSCs in CHF after ischemic event include the following: (1) the MSCs differentiate into functional cardiomyocytes [48,49]; (2) decrease the healing area [49,50] and increase the density of healing [48,50]; and (3) increase angiogenesis and neovascularization [49]. These mechanisms may result from the secretion of soluble factors that act in a paracrine manner [14] and have proangiogenic, antiapoptotic, antifibrotic, and immunoregulatory effects [27]. It is possible that MSCs can facilitate neural development through this pathway by inducing extracellular matrix remodeling [15]. However, it has been hypothesized that MSCs differentiate into nerve cells [51,52]. Nevertheless, the mechanisms underlying the beneficial effects of MSCs are not completely understood. In addition, several important questions regarding the delivery method and route, the amount of injected cells, and the timing for stem cell transplantation are among the factors that may limit cell-based therapies in the treatment of ischemic heart disease [9,10,53]. These issues, undoubtedly, require further studies to optimize the cell therapy approaches, and represent a focus of future research in this field [9,10,53].
In summary, MSC therapy is an important approach for maintaining normal physiological mechanisms during the development of CHF. After 5 weeks of MI and 4 weeks after MSC transplantation, MSCs improved the baroreflex sensitivity, autonomic modulation, DFA, extent of MI, and collagen density in the heart, demonstrating the beneficial effects of MSC therapy on heart failure.
Acknowledgments
The authors wish to thank Dr. Dimas Covas Tadeu and Ms. Patricia Vianna Bonini Palma, who are from the Regional Blood Center of Ribeirao Preto, for allowing us to use their flow cytometer. We also thank the following people from the Medical School of Ribeirao Preto (USP): Mr. Rubens Melo, Dr. Marcos A. Rossi (in memories), and Dr. Cibele Prado for histological support and Dr. Guillermo Andrey Ariza Traslaviña for assistance with the statistical analysis.
This submitted material was presented in the following conferences: 11th Annual Meeting of International Society for Stem Cell Research (ISSCR), June 12–15, 2013, Boston Convention and Exhibition Center, Boston, MA; Experimental Biology (EB) 2014, April 26–30, San Diego Convention Center, San Diego, CA.
Funding
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Núcleo de Insuficiência Cardíaca da Universidade de São Paulo (NIC-USP).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Felder RB, Francis J, Weiss RM, Zhang Z, Wei S. and Johnson AK. (2001). Neurohumoral regulation in ischemia-induced heart failure. Role of the forebrain. Ann N Y Acad Sci 940:444–453 [DOI] [PubMed] [Google Scholar]
- 2.Krieger EM, Salgado HC. and Michelini LC. (1982). Resetting of baroreceptors. Int Rev Physiol 26:119–146 [PubMed] [Google Scholar]
- 3.Dibona GF. and Sawin LL. (1994). Reflex regulation of renal nerve activity in cardiac failure. Am J Physiol 266:R27–R39 [DOI] [PubMed] [Google Scholar]
- 4.La Rovere MT, Bigger JT, Jr, Marcus FI, Mortara A. and Schwartz PJ. (1998). Baroreflex sensitivity and heart rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet 351:478–484 [DOI] [PubMed] [Google Scholar]
- 5.Porter TR, Eckberg DL, Fritsch JM, Rea RF, Beightol LA, Schmedtje JF. and Mohanty PK. (1990). Autonomic pathophysiology in heart failure patients: sympathetic-cholinergic interrelations. J Clin Invest 85:1362–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mark AL. (1995). Sympathetic dysregulation in heart failure: mechanisms and therapy. Clin Cardiol 18:I3–I8 [DOI] [PubMed] [Google Scholar]
