Abstract
Traumatic brain injury (TBI) is a significant cause of disability and death and a huge economic burden throughout the world. Much of the morbidity associated with TBI is attributed to secondary brain injuries resulting in hypoxia and ischemia after the initial trauma. Intracranial hypertension and decreased partial brain oxygen tension (PbtO2) are targeted as potentially avoidable causes of morbidity. Therapeutic hypothermia (TH) may be an effective intervention to reduce intracranial pressure (ICP), but could also affect cerebral blood flow (CBF). This is a retrospective analysis of prospectively collected data from 17 patients admitted to the Western General Hospital, Edinburgh. Patients with an ICP >20 mmHg refractory to initial therapy were randomized to standard care or standard care and TH (intervention group) titrated between 32°C and 35°C to reduce ICP. ICP and PbtO2 were measured using the Licox system and core temperature was recorded through rectal thermometer. Data were analyzed at the hour before cooling, the first hour at target temperature, 2 consecutive hours at target temperature, and after 6 hours of hypothermia. There was a mean decrease in ICP of 4.3±1.6 mmHg (p<0.04) from 15.7 to 11.4 mmHg, from precooling to the first epoch of hypothermia in the intervention group (n=9) that was not seen in the control group (n=8). A decrease in ICP was maintained throughout all time periods. There was a mean decrease in PbtO2 of 7.8±3.1 mmHg (p<0.05) from 30.2 to 22.4 mmHg, from precooling to stable hypothermia, which was not seen in the control group. This research supports others in demonstrating a decrease in ICP with temperature, which could facilitate a reduction in the use of hyperosmolar agents or other stage II interventions. The decrease in PbtO2 is not below the suggested treatment threshold of 20 mmHg, but might indicate a decrease in CBF.
Introduction
Traumatic brain injury (TBI) is a significant cause of disability and death and a huge economic burden on our society. The incidence of TBI is rising throughout the world and the World Health Organization estimates that TBI will become a primary cause of death by the year 2020 (Hyder et al., 2007). In the US, ∼1.7 million people suffer a TBI each year. Approximately 52,000 of these die and of those that survive to discharge, 43% have an ongoing disability 1 year after injury (Langlois et al., 2006; Corrigan et al., 2010). The financial cost of TBI in the US in the year 2000 was estimated to be $406 billion (Corso et al., 2006). In Europe there is an annual incidence of about 235 per 100,000 people and there are ∼7.7 million people suffering with disabilities due to TBI (Tagliaferri et al., 2006).
Much of the morbidity of TBI is attributed to secondary injuries, which occur after the initial injury and are associated with a failure to maintain adequate oxygen delivery to the injured brain (Chesnut et al., 1993; Chesnut, 1995). As part of the management of secondary brain injury, the Brain Trauma Foundation (BTF) guidelines from 2007 advocate intracranial pressure (ICP) monitoring and maintaining an ICP below a threshold of 20–25 mmHg (Brain Trauma Foundation et al., 2007). This is based upon evidence demonstrating worse outcomes in patients with intracranial hypertension above this threshold (Brain Trauma Foundation et al., 2007; Romner and Grande, 2013; Zeng et al., 2014).
In addition to monitoring ICP, many centers monitor partial brain oxygen tension (PbtO2), a marker of the oxygen available in the brain for adenosine triphosphate production, which also reflects the balance between oxygen delivery and consumption (De Georgia, 2014). Both raised ICP and low PbtO2 have been shown to be independent predictors of poor prognosis in severe TBI, and BTF guidelines advocate initiating therapy to increase PbtO2 if it falls below 15 mmHg (Jaeger et al., 2006; Jaeger et al., 2007; Chang et al., 2009; Jaeger et al., 2010; Oddo et al., 2011). However, contemporary articles suggest that a threshold of 20 mmHg may be more beneficial (Longhi et al., 2007; Chang et al., 2009; Oddo et al., 2011; Le Roux et al., 2014; Oddo and Bosel, 2014).
Therapeutic hypothermia (TH), the controlled lowering of core body temperature below 36°C, is currently used as a treatment modality for neonatal hypoxic ischemic encephalopathy and postcardiac arrest (Arrich et al., 2009; NICE, 2011). There is also emerging evidence that it may be beneficial in the management of ischemic stroke (van der Worp et al., 2010).
Despite conflicting evidence, TH is often used in intensive care units (ICUs) to manage patients following severe TBI (Sydenham et al., 2009; Hutchinson et al., 2013; Crossley et al., 2014). Neither the 2007 BTF guidelines nor a Cochrane review from 2009 support the use of TH in the management of severe TBI (Brain Trauma Foundation et al., 2007; Sydenham et al., 2009). However, a recent systematic review by Crossley et al. (2014) found some evidence to suggest that TH may be of benefit in the management of TBI. Both the Cochrane review and the review by Crossley et al. (2014) note that the evidence to support TH comes from low-quality trials, which have a tendency to overestimate the treatment effect, and state the need for more high-quality trials.
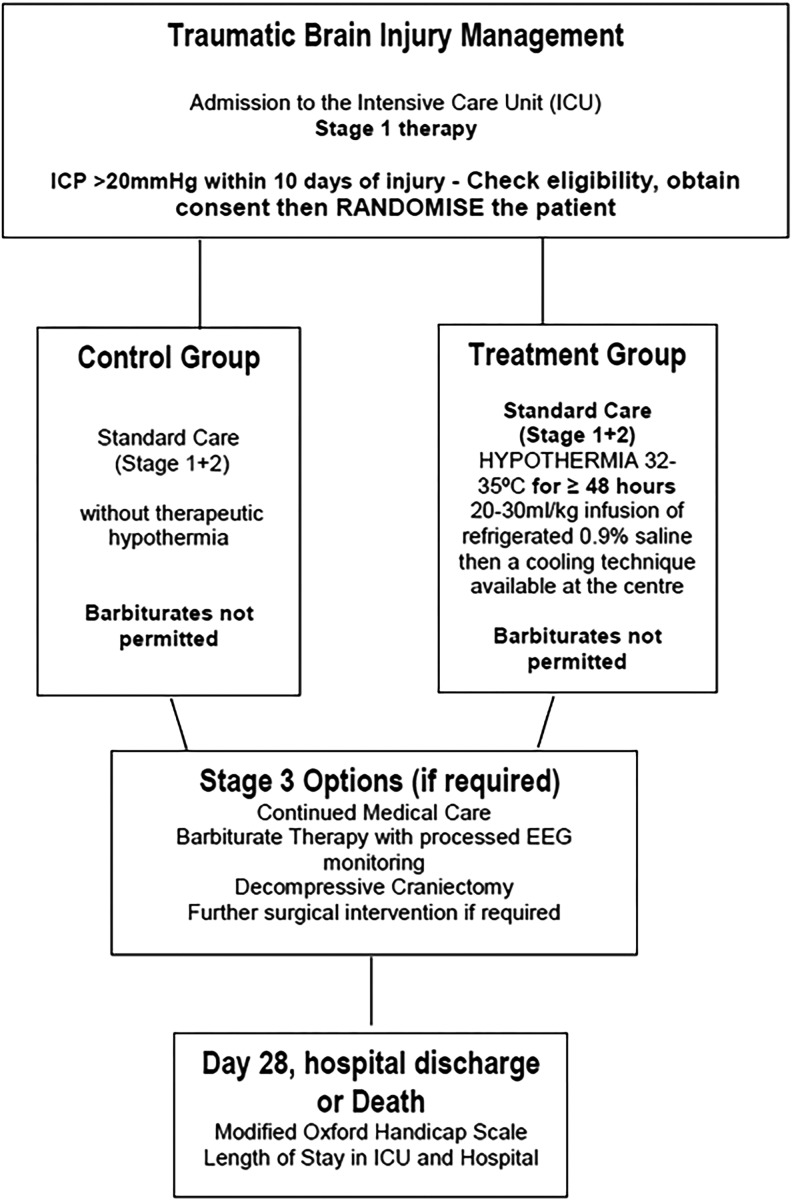
The Eurotherm3235 trial is a pragmatic multicenter randomized controlled trial investigating the effects of TH (32–35°C) on the outcome after TBI. TH is titrated to reduce ICP in patients following TBI who have an ICP >20 mmHg refractory to stage one treatment (Fig. 1) (Andrews et al., 2011; Andrews, 2012). Participants are randomized to either a control or intervention group and receive standard care without TH or standard care with TH, respectively as per the Eurotherm3235 trial protocol (Andrews et al., 2011).