- 7.Olshsnsky B, Sabbah HN, Hauptman PJ. and Colucci WS. (2008). Parasympathetic nervous system and heart failure: pathophysiology and potential implications for therapy. Circulation 118:863–871 [DOI] [PubMed] [Google Scholar]
- 8.da Silva Meirelles L. and Nardi NB. (2009). Methodology, biology and clinical applications of mesenchymal stem cells. Front Biosci 14:4281–4298 [DOI] [PubMed] [Google Scholar]
- 9.Mazhari R. and Hare JM. (2012). Translational findings from cardiovascular stem cell research. Trends Cardiovasc Med 22:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madonna R, Ferdinandy P, De Caterina R, Willerson JT. and Marian AJ. (2014). Recent developments in cardiovascular stem cells. Circ Res. 115:e71–e78 [DOI] [PubMed] [Google Scholar]
- 11.Berry MF, Engler AJ, Woo YJ, Pirolli TJ, Bish LT, Jayasankar V, Morine KJ, Gardner TJ, Discher DE. and Sweeney HL. (2006). Mesenchymal Stem cell injection after myocardial infarction improves myocardial compliance. Am J Physiol Heart Circ Physiol 290:H2196–H2203 [DOI] [PubMed] [Google Scholar]
- 12.Davani S, Marandin A, Mersin N, Royer B, Kantelip B, Hervé P, Etievent JP. and Kantelip JP. (2003). Mesenchymal progenitor cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a rat cellular cardiomyoplasty model. Circulation 108:II253–II258 [DOI] [PubMed] [Google Scholar]
- 13.Nagaya N, Kangawa K, Itoh T, Iwase T, Murakami S, Miyahara Y, Fujii T, Uematsu M, Ohgushi H, et al. (2005). Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation 112:1128–1135 [DOI] [PubMed] [Google Scholar]
- 14.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS. and Dzau VJ. (2005). Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med 11:367–368 [DOI] [PubMed] [Google Scholar]
- 15.Pak HN, Qayyum M, Kim Dt, Hamabe A, Miyauchi Y, Lill MC, Frantzen M, Takizawa K, Chen LS, et al. (2003). Mesenchymal stem cell injection induces cardiac nerve sprouting and increased tenascin expression in a Swine model of myocardial infarction. J Cardiovasc Electrophysiol 14:841–848 [DOI] [PubMed] [Google Scholar]
- 16.Kim SK, Pak HN, Park JH, Fang YF, Kim GI, Park YD, Hwang C, Kim YH. and Kim BS. (2010). Cardiac cell therapy with mesenchymal stem cell induces cardiac nerve sprouting, angiogenesis, and reduced connexin43-positive gap junctions, but concomitant electrical pacing increases connexin43-positive gap junctions in canine heart. Cardiol Young 20:308–317 [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Xue M, Xuan Y-L, Hu H-S, Cheng W-J, Suo F, Li X-R, Yan S-H. and Wang L-X. (2013). Mesenchymal stem cell therapy improves diabetic cardiac autonomic neuropathy and decreases the inducibility of ventricular arrhythmias. Heart Lung Circ 22:1018–1025 [DOI] [PubMed] [Google Scholar]
- 18.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop DJ. and Horwitz E. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8:315–317 [DOI] [PubMed] [Google Scholar]
- 19.Pfeffer MA, Pfeffer JM, Fishbein MC, Fletcher PJ, Spadaro J, Kloner RA. and Braunwald E. (1979). Myocardial infarct size and ventricular function in rats. Cir Res 44:503–512 [DOI] [PubMed] [Google Scholar]
- 20.Oliveira LF, Mejia J, Carvalho EE, Lataro RM, Frassetto SN, Fazan R, Jr, Salgado HC, Galvis-Alonso OY. and Simoes MV. (2013). Myocardial infarction area quantification using high-resolution SPECT images in rats. Arq Bras Cardiol 101:59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richman JS. and Moorman JR. (2000). Physiological time-series analysis using approximate entropy and sample entropy. Am J Physiol Heart Circ Physiol 278:H2039–H2049 [DOI] [PubMed] [Google Scholar]
- 22.Peng CK, Havlin S, Stanley HE. and Goldberger AL. (1995). Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time-series. Chaos 5:82–87 [DOI] [PubMed] [Google Scholar]