FIG. 1.
Stages of management of traumatic brain injury. Information for figure taken from BTF Guidelines (Brain Trauma Foundation et al., 2007). CPP, cerebral perfusion pressure; CSF, cerebrospinal fluid; ICU, intensive care unit; SOL, space-occupying lesion.
This study reports the retrospective analysis of prospectively collected data from the first 17 patients enrolled on the Eurotherm3235 trial in Edinburgh who were monitored using the Licox system. We examined the effect of induction of TH on ICP and PbtO2.
Materials and Methods
Ethical approval and consent
Ethical approval for the Eurotherm3235 trial was granted by the Scotland A Research Ethics Committee. Full consent was obtained from/for all patients, copies of which are retained within the patient's hospital notes and the Welcome Trust Clinical Research Facility, Edinburgh. The trial has been conducted in accordance with Good Clinical Practice guidelines.
Study design and patient selection
The study was a retrospective analysis of prospectively collected data from the first 17 patients enrolled into the Eurotherm3235 trial at the Western General Hospital, Edinburgh. This is a subgroup analysis of patients who received Licox monitoring from a single center, hence the small study number. Analysis of the Eurotherm3235 trial is ongoing but does not include multicenter analysis of PbtO2.
Patients were randomized to standard treatment based on the 2007 BTF guidelines or standard treatment and TH by an online randomization service (www.eurotherm3235trial.eu) as soon as possible after meeting the inclusion/exclusion criteria. Inclusion criteria lead to recruitment of patients less than 72 hours after TBI with a primary closed brain injury and an ICP >20 mmHg refractory to stage one treatment without obvious reversible causes and with an abnormal computed tomography (CT) head scan (Fig. 1). We excluded hypothermic patients (<36°C), those already receiving cooling therapy, those receiving barbiturates before randomization, and patients unlikely to survive the next 24 hours in the opinion of the admitting neurosurgeon.
Patients were managed according to the 2007 BTF guidelines, intubated and ventilated to achieve a PaCO2 of 4.5–5.0 kPa (at 37°C), sedated and nursed with 30° head elevation. Cerebral perfusion pressure was maintained at ≥60 mmHg by manipulating mean arterial pressure with fluids and noradrenaline and limiting ICP ≤20 mmHg. Hypothermia was initiated in the intervention group with 20–30 mL/kg of refrigerated 0.9% saline given intravenously and maintained with cooling blankets to an initial target temperature of 35°C. If ICP was not maintained below 20 mmHg, the depth of cooling was increased in 0.5°C increments to a maximum depth of 32°C. TH was maintained for a minimum of 48 hours and continued until ICP was no longer dependent upon hypothermia.
Refractory intracranial hypertension in either group lead to an escalation of therapy, including the use of 125 mL of 5% sodium chloride or 200 mL of 20% mannitol (approximately equimolar) as a bolus injection. Paralysis, further CT imaging and surgical intervention were also available.
Pyrexia in the control group (>38°C) was managed with paracetamol and cooling to normothermia (36.5–37.5°C). All patients received seizure prophylaxis with a loading dose of Phenytoin (20 mg/kg) and a maintenance dose (4–5 mg/kg) for 7 days postinjury. A comprehensive protocol was used to prevent and treat shivering in the cooled patients. Regular paracetamol was administered to patients and their peripheries were wrapped. Countercurrent surface warming and clonidine were both available according to the protocol and persistent shivering was not common in the Edinburgh center.
A Licox monitor (Integra, Lyon, France) recorded ICP, PbtO2, and brain temperature through fiber optic pressure catheter, oxygen electrode, and thermistor, respectively. The Licox® probe was inserted into the brain parenchyma through a dedicated triple-lumen bolt which was placed by a burr hole. The bolt was placed so that the monitors were inserted into the frontal white matter, in the nondominant hemisphere for diffuse injuries or on the side of maximal injury in focal injuries. The probe was not placed in nonviable tissue. Core body temperature was measured by rectal thermometer. The ICU Pilot software (CMA, Stockholm, Sweden) integrated data from the monitors to a bedside computer each minute until ICP monitoring was considered no longer required. See Figure 2 for study flow chart.
FIG. 2.
Study flowchart. Adapted from Eurotherm3235 trial (Andrews, 2012). EEG, electroencephalogram; ICP intracranial pressure.
Data and statistical analysis
The first 2 hours of PbtO2 data recorded were not included in the analysis to reduce artifact from the Licox monitor (Geukens and Oddo, 2012; De Georgia, 2014). Data from 17 patients, 9 intervention and 8 controls, were analyzed at four time points: Time 0, the hour before randomization (induction of cooling or control); Time 1, the first hour of hypothermia (<35°C); Time 2, the first episode of stable hypothermia (<35°C), defined as 2 consecutive hours of hypothermia; and Time 3, 6 hours of stable hypothermia. Values are given as the mean±standard error of the mean unless otherwise stated. One-way repeated measures of variance (analysis of variance [ANOVA]) were performed to identify differences within each group and paired Student's t-tests were performed to identify differences within the groups at the set time points. Independent Student's t-tests were performed to identify differences between the groups. All statistical tests were performed using Statistical Package for Social Sciences 20.0 (SPSS version 20; IBM, Inc., Armonk, NY).
Results
Control group demographics
The mean age of the participants was 34 years. All eight participants were male. Three patients suffered extradural hemorrhages (EDHs) as their primary injury, two subdural hemorrhages (SDHs), two traumatic subarachnoid hemorrhages, and one patient suffered a diffuse injury. Three participants had nondepressed skull fractures. The median Glasgow coma score (GCS) on admission to the emergency department was 7 (range 3–14, see Table 1).
Table 1.
Clinical Characteristics of Patients
| Patient No. | Age (year)/sex | GCS score on admission | CT classification |
|---|---|---|---|
| 1 | 26/M | 7 | EDH |
| 2 | 29/M | 6 | EDH |
| 3 | 25/M | 9 | SDH |
| 4 | 27/M | 3 | SAH |
| 5 | 43/M | 12 | EDH |
| 6 | 35/M | 12 | SDH |
| 7 | 48/M | 7 | Diffuse |
| 8 | 37/M | 14 | SAH |
| 9 | 48/M | 11 | EDH |
| 10 | 26/M | 14 | EDH |
| 11 | 30/F | 7 | Diffuse |
| 12 | 28/M | 3 | Diffuse |
| 13 | 55/F | 7 | Contusions |
| 14 | 55/M | 8 | EDH |
| 15 | 55/M | 6 | SDH |
| 16 | 25/M | 3 | Diffuse |
| 17 | 49/M | 6 | SDH |
CT, computed tomography; EDH, extradural hemorrhage; F, female; GCS, Glasgow coma score; M, male; SAH, subarachnoid hemorrhage; SDH, subdural hemorrhage.
Intervention group demographics
The mean age of the participants was 41 years. Two participants were female, seven were male. Three patients suffered EDH as their primary injury, three had diffuse injuries, one had contusions, and one had diffuse injuries and a SDH. Five patients had nondepressed skull fractures and one patient had a depressed skull fracture. The median GCS on admission to the emergency department was 7 (range 3–14, see Table 1).
Time to target temperature, hemoglobin, FiO2, PaO2, and PaCO2
The mean time to target temperature (<35°C) in the intervention group was 7 hours (421±72 minutes) after randomization, due to long lead times to initiation of TH following prerandomization hypertonic therapy.
There was no significant change in FiO2 from precooling to target temperature in the intervention group (median 0.35 [0.30–0.55] vs. 0.30 [range 0.30–0.55] p>0.05) and there was no significant difference in FiO2 between the two groups. PaO2 and PaCO2 values were not significantly different from precooling to target temperature: Precooling PaO2 16.1±0.9, target temperature 16.3±1.6 kPa, p>0.05; precooling PaCO2 4.5±0.1, target temperature 4.5±0.2 kPa, p>0.05. There was no statistically significant difference in PaO2 or PaCO2 values between the two groups. Hemoglobin values were not significantly different between intervention and control groups and were not different at precooling to target temperature in the intervention group (10.1±0.4 vs. 9.7±0.4, p>0.05).
There was no significant difference in the number of osmotic agents used between the control and intervention groups (1, range 0–4 and 0–5, respectively, p>0.05).
Intracranial pressure
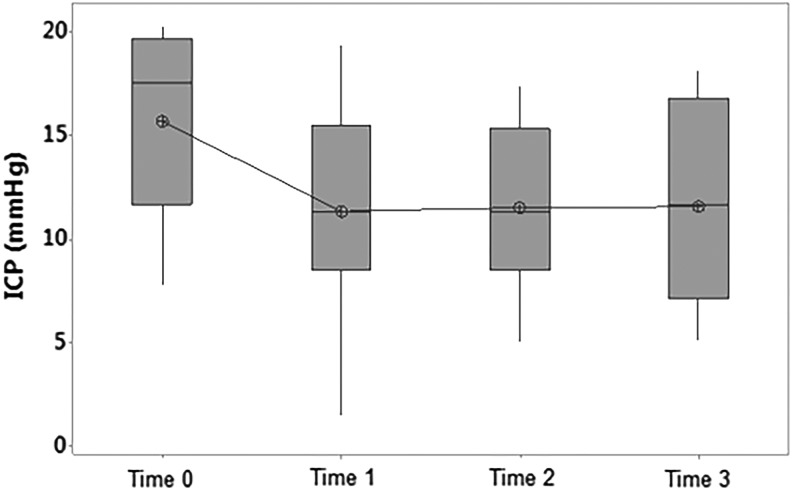
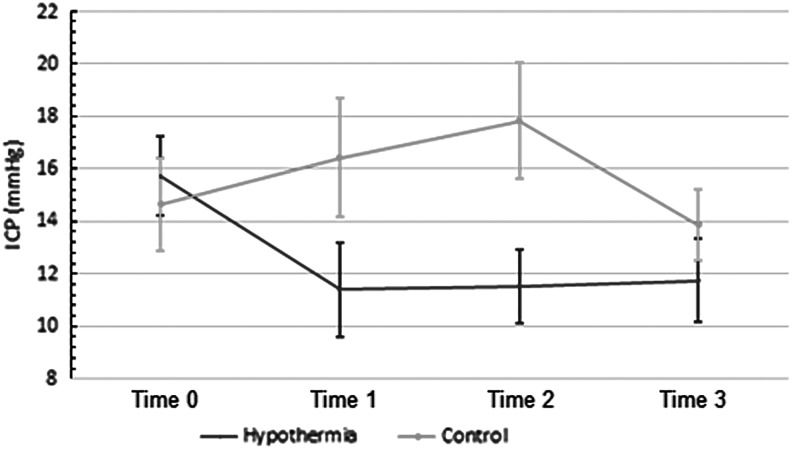
In the intervention group, the ICP decreased by a mean of 4.3±1.6 mmHg (p<0.04) from 15.7 to 11.4 mmHg from Time 0 to Time 1 (Figs. 3 and 4). This decrease in ICP was maintained from Time 0 to Times 2 and 3. A one-way repeated measures ANOVA in the intervention group was consistent with these results: F(3, 24)=6.13, p<0.01. There was no statistically significant difference in ICP in the control group between these times. A two-way repeated measures ANOVA reveals a difference between the two groups at these time points: F(3,45)=4, p<0.02.
FIG. 3.
Boxplot of ICP versus time in the intervention group. Time 0, prerandomization; Time 1, first hour of hypothermia (<35°C); Time 2, first episode of stable hypothermia (<35°C); Time 3, 6 hours of hypothermia (<35°C). Mean decrease in ICP from Time 0 to Time 1=4.3±1.6 mmHg (p<0.04).
FIG. 4.
Changes in ICP with time: intervention and control groups. Time 0, prerandomization; Time 1, first hour of hypothermia (<35°C); Time 2, first episode of stable hypothermia (<35°C); Time 3, 6 hours of hypothermia (<35°C). Two-way repeated measures ANOVA demonstrates a difference between the groups F(3,45)=4, p<0.02. ANOVA, analysis of variance.