- 23.Duque JJ, Silva LEV. and Murta LO. (2013). Open architecture software platform for biomedical signal analysis. In: 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka 2084–2087 [DOI] [PubMed] [Google Scholar]
- 24.Task Force Of The European Society Of Cardiology. (1996). The North American Society Of Pacing Eletrophysiology: heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 93:1043–1065 [PubMed] [Google Scholar]
- 25.Jugdutt BI. (2003). Ventricular remodeling after infarction and the extracellular collagen matrix: when is enough? Circulation 108:1395–1403 [DOI] [PubMed] [Google Scholar]
- 26.Murdoch CE, Zhang M, Cave AC. and Shah AM. (2006). NADPH oxidase-dependent redox signaling in cardiac hypertrophy, remodeling and failure. Cardiovasc Res 71:208–215 [DOI] [PubMed] [Google Scholar]
- 27.Meirelles L da S, Fontes AM, Covas DT. and Caplan AI. (2009). Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev 20:419–427 [DOI] [PubMed] [Google Scholar]
- 28.Guarita-Souza LC, Carvalho KA, Simeone BR, Franscisco JC, Miyague N. and Olandoski M. (2006). Functional outcome of bone marrow stem cells: mononuclear versus mesenchymal stem cell safer cellular therapy in myocardial scar in Wistar rats. Transplant Proc 38:1953–1954 [DOI] [PubMed] [Google Scholar]
- 29.Amado LC, Schuleri KH, Saliaris AP, Boyle AJ, Helm R, Oskouei B, Centola M, Eneboe V, Young R, et al. (2006). Multimodality noninvasive imaging demonstrates in vivo cardiac regeneration after mesenchymal stem cell therapy. J Am Coll Cardiol 48:2116–2124 [DOI] [PubMed] [Google Scholar]
- 30.Cheng Z, Liu X, Ou L, Zhou X, Liu Y, Jia X, Zhang J, Li Y. and Kong D. (2008). Mobilization of mesenchymal stem cells by granulocyte colony-stimulating factor in rats with acute myocardial infarction. Cardiovasc Drugs Ther 22:363–371 [DOI] [PubMed] [Google Scholar]
- 31.Osterziel KJ, Hänlein D, Willenbrock R, Eichhorn C, Luft F. and Dietz R. (1995). Baroreflex sensitivity and cardiovascular mortality in patients with mild to moderate heart failure. Br Heart J 73:517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li M, Zheng C, Sato T, Kawada T, Sugimachi M. and Sunagawa K. (2004). Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation 109:120–124 [DOI] [PubMed] [Google Scholar]
- 33.Du XJ, Cox HS, Dart AM. and Esler MD. (1998). Depression of efferent parasympathetic control of heart rate in rats with myocardial infarction: effect of losartan. J Cardiovasc Pharmacol 31:937–944 [DOI] [PubMed] [Google Scholar]
- 34.Gao L, Schultz HD, Patel KP, Zucker IH. and Wang W. (2005). Augmented input from cardiac sympathetic afferents inhibits baroreflex in rats with heart failure. Hypertension 45:1173–1181 [DOI] [PubMed] [Google Scholar]
- 35.Sabino JP, da Silva CA, Giusti H, Glass ML, Salgado Hc. and Fazan JR R. (2013). Parasympathetic activation by pyridostigmine on chemoreflex sensitivity in heart-failure rats. Auton Neurosci 179:43–48 [DOI] [PubMed] [Google Scholar]
- 36.Gheorghiade M, Vaduganathan M, Ambrosy A, Böhm M, Campia U, Cleland JG, Fedele F, Fonarow GC, Maggioni AP, et al. (2013). Current management and future directions for the treatment of patients hospitalized for heart failure with low blood pressure. Heart Fail Rev 18:107–122 [DOI] [PubMed] [Google Scholar]
- 37.Sapoznikov D, Backenroth R. and Rubinger D. (2010). Baroreflex sensitivity and sympatho-vagal balance during intradialytic hypotensive episodes. J Hypertens 28:314–324 [DOI] [PubMed] [Google Scholar]