Partial brain oxygen tension
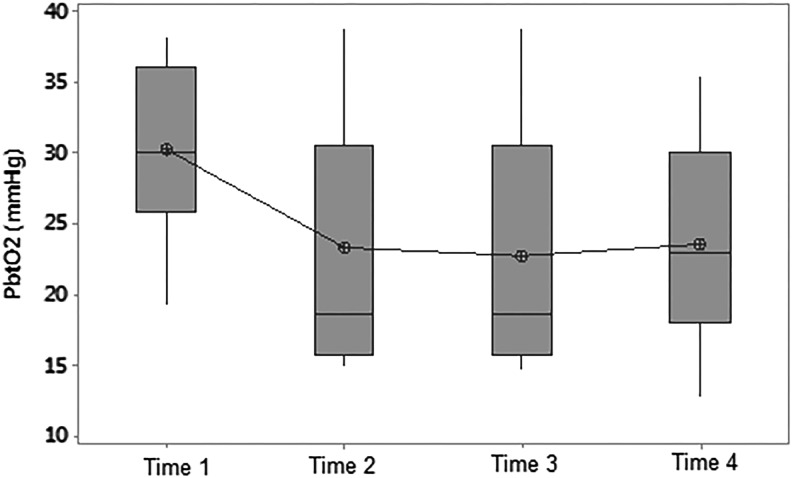
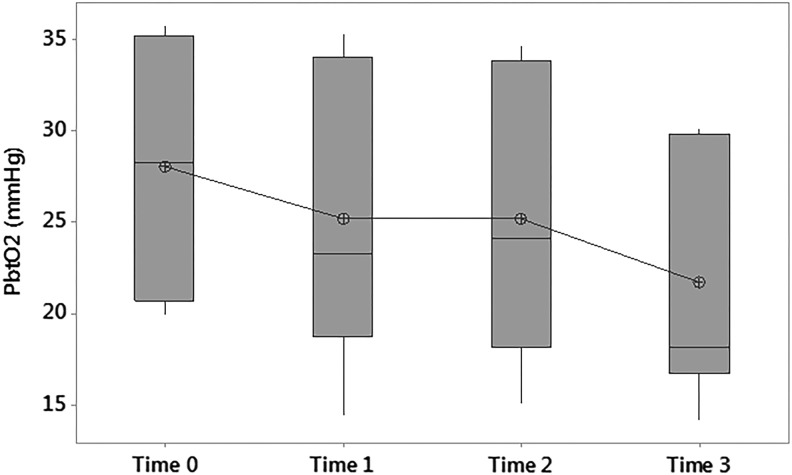
Paired t-tests demonstrated a statistically significant difference between Time 0 and Time 2, where there was a mean decrease in PbtO2 of 7.8±3.1 mmHg (p<0.05) from 30.2 to 22.4 mmHg (Fig. 5). A one-way repeated measures ANOVA for the intervention group also showed a difference in PbtO2 with time F(3,18) 4.60, p<0.02. Three patients in the intervention group had a decrease in PbtO2 from >25 mmHg at Time 0 to <20 mmHg at Time 3.
FIG. 5.
Boxplot of partial brain oxygen tension versus time in the intervention group. PbtO2, partial brain oxygen tension; Time 0, prerandomization; Time 1, first hour of hypothermia (<35°C); Time 2, first episode of stable hypothermia (<35°C); Time 3, 6 hours of hypothermia (<35°C). Mean decrease in PbtO2 from Time 0 to Time 2 of 7.8±3.1 mmHg (p<0.05) from 30.2 to 22.4 mmHg.
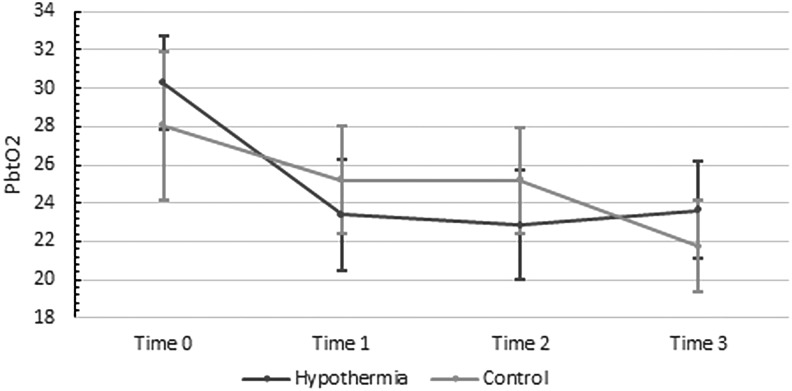
There was no statistically significant difference between Time 0 and any other times in the control group. However, paired t-tests demonstrated a mean decrease of 3.47±1.02 mmHg (p<0.02) between Time 1 and Time 3, and a mean decrease of 3.48±1.27 mmHg (p<0.03) between Time 2 and Time 3 in the control group (Fig. 6). There was no statistically significant difference between the two groups in terms of the change in PbtO2 (Fig. 7).
FIG. 6.
Boxplot of partial brain oxygen tension versus time in the control group. Time 0, prerandomization; Time 1, first hour of hypothermia (<35°C); Time 2, first episode of stable hypothermia (<35°C); Time 3, 6 hours of hypothermia (<35°C). There was no statistically significant difference between Time 0 and the other time points.
FIG. 7.
Changes in partial brain oxygen tension with time: intervention, and control groups. Time 0, prerandomization; Time 1, first hour of hypothermia (<35°C); Time 2, first episode of stable hypothermia (<35°C); Time 3, 6 hours of hypothermia (<35°C). There was no statistically significant difference between the two groups in terms of the change in PbtO2.
Discussion
Intracranial pressure
Starting ICP in this report was less than 20 mmHg (ET3235Trial protocol) because a single hypertonic treatment was given pending randomization which also resulted in long lead times to initiation of cooling. TH reduced ICP by a mean of 4.3±1.6 mmHg from precooling to the first episode of stable hypothermia (∼27%), despite pretreatment with hypertonic therapy. The reduction in ICP was maintained at 6 hours and there was no further decrease with prolonged hypothermia, which is consistent with previous studies (Shiozaki et al., 1993; Schwab et al., 1998). This is a comparable reduction to mannitol and hypertonic saline, which are reported to reduce ICP by 10–51% and have similar efficacy (James et al., 1977; James, 1980; Freshman et al., 1993; Berger et al., 1995; Qureshi et al., 1998, 1999a, 1999b; Sorani et al., 2008).
Neither of these therapies is without risk. Reported adverse effects of mannitol include renal failure, electrolyte abnormalities, acidosis, hypotension, and congestive heart failure. Reported adverse effects of hypertonic saline include renal failure and electrolyte abnormalities in addition to other theoretical concerns (Torre-Healy et al., 2012). Furthermore, hyperosmolar therapy is limited in that mannitol leads to a short-lived reduction in ICP with diminishing returns and the prolonged use of hypertonic saline over 72 hours has been shown to increase mortality (James et al., 1977; McGraw, 1978). Therefore, TH may be beneficial in providing a persistent reduction of ICP. It is not suggested that TH is used as an alternative to hyperosmolar therapies, but rather that it may help to reduce the number of therapies required when used in combination.
TH however, is not a risk-free intervention either, and can be associated with a number of adverse effects, including arrhythmias, electrolyte disturbances, and pneumonia. While pneumonia is often reported following TH, a Cochrane review from 2009 found that the trend toward an increased risk of pneumonia was not significant and the quality of evidence supporting it was poor (Sydenham et al., 2009).
This study supports others in finding that TH reduces ICP, but it does not suggest that outcomes are improved or that the use of hyperosmolar agents is reduced (Table 2). We await the full analysis of the Eurotherm3235 trial that will conclusively demonstrate whether TH improves outcomes or not.
Table 2.
Changes in Intracranial Pressure and PbtO2 with Temperature in Previous Studies
| Study | n | Mean decrease in ICP (mmHg) | Mean change in PbtO2 | Target temperature (°C) |
|---|---|---|---|---|
| Zhi et al. (2003) | 396 | 4.1–6.6 | 32–33 | |
| Clifton et al. (2001) | 392 | 1.75 | 33 | |
| Jiang et al. (2000) | 87 | 9.23 | 33–35 | |
| Marion et al. (1997) | 82 | 4.3 | 32–33 | |
| Qiu et al. (2007) | 80 | 1.2–2.1 | 33–35 | |
| Liu et al. (2006) | 45 | 5.33 | 33–35 | |
| Smrcka et al. (2005) | 38 | 8.07 | 34 | |
| Gal et al. (2002) | 30 | 6 | 32–34 | |
| Lavinio et al. (2007) | 24 | 4.8 | 34 | |
| Sahuquillo et al. (2009) | 23 | 7 | 32.5 | |
| Metz et al. (1996) | 10 | 9.5 | 32.5–33 | |
| Gupta et al. (2002)a | 30 | Decrease 1.0 kPa | <35 | |
| Zhang et al. (2002) | 18 | Increase 19.1 mmHg | 31.5–34.9 | |
| Current study | 17 | 4.3 | Decrease 7.8 mmHg | 32–35 |
Summary of change in ICP and PbtO2 with temperature from previous studies for comparison.
Estimate of results from graphed data.
ICP, intracranial pressure; n, number of participants in each study; PbtO2, partial brain oxygen tension.
Partial brain oxygen tension
Both groups in this report demonstrated a decrease in PbtO2 with time. In the intervention group there was a significant decrease of 7.8±3.1 mmHg from 30.2 to 22.4 mmHg. In the control group, no statistically significant decrease was seen from Time 0 to any other time points. However, it is worth noting that half of the control group were not included in the Time 0 analysis due to missing data (n=4/8). The first 2 hours of data from randomization were not included in the analysis because it coincided with the time we allowed for the oxygen electrode to stabilize. In the remaining patients, PbtO2 recording was already in place before randomization and the data were able to be included.
There was a statistically significant decrease in PbtO2 in the control group between Time 1 and 3 and Time 2 and 3 of 3.47±1.02 and 3.48±1.27 mmHg, respectively. It could be that a statistically significant difference between Time 0 and the other time periods would have been seen if all data were present at Time 0. There was no statistically significant difference between the two groups.
Previous studies have investigated the effect of hypothermia on PbtO2 with conflicting results. Gupta et al. (2002) saw a decrease in PbtO2 in a study of 30 patients cooled to a minimum of 33°C and concluded that decreasing brain temperature below 35°C may impair brain tissue oxygenation. However, it has been argued that the Paratrend 7 (Diametrics Medical, High Wycombe, United Kingdom) sensor used to measure PbtO2 in this study was not corrected for temperature and so did not reflect an accurate PbtO2. In contrast, Zhang et al. (2002) found that mild hypothermia (31.5–34.9°C) increased the mean (SD) PbtO2 from 9.6 (6.8) to 28.7 (8.8) mmHg in 18 patients with severe TBI. Patients in this study received TH within 20 hours following TBI and had PbtO2 recorded within the first 24 hours. Given the very low initial PbtO2 and subsequent increase within 24 hours following injury, it has been suggested that this increase was attributable to early changes in cerebral blood flow (CBF) rather than an effect of hypothermia (Andrews and Gupta, 2003).
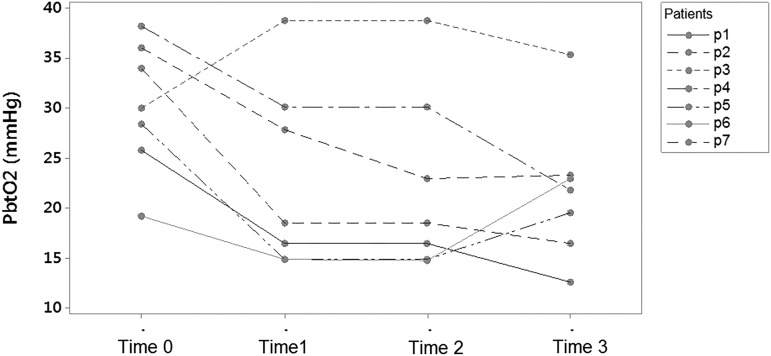
Decreases in PbtO2 have been associated with poor outcomes independent of raised ICP and the combination of both a raised ICP and decreased PbtO2 appears worse than intracranial hypertension in isolation (Chang, 2009). Normal levels of PbtO2 in neurosurgical patients are reported as between 23 and 35 mmHg, with lower values coming from probes sited deeper in brain tissue (Hoffman et al., 1996; Dings et al., 1998; Pennings et al., 2008). While the decrease of PbtO2 in the intervention group is significant, the mean value did not decrease below the threshold of 15 mmHg recommended for intervention by the 2007 BTF guidelines (Brain Trauma Foundation et al., 2007), nor did it decrease below 20 mmHg, the suggested threshold for compromised brain oxygen/moderate brain hypoxia (Le Roux et al., 2014). However, when looking at the individual value plots (Fig. 8) for the intervention group, one can see that three participants demonstrated a change in PbtO2 from above 25 mmHg before cooling to less than 20 mmHg after 6 hours of target temperature. It is unclear why certain patients exhibit this decrease in PbtO2 while others do not, but further investigation is warranted to identify those patients likely to benefit most from targeted temperature management.
FIG. 8.
Individual value plots of PbtO2 versus time. Time 0, prerandomization; Time 1, first hour of hypothermia (<35°C); Time 2, first episode of stable hypothermia (<35°C); Time 3, 6 hours of hypothermia (<35°C).
Arterial blood gases were analyzed using the alpha stat method to maintain autoregulation, which was consistent with current practice in the units that participated in the trial. This approach has been undertaken in other contemporary hypothermia trials, such as NABISH II and TTM (Clifton et al., 2011; Nielsen et al., 2013). Despite this, there was no significant difference in our precooling and target temperature PaCO2 and PaO2 values, which is important given the dependence of PbtO2 upon systemic oxygenation and transport (Rosenthal et al., 2008). If the decrease in PbtO2 was due to changes in PaCO2 and PaO2 with temperature (Gay-Lussac's Law), we would expect to see changes in PaO2 and PaCO2. Therefore, the decrease in PbtO2 may be due to reduced CBF.
Anemia has been associated with a decrease in PbtO2 following TBI (Oddo et al., 2012). The current study found no difference in hemoglobin levels between either the two groups, or in the intervention group at precooling or target temperature to account for the change in PbtO2. The hemoglobin of two patients in the control group and three patients in the intervention group was <9 g/dL, the level at which PbtO2 is thought to be compromised (Oddo et al., 2012). However, in those randomized to the intervention group, the decreased hemoglobin was present before the patients reached target temperature.