- 38.Kar S, Gao L, Belatti DA, Curry Pl. and Zucker IH. (2011). Central angiotensin (1–7) enhances baroreflex gain in conscious rabbits with heart failure. Hypertension 58:627–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flora JS. (2009). Sympathetic nervous system activation in human heart failure: clinical implications of an updated model. J Am Coll Cardiol 54:375–385 [DOI] [PubMed] [Google Scholar]
- 40.Eckberg DL, Drabinsky M. and Braunwald E. (1971). Defective cardiac parasympathetic control in patients with heart disease. N Engl J Med 285:877–883 [DOI] [PubMed] [Google Scholar]
- 41.Nolan J, Batin PD, Andrews R, Lindsay SJ, Brooksby P, Mullen M, Baig W, Flapan AD, Cowley A, et al. (1998). Prospective study of heart rate variability and mortality in chronic heart failure: results of the United Kingdom Heart Failure and Assessment of Risk Trial. Circulation 98:1510–1516 [DOI] [PubMed] [Google Scholar]
- 42.Voss A, Schulz S, Schroeder R, Baumert M. and Caminal P. (2009). Methods derived from nonlinear dynamics for analyzing heart rate variability. Philos Trans A Math Phys Eng Sci 367:277–296 [DOI] [PubMed] [Google Scholar]
- 43.Pincus SM. and Goldberger AL. (1994). Physiological time-series analysis: what does regularity quantify? Am J Physiol 266:H1643–H1656 [DOI] [PubMed] [Google Scholar]
- 44.Makikallio TH, Huikuri HV, Hintze U, Videbaek J, Mitrani RD, Castellanos A, Myerburg RJ. and Moller M. (2001). DIAMOND Study Group (Danish Investigations of Arrhythmia and Mortality ON Dofetilide). Fractal analysis and time- and frequency-domain measures of heart rate variability as predictors of mortality in patients with heart failure. Am J Cardiol 87:178–182 [DOI] [PubMed] [Google Scholar]
- 45.Porta A, Gnecchi-Ruscone T, Tobaldini E, Guzzetti S, Furlan R. and Montano N. (2007). Progressive decrease of heart period variability entropy-based complexity during graded head-up tilt. J Appl Physiol 103:1143–1149 [DOI] [PubMed] [Google Scholar]
- 46.Huikuri HV, Makikallio TH, Peng CK, Goldberger AL, Hintze U. and Moller M. (2000). Fractal correlation properties of R-R interval dynamics and mortality in patients with depressed left ventricular function after an acute myocardial infarction. Circulation 101:47–53 [DOI] [PubMed] [Google Scholar]
- 47.Tapanainen JM, Thomsen PE, Kober L, Torp-Pedersen C, Makikallio TH, Still AM, Lindgren KS. and Huikuri HV. (2002). Fractal analysis of heart rate variability and mortality after an acute myocardial infarction. Am J Cardiol 90:347–352 [DOI] [PubMed] [Google Scholar]
- 48.Shake JG, Gruber PJ, Baumgartner WA, Senechal G, Meyers J, Redmond JM, Pittenger MF. and Martin BJ. (2002). Mesenchymal stem cell implantation in a swine myocardial infarct model: Engraftment and functional effects. Ann Thorac Surg 73:1919–1925 [DOI] [PubMed] [Google Scholar]
- 49.Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM. and Itescu S. (2001). Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med 7:430–436 [DOI] [PubMed] [Google Scholar]
- 50.Tomita S, Li RK, Weisel RD, Mickle DA, Kim EJ, Sakai T. and Jia ZQ. (1999). Autologous transplantation of bone marrow cells improves damaged heart function. Circulation 100:II247–II256 [DOI] [PubMed] [Google Scholar]
- 51.Ortiz LA, Gambelli F, Mcbride C, Gaupp D, Baddoo M, Kaminski N. and Phinney DG. (2003). Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A 100:8407–8411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Azizi SA, Stokes D, Augelli BJ, Digirolamo C. and Prockop DJ. (1998). Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats—similarities to astrocyte grafts. Proc Natl Acad Sci U S A 95:3908–3913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vrtovec B, Poglajen G. and Haddad F. (2013). Stem cell therapy in patients with heart failure. Methodist Debakey Cardiovasc J 9:6–10 [DOI] [PMC free article] [PubMed] [Google Scholar]