Balancing oxygen delivery with demand
Tokutomi et al. (2003) have previously studied the optimal temperature to reduce ICP while resulting in minimal unfavorable outcomes in patients with TBI and concluded that this temperature is about 35°C. In their study from 2002, decreases in ICP were most noticeable between 35°C and 36°C and the incidence of jugular venous bulb oxygen desaturation was also decreased at these temperatures. However, oxygen consumption, measured through indirect calorimetry, and oxygen delivery also progressively decreased. Below 35°C Tokutomi et al. (2003) found that oxygen delivery decreased more than the decrease in oxygen demand resulting in an overall deficit.
A potential explanation for the decrease in ICP with hypothermia is a decrease in CBF (PaCO2 related), which could also explain the decrease in oxygen delivery (Marion et al., 1993; Shiozaki et al., 1993). Hypothermia is thought to decrease cerebral metabolic rate for oxygen (CMRO2) and there may be an associated decrease in oxygen demand (Keller, 2000). While we believe we can reduce ICP and CMRO2, perhaps we are yet to find the balance between decreasing CBF/ICP and maintaining adequate oxygen delivery and are causing unwanted decreases in PbtO2 because of this.
Limitations of the study
The present study has some limitations that need to be considered. Due to the nature of the patient population and TH, it is not possible to blind researchers from the administration of TH. In addition, the researchers were not blinded for the analysis of the two groups which could potentially lead to a bias of analysis.
Although we have attempted to correct the time taken to reach hypothermia by comparing prerandomization values with the different time periods, we must acknowledge that the mean time to target temperature was ∼7 hours. Given that early initiation of TH is considered beneficial in improving efficacy, it could be that a greater decrease in both ICP and PbtO2 would have been seen in the intervention group if the time to reach target temperature was reduced.
Finally, it should be noted that there were missing data. For example, data were not recorded due to a faulty connection attached to the PbtO2 monitor or a connection accidentally detached. Some of the data were missing in a nonrandom manner. An example is missing ICP and PbtO2 data when patients were transferred to the CT scanner because monitors were detached to facilitate patient transfer. Due to the small sample size and relatively small amount of data missing, statistical models to analyze the effect of missing data were not performed. The most apparent effect of the missing data is seen when analyzing PbtO2 at Time 0 in both the control and intervention groups as described above.
Conclusion
TH is an effective addition to the management of intracranial hypertension and could potentially reduce the number of hyperosmolar therapies required. It remains to be seen whether the use of TH, titrated to reduce ICP, will result in improved outcomes in patients following TBI. TH below 35°C could reduce oxygen delivery more than oxygen demand leading to reduced cerebral oxygenation and it is unclear why some patients exhibit a greater decrease in PbtO2 than others. Further analysis of patients enrolled on the Eurotherm3235 trial is required to assess the effects of TH on PbtO2.
Acknowledgments
The authors would like to thank Cat Graham for her help with designing the methods of statistical analysis. The Eurotherm3235 trial is funded by the NIHRH's Technology Assessment Program and J.R. has a Chief Scientists Office NHS research fellowship. Informed written consent was received from participants/their representatives to take part in the Eurotherm3235 trial and subsequent publication of information thereafter. Copies of the consent form are held in the patients' clinical records and in the Welcome Trust Clinical Research Facility, Edinburgh, and are available for review by the Editor-in-Chief upon request.
Author Disclosure Statement
P.J.D.A. is the Chief Investigator of the Eurotherm Trial, which is funded by the National Institute of Health Research's Health Technology Assessment Program. J.R. is the Principal Investigator of the Eurotherm Trial. J.R. and P.J.D.A. are on the speakers' panel for Integra and have participated in educational meetings for Bard Medical. Both of these companies manufacture cooling devices. L.M.C.F. has no competing interests to declare.
References
- Andrews P. Eurotherm3235 Trial PROTOCOL Version 8. 2014. Available at www.eurotherm3235trial.eu/Data/upload/Files/Eurotherm%20Protocol%20v8%209%205%2012%20FINAL.pdf, 2012, accessed April4, 2015
- Andrews P, Gupta AK. Effect of hypothermia on brain tissue oxygenation in patients with severe head injury. Br J Anaesth 2003;90:251–252 [DOI] [PubMed] [Google Scholar]
- Andrews PJ, Sinclair HL, Battison CG, Polderman KH, Citerio G, Mascia L, Harris BA, Murray GD, Stocchetti N, Menon DK, Shakur H, De Backer D; EurothermTrial Collaborators. European society of intensive care medicine study of therapeutic hypothermia (32–35°C) for intracranial pressure reduction after traumatic brain injury (the Eurotherm3235Trial). Trials 2011;12:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrich J, Holzer M, Herkner H, Mullner M. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev 2009;4:CD004128. [DOI] [PubMed] [Google Scholar]
- Berger S, Schurer L, Hartl R, Messmer K, Baethmann A. Reduction of post-traumatic intracranial hypertension by hypertonic/hyperoncotic saline/dextran and hypertonic mannitol. Neurosurgery 1995;37:98–107; discussion 107–108 [DOI] [PubMed] [Google Scholar]
- Brain Trauma Foundation, American Association of Neurological S, Congress of Neurological S. Guidelines for the management of severe traumatic brain injury. J Neurotrauma 2007;24 (Suppl 1):S1–S106 [DOI] [PubMed] [Google Scholar]
- Chang JJ, Youn TS, Benson D, Mattick H, Andrade N, Harper CR, Moore CB, Madden CJ, Diaz-Arrastia RR. Physiologic and functional outcome correlates of brain tissue hypoxia in traumatic brain injury. Crit Care Med 2009;37:283–290 [DOI] [PubMed] [Google Scholar]
- Chesnut RM. Secondary brain insults after head injury: clinical perspectives. New Horiz 1995;3:366–375 [PubMed] [Google Scholar]
- Chesnut RM, Marshall LF, Klauber MR, Blunt BA, Baldwin N, Eisenberg HM, Jane JA, Marmarou A, Foulkes MA. The role of secondary brain injury in determining outcome from severe head injury. J Trauma 1993;34:216–222 [DOI] [PubMed] [Google Scholar]
- Clifton GL, Miller ER, Choi SC, Levin HS, McCauley S, Smith KR, Jr., Muizelaar JP, Wagner FC, Jr., Marion DW, Luerssen TG, et al. . Lack of effect of induction of hypothermia after acute brain injury. N Engl J Med 2001;344:556–563 [DOI] [PubMed] [Google Scholar]
- Clifton GL, Valadka A, Zygun D, Coffey CS, Drever P, Fourwinds S, Janis LS, Wilde E, Taylor P, Harshman K, Conley A, Puccio A, Levin HS, McCauley SR, Bucholz RD, Smith KR, Schmidt JH, Scott JN, Yonas H, Okonkwo DO. Very early hypothermia induction in patients with severe brain injury (the National Acute Brain Injury Study: Hypothermia II): a randomised trial. Lancet Neurol 2011;10:131–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan JD, Selassie AW, Orman JA. The epidemiology of traumatic brain injury. J Head Trauma Rehabil 2010;25:72–80 [DOI] [PubMed] [Google Scholar]
- Corso P, Finkelstein E, Miller T, Fiebelkorn I, Zaloshnja E. Incidence and lifetime costs of injuries in the United States. Inj Prev 2006;12:212–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley S, Reid J, McLatchie R, Hayton J, Clark C, MacDougall M, Andrews P. A systematic review of therapeutic hypothermia for adult patients following traumatic brain injury. Crit Care 2014:18:R75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Georgia MA. Brain tissue oxygen monitoring in neurocritical care. J Intensive Care Med 2014. DOI: 10.1177/0885066614529254 [DOI] [PubMed] [Google Scholar]
- Dings J, Meixensberger J, Jager A, Roosen K. Clinical experience with 118 brain tissue oxygen partial pressure catheter probes. Neurosurgery 1998;43:1082–1095 [DOI] [PubMed] [Google Scholar]
- Freshman SP, Battistella FD, Matteucci M, Wisner DH. Hypertonic saline (7.5%) versus mannitol: a comparison for treatment of acute head injuries. J Trauma 1993;35:344–348 [PubMed] [Google Scholar]
- Gal R, Cundrle I, Zimova I, Smrcka M. Mild hypothermia therapy for patients with severe brain injury. Clin Neurol Neurosurg 2002;104:318–321 [DOI] [PubMed] [Google Scholar]
- Geukens P, Oddo M. Brain tissue oxygen monitoring in neurocritical care. In: Annual Update in Intensive Care and Emergency Medicine 2012. Vol. 2012, Vincent J-L. (ed). Berlin: Springer Berlin Heidelberg, 2012, pp. 735–745 [Google Scholar]
- Gupta AK, Al-Rawi PG, Hutchinson PJ, Kirkpatrick PJ. Effect of hypothermia on brain tissue oxygenation in patients with severe head injury. Br J Anaesth 2002;88:188–192 [DOI] [PubMed] [Google Scholar]
- Hoffman WE, Charbel FT, Edelman G. Brain tissue oxygen, carbon dioxide, and pH in neurosurgical patients at risk for ischemia. Anesth Analg 1996;82:582–586 [DOI] [PubMed] [Google Scholar]
- Hutchinson PJ, Kolias AG, Czosnyka M, Kirkpatrick PJ, Pickard JD, Menon DK. Intracranial pressure monitoring in severe traumatic brain injury. BMJ 2013;346:f1000. [DOI] [PubMed] [Google Scholar]
- Hyder AA, Wunderlich CA, Puvanachandra P, Gururaj G, Kobusingye OC. The impact of traumatic brain injuries: a global perspective. NeuroRehabilitation 2007;22:341–353 [PubMed] [Google Scholar]
- Jaeger M, Dengl M, Meixensberger J, Schuhmann MU. Effects of cerebrovascular pressure reactivity-guided optimization of cerebral perfusion pressure on brain tissue oxygenation after traumatic brain injury. Crit Care Med 2010;38:1343–1347 [DOI] [PubMed] [Google Scholar]
- Jaeger M, Schuhmann MU, Soehle M, Meixensberger J. Continuous assessment of cerebrovascular autoregulation after traumatic brain injury using brain tissue oxygen pressure reactivity. Crit Care Med 2006;34:1783–1788 [DOI] [PubMed] [Google Scholar]
- Jaeger M, Schuhmann MU, Soehle M, Nagel C, Meixensberger J. Continuous monitoring of cerebrovascular autoregulation after subarachnoid hemorrhage by brain tissue oxygen pressure reactivity and its relation to delayed cerebral infarction. Stroke 2007;38:981–986 [DOI] [PubMed] [Google Scholar]
- James HE. Methodology for the control of intracranial pressure with hypertonic mannitol. Acta Neurochir (Wien) 1980;51:161–172 [DOI] [PubMed] [Google Scholar]
- James HE, Langfitt TW, Kumar VS, Ghostine SY. Treatment of intracranial hypertension. Analysis of 105 consecutive, continuous recordings of intracranial pressure. Acta Neurochir (Wien) 1977;36:189–200 [DOI] [PubMed] [Google Scholar]
- Jiang J, Yu M, Zhu C. Effect of long-term mild hypothermia therapy in patients with severe traumatic brain injury: 1-year follow-up review of 87 cases. J Neurosurg 2000;93:546–549 [DOI] [PubMed] [Google Scholar]
- Keller E, Steiner T, Fandino J, Schwab S, Hacke W. Changes in cerebral blood flow and oxygen metabolism during moderate hypothermia in patients with severe middle cerebral artery infarction. Neurosurg Focus 2000;8:e4. [DOI] [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil 2006;21:375–378 [DOI] [PubMed] [Google Scholar]
- Lavinio A, Timofeev I, Nortje J, Outtrim J, Smielewski P, Gupta A, Hutchinson PJ, Matta BF, Pickard JD, Menon D, et al. . Cerebrovascular reactivity during hypothermia and rewarming. Br J Anaesth 2007;99:237. [DOI] [PubMed] [Google Scholar]
- Le Roux P, Menon DK, Citerio G, Vespa P, Bader MK, Brophy GM, Diringer MN, Stocchetti N, Videtta W, Armonda R, et al. . Consensus summary statement of the International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care: a statement for healthcare professionals from the Neurocritical Care Society and the European Society of Intensive Care Medicine. Intensive Care Med 2014;40:1189–1209 [DOI] [PubMed] [Google Scholar]
- Liu WG, Qiu WS, Zhang Y, Wang WM, Lu F, Yang XF. Effects of selective brain cooling in patients with severe traumatic brain injury: a preliminary study. J Int Med Res 2006;34:58–64 [DOI] [PubMed] [Google Scholar]
- Longhi L, Pagan F, Valeriani V, Magnoni S, Zanier ER, Conte V, Stocchetti N. Monitoring brain tissue oxygen tension in brain-injured patients reveals hypoxic episodes in normal-appearing and in peri-focal tissue. Intensive Care Med 2007;33:2136–2142 [DOI] [PubMed] [Google Scholar]
- Marion DW, Obrist WD, Carlier PM, Penrod LE, Darby JM. The use of moderate therapeutic hypothermia for patients with severe head injuries: a preliminary report. J Neurosurg 1993;79:354–362 [DOI] [PubMed] [Google Scholar]
- Marion DW, Penrod LE, Kelsey SF, Obrist WD, Kochanek PM, Palmer AM, Wisniewski SR, DeKosky ST. Treatment of traumatic brain injury with moderate hypothermia. N Engl J Med 1997;336:540–546 [DOI] [PubMed] [Google Scholar]
- McGraw CP, Alexander E, Jr., Howard G. Effect of dose and dose schedule on the response of intracranial pressure to mannitol. Surg Neurol 1978;10:127–130 [PubMed] [Google Scholar]
- Metz C, Holzschuh M, Bein T, Woertgen C, Frey A, Frey I, Taeger K, Brawanski A. Moderate hypothermia in patients with severe head injury: cerebral and extracerebral effects. J Neurosurg 1996;85:533–541 [DOI] [PubMed] [Google Scholar]
- NICE. Therapeutic Hypothermia with Intracorporeal Temperature Monitoring for Hypoxic Perinatal Brain Injury. Available at www.nice.org.uk/guidance/ipg347/resources/guidance-therapeutic-hypothermia-with-intracorporeal-temperature-monitoring-for-hypoxic-perinatal-brain-injury-pdf, 2011. (Retrieved 13th August, 2014)
- Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, Horn J, Hovdenes J, Kjaergaard J, Kuiper M, Pellis T, Stammet P, Wanscher M, Wise MP, Åneman A, Al-Subaie N, Boesgaard S, Bro-Jeppesen J, Brunetti I, Bugge JF, Hingston CD, Juffermans NP, Koopmans M, Køber L, Langørgen J, Lilja G, Møller JE, Rundgren M, Rylander C, Smid O, Werer C, Winkel P, Friberg H; TTM Trial Investigators. Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med 2013;369:2197–2206 [DOI] [PubMed] [Google Scholar]
- Oddo M, Bosel J. Monitoring of brain and systemic oxygenation in neurocritical care patients. Neurocrit Care 2014;21(Suppl 2):103–120 [DOI] [PubMed] [Google Scholar]
- Oddo M, Levine JM, Kumar M, Iglesias K, Frangos S, Maloney-Wilensky E, Le Roux PD. Anemia and brain oxygen after severe traumatic brain injury. Intensive Care Med 2012;38:1497–1504 [DOI] [PubMed] [Google Scholar]
- Oddo M, Levine JM, Mackenzie L, Frangos S, Feihl F, Kasner SE, LeRoux PD. Brain hypoxia is associated with short-term outcome after severe traumatic brain injury independently of intracranial hypertension and low cerebral perfusion pressure. Neurosurgery 2011;69:1037–1045; discussion 1045 [DOI] [PubMed] [Google Scholar]
- Pennings FA, Schuurman PR, van den Munckhof P, Bouma GJ. Brain tissue oxygen pressure monitoring in awake patients during functional neurosurgery: the assessment of normal values. J Neurotrauma 2008;25:1173–1177 [DOI] [PubMed] [Google Scholar]
- Qiu W, Zhang Y, Sheng H, Zhang J, Wang W, Liu W, Chen K, Zhou J, Xu Z. Effects of therapeutic mild hypothermia on patients with severe traumatic brain injury after craniotomy. J Crit Care 2007;22:229–235 [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Suarez JI, Bhardwaj A, Mirski M, Schnitzer MS, Hanley DF, Ulatowski JA. Use of hypertonic (3%) saline/acetate infusion in the treatment of cerebral edema: effect on intracranial pressure and lateral displacement of the brain. Crit Care Med 1998;26:440–446 [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Suarez JI, Castro A, Bhardwaj A. Use of hypertonic saline/acetate infusion in treatment of cerebral edema in patients with head trauma: experience at a single center. J Trauma 1999a;47:659–665 [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Wilson DA, Traystman RJ. Treatment of elevated intracranial pressure in experimental intracerebral hemorrhage: comparison between mannitol and hypertonic saline. Neurosurgery 1999b;44:1055–1063; discussion 1063–1054 [DOI] [PubMed] [Google Scholar]
- Romner B, Grande PO. Traumatic brain injury: intracranial pressure monitoring in traumatic brain injury. Nat Rev Neurol 2013;9:185–186 [DOI] [PubMed] [Google Scholar]
- Rosenthal G, Hemphill JC, 3rd, Sorani M, Martin C, Morabito D, Obrist WD, Manley GT. Brain tissue oxygen tension is more indicative of oxygen diffusion than oxygen delivery and metabolism in patients with traumatic brain injury. Crit Care Med 2008;36:1917–1924 [DOI] [PubMed] [Google Scholar]
- Sahuquillo J, Perez-Barcena J, Biestro A, Zavala E, Merino MA, Vilalta A, Poca MA, Garnacho A, Adalia R, Homar J, et al. . Intravascular cooling for rapid induction of moderate hypothermia in severely head-injured patients: results of a multicenter study (IntraCool). Intensive Care Med 2009;35:890–898 [DOI] [PubMed] [Google Scholar]
- Schwab S, Schwarz S, Spranger M, Keller E, Bertram M, Hacke W. Moderate hypothermia in the treatment of patients with severe middle cerebral artery infarction. Stroke 1998;29:2461–2466 [DOI] [PubMed] [Google Scholar]
- Shiozaki T, Sugimoto H, Taneda M, Yoshida H, Iwai A, Yoshioka T, Sugimoto T. Effect of mild hypothermia on uncontrollable intracranial hypertension after severe head injury. J Neurosurg 1993;79:363–368 [DOI] [PubMed] [Google Scholar]
- Smrcka M, Vidlak M, Maca K, Smrcka V, Gal R. The influence of mild hypothermia on ICP, CPP and outcome in patients with primary and secondary brain injury. Acta Neurochir Suppl 2005;95:273–275 [DOI] [PubMed] [Google Scholar]
- Sorani MD, Morabito D, Rosenthal G, Giacomini KM, Manley GT. Characterizing the dose-response relationship between mannitol and intracranial pressure in traumatic brain injury patients using a high-frequency physiological data collection system. J Neurotrauma 2008;25:291–298 [DOI] [PubMed] [Google Scholar]
- Sydenham E, Roberts I, Alderson P. Hypothermia for traumatic head injury. Cochrane Database Syst Rev 2009;2:CD001048. [DOI] [PubMed] [Google Scholar]
- Tagliaferri F, Compagnone C, Korsic M, Servadei F, Kraus J. A systematic review of brain injury epidemiology in Europe. Acta Neurochir (Wien) 2006;148:255–268; discussion 268 [DOI] [PubMed] [Google Scholar]
- Tokutomi T, Morimoto K, Miyagi T, Yamaguchi S, Ishikawa K, Shigemori M. Optimal temperature for the management of severe traumatic brain injury: effect of hypothermia on intracranial pressure, systemic and intracranial hemodynamics, and metabolism. Neurosurgery 2003;52:102–111; discussion 111–102 [PubMed] [Google Scholar]
- Torre-Healy A, Marko NF, Weil RJ. Hyperosmolar therapy for intracranial hypertension. Neurocrit Care 2012;17:117–130 [DOI] [PubMed] [Google Scholar]
- van der Worp HB, Macleod MR, Kollmar R; European Stroke Research Network for H. Therapeutic hypothermia for acute ischemic stroke: ready to start large randomized trials? J Cereb Blood Flow Metab 2010;30:1079–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J, Zheng P, Tong W, Fang W. Decreased risk of secondary brain herniation with intracranial pressure monitoring in patients with haemorrhagic stroke. BMC Anesthesiol 2014;14:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Zhi D, Lin X, Shang Y, Niu Y. Effect of mild hypothermia on partial pressure of oxygen in brain tissue and brain temperature in patients with severe head injury. Chin J Traumatol 2002;5:43–45 [PubMed] [Google Scholar]
- Zhi D, Zhang S, Lin X. Study on therapeutic mechanism and clinical effect of mild hypothermia in patients with severe head injury. Surg Neurol 2003;59:381–385 [DOI] [PubMed] [Google Scholar]